Identification and analysis of novel recessive alleles for Tan1 and Tan2 in sorghum
- Published
- Accepted
- Received
- Academic Editor
- Diaa Abd El-Moneim
- Subject Areas
- Agricultural Science, Genetics, Genomics, Molecular Biology, Plant Science
- Keywords
- Sorghum bicolor, Tannins, New alleles, Distribution
- Copyright
- © 2024 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Identification and analysis of novel recessive alleles for Tan1 and Tan2 in sorghum. PeerJ 12:e17438 https://doi.org/10.7717/peerj.17438
Abstract
Background
The identification and analysis of allelic variation are important bases for crop diversity research, trait domestication and molecular marker development. Grain tannin content is a very important quality trait in sorghum. Higher tannin levels in sorghum grains are usually required when breeding varieties resistant to bird damage or those used for brewing liquor. Non-tannin-producing or low-tannin-producing sorghum accessions are commonly used for food and forage. Tan1 and Tan2, two important cloned genes, regulate tannin biosynthesis in sorghum, and mutations in one or two genes will result in low or no tannin content in sorghum grains. Even if sorghum accessions contain dominant Tan1 and Tan2, the tannin contents are distributed from low to high, and there must be other new alleles of the known regulatory genes or new unknown genes contributing to tannin production.
Methods
The two parents 8R306 and 8R191 did not have any known recessive alleles for Tan1 and Tan2, and it was speculated that they probably both had dominant Tan1 and Tan2 genotypes. However, the phenotypes of two parents were different; 8R306 had tannins and 8R191 had non-tannins in the grains, so these two parents were constructed as a RIL population. Bulked segregant analysis (BSA) was used to determine other new alleles of Tan1 and Tan2 or new Tannin locus. Tan1 and Tan2 full-length sequences and tannin contents were detected in wild sorghum resources, landraces and cultivars.
Results
We identified two novel recessive tan1-d and tan1-e alleles and four recessive Tan2 alleles, named as tan2-d, tan2-e, tan2-f, and tan2-g. These recessive alleles led to loss of function of Tan1 and Tan2, and low or no tannin content in sorghum grains. The loss-of-function alleles of tan1-e and tan2-e were only found in Chinese landraces, and other alleles were found in landraces and cultivars grown all around the world. tan1-a and tan1-b were detected in foreign landraces, Chinese cultivars and foreign cultivars, but not in Chinese landraces.
Conclusion
These results implied that Tan1 and Tan2 recessive alleles had different geographically distribution in the worldwide, but not all recessive alleles had been used in breeding. The discovery of these new alleles provided new germplasm resources for breeding sorghum cultivars for food and feed, and for developing molecular markers for low-tannin or non-tannin cultivar-assisted breeding in sorghum.
Introduction
Sorghum (S. bicolor (L.) Moench) is the fifth largest food crop in the world and widely used for producing food, feed, brewed beverages and biofuel (Dahlberg, 2019; Zhao et al., 2019). Tannins (also known as condensed tannins or proanthocyanidins) are important for the perception of quality in sorghum, and the tannin content determines the use of sorghum grains. Tannins are widespread in fruits, nuts, vegetables and some cereals (He et al., 2008). During crop domestication and evolutionary processes, tannin production is removed from major cereal crops (such as rice, wheat, and maize) but is retained in finger millet, barley, and sorghum (Zhu, 2019). Tannins have diverse biological and biochemical functions. Higher contents of anthocyanins and tannin compounds in sorghum grains can prevent bird attacks. In China, high-tannin-producing sorghum grains are particularly important in liquor production, accounting for 80% of China’s total sorghum production. The well-known Moutaijiu, Langjiu, Luzhoulaojiao, Wuliangye and several other famous liquors are fermented by using high-tannin-producing sorghum grains as main feedstock (Zhang et al., 2022). Tannins not only inhibit the growth of miscellaneous bacteria but also produce syringic acid and syringaldehyde essential for the unique flavor during the brewing process. Moreover, distinct tannin content can brew different aroma, taste, and flavor liquors. However, high-tannin-producing grains and plants have negative impacts on nutritional value, such as decreasing protein digestibility and feed efficiency in humans and animals (Choi & Kim, 2020; Chung et al., 1998). Meanwhile, low tannin content can promote human health because of high antioxidant capacity and ability to fight obesity through reduced digestion (Cos et al., 2004; Habyarimana et al., 2019). Therefore, non-tannin-producing or low-tannin-producing sorghum cultivars are used in food and feeding production. Thus, it is very important to breed elite sorghum varieties with suitable tannin contents to meet different needs for food, feed, and liquor brewing industry. Tannin-producing and non-tannin-producing (or low-tannin-producing) sorghum cultivars are widely grown worldwide for their different applications and economic values. Evaluating the data on the presence of tannins in 11,557 cultivated sorghum accessions in Africa, approximately 55% are of the non-tannin-producing type and 45% are of the tannin-producing type (Wu et al., 2019). The coexistence of tannin-producing and non-tannin-producing (or low-tannin-producing) sorghum suggests that the elimination of this compound from sorghum grains during domestication is incomplete, exemplifying strong artificial selection against tannins in breeding and production.
Tannins (proanthocyanidins) and anthocyanins are major flavonoid end-products from a well-conserved family of aromatic molecules that have several biological functions in plant development and defense (Gutierrez, Avila & Torres, 2020; Huang et al., 2019; Xie et al., 2019; Xie & Xu, 2019). Tannins are derived from a branch of the flavonoid pathway, as well documented in Arabidopsis. AtTT2/AtTT8/AtTTG1 forms an MBW complex (MYB-bHLH-WD40) to regulate tannin synthesis (Baudry et al., 2004; Li et al., 2020; Schaart et al., 2013; Wang et al., 2017). Using genetic linkage mapping, Tannin1 (Tan1, Sobic.004G280800) and Tannin2 (Tan2, Sobic.002G076600) have been cloned in sorghum (Wu et al., 2019, 2012). Tan1 encodes a WD-40 repeat protein, and Tan2 encodes a bHLH domain protein, both have a regulatory function similar to that of Arabidopsis AtTTG1 and AtTT8. Three loss-of-function alleles each for Tan1 and Tan2 were identified in sorghum, including tan1-a, tan1-b, tan1-c, tan2-a, tan2-b, and tan2-c. Low or no tannins in sorghum grains can result from recessive alleles at one or both of Tan1 and Tan2 loci. A genome-wide association study (GWAS) was used to detect other tannin-related loci to explain natural variation in grain tannin content and pigmentation. Three highly significant association peaks spanning were observed, including 1.16–1.23 Mb (Chr1), 8.075–8.45 Mb (Chr2) and 57.9 Mb (Chr3) (Morris et al., 2013), were different from Tan1 and Tan2. The reported data showed that other genes controlling tannin production may exist.
To develop practical molecular markers for tannin breeding, more Tan1 and Tan2 alleles need to be detected. We used wild sorghum resources, as well as landraces and cultivars, to comprehensively identify the alleles of Tan1 and Tan2. We identified two novel recessive Tan1 alleles and four recessive Tan2 alleles by map-based cloning and sequencing Tan1 and Tan2 coding sequences. These new alleles will provide a solid foundation to study the evolution of Tan1 and Tan2 and their artificial selection in cultivar breeding and provide genetic resources for breeding non-tannin-producing or low-tannin-producing sorghum cultivars.
Materials and Methods
Plant materials
Sorghum accessions include wild sorghum resources, landraces and cultivars from all over the world, were collected from the Sorghum Research Institute, Liaoning Academy of Agricultural Sciences, China. Plants were grown at the experimental site of Liaoning Academy of Agricultural Sciences (Shenyang (41.8°N, 123.4°E)). Each sorghum accession was planted in a single 3-m-long plot with 0.6-m row spacing. Leaf tissue was collected, frozen in liquid nitrogen and stored at −80 °C for DNA extracting. Grains were harvested to determine the tannin contents.
DNA extraction
Leaves from each sorghum accession were sampled for genomic DNA extraction by the cetyltrimethylammonium bromide (CTAB) method as previously described with minor modifications (Allen et al., 2006). Add 2.5 volume of ethanol and incubate at room temperature for 10 min to precipitate DNAs. The mixtures were centrifuged under 13,000 g at room temperature for 10 min to pellet the DNAs. The DNA pellets were directly air-dried at room temperature for 5 min, and then dissolved in nuclease-free water.
PCR, DNA sequencing, and sequence analysis
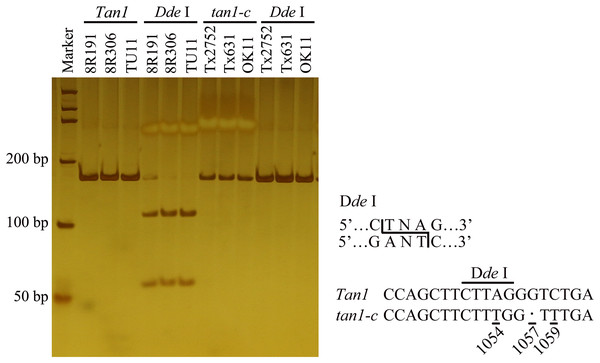
To genotype Tan1 and Tan2 alleles in different sorghum accessions, primers were designed (Table S1). The PCR products were sequenced by Beijing Tsingke Biological Technology Co., Ltd. (Beijing, China). The DNAMAN program (version 5.2.2) was used for sequence alignment and translation of nucleotides into amino acids. Tan1-1F/ Tan1-1R primers were designed for detecting tan1-a and tan1-b (Table S1). To develop the CAPS (cleaved amplified polymorphic sequence) marker to detect tan1-c allele, Tan1-2F/ Tan1-2R combined with Dde I were designed for tan1-c (Table S1). PCR products were digested with Dde I and analyzed by 8% polyacrylamide gel electrophoresis. Because tan1-c lost a Dde I restriction enzyme site as a result of A-to-T transversion at position 1054 in the coding sequence, PCR amplification with Tan1-2F/ Tan1-2R resulted in a 164 bp product (G deletion at position 1057); whereas dominant Tan1 contained a single Dde I site in the corresponding PCR product (165 bp) and was cut into 109 bp and 56 bp fragments. Primers of Tan2-1F/Tan2-1R to Tan2-6F/ Tan2-6R were designed for detecting Tan2 alleles (Table S1).
Determination of tannin content by reagent test kit
Tannin was determined according to the Tannin Microplate Assay Kit (Cohesion Biosciences, CAK1060). Five grams of grains were crushed into powder in a grinder. Tissue samples (0.1 g) were homogenized with 1 ml distilled water, placed in a water bath at 80 °C for 30 min, and centrifuged at 8,000 g at 4 °C for 10 min. The supernatant was placed into a new centrifuge tube for detection. 10 μl sample supernatant, 160 μl distilled water and 20 μl reaction buffer were mixed and incubated for 5 min at room temperature. Then, 10 μl dye reagent was mixed for 10 min, and the absorbance was measured and recorded at 650 nm to calculate the tannin content. The tannin contents were scored as low (≤0.5%), medium (0.5%> tannin content <1.0%) and high (≥1.0%). In this study, medium tannin content accessions were not exhibited.
Mapping population
8R191 was a Chinese landrace without tannins. 8R306 was a landrace from Africa and had tannins in the grains. The RIL population “8R306 × 8R191” was obtained by advancing random individual F2 plants to the F6 generation by single-seed descent with 557 lines.
Chlorox bleach test
Chlorox bleach test was performed previously described with minor modifications (Dykes, 2019). A total of 100 sorghum grains was placed into a 100 ml beaker, and 15 ml 6% NaClO was added to fully immerse sorghum grains. The beaker was left to sit for 20 min at room temperature, and the contents were swirled in the beaker every 5 min. The reaction solution, was discarded then it was rinsed with distilled water 2–3 times, and poured on filter paper to remove excess water. All bleach tests were repeated three times. The presence or absence of tannins in sorghum grains was evaluated based on grain color after dyeing. Sorghum grains were divided into three types: Type I grains were completely black and had tannins, Type II grains were lighter brown black or had small black spots, and Type III grains were white or lightly colored and had no tannins. In 557 RILs, Type I had 269 lines, Type II had 179 lines and Type III had 109 lines. For the accuracy of phenotypic identification, Type I and Type III lines were used to map the new Tannin locus. After dyeing, one grain was selected from one line to use for germinating and the seedling was sampled to extract the genomic DNAs.
Mapping and identification of the candidate gene
Genomic DNAs were extracted from 8R191, 8R306 and RIL population plants using the CTAB method. BSA (Bulked Segregant Analysis) was used to determine the new Tannin locus. Equal amounts of genomic DNAs from 50 tannin (Type I, 50/269) and 50 non-tannin (Type III, 50/109) plants were pooled to construct the tannin and non-tannin bulks, respectively. Whole-genome resequencing was performed using Illumina HiSeq2000 platform by Beijing PlantTech Biotechnology Co., Ltd (Beijing, China). The depths of two bulks and two parental lines were about 50× and 10×, respectively. The reads were aligned to the Sorghum bicolor v3.1.1 reference genome (https://phytozome-next.jgi.doe.gov/info/Sbicolor_v3_1_1) using Burrows-Wheeler Aligner (BWA) software bwa-0.7.10 (Li & Durbin, 2009), GATK toolkit used to detect and filter SNPs (McKenna et al., 2010). The SNPs were employed as input in the R package “QTLseqr” version 0.7.5.2 for subsequent analysis (Takagi et al., 2013). To perform the SNP-INDEX analysis, the window size was set at 1 Mb. The significance of ΔSNP-index was determined at a 99% confidence interval and at least 10 SNPs within the window size. Ten InDel markers within the region were used for fine mapping. Information on molecular markers for fine mapping was provided in Table S1.
Results
Relationships between Tan1 and Tan2 genotypes and tannin contents
The presence of tannins in sorghum grains is regulated by a pair of genes (Tan1 and Tan2), and both genes have three recessive alleles (Wu et al., 2019, 2012). Twenty accessions were used to determine the relationship between Tan1 and Tan2 genotypes and tannin contents in sorghum grains. Primers of Tan1-1F/ Tan1-1R, Tan1-2F/ Tan1-2R and Tan2-1F/Tan2-1R to Tan2-6F/ Tan2-6R were used to detect Tan1 and Tan2 alleles in different sorghum accessions. Tannin presence was scored by the Tannin Microplate Assay Kit for sorghum grains. As shown in Table 1, homozygous recessive genotypes at one or both genes can cause low-tannin phenotypes, and two wild sorghum resources had high tannin contents because they carry dominant alleles, which was consistent with the reported data (Wu et al., 2019). However, six accessions carrying Tan1 and Tan2 dominant alleles had low tannin contents in sorghum grains, indicating that there may be variation in unknown genes contributing to tannin production or new alleles of the known regulatory genes (Table 1).
| Accessions | Tan1 | Tan2 | Phenotype | Origin | Germplasm type |
|---|---|---|---|---|---|
| 8R156 | tan1-a | Tan2 | low-tannin | India | landrace |
| BTx623 | tan1-b | Tan2 | low-tannin | United States | cultivar |
| Tx2752 | tan1-c | Tan2 | low-tannin | United States | cultivar |
| 8R111 | Tan1 | tan2-a | low-tannin | Senegal | landrace |
| 8R035 | Tan1 | tan2-a | low-tannin | Mali | landrace |
| RTx430 | tan1-a | tan2-a | low-tannin | United States | cultivar |
| 8R374 | Tan1 | Tan2 | low-tannin | China | landrace |
| 8R336 | Tan1 | Tan2 | low-tannin | China | landrace |
| JS255 | Tan1 | Tan2 | low-tannin | China | landrace |
| JS257 | Tan1 | Tan2 | low-tannin | China | landrace |
| JS266 | Tan1 | Tan2 | low-tannin | China | landrace |
| JS273 | Tan1 | Tan2 | low-tannin | China | landrace |
| 8R245 | Tan1 | Tan2 | high-tannin | China | landrace |
| 8R249 | Tan1 | Tan2 | high-tannin | China | landrace |
| 8R284 | Tan1 | Tan2 | high-tannin | China | landrace |
| 8R243 | Tan1 | Tan2 | high-tannin | China | landrace |
| 8R446 | Tan1 | Tan2 | high-tannin | China | landrace |
| 8R312 | Tan1 | Tan2 | high-tannin | China | landrace |
| SV1-5 | Tan1 | Tan2 | high-tannin | NA | wild |
| TU11 | Tan1 | Tan2 | high-tannin | NA | wild |
A novel recessive allele of Tan1 in sorghum landrace
To identify new tannin genes or new alleles, a recombinant inbred line (RIL) population was built from 8R191 and 8R306. Sequencing and digestion were used to determine the Tan1 and Tan2 alleles. Amplifying and sequencing by Tan1-1F/ Tan1-1R indicated that 8R191 and 8R306 didn’t have tan1-a and tan1-b (Table S1). A CAPS marker was designed to detect the tan1-c allele. 165 bp PCR products with Tan1-2F/2R primers were digested by Dde I, 109 bp and 56 bp DNA fragments of dominant Tan1. Because of A-to-T transversion at position 1054 in the coding sequence of tan1-c, the PCR products of Tx2752, OK11 and Tx631 remained uncleaved (Table S1, Fig. 1) (Wu et al., 2019). The PCR product of 8R191 and 8R306 can cleave indicating that two parents did not have tan1-c. Six primers were used to detect Tan2 genotypes in 8R191 and 8R306 (Table S1). Tannin presence was scored by the chlorox bleach test for 8R191 and 8R306 grains. 8R191 is a non-tannin landrace and 8R306 is a tannin landrace, both of them carrying Tan1 and Tan2 dominant alleles.
Figure 1: Development of molecular marker for Tan1 and tan1-c in sorghum.
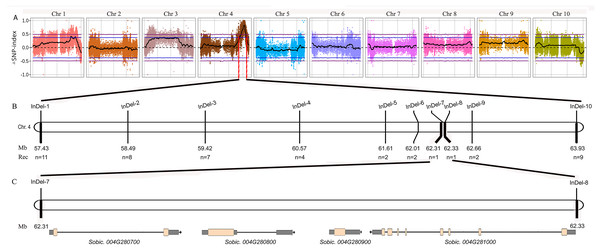
The marker is from Wuhan Servicebio Technology Co., Ltd (GN100bp DNA Ladder I, G3365-01). A-to-T transversion at position 1054 in the coding sequence of tan1-c results in loss of a Dde I restriction site, but that is present in Tan1. The 164 bp PCR product (G deletion at position 1057) from tan1-c is uncleaved, but the 165 bp product from Tan1 is cleaved into 109 bp and 56 bp fragments by Dde I. Tan1: 8R191, 8R306 and TU11 (wild sorghum); tan1-c: Tx2752, Tx631 and OK11 (Wu et al., 2019).We performed BSA using the 8R191/8R306 RIL population lines. Equal amounts of genomic DNAs from 50 tannin and 50 non-tannin plants were pooled to construct the tannin and non-tannin bulks, respectively. The 8R191(non-tannin parent), 8R306(tannin parent), and non-tannin, tannin bulks were subjected to Illumina high-throughput sequencing, from which, 70.99, 72.90, 290.71 and 280.82 million paired-end reads were produced, representing 14×, 14×, 54×, and 53× genome coverage, respectively (Table S2). Among them, 98.23%, 97.88%, 94.35% and 95.91% reads could be mapped to the Sorghum bicolor v3.1.1 reference genome, respectively, indicating good quality of the sequencing data (Table S2). Using the BSA-Seq method, we obtained only one region spanning 6.60 Mb on Chr4 between 57,400,000 to 64,000,000 was strongly associated with the tannin phenotype (Fig. 2A). Within this region, we developed 10 available InDel markers for fine mapping (Fig. 2B). Using a RIL population of 378 plants (Type I: 269 lines and Type III: 109 lines), the new Tannin locus was finally narrowed down to a 25.8 kb region defined by markers InDel-7 and InDel-8. We identified four candidate genes in this region, including Sobic.004G280700, Sobic.004G280800, Sobic.004G280900 and Sobic.004G281000 (Fig. 2C, Table S3). Sobic.004G280800 is Tan1, suggesting that Sobic.004G280800 is likely to be the causal gene. Primers (Tan1-F/Tan1-R; Table S1) were used to detect the sequence polymorphisms in Tan1 between 8R191 and 8R306. 8R306 had dominant Tan1, however, 8R191 had deletion and substitution in coding region of Tan1, and named as tan1-d. Therefore, Tan1 has another new genotype that affects its function.
Figure 2: Fine mapping of the tan1-d.
(A) ΔSNP index plot. ΔSNP index = SNP index (Tannin) − SNP index (Non-tannin). The purple dashed line represents the threshold (0.49) of ΔSNP index. The area above the purple dashed line is the rough mapping interval of tan1-d on Chr4. (B) Distribution of InDel markers in the rough mapping interval. The fine mapping interval is narrowed between InDel-7 and InDel-8. (C) Sobic.004G280700, Sobic.004G280800 (Tan1), Sobic.004G280900 and Sobic.004G281000 are found in the fine mapping interval.Identification and distribution of Tan1 and Tan2 other new alleles
To identify more different Tan1 and Tan2 alleles, we collected 396 sorghum accessions, including wild sorghum resources, landraces and cultivars (Table S4). DNA was extracted using leaves by CTAB method. The PCR primers Tan1-F/Tan1-R were designed for detecting Tan1 alleles. Primers from Tan2-1F/Tan2-1R to Tan2-6F/ Tan2-6R were designed for detecting Tan2 alleles (Table S1). Tannin content was determined according to the Tannin Microplate Assay Kit. According to the coding sequence variation and low-tannin content, the new variation types were determined for Tan1 and Tan2.
Another novel Tan1 recessive allele was found, named as tan1-e. Among 396 sorghum accessions, 10 wild sorghum resources and 200 high-tannin-producing accessions carried dominant Tan1, and 89 low-tannin-producing accessions carried tan1-a, tan1-b, tan1-c, tan1-d, and tan1-e alleles. A total of 97 accessions carried the Tan1 allele, but had low tannin contents, accounting for 24.5% (97/396) of the total sample size (Table 2, Table S4). Maybe there was allelic variation of Tan2 in low-tannin-producing accessions with dominant Tan1. Then seventy-two accessions carrying the Tan1 allele were used to detect different Tan2 alleles, including 38 low-tannin-producing accessions, 24 high-tannin-producing accessions and 10 wild sorghum resources. Ten wild sorghum resources and 24 high-tannin-producing accessions had dominant Tan1 and Tan2 (Table 3, Table S5). Four different recessive alleles of Tan2 were identified, named as tan2-d, tan2-e, tan2-f, and tan2-g (Table 3, Table S5). 24 sorghum accessions carried the dominant Tan1 and Tan2 alleles, but had low tannin contents, accounting for 63.2% of 38 low-tannin sorghum accessions (Table 3). Our analysis indicates that there may be existed other unknown loci involved in tannin production.
| Phenotype | Tan1 | Accession number |
|---|---|---|
| Low-tannin | Tan1 | 97 |
| tan1-a | 46 | |
| tan1-b | 18 | |
| tan1-c | 14 | |
| tan1-d | 9 | |
| tan1-e | 2 | |
| High-tannin | Tan1 | 200 |
| High-tannin (wild) | Tan1 | 10 |
| Total | 396 |
| Phenotype | Genotype | Accession number |
|---|---|---|
| Tan1/Tan2 | 24 | |
| Tan1/tan2-a | 3 | |
| Low-tannin | Tan1/tan2-d | 1 |
| Tan1/tan2-e | 6 | |
| Tan1/tan2-f | 1 | |
| Tan1/tan2-g | 3 | |
| High-tannin | Tan1/Tan2 | 24 |
| High-tannin (wild) | Tan1/Tan2 | 10 |
| Total | 72 |
More importantly, Tan1 and Tan2 recessive alleles had obvious regional distribution characteristics. Novel tan1-d, tan2-d, tan2-f and tan2-g alleles were distributed worldwide, including Ghana, France, Mexico, India, China and so on, but tan1-e and tan2-e were only found in Chinese landraces (Tables S4 and S5). The results implied that some Tan1 and Tan2 recessive alleles had different geographical distributions.
Functional variation of the newly identified Tan1 and Tan2 recessive alleles
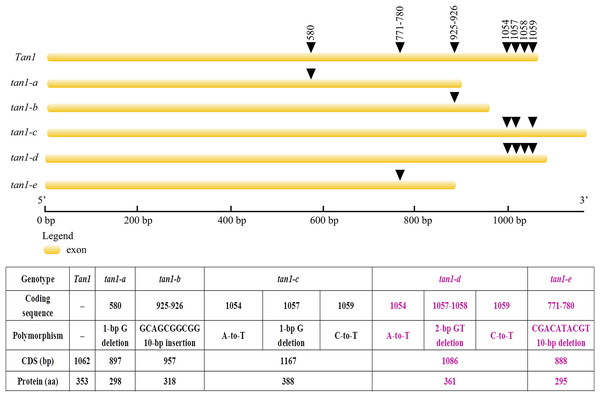
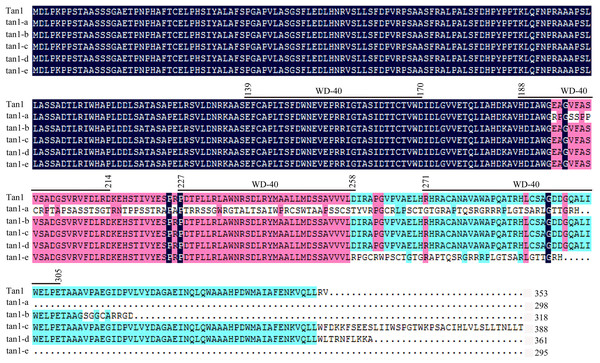
Tan1 and Tan2 are conserved regulatory factors in the plant tannin synthesis pathway and have higher nucleotide similarity within major cereal crops other than rice, wheat and maize, which do not produce tannins in their grains. Tan1 encodes a WD-40 repeat protein and has four WD-40 repeat domains. Deletion, substitution and insertion mutations in tan1-a, tan1-b, and tan1-c have caused frame shifts and premature stop codons, leading to disruption of the highly conserved region of WD-40 domain and C-terminus and resulting in the absence or low level of tannins in sorghum grains. Seven independent mutations of the TTG1 gene reveal that the truncation of the C-terminal region and WD-40 domain produced nonfunctional alleles in Arabidopsis, indicating that the C-terminal region and WD-40 domain are vital for the structure and function of WD-40 protein (Wu et al., 2012). In tan1-d, A-to-T transversion at position 1054, GT deletion at positions 1057 and 1058, and C-to-T transition at position 1059 in the coding sequence affected TGA (at positions 1060, 1061 and 1062) stop codon frameshift and led to nonfunctional protein. Because of mutation and deletion, tan1-d had 1086 bp coding sequence and 361 aa protein sequence. Sequence variation of tan1-d was similar to tan1-c and four WD-40 domains presented, but the C-terminal sequence had changed greatly in tan1-d. Compared to dominant Tan1, tan1-e had a 10-bp deletion (CGACATACGT) in the coding sequence between positions 771 and 780. The 10-bp deletion caused a frameshift mutation and resulted in a truncated protein with a length of only 295 aa. The fourth WD-40 domain and C-terminal region were dramatically changed in tan1-e (Figs. 3 and 4, Fig. S1).
Figure 3: Gene structures and mutation sites for Tan1 alleles.
tan1-a, tan1-b, and tan1-c were reported previously (Wu et al., 2012, 2019) and tan1-d and tan1-e were identified in this work.Figure 4: Amino acid sequence alignment for Tan1, tan1-a, tan1-b, tan1-c, tan1-d, and tan1-e.
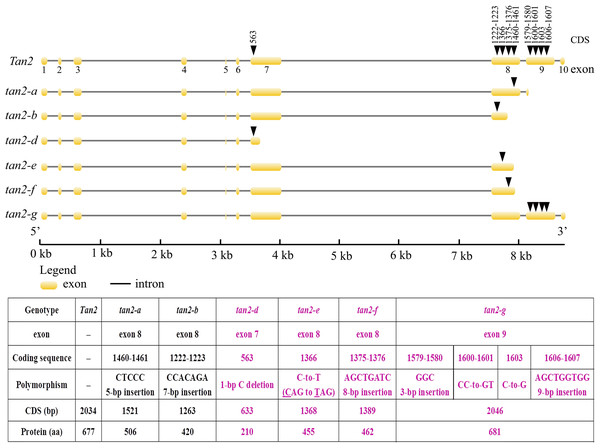
Four WD-40 domains are overlined. The 2nd, 3rd and 4th WD-40 domains are missed in tan1-a and 4th WD-40 domain is missed in tan1-e. Although four WD-40 domains present in tan1-b, tan1-c, and tan1-d, C-terminal sequences have dramatically changed.Tan2 encodes a bHLH transcription factor with 10 exons and nine introns in BTx623. tan2-a has a 5-bp (CTCCC) insertion in the 8th exon, tan2-b has a 7-bp (CCACAGA) insertion in the 8th exon and tan2-c has a 95-bp deletion removing the entire 8th intron (Fig. 5 and Fig. S2). Three mutations lead to frame shift, disrupt the bHLH domain and result in non-tannin-producing or low-tannin-producing phenotype (Wu et al., 2019). tan2-d, with the causal polymorphism of a 1-bp C deletion at position 563 in the coding region, led to a truncated protein with a length of only 210 aa. Because of the C-to-T transition at position 1366 (CAG to TAG) in the coding sequence, tan2-e resulted in premature termination and had a 455 aa protein. tan2-f contained a frameshift mutation and an early termination site because of an 8-bp (AGCTGATC) insertion between positions 1375 and 1376 in the coding region, resulting in a 462 aa protein sequence. tan2-d, tan2-e and tan2-f disrupted the bHLH domain structure and lost function. tan2-g, a null allele, had multiple substitutions and insertions from position 1579 to 1607 in the coding region and didn’t disrupt the bHLH domain structure (Figs. 5 and 6, Fig. S2).
Figure 5: Gene structures and mutation sites for Tan2 alleles.
tan2-a, tan2-b, and tan2-c were reported previously (Wu et al., 2019) and tan2-d, tan2-e, tan2-f, and tan2-g were identified in this work.Figure 6: Amino acid sequence alignment for Tan2, tan2-a, tan2-b, tan2-d, tan2-e, tan2-f, and tan2-g.
The conserved bHLH domain, which is missed in tan2-a, tan2-b, tan2-d, tan2-e, and tan2-f, is overlined. Although bHLH domain present in tan2-g, amino acid sequences between 526 and 537 have greatly changed.Tan1 and Tan2 alleles utilization in breeding programs
By investigating the distribution of Tan1 and Tan2 alleles in sorghum cultivars, we can determine which alleles have been used in breeding. 87 cultivars (from China and foreign countries), as well as 34 sterile lines and 43 restorer lines, were used to detect the different alleles of Tan1 and Tan2 (Tables S4 and S6). For Tan1, only tan1-a, tan1-b and tan1-c alleles were detected in Chinese cultivars, tan1-d and tan1-e may not have been inherited in low-tannin-producing cultivars (Tables S4 and S6). For Tan2, tan2-a, tan2-b and tan2-c were detected in cultivars in the reported data (Wu et al., 2019). In our study, tan2-f and tan2-g alleles were detected in cultivars, and tan2-d and tan2-e alleles were not (Tables S4 and S5). More importantly, tan1-e and tan2-e were only detected in Chinese landraces (Table S5). The results showed that only some Tan1 and Tan2 alleles were applied in breeding, leading to a decrease in the diversity of breeding resources.
Discussion
Wild sorghum resources generally show higher tannin contents than domesticated accessions due to selection during domestication (Dykes & Rooney, 2007). The apparent nutrient absorption and protein digestion issues were reduced by feeding sorghum grains with high tannin content. Breeders mainly rely on grain color to determine the contents of tannins in grains (Rhodes et al., 2014). Sorghum accessions with pigmented testa usually contain condensed tannins. The use of grain color as a proxy for tannin concentration is complicated by the need for varietal information, including pigmented testa and endosperm appearance, which are correlated with tannin levels (Dykes, 2019; Oliveira et al., 2017). In fact, grain color is not a reliable indicator of sorghum tannin contents. Using marker-assisted breeding can simplify and expedite breeding for determining the tannin content. Identifying tannin-related genes and alleles is very important for molecular selection and breeding.
AtTT2, AtTT8 and AtTTG1 form an MBW complex to regulate tannin synthesis (Baudry et al., 2004; Ha et al., 2018; Schaart et al., 2013). Nonfunctional AtTTG1 and AtTT8 proteins impact MBW complex function, which inhibits the expression of DFR, LAR and ANR and hinders tannin synthesis (Shan et al., 2019; Sun et al., 2022; Wei et al., 2019). Tan1 (homologous gene-AtTTG1) and Tan2 (homologous gene-AtTT8) are involved in regulating the tannin synthesis pathway in sorghum, and three recessive alleles each for Tan1 and Tan2 have been reported (Wu et al., 2019, 2012). In our study, two novel recessive alleles for Tan1 and four novel recessive alleles for Tan2 were identified, including tan1-d, tan1-e, tan2-d, tan2-e, tan2-f, and tan2-g (Figs. 3 and 5, Table S7). Because of insertion or deletion in the coding regions of five recessive Tan1 alleles and seven recessive Tan2 alleles, their corresponding Tan1 and Tan2 proteins are nonfunctional and show variable inhibition of tannin accumulation in sorghum grains. These alleles will be useful for marker-assisted breeding for the improvement of low-tannin-producing or non-tannin-producing sorghum cultivars.
The tannin contents of 186 out of 396 accessions were under 0.5%. These accessions were widely distributed in China, India, Africa and other countries (Tables S4 and S5). These low-tannin-producing accessions contain recessive Tan1 and Tan2 alleles or dominant Tan1 and Tan2 alleles. According to the reported data, sorghum accessions with dominant Tan1 and Tan2 alleles should contain high tannins, but in our study the tannin contents of some sorghum accessions with dominant Tan1 and Tan2 alleles were still low, indicating that there maybe unknown genes or new alleles of the known regulatory genes in the tannin synthesis pathway. Furthermore, the identified Tan1 and Tan2 alleles have certain characteristics of regional distribution; for example, tan1-e and tan2-e are only distributed in China (Tables S4 and S5).
There are many different characteristics among Chinese sorghum accessions, African sorghum accessions and Indian sorghum accessions. Heterosis in Chinese sorghum accessions is also different from that in African sorghum accessions and Indian sorghum accessions. However, these data cannot be regarded as evidence of a Chinese or foreign origin for sorghum but can indicate that Chinese sorghum accessions have high diversity and a strong evolutionary history. In our study, tan1-a and tan1-b were not detected in Chinese landraces (Tables S4 and S6). In Chinese sterile and restored lines, 16 materials contained tan1-a allele and 11 materials contained tan1-b allele, respectively (Table S6). Meanwhile, eight Chinese cultivars had tan1-a allele and five Chinese cultivars had tan1-b allele, indicating that tan1-a and tan1-b alleles may come from foreign accessions (Table S4).
The Tan1 genotypes were detected in 145 accessions of foreign sorghum (landraces and cultivars) with low tannin contents, however, there was no tan1-e allele in low-tannin-producing foreign accessions (Table S4). As shown in our data, tan1-e was only detected in two Chinese landraces. The two tan1-e landraces are from Jilin Province and Shanxi Province in China. The genetic background of these two accessions is quite different as there is 700 km between the two provinces. These results suggest that different alleles of Tan1 may have different geographic distributions and selective advantages in sorghum breeding. Tan2 and six recessive alleles (tan2-a, tan2-b, tan2-c, tan2-d, tan2-f, and tan2-g) were found in the United States, West Africa, Western Europe, North America, India, China and other parts of the world. However, the recessive tan2-e allele was only found in Chinese landraces (Table 3, Table S5). Therefore, Tan1 and Tan2 may serve as important clues to study the origin and evolutionary history of Chinese sorghum and foreign sorghum.
Conclusions
In our study, two new allelic variants of Tan1 and four new allelic variants of Tan2 were identified. Up to now, five recessive alleles of Tan1 and seven recessive alleles of Tan2 alleles were found, indicating that Tan1 and Tan2 had abundant allelic variants. This was because of loss-of-function recessive alleles in Tan1 and Tan2, which lead to low or no tannin content in sorghum grain. Only tan1-e and tan2-e were found, tan1-a and tan1-b were not found in Chinese landraces, and other alleles were found in landraces or cultivars worldwide. Some Tan1 and Tan2 alleles have not been used in breeding.
Supplemental Information
Coding sequence alignment for Tan1, tan1-a, tan1-b, tan1-c, tan1-d, and tan1-e.
A G deletion at position 580 nt in tan1-a. 10 bp (GCAGCGGCGG) insertion between 925 and 926 nt in tan1-b. A-to-T (1,054), G deletion (1,057), and C-to-T (1,059) are changed in tan1-c. A-to-T (1,054), GT deletion (1,057 and 1,058), and C-to-T (1,059) are changed in tan1-d, then TGA (1,060, 1,061 and 1,062) stop codon alters. Sequence variation of tan1-d is similar to tan1-c. In tan1-e, 10-bp (CGACATACGT) is deleted in the coding sequence between 771 and 780. Five Tan1 alleles identified so far are shown, including tan1-a, tan1-b, and tan1-c reported previously (Wu et al., 2012, 2019) and tan1-d and tan1-e identified in this work.
Coding sequence alignment for Tan2, tan2-a, tan2-b, tan2-d, tan2-e, tan2-f, and tan2-g.
The unlined sites of difference are synonymous mutations, which don’t affect protein function. 5 bp (CTCCC) insertion between 1,460 and 1,461 nt in tan2-a. 7 bp (CCACAGA) insertion between 1,222 and 1,223 nt in tan2-b. Although 95-bp intron between exon 8 and exon 9 deletion in tan2-c, tan2-c is a recessive allele. In tan2-d, a 1-bp C deletion at position 563 in the coding region led to terminate prematurely. C-to-T transition at position 1,366 ( C AG to T AG) in the coding sequence, tan2-e results in premature termination. 8-bp (AGCTGATC) insertion between positions 1,375 and 1,376 in the coding region, tan2-f has a frameshift mutation and an early termination. tan2-g has multiple substitutions and insertions, containing 3-bp (GGC) insertion between 1,579 and 1,580, CC-to-GT at position 1,600 and 1,601, C-to-G at position 1,603 and 9-bp (AGCTGGTGG) insertion between 1,606 and 1,607 in the coding region. Seven Tan2 alleles identified so far are shown, including tan2-a, tan2-b, and tan2-c reported previously (Wu et al., 2019) and tan2-d, tan2-e, tan2-f, and tan2-g identified in this work.
Genotype of the 77 accessions(34 sterile lines and 43 restorer lines).
A, sterile line; R, restorer line.