Occurrence patterns of sympatric forest wallabies: assessing the influence of structural habitat attributes on the coexistence of Thylogale thetis and T. stigmatica
- Published
- Accepted
- Received
- Academic Editor
- Donald Kramer
- Subject Areas
- Conservation Biology, Ecology, Zoology
- Keywords
- Habitat partitioning, Conservation, Rainforest wallaby, Macropods, Anthropogenic impacts, Forest ecology, Camera trapping, Threatened species management, Disturbance, Sympatry
- Copyright
- © 2024 Smith et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Occurrence patterns of sympatric forest wallabies: assessing the influence of structural habitat attributes on the coexistence of Thylogale thetis and T. stigmatica. PeerJ 12:e17383 https://doi.org/10.7717/peerj.17383
Abstract
Background
We studied the occurrence of two sympatric wallabies, the red-necked pademelon (Thylogale thetis) and the red-legged pademelon (T. stigmatica) in northeastern New South Wales, Australia in relation to structural habitat attributes. At our study site, both species inhabit closed forest environments and have overlapping distributions, but T. thetis leaves the forest at night to graze adjacent grassy forest edges whereas T. stigmatica remains within the forest and browses forest vegetation. The objectives of the study were to investigate how structural attributes of two forest types, wet sclerophyll forest and rainforest, relate to the fine-scale occurrence of these two wallaby species within the forested environment.
Methods
We gathered occurrence data from 48 camera trap stations divided equally between rainforest and wet sclerophyll forest. At each camera point, we also measured a range of structural habitat attributes to determine habitat affiliations for the two Thylogale species. Principal component analyses were used to describe major trends in habitat, and generalised linear models were used to describe the efficacy of the variables in predicting habitat occurrence of each species.
Results
The number of occurrences of Thylogale thetis was significantly greater than occurrences of T. stigmatica, which was driven by significantly greater occurrences of T. thetis in wet sclerophyll forest. There was both spatial and temporal partitioning between the two species; there was a significant difference in the occurrences of the two species at individual cameras and T. stigmatica had a different activity schedule than T. thetis in wet sclerophyll forest, where the latter reached its greatest rate of occurrence. At a finer (camera station) scale, occurrences of T. thetis increased with proximity to roads and grassy edges and at sites that were less rocky and less steep. T. stigmatica occurrence increased in the presence of rainforest elements like vines, palms and ferns, more ground-level cover and tree-fall gaps and at sites with fewer emergent eucalypts.
Conclusion
Our findings have implications for managing these pademelons and their habitats. T. thetis is a common species that was encountered more often than T. stigmatica, and it responded positively to human disturbance like roadsides and grassy edges, presumably because these areas provided good grazing opportunities. By comparison, T. stigmatica is a threatened species, and it responded to natural disturbance like tree-fall gaps where lateral cover was greater, and where rainforest food plants may be more abundant. Our results suggest, therefore, that conservation of the threatened T. stigmatica requires the preservation of intact rainforest.
Introduction
Ecological differences in species that allow for niche partitioning, and therefore coexistence, are manifested in three main ways. Species may differ in the resources on which they specialise (resource partitioning), conversely, they may partition their activity in time (temporal partitioning) and/or they may differ their activity in space (spatial partitioning) (Amarasekare, 2003). Habitat heterogeneity plays a key role in species richness and the ability of species with similar resource needs to co-occur, because an increase in the variety and structural complexity of available habitat types and the resources they support increases available niche space and allows more species to coexist (Stein, Gerstner & Kreft, 2014; Tews et al., 2004).
In forested ecosystems, plant communities largely determine the physical structure of the environment and therefore influence the structure of animal communities, species richness and the coexistence of species. Vegetation may drive the fine-scale spatial distribution of sympatric species by dictating the availability of resources at multiple scales (Kubiak, Galiano & de Freitas, 2015), both spatial and temporal. Drawing on markedly different studies in the same forest type highlights the importance of vegetation; in Brazil’s Atlantic Forest, bat diet was best explained by landscape composition, particularly vegetation density (Oelbaum et al., 2022), while in the same forest, the diversity of ants was explained by a combination of shrub leaf density and tree circumference (Sampaio et al., 2023). Among macropods, Le Mar & Mcarthur (2005) showed that sympatric wallabies in Tasmania had similar food requirements and foraged in the same habitats by night, but that their selection of daytime refuges differed markedly, probably due to their contrasting predator avoidance strategies.
Competitive interactions between species can also play an important role in shaping mammalian community assemblages. Competition may manifest in a number of ways, including behavioural changes, shifts in diet, or differential use of preferred habitat in space and time to avoid competition and other unfavourable encounters (Karanth et al., 2017). Historical competition between sympatric species may shape their current spatial distribution, resource use, and even phenotypic traits. For example, competitive interactions between sympatric bat species may lead to character displacement, where species exhibit phenotypic changes that reduce competition along one or more resource axes (Shi et al., 2018). Equally plausible, however, is that differences in resource use relate to evolutionary pressures developed in isolation before the two species were brought together. Regardless, populations of closely related species are considered to be sympatric even if they are ecologically distinct, provided a high proportion of each population encounters individuals of the other along adjacent or shared ecotones (Mallet et al., 2009).
Anthropogenic fragmentation can also play a role in shaping competitive interactions because fragmentation can alter habitat structure through edge effects and reduction of the overall amount of original habitat, but it can also create new opportunities for species that are pre-adapted to exploit the matrix of modified habitats in a fragmented landscape (Laurance, 1994; Laurance, 1997). Habitat fragmentation can therefore change the way closely related species interact (Valiente-Banuet et al., 2015). In Australian rainforests, fragmentation has a strong impact on mammalian assemblages (Laurance, 1997) and these changes might not be evident until many decades post-disturbance (Laurance, Laurance & Hilbert, 2008).
In Australia, two species of rainforest-dwelling wallaby occur in eastern Australia. The red-necked pademelon (Thylogale thetis) inhabits rainforest as well as other forest vegetation types with a dense understorey in eastern Australia’s subtropics and is most common at forest edges adjacent to pasture (Jarman & Phillips, 1989) where it grazes at night on pasture edge close to the forest. The red-legged pademelon (T. stigmatica) has a wider distribution than T. thetis, occurring from the extreme northern tropics at Cape York in northern Queensland to the mid north coast of New South Wales (Johnson & Vernes, 2008). In the northern part of its range where it occurs in the absence of T. thetis, T. stigmatica spatio-temporally partitions its range, spending diurnal hours resting and browsing within the forest interior and nocturnal hours grazing at the forest-pasture boundaries (Vernes, Marsh & Winter, 1995). When sympatric with T. thetis, T. stigmatica consumes only forest browse (Calaby, 1966; Jarman & Phillips, 1989; Vernes et al., 2006), and in northeastern New South Wales, our recent work has shown that they remain in the forest interior and avoid open grassy areas at the forest edge that are grazed by T. thetis (Smith, Andrew & Vernes, 2022). When sympatric, relative population densities can vary; Johnson (1977) reported T. stigmatica to be less abundant than T. thetis at an upland site at Dorrigo NSW, but McHugh et al. (2019) found the opposite to be the case in the North Coast Bioregion in far north-eastern New South Wales. T. stigmatica also appears to be less abundant in the south of its range (when in sympatry with T. thetis), compared to when it occurs as the sole pademelon species in the north of its range (Vernes, Elliott & Elliott, 2022). Diet and habitat usage by the different species and sub-species of pademelons in eastern Australia is also borne out in studies of dental morphology; while both T. thetis and T. stigmatica have a dental morphology suited to browsing (Sanson, 1989), differences in cranial morphology point towards T. thetis incorporating more grass in the diet than T. stigmatica generally, but for the northern sub-species of T. stigmatica to graze more than the southern sub-species (Mitchell et al., 2018).
In a recent study, we showed that T. thetis and T. stigmatica demonstrated strong spatiotemporal niche partitioning (Smith, Andrew & Vernes, 2022). The objectives of the current study were to investigate the broad-scale patterns of occurrence of these species in wet sclerophyll forest and rainforest, how the structural attributes of these forest types relate to their finer-scale occurrence, and whether these patterns are suggestive of habitat partitioning by these sympatric species.
Materials & Methods
Study animals and study site
Two forest-dwelling pademelons (T. thetis and T. stigmatica) were the focal species for this study. Both are medium-sized macropods (T. thetis: males 2.5–9.1 kg, females 1.8–4.3 kg; T. stigmatica: males 3.7–6.8 kg, females 2.5–4.2 kg) that require rainforest or other closed forest vegetation for shelter and diurnal browsing (Eldridge & Coulson, 2015; Johnson & Vernes, 2008). Pademelons occupy small, stable home ranges within which they make daily habitual movements between shelter and feeding sites (Johnson, 1980; Vernes, Marsh & Winter, 1995). Both species breed year-round (Johnson, 1977; Johnson & Vernes, 1994), and neither diel activity patterns (Smith, Andrew & Vernes, 2022) or diet (Johnson, 1977; Vernes, 1995) are influenced substantially by season. Although similar in overall appearance, these species are easily distinguished by their colour patterns when white-flash photography is used, as was done exclusively in this study. T. thetis has an unmistakable rufous-coloured neck and shoulders (without rufous-coloured body and legs) and a faint white cheek stripe, whereas T. stigmatica has conspicuous rufous face, flanks and hind legs (and no rufous-coloured neck and shoulders), and a prominent white cheek stripe.
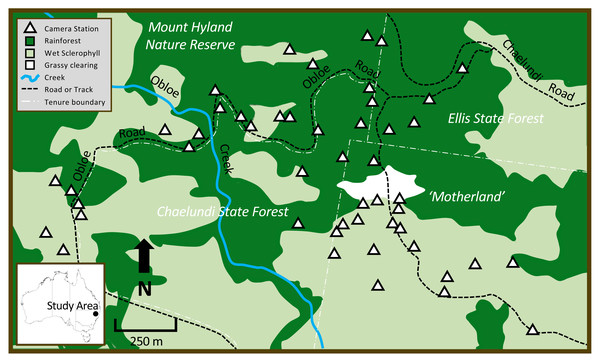
The study occurred in the Mount Hyland region of northeastern NSW, Australia, on the eastern slopes of the Great Dividing Range (study site centre: −30.165403°, 152.470407°; elevation range: 900–1,040 m). The area has a mild climate, with a mean maximum temperature of 20 °C and a mean minimum temperature of 10 °C (Australian Bureau of Meteorology, 2018). The study area of approximately 400 ha spanned private land, state forest and nature reserve (Fig. 1). Three major vegetation types occurred at the site: Northern Warm Temperate Rainforest (a wet closed forest of non-sclerophyllous tree species, with an open shrub layer, and some epiphytes and lianas; hereafter ‘rainforest’), Northern Hinterland Wet Sclerophyll Forest (a tall, open eucalypt forest with an open shrubby understorey; hereafter ‘wet sclerophyll forest’) and an anthropogenic grassy area clear of trees or shrubs (Fig. 1). At the centre of the study area, a private parcel of land called ‘Motherland’ (now part of Hyland Nature Reserve) comprised native forest vegetation situated around a grassy clearing; forest on the southern side of the clearing was predominantly wet sclerophyll forest with a rainforest understorey, while rainforest dominated the northern side of the clearing. This privately-owned forested area was continuous with the larger surrounding state forests and nature reserve comprising a mix of wet sclerophyll forest and rainforest (Fig. 1). At the time the research was undertaken, the study area was privately owned and not accessible to people other than those directly involved in the research.
Figure 1: The study area in northeastern New South Wales, showing patterns in vegetation type across different land tenures, major site features, and camera trap locations.
Dark green = rainforest, light green = wet sclerophyll forest, white = grassy clearing. White triangles show position of the 48 cameras that were deployed in rainforest and wet sclerophyll forest.Ethics approval for this research was obtained from the University of New England Animal Ethics Committee (Approval No. AEC-1708), and scientific licences for this research were issued by the New South Wales (NSW) Office of Environment and Heritage (Permit Nos. SL101721 and SL101837). While ethical approval specific to human subjects being caught incidentally on camera is not required in NSW, our standard procedure is to immediately delete any images of human subjects. However, as we set camera traps off-trail within a study site restricted by locked gates, no human subjects other than those directly associated with the project were captured.
Camera trap placement
Camera trapping methods employed were as previously described by Smith, Andrew & Vernes (2022). We set forty-eight Scoutguard and UOVision white flash cameras in total: 24 were in wet sclerophyll with a rainforest understorey and 24 were in rainforest (Fig. 1). Local fire trails allowed access to the forest habitat; these tracks were narrow and maintained an overstorey that limited the growth of non-forest vegetation. Random placement of cameras was achieved by partitioning access trails throughout the site into 100 m segments. At the end-point of each segment, we generated a random compass bearing (between 0–360°) and a corresponding random distance of between 0–300 m that directed us to where the camera would be placed. Cameras were positioned on the nearest tree to the randomly-allocated point, approximately 0.5 m above ground level and facing south. Baits were used with the purpose of prolonging the time an animal spent in front of a camera; these consisted of a porous PVC canister containing cotton wool soaked with truffle oil placed about 2 m in front of each camera. Baits were inaccessible to animals. Approximately 80% of the LED flash bulbs on each camera were covered with adhesive tape to prevent photos taken in low-light or at night being ‘washed out’ by excessive illumination of animal subjects at close range. Vegetation in the zone between camera and bait was pruned judiciously to avoid false triggers. Camera batteries, SD cards and baits were replaced every 8–12 weeks during a 425-day deployment from 20 January 2017 to 21 March 2018.
Independence of camera trap images
To ensure temporal independence of records of animals at a camera trap, we took a conservative approach by considering all of the photos taken of each species during the course of a 24-hour period as one independent record of the animal at that camera. We then corrected these records for uneven numbers of camera trap nights (due to the occasional camera that failed prior to the periodic battery replacements and downloading of data) to yield independent records per 100 camera trap days. In all subsequent analyses, therefore, with the exception of our representations of temporal activity (see below), ‘occurrence’ indicates the number of independent records (i.e., days) per 100 days of either T. thetis or T. stigmatica over the 425-day study. The only exception to this was in the calculation of temporal activity patterns; because an understanding of temporal activity patterns requires knowing all times of the 24-hour cycle an animal is active, we considered an ‘independent temporal event’ to be any photo of the same species at a camera separated temporally by a time gap of more than 30 min.
Habitat variables
Habitat variables were assessed at each camera point within a 5 m × 5 m plot centred on the camera trap (Table 1). The slope of each site was measured using a clinometer. The combined forest canopy and sub-canopy cover was measured using a ‘Model C’ concave spherical crown densiometer (Forest Densiometers, Rapid City, South Dakota) that estimated cover based on how many of the 24 cells on the densiometer were obscured by vegetation. Four readings were taken (one in each cardinal direction from the plot centre) and a mean value calculated. Sub-canopy foliage cover was measured using an ocular tube at random points around the perimeter of the 5 m × 5 m plot, with random whole numbers between 1 and 10 used to determine the number of steps between each of the 10 measurement points. At each point, the sub-canopy was viewed through the tube’s cross-hairs and scored as either ‘1’ (cross-hairs intersecting vegetation) or ‘0’ (cross-hairs intersecting open sky). Lateral density was estimated using a 1 × 1-m white sheet positioned on the perimeter of the plot at each of the four cardinal directions. For each measurement, an observer would stand with their back to the centre reference tree while the white grid sheet was held vertically with one edge in contact with the ground. Percentage cover was calculated by counting the number of squares obscured by vegetation. Leaf litter was measured on a 0–3 ranked scoring system according to depth. Vines, palms and ferns were measured on a 0–3 ranked scoring system according to density per square metre. The number of woody stems in two size classes were estimated according to 0–3 ranked scoring system. Trees were classed according to their diameter at breast height (DBH) then scored according to density. Rockiness of soil was also measured using a 0–3 ranked scoring system. Number of eucalypt emergents and any tree-fall gaps were counted within each plot. Distance to nearest road, forest edge and major water source (permanent creek) were also measured using a map and estimated to the nearest metre. The degree of decay of large logs and other fallen timber was scored according to a five-point scale outlined by Maser et al. (1979) who used bark characteristics, presence or absence of twigs, wood texture, log shape, wood colour and portion of log on the ground to estimate the degree of decomposition. No disturbances (e.g., fire, flood, domestic animal grazing) occurred in the study area during the study, and all measured variables were expected to have remained constant at any one site over the duration of the work.
| Measurement | Unit or score | Description |
|---|---|---|
| Leaf litter depth | 0–3 | 0: absent, 1: <5 cm, 2: 5–10 cm; 3: >10 cm |
| Vines, Palms, Ferns | 0–3 | 0: absent, 1: <3 per m2 , 2: 3–5 per m2 ; 3: >5 per m2 (for each) |
| Rockiness of soil | 0–3 | 0: absent, 1: <3 per m2 , 2: 3–5 per m2 , 3: >5 per m2 |
| Fallen Timber | 0–4 | see Maser et al. (1979) |
| Ground Cover | 0–4 | 0: absent, 1: 1–25%, 2: 26–50%, 3: 51–75%; 4: >75% |
| Lateral cover | % | % of 1 × 1 m white grid obscured by 0–1 m high vegetation at a distance of 5 m |
| Canopy cover | % | Estimated using concave spherical crown densitometer |
| Sub-canopy cover | % | calculated from 10 presence/absence random measurements using ocular tube |
| Small stem density (>10 cm dbh) | 0–3 | 0: absent, 1: <3, 2: 3–5; 3: >5 |
| Medium stem density (10–30 cm dbh) | 0–3 | As for small stem density |
| Slope | % | Evaluated using clinometer |
| Tree fall gaps | No. of gaps | in entire plot |
| Eucalypt emergent | No. of emergent | in entire plot |
| Distance to Nearest Road | m | Linear distance |
| Distance to edge | m | Linear distance |
Statistical analysis
All analyses were undertaken using R (R Core Team, 2020). A two-way ANOVA (using the package ‘stats’) was used to compare the occurrences of each species at cameras from the two forest sites. For this analysis, we only included those cameras that had detected at least one or the species during the study. Data were log transformed and residuals were analysed using a Shapiro–Wilk Normality Test to confirm assumptions of normality. Principal Component Analysis (PCA) was undertaken using the package ‘stats’ and visualized using the package ‘ggbiplot’. PCA examines quantitative associations between a group of variables, summarizing them parsimoniously into fewer variables, or ‘components’. We summarised the 17 habitat variables into components that described major trends in habitat. PCA loading scores, determined for each camera location, were used to establish the impact of individual variables on each principal component. Generalised linear modelling was then used to determine the efficacy of each component in predicting the fine-scale habitat occurrences of each Thylogale species, the latter expressed as the total number of independent detections made of T. thetis and T. stigmatica at each camera trap. The partitioning of occurrences of the two pademelon species in space were tested using a paired t-test with species occurrence data paired by camera location. Temporal activity patterns were visualised by creating polar plots of independent temporal events of pademelons on camera throughout the 24-hour cycle using the package ‘ggplot2’. Times were binned into 2-hour hourly bins (e.g., 00:00–02:00 h, 02:00–04:00 h).
Results
Structural variation of habitat types
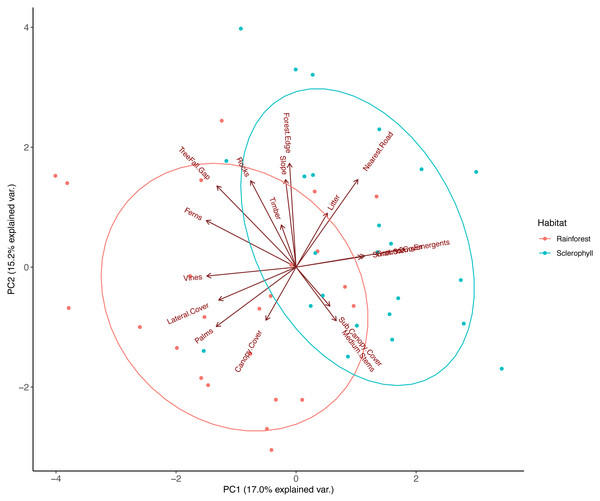
PCA on pooled data between wet sclerophyll forest and rainforest reduced habitat variables into three major principal components that explained 42% of the total variation in vegetation structure. PC1, which explained 17% of the total variance, was correlated negatively with vines, palms, ferns, lateral cover and tree fall gaps, and positively with eucalypt emergents (Fig. 2; Table 2). PC2, which explained 15.2% of the total variance, was positively correlated with increasing distance to the nearest road, distance to the grassy forest edge, the number of treefall gaps, increasing slope and increasing rockiness (Fig. 2; Table 2). PC3, which explained 10% of total variance, was positively correlated with decay class of fallen timber, sub-canopy cover, and medium stem density, and negatively correlated with number of emergent eucalypts and density of ground cover (Table 2). Ecologically, PC1 therefore described a transition from rainforest (more palms, vines, ferns and lateral cover) to more sclerophyll-dominated forest (e.g., more emergent eucalypts), PC2 described a trend from human disturbance (increasing distance to roads and grassy edges) to more natural disturbance (treefall gaps), and we interpreted PC3 to describe a trend in historical rainforest disturbance (perhaps from logging), where fallen timber in the plot was heavily decayed, medium stems density was high, and there was dense sub-canopy cover.
Figure 2: Principal component analysis (PCA) of habitat data measured at each camera trap location.
The components (PC1 and PC2) were extracted from an original dataset comprising 17 biotic and landform variables that were chosen to reflect fine-scale habitat differences across the study area, with each point representing the score for a camera trap location. Ellipses represent 95% confidence level for a multivariate t-distribution.| Measurement | PC1 | PC2 | PC3 |
|---|---|---|---|
| Leaf litter depth | |||
| Vines | * (–) | ||
| Palms | * (–) | ||
| Ferns | * (–) | ||
| Rockiness of soil | * (+) | ||
| Fallen Timber | ** (+) | ||
| Ground Cover | * (–) | ||
| Lateral cover | * (–) | ||
| Canopy cover | |||
| Sub-canopy cover | * (+) | ||
| Small stem density | |||
| Medium stem density | ** (+) | ||
| Slope | * (+) | ||
| Tree fall gaps | * (–) | * (+) | |
| Eucalypt emergent | ** (+) | * (–) | |
| Distance to Nearest Road | * (+) | ||
| Distance to edge | ** (+) |
Habitat correlates of T. thetis occurrence
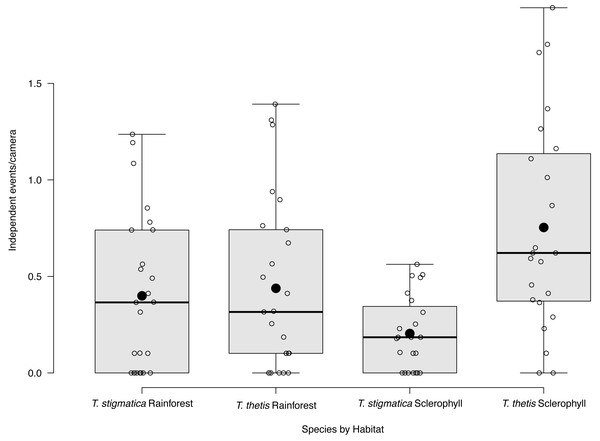
Eight of the 48 cameras did not capture images of T. thetis on any of the 425 days of the study. Nineteen cameras photographed T. thetis on 1–10 days, seven cameras photographed T. thetis on 11–20 days, 12 cameras photographed T. thetis on 20–100 days and two cameras photographed T. thetis on more than 100 days (186 and 280 days). T. thetis occurred in both forest types, with no significant different between the number of independent events at cameras located in rainforest versus wet sclerophyll forest (Fig. 3). The occurrence of T thetis was significantly negatively correlated with PC2 (t = −2.985; P = 0.005) suggesting that T. thetis were more likely to occur at sites near roads and grassy edges, with few treefall gaps.
Figure 3: Box and whisker plot of mean occurrence rate of red-necked pademelons (Thylogale thetis) and red-legged pademelons (T. stigmatica) at cameras located in rainforest or wet sclerophyll forest.
Open circles denote occurrence rate (days a species was encountered at a camera); closed circles show the mean. Boxes show the first to the third quartile, whiskers show the range, and black horizontal lines show the median.Habitat correlates of T. stigmatica occurrence
Fifteen of the 48 cameras did not capture images of T. thetis on any of the 425 days of the study. Cameras at a majority (N = 26) of the 48 sites photographed T. stigmatica on 1–10 days, three cameras photographed T. stigmatica on 11–20 days and four cameras photographed T. stigmatica on 20–100 days. The number of independent detections of T. stigmatica was not significantly different between cameras located in rainforest compared with those in wet sclerophyll forest (p = 0.15; Fig. 3). At a finer scale, independent detections of T. stigmatica were negatively correlated with PC1 (t = −2.643; P = 0.01), suggesting that T. stigmatica occupied forest containing elements more consistent with rainforest such as vines, palms and ferns that provided dense lateral cover. These sites also had few or no eucalypt emergents, but did contain natural treefall gaps.
Temporal and spatial partitioning
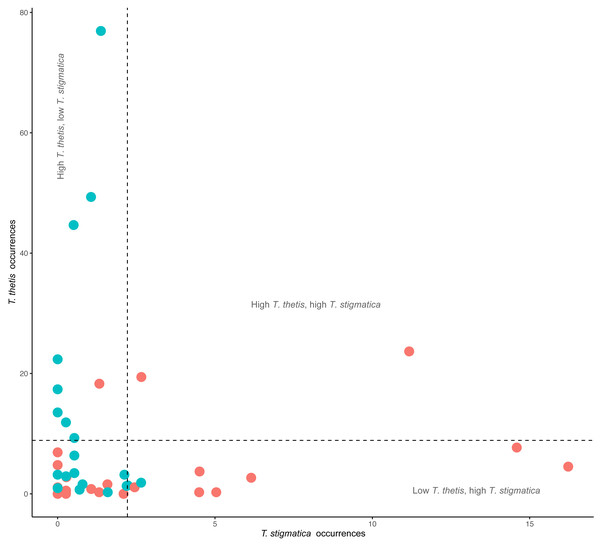
Comparisons of the occurrence of T. thetis and T. stigmatica at individual cameras at which at least one of the species was detected showed a significant negative relationship between the two pademelon species (t = −2.708, df = 41, P = 0.01); at cameras where T. thetis occurrence was relatively high, T. stigmatica occurrence was relatively low and vice versa (Fig. 4). Twenty-two cameras also returned low occurrence (less than the mean value) for both species, but only two had relatively high occurrences (greater than the mean value) of both species (Fig. 4).
Figure 4: Comparison of the occurrence rate of red-necked pademelons (T. thetis) and red-legged pademelons (T. stigmatica) at each of the 48 camera traps (24 in rainforest and 24 in wet sclerophyll forest).
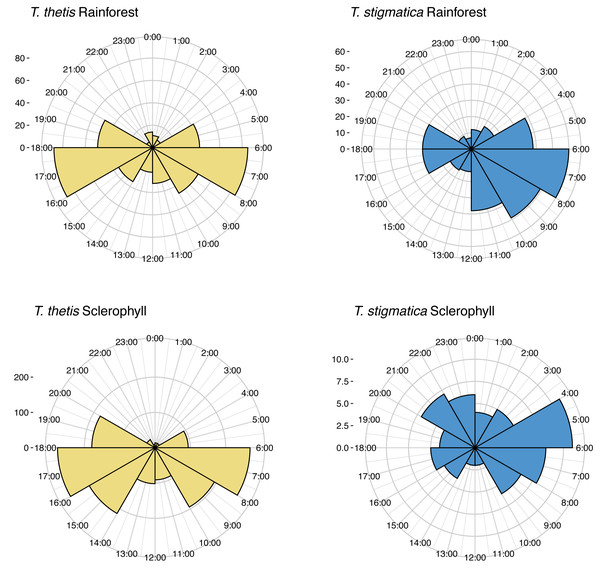
Blue circles are occurrence (total days a species was encountered at a camera) values for cameras located in wet sclerophyll forest; red circles are occurrence values from cameras located in rainforest. Dashed lines show the mean occurrence for each species, with text denoting zones of low (less than the mean) or high (greater than the mean) occurrence of each species.Across the study site, T. thetis had higher rates of occurrence than T. stigmatica (F1,80 = 13.06 p < 0.001); while the two pademelon species had similar rates of occurrence in rainforest, in wet sclerophyll forest the occurrence rate of T. thetis was significantly greater than that of T. stigmatica (p < 0.0001; Fig. 3). In both forest types, T. thetis maintained an activity pattern where activity peaked at dusk (16:00–18:00 h) and dawn (06:00–08:00 h; Fig. 5). In rainforest, T. stigmatica was also most active at dawn, but with activity that extended into the late morning (08:00–12:00 h). However, in wet sclerophyll forest (where T. thetis had significantly greater occurrences than T. stigmatica; see Fig. 3), T. stigmatica was most active before dawn (04:00–06:00 h) and after dusk (08:00–24:00 h); times that corresponded with low T. thetis activity (Fig. 5).
Figure 5: Polar plots of independent occurrences of red-necked pademelons (T. thetis) and red-legged pademelons (T. stigmatica) at cameras in rainforest or wet sclerophyll forest over the 24-hour cycle.
Occurrences are grouped into 2-hour bins for ease of comparisons. Independence for occurrences was defined as sets of photographs taken more than 30-mins apart. The ‘count’ on the y-axis provides a scale for the number of independent events comprising each graph.Discussion
T. thetis and T. stigmatica demonstrated a degree of habitat partitioning at our study site, with occurrences of each species aligning with particular combinations of habitat variables. T. thetis occurred more commonly in locations close to grassy forest edges and forest tracks rather than rainforest or wet sclerophyll forest. This result is easily interpretable in light of the occurrence of T. thetis at the abundant grassy resources found at forest edges (Johnson, 1980); by contrast, T. stigmatica at our study site has been shown by Smith, Andrew & Vernes (2022) to stay within the forest and not venture onto pasture. From our current study, we infer that T. stigmatica was more likely to occur in habitat containing rainforest elements (either rainforest, or sclerophyll forest with a rainforest understorey), with specific affiliation for vines, palms and ferns, and for sites with treefall gaps where vegetation at ground level was dense.
Home ranges typically scale with body size (Harestad & Bunnell, 1979); accordingly, small animals will perceive their environments at a fine scale and will be more sensitive to fine-scale vegetation structure and immediate landscape heterogeneity (Stirnemann et al., 2015). Sapling density, sub-canopy cover and medium stem density all provide sub-canopy cover for a ground-dwelling animal, and are examples of fine-scale measures (10s of metres) of vegetation heterogeneity. Because small-medium macropods shelter repeatedly at the same sites (Jarman, 1991), predator avoidance often depends on the ability to flee to a familiar location via known escape routes. Dingoes and wild dogs (Canis familiaris) are known to hunt pademelons (Vernes, 2000), and pademelons rely on dense vegetation to obscure them from such predators, particularly during the daylight hours (Le Mar & Mcarthur, 2005). Unlike other small macropods, T. stigmatica are active throughout much of the day and can move extensively throughout the forested parts of their range in daylight hours (Vernes, Marsh & Winter, 1995) in search of favoured rainforest browse (Vernes, 1995). Multilayered dense ground-layer rainforest vegetation would help to obscure T. stigmatica from predators, but would also offer them feeding opportunities for known food plants that include vines and ferns (Vernes, 1994). Treefall gaps would similarly offer browsing pademelons a diversity of pioneer species that thrive in high light conditions created by a treefall. T. stigmatica may frequent sites with these qualities more often due to the fitness benefits provided at a fine scale, such as cover from predators.
Our findings detected more independent events for T. thetis than T. stigmatica, however, T. thetis did not occur in one forest type more than another. Rather, detections of T. thetis were associated with disturbance attributes like edges and roads, where grasses grow in the greatest abundance. This is consistent with previous findings that T. thetis exploit forest-pasture boundaries (Jarman & Phillips, 1989; Johnson, 1980; Wahungu, Catterall & Olsen, 1999) and our related study (Smith, Andrew & Vernes, 2022) that showed T. thetis was strongly crepuscular, with periods of heightened activity corresponding with foraging excursions to and from the forest edge and adjacent pasture.
Effects of fragmentation include both declines and increases in the abundance of some species due to alterations to the microclimate within the fragment (Turton & Freiburger, 1997). Our findings are consistent with earlier research that showed T. stigmatica does not venture past the forest edge when in sympatry with T. thetis (Jarman & Phillips, 1989; Smith, Andrew & Vernes, 2022); by comparison, T. thetis makes regular foraging excursions beyond the edge into the adjacent pasture (Smith, Andrew & Vernes, 2022). Fragmentation increases the relative amount of edge to interior forest and significantly alters habitat at fragment edges (Laurance et al., 2002). Accordingly, T. thetis should occur at greater density than T. stigmatica at our study site because the site had a large grassy clearing at its centre, and roads and tracks that bisected the site, some of which offered grassy edges. Rather than associating with ubiquitous anthropogenic edges like roads and clearings, T. stigmatica may instead associate with tree-fall gaps that offer browsing opportunities for rainforest pioneer species away from grassy clearings.
When considering the conservation of species, understanding of habitat affiliation and niche utilisation is of obvious and paramount importance (Vernes, 2003). Competition avoidance in the form of altered use of space and temporal activity are likely employed by pademelons at our study site to facilitate co-occurrence. For example, T. stigmatica were detected on some cameras that were deployed very close to the forest edge, indicating that despite an apparent lesser association with disturbance and edge effects, T. stigmatica still utilised forest habitat all the way to the forest edge. However, cameras in the adjacent grassy clearing (see Smith, Andrew & Vernes, 2022) did not detect a single T. stigmatica, suggesting that they do not venture past the forest edge to graze pasture. Increased light penetration at forest edges can encourage an enriched understorey and a higher abundance of ground cover. This may at times attract T. stigmatica to edge-affected forest, however, competition with T. thetis probably excludes them from the adjacent pasture. In Australian vegetation communities, structural variation can govern the distribution of marsupials at various spatial scales (Kanowski et al., 2001). T. thetis appears to be spatio-temporally partitioning its habitat similarly to the way T. stigmatica does in the northern expanse of its distribution (Vernes, Marsh & Winter, 1995). Research on temporal activity of pademelons at our study site (Smith, Andrew & Vernes, 2022) also indicated some temporal partitioning between the species, suggesting that the two species are ecologically similar and subject to competitive interactions.
Prior to anthropogenic fragmentation of their habitat, pademelons (Thylogale spp.) are thought to have been edge-dwelling generalist species that exploited both rainforest browse and grasses in forested ecotones (Vernes, 1995; Vernes, Marsh & Winter, 1995). However, when sympatric with other forest-dwelling macropods, competition may force some species to narrow their niche breadth. In northern Australia, T. stigmatica occurs as the sole pademelon species, and there, habitat use and diet are very different from that seen in southern populations where T. stigmatica occurs in sympatry with T. thetis. These differences are also reflected in their cranial morphology; Mitchell et al. (2018) found that the southern subspecies of T. stigmatica (Thylogale stigmatica wilcoxi) had a broader cranium and a shorter and more robust muzzle—typical of browsing species, while the northern subspecies (Thylogale stigmatica stigmatica) possessed a more slender skull with a longer muzzle, a characteristic shared with T. thetis and that is commonly seen in grazing macropods. Direct competition for edge resources may have forced sympatric populations of T. stigmatica into a narrower niche and also driven their population density below what might be achieved in the absence of competition; our related work (Smith, Andrew & Vernes, 2022; Vernes, Elliott & Elliott, 2022) indicated that T. stigmatica occur at lower population densities when in sympatry with T. thetis than when they occur in isolation from them. Thus, when constrained within a narrower, more specialised niche, population density of T. stigmatica may be reduced.
Species that have adapted to forest edges benefit from the fragmentation process whereas forest specialists have a higher tendency towards extinction, particularly where the home range of the species is not significantly smaller than the available fragment (Harrington et al., 2001). Disturbances like the removal of rainforest at landscape scales and the impacts of fires in sclerophyll forest have the capacity to negatively affect T. thetis into the future (McHugh, Goldingay & Letnic, 2022) and presumably, also T. stigmatica. However, T. stigmatica would likely be more affected than T. thetis if fragmentation and other anthropogenic disturbances were to increase, because T. thetis appears better adapted to the interface between forests and cleared land. Further research into habitat selection, diet, and niche specialisation in southern populations of T. stigmatica is therefore important for understanding their ecology and to help ensure their continued persistence. Because this study presents some spatial and temporal limitations in understanding pademelon co-occurrence, we also recommend further research at a landscape scale in a range of landscape settings to build upon the results we present. Nevertheless, our results indicate that protection of large tracts of rainforest where edge effects are minimised would clearly be advantageous for the conservation of T. stigmatica in the southern temperate parts of their range.
Conclusions
The objectives of the current study were to investigate how structural attributes of two forest types, wet sclerophyll forest and rainforest, relate to the fine-scale occurrence of T. thetis and T. stigmatica. We found that T. thetis had similar occurrence in both forest types, but at a finer scale, was detected more at locations close to grassy forest edges and forest tracks where grasses were abundant. By comparison, T. stigmatica was more likely to be detected in rainforest habitat, with fine-scale affiliation for sites where vegetation at ground level was dense, including sites near tree-fall gaps. Our results therefore suggest that the threatened T. stigmatica requires tracts of undisturbed and unfragmented rainforest with fewer anthropogenic edges or incursions like roads.
Supplemental Information
R script for analyses of pademelon occurrences in relation to environmental and landscape variable at each camera trap site
R script for polar plots of independent occurrences of T. thetis and T. stigmatica at cameras located within either rainforest or wet sclerophyll forest throughout the 24-hour cycle
Vegetation and landscape variables for pademelon camera trap sites
Pademelon occurrences at each camera trap site, and PC score for PC1, PC2 and PC3
Headers: Location = trap site; Type = forest type; TTHET and TSTIG = Independent occurrences of Thylogale thetis (TTHET) and Thylogale stigmatica (TSTIG) at 30-min independence; thetday and stigday = number of days each species occurred at the camera; thet_cor and stig_cor = number of days each species occurred at a trap per 100 days, corrected by number of days each trap was operational.