Integrated analysis of lncRNAs, mRNAs, and TFs to identify network modules underlying diterpenoid biosynthesis in Salvia miltiorrhiza
- Published
- Accepted
- Received
- Academic Editor
- Douglas Domingues
- Subject Areas
- Bioinformatics, Genetics, Genomics, Molecular Biology, Plant Science
- Keywords
- LncRNA-mRNA/TF, Diterpenoid pathway, Salvia miltiorrhiza
- Copyright
- © 2023 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. Integrated analysis of lncRNAs, mRNAs, and TFs to identify network modules underlying diterpenoid biosynthesis in Salvia miltiorrhiza. PeerJ 11:e15332 https://doi.org/10.7717/peerj.15332
Abstract
Long non-coding RNAs (lncRNAs) are transcripts of more than 200 nucleotides (nt) in length, with minimal or no protein-coding capacity. Increasing evidence indicates that lncRNAs play important roles in the regulation of gene expression including in the biosynthesis of secondary metabolites. Salvia miltiorrhiza Bunge is an important medicinal plant in China. Diterpenoid tanshinones are one of the main active components of S. miltiorrhiza. To better understand the role of lncRNAs in regulating diterpenoid biosynthesis in S. miltiorrhiza, we integrated analysis of lncRNAs, mRNAs, and transcription factors (TFs) to identify network modules underlying diterpenoid biosynthesis based on transcriptomic data. In transcriptomic data, we obtained 6,651 candidate lncRNAs, 46 diterpenoid biosynthetic pathway genes, and 11 TFs involved in diterpenoid biosynthesis. Combining the co-expression and genomic location analysis, we obtained 23 candidate lncRNA-mRNA/TF pairs that were both co-expressed and co-located. To further observe the expression patterns of these 23 candidate gene pairs, we analyzed the time-series expression of S. miltiorrhiza induced by methyl jasmonate (MeJA). The results showed that 19 genes were differentially expressed at least a time-point, and four lncRNAs, two mRNAs, and two TFs formed three lncRNA-mRNA and/or TF network modules. This study revealed the relationship among lncRNAs, mRNAs, and TFs and provided new insight into the regulation of the biosynthetic pathway of S. miltiorrhiza diterpenoids.
Introduction
Long non-coding RNAs (lncRNAs) are defined as RNA transcripts with little or no potential for protein-coding capacity, and with at least 200 nucleotides (nt) in size (Ponting, Oliver & Reik, 2009; Nagano & Fraser, 2011; Palazzo & Koonin, 2020). They are usually transcribed by RNA polymerase II (Rinn & Chang, 2020). Functional analysis of eukaryotic lncRNAs has revealed that they act as molecular scaffolds, guide molecules, molecular sponges and decoys, precursors of microRNAs (miRNAs) and other small RNAs, or as miRNA target mimics (TMs) to regulate gene expression at multiple levels (epigenetic regulation, transcriptional regulation, and post-transcriptional regulation) (Franco-Zorrilla et al., 2007; Mercer, Dinger & Mattick, 2009; Wang & Chekanova, 2017; Rai et al., 2019). LncRNAs can also act on transcription factors (TFs); for example, lncRNA can be used as a TF-binding site to regulate its expression (Yu et al., 2019). LncRNAs are usually expressed at a low level and in a tissue-specific manner (Liu et al., 2012; Palazzo & Koonin, 2020). The subcellular localization of lncRNAs is the primary determinant of their molecular functions (Carlevaro-Fita & Johnson, 2019).
There is much evidence suggesting that lncRNAs play key roles in plant secondary metabolism. For example, mLncR8 putatively regulates terpenoid biosynthesis, and mLncR31 is involved in the biosynthesis of the isoprenoid side chain of ubiquinone and plastoquinone in Digitalis purpurea (Wu et al., 2012a). LncRNAs might regulate genes in the phenylpropanoid pathway of Populus tomentosa (Zhou et al., 2017). LncRNAs were also found to be involved in rubber biosynthesis in Eucommia ulmoides (Liu et al., 2018). LncRNAs were possibly involved in the biosynthesis of different fatty acids and lipid metabolism through post-transcriptional regulation in tree peony seeds (Yin et al., 2018). LncRNAs might be involved in the lignin biosynthetic pathway in Populus (Quan et al., 2019).
Studies have suggested that lncRNAs can act as local regulators, and lncRNA expression is correlated with the expression of nearby genes (Guil & Esteller, 2012; Engreitz et al., 2016). These correlations are attributed to sequence-specific functions of the mature lncRNA transcript, the transcription or splicing of an RNA, or DNA elements within the lncRNA promoter or gene locus, namely cis-regulation (Guil & Esteller, 2012; Engreitz et al., 2016; Kopp & Mendell, 2018).
Co-expressed genes are usually members of the same protein complex or metabolic pathway, and they are functionally controlled by the same transcriptional regulatory program. The genes or proteins within the co-expression network may have the same expression patterns (Liao et al., 2011; Rao & Dixon, 2019).
The medicinal plant S. miltiorrhiza produces a variety of diterpenoids (Ma et al., 2015). Tanshinones are the main bioactive compounds of S. miltiorrhiza, and they mainly accumulate in the roots of S. miltiorrhiza (Xu et al., 2015; Chang et al., 2019). Another diterpenoid in S. miltiorrhiza is the plant hormone gibberellin (GA), which is one of the five classic plant hormones (Brockdorff, 1998). In general, the biosynthetic pathway of terpenoids in S. miltiorrhiza can be divided into three stages (Ma et al., 2012). The first stage leads to the synthesis of the universal isoprene precursor isopentenyl diphosphate (IPP) and its isomer dimethylallyl diphosphate (DMAPP) through the 2-C-methyl-D-erythritol 4-phosphate (MEP) pathway and/or the mevalonate (MVA) pathway. In the second stage, the intermediate diphosphate precursors, including geranyl diphosphate (GPP), farnesyl diphosphate (FPP), and geranylgeranyl diphosphate (GGPP) are synthesized under the catalysis of isoprenyl diphosphate synthases (IDSs), including geranyl diphosphate synthase (GPPS), farnesyl diphosphate synthase (FPPS), and geranylgeranyl diphosphate synthase (GGPPS). The last stage involves the formation of diverse diterpenoids under the catalysis of terpene synthases (TPSs), such as copalyl diphosphate synthase (CPS) and kaurene synthase (KS) catalyze the formation of miltiradiene (Kai et al., 2010; Kai et al., 2011; Lu, 2021), ent-copalyl diphosphate synthase (ent-CPS) (Shimane et al., 2014) and ent-kaurene synthase (ent-KS) are involved in the conversion of GGPP to the tetracyclic hydrocarbon intermediate ent-kaurene (Yamaguchi, 2008; Shimane et al., 2014). Then tanshinones and GAs are formed by cytochrome P450 monooxygenases (P450s) and 2-oxoglutarate-dependent dioxygenases (2ODDs) modification.
Methyl jasmonate (MeJA) is a hormone involved in plant signal transduction, which is considered to play an indispensable role as a second messenger in the induction process leading to the accumulation of secondary metabolites. Therefore, it is often used as an inducer to explore the regulation mechanism of biosynthesis (Gundlach et al., 1992; Wasternack, 2007). MeJA, an effective elicitor, can enhance the accumulation of tanshinones and phenolic acids by inducing the expression of tanshinone biosynthesis- and phenolic acid biosynthesis-related genes in S. miltiorrhiza (Gao et al., 2009; Xiao et al., 2011; Liang et al., 2012; Luo et al., 2014). MeJA is used for genome-wide identification and characterization of novel terpenoid biosynthetic genes in S. miltiorrhiza (Ma et al., 2012).
Previous studies of lncRNAs in S. miltiorrhiza (Li, Shao & Lu, 2015) showed that 3,044 lncRNAs responded to Ag + solution and yeast extract (YE) in the roots of S. miltiorrhiza,15 lncRNAs differentially expressed in leaves under MeJA treatment. Jiang et al. identified the differential expression of natural antisense transcripts (NATs) with polyA tail in different tissues of S. miltiorrhiza, and some cis-NATs of SmKSL1 showed a high co-expression relationship and possible participation in tanshinone synthesis (Jiang et al., 2021). However, although lncRNAs reportedly play important roles in S. miltiorrhiza, the role of lncRNAs in the diterpenoid biosynthetic pathway of S. miltiorrhiza remains largely unclear.
Based on transcriptomic data from four varieties of S. miltiorrhiza, we carried out among lncRNAs, mRNAs, and TFs expression correlation analysis and genome loci analysis to obtain candidate lncRNA-mRNA/TF pairs, and to construct the co-expression network. To further explore the relationship among the candidate lncRNA-mRNA/TF pairs, we analyzed the time-series expression patterns of the candidate lncRNA-mRNA/TF pairs in MeJA-induced S. miltiorrhiza.
Materials & Methods
Plant materials and MeJA treatment
S. miltiorrhiza seedlings were cultured in the greenhouse under 23 °C–−25 °C. The plants were sprayed with 200 μM MeJA solution as mentioned in a previous report (Luo et al., 2014). After being treated with MeJA solution for 6, 12, 24, and 48 h, the treated and the non-treated (0 h) roots were collected and rinsed with water. These roots were dried gently and quickly with absorbent paper. The cleaned roots were immediately frozen in liquid nitrogen and stored at −80 °C until RNA isolation. Three biological replicates were carried out for each experiment.
S. miltiorrhiza genomic and transcriptomic data
The S. miltiorrhiza genome data were downloaded from NCBI (National Center for Biotechnology Information) Sequence Read Archive database (SRP051524, SRP051564, SRP028388) (Xu et al., 2016) and NCBI (National Center for Biotechnology Information) BioProject: PRJNA682867 (Ma et al., 2021). The transcriptomic data were obtained from our previous study (Zhou et al., 2021) with an accession number assigned to PRJNA712174. The transcriptomic data were gathered from four varieties of S. miltiorrhiza root tissues during the tanshinone accumulation stage and included Fragments Per Kilobase Million (FPKM) expression value and annotation information.
Pipeline for lncRNA identification
The following four filters were used to shortlist the bona fide lncRNAs from the transcriptomic data: (1) transcripts with annotation information in one of the Nr (Non-RedundantProtein Sequence), Swiss-Prot, and COG/KOG databases were removed; (2) transcripts shorter than 200 nt with an open reading frame (ORF) longer than 100 aa were discarded, found and extracted ORFs on getorf (http://emboss.bioinformatics.nl/cgi-bin/emboss/getorf), selection of ORF length <100 aa by R (version 4.2.1) script; (3) transcripts were searched against the Pfam database (Punta et al., 2012) (http://pfam.xfam.org/) by HMMER to remove transcripts possibly containing known protein domains; and (4) the protein-coding potential of each transcript was calculated using PLEK (Li, Zhang & Zhou, 2014) and Coding Potential Calculator 2 (CPC2, http://cpc2.cbi.pku.edu.cn) (Kang et al., 2017), and these with PLEK and CPC2 scores >0 were discarded. Through the above process, identified lncRNAs were obtained. FPKM value less than 0.05 was used as the standard for low expression levels (Li et al., 2016). The transcripts that remained were regarded as candidate lncRNAs.
Characterization and conservation analysis of S. miltiorrhiza lncRNAs
To gain more understanding of these lncRNAs in S. miltiorrhiza, we compared several different features of the lncRNAs and mRNAs: GC content and transcript length. GC content and transcript length were determined by R (version 4.2.1), and statistical analysis was carried out by Excel.
We aligned the lncRNA sequences identified here with BLAST+ (Camacho et al., 2009) (blast−2.11.0+) against the genome sequences of the Lamiaceae family: Salvia splendens, Salvia hispanica, Mentha longifolia, Scutellaria baicalensis, Pogostemon cablin, and Sesamum indicum, of which Salvia splendens and Salvia hispanica both belong to the Salvia genus; Solanaceae family: Nicotiana tabacum, Brassicaceae family: Brassica napus and Arabidopsis thaliana; and Selaginellaceae family: Selaginella moellendorffii. A cutoff E-value <1e−10 was used. The genomes were downloaded from the NCBI databases: GCF_004379255.1 (SspV2), GCF_023119035.1 (UniMelb_Shisp_WGS_1.0), GCA_001642375.2 (Mlong_CMEN585_v), GCA_005771605.1 (ASM577160v1), GCA_023678885.1 (GZUCM_PCab_1.0), GCF_000512975.1 (S_indicum_v1.0), GCF_000715135.1 (Ntab-TN90), GCF_020379485.1 (Da-Ae), GCF_000001735.4 (TAIR10.1), and GCF_000143415.4 (https://www.ncbi.nlm.nih.gov/data-hub/genome/GCF_000143415.4/). The lncRNAs that had coverage of >20% of matched regions were defined as conserved lncRNAs.
Precursors of miRNA and miRNA target prediction in S. miltiorrhiza lncRNAs
The candidate lncRNAs may act as the precursors of miRNAs, the S. miltiorrhiza miRNAs in miRBase (Kozomara, Birgaoanu & Griffiths-Jones, 2019) (Release 22.1, http://www.mirbase.org/) and PmiREN2.0 (Guo et al., 2022) (https://pmiren.com/) were aligned to the sequences of the candidate lncRNAs. The secondary structure of lncRNAs was predicted by RNAfold (Gruber et al., 2008) (http://rna.tbi.univie.ac.at/). LncRNAs with classical stem-loop hairpins were regarded as the putative precursors of miRNA (Zhou et al., 2017). Given that miRNA targets and lncRNAs have highly similar miRNA-binding sites, miRNA can be sequestered by lncRNA (Paschoal et al., 2017). Three kinds of prediction software were used to determine the miRNAs targeted to candidate lncRNAs. The first was TAPIR (Bonnet et al., 2010) (http://bioinformatics.psb.ugent.be/webtools/tapir); this server offers the possibility to search for plant miRNA targets using a fast and precise algorithm (score ≤ 4, free energy ratio ≥ 0.7). The second was psRobot (Wu et al., 2012b) (Version 1.2) with default parameters, which is a widely used online miRNA target prediction tool. The third was psRNATarget (2017 release) with default settings, and psRNATarget was developed to identify plant sRNA targets by (i) analyzing complementary matching between the sRNA sequence and target mRNA sequence using a predefined scoring schema and (ii) by evaluating target site accessibility (Dai, Zhuang & Zhao, 2018), targets with an E value less than 5.0 were retained as potential miRNA targets.
Subcellular localization of lncRNAs
The subcellular localization of S. miltiorrhiza lncRNAs was predicted by lncLocator (Cao et al., 2018). LncLocator is an ensemble classifier-based predictor, which adopts both k-mer features and high-level abstraction features generated by unsupervised deep models and constructs four classifiers by feeding these two types of features to support vector machine and random forest, respectively. The current lncLocator can predict five subcellular localizations of lncRNAs, namely, cytoplasm, nucleus, cytosol, ribosome, and exosome.
Predicted lncRNAs related to the diterpenoid biosynthesis in S. miltiorrhiza
Diterpenoid biosynthetic genes and the genes encoding TFs involved in diterpenoid biosynthesis were screened from the transcriptomic annotation data of S. miltiorrhiza. Our research focused on the downstream of the diterpenoid biosynthetic pathway (Fig. S1).
The functions of lncRNA can be performed on diterpenoid biosynthetic genes/TFs in a cis manner (Kopp & Mendell, 2018), and the lncRNAs and their target genes are considered lncRNA-mRNA/TF pairs. Pearson correlation coefficient (PCC) of the expression of lncRNA and its target gene pair was calculated using the psych (Revelle, 2022) package in R, requiring co-expressed gene pairs with —PCC— ≥ 0.4 and p ≤ 0.05. The basis for predicting cis-regulation was related to the positional relationship of lncRNA genes and coding genes on the genome (Liu et al., 2019). It was determined to be cis-regulatory if the lncRNA gene was within 100 kilobases (kb) upstream or downstream regions of the target genes used (Huang et al., 2018; Wang et al., 2021). used. For genomic location analysis, the co-expressed gene pairs were aligned against two S. miltiorrhiza genomes (Xu et al., 2016; Ma et al., 2021) by BLAST+ (BLAST−2.11.0+) with a cutoff of E value <1e−5. Finally, the candidate lncRNA-mRNA/TF pairs related to the diterpenoid biosynthesis of S. miltiorrhiza were obtained.
Construction of the diterpenoid biosynthesis-related lncRNA-mRNA-TF networks and identification of hub genes
Cytoscape 3.2 (Shannon et al., 2003) was utilized to draw the putative lncRNA-mRNA-TF co-expression network with —PCC— ≥ 0.8 (p ≤ 0.05). Genes with a degree ≥ 10 were considered hub genes.
Candidate lncRNA-mRNA/TF pairs expression profile in MeJA-induced S. miltiorrhiza
MeJA is an effective elicitor for the production of diterpenoid tanshinones in S. miltiorrhiza (Luo et al., 2014). To further explore whether candidate lncRNA-mRNA/TF pairs have a response to the diterpenoid biosynthetic pathway, we performed the quantitative real-time polymerase chain reaction (qRT-PCR) to analyze expression patterns of the detected lncRNA-mRNA/TF pairs under MeJA induction in S. miltiorrhiza. Plant materials treated with MeJA dissolving media were used as a control (0 h), and three biological replications were carried out. The 2(-delta-delta CT) method was used for calculating the relative expression levels of genes. ANOVA was calculated using SPSS (Version 23.0, IBM, USA), and p < 0.05 and p < 0.01 were considered statistically significant. The hub genes were identified by using the cytoHubba (Chin et al., 2014) plug-in of Cytoscape 3.2. To increase the sensitivity and specificity, we proposed Maximal Clique Centrality (MCC) to discover featured nodes. Subsequently, we constructed a network module of these hub genes.
RNA extraction and qRT-PCR
Total RNA was extracted from plant tissues using the RNeasy plant kit (PH-01013-B, Foregene, Chengdu, China). RNA degradation and contamination were monitored using 1% RNase-free agarose gel electrophoresis, and the RNA purity was analyzed using a NanoPhotometer™ -N60 ultra-micro spectrophotometer. The reverse transcription reaction was used RT Easy™ II (With gDNase) kit (Version Number: 1.0-1904, Foregene, Chengdu, China) following the instruction manual. qRT-PCR was performed in a 20 μL reaction volume containing primers by Real-Time PCR EasyTM-SYBR Green I kit (Cat. No. QP-01011/01012/01013/01014, Foregene, Chengdu, China) following the instruction manual. qRT-PCR was carried out in triplicate reactions in the LineGene K Plus real-time PCR detection system (Bioer, Hangzhou, China). Primers were designed using the Primer-BLAST (Ye et al., 2012). Primer sequences were listed in Table S1. β-actin (Yang et al., 2010) gene was selected as a reference since it showed stable expression in the S. miltiorrhiza tissues analyzed compared to others. The reaction program was as follows: 3 min at 95 °C, 10 s at 95 °C, 30 s at 60 °C, and 40 cycles. The temperature was then gradually increased to produce melting curves for amplification specificity verification. The mean value of three replicates was normalized using β-actin as the endogenous control. The 2(-delta-delta CT) method was used for calculating the relative expression of genes.
Results
Identification of candidate lncRNAs
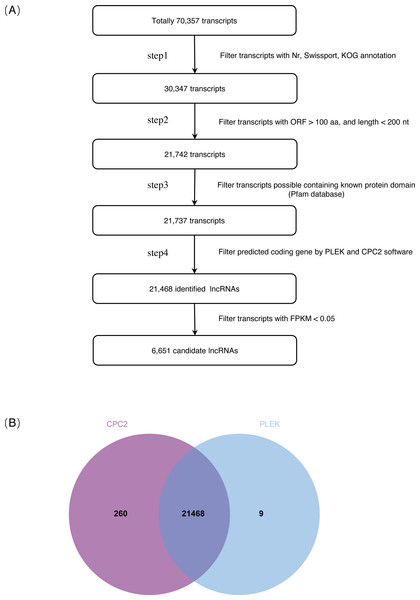
On the basis of the strict lncRNA identification pipeline, a total of 30,347 transcripts with no annotation information were obtained. A total of 21,742 transcripts with length >200 nt and ORF <100 aa were obtained. Subsequently, a total of 21,468 identified lncRNAs were obtained from the intersection of PLEK and CPC2 software prediction results (Fig. 1, Tables S2, S3). Expression transcripts with FPKM ≤ 0.05 were filtered, and a total of 6,651 candidate lncRNAs were selected for further analysis (Table S4).
Figure 1: Identification of lncRNAs in S. miltiorrhiza.
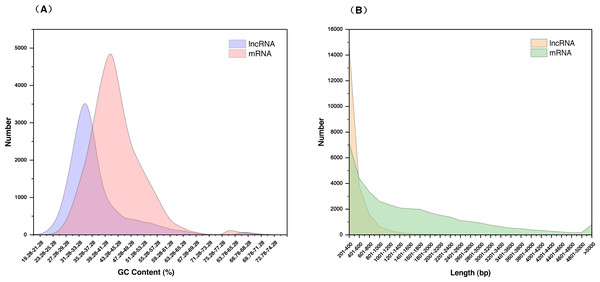
(A) Pipeline for lncRNAs identification in S. miltiorrhiza. Step1: transcripts with annotation information in one of the Nr, Swiss-Prot, and COG/KOG databases were removed; step2: transcripts shorter than 200 nt with an ORF longer than 100 aa were discarded; step3: transcripts were searched against the Pfam database to remove transcripts possibly containing known protein domains; and step4: the protein-coding potential of each transcript was calculated using PLEK and CPC2, transcripts with PLEK and CPC2 scores >0 were discarded. nt nucleotide, ORF open reading frame, aa amino acid. (B) Venn diagram showing the number of lncRNAs identification results in PLEK and CPC2 software, and their overlap.The 21,468 identified lncRNAs ranged from 201 nt to 4,340 nt in length, the majority of which (approximately 68.95%) were 200–400 nt. The mean length was 392 nt, which was lower than the values observed in S. miltiorrhiza mRNAs (mean length = 1,515 nt). The GC content of lncRNAs was mainly concentrated at 31.28%–37.28% (accounting for 46.32%), whereas mRNAs were mainly concentrated at 39.28%–41.28% (accounting for 36.00%). The mean GC content of lncRNAs was approximately 36.86%, which was slightly lower than that of mRNA sequences (approximately 43.42%) (Fig. 2, Table S5).
Figure 2: Characterization of lncRNAs in S. miltiorrhiza.
(A) GC% distribution of the identified lncRNAs and mRNAs in S. miltiorrhiza. (B) Size distribution of the identified lncRNAs and mRNAs in S. miltiorrhiza.Characterization and conservation of lncRNAs in S. miltiorrhiza
Conservation analysis showed that 21.02% and 15.40% of S. miltiorrhiza lncRNAs (6,651 candidate lncRNAs) were conserved compared with Salvia splendens and Salvia hispanica of the same genus, respectively. However, in the same family of the different genera of Mentha longifolia, Scutellaria baicalensis, Pogostemon cablin, and Sesamum indicum, 6.54%, 0.96%, 0.83%, and 0.51% of S. miltiorrhiza lncRNAs were conserved, respectively. Among plants of different families, only 0.05%, 0.02%, and 0.02% of S. miltiorrhiza lncRNAs were conserved in Nicotiana tabacum, Arabidopsis thaliana, and Brassica napus, respectively. There was no match in Selaginella moellendorffii. The results of the conservation analysis are presented in Table S6.
LncRNAs might be used as precursors or target mimics of S. miltiorrhiza miRNAs
In this study, a total of 17 lncRNAs were identified as potential precursors of 41 S. miltiorrhiza miRNAs (Table S7). To improve the prediction accuracy, the intersection of three kinds of software prediction results was selected. A total of 14 lncRNAs were identified as potential targets of 66 S. miltiorrhiza miRNAs from 18 families according to PmiREN2.0 (Table S7).
Subcellular localization predictions indicated that most S. miltiorrhiza lncRNAs were found in the cytoplasm and nucleus
Subcellular localization of lncRNA is closely related to its function. At present, many studies have indicated that lncRNA contains specific RNA motifs with nuclear localization (Zhang et al., 2014). Meanwhile, in the subcellular localization of lncLocator (Cao et al., 2018), a total of 3,381 cytoplasms, 3,316 nucleus, 83 exosomes, 51 cytosols, and 20 ribosomes were obtained from 6,651 candidate lncRNAs. Among our candidate lncRNA-mRNA/TF pairs, 13 lncRNAs were found in the nucleus, 9 lncRNAs were found in the cytoplasm, and 1 lncRNA was found in the exosome (Table S8).
Genes and TFs involved in diterpenoid biosynthesis from transcriptomic data
To investigate the potential lncRNAs involved in diterpenoid biosynthesis, we predicted the potential targets of lncRNAs in cis-regulatory relationships. In transcriptomic annotation, we obtained 46 diterpenoid biosynthetic genes: GPPS (Van (Schie et al., 2007), FPPS, GGPPS, CPS, ent-CPS, KS, ent-KS, CYP76AH1 (Guo et al., 2013; Ma et al., 2016), CYP76AK2, CYP76AK3 (Li et al., 2021), CYP76AK5 (Xiangdong & Lizhi, 2017), ent-kaurene oxidase (KO) (Hedden & Thomas, 2012), ent-kaurenoic acid oxidase (KAO) (Helliwell et al., 2001), GA 2-oxidase (GA2ox), GA 3-oxidase (GA3ox), GA 20-oxidase (GA20ox) (Hedden & Thomas, 2012; Du et al., 2015), and 11 TFs: bHLH148, ERF6, GRAS1, MYB36 and WRKY2 (Li & Lu, 2014; Zhang et al., 2015; Li et al., 2015; Li et al., 2019; Ji et al., 2016), belonging to five TF families: bHLH, ERF, GRAS, MYB, and WRKY. The list of the above genes is shown in Table S9.
LncRNAs related to the diterpenoid biosynthetic pathway
We detected 6455 lncRNAs that were co-expressed with 46 genes and 11 TFs involved in diterpenoid biosynthesis. In the PCC matrix, we obtained 45,198 correlation gene pairs with —PCC—≥ 0.4 (p ≤ 0.05), of which 47,946 pairs were positively correlated and 5,775 pairs were negatively correlated. At the positional level, combining the results from the two S. miltiorrhiza genomes (Xu et al., 2016; Ma et al., 2021), a total of 180 pairs of lncRNA-mRNA/TF were located adjacent to each other within 100 kb (including ±3 kb), of which 23 pairs were co-located and co-expressed. A total of 23 candidate lncRNA-mRNA/TF pairs were obtained (Table S10).
LncRNA-mRNA-TF networks and hub genes
The gene co-expression network was constructed with —PCC— ≥ 0.8 (p ≤ 0.05) as the threshold, the visualization is shown in Fig. S2A. We obtained 1,402 gene pairs with —PCC—≥ 0.8. We obtained 24 mRNAs with a degree > 10, which were considered hub genes (Table S11). In the 23 gene pairs of lncRNA-mRNA/TF, 8 mRNAs were present in these hub genes. Only two gene pairs Smlnc0032870-Sm0012648 (GA2OX) and Smlnc0018769-Sm0037093 (KS2) existed in the network with high expression correlation of 0.85 and 0.80, respectively. The hub genes Sm0012648 and Sm0037093 in these two gene pairs were used to construct the subnetworks (Figs. S2B, S2C).
Tissue specificity of lncRNA expression
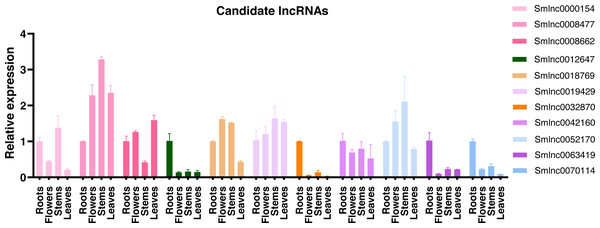
Using the qRT-PCR, expression patterns of lncRNAs in the candidate lncRNA-mRNA/TF pairs were analyzed in roots, stems, leaves, and flowers of 2-year-old field greenhouse-grown S. miltiorrhiza plants. Of them, 11 lncRNAs were detected in at least one tissue and showed tissue-specific expression. The other lncRNAs were undetected, suggesting that they could be not expressed or expressed at a low level in the tissues analyzed. Smlnc0012647, Smlnc0032870, Smlnc0042160, Smlnc0063419, and Smlnc0070114 exhibited the highest expression in root tissue. Smlnc0000154, Smlnc0008477, Smlnc0019429, and Smlnc0052170 were more stem-specific. Smlnc0018769 was expressed mainly in flowers and stems. Smlnc0008662 showed high expression in roots, flowers, and leaves and low expression in stems (Fig. 3). Thus, lncRNAs showed obvious differential expression in different tissues, which may be related to their regulatory function. These results suggest that the expression of lncRNAs may be limited to specific tissue types or regulated by development in S. miltiorrhiza.
Figure 3: Tissue specificity of lncRNA expression.
Validation of the expression level of 11 lncRNAs in roots, flowers, stems, and leaves tissues of S. miltiorrhiza. Fold changes of lncRNA levels were shown. The level of transcripts in roots was arbitrarily set to 1 and the level in other tissues was given relative to this. Error bars represent the standard deviations of three qRT-PCR replicates.Time-series expression pattern of candidate lncRNA-mRNA/TF pairs induced by MeJA and module detection in S. miltiorrhiza
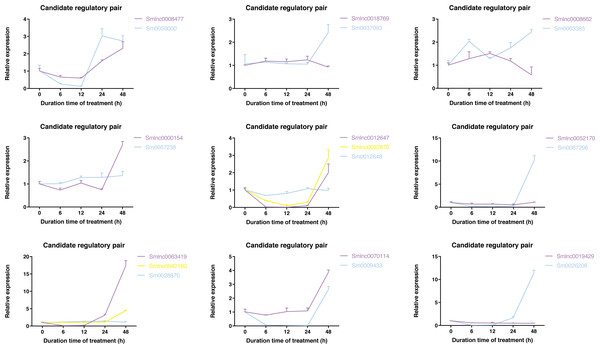
To validate whether the candidate lncRNA-mRNA/TF pairs are related to diterpenoid biosynthesis, we analyzed the time-series expression patterns of candidate gene pairs in response to MeJA treatment. MeJA treatment significantly changed the expression of 19 genes in S. miltiorrhiza at least a time-point of MeJA treatment (Fig. S3). As shown in Fig. 4, the expression of most genes showed a downward trend at the 6 h time point, and only the Smlnc0018769-Sm0037093 pair and Smlnc0008662-Sm0063385 pair had an upward trend. The Smlnc0008477-Sm0056000 pair and Smlnc0012647-Smlnc0032870 pair had the same expression trend. The Smlnc0042160-Sm0028870 pair had the same expression trends at 6, 12, and 24 h. The Smlnc0018769-Sm0037093, Smlnc0008662-Sm0063385, and Smlnc0052170-Sm0067296 pairs had opposite expression trends at 48 h, whereas the Smlnc0019429-Sm0026208 pair had opposite trends at 24 and 48 h. Interestingly, the two gene pairs in the co-expression network, Smlnc0032870-Sm0012648 and Smlnc0018769-Sm0037093, which were positively correlated, showed complex expression correlation under the induction of MeJA, even showed opposite expression trends after 24 h. The hub genes: Sm0056000, Sm0037097, Sm0063385, and Sm0067296 in the co-expression network showed significantly differential expression in MeJA-induced S. miltiorrhiza.
Figure 4: Time-series expression pattern of candidate lncRNA-mRNA/TF pairs in MeJA-induced S. miltiorrhiza.
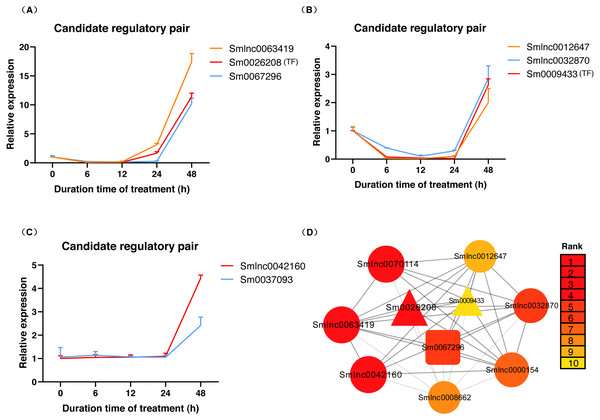
Expression of Smlnc0008477, Smlnc0018769, Smlnc0008662, Smlnc0000154, Smlnc0012647, Smlnc0032870, Smlnc0052170, Smlnc0063419, Smlnc0042160, Smlnc0070114, Smlnc0019429 and the target gene: Sm0056000, Sm0037093, Sm0063385, Sm0067238, Sm0012648, Sm0067296, Sm0028870, Sm0009433, Sm0026208 in MeJA-induced S. miltiorrhiza at five time points (0 h, 6 h, 12 h, 24 h, and 48 h). Fold changes of expression levels were shown. The relative expression levels were normalized against β-actin levels. The level of lncRNA, mRNA, and TF genes in untreated roots (0 h) was arbitrarily set to 1. Error bars represent the standard deviations of three qRT-PCR replicates.The data in Fig. 5A showed that Smlnc0063419, Sm0026208, and Sm0067296 had similar expression patterns, which formed the lncRNA-mRNA-TF module. Sm0026208 and Sm0067296 were annotated with TF WRKY2 and mRNA GA3ox2, respectively. Smlnc0012647, Smlnc0032870, and Sm0009433 had similar expression patterns, as shown in Fig. 5B. Sm0009433 was annotated with TF MYB36. Smlnc0042160 and Sm0037093 had similar expression patterns, as shown in Fig. 5C. Sm0037093 was annotated with mRNA KS2. Through the plug-in cytoHubba, we calculated the top 10 hub genes (Table S12 and Fig. 5D). The modules of Smlnc0063419-Sm0026208-Sm0067296 and Smlnc0012647-Smlnc0032870-Sm0009433, in which all genes were present in the top 10 hub genes.
Figure 5: LncRNA-mRNA-TF module, lncRNA-TF module, lncRNA-mRNA module and hub genes in S. miltiorrhiza.
(A–C) Gene expression pattern analysis: three types of network modules, namely, lncRNA-mRNA-TF module, lncRNA-TF module and lncRNA-mRNA module. (D) Network module analysis of lncRNAs, mRNAs, and TFs by cytoHubba. The circle node represents lncRNA, the square node represents mRNA, and the triangle node represents TF gene. Rank 1 indicates the highest rank, and Rank 10 indicates the lowest rank.Discussion
In this study, the transcriptomic data of S. miltiorrhiza during the tanshinone accumulation stage were used for exploring the lncRNAs and their target transcripts involved in the diterpenoid biosynthetic pathway. A total of 6,651 lncRNAs were obtained by a strict bioinformatic pipeline. We found some common features of lncRNAs, which may be related to their function. Among the candidate lncRNAs, diterpenoid biosynthetic genes/TFs in S. miltiorrhiza, we detected 23 candidate lncRNA-mRNA/TF pairs with a cis-regulatory relationship. To further verify their relationship, we used MeJA as an inducer to observe the gene expression of these candidate lncRNA-mRNA/TF pairs with MeJA treatment for 6, 12, 24, and 48 h. Through the expression data of time series, which provided an exploratory method for the role of lncRNA, three lncRNA-mRNA and/or TF network modules were finally obtained.
In the present study, the identified lncRNAs of S. miltiorrhiza were found to be shorter in length compared with protein-coding transcripts, which was consistent with the previous reports (Hao et al., 2015; Liu et al., 2018; Shen et al., 2018). The mean GC content of lncRNAs was slightly lower than that of mRNAs, which has also been reported in Populus tomentosa (Zhou et al., 2017). Conservation analysis showed that plants of the same genus are more conservative than those of different families. A previous study also showed that the majority of lncRNAs have high sequence conservation at the intra-species and sub-species levels (Deng et al., 2018). Polymerase C-terminal domain (CTD) modifications [e.g., CTD modification threonine 4 phosphorylation (CTD-T4P)] are on the promoters of lncRNAs, which leads to the decrease in the polymerase pause and the advance of the termination in the whole lncRNA genome. The transcription rate of lncRNAs is very fast, which means that they can quickly act on the regulatory target and respond to the signal. Therefore, transcription accuracy and sequence conservation were low (Rinn & Chang, 2020). The lncRNA function was maintained across large evolutionary distances even when the lncRNA sequence substantially diverged (Ulitsky, 2016). Another possible explanation for this is that RNA secondary structures may be the units of lncRNA words rather than the primary sequence, and disparate sequences form similar structure–function relationships to transmit symbolic language like hieroglyphics, thereby forming the molecular grammar of lncRNAs (Rinn & Chang, 2020).
LncRNAs have been proposed to carry out their functions by cis or trans, transcriptional regulation cis-acting lncRNAs influence the expression and/or chromatin state of nearby genes. We predicted lncRNA-mRNA pairs in the cis-acting relationship. The results of our study showed that 45,198 and 180 lncRNA-mRNA/TF pairs were co-expressed (—PCC— ≥ 0.4) or co-localized, respectively, and 23 lncRNA-mRNA/TF pairs were both co-localized and co-expressed (Table S10). The results indicated that most lncRNAs were not co-expressed with their nearby coding genes and were transcribed independently in S. miltiorrhiza (Liao et al., 2011). Two pairs were both co-expressed and co-localized in the high correlation co-expression network with —PCC— ≥ 0.8. Thus, the modes of action of lncRNAs were not limited to local regulators. Another possible explanation is that the strength of the required gene co-expression may depend on the stability or toxicity of the metabolites, and strong co-expression should only be required for unstable monomers (Obayashi & Kinoshita, 2009).
Although advances have been made in the miRNA and miRNA target prediction fields, the precision of miRNA target prediction needs to be improved (Akgül et al., 2022). To reduce false positives, we used three kinds of prediction software to predict miRNA targets. Although many miRNA databases and prediction software are published for plants, few of them are available (De Amorim, Pedro & Paschoal, 2022). This limitation reduced our chance to find miRNAs associated with the lncRNA-mRNA/TF pairs in the diterpenoid biosynthetic pathway. On the basis of the relationship between miRNAs and lncRNAs, we predicted that 14 lncRNAs were potential targets or TMs of 66 miRNAs in S. miltiorrhiza, and no one existed in the candidate lncRNA-mRNA/TF pairs. However, all the results were predicted preliminarily based on bioinformatic analyses and need to be further validated.
Building gene regulatory networks from transcriptomic studies often results in a static view of gene expression, which can make it difficult to disentangle the regulatory pathway structure response to a stimulus. Time-series expression analysis may uncover the temporal transcriptional logic for plant response systems, and provide more accurate predictions for targeted breeding (Greenham & McClung, 2018). By studying the time-series expression of some candidate regulatory pairs inducted by MeJA, we observed a more detailed landscape. We found that some lncRNAs were downregulated in the early stage. The expression of the corresponding mRNAs was upregulated in the later stage between the regulatory pairs, and plants’ response to the signal had a time delay. For example, in response to vernalization, COOLAIR is transiently induced by prolonged cold, reaching a maximum expression level after 2 weeks (Swiezewski et al., 2009). Meanwhile, we found that some genes showed different expression patterns within a period, namely, both upregulated and downregulated, this phenomenon may be because gene regulatory networks are inherently complex, with multiple feedback and feedforward loops (Wils & Kaufmann, 2017).
Nineteen genes were differentially expressed significantly under MeJA-induced, and some of them were present in hub genes of the co-expression network. These results indicated that these genes might participate in the biosynthesis of secondary terpenoids such as tanshinone and also play an important role in the defense response of S. miltiorrhiza. Our results indicated that the expression levels of Sm0056000 (CPS) and Sm0037093 (KS) were upregulated under MeJA induction, which was also observed in the previous report (Luo et al., 2014). However, the levels of these two genes Sm0056000 (CPS) and Sm0037093 (KS) started to increase at 12 h and 24 h in our study, respectively. The response of TFs to MeJA was also observed in this study (Luo et al., 2014), which was consistent with the TF WRKY we studied. For MeJA treatment, the expression of CYP76AH1 was up-regulated over time and reached a peak at 12 h in the previous study (Li et al., 2021), however, after peaking at 6 h, our Sm0063385 (CYP76AH1) expression was down-regulated, and then it began to up-regulate at 12 h and peaked at 48 h. Previous research has suggested that WRKYs might regulate the development of bast fiber in response to GA3 stress in jute (Corchorus capsularis) (Zhang et al., 2020), the Smlnc0063419-Sm0026208-Sm0067296 module (Sm0026208: TF WRKY2, Sm0067296: GA3ox2) in our study may form a similar response module in S. miltiorrhiza.
The tissue specificity and subcellular localization of lncRNAs may suggest their function. We obtained 3 lncRNA-mRNA and/or TF modules: Smlnc0063419-Sm0026208 (TF)-Sm0067296, Smlnc0012647-Smlnc0032870-Sm0009433 (TF), and Smlnc0042160-Sm0037093, among of lncRNAs: Smlnc0063419, Smlnc0012647, Smlnc0032870, and Smlnc0042160 mainly expressed in roots (Figs. 3 and 5), which was consistent with the place where tanshinones accumulated of S. miltiorrhiza (Chang et al., 2019). In the prediction of subcellular localization, Smlnc0012647, Smlnc0042160, and Smlnc0063419 were predicted to be located in the cytoplasm, this suggests that these predicted cytoplasmic lncRNAs may interfere with protein post-translational modifications or regulate mRNA export (Chen, 2016). Although Smlnc0012647 and Smlnc0032870 existed in the same module, Smlnc0032870 was predicted to be located in the nucleus, a possible explanation for this result may be that some lncRNAs are located in both the nucleus and cytoplasm (Cabili et al., 2015).
Conclusions
This study set out to investigate the possible functions of the lncRNAs of S. miltiorrhiza related to diterpenoid biosynthesis, we predicted the potential targets of lncRNAs in cis-regulatory relationships, a summarizing figure is shown in Fig. S4. Through a strict bioinformatic pipeline, we identified 6,651 candidate lncRNAs, and obtained three lncRNA-mRNA and/or TF network modules. This study revealed the possible roles of the lncRNAs of S. miltiorrhiza related to diterpenoid biosynthesis. These findings indicated that lncRNA is generally complex in regulating mRNA and/or TF. This study provides useful information to deepen our understanding of the function and regulatory mechanisms of lncRNAs in the diterpenoid biosynthetic pathway of S. miltiorrhiza.
Supplemental Information
Biosynthesis pathway of diterpenoids in S. miltiorrhiza
GGPPS, geranylgeranyl diphosphate synthase; GPPS, geranyl diphosphate synthase; FPPS, farnesyl diphosphate synthase; CPS, copalyl diphosphate synthase; ent-CPS, ent-copalyl diphosphate synthase; KS, kaurene synthase; ent-KS, ent-kaurene synthase; CYP76AH1; CYP76AK2; CYP76AK3; CYP76AK5; KO , ent-kaurene oxidase; KAO, ent-kaurenoic acid oxidase; GA2ox, GA 2-oxidase; GA3ox, GA 3-oxidase; GA20ox, GA 20-oxidase.
Co-expression network of lncRNAs, mRNAs, and TFs
(A) Co-expression network with —PCC—≥ 0.8. (B) Co-expression network of the Sm0012648 hub genes. (C) Co-expression network of the Sm0037093 hub genes. The circle node represents lncRNA, the square node represents mRNA, and the triangle node represents TF gene ( p ≤ 0.05).
The expression levels of candidate lncRNA-mRNA/TF pairs induced by MeJA in S. miltiorrhiza
The expression levels of 11 lncRNAs, 6 mRNAs, and 3 TFs of S. miltiorrhiza treated with MeJA at 6, 12, 24, and 48 h were shown. The expression level of non-treated (0 h) roots of S. miltiorrhiza was arbitrarily set to 1 and the levels induced by MeJA were given relative to this. Fold changes of lncRNA levels were shown. Error bars represent the standard deviation. p value was determined by an unpaired t-test using SPSS (Version 23.0, IBM, USA), p < 0.05 ( ∗) and p < 0.01 ( ∗ ∗) were considered statistically significant.
A summary workflow of our study
The figure shows the process of lncRNA function analysis in this study.
Primer sequences used in real-time PCR analysis
Primer sequences needed for tissue-specific analysis and MeJA treatment analysis.
Functional annotation of transcripts
The functional annotation of S. miltiorrhiza transcripts in public database.
The results of lncRNA identification by bioinformatics pipeline in S. miltiorrhiza
The ORF prediction, Pfam database annotation, CPC2 software and PLEK software prediction results.
A total of 6,651 candidate lncRNAs were obtained from the lncRNA prediction pipeline
Conservation analysis of S. miltiorrhiza lncRNAs in ten species genomes
Salvia splendens, Salvia hispanica, Mentha longifolia, Scutellaria baicalensis, Pogostemon cablin, Sesamum indicum, Nicotiana tabacum, Brassica napus, Arabidopsis thaliana, and Selaginella moellendorffii. The number of conserved lncRNAs in S. miltiorrhiza appears in the second column.
Precursors of miRNA and miRNA targets prediction in S. miltiorrhiza lncRNAs
Worksheet 1 represents the BLAST result. Worksheet 2 shows the secondary structure of lncRNAs predicted by RNAfold. Worksheets 3, 4 and 5 represent the prediction results of TAPIR, psRobot, and psRNATarget software respectively. Worksheet 6 represents the intersection of three kinds of prediction software results.
Subcellular localization prediction in lncLocator
All results include cytoplasm, nucleus, cytosol, ribosome, and exosome.
Diterpenoid biosynthetic pathway genes and TFs list
All the 46 diterpenoid biosynthetic pathway genes and 11 TFs related to diterpenoid biosynthetic pathway were used to study in this work.
Co-expression relationships analysis results, co-location relationships analysis results, and the candidate lncRNA-mRNA/TF pairs
Worksheet 1 is pearson correlation coefficient analysis result of candidate lncRNAs and diterpenoid biosynthesis related mRNAs/TFs, raw.r represents correlation coefficient, raw.p represents p value, and correction represents positive or negative correlation. Worksheet 2 is the result of genome co-localization (within 100 kb). Worksheet 3 is the candidate lncRNA-mRNA/TF pairs identified in our studies, the third and fourth column contain the gene sequences, the fifth column represents the annotation information of mRNAs/TFs, the seventh column represents PCC value among pairs (p ≤ 0.05), the eighth column represents the p value, the ninth and tenth column represent the starting and ending genomic location of lncRNAs respectively, the eleventh and twelfth columns represent the starting and ending genomic location of mRNAs/TFs, and the last column is the distance between each gene pair.
Hub genes in co-expression network (—PCC—≥ 0.8)
Worksheet 1 is PCC result, raw.r represents correlation coefficient, raw.p represents p value, and correction represents positive or negative correlation. Worksheet 2 is the hub genes with degrees ≥ 10.
The top 10 hub genes rank in cytoHubba
Top 10 hub genes in network ranked by MCC method.
qPCR raw data
Sheet1 contains tissue-specific expression raw data; Sheet2 contains lncRNA-mRNA/TF pairs expression raw data in MeJA-induced S. miltiorrhiza.