A new species of Languidipes Hubbard (Ephemeroptera, Polymitarcyidae) from Borneo
- Published
- Accepted
- Received
- Academic Editor
- Daniel Erasmus
- Subject Areas
- Biodiversity, Entomology, Taxonomy, Freshwater Biology
- Keywords
- Povilla, Burrowing mayfly, Southeastern Asia, Asthenopodinae
- Copyright
- © 2024 Hankel and Molineri
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. A new species of Languidipes Hubbard (Ephemeroptera, Polymitarcyidae) from Borneo. PeerJ 12:e17327 https://doi.org/10.7717/peerj.17327
Abstract
The genus Languidipes is currently represented by three species distributed in southeastern Asia, India, and Sri Lanka. Languidipes corporaali is the most widely distributed species, and both, male and female imagos, as well as nymphs, are known. In contrast, the other species, L. taprobanes and L. lithophagus, are only known from nymphs. Here, we describe a new species, Languidipes janae sp nov, based on male imagos collected from Borneo, Indonesia. This new species is characterized by the presence of ommation on mesonotum, and penis almost completely divided, with sub-quadrate base and a small outer projection basal to the long and slender distal arms. This constitutes the first record of the genus for Borneo. A cladistic analysis of the subfamily Asthenopodinae supports its taxonomic status.
Introduction
Polymitarcyidae (Ephemeroptera), with a worldwide distribution, includes large to medium-sized mayflies with burrowing nymphs (Kluge, 2004; McCafferty, 2004). The mandibular tusks of the immature forms are used to dig tunnels in a variety of underwater sediments, including mud, clay and even siliceous rocks (Molineri, Salles & Peters, 2015; Bolotov et al., 2022). In addition they produce silk from the malpighian ducts, allowing them to coat their tunnels with a thin mesh of this material (Sattler, 1967), or even to construct silk cases where tunnels are impossible to dig (Molineri & Emmerich, 2010; Pai et al., 2023). Furthermore, adults are so short-lived that they do not present functional legs (except for the male forelegs, used to grasp females during copula), spending their entire life in flight. This forces them to make their subimaginal molt in a unique manner, not shading their cuticle in the classic form (as an entire piece) but in flakes that come off the body and wings (Molineri, 2010). Because of their unique biology, including nymphs hidden in the substrates and extremely short-lived adults, specimens of this group are infrequently collected.
The genus Languidipes was originally described as Asthenopus corporaali (Lestage, 1922) from Java, Indonesia. Languidipes corporaali (Lestage) was subsequently recorded from other Indonesian localities (Sumatra and Simeulue), as well as from Malaysia and Thailand (Baumgardner et al., 2012). The genus Languidipes also includes the species L. taprobanes (Hubbard, 1984) (Hubbard, 1984; Rathinakumar, Kubendran & Balasubramanian, 2019; Pai et al., 2023), from India and Sri Lanka, and the recently described L. lithophagus (Bolotov et al., 2022) from Myanmar.
A phylogenetic framework has been proposed for the subfamily Asthenopodinae, where Languidipes is included together with partially sympatric Povilla and other three South American genera (Molineri, Salles & Peters, 2015).
Here we describe a new species of Languidipes based on male imagos from Borneo, Indonesia, and test its phylogenetic relationships inside the subfamily.
Materials & Methods
Specimens were fixed in 70° % (v/v) ethanol. One wing was removed and mounted dry on microscope slides. Genitalia was dissected and temporarily mounted in gel alcohol for study and drawings with a camera lucida attached to an Olympus BX51 microscope. Photographs were taken with a Zeiss Axiocam ICc5 attached to a Zeiss Stemi 508 stereo microscope. Some images were processed with CombineZP software (Hadley, 2010) to improve focus.
Material is deposited in the following Institution: IBN (Instituto de Biodiversidad Neotropical, Tucumán), and FAMU (Florida A&M University, Tallahassee, FL).
The morphological matrix published in Molineri, Salles & Peters (2015) was revised, the new species amended, and some characters of L. corporaali were modified following the description of Baumgardner et al. (2012). All other taxa and characters in the matrix were not modified (Appendix 1).
The TNT program (Goloboff, Farris & Nixon, 2008) was used to set up the most parsimonious trees. Heuristic searches were conducted under implied weights (Goloboff, Mattoni & Quinteros, 2006) with k = 3 and 100 replicates of tree bisection and reconnection. All characters were treated as non-additive except for continuous characters (chars. 0 to 26), for additional details see Molineri, Salles & Peters (2015). Group support was calculated with the method of frequency difference (Goloboff et al., 2003), using 1,000 replications of symmetric jackknifing.
The electronic version of this article in Portable Document Format (PDF) will constitute a published work as defined by the International Commission on Zoological Nomenclature (ICZN). Consequently, the new names introduced in the electronic version are deemed effectively published under the Code solely from the electronic edition. This published work, along with the associated nomenclatural acts, has been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be accessed and the relevant information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: [LSIDurn:lsid:zoobank.org:act:048403BC-2E75-4C1B-AE70-8DDF826FF9CA]. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE, and CLOCKSS.
Results
Type material. Holotype male imago from Indonesia (Borneo): Kalimantan, Timur Prov., Lake Semayang, nr. Kota Bangun, attracted to light on boat, 3.vii.1985, M. Christensen, specimen number IBN–E 6370. Paratypes: four male imagos, same data, all deposited in IBN (IBN–E–6371, IBN–E–6372, IBN–E–6373 and IBN–E–6374).
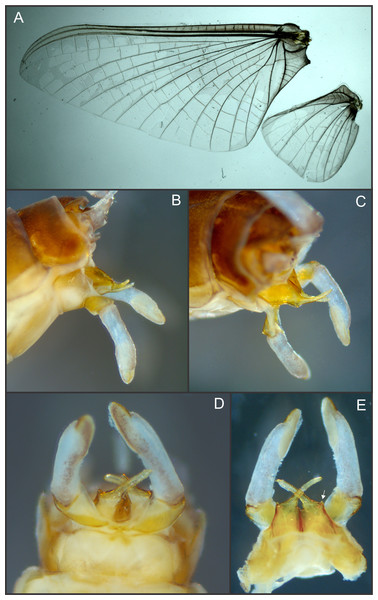
Figure 1: Languidipes janae sp. nov., male imago.
(A) Lateral habitus; (B) foreleg, dorsal; (C) dorsal habitus (wings removed); (D) ventral habitus (wings removed). Photo by Carlos Molineri.Additional material. We also examined 1 larvae of L. taprobanes, paratype, FAMU E2109, from Ceylon, Kollonawe, iv.1954 (no more data).
Diagnosis. The male imago of this species is characterized by the presence of ommation on mesonotum, and penis divided almost completely, with sub-quadrate base, small outer projection basally to the long and slender distal arms; distal arms with pointed apex.
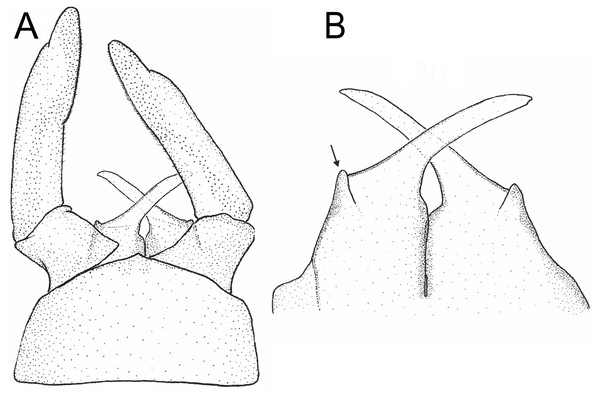
Male imago. Length (mm): body, 10.0–14.0; forewing, 12.2–13.0; hind wing, 4.0–5.0; cercus, 26.0, parecercus, 0.5−1.1. Head. Compound eyes large, black, covering most of head, separated in the middle of head by a distance equal to 1/3 of the width of an eye (Figs. 1A, 1C); lateral ocelli large and pedunculated (Fig. 1C). Head brown dorsally, shaded with black mainly at the base of ocelli; ventrally much paler. Remnants of mouthparts whitish yellow. Antenna: scape and pedicel yellowish (flagellum broken-off and lost). Thorax. Pronotum reddish brown with black stippling on central area; anterior membranous portion blackish, posterior margin withish; sternum and pleura whitish. Mesonotum reddish brown slightly paler medially, shaded with black between the posterior scutal protuberances; ommation (oval whitish median area in anterior of mesonotum) present (arrow in Fig. 1C); pleura and sternum light yellowish brown, furcasternal median impression translucent. Metanotum reddish brown shaded with black on median area and posterior margin, pleura yellowish, sternum whitish translucent. Forelegs relatively short (slightly shorter than of body length), yellowish white (Fig. 1B). Middle and hind legs whitish, weak (Fig. 1D). Forewings (Fig. 2A) hyaline shaded with gray along costal margin and on membrane basal to vein A. Hindwings (Fig. 2A) hyaline, shaded with gray at costal and basal half of subcostal areas, and at base. Veins of both wings brownish, lighter toward apex, except cross veins on apical half of wing, translucent. Abdomen. Dorsum brownish shaded with black, ventrally whitish. Genitalia (Figs. 2B to 2E, 3A and 3B): forceps one-segmented, robust, distally with a patch of short and curved setae along the inner margin. Penis divided almost completely, penis base sub-quadrate with a small outer projection (arrow in Figs. 2E and 3B), distal arms long and slender with pointed apex. Cerci: whitish, shaded with light gray basally. Paracercus as long as tergum X, whitish and thin.
Figure 2: Languidipes janae sp. nov., male imago.
(A) Wings; (B) genitalia, lateral; (C) same, latero-dorsal; (D) ventral; (E) dorsal. Photo by Carlos Molineri.Figure 3: Languidipes janae sp. nov., male imago.
(A) Genitalia, ventral; (B) penis, dorsal. Illustration by Carlos Molineri.Etymology. The specific name (noun in the genitive case) is a tribute to Janice Peters (“Jan”), who facilitated the material of the new species, and for her constant support.
Notes. In forewings, ICu veins presented variations among specimens. Frequently ICu1 is basally fused to CuA but may be basally free or joined to ICu2, additionally ICu2 may be basally free or fused to CuP.
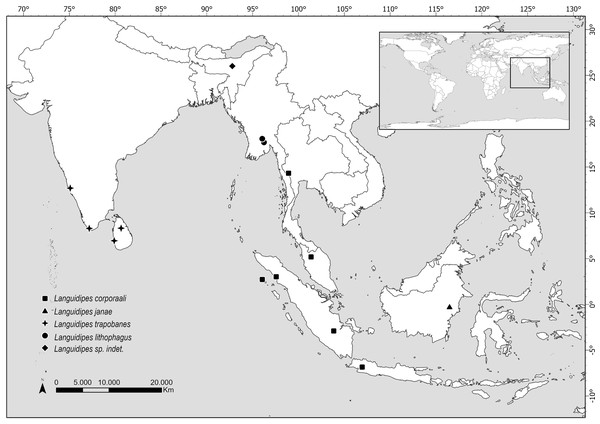
Distribution. Data here presented constitute the first record of a Languidipes species in Borneo Island (Fig. 4).
Figure 4: Map showing the distribution of all known Languidipes species.
Map elaborated by Luciana Cristobal. Map elaborated with QGIS 3.34. Made with Natural Earth, Free vector and raster map data (https://www.naturalearthdata.com).Phylogenetic study
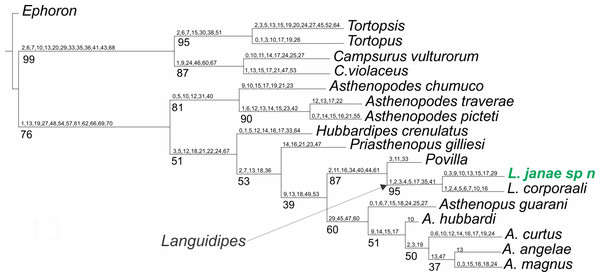
Only one shortest tree was recovered (Fig. 5), with a tree length of 270.8, a total fit of 5.8, and an adjusted homoplasy of 15.2. A high support was obtained for Languidipes (95%) and for the sister group Languidipes + Povilla (87%). The synapomorphies supporting the genus Languidipes (two species included) are: 1) ratio length second foretarsite/foretibia (char. 1 changes from 0.584−0.645 to 0.480), 2) ratio FW/foreleg length (char. 2, from 1.661−1.736 to 2.800), 3) ratio FW/cercus length (char. 3, from 0.339−0.347 to 0.375−0.464), 4) FW ratio length/width (char. 4, from 2.000−2.214 to 2.265), 5) ratio length FW/HW (char. 5, from 2.302−2.447 to 2.790), 6) penes, ratio basal width/subapical width (char. 17, from 1.300 to 2.000), 7) FW Cu sector, ICus joinning hind margin on different sides of tornus (char. 35): ICu1 close to tornus, ICu2 on basitornal margin, and 8) median plate of styliger (char 41) absent. The autapomorphies found for Languidipes janae are: 1) ratio subapical width of foretibia/subbasal width of tarsite 2 (char. 0, from 1.700 to 1.040), 2) ratio FW/cercus length (char. 3, from 0.375−0.464 to 0.500), 3) ratio marginal length between main longitudinal veins/imv length (mean of all values in a wing) (char. 9, from 1.653 to 1.745), 4) Rs stem length (FW male)/Rs from fork to margin (char. 10, from 0.235−0.241 to 0.220), 5) ratio total length of forceps/basal width (char. 13, from 4.545 to 4.300−4.500), 6) ratio length/basal width of penile lobe (char. 15, from 4.706−5.200 to 2.600), 7) penes, ratio basal width/subapical width (char. 17, from 2.000 to 3.125), and 8) male foretarsite 1 subrectangular (char. 29).
Figure 5: Phylogenetic tree of the Asthenopodinae subfamily, incorporating Languidipes janae.
Discussion
The species of Languidipes seem restricted to southeastern Asia (Fig. 4). The range of Languidipes corporaali is the widest of the genus, being recorded in some Indonesian islands (Java, Sumatra, and Simeulue), Thailand, and Malaysia; with a doubtful record for Assam, India (Chopra, 1927 cited in Hubbard, 1984). Hubbard (1984) affirms that probably this last record will be a new species.
Most species of Languidipes are only known from nymphs. Languidipes taprobanes is known from Sri Lanka and the south of India, while L. lithophagus was recently described from Myanmar (Bolotov et al., 2022). It is possible that the males described here as L. janae represent the adult stage of one of them, but this seems unlikely. Nevertheless, we prefer to describe the new species because it constitutes the unique record from Borneo, and its size is relatively smaller than the other species (Hubbard, 1984; Rathinakumar, Kubendran & Balasubramanian, 2019; Bolotov et al., 2022; Pai et al., 2023). While we believe that obtaining a DNA barcode would have been very helpful for the future association of nymphs or female adults, this technique couldn’t be pursued due to the nature of the available material. The material is scarce and has been collected over a long period (more than 30 years), preserved in a manner that does not support genetic material preservation. Therefore, the utilization of this technique was ruled out.
Styliger in Languidipes is reduced to pedestals, which appear to be the basal segment of forceps. Median plate of styliger is not present, contrary to Povilla and other Asthenopodinae, but similar to Campsurinae (Kluge, 2004; Molineri, Salles & Peters, 2015). Following this interpretation, forceps of Languidipes are one-segmented, and the diagnosis proposed by Baumgardner et al. (2012) including the statement “male genitalia without a remnant of styliger plate” should be amended to “male genitalia without a remnant of the median plate of styliger”.
Surprisingly, a weak small circular area in the center of the mesonotum (Fig. 1C) is present in the specimens here studied. This structure, much resembling the ommation of Caenidae and Neoephemeridae (Wang, McCafferty & Bae, 1997), is unique in the family Polymitarcyidae, and most probably is an independent acquisition.
Among the species of Languidipes, only L. corporaali is known from the male adult, and it presents a penis structure strongly different to L. janae sp. nov. The basal portion of the penis are wide and laterodistally rounded in L. corporaali but is sub-quadrate and with an acute projection in outer margin in L. janae. Penis arms in L. corporaali end more acutely than in the species described here. Finally, the penis is divided from the base of the arms to the apex in L. corporaali, but L. janae presents a much deeper division including most of the basal portion of the penis.
The previous phylogenetic hypothesis (Molineri, Salles & Peters, 2015) is not modified by the inclusion of Languidipes janae. As expected, this species is grouped with L. corporaali in a well-defined group, sister to Povilla.
Supplemental Information
Characters matrix
A matrix containing the characters used for phylogenetic analysis.