Dose-response association of sleep duration with anxiety symptoms in Chinese type 2 diabetes mellitus
- Published
- Accepted
- Received
- Academic Editor
- Mohsen Khosravi
- Subject Areas
- Diabetes and Endocrinology, Public Health, Mental Health
- Keywords
- Anxiety, Diabetes mellitus, type 2, Sleep duration
- Copyright
- © 2024 Shang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Dose-response association of sleep duration with anxiety symptoms in Chinese type 2 diabetes mellitus. PeerJ 12:e16954 https://doi.org/10.7717/peerj.16954
Abstract
Objectives
Anxiety is a disorder with a high prevalence in T2DM, and some studies have found that sleep problems can cause anxiety. Therefore, this study explored the independent effects of night sleep duration on anxiety symptoms in T2DM patients.
Research Design and Methods
A cross-sectional population-based study was conducted using self-reported questionnaires and taking into account several socio-demographic, lifestyle and health related characteristics. The 1,611 participants enrolled in our study. Anxiety was assessed by the Zung self-rating anxiety scale (SAS). A multivariate logistic regression model and restricted cubic spline with anxiety symptoms as the dependent variable were fitted.
Results
Of the T2DM patients in this study, 891 (55.31%) were male, 720 (44.69%) were female and 207 (12.85%) had anxiety symptoms. Controlling for potential confounders and intermediates, sleep duration >8 h relative to intermediate sleep (7–8 h) was significantly associated with anxiety syndrome (OR: 1.46, 95% CI [1.06–2.02], p = 0.02) and a J-shaped association was found between sleep duration and anxiety. The prevalence of anxiety symptoms was higher in the male group (>8 h/night) compared to the female. Study participants under the age of 50 who had a shorter sleep duration had a higher prevalence of anxiety compared to those between the ages of 50 and 60.
Conclusion
Among Chinese T2DM patients, there is a dose-response relationship between night sleep duration and anxiety, these findings may propose important public health implications for diabetes management.
Introduction
The International Diabetes Federation released an epidemiological report in 2021 showing that more than one in ten adults worldwide has diabetes and that China has the highest number of people with this disease, at 140 million (Sun et al., 2022). Type 2 diabetes mellitus (T2DM) is the most common form of diabetes, representing over 90% of all cases. It is characterized by high blood glucose levels in an insulin-resistant due to the combination of insulin resistance and insufficient insulin production (Karalliedde & Gnudi, 2016). To maintain blood glucose and reduce complications, T2DM patients need lifelong restrictions on diet, exercise, medication and blood glucose monitoring (Lee et al., 2014). Throughout the extended period of self-management, many patients experience tremendous stress in their daily life due to the threat of complications, the daily administration of their diabetes, and uncontrolled blood glucose (Pouwer, 2009; Snoek et al., 2000). Hence, T2DM patients are susceptible to developing psychiatric illnesses (Borovcanin et al., 2023). Anxiety is a common psychological comorbidity among T2DM patients, and a previous study (Collins, Corcoran & Perry, 2009) found that the prevalence of anxiety symptoms in diabetics was 22.4%–75%, about twice as many as the general population (Roy & Lloyd, 2012). As well as increases the risk of cardiovascular disease (Allgulander, 2016; Vogelzangs et al., 2010), anxiety even increases the likelihood of suicide (Anderberg et al., 2016). In addition to the common risk factors for anxiety in the general population, such as stressful tension (Meyer & Curry, 2017), hormone levels (Davey et al., 2016), and adverse childhood experiences (Poole, Dobson & Pusch, 2017), T2DM patients have diabetes-specific emotional problems. A study in Japan (Hayashino et al., 2012) found that anxiety symptoms and poor glycemic control were associated in diabetic patients. Another study in China found that glycemic fluctuations and sleep quality were associated with an increased prevalence of anxiety in T2DM patients (Wang et al., 2015b). Sleep problems are significantly more common in diabetic patients compared to non-diabetic (Anderson et al., 2001; Egede, Zheng & Simpson, 2002). Various factors may contribute to sleep problems in diabetic patients, including discomfort or pain associated with peripheral neuropathy and rapid changes in blood glucose levels during the night, leading to hypoglycemic and hyperglycemic episodes (Resnick et al., 2003).
We were motivated to investigate the correlation between sleep duration and anxiety symptoms due to their high occurrence in T2DM patients. The previous study of the general population in Henan, China, showed an association between sleep duration as well as sleep onset time and anxiety (Zhou et al., 2020). Existing research on sleep and anxiety has focused on correlational studies (Kim et al., 2020), in which sleep quality is evaluated using The Pittsburgh Sleep Quality Index (PSQI) scale, which combines sleep duration and subjective state during sleep to assess sleep quality (Del Rio João et al., 2018; Pilz et al., 2018). In this study, only the objective part of the scale—sleep duration, were used to observe a more direct effect of sleep.
The Chinese are particularly vulnerable to T2DM due to physical, cultural, and social factors (The Lancet Diabetes & Endocrinology, 2014). Because of the large number of patients, it is imperative to manage their blood glucose levels, addressing their emotional concerns and anxiety which could affect the progress and results of diabetes management. Anxiety in diabetic patients might prompt doctors to contemplate the potential influence of sleep issues on treatment results in order to address patients’ physical and mental well-being better. Current findings in the same field of research are mainly about the interaction of poor sleep quality and anxiety symptoms reduces quality of life in T2DM patients (Wang et al., 2015a; Zhang, Chen & Chen, 2008); however, there are few studies on the sleep duration. Therefore, this study aimed to explore the dose–response relationship between night sleep duration and anxiety symptoms in T2DM patients in Ningxia, China, and to explore the nonlinear relationship between patients and anxiety symptoms in different gender and age groups.
Methods
Participants
We carried out a cross-sectional study of 1,611 people, selected from 10 public hospitals (including three tertiary class A hospitals, three secondary class A hospitals, three tertiary class B hospitals, and one secondary class B hospital) from five cities in Ningxia based on geographical distribution and hospital level from August 2019 to November 2020. A probability sampling method proportional to size was used to recruit participants for this study. According to a previous study, the prevalence of quality of life in Chinese diabetes patients was 43.6% (Sun et al., 2016). Therefore, sample size was calculated to be 1,083 using the cross-sectional research sample size calculation. To prevent an invalid survey sample, we increased the sample by 20%, which made the minimum sample size in the survey 1,353. The final data collected met the sample size requirements.
Participants who diagnosed with T2DM by physicians and age ≥18 years old, living at the current address for at least 6 months and informed consent were recruited in our study. T2DM was defined as having been prescribed medication for diabetes by physicians or with a fasting plasma glucose ≥7.0 mmol/l or OGTT-2 h plasma glucose ≥11.1 mmol/l or glycated hemoglobin A1c (HbA1c) ≥48 mmol/mol (6.5%). Participants who met the following criteria were not included in the analysis: (1) with severe mental disorders or serious illnesses, or language barriers that prevented communication, (2) pregnant or lactating women, (3) with diabetic ketoacidosis, (4) with malignant tumors or (5) refused to sign the informed consent form.
In this study, using a standard questionnaire and face-to-face interviews, staff collected information on sociodemographic characteristics and lifestyle information and if there were problems with the poor quality of the collected questionnaires, a telephone survey method was used to supplement the questionnaires. Research procedures of this study involving human participants conformed with the ethical standards of the Yinchuan Hospital of Traditional Chinese Medicine Chinese Medicine Research Ethics Committee and with the 1964 Helsinki declaration and the later amendments or similar ethical standards.
Sleep variable
Night sleep duration were assessed by asking the following questions: “What time do you go to bed?” and “What time do you wake up?”, and night sleep duration was computed as the duration between sleep onset time and waking up time (Zhang et al., 2020). We analyzed the continuous variables and then classified them. The night sleep duration was divided into three categories: <7 h, 7–8 h, and >8 h, and 7–8 h was taken as the reference group.
Definition of anxiety symptoms
Anxiety symptoms in the past week were assessed using the Zung Self-Assessment of Anxiety (SAS) scale, which consists of 20 items, each rated on a scale of 1–4 (Zung, 1971), and the summed total score was multiplied by 1.25 to obtain a standardized score. Standardized scores ranged from 25–100, with a score of ≥50 representing the presence of anxiety symptoms, and the higher the score, the higher the level of anxiety.
Assessment of covariates
Covariates include numerical variables such as age. Binary variables were gender, napping habits (Yes/No), physical activity (including normal daily activities) of at least 30 min per day at work and/or leisure time (Yes/No), complications of T2DM (Yes/No). Multiple categorical variables were education levels (illiteracy, primary school, senior high school, College degree or above), duration of T2DM (3 months-1 year, 1–5 years, 6–10 years, more than 10 years), family history of diabetes (Yes/No/Not clearly),
Statistical analysis
Statistical analyses were performed with R version 4.2.2 (R Core Team, 2022), and a value of P < 0.05(two-tailed) indicated statistical significance. Continuous variables are expressed as mean ± standard deviation (SD) and categorical variables as frequency (percentage). Analysis of variance (ANOVA) and chi-square tests were used to compare differences in continuous and categorical variables, respectively. Logistic regression analysis was used to determine the relationship of night sleep duration and anxiety symptoms. Two sets of models were used: an unadjusted model, and a multivariate-adjusted model, adjusted for age, sex, education, family history of diabetes, nap time, complications, exercise, duration of T2DM. Results of logistic regression analysis are reported as odds ratios (ORs) and 95% confidence intervals (CIs). To show the relationship between sleep duration and anxiety symptoms, we have drawn a restricted cubic spline (RCS) by using spline regression. To examine the correlation between sleep duration and anxiety across various gender and age categories, we divided the population into subgroups and using logistic regression and RCS curves to analyze them individually.
Sensitivity analyses
Sensitivity analyses were conducted to examine the reliability of the dose relationship results for anxiety and sleep duration derived using RCS. We preformed the nonlinear association between sleep duration and anxiety after decreasing and increasing the patients’ sleep by one hour.
Results
The demographic characteristics of subjects
Basic characteristics of the population appear in Table 1. The mean age was 58.5 years, and 720 (44.69%) were women, and 207 (12.85%) had anxiety symptoms. T2DM patients with long sleep duration (>8 h) had more comorbidity and anxiety symptoms, less education level, more exercise behavior, more napping behavior than T2DM patients with short sleep duration (<7 h). Age and gender differences between the three sleep duration groups was observed. There were significant differences in age, sex, education levels, physical activity, family history of diabetes, complications and anxiety between three sleep duration levels (P > 0.05) (Table 1).
| Characteristics | Sleep duration total (n = 1611) | Short sleep duration (<7 h/day) (n = 123) | Normal sleep duration (7–8 h/day) (n = 784) | Long sleep duration (>8 h/day) (n = 704) | p |
|---|---|---|---|---|---|
| Age, mean ± SD | 58.5 ± 12.11 | 53.4 ± 1.12 | 57.3 ± 0.43 | 60.7 ± 0.45 | <0.001 |
| Female, n (%) | 720(44.7) | 45(36.6) | 330(42.1) | 345(49.0) | 0.005 |
| Education levels, n (%) | <0.001 | ||||
| Illiteracy | 307(19.1) | 15(12.2) | 118(15.0) | 174(24.7) | |
| Primary school | 357(22.1) | 19(15.5) | 148(18.9) | 190(27.0) | |
| Senior high school | 572(35.5) | 47(38.2) | 297(37.9) | 228(32.4) | |
| College degree or above | 375(23.3) | 42(34.1) | 221(28.2) | 112(15.9) | |
| Have a napping habit, yes, n (%) | 796(49.4) | 62(50.4) | 407(51.9) | 327(46.4) | 0.106 |
| Physical activity, yes, n (%) | 668(41.5) | 44(35.8) | 304(38.8) | 320(45.5) | 0.014 |
| Family history of diabetes, yes, n (%) | 484(30.0) | 48(39.2) | 260(33.2) | 176(25.0) | 0.002 |
| Duration of T2DM, n (%) | 0.071 | ||||
| 3 months-1 year | 270(16.8) | 32(26.0) | 136(17.4) | 102(14.5) | |
| 1–5 years | 461(28.6) | 32(26.0) | 223(28.4) | 206(29.3) | |
| 6–10 years | 348(21.6) | 25(20.3) | 160(20.4) | 163(23.1) | |
| More than 10 years | 532(33.0) | 34(27.7) | 265(33.8) | 233(33.1) | |
| Complications, yes, n (%) | 977(60.7) | 63(51.2) | 468(59.7) | 446(63.4) | 0.030 |
| Anxiety, n (%) | 207(12.9) | 13(10.6) | 76(9.7) | 118(16.8) | <0.001 |
Notes:
Association between night sleep duration with anxiety symptoms in T2DM patients
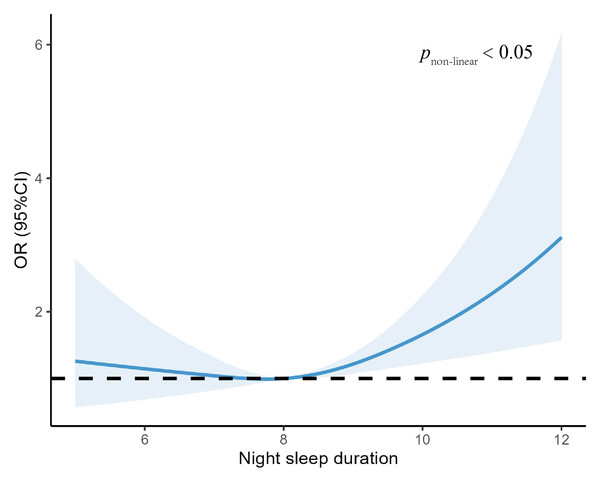
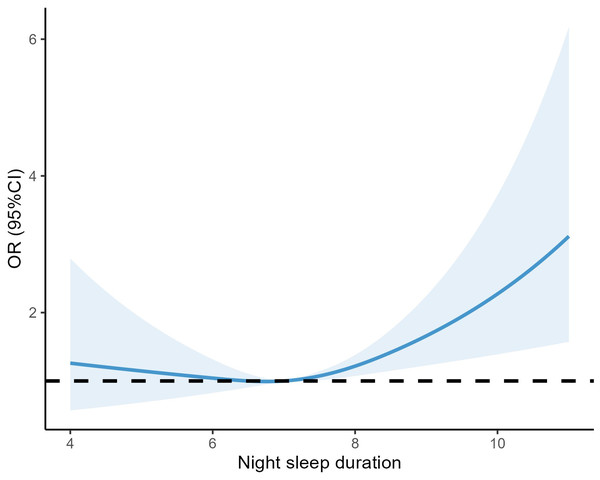
Table 2 shows the relationship between night sleep duration and anxiety symptoms in patients with T2DM. In the adjusted model, we found that those who with long sleep duration were 46% more likely to experience anxiety symptoms compared to the reference group with normal sleep duration (7–8 h) (OR: 1.46, 95% CI [1.06–2.02], P = 0.02), and similarly, those who with short sleep duration were 33% more likely to observe generalized anxiety symptoms (OR: 1.33, 95% CI [0.67–2.46], P = 0.387), which was not statistically significant. Among all participants, a J-shaped relationship between sleep duration and anxiety was observed before or after multivariable adjustment (Table 2). In addition, in Fig. 1, we flexibly modelled and visualized the relationship between night-time sleep duration and anxiety symptoms in T2DM patients using restricted cubic spline curves. The risk of developing anxiety increased rapidly when sleep duration was higher than 8 h, showing a j-shaped curve (Fig. 1).
Figure 1: Association between night sleep duration with anxiety symptoms.
Comparison of sleep duration and prevalence of anxiety disorders in T2DM patients across age and gender groups
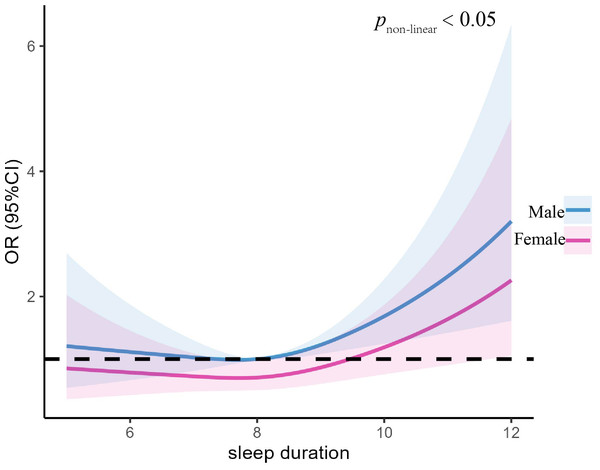
Table 3 displays the correlation between sleep duration and anxiety in various subgroups genders. We observed that long sleep duration and anxiety was positively correlated in the male after adjusting for demographic and lifestyle factors and the OR was 2.89 times higher than normal sleep duration. (Male: OR =1.76, 95%CI [1.07–2.93], P = 0.026). The female with long sleep duration had more susceptible to anxiety while the findings did not reach statistical significance (Female: OR =1.27, 95%CI [0.83–1.95], P = 0.278).
| Variable | Anxiety/N | Model 1a | P value | Model 2b | P value |
|---|---|---|---|---|---|
| Male | |||||
| <7 | 6/78 | 1.18 (0.43–2.75,) | 0.725 | 1.45 (0.52–3.50) | 0.436 |
| 7–8 | 30/454 | 1 (reference) | 1 (reference) | ||
| >8 | 46/359 | 2.08 (1.29–3.39) | 0.003 | 1.76 (1.07–2.93) | 0.026 |
| Female | |||||
| <7 | 7/45 | 1.14 (0.44–2.56) | 0.770 | 1.22 (0.46–2.87) | 0.673 |
| 7–8 | 46/330 | 1 (reference) | 1 (reference) | ||
| >8 | 72/345 | 1.63 (1.09–2.46) | 0.018 | 1.27 (0.83–1.95) | 0.278 |
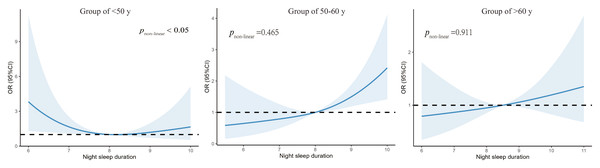
Table 4 shows the relationship between sleep duration and anxiety in subgroups of different age groups. We observed that who with short sleep duration had a higher risk of suffering from anxiety compared to those who with normal sleep duration in <50 ages group and the OR of short sleep duration was 5.86 times higher than normal sleep duration (<50 years: OR:5.86, 95%CI [1.47–23.26], P = 0.010). In 50–60 ages group, the longer sleep duration associated with a higher risk of anxiety and the OR of long sleep duration was 1.88 times higher than the normal (50–60 y: OR:1.88, 95%CI [1.02–3.53], P = 0.046).
| Variable | Anxiety/N | Model 1a | P value | Model 2b | P value |
|---|---|---|---|---|---|
| Group of <50 y | |||||
| <7 | 6/49 | 2.34 (0.69–7.11) | 0.145 | 5.86 (1.47–23.26) | 0.010 |
| 7–8 | 9/194 | 1 (reference) | 1 (reference) | ||
| >8 | 11/117 | 2.13 (0.86–5.45) | 0.104 | 2.16 (0.78–6.19) | 0.139 |
| Group of 50-60 y | |||||
| <7 | 7/37 | 0.32 (0.02–1.62) | 0.278 | 0.25(0.01–1.39) | 0.201 |
| 7–8 | 21/273 | 1 (reference) | 1 (reference) | ||
| >8 | 34/218 | 2.22 (1.26–4.00) | 0.007 | 1.88 (1.02–3.53) | 0.046 |
| Group of >60 y | |||||
| <7 | 7/36 | 1.42 (0.55–3.28) | 0.434 | 1.49 (0.56–3.51) | 0.392 |
| 7–8 | 46/317 | 1 (reference) | 1 (reference) | ||
| >8 | 73/369 | 1.45 (0.97–2.19) | 0.070 | 1.27 (0.84–1.94) | 0.262 |
Figure 2 illustrates the nonlinear association between the anxiety symptoms and the sleep duration in different genders of T2DM patients. We found a right-high and left-low J-shaped curve for both men and women after controlling for variables, with the highest predictive value on the right side of the curve being higher for men than women (pnon−linear = 0.05) (Fig. 2). Figure 3 illustrates the nonlinear association between anxiety and sleep duration in participants of different ages. After controlling for variables, we observed that both the age group less than 50 years and the age group between 50–60 years exhibited J-shaped curves. Upon taking into account factors, we observed that both the age group less than 50 years and the age group between 50 and 60 years exhibited J-shaped curves. Specifically, the former displayed a J-shaped curve with a peak on the left and a trough on the right, while the latter displayed a J-shaped curve with a trough on the left and a peak on the right (Fig. 3).
Figure 2: Association between night sleep duration with anxiety symptoms in different sex group.
Figure 3: Association between night sleep duration with anxiety symptoms in different age group.
T2DM patients were grouped by age into three cohorts to observe the relationship between sleep duration and anxiety in different age groups.Sensitivity analyses
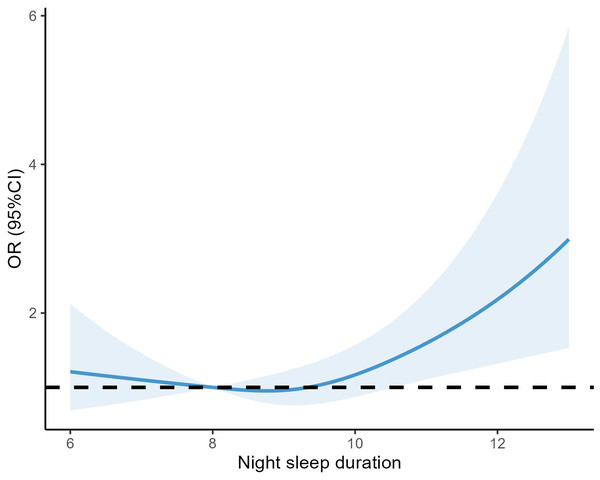
Conducting sensitivity studies revealed that even when sleep duration was increased or decreased by one hour, the association between sleep duration and anxiety remained consistent. Specifically, there was a greater prevalence of anxiety associated with longer sleep duration (Figs. 4 and 5).
Figure 4: RCS curves between sleep duration and anxiety after extending sleep duration by one hour overall.
Figure 5: RCS curves between sleep duration and anxiety after reducing sleep duration by one hour overall.
Discussion
The present study was designed to evaluate the potential associations of sleep duration and anxiety using a T2DM patients’ sample from the west of China. The study showed that T2DM patients who had longer sleep duration exhibited higher occurrence of anxiety symptoms. Furthermore, females demonstrated a higher prevalence of anxiety symptoms compared to males. Additionally, patients under the age of 50 were more inclined to experiencing anxiety when they had shorter sleep duration.
The prevalence of anxiety symptoms was 12.8% in our analysis, which is lower than the 22%–75% identified in earlier studies (Collins, Corcoran & Perry, 2009). The discrepancy may be attributable to the fact that patients with type 1 diabetes mellitus were included in that investigation. In contrast to patients who slept 7–8 h per night, our study found that patients who slept more than 8 h at night had a higher risk of developing anxiety symptoms. The review of the literature showed that sleep and physical in T2DM patients are inversely correlated. In T2DM patients with obesity, it has been discovered that hypertrophic adipocytes and NK cells (natural killer cell) produce pro-inflammatory cytokines that lower T-regulatory cells (Tregs) and B-regulatory cells (Bregs), aggravating the pro-inflammatory state of the adipose tissue and causing inflammation (Prasad et al., 2020). Peak circulation levels of the cellular inflammatory factor IL-6 are also detected during nocturnal sleep (Dimitrov et al., 2006), driven by circadian cycles, and circulating levels of IL-6R are also markedly elevated. As a result, more sleep is observed (Dimitrov et al., 2006; Irwin & Opp, 2017; Opp, 2009). Additionally, there is proof that inflammatory stimuli have an impact on anxiety-related behaviors and brain function (Felger, 2018). Overall, T2DM patients are more likely to have inflammatory disorders. Additionally, in those with T2DM, blood glucose variations and sleep quality are positively connected, and longer sleep durations may affect insulin secretion more than shorter ones (Brady et al., 2018; Wang et al., 2015b). Blood glucose fluctuations are associated with a higher incidence of anxiety problems in T2DM patients (Yang et al., 2022).
In addition to the effects of T2DM patients’ own inflammation, there may be a bidirectional causal relationship between anxiety and sleep, with some studies finding that chronic insomnia may be a precursor to anxiety symptoms (Neckelmann, Mykletun & Dahl, 2007), and others suggesting that anxiety disorders precede sleep problems (Ohayon & Roth, 2003); it is difficult to determine if sleep problems play a role in the development of anxiety symptoms or if sleep problems are a result or symptom of anxiety. The impact of co-morbidities of depression is usually considered in studies of anxiety, and one study found that sleep problems pose a higher risk of anxiety even in depressed populations (Nyer et al., 2013).
In this study, the prevalence of anxiety disorders was 10.13% in men and 21% in women, which is consistent with a cross-sectional study in Xuzhou, China (Sun et al., 2016), which found that among patients with T2DM, the risk of anxiety disorders was higher in women than men. Women have twice the lifetime chance of developing anxiety disorders as men (Gater et al., 1998).There are structural and functional differences in the brain between females and males that result in females being more sensitive to rejection, criticism and separation, which are key features of psychological problems such as anxiety disorders (Martel, 2013). In addition, fluctuations in gonadal steroid and HPA axis regulation during the menstrual cycle and pregnancy are necessary for conception and pregnancy, but also expose women to more intense perturbations of gonadal steroid and glucocorticoid-responsive brain systems (Altemus, Sarvaiya & Neill Epperson, 2014). A study on sleep and anxiety in Korean adults (Kim et al., 2020) indicated that the relationship between sleep duration and anxiety was gender-neutral and had a consistent nonlinear curve, which is in line with the findings of the current study. Intriguingly, this study discovered that males with T2DM were more likely than women to experience anxiety following long sleep.
The non-linear link between sleep duration and anxiety in various age groups was also examined in this study. In the age group of T2DM under the age of 50, we discovered that short sleep duration predicted anxiety prevalence, this results are similar to those of other studies, which have shown that short sleep, but not insomnia, increases the risk of anxiety emergence (van Mill et al., 2014), whereas long sleep duration predicted anxiety prevalence in the group of T2DM between the ages of 50 and 60. These findings are similar to another study on the relationship between long sleep and anxiety (Zhou, Li & Xu, 2022). Inflammation and sleep appear to be influenced by different mechanisms over the lifetime. Elderly people tend to sleep longer than young, and this trend may be related to aging and deteriorating health (Grandner & Drummond, 2007). According to some research (Biddle et al., 2019), sleep deprivation increases the chance of developing chronic mental health symptoms, middle-aged adults are physically healthier than older age groups, and inflammation may have a less significant impact on patients.
A study by van Mill et al. (2014) on Dutch individuals indicated that extra sleep duration predicted anxiety symptoms two years later in terms of the link between sleep duration and anxiety. Long periods of sleep at baseline, however, were not linked to anxiety at the follow-up, according to a study of university students in Bangladesh (Hossain et al., 2019). We propose that discrepancies in how sleep duration was defined in the research and how data on sleep duration. were collected may be to blame for the contradictory findings of these investigations. Whether there is a causal relationship between sleep duration and anxiety is unclear, as the number of relevant experimental studies is still limited.
Due to a number of restrictions, the results of this study should be closely examined. First, the majority of the T2DM patients we gathered were middle-aged and older, who usually have the habit of going to bed early, which may lead to biased results, and as the diabetic population gets younger, study results may have limited the applicability of our findings to the youth population. Also, we did not include a group of healthy people to comparatively observe the problems highlighted in diabetics. The findings are further affected by the rhythmic variations caused by the later sunset times in northern China, which impacts its external validity. Secondly, since sleep duration and anxiety symptoms were measured using a self-report questionnaire, it may involve information bias despite that it is commonly used in the epidemiological study due to the feasibility consideration. Although we collected sleep/wake schedules, there was still a lack of information on sleep disorders, and symptoms such as sleep disorders and insomnia should be discussed separately from sleep duration as they are differently associated with psychopathology. Thirdly, this was a cross-sectional study with limitations to conducting causal links of anxiety and sleep duration.
Conclusion
The present study’s findings indicate that T2DM patients are at high risk for anxiety, and there is a dose–response relationship between night sleep duration and anxiety, with the risk of anxiety associated with sleep duration longer than 7–8 h. While doctors are managing diabetes in a more balanced way with their patients, psychological distress issues and glycemic control need to be managed with equal attention, and in addition to paying attention to sleep problems such as insomnia or drowsiness in their patients, the sleep duration is also one of the risk factors of concern. Such low-cost interventions can be incorporated into current strategies to prevent diabetes and other chronic diseases.