Role of microRNA miR171 in plant development
- Published
- Accepted
- Received
- Academic Editor
- Diaa Abd El-Moneim
- Subject Areas
- Agricultural Science, Developmental Biology, Genetics, Plant Science
- Keywords
- miR171, GRAS, Growth and development, Environment stress
- Copyright
- © 2023 Pei et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Role of microRNA miR171 in plant development. PeerJ 11:e15632 https://doi.org/10.7717/peerj.15632
Abstract
MicroRNAs (miRNAs) are endogenous non-coding small RNA with 19–24 nucleotides (nts) in length, which play an essential role in regulating gene expression at the post-transcriptional level. As one of the first miRNAs found in plants, miR171 is a typical class of conserved miRNAs. The miR171 sequences among different species are highly similar, and the vast majority of them have both “GAGCCG” and “CAAUAU” fragments. In addition to being involved in plant growth and development, hormone signaling and stress response, miR171 also plays multiple and important roles in plants through interactions with microbe and other small-RNAs. The miRNA functions by regulating the expression of target genes. Most of miR171’s target genes are in the GRAS gene family, but also include some NSP, miRNAs, lncRNAs, and other genes. This review is intended to summarize recent updates on miR171 regarding its function in plant life and hopefully provide new ideas for understanding miR171 function and regulatory mechanisms.
Introduction
MicroRNAs (miRNAs) are endogenous non-coding small RNA transcripts of 19–24 nucleotides (nts) that play essential roles in the growth, development, and stress tolerance of plants and animals by regulating gene expression (Yang et al., 2017; Huang et al., 2021). Lee, Feinbaum & Ambros (1993) discovered the first members of the miRNA family in Caenorhabditis elegans in 1993. . Since then, miRNAs have been the subject of several studies.
MiRNAs are the end products of miRNA-encoding genes that undergo a series of processing processes. The genes encoding miRNA are transcribed by RNA polymerase II to form a primary transcript, pri-miRNA, which is a few hundred nts long (Lee et al., 2004). DCL1, in association with HYL1 and SE proteins, processes pri-miRNAs at the bottom of the stem to liberate pre-miRNAs (Kurihara & Watanabe, 2004; Kurihara, Takashi & Watanabe, 2006; Lobbes et al., 2006). DCL1 continues to act on pre-miRNA to form miRNA-miRNA∗ duplex (Kurihara & Watanabe, 2004). HEN1 then proceeds to methylate the duplex; these 3′-terminal modifications do not occur in animal miRNAs (Yang et al., 2006). The miRNA duplex binds with the cytoplasmic Argonaute (AGO) protein, leading to the removal and degradation of the miRNA* strand, ultimately forming the miRNA-induced silencing complex (miRISC) (Schwarz et al., 2003). The miRISC complex plays a crucial role in post-transcriptional gene regulation.
MiRNAs negatively regulate their target genes by cleaving complementary mRNA or inhibiting translation at the post-transcriptional level in many biological processes. Studies also demonstrate that several miRNAs mediate DNA methylation to silence genes (Huang et al., 2021; Zhang et al., 2006; Jover-Gil, Candela & Ponce, 2005). Most of the target genes of miRNAs are transcription factors involved in a wide range of plant processes, including growth and development, metabolism, abiotic and biotic stress, among others (Bernardi et al., 2022; Jiang et al., 2018b; Huang et al., 2016; Liu et al., 2018; Luis et al., 2012; Zhang et al., 2010).
The first plant miRNA in Arabidopsis thaliana was discovered by researchers in 2002. Reinhart et al. (2002) identified 16 miRNAs in Arabidopsis, including miR171 and its homologs in rice (Reinhart et al., 2002). With advancements in research methods, many plant miRNAs have been discovered and characterized. MiR171 is a conserved 21-nt miRNA found in several plant species, such as Arabidopsis thaliana, Oryza sativa, Solanum lycopersicum, Lilium pumilum, Hordeum vulgare, Morus alba, and others (Mahale et al., 2014; Yang et al., 2017; Kravchik et al., 2019; Yan et al., 2022; Curaba et al., 2013; Sun et al., 2022). As research has progressed, the miR171 functions have been discovered in plant growth, development, and stress responses, such as flowering time, phase transition, drought, extreme temperature, virus, and so on (Sun et al., 2022; Huang et al., 2019; Huang et al., 2016; Curaba et al., 2013; Um et al., 2022; Huang et al., 2016; Jiang et al., 2018b; Shi et al., 2022; Yan et al., 2022). In recent years, scientists have made many advances in their understanding of the miR171 family in plants, but the analysis and classification of these developments are missing. This review concentrates on the roles of miR171 and its target genes in plant life and aims to elucidate the possible functional mechanisms of miR171. The findings should be useful for researchers working on plant microRNAs, particularly the miR171 family. It may also serve as a theoretical basis for future developments in the study of other miRNAs, the study of miR171 function in other species, or even novel functional aspects of miR171.
Survey methodology
In this article, the PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofscience.com/wos/alldb/basic-search), Baidu Academic (https://xueshu.baidu.com/) and sci-hub (https://sci-hub.se/) to search for literature. The search keywords and their combinations included “miRNA”; “miR171”; “water stress”; “plant miRNA”; “GRAS Family”; “drought stress”; “plant development and growth”; “abiotic stress”; “biotic stress”; “target gene of miR171”; “phylogenetic tree”; “Scarecrow-like”; “small-RNA”; “miR171 interaction with”; “microRNA171”; “Embryogenesis-Associated microRNAs”; “abscisic acid”; “auxin”; “miR171 and its target gene”. We collected and screened a large number of related studies based on their relevance to the topic, and excluded those unrelated. We collected and reviewed several related studies for relevance to the topic and excluded the unrelated ones. To examine the conservation and specificity of miR171 mature sequences across different species, we obtained the sequences from the PmiREN2.0 database (https://www.pmiren.com/) and carried out a sequence alignment using DNAMAN software. Moreover, we generated a maximum likelihood evolutionary tree of miR171’s mature and precursor sequences utilizing MEGA software, considering representative species for this analysis. Our goal was to describe the function of miR171 and its target genes in plant life and discuss the possible functional mechanisms of miR171. Therefore, we also excluded articles with less relevance after determining their focus by reading the abstract.
MiR171 and its target genes in plants
Members of the miR171 family
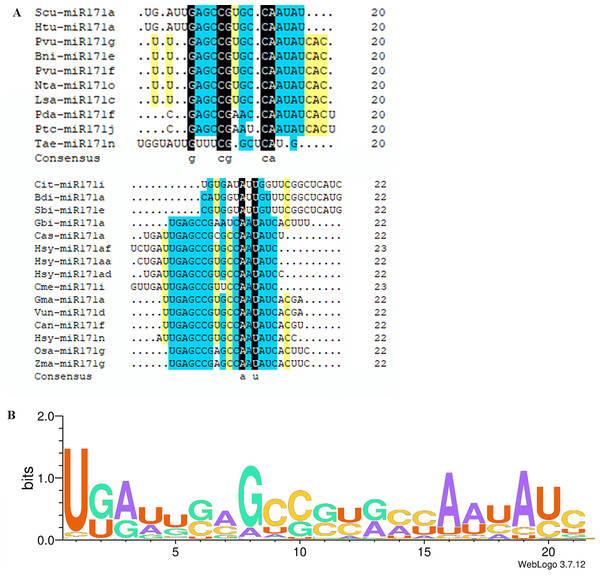
The miR171 family currently comprises eight hundred and seventeen members. The Plant MicroRNA Encyclopedia (https://www.pmiren.com/) lists their mature sequences in one hundred eight species. The miR171 family is the second-largest miRNA family (Zhang et al., 2021a; Zhang et al., 2021b). In most plants, the members appear in the 21 nts form; however, some 20 nts, 22 nts, and 23 nts miR171 families were also present in other species (Fig. 1A). We observed twenty-nine miR171 members in 20 nts form in eleven species (Bni-miR171e, Pda-miR171f, Jre-miR171a, Rsa-miR171a-d, Scu-miR171a-l, Tae-miR171n/o, Hlu-miR171b, Lsa-miR171c, Lja-miR171e-g, Nta-miR171o, Pvu-miR171f/g); twenty-two 22 nts in seventeen species (Bdi-miR171a, Can-miR171f, Cit-miR171i, Gbi-miR171a, Gma-miR171a/i, Han-miR171a, Hsy-miR171n/aa/ae/ad, Lsa-miR171e, Mdo-miR171b, Osa-miR171 g, Pdu-miR171a, Pca-miR171b/d, Sbi-miR171e, Scu-miR171a, Tha-miR171a, Vun-miR171d, Zma-miR171 g); and six 23 nts in two species (Cme-miR171i, Hsy-miR171a/y/af/ai/al). Notably, both sugarcane and radish miR171 mature sequences are 20 nts long, suggesting that they may have a closer genetic relationship and similar functional properties.
Figure 1: Multi sequence alignment of specially mature miR171.
(A) Multiple sequence alignment of the non-21 nts mature miR171s; (B) Sequence logo showing a consensus sequence generated from multiple alignments of mature miR171s from different plant species. Black indicates a homology level of 100%, yellow indicates a homology level above 75%, and blue indicates a homology level above 50%.Conservation of miR171
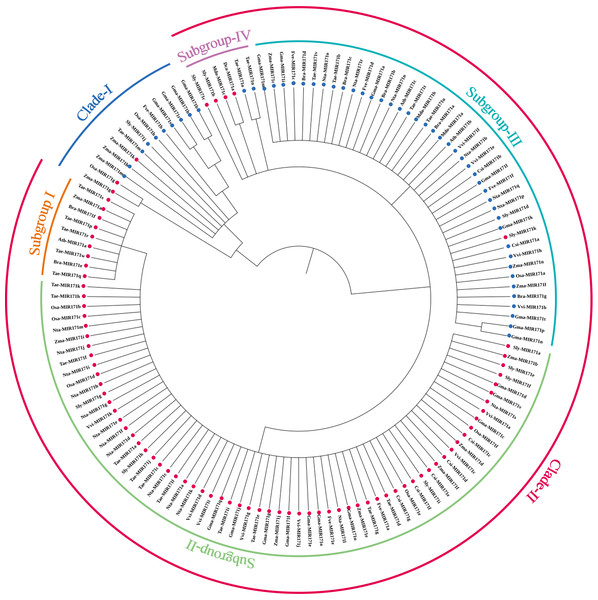
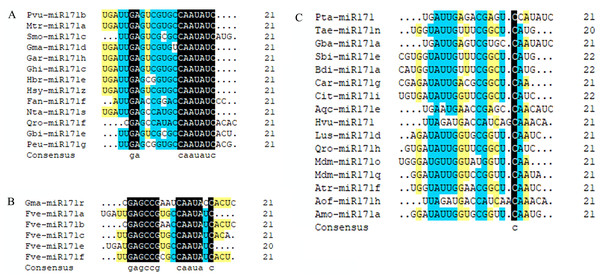
We observed that mature sequences of miR171 in the same species have high sequence similarity and that some identical sequences exist between different species by sequencing all miR171 family members (Fig. 1B). Further, to gain an insight into the evolutionary process of the miR171 family, several representative species were selected (Arabidopsis thaliana, Oryza sativa, Zea mays, Triticum aestivum, Nicotiana tabacum, Solanum lycopersicum, Fragaria vesca, Vitis vinifera, Malus domestica, Glycine max, Citrus sinensis, Brassica rapa, and Daucus carota) to construct the ML phylogenetic tree of miR171 family members (Fig. 2). The ML phylogenetic tree obtained grouped miR171 into two clades. Clade-I comprised 13 mature miRNAs, while the remaining 122 formed Clade-II. In Clade-II, the sequences of the same subgroups varied only by 4–6 bases, whereas those in Clade-I were identical. The low degree of sequence similarity within Clade-I implies higher sequence variation, and the low degree of sequence divergence within and between the two subgroups of Clade-II suggests that they have undergone consistent or similar evolutionary processes. The presence of miR171 sequences from different species in each clade and subgroup further supports this observation. We also noted the occurrence of “GAGCCG” and “CAAUAU” segments in all clades. Even without feature fragments, there was some sequence similarity between the sequences (Fig. 3), which confirms the high conservation of miR171 across different species.
Figure 2: The maximum likelihood phylogenetic relationships among miR171 family members in diûerent species.
(A) Thaliana (Ath), rice (Osa), strawberry (Fve), tomato (Sly), maize (Zma), wheat (Tae), cabbage (Bra), citrus (Csi), radish (Dca), soybean (Gma), apple (Mdo), grape (Vvi) and tobacco (Nta). Dots indicate where the corresponding precursors are Clades.Figure 3: Multi sequence alignment of specially mature miR171.
(A) Multiple sequence alignment of the mature miR171s without “GAGCCG”; (B) Multiple sequence alignment of the mature miR171s without “CAAUAU”; (C) Multiple sequence alignment of the mature miR171s without “GAGCCG” and “CAAUAU”. Black indicates a homology level of 100%, yellow indicates a homology level above 75%, and blue indicates a homology level above 50%.Target genes of miR171
MiRNAs function by binding to complementary sequences on target genes, which regulate gene expression through cleavage or translational repression. Various methods, such as degradome sequencing, 5’RACE, and site prediction, have been used to identify miR171 target genes. It has been discovered that most of the target genes of miR171 belong to the GRAS family through inductive summation. The GRAS gene family is exclusive to plants and comprises transcription factors containing a C-terminal GRAS domain (Waseem et al., 2022). The GRAS family has diverse functions and is widely distributed across plants. In addition to its role in regulating growth and development, its subfamilies are also involved in crucial processes such as signal transduction, stress response, and plant-microbe interaction (Silverstone, Ciampaglio & Sun, 1998; Schulze et al., 2010; Zhou et al., 2018a; Zhou et al., 2018b; Zhang et al., 2020; Gao, Wan & Wong, 2013).
The GRAS family member LOST MERISTEMS 1 (LOM1/2/3), also known as HAM1/2/3 or SCL6-II/III/IV in different species, regulates shoot apex indeterminacy and influences shoot branching (Engstrom et al., 2011; Llave et al., 2002; Xue et al., 2014; Sunkar & Zhu, 2004; Geng et al., 2021; Zhang et al., 2010; Stuurman, Jäggi & Kuhlemeier, 2002; Wang et al., 2010). When LOM1 is combined with ath-miR171c and cleaved, resulting in its reduced expression, the plant exhibits a reduced branching phenotype (Wang et al., 2010). Unigene83401, a predicted target gene of Pde-miR171, was discovered to have a cleavage site and exhibited significant homology with AtSCL6-III. In addition, Pde-miR171 can also direct target AtSCL-III and AtSCL- IV; to regulate leaf shape and root length (Hai et al., 2018; Wang et al., 2010). In addition, SCL6 has been identified as a target gene of miR171 in several plant species, including Pinus densata, Larix kaempferi, Lilium pumilum, Hordeum vulgare, Oryza sativa, Glycine max, Pyrus communis, and Brassica oleracea (Hai et al., 2018; Li et al., 2014; Yan et al., 2022; Curaba et al., 2013; Um et al., 2022; Hossain et al., 2019; Jiang et al., 2018b; Li et al., 2018).
Degradome sequencing has identified three SCLs (CsSCL1/2/3) as miR171c target genes in Citrus callus (Wu et al., 2015), and overexpression of miR171c in citrus reduced the expression of these genes and promoted somatic embryogenesis (Shi et al., 2022). Other targets of miR171 include PylSCL22, BolSCL27, SlGRAS24, MnoLOM2, MnoLOM3, GmNSP2, MtNSP2, LjaNSP2, and lncRNA (Jiang et al., 2018a; Li et al., 2018; Huang et al., 2016; Sun et al., 2022; Hossain et al., 2019; Lauressergues et al., 2012; Luis et al., 2012; Yang et al., 2022).
miRNA/miRNA* is a duplex product after Dicer processing the pre-miRNA (Chen, 2005). The miRNA is named the guide strand and the miRNA* is named the passenger strand. During RNA-induced silencing complex (RISC) maturation, the miRNA/miRNA* duplex associates with the catalytic subunit, an ARGONAUTE (AGO) protein. The miRNA in the duplex directs gene silencing and is retained and incorporated into RISC, while the passenger strand degrades due to its low stability (Chen, 2005; Zuo et al., 2011). However, studies have shown the presence of active AGO1-miR171a * complexes. Although there are five mismatches between miR171* and SUVH8, SUVH8 is identified as the target gene of miR171* in Arabidopsis thaliana using poly (A+) RNA from pistils and 5’RACE analysis (Manavella et al., 2013).
In addition, many other target genes have been predicted but not confirmed. We used the psRNATarget prediction website (http://www.zhaolab.org/psRNATarget/home) to analyze potential target genes for miR171 in three representative plants: Arabidopsis, tobacco, and tomato. It was found that miR171 can regulate pre-mRNA splicing factor-like proteins, programmed cell death 4, ABC transporter type 1, kinase, and other coding genes in addition to the above target genes (Table S1). These results provide new information about miR171 target genes and their corresponding functions.
Functions of miR171
Plant growth and development
Embryogenesis
Somatic embryogenesis (SE) is an important method for in vitro regeneration of plants (Shi et al., 2022). In recent years, many miRNAs involved in the SE systems of plants, including Arabidopsis, rice, and maize, were identified (Szyrajew et al., 2017; Luo et al., 2006; Chávez-Hernández et al., 2015). Shi et al. (2022) predicted that csi-miR171 might play an important role in the embryogenic callus (EC) and during SE of citrus because of its abundant expression only when embryogenic callus was induced (Wu et al., 2011; Shi et al., 2022). At relatively low expression levels of the target gene SCLs, transgenic citrus plants overexpressing csi-miR171c produced massive somatic embryos earlier than wild-type plants. The number was proportional to the expression level (Shi et al., 2022). In larch, miR171 was highly expressed in embryogenic cultures, but its expression was nearly undetectable in non-embryogenic cultures, whereas its target gene LaSCL6 showed an opposite pattern (Li et al., 2014). Another study discovered that more LaSCL6 transcripts were cleaved in EC, indicating that miR171-SCL6 may have a post-transcriptional role in maintaining embryogenic potential (Zhang et al., 2010). The mismatch between Lka-miR171a-e and SCL6 ranged from 0.5 to 2.5 (Li et al., 2014). Compared to the other members of the Larch miR171 family, Lka-miR171d has the highest frequency, indicating that it may be more critical for maintaining embryogenic potential (Zang et al., 2021).
Different members of the miR171 family show differential expression in the two lily species at different EC stages, suggesting their complex regulation of SCL6 (Li et al., 2017). Silencing Lpu-miR171a/b and overexpressing LpSCL6-II/I speed up the development of Lilium somatic embryos (Yan et al., 2022). It is worth mentioning that, unlike other miRNAs, Lda-miR171a/c and their target genes have a positive correlation (Li et al., 2017). MiR171 expression is downregulated in radish and rice during embryogenesis, while it is predominantly expressed in EC before initiating rice plant differentiation (Zhai et al., 2014; Luo et al., 2006). In the EC of Japanese Larch, the expression of Ptc-miR171c/d/j/k is significantly increased compared to the non-embryogenic callus (Zhang et al., 2010). These conclusions suggest that miR171 is essential for the development of plant regeneration, but its specific regulatory mechanism needs further investigation.
Vegetative development
Many studies have demonstrated that miR171 plays a diverse and indispensable role in vegetative development. The Hairy Meristem (HAM) transcription factors, members of the GRAS family, have been identified as miR171 targets and have been shown to significantly regulate shoot apical meristem and axillary meristem formation. AtHAM interacts with WUS/WOX, which plays a vital role in stem cell differentiation and maintenance in all meristem cells. HAM and WUS regulate common target genes that promote sprout stem cell proliferation. HAM and WOX are widely distributed throughout the plant, where their functions overlap, and they work together as regulators in different stem cell niches (Zhou et al., 2015). The combination of WUS and the CLV3 promoter activates the expression of CLV3, forming a negative feedback regulatory loop that ensures the normal maintenance and transformation of Arabidopsis thaliana, but this activation is only effective in the absence of HAM. Ectopic miR171 expression results in anomalous SAMs and dysregulated CLV3 expression (Brand et al., 2000; Schulze et al., 2010; Zhou et al., 2018a; Zhou et al., 2018b). Besides its role in Arabidopsis meristem, HAM has also been reported to regulate meristem in Petunia hybrida, Dendrocalamus latiflorus, Solanum lycopersicum, and other plants (Stuurman, Jäggi & Kuhlemeier, 2002; Yang et al., 2014; Hendelman et al., 2016), as a target gene for miR171.
Furthermore, the epidermis-specific transcription factors ATML1 and PDF2 directly bind to the L1 box of the miR171 promoter, forming a HAM concentration gradient, which regulates shoot development (Han et al., 2020). Overexpression of Hvu-miR171a in barley strongly represses the function of the target gene HvSCL, resulting in indeterminate initiation of the axillary meristem (Curaba et al., 2013). Similarly, increased expression of the target gene SlGRAS24 leads to an abnormal axillary bud phenotype (Huang et al., 2016). PpGRAS12, the miR171 target in Physcomitrium patens, leads to the formation of multiple apical meristems in the vegetative stage of the gametophyte when its transcript level increases (Beheshti et al., 2021). Thus, the miR171-HAM pathway plays an essential role in regulating the formation of apical and axillary meristems in plant shoots.
Overexpression of miR171c in Arabidopsis inhibits shoot branching, root elongation, and trichome distribution while promoting plant height, chlorophyll accumulation, and altered leaf shape and patterning, consistent with the target genes in the scl6 triple mutant plants (Wang et al., 2010; Xue et al., 2014). Further research revealed that the target genes of the miR171a gene activate its expression, forming a feedback regulatory loop (Xue et al., 2014). Transgenic plants overexpressing Ath-miR171a and down-regulating Sly-miR171 showed the opposite phenotype with increased branching (Song, Axtell & Fedoroff, 2010; Kravchik et al., 2019). Transgenic Arabidopsis also showed defects in cauline and rosette leaf patterns, and transgenic tomatoes produced irregular compound leaves (Song, Axtell & Fedoroff, 2010; Kravchik et al., 2019). Overexpression of Hvu-miR171a in barley resulted in shorter plants, fewer tillers, and more leaves due to repression HvSCL (Curaba et al., 2013).
The diameter of stems and the number of nodes increased significantly upon overexpression of Osa-miR171c mutants, while the leaf blades became irregular compared to the wild-type plants (Fan et al., 2015). In sugarcane, the length of internode increased along with the increase of miR171 expression (Sternes & Moyle, 2014). Transgenic tomatoes with increased expression of SlGRAS24, the target of Sly-miR171, have shorter and narrower leaves without serrated edges and more branches. Additionally, transgenic plants showed significantly suppressed root length compared to wild-type plants (Huang et al., 2016). Excessive accumulation of miR171 in Medicago truncatula promotes primary root development and reduces lateral root growth by targeting NSP2 (Lauressergues et al., 2015).
In contrast to its target SCL6, the expression of miR171 in Arabidopsis increased significantly during the light period while decreasing quickly during the dark period (Siré et al., 2009). However, the circadian clock does not regulate the accumulation of miR171 (Siré et al., 2009). Many cis-acting regulatory elements for photosensitivity are involved in the 2000 bp promoter sequences of Ptc-miR171, suggesting that its members may participate in light signal transduction and light morphogenesis in diverse ways (Liu et al., 2014).
Phase transition
MiR171 is also involved in the regulation of plant phase transitions. Phase transitions in plants are crucial stages in their growth and development and are closely related to plant resistance to disease and insects, yield, quality, and other agronomic traits. This process is generally non-directional and irreversible (Hu et al., 2022). Therefore, identifying the variables in this process is necessary.
Transgenic barley overexpressing Hvu-miR171a showed delayed phase transitions under long-day conditions, with more leaves, shorter internode lengths, and later flowering compared to wild-type plants. These phenotypic changes are partly due to the upregulation of miR156 in transgenic plants (Curaba et al., 2013). The mutant of rice, which up-regulated the expression of the Osa-miR171c, continued to produce new leaves. At the same time, the wild-type plant converted into an inflorescence meristem, indicating the phase transition’s delayed was because of the changes of Osa-miE171c and its target gene OsHAM expression (Fan et al., 2015). Overexpression of Sly-miR171 in tomatoes accelerates the phase transition and plant height (Huang et al., 2016). Fluctuations in miR171 levels during phase transition may be an effective pathway for yield improvement, and it also provides new ideas for plant breeding.
Reproduction development
Several studies have demonstrated that miRNAs are involved in reproductive development. Overexpression of miR171 in barley results in late flowering and sterile spikes due to delayed differentiation of spikelet meristems into floral organs (Curaba et al., 2013). Overexpression of 35Spro-miR171c in Arabidopsis resulted in the formation of abnormal and late flowers under long-day conditions (Wang et al., 2010). In rice, upregulation of Osa-miR171c significantly delayed flowering time, caused pleiotropic morphological abnormalities in the panicle, and affected SAM maintenance (Fan et al., 2015). When SlGRAS24, the target gene of Sly-miR171, was up-regulated (OE-SlGRAS24), flower opening was late, and the fruits were smaller with fewer seeds due to damaged pollen sacs and fewer viable pollen grains (Huang et al., 2016). Consistent with the above observation, the transgenic tomato with down-regulated Sly-miR171 showed male sterility because of malformed and nonviable pollen (Kravchik et al., 2019). Overexpression of miR171 and down-regulation of its target gene, SCL, also contributes to the reproductive development of rice. MiR171 up-regulated transgenic rice plants had thicker tillers, longer panicles, and more spikelets than wild-type plants (Tong et al., 2017).
Phytohormone response
According to several studies, miR171 also plays a role in the phytohormone response of plants. In Arabidopsis, the miR171 target gene SCL2 7 can reverse-regulate miR171 and create a feedback loop essential for mediating gibberellin signaling and regulating chlorophyll biosynthesis and leaf growth (Ma et al., 2014). Furthermore, the 1,000-bp upstream promoter sequence of miR171 was analyzed, which revealed the presence of several cis- and trans-acting elements for phytohormone response, including TGA-element for auxin, GARE motif for gibberellin, ERE for ethylene, and TCA-element for salicylic acid (Liu et al., 2008). Likewise, the 2000-bp upstream promoter sequence of Pts-miR171 also contains cis-acting elements for salicylic acid (TCA-element) and abscisic acid (ABRE) responsiveness (Liu et al., 2014). In pear, IAA-induced miR171 negatively regulates the IAA signaling cascade by targeting PyrSCL6/22; thus, promoting shoot growth and maintaining apical dominance (Jiang et al., 2018b). SlGRAS24, the target gene of Sly-miR171, also responds to auxin, suggesting that Sly-miR171 is involved in tomato growth and development via the auxin-signaling pathway (Huang et al., 2016).
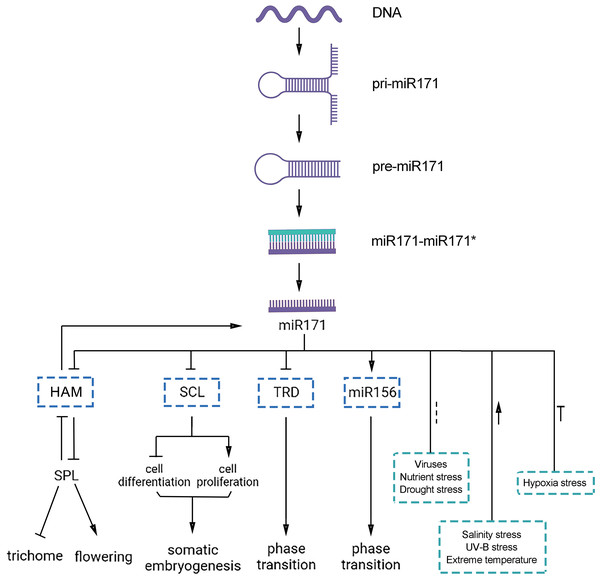
Overall, miR171 is critical for all stages of plant growth and development. MiR171 and its target genes play essential regulatory roles throughout the life cycle of a plant, from embryonic development to vegetative growth to reproductive growth (Table 1, Fig. 4). Therefore, understanding the mechanisms by which miR171 and its target genes influence plant growth and development is crucial for crop breeding and enhancing crop yield and quality.
| Species | Biological processes and the expression changes of miR171 (↑up, ↓ down) | Target gene | References |
|---|---|---|---|
| Arabidopsis thaliana | Root and leaf development, meristem formation, trichome distribution, diurnal cycle, chlorophyll biosynthesis, flower development, phytohormone |
SCL6-II/III/IV SUVH8 SCL27 |
Wang et al. (2010), Xue et al. (2014), Han & Zhou (2022), Manavella et al. (2013), Ma et al. (2014), Siré et al. (2009), Liu et al. (2008) |
| Oryza sativa | Somatic embryogenesis, phase transition, root development, chlorophyll accumulation |
SCL |
Luo et al. (2006), Fan et al. (2015), Cho & Paszkowsk (2017), Tong et al. (2017) |
| Citrus | Somatic embryogenesis | SCL | Shi et al. (2022) |
| Larix leptolepis | Somatic embryogenesis | SCL | Zhang et al. (2010) |
| Lilium | Somatic embryogenesis | SCL6 |
Li et al. (2017), Yan et al. (2022) |
| Raphanus sativus | Somatic embryogenesis | SCL6 | Zhai et al. (2014) |
| Larix kaempferi | Somatic embryogenesis | SCL6 | Zang et al. (2021) |
| Solanum lycopersicum | Anther development, shoot branching, leaf architecture, plant height, flowering time, root development, fruit set and development, phytohormone, phase transition |
HAM, NSP2L, GRAS24 |
Kravchik et al. (2019), Huang et al. (2016), Hou et al. (2019) |
| Hordeum vulgare | Plant height, leaf numbers, phase transition, floral meristem determinancy |
SCL | Curaba et al. (2013) |
| Saccharum officinarum | Stem | SCL6 | Sternes & Moyle (2014) |
| Physcomitrium patens | Meristem formation | GRAS12 | Beheshti et al. (2021) |
| Medicago truncatula | Root development | NSP2 |
Lauressergues et al. (2015), Branscheid et al. (2011) |
| Populus trichocarpa | phytohormone |
GRAS MYB |
Liu et al. (2014) |
| Pyrus | Phytohormone shoot growth |
SCL6/22 | Jiang et al. (2018b) |
| Pinus densata | Needle, stem, root, leaf, flower |
SCL6 | Hai et al. (2018) |
| Dendrobium officinale | SCL | Yang et al. (2015) | |
| Litchi chinensis | GRAS8/9/24/27 | Chen et al. (2021) |
Environmental stress
Abiotic stress response
A plant will inevitably experience a variety of environmental stresses throughout its life cycle, such as drought, excessive heavy metal exposure, nutrient deficiency, etc., which significantly affect plant growth and development. Microarray data identified 14 stress-inducible miRNAs, including miR171, in Arabidopsis under drought, low temperature, and high salinity conditions (Liu et al., 2008). However, after eight hours of drought stress, wheat roots displayed an opposite trend with the down-regulation of miR171 (Liu et al., 2008; Kantar, Lucas & Budak, 2011; Sunkar & Zhu, 2004). The expression pattern of miR171 in rice during drought stress is stage- dependent; the expression is higher during the tillering stage and lower during the reproductive stage (Zhou et al., 2010). While Osa-pre-miR171a abundance decreases under drought stress, Osa-miR171f expression levels rise, and it cleaves SCL6-I/II transcripts to alleviate drought symptoms (Chung et al., 2016; Um et al., 2022). The expression of Osa-miR171i decreases after ten days of water withholding to cope with water scarcity, as evidenced by Zhou et al. (2010). Although miR171 expression levels in tobacco cultivars vary, no clear pattern of change is observed under drought stress (Deniz, 2015). Drought stress leads to reduced miR171 expression in Triticum dicoccoides, Medicago truncatula, Populus tomentosa, and Ipomoea campanulata, increased expression in Morus alba, and a mixed pattern of expression in Solanum tuberosum and Prunus persica, as reported by Kantar, Lucas & Budak (2011), Wang et al. (2011), Ren et al. (2012), Ghorecha et al. (2017), Sun et al. (2022), Hwang et al. (2011), Eldem et al. (2012), and Ferdous, Hussain & Shi (2015). These findings suggest that miR171 plays an essential role in regulating multiple signaling pathways related to drought response.
Tomatoes exhibit leaf chlorosis, chlorophyll and starch degradation, and a decline in photosynthetic efficiency when exposed to carbon starvation; however, the symptoms alleviate when melatonin-induced miR171b is overexpressed and targets the GWD gene (Wang et al., 2022). Zinc oxide nanoparticles (ZnONPs) are the most widely used nanomaterials that can improve plant resistance to drought, cadmium, arsenic, etc. (Niazi et al., 2022; Semida et al., 2021; Hussain et al., 2018; Wu et al., 2020). The down-regulation of miR171 was observed in wheat when treated with 10 mg/L ZnONPs, whereas the up-regulation of miR171 was observed when the dosage was increased to 50 mg/L (Niazi et al., 2022). The expression of miR171 in leaf tissues of Arabidopsis thaliana was up-regulated with a rise in temperature from 35 °C to 45 °C (Mahale et al., 2014), suggesting that temperature is also a factor that affects miR171 expression levels. Ath-miR171 is also induced upon nitrogen starvation and suppresses SCL6-II/III/IV in response to better development of the primary root system (Liang, He & Yu, 2012). Boron treatment reduces the number of root tips, which further affects the architecture of the root system. B-toxic treatment in citrus reduces the expression levels of miR171 while increasing SCL expression to protect against stressful environments (Huang et al., 2019). Chromium stress initially decreased the expression of miR171 in rice, but after 24 hours, it began to increase (Dubey et al., 2020). A study showed that wheat miR171 reaches its maximum expression 2 h after UV-B treatment and interacts with other miRNAs in response to a stressful environment (Wang et al., 2013). Hypoxia treatment decreases the expression of miR171 in tomato roots and increases the length and quantity of lateral roots (Hou et al., 2019). When GSNO was used to mimic environmental NO, an increase in the abundance of both mature and precursor miR171 was observed (Santos et al., 2022). Since NO is essential for root hair development in Arabidopsis, it is plausible that miR171 will encourage root hair development when GSNO is present (Moro et al., 2017; Santos et al., 2022).
Figure 4: Functions of miR171 and its target genes in plants.
Arrows indicate upregulation, blunted lines indicate downregulation, and dotted lines indicate undefined correlations or functions.Several investigations have explored the involvement of miR171 and its target genes in the plant’s response to abiotic stress (as shown in Table 2 and Fig. 4). Nonetheless, some questions remain unanswered, such as how miR171 collaborates with other miRNAs to regulate the same stress and whether there is any correlation among the various target genes controlled by miR171.
| Species | Biological processes and the expression changes of miR171 (↑up, ↓ down) | Target gene | References |
|---|---|---|---|
| Arabidopsis thaliana | Nitrogen starvation ↑, heat stress ↑, high salinity stress ↑, drought stress ↑, temperature stress ↑, GSNO stress ↑ |
SCL6 |
Liang, He & Yu (2012), Mahale et al. (2014), Liu et al. (2008), Santos et al. (2022) |
| Oryza sativa | Drought stress ↑↓, bacterial infection ↑, chromium stress ↓↑ |
SCL; ACP1 |
Zhou et al. (2010), Tong et al. (2017), Dubey et al. (2020), Sasani et al. (2020) |
| Solanum lycopersicum | Hypoxia stress ↓, arbuscular mycorrhizal symbiosis ↑ |
GRAS24 |
Hou et al. (2019), Wu et al. (2016) |
| Populus tomentosa | Drought stress ↓, flooding stress ↓ |
Ren et al. (2012) | |
| Medicago truncatula | Drought stress ↓, arbuscular mycorrhizal symbiosis ↑ |
GRAS; NSP2 |
Wang et al. (2011), Lauressergues et al. (2012) |
| Prunus persica | Drought stress ↑ | Eldem et al. (2012) | |
| Solanum tuberosum | Drought stress ↓↑ | GRAS | Hwang et al. (2011) |
| Spartina alterniflora | Salinity stress ↓ | Qin et al. (2015) | |
| Ipomoea campanulata | Drought stress ↓ | Ghorecha et al. (2017) | |
| Morus alba | Salinity stress ↑, drought stress ↑ |
LOM2; LOM3 | Sun et al. (2022) |
| Citrus | Boron toxicity ↓, bacterial infection ↓ |
SCL |
Huang et al. (2019), Reyes et al. (2015) |
| Triticum aestivum | UV-B stress ↑, Zinc oxide nanoparticles ↓↑ |
GRAS |
Wang et al. (2013), Niazi et al. (2022) |
| Nitotiana tabacum | Drought stress ↓ | SCL | Adalı (2015) |
| Glycine max | Bacterial infection ↑↓ | Hossain et al. (2019) | |
| Hibiscus cannabinus | Bacterial infection | SCL1 | Gao, Wan & Wong (2013) |
| Lotus japonicus | Arbuscular mycorrhizal symbiosis ↑ | NSP2 | Luis et al. (2012) |
Plant-microbe interactions
Studies on viral infection and symbiosis have revealed a complex reciprocal relationship between plants and microbes, and miRNA plays a crucial role in these processes. For instance, interactions between soybean and Bradyrhizobium japonicum led to the overexpression of miR482 and miR1515, causing an increase in the number of nodulations in soybean (Li et al., 2010). Several other reports have investigated the role of mir171 in plant-microbe interactions. In response to infection with the bacterium B. japonicum, Gma-miR171o, and Gma-miR171q display opposite expression patterns; Gma-miR171q shows up-regulation while Gma-miR171o shows down-regulation (Hossain et al., 2019). Rhizoctonia solani infection, which simulates sheath blight disease in rice, leads to an increase in Osa-miR171 expression in the later stages of stress (Sasani et al., 2020).
On the other hand, infection with the rice stripe virus results in down-regulation of Osa-miR171b. At the same time, overexpression of this miRNA confers resistance to the virus and mitigates virus-induced symptoms (Tong et al., 2017). Compared to control plants, Hibiscus chlorotic ringspot virus infection of kenaf up-regulates miR171, while the corresponding target gene SCL1 shows opposite changes in expression (Gao, Wan & Wong, 2013). The miR171 in sweet orange shows almost 3-fold lower expression in plants infected with Citrus psorosis virus and a corresponding enrichment of the target gene SCL6 (Reyes et al., 2015). This change led to the relative accumulation of the target gene SCL6 (Reyes et al., 2015). Further immunoprecipitation experiments showed the interaction between pre-miR171a and viral protein (Reyes et al., 2015).
Previous research has also investigated the function of miR171 in regulating the symbiotic relationship between plants and microbes. For instance, in Lotus japonicas, the expression of Lja-miR171c is higher in infected nodules than in healthy ones, indicating its potential involvement in bacterial infection (Luis et al., 2012). In Medicago truncatula, overexpression of miR171 h results in a nearly 50% decrease in arbuscular mycorrhizal colonization, similar to that observed in the nsp2 mutant (Lauressergues et al., 2012). In addition, miR171 h-NSP2 is crucial for the Myc-LCO signaling pathway (Lauressergues et al., 2012). Interestingly, miR171b enhances mycorrhization by promoting the expression of the target gene LOM1 and shielding it from cleavage by other miR171 family members (Couzigou et al., 2016). Tomato miR171 g, which shares a high sequence similarity with Mtr-miR171 h, was also up-regulated upon Rhizophagus irregularis inoculation treatment. Moreover, the same trend was observed for miR171i, suggesting a role in regulating arbuscular mycorrhizal symbiosis (Wu et al., 2016). Thus, different miR171 members play different roles in the same biological process, controlling plant-microbe symbiosis through many targets.
Interaction between the miR171 network and other small-RNAs
Several studies have shown the complex interactions between the miR171 regulatory network and other miRNAs. Transgenic maize with overexpressing miR171 and miR156 has a similar phenotype of prolonged juvenile development (Chuck et al., 2007a; Chuck et al., 2007b). Since miR156b/c overexpression in maize down-regulates miR172, miR171 may also affect the miR156-miR172 pathway (Chuck et al., 2007b; Chuck, Meeley & Hake, 2008). Barley also showed similar results. Transgenic plants with up-regulation of miR171 (OE171) showed a similar phenotype as transgenic plants with down-regulation of miR172 (Curaba et al., 2013; Brown & Bregitzer, 2011). Moreover, in the inflorescence tissue of OE171, the expression of miR156 increases but not that of miR172, whereas the target gene of miR156, SCL is also down-regulated (Curaba et al., 2013). Interestingly, in young leaves of OE171, increased miR156 accumulation was accompanied by decreased miR172 expression, suggesting that miR171 affects the phase transition through the regulation of miR156 and miR172 in a spatiotemporally dependent manner (Curaba et al., 2013). In Arabidopsis, overexpression of miR171 significantly reduced trichomes on the stem, and its target genes, LOMs, had an antagonistic effect on the SPLs, the target genes of miR156 (Xue et al., 2014). It altogether suggests an indirect interaction between miR171 and miR156. The stem cell niche (SCN) is composed of the quiescent center (QC), also known as the root stem cell niche, and the surrounding initial stem cells (Pardal & Heidstra, 2021). SCARECROW(SCR) is involved in the regulation of the SCN and plays a role in the specification and maintenance of the QC. In addition, miR396 also plays a role in maintaining the SCN (Pardal & Heidstra, 2021). Based on these studies, we hypothesize that some degree of interaction exists between miR171 and miR396 for root system development.
The construction of a ceRNA network associated with drought resistance in rice identified a lncRNA (MSTRG.28732.3) that functions as a target gene for miR171. According to RT-qPCR analysis, the expression patterns of both genes were found to be inversely correlated (Yang et al., 2022). In response to salt stress, the screening of differentially expressed lncRNAs using ssRNA sequencing on duckweed with and without salt treatment revealed that some lncRNAs might act as target genes that interact with miR171 to provide protection against salt stress (Fu et al., 2020). Additionally, studies indicate that specific lncRNAs are also targeted by multiple miRNAs; for instance, TCNOS_00033722 is targeted by miR156, miR169, and miR393, while TCONS_00044328 and TCONS_00059333 are targeted by miR171, miR167, and miR168 (Fu et al., 2020). Interestingly, the lncRNA TCONS_00155383 acts as a precursor of miR171 in polyploid switchgrass (Yan et al., 2018). Mutations in SCR, a predicted target gene for miR171, result in proliferation and abnormal differentiation of the bundle sheath cells, which suggests that both miR171 and lncRNA are involved in stem cell development (Yan et al., 2018; Slewinski et al., 2012).
Conclusions
MiR171, one of the earliest miRNAs discovered in plants, has a length of 20 to 23 nts and is one of the oldest and most evolutionarily conserved miRNAs. The fragments “GAGCCG” and “CAAUAU” are mostly conserved across all miR171 sequences. MiR171 plays a role in plant growth and development, hormone signaling, stress responses, and microbial interactions (Xue et al., 2014; Huang et al., 2016; Sun et al., 2022; Sasani et al., 2020). Its target genes are present across different gene families; however, the most common targets belong to the GRAS family of transcription factors, along with a small number of miRNAs, lncRNAs, and other genes (Han & Zhou, 2022; Xue et al., 2014; Yang et al., 2022; Luis et al., 2012). The wide range of its target genes can also account for the functional diversity of miR171.
As shown in the 1st, 3rd, 4th, and 12th rows of Table 1, miR171 can act on different target genes and play different roles in the same species. Moreover, miR171 has been found to target the same or different genes to achieve similar effects across various species. In Arabidopsis, miR171 cleaves SCL6-II/III/IV and SCL27 to regulate chlorophyll biosynthesis and organ development (Xue et al., 2014; Ma et al., 2014). Even ath-miR171* has been observed to target SUVH8, thereby influencing plant height and leaf type (Manavella et al., 2013). In both Citrus and Larix leptolepis, miR171 targets SCL to regulate somatic embryogenesis (Shi et al., 2022; Zhang et al., 2010). In Medicago truncatula, miR171 targets NSP2 to regulate root development, but in Arabidopsis, miR171 exerts the same function by regulating another target gene, SCL6 (Lauressergues et al., 2015; Wang et al., 2010). In addition, different propagation patterns of the same species can also lead to differences in miR171 levels. For example, micro-propagated D. officinale has much higher levels of miR171 than conventional cultivars, which also accounts for the difference in their growth rates (Yang et al., 2015). In addition, miR171 and its target genes may exhibit varying patterns of accumulation in response to different levels of stress. For instance, the expression of miR171 is decreased at low concentrations of ZnONPs, while high concentrations lead to its upregulation, indicating the existence of a minimum threshold for miR171 expression (Niazi et al., 2022). The same stress in different species also causes variation in expression levels of miR171. In Table 2, 1st, 4th to 7th, 10th, 11th and14th rows demonstrate that, in response to drought stress, miR171 expression is down-regulated in Triticum dicoccoides, Populus tomentosa, Medicago truncatula, and up-regulated in Arabidopsis, Prunus persica, and Morus alba (Kantar, Lucas & Budak, 2011; Ren et al., 2012; Wang et al., 2011; Liang, He & Yu, 2012; Eldem et al., 2012; Sun et al., 2022).
Overall, this review summarizes the information on the role of miR171 in plant development and its response to external negative stimuli. However, a clear picture of how different miR171 members interact with their common targets in the same plant species while participating in biological processes is still lacking. In addition, the relevant regulatory mechanisms for the passenger strand and uplink-downlink networks are still unclear and need further exploration. Despite our limited understanding of the miR171 association network and its interacting genes or proteins, the collective functions of miR171, such as somatic embryogenesis, phytohormone signaling responses, and plant-microbe interactions, are gradually becoming clear.