Anthropogenic noise decreases activity and calling behavior in wild mice
- Published
- Accepted
- Received
- Academic Editor
- Sebastian Oberst
- Subject Areas
- Animal Behavior, Conservation Biology, Ecology, Zoology, Environmental Impacts
- Keywords
- Foraging, Napaeozapus, Noise pollution, Peromyscus, Bioacoustics, Wild, Ultrasonic vocalizations, Woodland jumping mouse USV, Deer mouse USV, Mouse calls
- Copyright
- © 2023 Petric and Kalcounis-Rueppell
- Licence
- This is an open access article, free of all copyright, made available under the Creative Commons Public Domain Dedication. This work may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose.
- Cite this article
- 2023. Anthropogenic noise decreases activity and calling behavior in wild mice. PeerJ 11:e15297 https://doi.org/10.7717/peerj.15297
Abstract
Background
Animals rely on sound for daily activities, and anthropogenic noise is a pollutant that alters the natural soundscape within which they are active. As human infrastructure expands, broadband anthropogenic noise increases, which can affect behaviors of nocturnal animals. Mice are nocturnal animals that produce ultrasonic calls as part of their behavioral repertoire.
Methods
We assessed effects of anthropogenic and natural noise on the behaviors of wild deer mice (Peromyscus maniculatus) and woodland jumping mice (Napaeozapus insignis), two species of mice that produce ultrasonic calls. We measured activity, foraging behavior at a foraging tray, and calling behavior to broadcasts of natural and anthropogenic noise, compared to a baseline with no broadcasting, at 25 focal areas in the Southern Appalachian Mountain Range of North Carolina, USA.
Results
Deer mice exposed to anthropogenic noise spent less time in focal areas with broadcasted anthropogenic noise. Mice took longer to begin foraging in the presence of anthropogenic noise, they spent less time at the foraging tray, and left fewer husks but consumed the same number of seeds as mice exposed to natural noise. Deer mice were less likely than woodland jumping mice to be the first to enter the focal area and approach food when in the presence of anthropogenic noise. Both species produced few ultrasonic calls in the presence of broadcasted natural and anthropogenic noise compared to their baseline level of calling. We present the first calls recorded from woodland jumping mice.
Conclusion
Anthropogenic noise affects activity, foraging behavior, and calling behavior of nocturnal mice. Natural noise also affects the calling behavior of mice. Mouse species respond differently to anthropogenic noise, with deer mice appearing more sensitive to anthropogenic noise than woodland jumping mice. Responses to noise could have important effects on the ecology of mice and these two species respond differently. Species differences should be considered when mitigating the effects of noise in conservation ecology.
Introduction
Anthropogenic noise is a global pollutant that alters the natural soundscape which animals rely on for foraging, navigation, exploration, predator avoidance and communication (Cox et al., 2018; Barber, Crooks & Fristrup, 2010; Shannon et al., 2016). To meet the high transportation demands of the expanding human population, the development of urban and agricultural areas with transportation networks are rapidly increasing (Dulac, 2013). Road traffic and construction noises are recognized as the most prominent sources of noise pollution that affect a variety of animal species (Cox et al., 2018; Jamrah, Al-Omari & Sharabi, 2006; McGregor et al., 2013; Shannon et al., 2016). For example, non-breeding wood mice (Apodemus sylvaticus) living within 50m of motorways in Spain showed increased stress hormone metabolites, through the measurement of fecal corticosterone metabolites, relative to conspecifics living 1000m from the motorways (Navarro-Castilla et al., 2014). Anthropogenic noise associated with traffic and construction activities spans audible and ultrasonic ranges (from 1 up to 50 kHz; Raboin & Elias, 2019).
Anthropogenic noise can negatively affect animal communication by directly and indirectly influencing the production, transmission, and reception of acoustic signals, which alters the behavior, physiology, and survival of both senders and receivers (Babisch, 2003; Jarup et al., 2008; Radford, Kerridge & Simpson, 2014; Slabbekoorn & Ripmeester, 2008). Noise can negatively affect communication in two ways: (1) by eliciting energetically costly anti-predator behaviors which shift energy away from exploring, foraging, and vocalizing (McLaughlin & Kunc, 2013), and (2) by masking acoustic signals (Slabbekoorn & Ripmeester, 2008) which prevent successful propagation, detection, and interpretation of signals (Frid & Dill, 2002; Bee & Swanson, 2007). It is also possible that anthropogenic noise can lead to misinterpretation of sounds for biologically relevant signals (Shier, Lea & Owen, 2012). Animals can deal with the masking effects of noise, by either leaving the area or adjusting their behavior (McLaughlin & Kunc, 2013).
In some species, senders have the vocal plasticity to cope with the masking effects of noise by adjusting spectral and/or temporal characteristics of vocalizations (Nelson et al., 2017; Brumm et al., 2004) or increasing their signal amplitude (Brumm & Todt, 2002; Lowry, Lill & Wong, 2012). Animals may avoid masking by producing calls above the frequency of the overlapping anthropogenic noise (McLaughlin & Kunc, 2013; Rabin et al., 2003). If anthropogenic background noise is not constant, such as traffic noise, then animals may use the “quiet” times to call (Fuller, Warren & Gaston, 2007). Calling may cease altogether if background noise levels are high and adjusting frequency, amplitude, or duration of the call does not influence successful signal transmission (Ophir, Schrader & Gillooly, 2010; Warren et al., 2006) or if animals lack vocal plasticity (Nelson et al., 2017).
Animals use sounds as cues to listen for approaching predators or to hunt their prey (Knudsen & Konishi, 1979) and noise can mask these cues thereby altering foraging ecology (Muhly et al., 2011). If a cue is masked by noise, it could lead to a missed feeding opportunity for the predator or a loss in predator detection by the prey (Leighton, Horrocks & Kramer, 2010; Muhly et al., 2011; Schaub, Ostwald & Siemers, 2008). Greater mouse-eared bats (Myotis myotis) rely on cues to passively locate invertebrates and traffic noise increases their search duration with decreased feeding success (Siemers & Schaub, 2010). Fringe-lipped bats (Trachops cirrhosus) shift from passively listening for their prey to actively echolocating, in the presence of noise, thereby changing their sensory modality for feeding (Gomes et al., 2016). In prey species, there is a trade-off between food acquisition and predator avoidance (Bleicher, Kotler & Brown, 2019) and anthropogenic noise leads to decreased activity and foraging effort (Bunkley et al., 2015; Shannon et al., 2014; Waynert et al., 1999). The shift in the allocation of time and energy toward being alert in the presence of noise can directly lead to an increase in time spent hiding and a decrease in time spent foraging, mating, and calling, or can indirectly affect communication by altering spatial behavior, which results in altered individual, social, and reproductive behaviors (Barber, Crooks & Fristrup, 2010; Brown et al., 2012; Bunkley et al., 2017; Habib, Bayne & Boutin, 2007).
Anthropogenic noise is pervasive in the audible range during the day and our understanding of the effects of noise on animals comes mainly from studies during the day, for example, from birds (Jerem & Mathews, 2020). However, noise extends into the ultrasonic range (Raboin & Elias, 2019) and many vocalizing animals are nocturnal, including Peromyscus mice (Kalcounis-Rueppell, Pultorak & Marler, 2018a). Given that ultrasonic calling, which mediates social interactions, is a component of the behavioral repertoire of nocturnal Peromyscus mice, the objective of our study was to understand how broadband (sound energy that is distributed over multiple frequencies) anthropogenic noise influences behavioral responses of mice. We focused our study on the deer mouse (Peromyscus maniculatus) and were additionally able to measure the woodland jumping mouse (Napaeozapus insignis), in the wild. Woodland jumping mice are found throughout northeastern North America and deer mice are the most abundant and widely distributed native rodent species in North America. We hypothesized that broadband anthropogenic noise would alter the allocation of time that mice spent foraging and/or in their main area of activity. We also hypothesized an effect of noise on both call structure and the calling behavior of mice. We further predicted that the effects of anthropogenic noise would be greater than the effects of natural noise.
Materials & Methods
Study design
Our field work was conducted from June to August 2016, 2017, and 2018 at 25 focal areas (defined below) at three different sites across the Highlands-Cashiers Land Trust Property (Brushy Face; 35°054′N, 83°188′W), Turtle Pond in the Nantahala National Forest (35°234′N, 83°559′W), and Highlands Biological Station (35°054′N, 83°188′W), in the Southern Appalachian Mountains in Macon County, North Carolina, USA. All sites were > 610 m above sea level (ASL). At each site, we determined resident deer mice and their main areas of activity which we call ‘focal areas’ through standard capture-mark-recapture live trapping. We then used radio telemetry, a foraging tray, full-spectrum audio recording, and a thermal imaging camera to measure behaviors. Our study initially focused only on deer mice, but woodland jumping mice were common at our sites. We were able to measure and report on some of the behavioral responses for both mouse species because woodland jumping mice are easily identified on video based on their jumping gait (see Supplement 1a and Supplement 1b for videos). We used radio telemetry to collect individual activity data from deer mice. Foraging trays were used to collect foraging data and assess risk perception from deer mice and woodland jumping mice, combined. The audio recording was used to collect vocalization data from deer mice and woodland jumping mice, combined and separately. We also used thermal video to determine which species first entered the focal area, first visited the foraging tray, and how long each species stayed in the focal area and at the foraging tray.
All focal areas were in close proximity (less than 20m) to a creek in dense rhododendron (Rhododendron catawbiense) riparian habitat within steep mountain topography. We used capture-mark-recapture live trapping to determine which deer mice were resident in focal areas. The preferred habitat type for deer mice and woodland jumping mice in this region is old growth hemlock forest (Tsuga canadensis) with a dense rhododendron understory. Golden mice (Ochrotomys nuttalli), although not part of our study, were present in the three study sites and were more likely to be captured away from the creek with mountain laurel (Kalmia latifolia) as the preferred understory (unpublished data). Due to microhabitat selection by deer mice and golden mice in this region, we were able to specifically target sections of our study sites to focus on deer mice.
Trap stations across all focal areas were set with 10 meter (m) spacing between stations. At each study site, we were either able to set a grid or transect based on the steepness of the topography and narrowness of the riparian zone. At the Brushy Face study site we were able to set up a grid configuration (50 m × 200 m). Due to the steepness at Turtle Pond and Highlands Biological station we set up transects. At Turtle Pond, trap stations were set along a 650m transect, and at Highlands Biological Station trap stations were set along a 1000m transect. At each trap station, we set two Sherman traps (7.6 cm × 8.9 cm × 22.9 cm) baited with a rolled oat and sunflower seed mixture. On cold nights we also added a small amount of pillow stuffing for bedding. Traps were set an hour before sunset and checked at midnight and reset, and then checked again and closed two hours before sunrise. Upon capture of any mouse species, we recorded age, sex, reproductive condition, and mass (for details see Petric & Kalcounis-Rueppell, 2013)). All mice captured for the first time were marked with a unique numbered ear tag (Monel #5). The majority of mouse captures were of the deer mouse (Peromyscus maniculatus; 49% of individuals captured). We also captured the woodland jumping mouse (Napaeozapus insignis; 28% of individuals captured) and the golden mouse (Ochrotomys nuttalli; 23% of individuals captured). Any species captured that was not a mouse was released immediately.
We identified resident deer mice as adult individuals captured two or more times at the same trap station or the surrounding five trap stations in any direction over five trapping nights. We then selected a resident deer mouse and outfitted that deer mouse with a 0.55 g M1450 mouse style transmitter (Advanced Telemetry System, ATS) as in Petric & Kalcounis-Rueppell (2013). We outfitted and followed one resident deer mouse at a time to determine its focal area. We could only follow one resident deer mouse in its focal area at a time at any given site because of equipment and logistics. Using handheld radio telemetry, we determined the main area of activity for the resident deer mouse, and this was its focal area (approximately 3 m × 5.5 m area across all resident mice). To measure the total time the radio-tagged deer mouse spent in the focal area, we deployed automated radio telemetry equipment (R4500S DCC receiver/datalogger; ATS, Isanti, MN, USA) that continually scanned for the radio signal of the radio-tagged deer mouse and allowed us to measure the time (number of minutes) the deer mouse was in the focal area. The datalogger was connected to an antenna in the center of the focal area and was programmed to detect and record the transmitter frequency of the mouse through the night. The following morning, the datalogger was removed from the field and brought to the lab to download the data and recharge the battery.
In the center of the focal area, we also set up a single seed tray to examine foraging behavior. Each tray had 20 whole sunflower seeds mixed into five cups of sand in a plastic plant saucer (30.48-cm diameter, 5.08-cm deep). The seed/sand mixture was protected from the rain with a clear, 40.64-cm diameter plate that was supported by four stakes and suspended eight cm above the mixture. The following morning, we used a mesh strainer to separate the sand from the leftover seeds/husks. The intact seeds were collected and counted. We also assessed and classified leftover husks into three categories: (1) zero husk pieces, (2) one to five husk pieces and (3) >10 husk pieces remaining after each night. A zero in leftover husks showed that mice collected the seeds but did not take the time to consume the seeds at the foraging tray, indicating that risk perception is high, whereas >10 leftover husks showed that mice took the time to consume the seeds at the foraging tray, indicating that risk perception is low. We could not determine which mouse species had consumed the seeds, so species are combined for this measure.
We recorded the focal area, through the night, using video recordings with a thermal lens (Photon 320 14.25 mm; FLIR/Core & Indigo, Wilsonville, OR, USA). The lens was mounted on a 2-meter tripod to capture the entire focal area in the field of view. The lens was connected to a JVC Everio HDD camcorder and powered by an external dry cell car battery connected to an inverter. Although not the initial focus of this study, we also captured jumping mice who were active in the area. From the video, we were able to identify the species of the mouse, a deer mouse or a woodland jumping mouse, based on gait differences (Supplement 1a and Supplement 1b).
To measure mouse calling in the focal area, we used 12 ultrasonic microphones (Emkay FG Series; Avisoft Bioacoustics, Berlin, Germany) connected to an UltraSoundGate system 1216H (Avisoft Bioacoustics, Berlin, Germany), which was connected to a small laptop (DELL Latitude E6230). The microphones were arranged in a 3x4-meter grid configuration approximately 1 − 2 − m apart with the grid centered in the focal area. All microphones were calibrated prior to deployment. Using Avisoft RECORDER Software, the system was set to record when sonic and ultrasonic sounds were detected by the microphone(s). Recording settings were as in Briggs & Kalcounis-Rueppell (2011) and Petric & Kalcounis-Rueppell (2013). Microphones were triggered and a .wav file was recorded when sounds were detected. Each morning, files were downloaded. All files recorded were examined, and all mouse recording were analyzed and counted, using Avisoft SAS Lab Pro (Avisoft Bioacoustics). Using the time of the recording of the call we matched the vocalization with the time of the activity on the video footage (as in Briggs & Kalcounis-Rueppell, 2011; Petric & Kalcounis-Rueppell, 2013), allowing us to assign the vocalization to species (either a deer mouse or a woodland jumping mouse).
We have described calls produced under laboratory conditions by adult Peromyscus from seven different species, including calls from Peromyscus maniculatus (Kalcounis-Rueppell, Petric & Marler, 2018b). We have also described calls produced by Peromyscus under free living, natural conditions in the wild. In the wild and laboratory, Peromyscus spp. most commonly produce sustained vocalizations (SVs) as summarized in Kalcounis-Rueppell, Pultorak & Marler (2018a) and as reported in Petric & Kalcounis-Rueppell (2013), Briggs & Kalcounis-Rueppell (2011), Timonin, Kalcounis-Rueppell & Marler (2018), Petric, Kalcounis-Rueppell & Marler (2022). The SVs we record are loud and used for conspecific contact and travel 3 to 7m in the wild (Timonin, Kalcounis-Rueppell & Marler, 2018). Our equipment setup was similar to the setup in our previous studies and involved the same equipment and approach to microphone placement (on the ground with a small tripod with a rain guard) and with cables carefully placed and tested prior to each experiment.
Broadcasting noise experiment
We used a “before-during” experimental design where the “before” sampling served as our control. For the “before” behaviors, we recorded the resident mice for three consecutive nights (nights one, two, and three) without any noise manipulations in the focal area. We consider these first three nights without any noise broadcasts as our control. At the same focal area, on nights four, five, and six, we introduced noise manipulation via a broadcast speaker and recorded the resident mice. At each focal area, the noise manipulation was only one of two types: either anthropogenic noise or natural noise. We consider these last three nights with noise broadcast as our treatment and there were two treatment types, anthropogenic noise and natural noise. We recorded for three consecutive nights before broadcasting and for three consecutive nights during broadcasting to make sure that there was no habituation to our equipment, which we would have observed as a night effect in our analysis. Our goal was to capture natural variation in the behaviors we measured, and we were more likely to do that across multiple nights. The broadcasting treatment type (anthropogenic noise or natural noise) was randomly assigned to each focal area. Our anthropogenic noise was pre-recorded from a diesel automatic standby generator (ranging from 1-42 kHz). Our natural noise was pre-recorded from a free-flowing creek in our study area (ranging from 1-25 kHz). We chose these particular sounds because they were present in the study area; streams represent a natural noise that would be regularly encountered by mice in the soundscape whereas the generator would not be regularly encountered but represents a loud anthropogenic noise that is possible within the soundscape.
Anthropogenic and natural noise was recorded using a Zoom H2 Handy Portable Stereo Recorder. The recorder was mounted on a mini tripod or a box between 30 cm–40 cm off the ground 1m from the source. We recorded noise continuously from sunset to sunrise for three nights in .wav format at a sampling rate of 96 kHz/24-bit (see Supplement 2 for spectrograms of exemplar recordings). We used these noise recordings to create files for our noise broadcast experiments. We randomly selected four 15-minute exemplars for each noise type (anthropogenic or natural) from across the three nights and created a single 1-hour sound file by randomly stringing the four 15-minute exemplars together using Avisoft SAS Lab Pro.
For our broadcast experiments, we set up an AT-100 wide bandwidth speaker (Binary Vocal Technology LLC. Tucson, AZ, USA). The speaker was set 2m from the edge of the focal area, and 40 cm off the ground. The 1hr sound file was then broadcasted on a continuous loop for eight hours per night, during nights four, five, and six, at a volume of approximately 80-90 dBA at 1-meter following Oliveira et al. (2009). Each night we adjusted our amplitude to match the source amplitude from our recordings at 1m from the source. Prior to the start of the experiment, we measured amplitude for five minutes at a distance of 1m from the speaker using a calibrated Avisoft USG Electret Ultrasound Microphone and matched amplitude by adjusting the volume on the speaker. The speaker was connected to a small laptop (DELL Latitude E6230) and operated using G’Tools version 1.6 PLAY’R ultrasonic generation software (Binary Vocal Technology LLC, Germantown, MD, USA).
We measured seven response variables to our experiment as follows and for each variable, we calculated the differences between treatment nights (nights four, five, and six) and control nights (nights one, two, and three). We calculated differences, as opposed to using raw data, so that we could examine the effects of noise independent of absolute levels of activity. The difference was calculated within each focal area in the following way: night four minus night one; night five minus night two; night six minus night three. The difference was a measure of the effect of noise allowing for an effect of night, with a zero-difference indicating no effect of noise. We measured the following variables:
(1) Difference in total time spent in the focal area by deer mice—number of minutes the radio-collared deer mouse spent in the focal area from sunset to sunrise from radio telemetry data.
(2) Difference in total seeds consumed by both species—2a) number of seeds consumed in the foraging tray and 2b) the number of husks leftover at the foraging tray.
(3) Difference in latency to enter the focal area by either species—from video recordings, the number of minutes post-sunset until the first mouse (deer mouse or a woodland jumping mouse) appeared in the focal area.
(4) Difference in time spent in the focal area on the first visit by either species—from video recordings, the number of seconds the first mouse (deer mouse or a woodland jumping mouse) spent in the focal area after first entering the focal area.
(5) Difference in latency to start foraging at the tray by either species—from video recordings, number of minutes post-sunset until the first mouse (deer mouse or a woodland jumping mouse) entered a foraging tray.
(6) Difference in time spent foraging on first appearance at the tray by either species—from video recordings, the number of seconds the first mouse (deer mouse or a woodland jumping mouse) spent in the foraging tray.
(7) Calling behavior of each species—number of calls produced by each species of mouse through audio recordings identified to species (deer mouse or a woodland jumping mouse) through video.
All field work was approved through the following permissions: The University of North Carolina Institutional Animal Care and Use Commitee (IACUC) 16-002, NC Wildlife Resource commission 17-ES00336 and 17-SC00162, North Carolina Park Services 2720, Highlands Biological Station IACUC 16-08, and Highlands-Cashiers Land Trust.
Statistical analyses
Our statistical analyses and data visualization were conducted in R software version 3.2.2 (R Core Team, 2018). We used an alpha level of p < 0.05 for all analyses. Data for all dependent variables were checked for normality using a Shapiro–Wilk test. We used the package MASS to fit Generalized Linear Mixed Models (GLMM) with focal area as the random term, noise type as the fixed term, and night as a covariate to the following variables: difference in total time spent in the focal area, difference in total food consumed, difference in number of husks leftover, difference in total calls produced, difference in latency to enter the focal area, difference in time spent in the focal area on the first visit, difference in latency to start foraging, and difference in time spent foraging. Although we were interested in the effect of noise, and noise type, on behaviors, we were also interested in night effect to determine if mice were habituated to our equipment. Thus, we used night as a sample unit and we account for the repeated samples across nights by including focal area ID in the GLMM as the random term. Due to our small sample size and non-overlapping distribution among dependent variables, we were only able to run one dependent variable at a time. In our constructed generalized linear models, natural noise served as the reference level for noise effects and night four served as the reference for night effects. The generalized linear models were fitted with either a Poisson or Gaussian distribution and reported as GLMM Estimate ±SE. The Poisson distribution does not allow for any negative values therefore, the data were transformed by using the scaling method (adding the absolute smallest number to every value in the array). All GLMM models are listed in Supplement 3. All data are represented using box plots to show skewness and outliers.
We were also interested in whether noise would influence which species was first to arrive at the focal area and feeding tray. We used the Chi-squared test of Independence to examine the relationship between the first species to appear (deer mouse or woodland jumping mouse) and the presence of noise (“before” or “during” the noise broadcast).
Results
Sample size
We tagged 96 deer mice, 54 woodland jumping mice, and 46 golden mice over 464 captures from June to August 2016, 2017, and 2018 at 25 different focal areas across the three years. The value of 464 captures included a large number of recaptures that facilitated our identification of residents. We additionally captured and immediately released the following species: southern flying squirrel (Glaucomys volans), red backed vole (Myodes gapperi), masked shrew (Sorex cinereus) and Northern short-tailed shrew (Blarina brevicauda). We radio-collared and broadcasted sounds in 21 focal areas with radio-collared deer mice (anthropogenic noise=13, natural noise=8). Of our 21 radio-collared mice, 13 were male and 8 were female. We broadcasted noise to an additional 4 focal areas where we were not able to radio-collar the resident deer mouse because we ran out of radio collars, but where we had a focal area with mice (anthropogenic noise=2, natural noise=2). Thus, for all variables that did not require individually radio-tagged deer mice, we have a total of 25 focal areas (anthropogenic noise=15, natural noise=10). The thermal imaging equipment was set-up in all 25 focal areas (anthropogenic noise=15, natural noise=10) but due to the intensive, real time, analysis of thermal video we were only able to analyze a random subset of 10 focal areas (anthropogenic noise=5, natural noise=5), and only the first mouse appearing in the focal area and at the foraging tray. The foraging tray was set-up at all 25 focal areas (anthropogenic noise=15, natural noise=10). Due to raccoon disturbance, we did not collect data from one night at two different anthropogenic broadcasting focal areas. Our final foraging dataset consisted of 148 recording nights from 25 focal areas (anthropogenic noise=15, natural noise=10).
Difference in total time spent at the focal area by deer mice
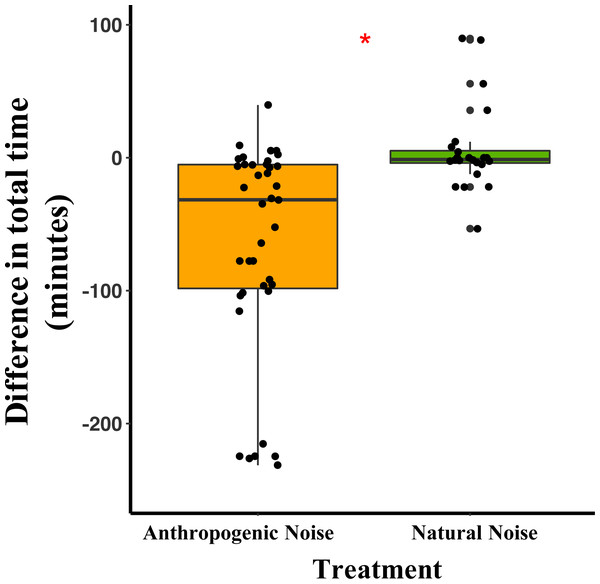
In the anthropogenic noise focal areas, deer mice spent an average of 64.7 fewer minutes/night during broadcast of anthropogenic noise than during the nights with no broadcast of noise. In the natural noise focal areas, mice spent an average of 5.9 more minutes/night during the broadcasting of natural noise than during the nights with no broadcast of noise (Table 1). The difference in total time spent by deer mice at the focal area was significantly different between anthropogenic and natural noise focal areas (Anthropogenic-GLMM Estimate 0.21 ± 0.08, df = 19, p = 0.0138; Fig. 1; Table 1). Difference in time spent in the focal area was not influenced by night (night five GLMM Estimate −0.05 ± 0.10, df = 40, p = 0.6108; night six GLMM Estimate 0.03 ± 0.09, df = 40, p = 0.7186).
| Broadcasting | n(nights) | Mean | SE | Median | Min | Max | ||
|---|---|---|---|---|---|---|---|---|
| Time at the Focal Area | Anthropogenic Noise- Control | No | 39 | 80.5 | 19.1 | 34 | 0 | 641 |
| Anthropogenic Noise- Treatment | Yes | 39 | 15.3 | 4.3 | 7 | 0 | 125 | |
| Natural Noise - Control | No | 24 | 22.8 | 8.3 | 6 | 0 | 153 | |
| Natural Noise - Treatment | Yes | 24 | 28.7 | 11.1 | 2 | 0 | 205 | |
| Time at the Focal Area by Night | Anthropogenic Noise- Night One | No | 13 | 101.5 | 48.3 | 34 | 0 | 641 |
| Anthropogenic Noise- Night Two | No | 13 | 72.8 | 21.8 | 43 | 0 | 244 | |
| Anthropogenic Noise- Night Three | No | 13 | 67.3 | 24.5 | 24 | 0 | 294 | |
| Anthropogenic Noise- Night Four | Yes | 13 | 12.1 | 4.9 | 9 | 0 | 67 | |
| Anthropogenic Noise- Night Five | Yes | 13 | 11.2 | 4.0 | 5 | 0 | 53 | |
| Anthropogenic Noise- Night Six | Yes | 13 | 22.8 | 11.3 | 5 | 0 | 125 | |
| Natural Noise - Night One | No | 8 | 27.1 | 14.7 | 6 | 0 | 118 | |
| Natural Noise - Night Two | No | 8 | 17.5 | 10.1 | 5 | 0 | 78 | |
| Natural Noise - Night Three | No | 8 | 23.6 | 18.6 | 5 | 0 | 153 | |
| Natural Noise - Night Four | Yes | 8 | 36.3 | 21.1 | 2 | 0 | 152 | |
| Natural Noise - Night Five | Yes | 8 | 13 | 7.7 | 4 | 0 | 63 | |
| Natural Noise - Night Six | Yes | 8 | 36.8 | 25.6 | 2 | 0 | 205 |
Notes:
- n
-
number of recording nights
Figure 1: The difference between the total time/night that deer mice spend in their focal areas of activity between control and broadcasting nights at focal areas with anthropogenic noise and natural noise.
Median and quartiles of the difference in total time the radio-collared deer mouse spent in the focal area between a night with broadcasting and a night without broadcasting. Whiskers are minimum and maximum values. Distribution was a Poisson distribution. Red line and asterisk indicate a significant difference at p < 0.05. Broadcasts were of natural noise and anthropogenic noise. Each point represents a difference between a pair of nights (during broadcasting minus before broadcasting). Data were collected at and near the Highlands Biological Station in the Southern Appalachian Mountains, North Carolina, USA from 2016–2018.Difference in total seeds consumed by both species
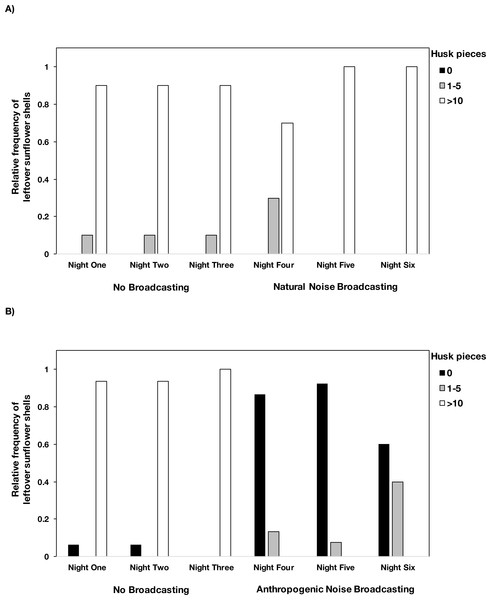
In the anthropogenic noise focal areas, mice consumed an average of 4.7% fewer seeds during the broadcast of anthropogenic noise (Table 2). In the natural noise focal areas, mice consumed an average of 2.5% more seeds during the broadcast of natural noise (Table 2). The difference in the number of seeds consumed did not large significantly differ between natural and anthropogenic noise broadcasts (Anthropogenic-GLMM Estimate 0.07 ± 0.047, df = 23, p = 0.1389) and there was no night effect (night five GLMM Estimate 0.01 ±0.06, df = 46, p = 0.8852; night six GLMM Estimate −0.08 ± 0.06, df = 46, p = 0.1578). However, there was a difference in the number of husks left by mice when anthropogenic noise was broadcast compared to when natural noise was broadcast (Anthropogenic-GLMM Estimate 3.22 ± 0.51, df = 23, p = 0.0000). Mice left husks in the tray during the control nights, and during the broadcast of natural noise, but not during the broadcasts of anthropogenic noise (Fig. 2). There was no night effect for the difference in the number of husks leftover (night five GLMM Estimate −0.10 ± 0.20, df = 46, p = 0.6163; night six GLMM Estimate −0.20 ± 0.20, df = 46, p = 0.2201).
| Broadcasting | n(focal area) | Mean | SE | Min | Median | Max | ||
|---|---|---|---|---|---|---|---|---|
| Total Seeds Consumed (%) | Anthropogenic Noise- Control | No | 15 | 99.56 | 2.10 | 90 | 100 | 100 |
| Anthropogenic Noise- Treatment | Yes | 15 | 94.88 | 0.27 | 35 | 100 | 100 | |
| Natural Noise- Control | No | 10 | 94.50 | 2.83 | 0 | 100 | 100 | |
| Natural Noise- Treatment | Yes | 10 | 97.00 | 3.92 | 15 | 100 | 100 |
Notes:
- n
-
number of recording nights
Figure 2: The number of husk pieces leftover in the foraging tray by night and treatment type.
Mice left >10 husk pieces in the foraging tray during the control nights at both (A) natural noise and (B) anthropogenic noise focal areas. During the broadcasting of natural noise, >10 husk pieces were left in the tray however during the broadcasting of anthropogenic noise there were relatively few or no husk pieces left in trays. There were no instances where 6–10 husks were left so we did not include that category. Anthropogenic noise n = 15 sites, natural noise n = 10 sites. Data were collected at and near the Highlands Biological Station in the Southern Appalachian Mountains, North Carolina, USA from 2016–2018.Difference in latency to enter the focal area and time spent in the focal area on the first visit by both species
In the anthropogenic noise focal areas, the average latency for mice to enter the focal area was 18.4 min longer than the control during broadcasting of anthropogenic noise (Table 3). In the natural noise focal areas, the average latency for mice to enter the focal area was 16 min longer than the control during broadcasting of natural noise (Table 3). The difference in latency to enter the focal area between anthropogenic and natural noise focal areas was not significantly different (Anthropogenic-GLMM Estimate −0.02 ± 0.20, df = 8, p = 0.9211), nor was there a night effect (night five GLMM Estimate 0.04 ± 0.23, df = 18, p = 0.8522; night six GLMM Estimate −0.21 ± 0.24, df = 18, p = 0.4014). However, in the anthropogenic noise focal areas but not the natural noise focal areas, the first mouse to appear on the control nights was more likely to be a deer mouse, whereas, during the broadcasting nights the first mouse to appear was more likely to be a woodland jumping mouse (anthropogenic noise χ2 =9.5, df = 1, p =0.0179; natural noise χ2 =0.14, df = 1, p =0.3524).
| Broadcasting | n(nights) | Mean | SE | Min | Median | Max | ||
|---|---|---|---|---|---|---|---|---|
| Latency to Enter the Focal Area (minutes) | Anthropogenic Noise—Control | No | 15 | 34.13 | 3.34 | 15 | 34 | 55 |
| Anthropogenic Noise—Treatment | Yes | 15 | 52.47 | 5.25 | 26 | 55 | 101 | |
| Natural Noise—Control | No | 15 | 58.87 | 11.33 | -1 | 50 | 201 | |
| Natural Noise—Treatment | Yes | 15 | 74.93 | 17.84 | 31 | 53 | 312 | |
| Time Spent in the Focal Area (seconds) | Anthropogenic Noise—Control | No | 15 | 75.27 | 30.67 | 4 | 60 | 480 |
| Anthropogenic Noise—Treatment | Yes | 15 | 22.33 | 4.11 | 2 | 22 | 60 | |
| Natural Noise—Control | No | 15 | 221.20 | 90.60 | 7 | 62 | 1220 | |
| Natural Noise—Treatment | Yes | 15 | 318.93 | 95.14 | 17 | 146 | 1200 | |
| Latency to Start Foraging (minutes) | Anthropogenic Noise—Control | No | 15 | 56.33 | 11.91 | 9 | 41 | 155 |
| Anthropogenic Noise—Treatment | Yes | 15 | 118.33 | 35.18 | 29 | 52 | 379 | |
| Natural Noise—Control | No | 15 | 98.33 | 24.62 | 41 | 66 | 399 | |
| Natural Noise—Treatment | Yes | 15 | 80.60 | 18.28 | 31 | 61 | 317 | |
| Time Spent Foraging (seconds) | Anthropogenic Noise—Control | No | 15 | 291.67 | 80.26 | 60 | 140 | 1140 |
| Anthropogenic Noise—Treatment | Yes | 15 | 91.13 | 32.43 | 14 | 50 | 420 | |
| Natural Noise—Control | No | 15 | 157.53 | 52.72 | 7 | 43 | 700 | |
| Natural Noise—Treatment | Yes | 15 | 179.73 | 47.29 | 8 | 116 | 516 |
Notes:
- n
-
number of recording nights
In the anthropogenic noise focal areas, the average length of the first bout of mouse activity was 53 s shorter during broadcasting of anthropogenic noise than the control (Table 3). In the natural noise focal areas, the average length of the first bout of mouse activity was 97.7 s longer during broadcasting of natural noise than the control (Table 3). The difference in length of the first bout of mouse activity in the focal area between anthropogenic and natural noise focal areas was not significantly different (Anthropogenic-GLMM Estimate 0.13 ± 0.14, df = 8, p = 0.3580), nor was there a night effect (night five GLMM Estimate 0.16 ± 0.16, df = 18, p = 0.3139; night six GLMM Estimate 0.12 ± 0.16, df = 18, p = 0.4623).
Difference in latency to start foraging and time spent foraging by both species
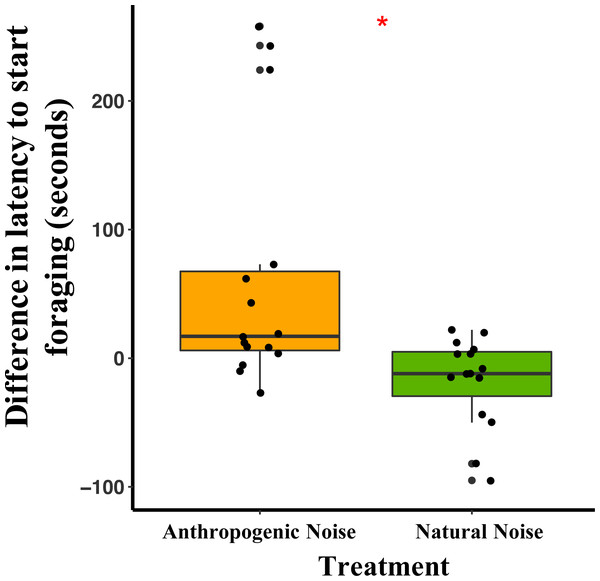
In the anthropogenic noise focal areas, the average latency for a mouse to start foraging was 62 min longer during broadcasting of anthropogenic noise than the control (Table 3). In the natural noise focal areas, the average latency for a mouse to start foraging was 17.7 min shorter during broadcasting of natural noise than the control (Table 3). The difference in latency to start foraging between anthropogenic and natural noise focal areas was significantly different (Anthropogenic-GLMM Estimate −0.71 ± 0.24, df = 8, p = 0.0183; Fig. 3). There was no effect of night on difference in latency to start foraging (night five GLMM Estimate −0.17 ± 0.23, df = 18, p = 0.4942; night six GLMM Estimate −0.19 ± 0.24, df = 18, p = 0.4325). Furthermore, in the anthropogenic noise focal areas but not the natural noise focal areas, the first mouse to start foraging on control nights was more likely to be a deer mouse, whereas, during the broadcasting treatment nights the first mouse to start foraging was more likely to be a woodland jumping mouse (anthropogenic noise χ2 =9.5, df = 1, p =0.0179; natural noise χ2 =0.60, df = 1, p = 0.2193).
Figure 3: The difference in latency to start foraging at the foraging tray for mice in focal areas between control and broadcasting nights at focal areas with anthropogenic noise and natural noise.
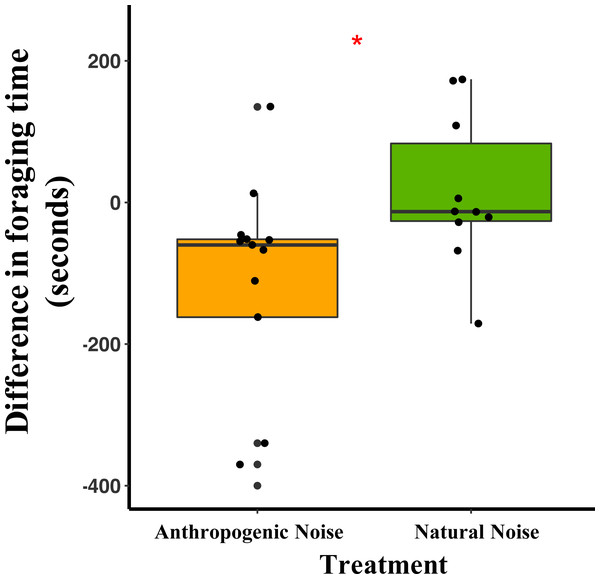
Median and quartiles of the difference in latency of a mouse to start foraging at the foraging tray by broadcasting type. Whiskers are minimum and maximum values. Distribution was a Poisson distribution. Red line and asterisk indicate a significant difference at p < 0.05. Latency to forage was similar during the broadcast of natural noise but longer during the broadcast of anthropogenic noise (anthropogenic noise n = 5 sites, natural noise n = 5 sites). Each point represents a difference between a pair of nights (during broadcasting minus before broadcasting). Data were collected at and near the Highlands Biological Station in the Southern Appalachian Mountains, North Carolina, USA from 2016–2018.In the anthropogenic noise focal areas, the average time a mouse spent foraging at the tray was 200.6 s shorter during broadcasting of anthropogenic noise (Table 3). In the natural noise focal areas, the average time a mouse spent foraging at the tray was 22.2 s longer during broadcasting of natural noise (Table 3). There was a trend in the difference in time spent foraging between anthropogenic and natural noise focal areas (Anthropogenic-GLMM Estimate 222.73 ± 97.29, df = 8, p = 0.0553; Fig. 4). There was no effect of night for the difference in time spent foraging at the tray (night five GLMM Estimate −110.30 ± 121.72, df = 18, p = 0.3768; night six GLMM Estimate −43.50 ± 121.72, df = 18, p = 0.7250).
Figure 4: The difference in foraging time at the foraging tray for mice in focal areas of between control and broadcasting nights at focal areas with anthropogenic noise and natural noise.
Median and quartiles of the difference in foraging time of a mouse (of either species) at the foraging tray by broadcasting type. Whiskers are minimum and maximum values. Distribution was a Gaussian distribution. Red line and asterisk indicate a significant difference at p < 0.05. Foraging time was similar during the broadcast of natural noise but shorter during the broadcast of anthropogenic noise (anthropogenic noise=5, natural noise=5). Each point represents a difference between a pair of nights (during broadcasting minus before broadcasting). Data were collected at and near the Highlands Biological Station in the Southern Appalachian Mountains, North Carolina, USA from 2016–2018.Vocalization production
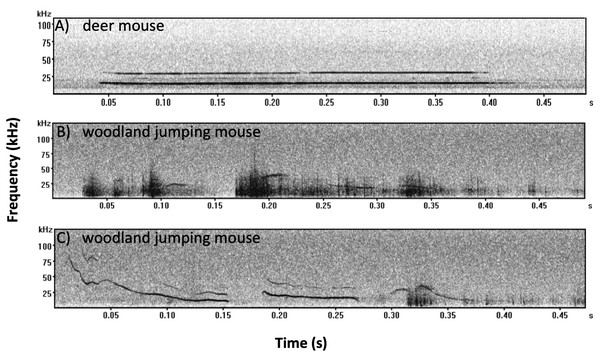
We recorded 353 mouse calls and assigned 204 calls to deer mice and 149 calls to woodland jumping mice. All calls recorded from deer mice were of the SV type (Kalcounis-Rueppell, Pultorak & Marler, 2018a; Fig. 5A). All calls recorded from woodland jumping mice were of two types (Figs. 5B and 5C) and these are the first reports of ultrasonic vocalizations from this species. Spectral and temporal characteristics of both the deer mouse and the woodland jumping mouse calls will be described in a separate publication but we present measures from 15 randomly selected calls from each species in Supplement 4 to describe basic temporal and spectral parameters of the calls.
Figure 5: Example spectrograms recorded from the deer mouse and woodland jumping mouse.
Examples of characteristic spectrograms of ultrasonic vocalizations produced by the (A) deer mouse (Peromyscus maniculatus) and (B and C) woodland jumping mouse (Napaeozapus insignis). All deer mouse calls were of the SV type as shown in A. There were two general types of calls recorded from the woodland jumping mouse as represented by B and C. All files were recorded using Avisoft UltraSoundGate 1216H with 12 balanced analog inputs and Avisoft Bioacoustics Knowles FG ultrasonic microphones. Sound files were visualized and analyzed using Avisoft SAS Lab Pro. Each spectrogram was generated with a 256 FFT (Fast Fourier Transform), and a 100-frame size with Hamming window. All recordings were recorded at Brushy Face Study Site (A) 18-July-2016 file #270; (B) 8-July-2016 file #1395; (C) 10-June-2016 file #415.We only recorded six calls during the broadcasting treatment nights (anthropogenic noise=2, deer mouse=1, woodland jumping mouse=1; natural noise=4, deer mouse=3 woodland jumping mouse=1) and the remaining 347 were recorded during the control nights before broadcasting (anthropogenic noise=300, deer mouse=161, woodland jumping mouse=133, natural noise=53, deer mouse=39, woodland jumping mouse=14). At one of the anthropogenic noise sites we recorded 183 calls in a single before-broadcasting night, and there was no significant difference with or without the outlier, therefore, we include the outlier.
There was no difference in the number of calls produced between before and during broadcasting nights (Anthropogenic - GLMM Estimate 0.75 ± 0.73, p = 0.3190) and there was no night effect (night five GLMM Estimate 1.74 ± 0.42, p = 0.0860; night six GLMM Estimate −0.12 ± 0.54, p = 0.8181).
Discussion
Our study is the first to demonstrate a direct impact of noise on individual behaviors of nocturnal and free-living deer mice. We also found that noise altered the calling behaviors of deer mice and woodland jumping mice in the wild. Both species produced few ultrasonic calls in the presence of broadcasted natural and anthropogenic noise compared to their control, or baseline, level of calling. Deer mice also spent less time in focal areas with anthropogenic noise. In the presence of anthropogenic noise, mice took longer to begin foraging, they spent less time at the foraging tray, and left fewer husks but consumed the same number of seeds. Across all behavioral variables analyzed with our GLMMs there was no night effect suggesting that there was no habituation to our equipment. Deer mice were less likely than woodland jumping mice to be the first to enter the focal area and approach food when in the presence of anthropogenic noise. Natural noise did not affect time spent in the study area or foraging behavior, but it did alter vocalization production. Thus, there are effects of noise as well as noise type on mouse behavior. Furthermore, behavioral responses to anthropogenic noise vary by species.
Our results contrast with two recent studies (Giordano, Hunninck & Sheriff, 2022; Willems et al., 2021) that show limited impacts of noise on Peromyscus species activity and foraging behavior. Using an existing noise gradient associated with energy extraction in New Mexico, Willems et al. (2021) found no effects of noise on pinon mouse (P. truei) trapping activity; however, at the beginning of their experiment, mice in noisy environments had lower body conditions than in other environments. Using predator and road noise broadcast stimuli, Giordano, Hunninck & Sheriff (2022) determined that road noise did not influence the number of seeds eaten, number of foraging visits, nor time spent foraging by the white-footed mouse (P. leucopus) both with and without the risk of predation. Both studies suggest limited influence of anthropogenic noise on mouse activity and foraging behavior and highlight the complex interaction between noise and other stimuli like light and predation. Our results may differ because we were measuring activity at different scales using telemetry and video across the entire area of activity instead of trapping either through live traps or camera traps. This methodological difference is important especially given that live traps (Willems et al., 2021) and camera traps (Giordano, Hunninck & Sheriff, 2022) were baited and effectively measured whether mice were coming to feed as opposed to overall presence in the area. In contrast, we were measuring total time spent in the area and had we set up traps as our measure of activity we likely would have had high trapability even though time spent in the area was less in the presence of anthropogenic noise. These methodological details are nuanced but important, especially when attractants like bait are involved. It is also important to recognize, as we have shown in our study, that different mouse species may respond to noise differently and Peromyscus truei (Willems et al., 2021), Peromyscus leucopus (Giordano, Hunninck & Sheriff, 2022), and Peromyscus maniculatus (our study) may have specific behaviors, morphologies, and physiologies responsible for the differences in responses among these studies. Critically, neither Willems et al. (2021) nor Giordano, Hunninck & Sheriff (2022), considered the effects of noise on the acoustic behaviors of the Peromyscus species they studied, and this is where we saw a marked influence of noise. It would be fascinating to replicate our study across all three Peromyscus species to eavesdrop on their activity and measure acoustic behavioral responses in the presence of the interaction of both predators and light.
Consistent with studies in other animals, we found that mice experience disruption and avoid environments with high noise (Finch, Schofield & Mathews, 2020; Reijnen et al., 1995; Sørensen et al., 2020). In the presence of anthropogenic noise, but not natural noise, deer mice spent less time in their focal areas, likely shifting spatial preferences and allocating less time in areas with anthropogenic noise. Focal areas with anthropogenic noise may hinder the ability for individual deer mice to detect predators, communicate, defend a territory, attain a mate, and reproduce (Barber, Crooks & Fristrup, 2010). Noisy environments can reduce individual awareness of predators as observed in Ambon damselfish (Pomacentrus amboinensis) where the fish were twice as likely to be preyed upon in the presence of noise (Simpson et al., 2016) and as was found by Giordano, Hunninck & Sheriff (2022) for white-footed mice. Animals must mitigate the negative effects of noise by altering space use or by dealing with the noise or they risk becoming locally extinct (Slabbekoorn & Ripmeester, 2008).
As highlighted by other anthropogenic noise studies, latency to begin activity and begin foraging, and time spent being active and foraging, are important measures of the initial behavioral response which could have direct consequences for individual reproductive success and survival (Barber, Crooks & Fristrup, 2010; Chan et al., 2010; Miller & Degn, 1981; Schaub, Ostwald & Siemers, 2008). We found that deer mice exhibit spatial avoidance by spending less time in the physical environment when broadcasted anthropogenic sound is present. We were not able to determine where mice went when they were not in their focal areas, and future studies should determine whether they are simply retreating to their nests or allocating more time to different areas. In any case, the results that we found for both latencies to be active and forage, and lower total time spent active and foraging, in the presence of noise could result in lower body condition of mice exposed to noise as was found for P. truei at noisy sites in NM (Willems et al., 2021). Our design did not allow us to measure the effects of noise on body condition because we only broadcast for three days but it would be interesting to examine body condition of mice at our sites near chronic road noise.
Although the number of seeds consumed did not differ between broadcast sound type or night, there was a difference in the amount of time a mouse spent at the foraging tray. In the presence of anthropogenic noise, but not natural noise, the number of leftover shells decreased, which also corresponded to mice spending less time in the foraging tray during nights with anthropogenic noise. Together, these results suggest that anthropogenic noise alters the perception of risk at the foraging tray. In the presence of anthropogenic noise, but not natural noise, mice did not stay at the foraging tray to consume seeds and leave husks but rather took seeds elsewhere. Our foraging and video data demonstrate that in the presence of noise, mice also took longer to approach a food source and spent less time foraging, consistent with the risk disturbance hypothesis in which animals exposed to noise spend less time foraging and more time being cautious (Brown et al., 2012; Evans, Dall & Kight, 2019; Morris-Drake et al., 2017; Rabin, Coss & Owings, 2006; Shannon et al., 2014). Alternatively, noise can interfere with central information processing and cognitive performance which could alter foraging patterns (Halfwerk & Van Oers, 2020). If individuals are spending more time being cautious, more energy is diverted to listening and looking for potential threats than to other behaviors.
Interestingly, both natural and anthropogenic noise decreased call production in both species of mice. Our results are consistent with other studies which found that calling behavior decreases in the presence of noise (Bermúdez-Cuamatzin et al., 2020; Fuller, Warren & Gaston, 2007; Gil et al., 2014). It is unlikely that the lack of call detection was a result of masking by our noise playbacks because we recorded several mouse calls during broadcasting, and we also recorded hundreds of bat calls in the same frequency range as the mouse calls. We also observed from our video data (not shown), that there is no evidence that the presence of noise stunned the mice, as they appeared to behave with normal movements in the focal area. They simply did not vocalize while active in the focal area if noise was being broadcast. Our results suggest that the production or reception of mouse calls are potentially sensitive to signal degradation, and that individuals may recognize noise as creating unsuitable conditions for successful acoustic communication.
Previous studies in other animals such as birds, that compared equivalent behaviors of different species to the same noise stimulus, found species-specific behavioral responses (Bayne, Habib & Boutin, 2008; Shonfield & Bayne, 2017; Francis, 2015; Voellmy et al., 2014). For example, Bayne, Habib & Boutin (2008) examined the density and occupancy rate of passerine bird species at noiseless natural gas well pads and noisy compressor stations and compared those to forest interiors in the boreal forest in Alberta, Canada. Although there was an overall effect of noise on density when considering all species combined, they found species specific responses to noise with densities of the Yellow-rumped warbler (Dendroica coronata) and Red-eyed Vireo (Vireo olivaceus) being lowest near the compressor stations whereas there was no difference in density of Tennessee Warblers (Vermivora peregrina) (Bayne, Habib & Boutin, 2008). A study of three species of owls, on the other hand, showed no effect of noise on the likelihood of occupancy of all three species at noiseless vs noisy sites (Shonfield & Bayne, 2017).
The woodland jumping mouse appears to be more tolerant of noise than the deer mouse. During the broadcasting of anthropogenic noise but not natural noise, woodland jumping mice were more likely to approach the foraging tray than deer mice. Why did we find a species difference in response to the same stimulus between woodland jumping mice and deer mice? As suggested by Bayne, Habib & Boutin (2008) there are likely reasons associated with the effects of noise on both particular acoustic cues and life history attributes of each species which would require further examination. An explanation may be the ear morphology differences between deer mice and woodland jumping mice (Blair, 1950; Preble, 1956) that could lead to a difference in sensitivity across the frequency spectra of the noises we broadcasted. Another potential explanation for the differences between woodland jumping mice and deer mice is boldness. Activity during the presence of anthropogenic noise is risky behavior (Chan et al., 2010), and boldness is the inclination of an individual to engage in risky behavior. We collected anecdotal data from trap releases during our last field season by examining latency to exit a live trap in the presence of a potential threat. We found that deer mice took longer to exit the trap than woodland jumping mice suggesting that there are boldness differences between species and consistent with Kalcounis-Rueppell, Petric & Marler (2018b) may link boldness to bioacoustics in mice. However, this and any link to responses to noise would require further investigation. In any case, determining the mechanism by which mouse species respond differently to noise will improve our understanding of why and how changes in the soundscape lead to altered time allocation, foraging and vigilance, and this is important for mitigation or conservation action.
There should be a cost for individuals that engage in cautious behavior or vigilance because they are more likely to mount a stress response and coupled with decreased foraging, the overall energetic intake and nutrition would be altered (discussed in Willems et al., 2021). For example, in tree swallows (Tachycineta bicolor), traffic noise-induced greater oxidative stress, lower body weight and delayed fledging (Injaian, Taff & Patricelli, 2018). Whereas, in other species like great tits (Parus major), noise decreases the clutch size and the number of chicks that fledge (Halfwerk et al., 2011), showing a direct fitness cost. Future studies should measure the physiological response of wild deer mice and woodland jumping mice to noise and determine if there are any long-term effects on survival and reproduction.
We did not have sufficient sample sizes in each of our groups to add effects such as sex, year, and site to our analysis, nor were we able to consider environmental covariates such as nightly precipitation, temperature, or moonphase. In our study we were careful to not sample on any nights with extreme weather, like heavy rain events. Furthermore, the riparian rhododendron understory where these mice were resident provided dense cover that mitigated moonphase, at least from our human perspective. Lastly, we have demonstrated in other Peromyscus spp. that there are no differences in SV calling behaviors between males and females in the wild (Briggs & Kalcounis-Rueppell, 2011; Petric & Kalcounis-Rueppell, 2013). We encourage future studies to examine environmental covariates and demographics on noise effects on wild rodent bioacoustics.
Conclusions
Overall, broadband anthropogenic noise reduces activity and call production in deer mice and woodland jumping mice, two species of rodents which have not been previously studied in relation to anthropogenic noise. Broadband anthropogenic noise also changes the foraging behavior of mice. Our results are consistent with previous research from other taxonomic groups, which demonstrate that anthropogenic noise negatively impacts multiple aspects of animal behavior (Barber, Crooks & Fristrup, 2010; Cox et al., 2018; Shannon et al., 2016). Rodents provide important ecosystem services by directly and indirectly influencing the abundance and distribution of other species, from plants to carnivores (Davidson, Lightfoot & McIntyre, 2008; Fischer et al., 2018; Tschumi et al., 2018; Zhang, Zhang & Liu, 2003) and we show they are negatively impacted by anthropogenic noise. Furthermore, the species examined in this research respond differently to the same stimulus and the species-specific differences are important for consideration when implementing conservation efforts to mitigate the negative effects of noise. Overall, our results highlight the importance of assessing the effects of noise across the frequency spectrum on multiple species within a community.
Supplemental Information
Video of deer mice recorded from a focal area in the study
Note that the gait is mouse like without jumping or hopping as seen in the video of the woodland jumping mice.
A short clip of woodland jumping mice recorded from a focal area in the study
Note the jumping or hopping gait.
Spectrograms of exemplars of anthropogenic (top) and natural (bottom) noise
Noise was recorded using a Zoom H2 Handy Portable Stereo Recorder. The recorder was mounted between 30 to 40-cm off the ground at the source. Our anthropogenic noise was pre-recorded from a diesel automatic standby generator (ranging from 1–42 kHz). Our natural noise was from a free-flowing creek recorded in our study area (ranging from 1–25 kHz;). We recorded noise continuously from sunset to sunrise for three nights in .WAV format at a sampling rate of 96 kHz/24-bit. We used these noise recordings to create files for our noise broadcast experiments. We randomly selected four 15-minute exemplars for each noise type (anthropogenic or natural) from across the 3 nights and created a single 1-hour sound file by randomly stringing the four 15-minute exemplars together using Avisoft SAS Lab Pro (Avisoft Bioacoustics).
All Generalized Linear Mixed Models (GLMM) models tested
Site was the random term, noise type was the fixed term, and night was a covariate. Variables tested were: difference in total time spent in the focal area, difference in total seeds consumed, difference in number of husks leftover, difference in total USVs produced, difference in latency to enter the focal area, difference in time spent in the focal area on the first visit, latency to start foraging, and difference in time spent foraging.
Spectral and temporal characteristics of select mouse calls
We selected 15 random calls recorded from the deer mouse (Peromyscus maniculatus) and the woodland jumping mouse (Napeozapus insignias) to present some basic spectral and temporal characteristics of the calls. Some recordings included a single call as in A and others were bouts of calls as in B and C. Spectral and temporal characters were derived from the first call in each bout. For the deer mouse we list how many calls were in the SV call (1call = 1SV, 2calls = 2SV). Data were extracted from sound files visualized and analyzed using Avisoft SAS Lab Pro.
Data for difference in latency to enter the focal area, time spent in the focal area on the first visit, and latency to start foraging at the tray (both species)
Data used in analyses to examine the difference in latency to enter the focal area by either species (minutes), time spent in the focal area on the first visit by either species (seconds), and latency to start foraging at the tray by either species (minutes), on nights with broadcasting of noise compared to nights without broadcasting of noise.
Total seeds consumed and husk remains (both species)
Data used in analyses to examine the difference in total seeds consumed by both species based on left over husks, on nights with broadcasting of noise compared to nights without broadcasting of noise.
Number of calls
Data used in analyses to examine the difference in number of calls produced by each species, on nights with broadcasting of noise compared to nights without broadcasting of noise.
Time spent foraging (both species)
Data used in analyses to examine the difference in time spent foraging on first appearance at the tray by either species (seconds), on nights with broadcasting of noise compared to nights without broadcasting of noise.
Total time spent in the focal area by deer mice
Data used in analyses to examine the total time spent in the focal area by deer mice (minutes), on nights with broadcasting of noise compared to nights without broadcasting of noise.