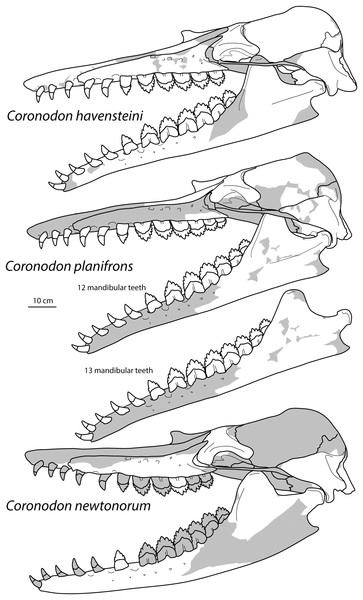
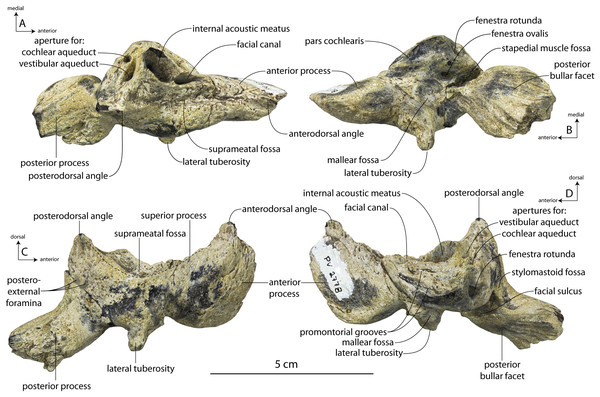
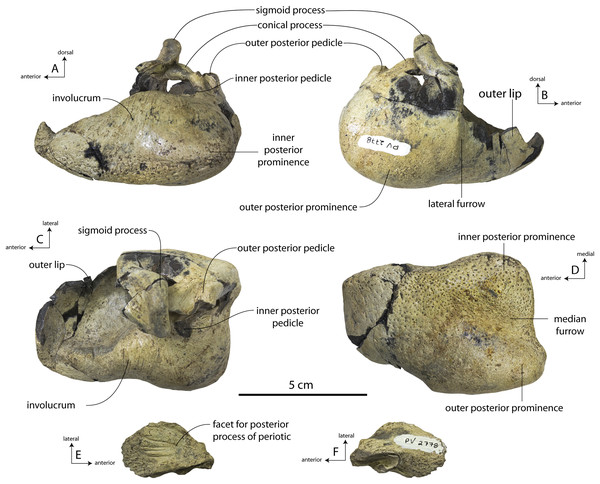
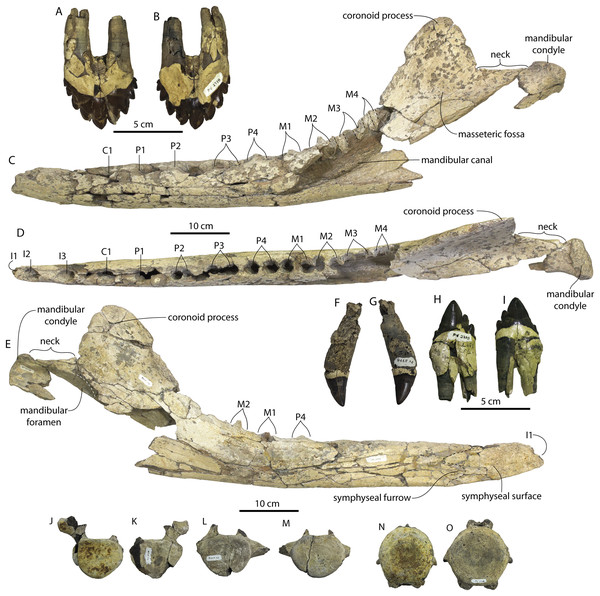
New specimens and species of the Oligocene toothed baleen whale Coronodon from South Carolina and the origin of Neoceti
- Published
- Accepted
- Received
- Academic Editor
- Matthew McCurry
- Subject Areas
- Evolutionary Studies, Marine Biology, Paleontology, Zoology
- Keywords
- Mysticeti, Neoceti, Cetacea, Oligocene, North Atlantic, Phylogeny
- Copyright
- © 2023 Boessenecker et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2023. New specimens and species of the Oligocene toothed baleen whale Coronodon from South Carolina and the origin of Neoceti. PeerJ 11:e14795 https://doi.org/10.7717/peerj.14795
Abstract
Baleen whales (Mysticeti) are gigantic filter-feeding cetaceans possessing the unique soft tissue structure baleen and lacking adult teeth; Oligocene fossils have revealed a wealth of early diverging tooth-bearing mysticetes highlighting the transition from archaeocete ancestors to early toothless baleen-bearing eomysticetid whales. The archaeocete-like, toothed mysticete Coronodon havensteini from the lower Oligocene Ashley Formation of South Carolina possesses a number of peculiar aspects of feeding morphology suggesting dental filter-feeding in the earliest diverging mysticete lineage. New fossils of Coronodon are described in detail, including (1) supplementary description of the holotype skull and skeleton of Coronodon havensteini; (2) description of two new juvenile skulls of C. havensteini and a partial skull and postcranial skeleton of an adult; (3) description of the new species Coronodon planifrons n.sp.; and (4) description of the new species Coronodon newtonorum. New specimens of Coronodon havensteini include a partial adult skeleton preserving new elements for the species including incisors, numerous upper premolars and molars, lower m4, scapula, lumbar, and caudal vertebrae, and two juvenile skulls with tympanoperiotics and teeth. Fossils from the overlying unit, the Chandler Bridge Formation, represent two new species: Coronodon newtonorum n. sp. and Coronodon planifrons n. sp. Coronodon newtonorum possesses a concave-up alveolar profile, a mandibular condyle elevated far above the toothrow, and a gracile periotic resembling those of juvenile C. havensteini. Coronodon planifrons n. sp. possesses a horizontal supraorbital process, successively smaller upper molars, massively inflated periotic, and longer intertemporal region. Coronodon planifrons n. sp. preserves one of the most complete vertebral columns among toothed mysticetes, indicating nine thoracic vertebrae, ten lumbar vertebrae, and at least 20 caudal vertebrae. The column exhibits a somewhat stabilized caudal peduncle with enlarged lumbocaudal vertebrae, and rectangular terminal caudals indicate the presence of tail flukes. Juvenile skulls reveal several ontogenetic trends in Coronodon havensteini, including the anterior migration of the orbitotemporal crest, anteroposterior elongation of the intertemporal region, inflation of the body of the periotic, enlargement of the tympanic bulla, and continued postnatal emergence of the premolars and molars from their alveoli. Disarticulated skulls suggest a degree of rostral kinesis in this genus. Phylogenetic analysis of the largest assembled supermatrix of Mysticeti (n =138 OTUs; four archaeocetes, 10 odontocetes, 124 mysticetes; 391 morphological and 27,225 molecular characters) confirms placement of Coronodon as the earliest diverging lineage of Mysticeti under equally weighted analyses whereas implied weighting places Coronodon and similar taxa outside Neoceti, prompting a review of character transformations at the base of Neoceti.
Introduction
The terrestrial to aquatic transition in whales is one of the most dramatic and compelling examples of macroevolution, and a series of well-preserved skulls and skeletons of Eocene archaeocete whales have illuminated changes in brain size, hearing, olfaction, locomotion, feeding morphology, and even reproduction (Gingerich, Smith & Simons, 1990; Gingerich et al., 1994, 2001, 2009; Godfrey, Geisler & Fitzgerald, 2013; Marino, McShea & Uhen, 2004; Nummela et al., 2007; Thewissen, Hussain & Arif, 1994; Thewissen et al., 2001; Uhen, 2004a). While many gaps in our knowledge have been filled, the divergence of the Neoceti—the clade including modern and extinct toothed whales (Odontoceti) and baleen whales (Mysticeti) and their common ancestor-is relatively understudied. The origin of toothed whales has been the focus of some studies evaluating the early adaptations (or lack thereof) for echolocation (Geisler, Colbert & Carew, 2014; Churchill et al., 2016; Racicot et al., 2019), feeding morphology (Boessenecker, Ahmed & Geisler, 2017) and locomotion (Boessenecker et al., 2020), although the earliest odontocetes remain unnamed and only partially described (Barnes, Goedert & Furusawa, 2001).
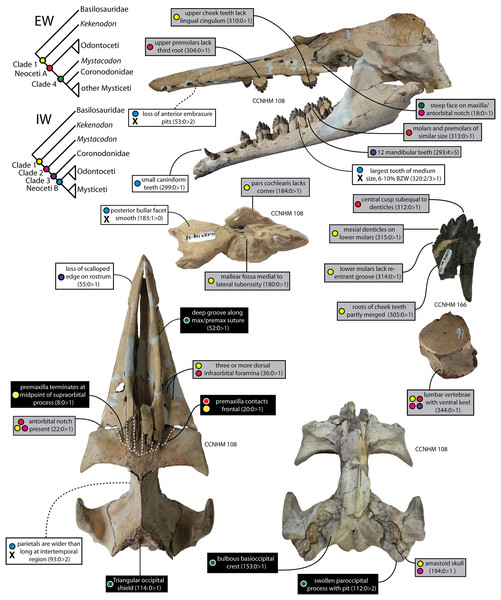
The transition from archaeocetes to early mysticetes, on the other hand, has attracted extensive study in recent years. Early discoveries of toothed mysticetes were formerly confused with or considered to be archaeocetes (Pritchard, 1939; Emlong, 1966; Russell, 1968), or known from poorly preserved material too incomplete to reveal morphological transformations in the earliest members of the group (Mitchell, 1989). The recognition of aetiocetids as toothed mysticetes was a key development in this field of study (Barnes et al., 1995), followed later by the recognition of small, large-eyed raptorial feeding forms like Janjucetus (Fitzgerald, 2006). These discoveries suggested a degree of diversity among toothed mysticetes that had not been previously appreciated. The identification of lateral palatal foramina in Aetiocetus weltoni by Deméré et al. (2008), thereby suggesting the simultaneous presence of baleen and teeth, proved to be surprisingly provocative and triggered a number of critical responses (Fitzgerald, 2010; Fordyce & Marx, 2018; Marx, 2011; Marx et al., 2016; Peredo, Pyenson & Boersma, 2017; Peredo et al., 2018; Peredo, Pyenson & Uhen, 2022). Among the flurry of research published in the wake of Fitzgerald (2006) and Deméré et al. (2008), is research on the diverse feeding adaptations in the dentition, mandibles, and skulls of toothed mysticetes including articles proposing (1) benthic suction feeding (Fitzgerald, 2010; Marx et al., 2016; Fordyce & Marx, 2016; Lambert et al., 2017); (2) macrophagy (Fitzgerald, 2006; Marx & Fordyce, 2015; Hocking et al., 2017); (3) filter feeding using baleen (Ekdale & Deméré, 2022) or even (4) dental filtering (Geisler et al., 2017); the (5) possible retention of teeth in the early chaeomysticete clade Eomysticetidae (Boessenecker & Fordyce, 2015a); (6) recognition of a mammalodontid clade (Fitzgerald, 2010; Marx, 2011); (7) the early evolution of baleen and associated (or non-associated) neurovascular plumbing (Ekdale & Deméré, 2022; Peredo, Pyenson & Uhen, 2022) or alternatively (8) thickened gums (Marx et al., 2016; Fordyce & Marx, 2018); (9) the evolution of tooth loss (Meredith et al., 2009, 2011; Peredo, Pyenson & Boersma, 2017; Mu et al., 2021; Randall, Gatesy & Springer, 2022; Gatesy et al., 2022), and (10) the origin of low frequency hearing (Ekdale & Racicot, 2015; Park et al., 2017). In addition, two long-standing but (until recently) unpublished toothed mysticetes—Llanocetus and Coronodon—were finally described in full (Geisler et al., 2017; Fordyce & Marx, 2018).
Despite this research effort, many disagreements remain over the origin and interpretation of baleen, dental filtration, and the phylogenetic placement of various toothed mysticetes. Virtually every published matrix resolves different topologies at the base of Mysticeti (e.g., mammalodontids as the earliest diverging clade, followed by Coronodonidae and Llanocetus, Marx & Fordyce, 2015; Mystacodon as the earliest diverging clade, Muizon et al., 2019; Coronodonidae fam. nov. most basal, followed by Llanocetus and then Mammalodontidae, Fitzgerald, 2010; Fordyce & Marx, 2018; Coronodonids most basal, followed by mammalodontids, and then Llanocetus, Geisler et al., 2017). Otherwise, little has advanced regarding the evolution of rostral kinesis and mandibular kinesis (see Gatesy et al., 2022), locomotor adaptations (see Muizon et al., 2019), taphonomic patterns, ontogenetic changes, or the divergence of mysticetes from odontocetes from their archaeocete ancestors. More recently, one phylogenetic analysis even suggested that many toothed mysticetes (including Coronodon, Llanocetus, Mystacodon, and mammalodontids) may be placed outside the odontocete-mysticete clade, suggesting that only the Aetiocetidae are actually toothed mysticetes (Corrie & Fordyce, 2022).
A consensus has yet to emerge for even the most intensely studied aspects of early mysticete evolution, and many questions remain to be answered—and others have not yet been asked. Likely contributing to these disagreements is the fossil record of toothed mysticetes, which chiefly consists of isolated skulls, occasionally preserved with the phylogenetically informative earbones, teeth, and mandibles. Few specimens preserve postcrania, with some exceptions (e.g., Mystacodon; Lambert et al., 2017; Muizon et al., 2019), and virtually all nominal toothed mysticete species are represented solely by a holotype skull, with only a single exception—Fucaia goedertorum, also known from a paratype skull (Barnes et al., 1995). Biases in the mysticete fossil record limit phylogenetic coding, assessment of locomotion, and in particular, assessment of individual variation and ontogenetic variation—both of which are virtually unstudied amongst early Neoceti.
Archaeocete-like fossils with some features of Neoceti and Mysticeti were first discovered from Oligocene sediments (Ashley and Chandler Bridge formations) in the vicinity of Charleston, South Carolina (USA) in the 1970s, and first formally studied in the 1990s (Barnes & Sanders, 1996a, 1996b). These specimens housed in The Charleston Museum (ChM PV 2778, 4745, and 5720) were widely acknowledged and studied by mysticete specialists and colloquially referred to as ‘archaeomysticetes’ or the ‘Charleston toothed mysticetes’, though they remained unpublished. Early conference presentations remarked that these fossils were more archaic than previously discovered toothed mysticetes and demonstrated the derivation of early mysticetes from “dorudontine” basilosaurids (Barnes & Sanders, 1996a, 1996b). A virtually complete skull (CCNHM 108), clearly closely related to ChM PV2788, 4745, and 5720, was collected from exposures of the Ashley Formation (late Rupelian) in 2002 and subsequently became the holotype of Coronodon havensteini (Geisler et al., 2017). Coronodon havensteini possesses large, basilosaurid-like teeth, a wide and somewhat flattened, partly kinetic rostrum, large basioccipital crests, and a veritable mix of basilosaurid-like and mysticete-like features, though admittedly more plesiomorphic than all other described toothed mysticetes (Geisler et al., 2017). A number of strange craniomandibular features, unique amongst toothed mysticetes, led to the novel proposal that Coronodon represented an early stage of toothed mysticetes that evolved the ability to filter feed with their cheek teeth (Geisler et al., 2017). This interpretation was based on worn, mesially-facing cusps; a lack of apical wear on many of the highest cusps on the cheek teeth; highly emergent lower cheek teeth that overlapped labiolingually to form posterolaterally-directed, interdental slots, and a near homodont battery of cheek teeth (premolars and molars of near identical size and morphology) with accessory cusps subequal to the primary cusp (Geisler et al., 2017). This interpretation was subsequently challenged on the basis of a single dental metric (Hocking et al., 2017).
New material of Coronodon includes partial skeletons of two new species of Coronodon from the younger Chandler Bridge Formation as well as new specimens, including young juveniles, of Coronodon havensteini from the Ashley Formation that, for the first time, shed light on the ontogeny, individual variation, and locomotor adaptations of a single species of early mysticete. This bountiful sample of an early neocete includes virtually complete skulls, earbones, teeth, mandibles, and postcrania of multiple individuals, permitting evaluation of (1) many characters identified as synapomorphies of Neoceti and Mysticeti, as well as (2) the hypothesis that Coronodon and other toothed mysticetes might fall outside crown Cetacea, and (3) paleoecological inferences of the functional morphology of Coronodon.
Materials and Methods
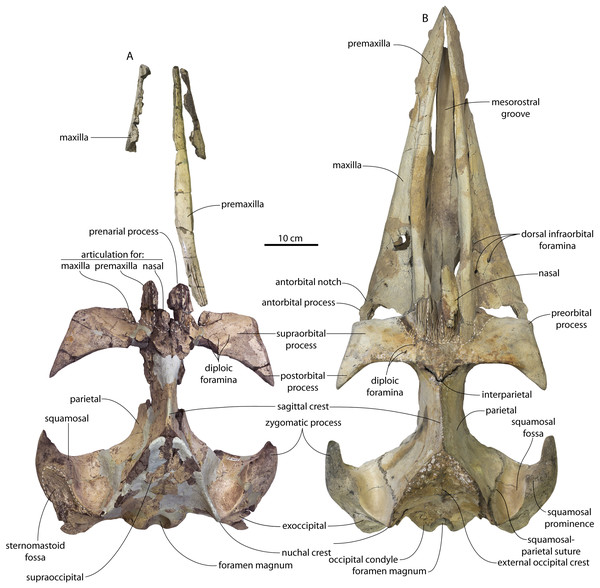
Descriptive methods and anatomical terminology
Anatomical terminology follows Mead & Fordyce (2009) with some additions from Boessenecker & Fordyce (2015b); notable changes include use of periotic fossa and cranial hiatus of the former, despite changes introduced by the latter (e.g., pit for the periotic). Photographs were taken with a Canon Rebel Eos T5 and a 18–55 mm zoom lens or a 100 mm f/2.8 macro lens. Measurements were recorded using large calipers to the nearest millimeter and digital calipers for smaller (<30 cm) measurements to the nearest tenth of a millimeter.
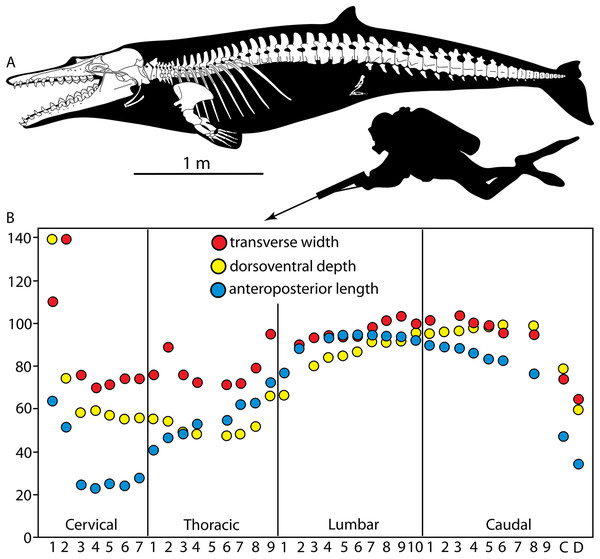
We estimated the body length of Coronodon by using three methods: the bizygomatic skull width and partial least square equations from Pyenson & Sponberg (2011) for stem Mysticeti, and using a composite skeletal length using the holotype skull and cervical vertebrae of Coronodon havensteini, the thoracic vertebrae of the referred Coronodon havensteini specimen CCNHM 164, and the holotype lumbocaudal vertebrae Coronodon planifrons n. sp. (under the assumption that both species shared similar vertebral counts), along with estimated intervertebral disc lengths based on Long et al. (1997).
Taxonomy
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:796ED3F3-33A1-46E3-A6A0-F3898EA5C094. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central SCIE and CLOCKSS.
Ontogeny
We assessed relative ontogenetic status in Coronodon using the following criteria: (1) increasing skull size, (2) degree of tooth eruption, (3) closure of pulp cavities in tooth roots, (4) tooth wear, (5) development of embrasure pits, (6) closure and obliteration of posterior skull sutures (median frontal, frontoparietal, median parietal, squamosal-parietal, parietal-occipital sutures), (7) occipital synchondroses, (8) development of sagittal and nuchal crests, and (9) vertebral epiphyseal fusion. Owing to the lack of neonates and small sample sizes, individual specimens in this study were simply identified as juveniles or adults, with tentative assignments to the ontogenetic classes of Perrin (1975).
Phylogenetic methods
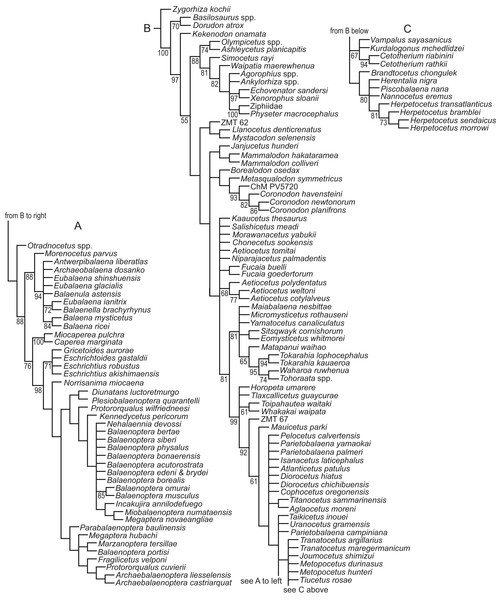
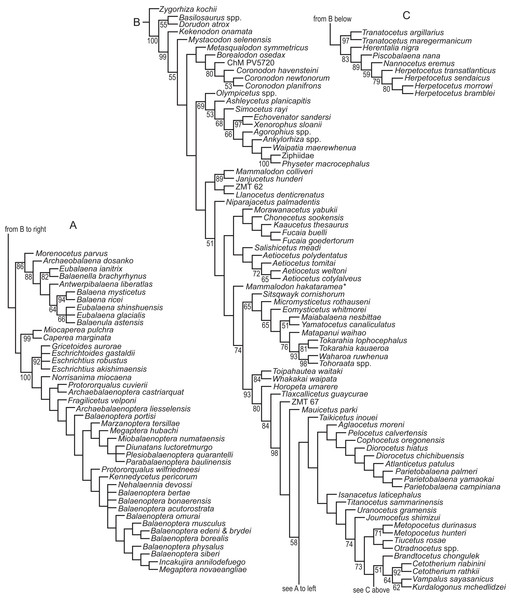
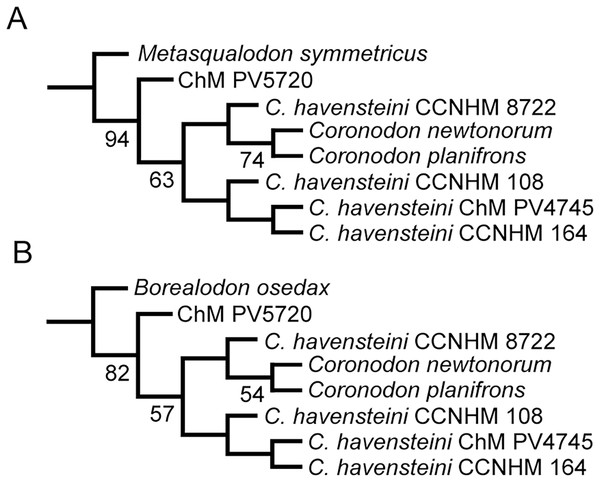
We revisited the phylogenetic position of Coronodon havensteini, as well as determined the positions of Coronodon newtonorum n. sp. and C. planifrons n. sp., using a supermatrix of 27,617 characters (Data S1). The morphological partition of this supermatrix was based on the dataset of Boessenecker & Fordyce (2017), to which we added 29 new morphological characters, ordered 81 multistate characters (Boessenecker & Fordyce, 2017 treated all multistate characters as unordered), and added 53 taxa (Data S2). Ordering allows for similarity among character states to be included as data in phylogenetic analyses (Wilkinson, 1992), and multistate characters that have equally dissimilar states were left unordered. The number of odontocete outgroups was increased from two to 10, now including Olympicetus, Ashleycetus, Ankylorhiza tiedemani, the xenorophids Echovenator and Albertocetus, and two extant odontocetes (Ziphiidae, based primarily on Tasmacetus shepherdi, and Physeter macrocephalus). The enigmatic and recently redescribed Kekenodon onamata, a late-surviving archaeocete, was also added (Corrie & Fordyce, 2022). Five specimens in the genus Coronodon were coded separately in the matrix. Four (i.e., CCNHM 108, 164, 8722, ChM PV4775) represent C. havensteini, and were combined to create a species-level operational taxonomic unit (OTU). Differences among the four specimens were coded as polymorphisms for the composite OTU, but majority-rule coding was employed where PV4775 and or CCNHM 8722 were different from the others and the difference could be explained by a young ontogenetic stage. Other noteworthy taxonomic additions (citations indicate taxa coded from the literature or photographs, otherwise specimens were examined directly) to the matrix of Boessenecker & Fordyce (2017) include the toothed mysticetes Aetiocetus tomitai (Barnes et al., 1995), Borealodon osedax, Chonecetus sookensis, Fucaia buelli, Kaaucetus thesaurus (Hernández Cisneros, 2022), Llanocetus denticrenatus, Mammalodon hakataramea (Fordyce & Marx, 2016), Metasqualodon symmetricus (Okazaki, 1982), Mystacodon selenensis (Muizon et al., 2019), Morawanocetus yabukii (Barnes et al., 1995), Niparajacetus palmadentis (Solis-Añorve, González-Barba & Hernández-Rivera, 2019), Salishicetus meadi (Peredo & Pyenson, 2018), and the basal toothless or nearly toothless mysticetes Maiabalaena nesbittae and Sitsqwayk cornishorum. Several additional crown mysticetes were also coded. The resulting morphological dataset has 130 distinct OTU’s, and one additional composite OTU for Coronodon havensteini, that are coded for 392 morphological characters (Data S3). This morphological matrix was then combined with the molecular partition published by Deméré et al. (2008).
The morphological dataset was constructed in the application Mesquite (Maddison & Maddison, 2021), exported to TNT format (Goloboff, Farris & Nixon, 2008), and then manually combined with the molecular partition in a text editor. Most parsimonious trees were discovered using a “new technology search” in the computer application TNT. Two separate analyses were conducted; one with all characters equally weighted, referred to as the equal weights analysis (EW), and another using implied weighting (IW), with the constant k = 3 (Goloboff, 1993). The shortest or best-fit trees from these analyses are referred throughout the text as the EW trees or the IW trees, respectively. Default settings were used in both analyses except that the search was ended after the most parsimonious trees were found 1,000 times and the memory was set to save up to 10,000 shortest trees. The EW phylogenetic analysis initially found 3,836 most parsimonious trees, and then subsequent TBR branch swapping recovered another 10,000 trees. It is unclear if the strict consensus from those trees is representative of the strict consensus of all most parsimonious trees, both saved and unsaved. Thus, the strict consensus was compared to an estimated consensus that was derived from a driven search, which used default settings except that the consensus was stabilized 300 times. Nodal support was measured using the bootstrap in TNT. Default search settings were used except for the following: (1) bootstraps were done with replacement, (2) absolute frequencies were reported, and (3) each replicate included a new technology search, with the search ended after the shortest trees for that replicate were recovered five times. Optimization of characters onto individual trees was explored in Mesquite, but summaries of all synapomorphies were saved to output files using TNT (optimize > list common synapomorphies). To investigate the lengths of individual characters on all trees from the EW analyses (i.e., >10,000 trees), all but the character of interest was excluded from the calculation of tree length, all trees were sorted by length (trees > tree buffer > sort trees), and then the longest and shortest trees were viewed to get the range of length across all trees. If the range of lengths for a specific character from the trees obtained with implied weights (IW trees) overlapped with the range from trees obtained without implied weighting (EW trees), then we considered this character to support both sets of trees equally. In these comparisons, the support of a character was measured by steps according to equal weights, not the fit of the character as measured by implied weighting.
We also conducted phylogenetic analyses that treated each specimen as an OTU to test our assignment of individual specimens to C. havensteini. We did not select these analyses are our primary analyses because two of these specimens (i.e., ChM PV4745, CCNHM 8722) are immature, and thus some “relationships” recovered may reflect ontogenetic stage instead of recency of ancestry. The same methods described above were employed for these additional analyses.
Geologic background
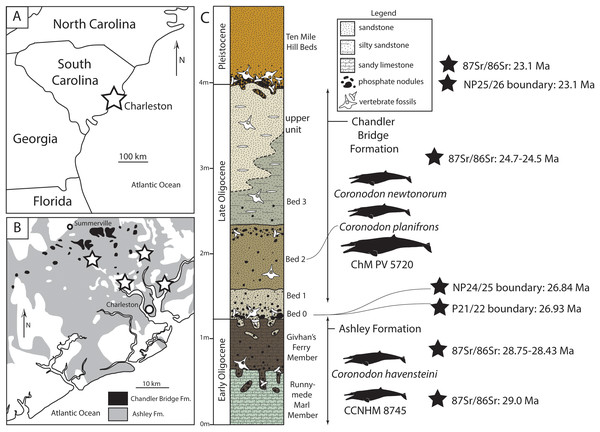
Fossils of Coronodon have only been discovered in the Oligocene Ashley and Chandler Bridge formations of the Charleston embayment in South Carolina, USA (Fig. 1). The Ashley Formation is a lightly consolidated, quartzose to phosphatic calcarenite ranging from yellow to tan, light gray, and olive brown in color (Weems et al., 2016). The Ashley Formation is up to 38 m thick, and unconformably overlies the uppermost Eocene Harleyville Formation. The Ashley Formation is sparsely to richly fossiliferous and frequently contains isolated mollusks and barnacles, occasionally concentrated into pavements. Phosphatic molds of small solitary corals (Flabellum, Balanophyllia) as well as steinkerns and phosphate pebbles are common; common invertebrates include the wentletrap Epitonium, the oyster Cubitostrea, and the barnacle Concavus (Fallon & Boessenecker, 2020). Vertebrate fossils are uncommon within the Ashley Formation, but include sharks (Miller, Gibson & Boessenecker, 2021), bony fish (Fierstine & Weems, 2009), sea turtles (Ashleychelys, cf. Euclastes, Natemys, cf. Psephophorus; Weems & Sanders, 2014; Fallon & Boessenecker, 2020), sirenians (Crenatosiren, Dioplotherium, Priscosiren, Stegosiren; Domning, 1989, 1997; Domning & Beatty, 2019; Velez-Juarbe & Domning, 2014), toothed whales (Albertocetus, Agorophius, Ankylorhiza, Ediscetus, Inermorostrum, Xenorophus; Albright, Sanders & Geisler, 2018; Albright et al., 2019; Boessenecker et al., 2017; Boessenecker et al., 2020; Churchill et al., 2016; Geisler, Colbert & Carew, 2014; Godfrey et al., 2016; Kellogg, 1923; Sanders & Geisler, 2015), an eomysticetid baleen whale (Micromysticetus; Sanders & Barnes, 2002a), and Coronodon (Geisler et al., 2017). Extensive bioturbation, grain size (fine-medium sand), and phosphatic bonebeds indicate middle shelf deposition (Fallon & Boessenecker, 2020). Fossils of the billfish Aglyptorhynchus suggest relatively warm conditions, with sea surface temperatures ranging 20–24 °C, similar to the overlying Chandler Bridge Formation (Fierstine & Weems, 2009). The Ashley Formation has produced microfossils corresponding to calcareous nannofossil zone NP24 (29.63–26.84 Ma; Gradstein et al., 2012) and foraminiferal zone P21 (29.18–26.93 Ma; Gradstein et al., 2012), as well as 87Sr/86Sr dates of 28.4–29.0 Ma for the Runnymede Marl and Givhan’s Ferry members (Weems et al., 2016), summarized here as 29–27 Ma. These dates indicate that the unconformity separating Oligocene rocks and cetaceans from the uppermost Eocene Harleyville Formation represents approximately 5 my, given that the basilosaurid-producing Harleyville Formation has produced microfossils corresponding to the Eocene portion of calcareous nannofossil zone NP21 (34.44–33.9 Ma; Weems et al., 2016).
Figure 1: Geologic and stratigraphic context of Coronodon.
(A) Regional map showing location of Charleston, South Carolinal USA. (B) Simplified geologic map (after Weems & Lewis, 2002) showing the extent of the Oligocene Ashley and Chandler Bridge formations and Coronodon localities (stars). (C) Sedimentary column of the uppermost Ashley Formation and Chandler Bridge Formation in the vicinity of Summerville, South Carolina (modified from Fallon & Boessenecker, 2020), showing the stratigraphic origin of coronodonid fossils and age determinations (see Geologic Background for summary).The Chandler Bridge Formation unconformably overlies the Ashley Formation; it is patchy in distribution, apparently being eroded away or only deposited along paleotopographic highs (Katuna, Geisler & Colquhoun, 1997). It consists of under 1 m (typically 40–60 cm thick, and rarely up to 2.5 m thick) of massive poorly lithified siltstone with some sand and is rich in phosphatic pebbles; the siltstone is typically khaki to olive green at the base (Bed 0–1) and brown to tan in the upper part (Bed 2); where exposed, the rare uppermost bed (Bed 3) is gray to tan and lightly consolidated and yields scattered discoidal quartz pebbles (Sanders, Weems & Lemon, 1982). The Chandler Bridge Formation is in turn unconformably overlain by the even thinner and patchier Edisto Formation, which straddles the Oligocene-Miocene boundary (Weems et al., 2016). Fossil vertebrates from the Chandler Bridge Formation have been more intensely studied, relative to the Ashley Formation, and include sharks (Cicimurri & Knight, 2009; Miller, Gibson & Boessenecker, 2021), bony fish (Fierstine & Weems, 2009; McCuen, Ishimori & Boessenecker, 2021), sea turtles (Carolinachelys, cf. Egyptemys, Natemys, Procolpochelys, cf. Psephophorus; Hay, 1923; Weems & Sanders, 2014, Weems & Brown, 2017; Fallon & Boessenecker, 2020), sea birds (Pelagornis, Sulidae; Ksepka, 2014), toothed whales (Agorophius, Ankylorhiza, Cotylocara, Echovenator, Xenorophus; Geisler, Colbert & Carew, 2014; Churchill et al., 2016; Godfrey et al., 2016; Boessenecker & Geisler, 2018; Boessenecker et al., 2020), an eomysticetid baleen whale (Eomysticetus; Sanders & Barnes, 2002b), and sirenians (Crenatosiren, Metaxytherium; Domning, 1997; Velez-Juarbe & Domning, 2014). Dinoflagellates and vertebrate taphonomy initially suggested that Bed 1 represented fully marine conditions followed by shallower deposition within a protected embayment or estuary with Beds 2 and 3 (Katuna, Geisler & Colquhoun, 1997). Studies of the ichthyofauna suggest continuous open marine conditions throughout deposition (Cicimurri & Knight, 2009), though these authors did not report sharks from individual beds. The occurrence of warm water sharks and the billfish Aglyptorhynchus indicates sea surface temperatures of approximately 20–24 °C (Fierstine & Weems, 2009). Dinoflagellates from the Chandler Bridge Formation indicate assignment to zones NP24-25, indicating an age of 29.6–23.1 Ma (Gradstein et al., 2012), and 87Sr/86Sr ratios from oyster shells ranging from 24.7–24.5 Ma (Weems et al., 2016). A minimum age for the Chandler Bridge Formation is provided by 87Sr/86Sr dates of 23.5 Ma from the overlying Edisto Formation (Weems et al., 2016), in concert indicating an age range of 24.7–23.5 Ma (e.g., McCuen, Ishimori & Boessenecker, 2021).
Results
Systematic paleontology
Mammalia Linnaeus, 1758
Cetacea Brisson, 1762
Neoceti Fordyce & Muizon, 2001
Mysticeti Gray, 1864
Coronodonidae New Family LSID urn:lsid:zoobank.org:act:1FE35563-5AD1-447E-A3AC-280C4A9BB2D0
Diagnosis
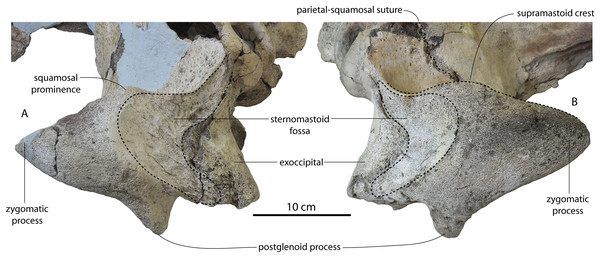
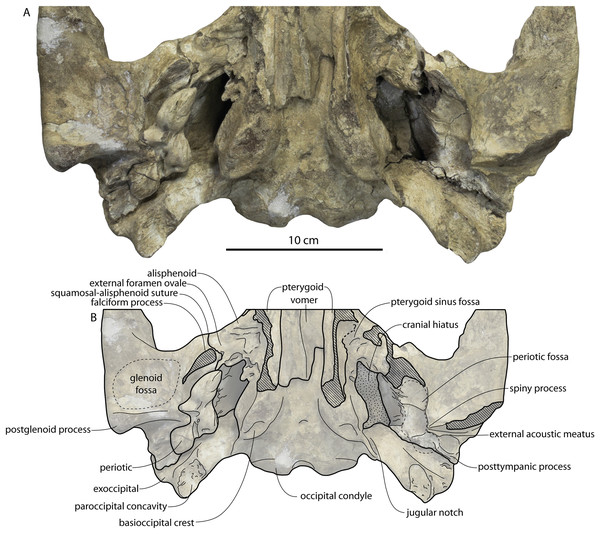
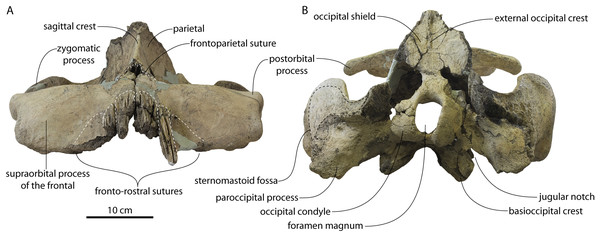
Large toothed mysticetes (BZW = 40–60 cm, estimated body length 5–8 m) with incipient polydonty (11 upper, 12 lower teeth); wide rostra with loose premaxilla-maxilla and maxillofrontal sutures; edentulous and transversely narrow blade-like premaxilla anterior to I1; dorsally curved nasal apex; long intertemporal constriction with high sagittal crest and parallel dorsolateral margins; steeply sloping to nearly vertical occipital shield with occipital apex thrust to level of supramastoid crest; tall and vertical nuchal crest; squamosal with short, dorsoventrally deep zygomatic process bearing facet for jugal, enlarged squamosal prominence, large sternomastoid fossa; amastoid periotic with triangular anterodorsal and posterodorsal angles but highly reduced superior ridge and shallow trough-like suprameatal fossa, low and anteriorly narrow pars cochlearis with narrow anterior cochlear ridge and separated from anterior process by obtuse angle (160°–180°), and distally widening posterior bullar facet; wide non-rotated bulla with flattened ventral surface and median furrow, step-like profile of involucrum with flat medial face; dentition with thin smooth enamel (some lingual ridging on caniniform teeth and p1-2 only), pseudoserrations on proportionally large postcanine teeth; double rooted postcanines (P3-M3) with long root isthmus, demi-roots (except C. newtonorum), overlapping lower cheek teeth, five or more mesial denticles on premolars; posterior upper cheek teeth distally inclined; mandible with faint sutural surface for symphysis, elevated molars, lobate but subtriangular and vertical coronoid process and a mandibular condyle separated far from coronoid process.
Included taxa
Coronodon; unnamed genera represented by ChM PV 5720 (and CCNHM 214), and CCNHM 8745.
Remarks
The name Coronodontidae is unavailable as it is preoccupied by Coronodontidae Harris 1951. In accordance with ICZN articles 29.2 and 29.6, Coronodonidae is available. At present this clade includes only one genus, Coronodon. However, naming this clade is warranted as an unnamed toothed mysticete, ChM PV 5720, has been used in a number of cladistic analyses (Geisler & Sanders, 2003; Geisler et al., 2011; Fitzgerald, 2006, 2010; Boessenecker & Fordyce, 2015a, 2015b, 2015c, 2017; Marx & Fordyce, 2015; Sanders & Geisler, 2015; Lambert et al., 2017; Martinez-Caceres, Lambert & Muizon, 2017; Fordyce & Marx, 2018; Peredo et al., 2018; Muizon et al., 2019). Unpublished specimen CCNHM 214 appears to represent a juvenile of the same taxon as ChM PV 5720. CCNHM 8745 is described below. A comparative diagnostic table for different coronodonid taxa is presented in Table 1.
| Coronodon havensteini | Coronodon planifrons | Coronodon newtonorum | ChM PV 5720 | CCNHM 8745 | |
|---|---|---|---|---|---|
| Alignment of molars/diastemata | Anteroposterior, no diastemata | ? | Overlapping | Anteroposterior, short diastemata | ? |
| Upper molars | Subequal | M2 and m3 successively smaller | Subequal? | Subequal? | ? |
| Embrasure pits | Present along toothrow | ? | Absent posterior to P2 | Present along toothrow | ? |
| Ventral margin of maxilla | Straight | ? | Convex | Straight | ? |
| Ventral margin of mandible | Straight | Straight | Convex | Straight | ? |
| SOPF angle in anterior view | Ventrolateral | Horizontal | Ventrolateral | Ventrolateral | Ventrolateral |
| Rostrofrontal overlap v. SOPF length (ant. Frontal to ant. Orbitotemporal crest) | 72.7% | 64% | 89.9% | 100%? | 65% |
| Dorsal profile of nasals | Upturned | Upturned | ? | Upturned | Horizontal |
| Prenarial triangle | Absent | Absent | Absent | Present, 62% of nasal length | Present, 44% of nasal length |
| Preorbital v. postorbital process | Thick, subequal; postorb = 82% of preorb depth | Postorbital process thicker, postorb = 194% of preorb depth | Preorbital process thicker, postorb = 64% of preorb depth | Postorb slightly thicker, postorb = 135% preorb depth | Preorbital process thin (23 mm) |
| Intertemporal constriction length v. postorbital width | Long, 49% | Moderate, 40.8% | ? | Short, 35% | Very long, 54% |
| Sternomastoid fossa | Does not ascend nuchal crest | Ascends nuchal crest | ? | Does not ascend nuchal crest | ? |
| Inflation of periotic body | Moderately to strongly inflated, 155–175% | Strongly inflated, 162% | Slightly inflated, 140% | Slightly inflated, 133% | ? |
| Posterior process length as % of periotic length | Long, 48.2–50.3% of periotic length | Long, 44.6% of periotic length | Short, 41% of periotic length | Short, 38.5% of periotic length | ? |
| Lateral tuberosity length | Short, does not extend beyond body (except in juvenile) | Long, extends beyond body | Long, extends beyond body | Short, does not extend beyond body | ? |
Coronodonidae indeterminate
Referred specimen
CCNHM 8745, a partial braincase probably collected ex situ from the bottom of the Cooper River (or possibly from the Wando River), Ashley Formation, Berkeley County, South Carolina, USA, discovered in the early 2000s by an unknown amateur collector. Additional locality data is available on file at CCNHM.
Description
Frontal, nares, and orbit
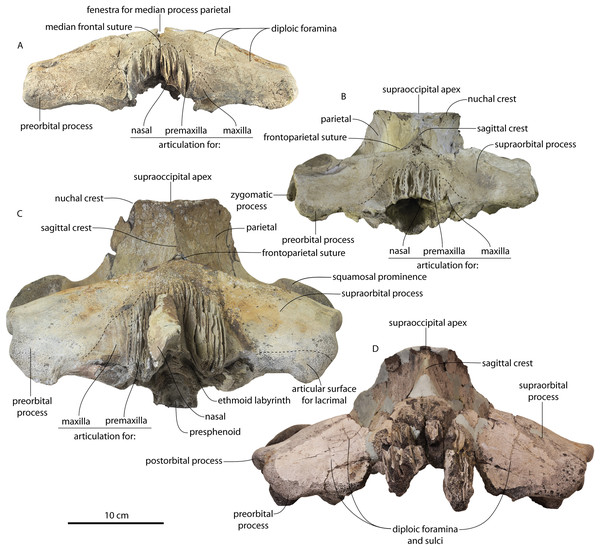
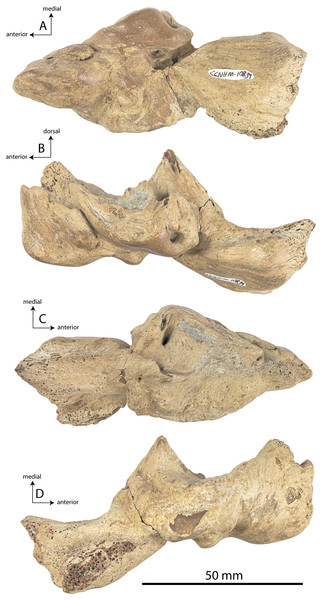
CCNHM 8745 (Fig. 2; Table 2) generally resembles Coronodon spp. and Basilosauridae in possessing a narrow and posteriorly positioned vertex, long intertemporal constriction, and a supraorbital process of the frontal that is only slightly wider than long. CCNHM 8745 has a nearly complete and rectangular supraorbital process of the frontal on the right side, missing just the postorbital process. Judging from a preorbital width of 340 mm, CCNHM 8745 is approximately the same size as Coronodon havensteini and Coronodon planifrons n. sp., likely having a bizygomatic width of around 450–460 mm.
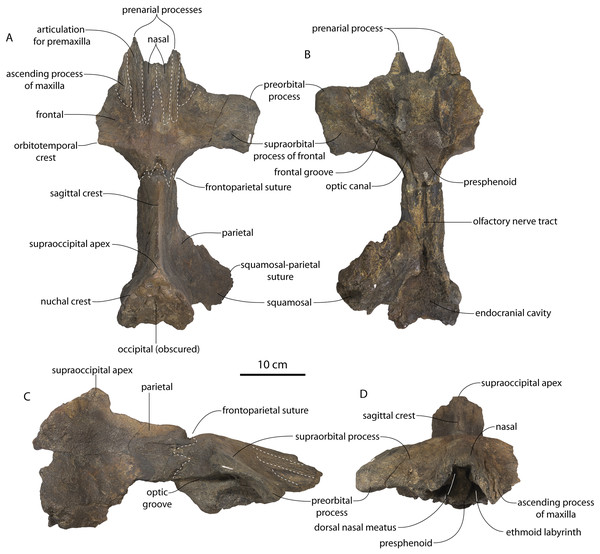
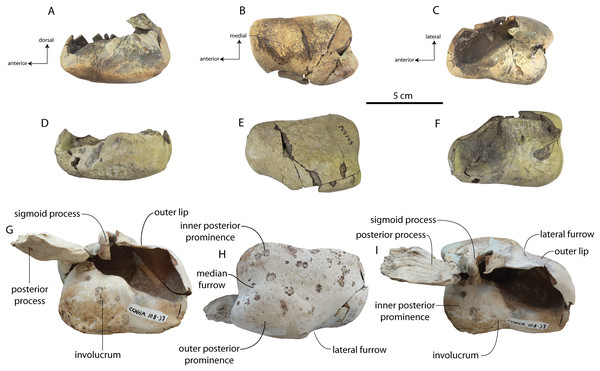
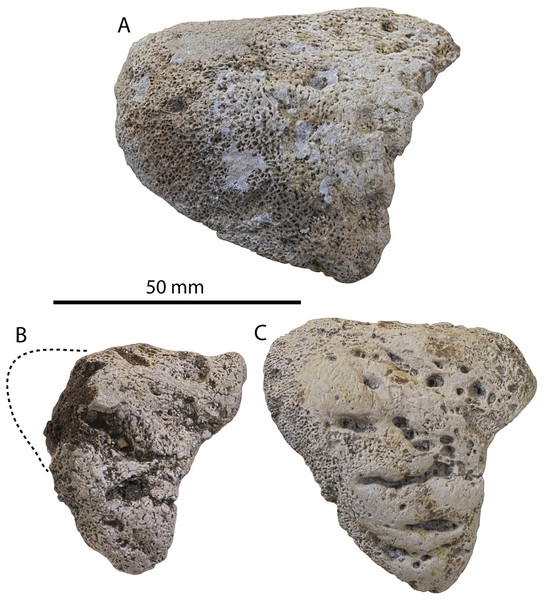
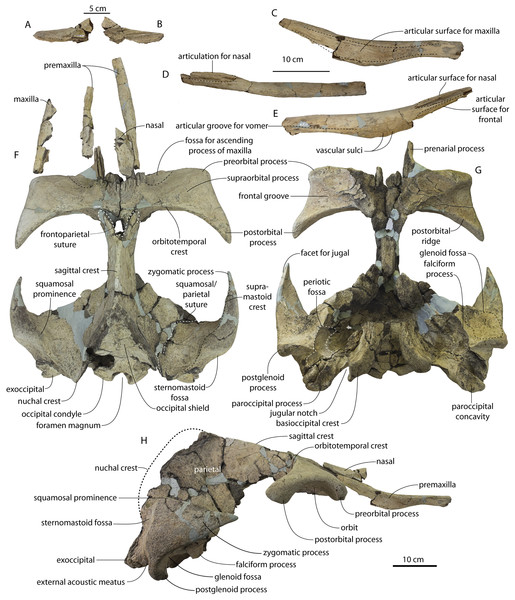
Figure 2: Skull of Coronodonidae indet., CCNHM 8745.
Skull in dorsal (A), ventral (B), lateral (C), and anterior (D) view.| Coronodon havensteini | C. newtonorum | C. planifrons | Coronodon-idae indet. | ||||
|---|---|---|---|---|---|---|---|
| Measurement | CCNHM 8722 | ChM PV 4745 | CCNHM 108 | CCNHM 164 | ChM PV 2778 | CCNHM 166 | CCNHM 8745 |
| Skull length without pmx | ? | 640e | 809 | 800+ | 820e | ? | ? |
| Skull width at c1 | ? | 53 | 118 | ? | 100.6 | ? | ? |
| Skull width at p2 | 80–85e | 83.2 | 169 | ? | 165e | ? | ? |
| Skull width at antorb. notch | ? | 200e | 301 | ? | 312e | ? | ? |
| Skull width at preorb. Proc. | 263 | 265.4 | 347 | 335 | 388e | 352 | 330 |
| Min. interorb. width | 270 | 264.6 | 351 | 342 | 402e | 349 | 324 |
| Skull width at postorb. Proc. | 294 | 294.1 | 402 | 406 | 414e | 414 | ? |
| Skull width at zyg. proc. | 330e | 347 | 463 | 457 | ? | 463 | ? |
| Min intertemp. width | 40e | 66.2 | 88 | 86 | ? | ? | 65e |
| Exocc. width | ? | 258 | 356 | 380e | ? | 358 | ? |
| Neurocranium height (basiocc. to vertex) | ? | 147e | 237 | 220 | ? | 249 | ? |
| Min distance nasals to supraocc. | 136 | 132 | 212 | 192 | ? | 227 | 240e |
| Dorsal length parietals (excluding interparietal) | 80.9 | 85 | 129 | 130e | ? | 143 | 145e |
| Dorsal length of frontals at midline | 64.9 | 53.4 | 70.5 | 80e | 603 | 87 | 136.6 |
| Ant/post length of parietal/frontal overlap | 57 | 18.7 | 45.5 | ? | 32.8 | 63 | 70 |
| Anterior length from orbitotemp. crest to post nuchal crest | 225 | 221 | 306/290+ | 290+ | ? | 330+ | 280+ |
| Max (diagonal) length of temporal fossa ventral view | 169 | 166 | 208/210 | 225/230e | ? | 250/258 | ? |
| Antpost length from anteriormost postorb ridge to post edge subtemporal crest | 180e | 139 | 217/212 | ?/185e | ? | 229/234 | 180–190 |
| Length max on rostrum | 320+ | 36e | 388 | ? | 42.3 | ? | ? |
| Upper toothrow length | 310+ | ? | 593/595 | ? | 58.5e | ? | ? |
| Depth palate max-pal suture | 17e | 9.5 | 16/16 | ? | 11 min | ? | ? |
| Gap between premax. at nares | ? | ? | 56.5 | ? | ? | ? | ? |
| Max width bony nares | ? | ? | 67 | 70–80e | ? | 77e | ? |
| Depth nasals ant edge | ? | ? | 4.7/? | 8.3/? | ? | ?/6–7e | 6.3 |
| Width nasals ant edge | ? | ? | 29.5/? | 28.6/? | ? | 33 | 45 |
| Max width nasals | 45–55e | ? | 63.6 | 71e | ? | 66 | 25 |
| Max length nasals | ? | ? | 140 | ? | ? | 130e | 106.5 |
| Width post nasals | ? | ? | 33.3 | ? | ? | 20e | 43 |
| Max length frontonasal suture (if nasals missing) | 59e | 53 | 105e | 80 | 100 min | 83 | 106.5 |
| Min distance nasals to orbitotemp crest | 31e | 32 | 33e | 31.5 | 21.4 | 35 | 41.6 |
| Width of pmx at antorbital notch | ? | ? | 101.4 | 104.4 | ? | 96 | 22 |
| d/v depth preorb | 19.7/20.5 | 25 | 35.5/41.6 | 28/29.2 | 39.9 | 32.5/33 | 25.8 |
| d/v depth postorb | ?/26 | 29 | 29.9/33.7 | 21.9/25.7 | 34 | 38.5/42.7 | ? |
| Expanse of frontal anterior to preorb ridge | 33/35 | 30.6 | 51/54 | 49/57 | 76.5 | 59/59 | 48.6 |
| Orbit length | 75e | 88.4 | 105.7/105 | 105/105 | 102.3 | 108/100.5 | 80+ |
| Depth of em pit post to C1 | ? | ? | 14/? | ? | 6.7 | ? | ? |
| Length of em pit post to C1 | ? | ? | 18/? | ? | 8 | ? | ? |
| Depth of em pit post to P1 | ? | 5 | 19/14 | ? | 13.5 | ? | ? |
| Length of em pit post toPC1 | ? | 11.2 | 21/18 | 21 | 17e | ? | ? |
| Depth of em pit post to p2 | ? | 11.5 | 8-Oct | ? | 11.4 | ? | ? |
| Length of em pit post to p2 | ? | 16 | 32/? | 29+ | 17 | ? | ? |
| Depth of em pit post to p3 | ? | 3.3 | 16/? | ? | ? | ? | ? |
| Length of em pit post to p3 | ? | 6.5 | 35/? | 30+ | ? | ? | ? |
| Depth of em pit post to p4 | ? | 5.5 | 20/16+ | ? | ? | ? | ? |
| Length of em pit post to p4 | ? | 16 | 40/40 | 45 | ? | ? | ? |
| Depth of em pit post to m1 | ? | 12 | 25/23 | ? | ? | ? | ? |
| Length of em pit post to m1 | ? | 19 | 46/48 | ? | ? | ? | ? |
| Depth of em pit post to m2 | ? | ? | ? | ? | ? | ? | ? |
| Length of em pit post to m2 | ? | ? | ? | ? | ? | ? | ? |
| Height or orbit above lat edge rostrum | 58e | 45e | 60 | ? | 76 | ? | ? |
| Width of squamosal lat to exocc | 28e/21e | 37 | 47.3/59.6 | 44.3/? | 25e | 41.2/35 | ? |
| Half exoccip width | 125e | 132 | 174.2/176.3 | 184 | ? | 179 | ? |
| Occipital condyle breadth | ? | 95 | 115 | 111 | ? | 111.5 | ? |
| Condyle depth | ? | 59.7 | 78.9 | 79 | ? | 80e | ? |
| Foramen magnum max width | ? | 41.7 | 46.4 | 46.1 | ? | 36e | ? |
| Foramen magnum max depth | ? | 33.4 | 46 | 47 | ? | 50 | ? |
| Depth of squamosal fossa | 24/24 | 45.1 | 48/46 | 60.1/67e | 46.5 | 52/53 | ? |
| Squamosal fossa to supramastoid crest | 35/35 | 33 | 30/26 | 41/38.6 | 39 | 42/38 | ? |
| Width glenoid fossa | 54/52 | 58 | 70/70 | /7778 | 88.5 | 77/73 | ? |
| Postglenoid to zyg apex, ant post plane | ? | 132 | 151.9/? | ? | 151 | 166/196 | ? |
| Max width single basioccipital crest | ? | 32.5 | 52/52 | 44+/51 | 38.4 | 54.6/53.5 | ? |
| Max width across basioccipital (lateral edge in cranial hiatus) | ? | 111.8 | 190.4 | ? | ? | 147.5 | ? |
| Max width across basioccipital crests | ? | 99.6 | 167.9 | 150+ | ? | 167.3 | ? |
| Anto/post length from anterior pterygoid sinus to subtemporal crest | ? | ?/23 | 39/38e | ? | ? | ? | ? |
| Max length of mastoid gap, periotic to lat edge of squamosal | ? | 18.6/− | 22.3/21.8 | 22.9/? | 35.1 | 36e/40.3 | ? |
| Max length sternomastoid fossa | 54/50 | 47 | 60e/58e | 82/82 | 56.3 | 76/88 | ? |
| Max depth sternomastoid fossa (to lowest point supramastoid crest) | 62/59 | 46.5 | 91/85e | 76/72 | 96.9 | 95/92 | ? |
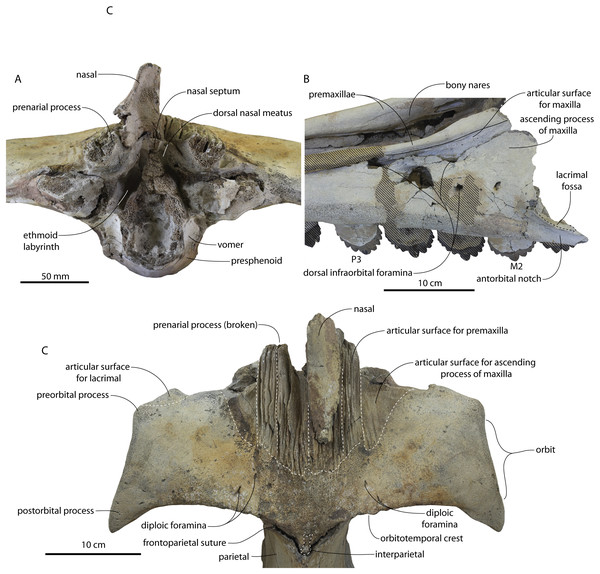
The supraorbital process is dorsoventrally shallow and delicate at the orbital margin, and the preorbital process is dorsoventrally thin (23 mm) compared to Coronodon havensteini (41 mm; CCNHM 108). The preorbital process is squared off and the anterior edge of the supraorbital process is transversely oriented; the posterior margin of the supraorbital process is concave like Coronodon spp. The orbitotemporal crest is positioned dorsally to the postorbital ridge so that the surface of the frontal between these is vertical and faces posteriorly (intermediate between Basilosauridae and Kinetomenta). A single large ?diploic foramen is positioned 10 mm ventral to the orbitotemporal crest and 7.5 cm lateral to the midline on this posterior face of the frontal, as in Coronodon spp. and some Basilosauridae (e.g., Basilosaurus isis).
The dorsal surface of the supraorbital process faces somewhat anterodorsally (like Coronodon spp.) but is otherwise planar. The middle of the frontal, where it bears sutural articulations with the nasal, premaxilla, and maxilla, is transversely arched and raised 5 cm above the supraorbital process. This is more greatly arched than in Coronodon. At the base of this arch is a deep triangular fossa for the ascending process of the maxilla on the right side; on the more incomplete left side, much of the ascending process of the maxilla is preserved in articulation with the frontal. It is triangular and covers the anterior 50% of the frontal, terminating at the anteroposterior midpoint. The maxillofrontal suture is mortised with four to five parallel longitudinal grooves/ridges (on the right side), unlike the flat butt joint in Coronodon. These ridges are discontinuous and about 3–4 cm long.
The ascending process of the maxilla contacts the frontal ventrally but not medially; there is a transversely narrow gap between these elements occupied by a thin vertical sheet of the nasal process of the premaxilla separating the maxilla from the medial ‘arched’ portion of the frontal. The premaxilla and maxilla share a slightly mortised suture. The nasal process of the premaxilla extended about 3 cm posterior to the maxilla, sharing a direct contact with the frontal posteriorly, like Coronodon (and differing from Protocetidae and Basilosauridae).
Both nasals are preserved and the left is nearly complete; the nasal is nearly flat and has a straight dorsal margin, lacking the upturned anterior tip seen in Coronodon spp. and ChM PV 5720. The nasal is triangular in dorsal view, and slightly transversely convex in cross-section, though generally conforming to the transverse arching of the underlying frontal. The nasal is small, only 85 mm long and 18.5 mm wide, v. 140 and 31.8 mm in Coronodon havensteini (CCNHM 108) despite nearly identical absolute skull size. The nasal gradually narrows posteriorly, and it is unclear if the nasals contacted medially or were separated along their entire length by a narrow strip of frontal owing to incompleteness. Judging from articular sutures on the underlying frontal, the nasals most likely contacted medially only along the anterior 30–40 mm of their length, and at least the posterior half of the nasals were separated by a triangular exposure of the frontal as in Basilosauridae and ChM PV 5720 (differing from Coronodon). Posterior to the termination of the premaxilla are paired (bilateral) 2 cm wide, 4 cm long shallow troughs on the frontal flanked by a low, longitudinal ridge that extends posteriorly from the premaxilla-maxilla suture; such a pair of median troughs and/or ridges characterizes some Basilosauridae (Basilosaurus cetoides, USNM 4674; Dorudon atrox, UM 101222; Zygorhiza kochii, USNM 11962; R. W. Boessenecker, 2021, personal observation).
The anterior part of the frontal bears a triangular prenarial process on either side of the external nares, which serves as an articular buttress for the nasal and premaxilla; the process is transversely narrow and near vertical with the lateral surface formed by the premaxilla-frontal suture and the dorsal surface overlapped by the nasals The prenarial process extends at least 4 cm anterior to the nasal. Each nasal bears a longitudinal trough leading to the common fissure for the dorsal nasal meatus (dorsal end) and the ethmoid labyrinth (ventral end). These fissures (Fig. 2D) are sigmoidal in shape, and the dorsal nasal meatuses are close to the midline and separated by only 12 mm. Ventrally and medially to the common fissures is the highly cancellous presphenoid, which is dorsoventrally thick, transversely narrow, oval in cross-section and narrowing somewhat dorsally. The presphenoid is flanked on either side by the choanae, which descend posteroventrally 25° from the horizontal plane. The choana is separated from the ethmoid labyrinth by a thin subhorizontal shelf. A deep laterally facing fossa is present dorsal to the choana but ventral to the frontal groove.
The frontal groove and optic canal is exposed along its entire length from the braincase, the left and right canals together forming a Y-shape; the canals are never confluent but diverge gradually just posterior to the frontoparietal suture and curve anterolaterally; the groove widens into a broad anterolaterally directed frontal groove on the ventral side of the supraorbital process. Two laterally directed ethmoid foramina are present within the proximal part of the frontal groove. The postorbital ridge is low and formed as a corner in cross-section; the optic foramen is positioned posteriorly. Small diploic foramina are present laterally within the frontal groove; a few scattered diploic foramina are also present dorsally on the supraorbital process within 5 cm of the midline near the apices of the premaxillae and maxilla.
Posteriorly, each optic canal is 13 mm wide and separated from one another by a 21 mm wide gap. Dorsomedial to these is a long olfactory nerve tract with a thin (~1 mm) median bony septum; the combined olfactory nerve tracts are 9 mm wide and 10 mm deep. If the cribriform plate is positioned at approximately the level of the ethmoid foramen, the entire olfactory nerve tract would be at least 200 mm long.
Intertemporal constriction and vertex
The intertemporal constriction is long, measuring approximately 183 mm long and constituting 54% of preorbital width, compared with a maximum of 49% in Coronodon havensteini; the constriction is quite narrow and measures approximately 65 mm wide or 19.1% of preorbital width, compared to 25% in Coronodon havensteini. In each of these regards CCNHM 8745 is plesiomorphic relative to Coronodon. Like Coronodon the sagittal crest is tall and sharp; the dorsal margin of the crest is concave where it rises abruptly in its posterior third towards the highly elevated vertex, unlike in Coronodon where the crest has a straight dorsal margin.
The frontoparietal suture appears approximately transverse owing to breakage, though grooves on the frontal suggest the presence of anterolateral wings of the parietal that would overlap the frontal on the anterior part of the constriction; these wings give the frontoparietal suture the posteriorly pointing V shape in Coronodon and this condition likely occurred in CCNHM 8745. If true, sutures on the frontal suggest that the frontals would penetrate 2–3 cm between the parietals in this specimen. The intertemporal portion of the parietal is laterally flat and nearly vertical. Posteriorly, the parietal is broadly concave. Like Coronodon, and differing from Basilosauridae, no postparietal foramina are developed.
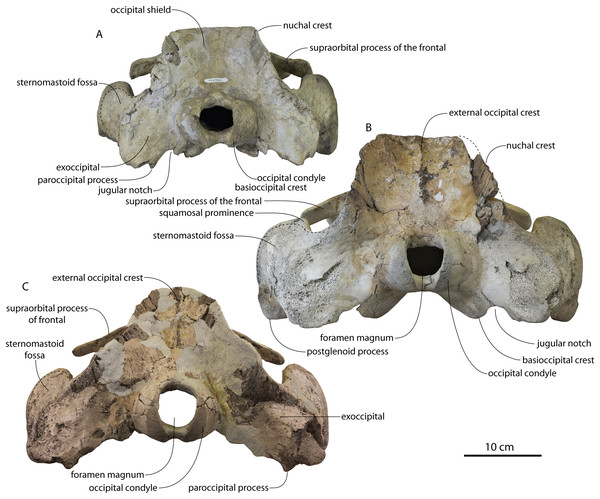
The vertex (defined herein as the supraoccipital apex and its contact with the parietals) is elevated 3 cm above the level of the sagittal crest; in dorsal view, the nuchal crests diverge at approximately 77°–80°. The occipital shield is obscured by matrix but appears to have been flat to slightly concave, and faces posterodorsally at approximately a 45°–50° angle from horizontal. The nuchal crests are tall, vertical, and do not overhang the braincase in dorsal view. The occipital shield is triangular and narrow, with a triangular rather than rounded apex.
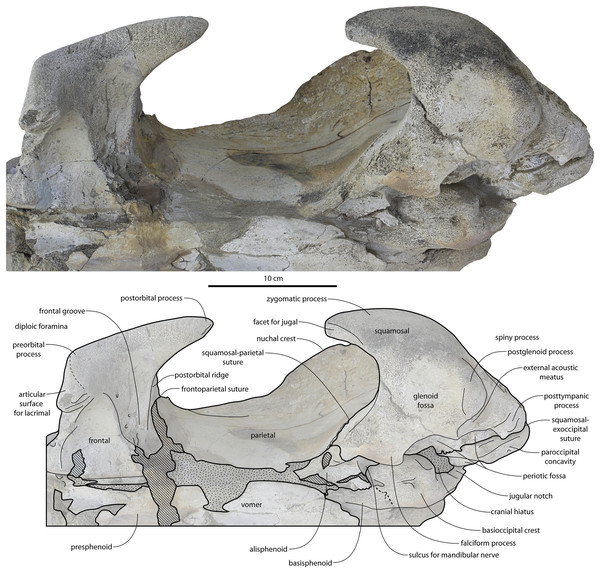
Braincase
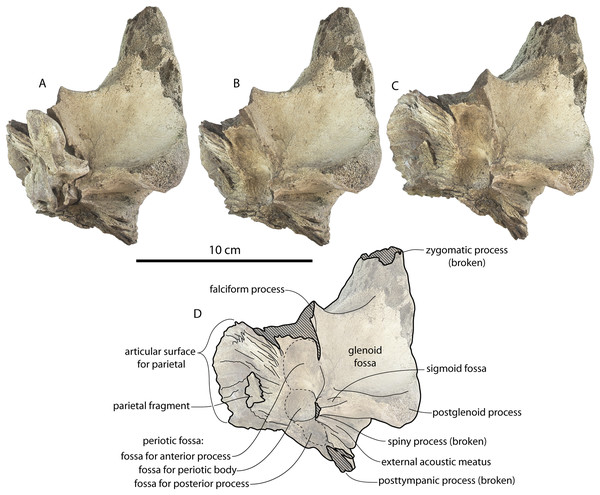
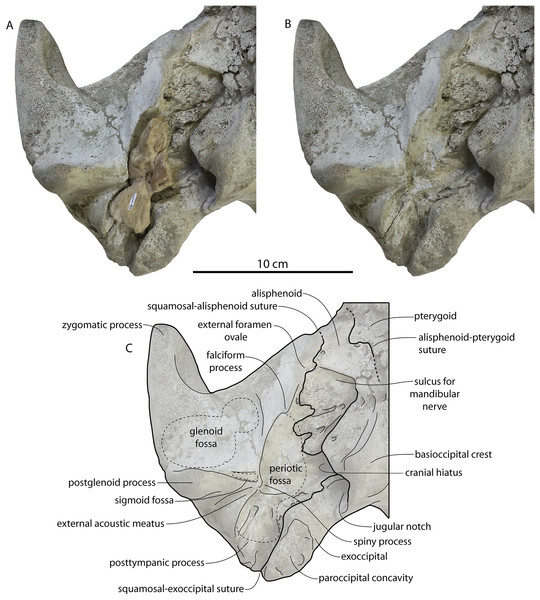
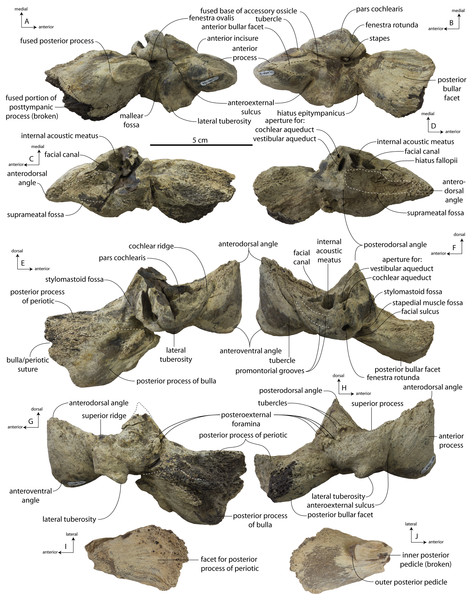
The squamosal is mostly missing but nearly the entire suture with the parietal is preserved. The suture is laterally more convex than in Coronodon and the lateral apex of the suture is positioned about halfway up the side of the braincase, whereas in Coronodon spp. it is low and just posterodorsal to the subtemporal crest. The dorsal half of the suture is nearly transverse in CCNHM 8745 whereas it is approximately anteroposterior in Coronodon. A small fragment of the squamosal is preserved ventrally, and bears a smooth lunate trough as in CCNHM 164 (Coronodon havensteini) and CCNHM 166 (Coronodon planifrons n. sp.), identified as receiving the dorsal part of the alisphenoid.
The endocranial cavity is similar to Coronodon (e.g., CCNHM 164), being broadly pyramidal in shape with a deep fissure anterodorsally for the posterior terminus of the olfactory nerve tract. The fossae for the cerebral hemispheres are 12 cm wide and posteriorly flanked by a large fossa for an endocranial rete situated dorsal to the cerebellum; these fossae suggest a posterior cranial fossa that is 155 mm across. A low median ridge subdivides the dorsal side of the posterior cranial fossa.
Ontogeny, identification and remarks
CCNHM 8745 lacks teeth and postcrania but is relatively large and similar in size to adult specimens of Coronodon havensteini and possesses tall sagittal and nuchal crests, closed (but not obliterated) frontoparietal and parietal-occipital sutures, and obliterated frontonasal and median frontal sutures, altogether suggesting adult status for this specimen, perhaps equivalent to Class 5 or 6 of Perrin (1975).
CCNHM 8745 is seemingly slightly more plesiomorphic than Coronodon, with a slightly longer and narrower intertemporal constriction and prenarial exposure of the frontal between the nasals. Despite these features, it does not represent a basilosaurid as it possesses several features typical of basal neocetes, including dorsal contact of the premaxilla and frontal, a triangular apex of the occipital shield, as well as a somewhat telescoped vertex that is at the approximate level of the subtemporal crest with an occipital shield facing posterodorsally (e.g., Martinez-Caceres, Lambert & Muizon, 2017). Amongst all nominal Neoceti, the shape of the supraorbital process and length and width of the intertemporal constriction in CCNHM 8745 are present only in the Coronodonidae. Owing to incompleteness it is not coded into our cladistic matrix, but is similar enough to Coronodon to warrant referral to the Coronodonidae.
This specimen exhibits adhering matrix most consistent with derivation from one of the members of the Ashley Formation. This specimen was collected from the Cooper River along with CCNHM 552, an isolated lower beak of the sea turtle Euclastes sp. described by Weems & Brown (2017), and CCNHM 4294, an isolated atlas vertebra of Ankylorhiza (M. Brown, personal communication, 2016). Weems & Brown (2017: 6), influenced by the archaic morphology of CCNHM 552 and its association with fossils identified as Dorudon serratus, considered CCNHM 552 and associated material likely to have been derived from the uppermost Eocene Parkers Ferry Formation. However, no fossils of Dorudon serratus exist in CCNHM collections aside from those collected in situ from quarries in the Harleyville region further inland. It is possible that, owing to the incomplete nature of CCNHM 8745, this braincase was initially misidentified as Dorudon serratus. Regardless, the Cooper River in the vicinity plotted by Weems & Brown (2017: fig. 1) bottoms out in the Ashley Formation (Weems, Lemon & McCartran, 1985; Weems & Lemon, 1993), and these specimens (CCNHM 552, CCNHM 4294, and CCNHM 8745) are best interpreted as being derived from the Ashley Formation. This is surprising as it would extend the already surprisingly young late Eocene age for the archaic Euclastes lineage proposed by Weems & Brown (2017) well into the Oligocene epoch.
CCNHM 8745 differs from Coronodon spp. and ChM PV 5720 in having absolutely and proportionally tiny and flat nasals, parallel troughs and ridges on the frontal posterior to the nasals and premaxillae (shared with some Basilosauridae), a concave dorsal margin of the sagittal crest, a longer sagittal crest (much longer than in ChM PV 5720), and a dorsally shallow preorbital process (Tables 1and 2). CCNHM 8745 shares with Basilosauridae and ChM PV 5720 a triangular median wedge of frontals separating the nasals, differing from continuous medial contact in Coronodon spp. The apex of the occipital shield is narrower and more acutely triangular than in Coronodon, ChM PV 5720, or CCNHM 214. Based on the small and flat nasals and other basilosaurid-like symplesiomorphies, CCNHM 8745 may lie as sister to the Coronodon + ChM PV 5720 clade rather than sister to either coronodonid. Regardless, probable derivation from the Ashley Formation indicates that at least two coronodonids are present in the Rupelian, paralleling three in the Chattian based on the assemblage from the Chandler Bridge Formation (Coronodon newtonorum n. sp., Coronodon planifrons n. sp., and third taxon represented by ChM PV 5720 and CCNHM 214).
Coronodon Geisler et al., 2017
Type species
Coronodon havensteini, Geisler et al., 2017
Referred species
Coronodon newtonorum n. sp.
Coronodon planifrons n. sp.
Amended diagnosis
Species of Coronodon are large toothed mysticetes (ca. BZW = 460 mm) differing from unnamed coronodonid genus (represented by ChM PV 5720 and CCNHM 214) in slightly smaller size, possessing more elongate intertemporal constriction with tall sagittal crest (length of crest = 34% of BZW, v. 20% of BZW in ChM PV 5720), wider and dorsoventrally shallower maxilla with straight (rather than concave) lateral edge; periotic with multiple (rather than single) posteroexternal foramina.
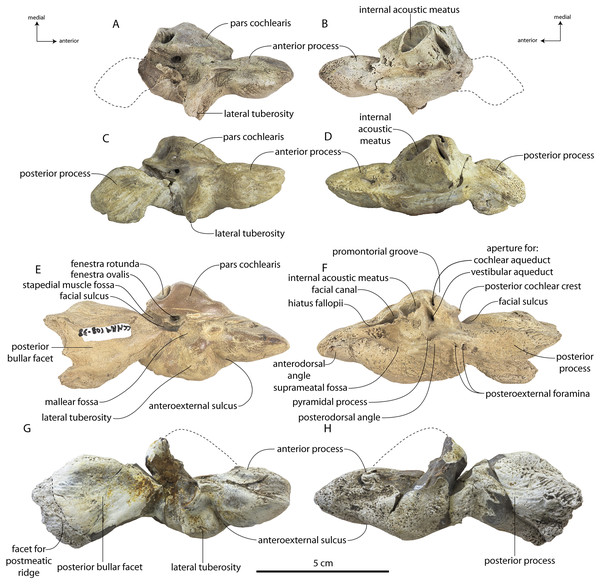
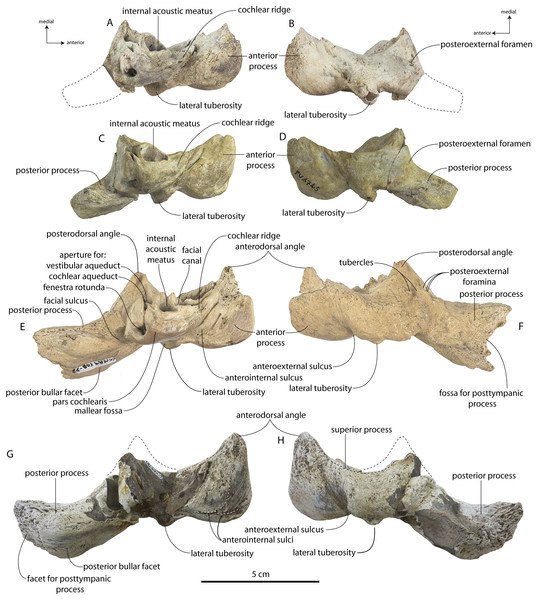
Coronodon havensteini Geisler et al., 2017
Type specimen
CCNHM 108, partial skeleton including virtually complete skull with left and right periotics and tympanic bullae, left and right mandibles, 16 teeth, seven cervical vertebrae, seven thoracic vertebrae, and eight ribs, collected by Mark Havenstein and others, summer 2002 (Geisler et al., 2017).
Referred specimens
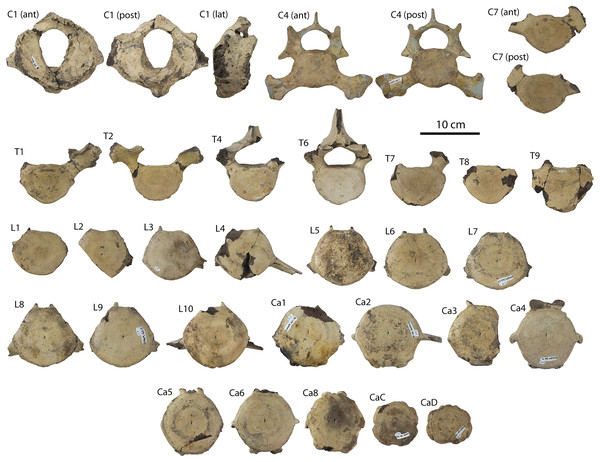
CCNHM 164, partial skeleton including rostrum fragments, braincase, fragmentary periotic, 19 teeth, five cervical vertebrae, nine thoracic vertebrae, three lumbar vertebrae, rib fragments, and partial scapula, collected summer 2007 by Paul Bailey from the Ashley Formation in the vicinity of North Charleston, Dorchester County, South Carolina; CCNHM 8722, partial skull including partial maxilla and braincase, left periotic, and right tympanic bulla, collected spring 2019 by Jeremmiah Volcko from the vicinity of North Charleston, Dorchester County, South Carolina; ChM PV 4745, nearly complete skull, four teeth, periotics, right tympanic bulla, collected May 1986 by Steve Faust from a drainage ditch exposure of the Ashley Formation in the vicinity of Summerville, Dorchester County, South Carolina, USA. Detailed locality data on file at CCNHM and ChM.
Type locality
The holotype of Coronodon havensteini was collected from subaqueous exposures of the Ashley Formation in the Wando River, Charleston/Berkeley County, South Carolina. Detailed locality data on file at CCNHM.
Horizon and age
Ashley Formation, late early Oligocene (28–30 Ma).
Amended diagnosis
A species of Coronodon possessing frontal with preorbital and postorbital processes of equal depth, ventrolaterally sloping supraorbital processes of the frontal in anterior view (horizontal in C. planifrons n. sp.), a periotic with a distally widening posterior bullar facet with large spurs on distal edge, upper molars of identical size (differing from C. planifrons n. sp.), lack of overlapping of the upper cheek teeth (differing from C. newtonorum n. sp.), maxilla with embrasure pits along length of toothrow and straight ventral edge (differing from C. newtonorum n. sp.), mandible with straight ventral edge and condyle not elevated above m4 alveolus (differing from C. newtonorum n. sp.).
Ontogenetic status
Specimens of Coronodon havensteini represent several ontogenetic stages. Referred specimens CCNHM 8722 and ChM PV 4745 were identified as juveniles (=Class III of Perrin, 1975) owing to their small skull size, incompletely erupted teeth lacking wear and possessing open pulp cavities, shallow embrasure pits, some open skull sutures and persistent (closed but not obliterated), low sagittal and nuchal crests, and small vertebrae lacking epiphyses. The holotype specimen CCNHM 108 represents a subadult or young adult (likely class V of Perrin, 1975, but possibly class IV) in its larger size, completely erupted teeth with closed pulp cavities and minimal tooth wear, deep embrasure pits, obliterated median frontal suture, closed (but not obliterated) skull sutures (frontoparietal, parietal-occipital, squamosal-parietal), higher sagittal and nuchal crests, and near-complete epiphyseal fusion in cervicals and thoracics except for T6. Referred specimen CCNHM 164 is approximately the same size based on bizygomatic width as the holotype but evidently represents a more mature individual (=Class V or six of Perrin, 1975) owing to severe tooth wear and complete epiphyseal fusion in vertebrae.
Description
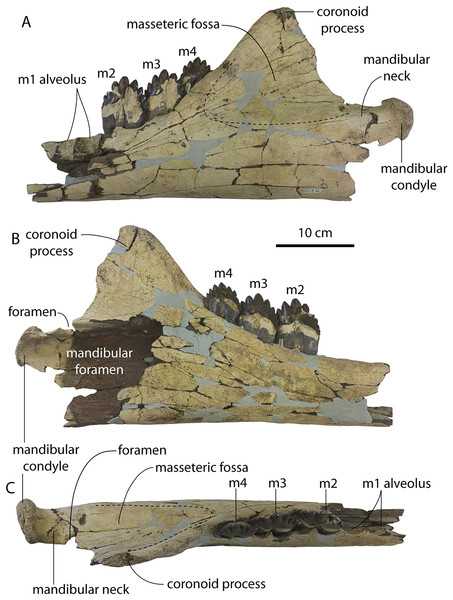
A complete description of the holotype specimen of Coronodon havensteini was provided by Geisler et al. (2017: supporting information). Accordingly, this description will emphasize new aspects of the morphology of C. havensteini revealed by the new specimens rather than repeating the published description. New details fall into three categories: (1) features not preserved in the holotype or details reinterpreted in light of insights gained from new specimens; (2) polymorphic features; and (3) morphological differences between juvenile and adult specimens that represent ontogenetic changes.
Rostrum
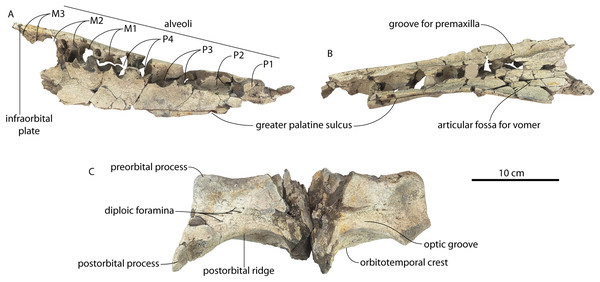
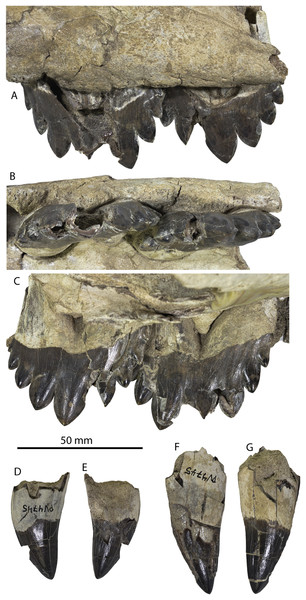
The left and right maxillae and a partial vomer are preserved in juvenile ChM PV 4745 (Figs. 3–5; Table 2), including the alveoli for C1-M2. This specimen was collected long before the Coronodon havensteini holotype and prepared as best as was possible at the time, with the descending processes of the maxillae meeting at the midline. The more complete rostrum of the adult holotype CCNHM 108 (Figs. 4–7) indicates that the maxillae did not medially contact and that there was a continuous strip of vomer present; owing to this, and to the curvature of the medial margin of the maxilla, the rostrum of ChM PV 4745 is likely too narrow. A corrected reconstruction is shown in Fig. 8.
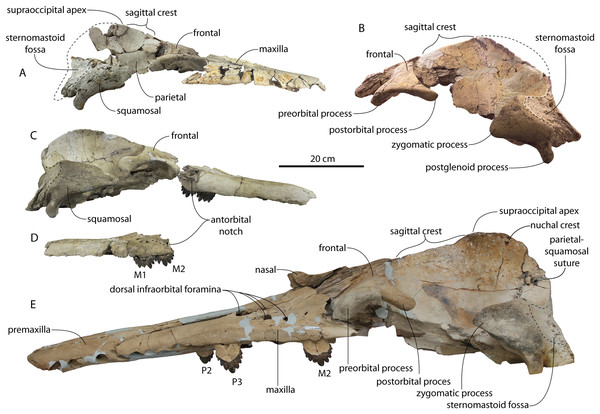
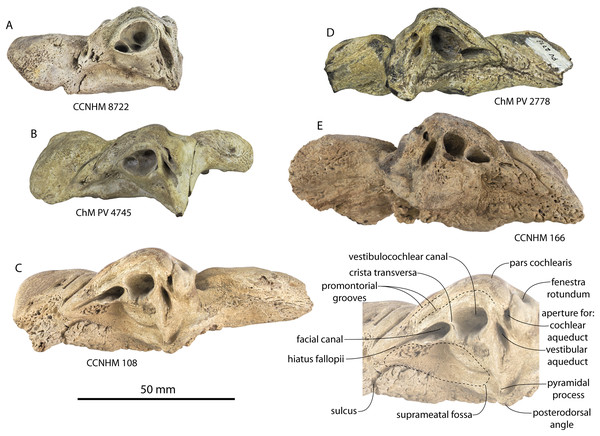
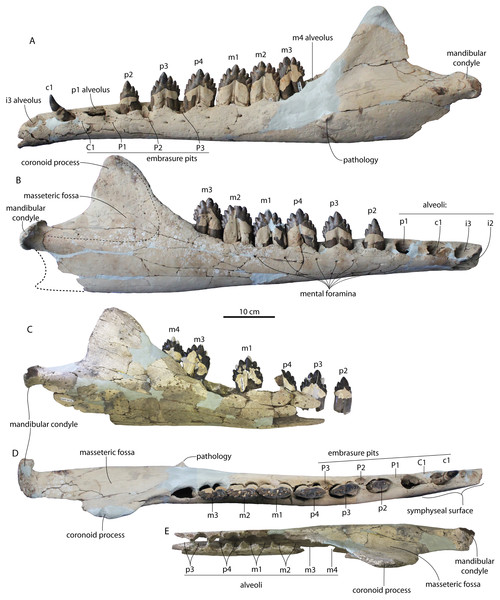
Figure 3: Skulls of juvenile Coronodon havensteini in dorsal view.
(A) Referred juvenile specimen CCNHM 8722 and (B) referred juvenile specimen ChM PV 4745.Figure 4: Skulls of Coronodon havensteini in ventral view.
(A) Referred juvenile specimen ChM PV 4745, (B) adult holotype specimen CCNHM 108, and (C) referred adult specimen CCNHM 164.Figure 5: Skulls of Coronodon havensteini in lateral view.
(A) Referred juvenile specimen CCNHM 8722 (right), (B) referred adult specimen CCNHM 164 (left), (C) referred juvenile specimen ChM PV 4745 (left), (D) rostrum of referred adult specimen CCNHM 164 (right), and (E) adult holotype specimen CCNHM 108.Figure 6: Skull and mandible of Coronodon havensteini holotype in lateral view.
(A) Holotype skull prior to attachment of cast teeth. (B) Holotype skull with cast teeth attached. (C) Holotype skull and mandible in occlusion.Figure 7: Skulls of adult Coronodon havensteini in dorsal view.
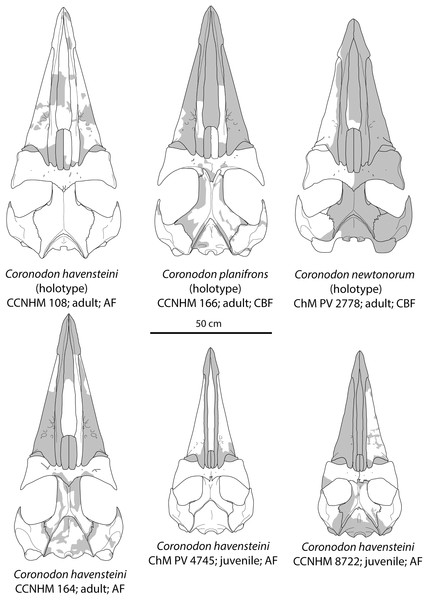
(A) Referred adult specimen CCNHM 164 and (B) adult holotype specimen CCNHM 108.Figure 8: Reconstruction of holotype and referred skulls of Coronodon havensteini, Coronodon planifrons, and Coronodon newtonorum.
Abbreviations: AF, Ashley Formation; CBF, Chandler Bridge Formation.The right premaxilla is nearly completely preserved in CCNHM 164 (Fig. 7; Table 2), and is missing only the incisor-bearing portion. The premaxilla is nearly longitudinally straight in dorsal view, lacking the slight lateral bowing in the reconstructed holotype (Fig. 7B). The posterior half of the premaxilla is nearly identical to the loose premaxilla of Coronodon planifrons n. sp. The lateral surface is undulatory in places and anteriorly bears a sharp horizontal ridge that descends anteroventrally; ventral to this is a deep longitudinal furrow to receive the anterodorsal edge of the maxilla.
The lateral edge of the maxilla is straight in CCNHM 8722, ChM PV 4745, and CCNHM 108 (Figs. 3, 4 and 7). CCNHM 8722 appears to have had a triangular rostrum of nearly identical proportions to the adult holotype (CCNHM 108), based on the length of the maxilla from the P1 alveolus to the antorbital notch relative to postorbital width; the length of the maxilla posterior to P1 is approximately 90% of postorbital width in all specimens of Coronodon havensteini.
In CCNHM 8722 the maxilla is dorsoventrally deeper than and lacks the dorsal, horizontal surface on the posterior half of the maxilla of the holotype (Fig. 5); instead, the maxilla is gently sloping along its entire length with a small subhorizontal platform adjacent to the dorsal infraorbital foramina. This results in a shallowly triangular cross-section at the base of the rostrum in juvenile CCNHM 8722, whereas the cross-section is more sinuosoidal in juvenile ChM PV 4745 and the adult holotype (CCNHM 108), the latter of which is more dorsoventrally flattened. This subhorizontal platform adjacent to the dorsal infraorbital foramina is somewhat larger in ChM PV 4745 and more similar to the holotype, and is present along the posterior half; it bears three dorsal to dorsolaterally opening dorsal infraorbital foramina at the level of M1. The maximum depth of the maxilla is equivalent to 18–19% of antorbital width in the juvenile skulls (CCNHM 8722, ChM PV 4745) compared to 13% in the holotype. The external nares (based on the widest part of the mesorostral canal) seem to have been located at the level of P4 in these juveniles. The dorsal infraorbital foramina are positioned anteromedial to the antorbital notch in referred juvenile ChM PV 4745 and the holotype (CCNHM 108). The juvenile possesses three large, laterally directed foramina on the left and four large foramina on the right (the medial two pointing anteriorly, the lateral one pointing laterally, and the posteriormost one directed posterolaterally). The dorsal infaorbital foramina of the holotype consist of anteriorly placed clusters or confluent foramina anteriorly with shallow anterior and anterolaterally directed sulci emanating and smaller lateral to posterolaterally directed foramina posteriorly; four or five are present on the left maxilla, and four are present on the right maxilla. A more detailed description of the dorsal infraorbital foramina of the holotype is presented in Geisler et al. (2017: supporting information).
The morphology of the premaxilla-maxilla articulation is obscured in the holotype owing to the articulation of these elements and the vomer, but the medial side of the maxilla (Fig. 9B) is well preserved in juvenile specimens (ChM PV 4745, CCNHM 8722) and the lateral surface of the premaxilla is exposed in the referred adult (CCNHM 164) with a disarticulated (and fragmentary) rostrum. The medial surface of the maxilla in juvenile specimens (Fig. 9B) preserves four major surfaces—two ventral surfaces below a horizontal ridge that underlies the premaxilla, and two dorsally positioned surfaces above this ridge. The first is a deep trough for the premaxilla positioned along the posterior 2/3 of the maxilla; this trough is deepest anteriorly and posteriorly but shallows around the level of the P4. The sutural surface is smooth and lacks a mortised articulation. Anteriorly, the second surface is developed along the anterior 2/3 of the maxilla; this surface is a vertical, flat butt joint articulation between the premaxilla and maxilla. The third is a long, smoothly concave ventromedial trough for the palatal part of the vomer, positioned on the dorsomedial surface of the descending plate of the maxilla. The fourth is a fossa for the wing of the vomer; it is dorsoventrally deep posteriorly, transversely concave and smooth; this accommodates the choanae and would have been lined by the vomerine wing when complete. The maxillae of each juvenile specimen (CCNHM 8722, ChM PV 4745) preserve a delicate dorsomedial ridge that forms a lip along the lateral edge of the premaxilla (Figs. 3A, 9B); medial to the dorsal infraorbital foramina this ridge overhangs laterally somewhat.
Figure 9: Maxilla and frontal of juvenile specimen of Coronodon havensteini.
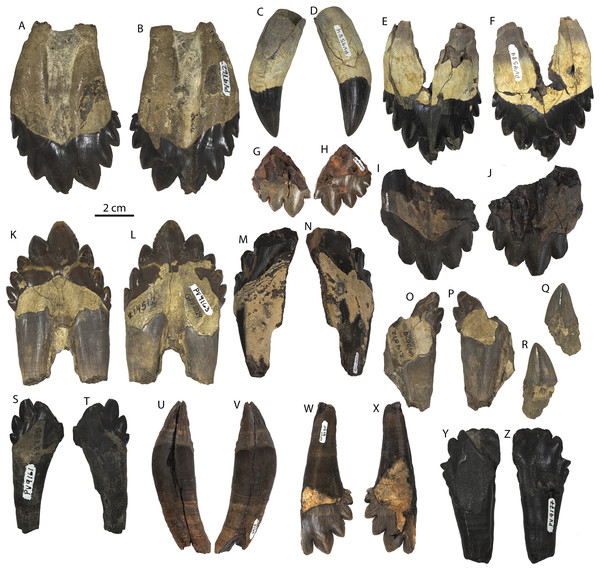
Referred juvenile specimen CCNHM 8722 maxilla in ventral (A) and medial (B) view, and frontal in ventral view (C).Demi-alveoli for the ‘demi-roots’ are present in juvenile specimens (Figs. 4B, 9A), but fewer than in the adult holotype, where demi-roots (a small third root present in between the mesial and distal root lobes) or alveoli for them are present on P3 through M2. In juvenile CCNHM 8722, there are only alveoli for demi roots in M1 and M2. In ChM PV 4745 there are quadrate to circular pedestals in between the root alveoli for P3 through M2 (Fig. 4A), which may correspond to demi-root alveoli later in ontogeny.
In the juvenile specimen CCNHM 8722, embrasure pits are present on the palate labially between P1 and P2 (for p1), and medial to P4, M1, and M2, but not medial to P3 (Fig. 9A). These pits are much shallower than in CCNHM 108. In ChM PV 4745, more embrasure pits are present and are deeper than in CCNHM 8722, but fewer and shallower than in the holotype (Figs. 4A and 4B, 9A). These include labial pits for the p1 (between C1/P1), p2 (between P1/P2), and lingual pits for the p3 (anteromedial to P2), p4 (just medial to P3/P4), m1 (medial to the anterior root of M1), and m2 (medial to anterior root of m2). The m2 embrasure pit is the deepest. Fragments of the maxilla in CCNHM 164 include labially-facing embrasure pits anterior to P1 and P2, and lingual embrasure pits medial to the P3-M2 alveoli (Fig. 7A). The well-preserved palate of the holotype (CCNHM 108) preserves labial embrasure pits between teeth from I1 to P1, a deep embrasure pit in line with the toothrow between P1 and P2, and deep lingual embrasure pits medial to the anterior roots of P3, P4, M1, and M2 (Fig. 4B); the pits medial to P3-M1 (P4 in particular) are the deepest. Each of these is shallowly conical and of sufficient anteroposterior diameter to accommodate the entire mesiodistal crown length of the corresponding mandibular tooth, with the bony bridges between the pits corresponding to the gaps between crown apices of the mandibular cheek teeth. Bone remodeling (resorption) on the labial edge of these pits has broadly exposed the lingual side of the roots of P3-M2. The same is likely true of M3, but the medial part of the maxilla is missing and only the lateral edge of the reduced infraorbital plate of maxilla is present in CCNHM 108 and 8722, and the reduced infraorbital plate and M3 alveolus is missing completely in ChM PV 4745.
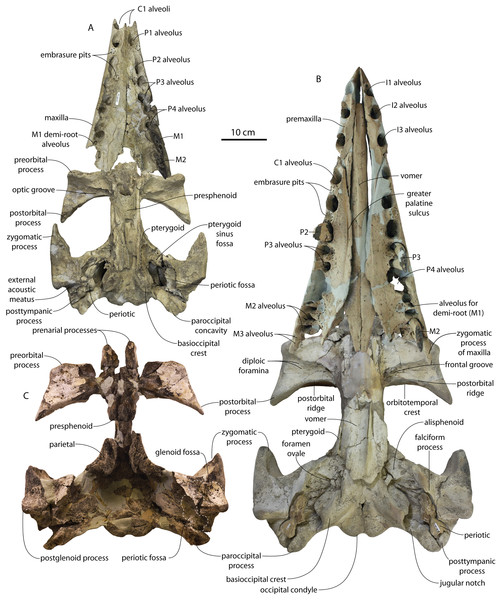
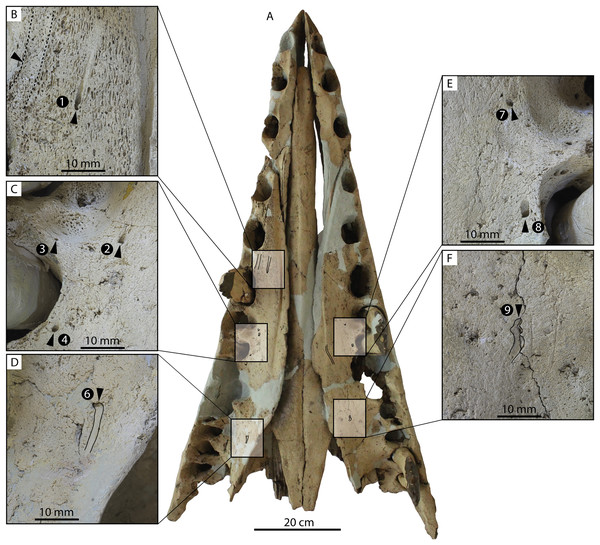
Several lateral palatal foramina are present in the maxillae of the Coronodon havensteini holotype (CCNHM 108; Fig. 10), some of which were noted in the original supplementary description (Geisler et al., 2017: Data S1, p. 11). A more thorough description is provided here of all palatal foramina measuring over 1 mm in diameter after Peredo, Pyenson & Uhen (2022). In addition, many foramina between 0.5 and 1 mm are present, especially around the alveoli of posterior premolars and molars. The first and clearest foramen, foramen 1, is positioned anteromedial to RP2, opens anteriorly, and is 1.3 mm wide and bears a 14–15 mm long sulcus (Fig. 10B). Foramen 2 is positioned medial to RP3, is anteromedially opening, 1.6 mm wide, and has a 6 mm long sulcus; an unnumbered foramen only 0.8 mm wide opens just anterior, with a short 2 mm long sulcus (Fig. 10C). Foramen 3 is also medial to RP3, 1.6 mm wide, vertically oriented, and lacks a sulcus (Fig. 10C). Foramen 4 is medial to RP3, anteroventrally opening, 1.3 mm wide, and bears a 2 mm long sulcus (Fig. 10C). Foramen 5 is positioned medial to the RP3/RP4 diastema, opens anterolaterally, is 1.2 mm wide, and bears a 4 mm long sulcus. Foramen 6 is positioned further posteriorly and far medially to RM2 only 15 mm from the medial edge of the maxilla; it opens posteriorly, is 1.9 mm wide, and has a 5.3 mm long sulcus (Fig. 10D). Foramen 7 is positioned medial to LP3, is vertical, 1.2 mm in width, and lacks a sulcus (Fig. 9E). Foramen 8 is medial to the posterior root of LP3, anteriorly opening, 1.9 mm wide, and has a 5.3 mm long sulcus (Fig. 10E). Foramen 9 is similar to Foramen 6 in position (but somewhat further anterior) and morphology, and is medial to the anterior root of LM1, opens posteriorly, positioned 3 cm from the medial margin of the maxilla, 1.8 mm in width, and bears a 9.3 mm long sulcus (Fig. 10F). Re-examination of CT data indicates that in the anterior part of the rostrum, the interior is quite damaged, and the infraorbital canal is only well-preserved posteriorly. However, foramina 1–3 and 7–8 can be traced internally trending posterodorsally to dorsolaterally and towards the roots of the teeth rather than medially.
Figure 10: Palate and lateral palatal foramina in the holotype of Coronodon havensteini.
(A) Rostrum in palatal view, (B) foramen 1, (C) foramina 2-4, (D) foramen 6, (E) foramina 7-8, and (F) foramen 9.In addition to these, there is a deeply entrenched greater palatine sulcus along the medial edge of the maxilla, originating from near the premaxilla-maxilla suture and P1 alveolus and becoming more faintly excavated posteriorly, giving way to a complately flat palatal surface by the level of P4 to M1 (Fig. 10B). A greater palatine foramen is not preserved. Additionally, within the right greater palatine sulcus, there is a single 1.5 mm wide foramen within the larger sulcus medial to RP3. Though obscured on the left side, there seems to be an equivalent foramen. Several additional anteroposteriorly oriented sulci lacking foramina are present medially within the diastema between RC1 and RP1 and RP1 and RP2, just medial to the embrasure pits. Another sulcus without a foramen is positioned medial to LP3 and lateral to the greater palatine sulcus and trends anteromedially for approximately 4 cm (Fig. 10A).
Orbit, supraorbital process, and interorbital region
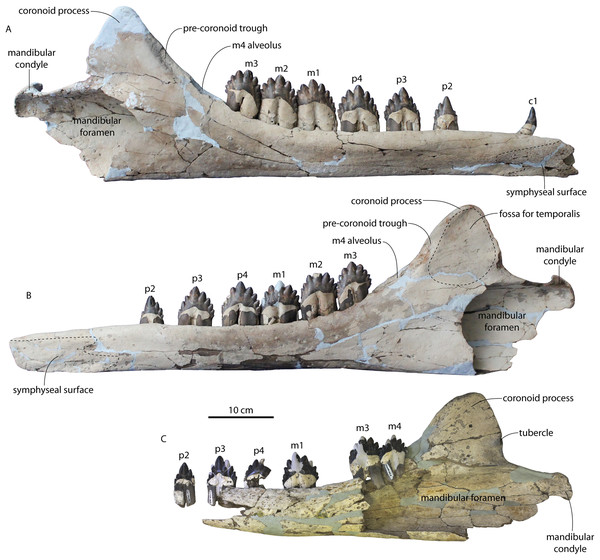
In anterior view, the supraorbital process of the frontal descends ventrolaterally in all specimens of different ontogenetic stages (Fig. 11); it descends at a 16° angle in the holotype, only 10° in juvenile ChM PV 4745, and 18° in adult CCNHM 164. The postorbital process is longer and more acutely pointed in ChM PV 4745 and CCNHM 164 than in the holotype, though CCNHM 8722 is similar to the holotype. The median frontal suture is open and planar to slightly sinuous in juvenile specimen CCNHM 8722 (Fig. 3A), whereas it is closed and partially obliterated in ChM PV 4745 (Fig. 3B); it is completely obliterated in CCNHM 108 (Figs. 7, 12). A furrow is present at the frontal midline in CCNHM 164, but owing to poor preservation, it is unclear whether or not the suture was persistent or obliterated. The supraorbital process of the frontal is anteroposteriorly shorter in the juvenile specimens than in the holotype (Figs. 3, 7, 8; Table 2), approximately 26.5% of postorbital width in CCNHM 8722, 26.4% in ChM PV 4745, and 31.5% in the holotype. However, the supraorbital process is somewhat shorter in adult specimen CCNHM 164 as well, 25.5% of postorbital width. The posterior margin in CCNHM 8722 is more concave (Fig. 3) and there is a stronger angle between the orbitotemporal crest and the postorbital process. The preorbital and postorbital processes of CCNHM 8722, ChM PV 4745, CCNHM 108, and 164 are nearly equivalent in dorsoventral depth (Figs. 5, 6; Table 1), unlike Coronodon newtonorum n. sp. and Coronodon planifrons n. sp.
Figure 11: Braincases of Coronodon havensteini in anterior view.
(A) Referred juvenile specimen CCNHM 8722, (B) referred juvenile specimen ChM PV 4745, (C) adult holotype specimen CCNHM 108 and (D) referred adult specimen CCNHM 164.Figure 12: Frontals, antorbital region, and ethmoid region of Coronodon havensteini holotype (CCNHM 108).
(A) Ethmoid region in anterior view, (B) antorbital region of left maxilla in dorsolateral view, and (C) interorbital region in dorsal view.The frontonasal and frontal-premaxilla sutures are anteroposteriorly shorter in juvenile specimens, measuring approximately 55% of anteroposterior supraorbital length in CCNHM 8722 and 54% in ChM PV 4745 v. 70% in the holotype. In ChM PV 4745, a median triangular extension of the frontals was present between the posterior ends of the nasals (Fig. 3B); a similar condition is present in CCNHM 8722, though the frontal extended less far anteriorly (Fig. 3A). In adult specimens CCHM 108 and 164, the sutural ridges for the frontonasal suture are too elongated to evaluate and no smooth triangular surface is evident (Figs. 7, 12). The fossa for the ascending process of the maxilla is slightly more excavated in CCNHM 8722 and ChM PV 4745, whereas this surface is nearly flat in CCNHM 108 (Fig. 7C). Preserved articular surfaces on the frontal in all specimens suggests that it terminated anterior to the posterior apex of the premaxilla. A clue lies in the coloration and staining of the frontal in CCNHM 108; if this is a stain from the ascending process of the maxilla, it would indicate a roughly triangular ascending process with a blunt or lobate apex extending to nearly the posterior edge of the preserved part of the premaxilla and nasal, and terminating just anterior to the preserved articular grooves on the frontal for these elements (Fig. 7C). In CCNHM 164, the fossa for the maxilla is somewhat more defined, and based on this feature the ascending process of the maxilla overlapped the anterior 48 mm of the frontal (45% of the length of the frontal), terminating just anterolateral to the posterior apex of the premaxilla. Lateral to the sutures for the premaxilla there are scattered diploic foramina in all specimens. In CCNHM 8722, ChM PV 4745, and CCNHM 108 they are small dorsally to posterodorsally opening pores. However, in CCNHM 164, they are confluent with roughly transversely oriented, shallow, 1.5–2 mm wide sulci. Some anteroposteriorly oriented sulci cross-cut these. In the holotype there are an additional pair of diploic foramina positioned near the posterior margin and open posteriorly but lack sulci.
The orbit is 67 mm long in CCNHM 8722 (Figs. 9C, 13) and corresponding to 21% of postorbital width, which is proportionally smaller than in the adult holotype (25% of postorbital width); however, in ChM PV 4745 the orbit is proportionally larger, approximately 85 mm and 28% of postorbital width (Fig. 4C). Scattered diploic foramina are present in the optic canal of all specimens halfway from the midline to the orbital margin. In both juveniles there is a low curved ridge on the dorsal surface of the supraorbital process that extends from the middle of the orbit to the medial part of the orbitotemporal crest; it is more clearly defined in ChM PV 4745, but diffuse and nearly absent in adult specimens CCNHM 108 and 164. In CCNHM 8722, a shallow fossa parallels the posterior margin of this low crest medial to the postorbital process. In CCNHM 8722, a fossa is present medial to the middle of the orbit on the right frontal, and is floored by cancellous bone; since it is absent on the left, and in all other Coronodon specimens, it is best interpreted as pathological in origin. In CCNHM 8722 the postorbital ridge is low and medially sharp. In both CCNHM 8722 and ChM PV 4745, the supraorbital process is anteroposteriorly shorter (~25% of postorbital width at mid-frontal) than in the holotype; in CCNHM 164, it is longer than in the juveniles, but still somewhat shorter than the holotype. Juvenile specimens possess the longest and narrowest postorbital processes.
Figure 13: Temporal fossa, and basicranium of Coronodon havensteini holotype in ventrolateral view.
The frontoparietal suture is V-shaped and posteriorly-pointing in all specimens (Figs. 3, 7, 8, 12), but in ChM PV 4745 there is a transversely narrow median process of the parietal or separate midline ossification that extends anteriorly between the frontals; the parietals of CCNHM 8722 are incomplete, but the frontals possess a narrow median embayment and likely received a projection of the parietal (Fig. 3A). In CCNHM 108, the suture is V-shaped without a median parietal process; this region is fractured in CCNHM 164. Upon closer examination of the holotype, a similar condition is present in CCNHM 108 that eluded the initial description. An oval-shaped median ossification (Fig. 12C) is present just anterior to the frontoparietal suture (and separated from the parietal by the frontoparietal suture), corresponding to the complete element in ChM PV 4745 and the gap in the frontal in CCNHM 8722. This element is not fused to the parietal and has clear sutures laterally for the frontal, and anteriorly is fused to the frontal at the midline. This element appears to be homologous with the interparietal (Roston, Boessenecker & Geisler, In Press).
Lateral to the sutural surface for the premaxilla, the dorsal surface of the frontal is smooth and shallowly concave, corresponding to the articulation for the ascending process of the maxilla. The exact shape is unclear, and only the holotype preserves a partial ascending process, which is approximately 31 mm wide (Fig. 6B). Medially there is a triangular prong of frontal in ChM PV 4745, CCNHM 108, and CCNHM 164 (Figs. 3B, 7, 12); this feature is not developed in CCNHM 8722 (Fig. 3A) but may be obscured by fracturing. This structure forms the articular buttress ventral to the nasal and premaxilla. The olfactory region of the holotype is exposed (Fig. 12) and is broadly similar to that of CCNHM 8745, possessing proportionally larger and straight (rather than sigmoidal) common fissures for the dorsal nasal meatus and ethmoid labyrinth. Unlike CCNHM 8745, the fissure is expanded rather than transversely constricted at mid-height. Unlike CCNHM 8745, the nasal passages curve anterodorsally after emanating anteriorly from the dorsal nasal meatus, conforming to the anterodorsally flaring profile of the nasal bone.
Intertemporal region
The intertemporal region is transversely narrow and anteroposteriorly elongate in all specimens of Coronodon havensteini, generally resembling basilosaurids (Figs. 3, 7, 8; Table 2). A tall sagittal crest is developed in all specimens, though in the ontogenetically youngest (CCNHM 8722) the apex of the crest is flat but narrow, with a minimum width of 4.1 mm, widening slightly anteriorly and posteriorly; the crest is narrow in the larger juvenile ChM PV 4745 and both adult specimens (CCNHM 108 and 164). However, in ChM PV 4745, it is only sharp along its posterior half and it dissipates anteriorly toward the frontoparietal suture (Fig. 3B). The length of the intertemporal constriction (gap between the supraoccipital apex and the anteriormost point on the orbitotemporal crest) is relatively shorter in CCCNHM 8722, where it measures only 33% of postorbital width; in ChM PV 4745, it measures 31% of postorbital width. In adult specimens, it measures 46% (CCNHM 108) and 40% (CCNHM 164). However, owing to some uncertainty with reassembled fractures in the intertemporal region of CCNHM 164, the intertemporal constriction could have been somewhat longer as in CCNHM 108; 40% of postorbital width should therefore be viewed as a minimum value in CCNHM 164. In all specimens the median parietal suture is obliterated (Figs. 3, 7) and there is no sign of it in broken specimens (CCNHM 164, 8722). The medial wall of the temporal fossa is continuously concave in CCNHM 8722, unlike the straight/flat lateral margin in the holotype; larger juvenile ChM PV 4745 is intermediate, with a slightly longer intertemporal region with a short anterior portion with parallel sides and widening posteriorly (Figs. 3, 7). Despite breakage in CCNHM 164, the lateral surface of the intertemporal constriction was straight-sided with parallel margins for at least the anterior 2/3 of its length and likely no wider than 90 mm (Fig. 7A).
Vertex, dorsal braincase, and occiput
The apex of the supraoccipital is triangular in CCNHM 8722, ChM PV 4745, and CCNHM 108, and is incompletely preserved but likely triangular in CCNHM 164 (Figs. 3, 7, 14). The apex of the occipital is positioned at the level (in the anteroposterior plane) of the subtemporal crest in CCNHM 108 and 8722, slightly anterior to the crest in ChM PV 4745, and far anterior in CCNHM 164. Interpretation of this condition is sensitive to skull orientation, but in CCNHM 164, it is possible that the intertemporal constriction was reconstructed too far ventrally during preparation, and a condition closer to the holotype is possible owing to incompleteness and lack of clear contacts.
Figure 14: Skulls of Coronodon havensteini in posterior view.
(A) Referred juvenile specimen ChM PV 4745, (B) adult holotype specimen CCNHM 108, and (C) referred adult specimen CCNHM 164.The occipital/parietal suture is open but mortised in CCNHM 8722 and ChM PV 4745, and more tightly mortised in the holotype, measuring at least 5–6 cm deep dorsoventrally in CCNHM 8722 (Figs. 3, 7). The nuchal crests diverge posteriorly at an 82° angle in CCNHM 8722 and 95° in ChM PV 4745, similar to the holotype (85°); this angle may be affected by some deformation of the vertex in ChM PV 4745. The occipital shield (Fig. 14B) of the holotype is nearly vertical, whereas in the juvenile specimens it is slightly more anterodorsally sloping. The more completely preserved nuchal crests of ChM PV 4745 slightly overhang the lateral walls of the braincase more than in the holotype (Figs. 3B, 7B), a result of the more sloping occipital shield earlier in ontogeny. Such a degree of nuchal crest overlap can be duplicated in the holotype by viewing in posterodorsal view, instead of dorsal view. In the juveniles (CCNHM 8722, ChM PV 4745) the shield bears a short (albeit lower) external occipital crest (Fig. 14) like the adult (CCNHM 108); such a crest is absent in Coronodon planifrons n. sp. Lateral to the crest, the supraoccipital bears a faintly rugose surface for the attachment of neck muscles, likely the semispinalis (Schulte, 1916).
The squamosal-parietal suture in all specimens of Coronodon havensteini is sinusoidal in dorsal view (Figs. 3, 7) with an anterolaterally convex curve anteriorly, differing from the sharp corner present in Coronodon planifrons n. sp. and Coronodon newtonorum; juvenile specimens CCNHM 8722 and ChM PV 4745 exhibit a more sinuous suture than the holotype (Fig. 8). The subtemporal crest is sharp and approximately transverse in CCNHM 8722, but trends anteromedially in larger juvenile ChM PV 4745 and adults (CCNHM 108, 164). The subtemporal crest is sharp laterally near the base of the zygomatic process but medially becomes rounded in cross-section in the adult holotype (CCNHM 108).
Ventromedially the parietal of CCNHM 8722 bears a smooth and somewhat rectangular facet for the alisphenoid; the alisphenoid-parietal suture seems to be an open fissure in the larger juvenile (ChM PV 4745). In CCNHM 164, the facet is instead crescentic and unlike the juveniles, the articular surface is somewhat rugose indicating postnatal transition from a planar butt joint to a firmer suture. The anterior half of the parietal in CCNHM 8722 is composed entirely of bone with a strong longitudinal grain. The incomplete condition of CCNHM 8722 permits some observations of the endocranial cavity. Internally there is a smooth (possibly eroded) impression of the right cerebral hemisphere and a low ridge, perhaps the location where the tentorium cerebelli attaches and separates the cerebral hemisphere from the cerebellum.
Basicranium
The squamosal in Coronodon spp. is distinctive in possessing an unusually deep and anteroposteriorly shortened zygomatic process (Figs. 3–7, 15). The zygomatic process is laterally inflated, medially excavated, and triangular in lateral view. It bears an enlarged squamosal prominence (Figs. 3, 7, 15), much larger than in all other toothed mysticetes. In CCNHM 8722 and ChM PV 4745, the squamosal prominence is proportionally smaller than the holotype and positioned further laterally from the squamosal-parietal suture (Figs. 3, 8). The squamosal bears a dorsoventrally deep and proportionally large sternomastoid fossa that faces posterolaterally (Fig. 15; Table 2). The squamosals of juvenile specimens CCNHM 8722 and ChM PV 4745 are similar to the adult, though these specimens bear zygomatics that are dorsoventrally shallower at mid-length (19% of bizygomatic width in CCNHM 8722, 17% of BZW in ChM PV 4745, compared with 26% in CCNHM 108). In ChM PV 4745, the zygomatic processes are much closer (less than 10 mm) to the postorbital processes of the frontals than in the holotype (Figs. 3, 7, 8) where they are separated by a large gap (8–9 cm); although some of this difference could be ontogenetic, it is at least partly due to the tips of the zygomatic processes being broken off in CCNHM 108. The zygomatic processes in ChM PV 4745 are pinched anteriorly giving the entire process a ‘spindle’ shape in lateral view like in Basilosauridae, Llanocetus, Mystacodon, and Coronodon planifrons n. sp.—though the entire zygomatic process anteroposteriorly much shorter in Coronodon spp. Anteroventrally the zygomatic of ChM PV 4745 further possesses a clear facet for the posterior end of the jugal as in Coronodon planifrons n. sp. The zygomatic of CCNHM 8722 is composed of more extremely cancellous bone than the rest of the squamosal and in lateral view it is more rectangular; this cancellous bone is preferentially worn away in CCNHM 8722, the holotype, and CCNHM 164, but unabraded in CCNHM 8722.
Figure 15: Comparison of the squamosal and sternomastoid fossae of Coronodon.
Squamosal of Coronodon planifrons holotype CCNHM 166 (A) and Coronodon havensteini holotype CCNHM 108 (B) in dorsolateral view.The sternomastoid fossae in the juvenile skulls are large but slightly smaller than in the holotype (Fig. 5); their depth is 52% of the maximum depth of the squamosal, v. 60% in the holotype (measured from the squamosal prominence to the postglenoid process). In CCNHM 164, the sternomastoid fossa is larger even than the holotype, and extends further anteriorly (Fig. 7); the maximum length of the fossa is equivalent to 20% of bizygomatic width, v. 12% in the holotype. In the juvenile specimens, the fossa faces more laterally than in the holotype and CCNHM 164. The fossa is shallowly concave and faintly rugose with a somewhat cancellous and radiating surface texture in CCNHM 8722; in the holotype and CCNHM 164 the surface is more deeply pitted and composed entirely of cortical bone at the surface. It is unclear how far dorsally the fossa extended in CCNHM 8722, but does not appear to have extended dorsally as a thin strip along the lateral edge of the nuchal crest as in Coronodon planifrons n. sp. In ChM PV 4745, it terminates at the base of the nuchal crest as in CCNHM 108 (Fig. 15B) and 164, and lacks a dorsal extension like Coronodon planifrons n. sp. Ventrally the fossa transitions into a rugose and deeply fissured posttympanic ridge in juveniles CCNHM 8722 and ChM PV 4745; these deep fissures are not present in ontogenetically older specimens (CCNHM 108, 164).
The periotic fossa (Figs. 13, 16–18) is oval in juvenile specimens and approximately twice as long as transversely wide, and bilobate as in adults. In juveniles CCNHM 8722 and ChM PV 4745, the periotic fossa is oriented nearly parasagittally (Figs. 16–18) whereas in both adult specimens (CCNHM 108, 164) the pit is oriented anteromedially and deviates ~30° from the sagittal plane (Figs. 4B, 18). A smooth, low transverse ridge is present in the juvenile specimens, but is lower than in the holotype (Figs. 16–18); in CCNHM 164, this transverse ridge is higher and sharp. In CCNHM 8722, the fossa posterior to this ridge is punctate. A foramen spinosum is not present in any specimen. The spiny process is broken in CCNHM 8722, but periotic fossa was excavated dorsomedial to the process; a shallow oval sigmoid fossa is present laterally. There is a 10 mm gap between the spiny process and the falciform process to accommodate the lateral tuberosity of the periotic. When placed in articulation, nearly the entire lateral surface of the periotic is separated from the wall of the periotic fossa by a gap, nearly 10 mm wide at the base of the anterior process (Fig. 16A). In juvenile CCNHM 8722, the transverse ridge does not conform to the morphology of the lateral surface of the periotic. The posterodorsal angle of the periotic articulates with a dorsally ascending triangular furrow opposite from the spiny process; a sheet of parietal appears to have been received by the trough-like suprameatal fossa as in some early odontocetes (e.g., Xenorophidae; CCNHM 1838). The medial edge of the periotic fossa is defined by a sharp ridge formed by the squamosal at the squamosal-parietal suture (Fig. 16). The tip of the anterodorsal angle of the periotic appears to have articulated with the squamosal at the anteriormost end of the periotic fossa; most of the surface contact for the periotic-squamosal articulation appears to be where the epitympanic recess of the periotic received the spiny process of the squamosal as well as the gap between the spiny process and the falciform process, which received the lateral tuberosity of the periotic. Despite breakage there seems to have been a 1.5–2 cm gap between the posterior process of the periotic and the lateral edge of the posttympanic ridge in juvenile ChM PV 4745, indicating that Coronodon havensteini possessed an amastoid condition at all stages of ontogeny (Figs. 17 and 18). The pit for the posterior process of the periotic is much larger in CCNHM 164 to accommodate the larger posterior process. The periotic of CCNHM 164 articulates tightly with the periotic fossa along the posterior half of the body and the posterior process, but the anterior process is separated from the lateral wall by an anteriorly widening gap.
Figure 16: Basicranium and periotic of Coronodon havensteini referred juvenile (CCNHM 8722).
(A) Left squamosal and periotic in ventral view; (B) left squamosal in ventral view with periotic removed; (C) left squamosal in ventromedial view, (D) line drawing of squamosal in ventromedial view.Figure 17: Basicranium and periotic of Coronodon havensteini referred juvenile (ChM PV 4745).
(A) Basicranium and right periotic in ventral view; (B) line drawing.Figure 18: Basicranium and periotic of Coronodon havensteini holotype.
(A) Right squamosal and periotic in ventral view; (B) right squamosal with periotic removed; (C) line drawing of squamosal with periotic removed.The glenoid fossa (Figs. 4, 16–18) is smoothly concave in the anteroposterior (but not transverse) plane in CCNHM 8722, ChM PV 4745, and CCNHM 108, but in CCNHM 164 the fossa is bilobate and consists of a smooth posterolateral fossa and a smaller, highly rugose anteromedially positioned pit. The secondary pit is positioned just lateral to the falciform process; each side is broken but the left secondary fossa is 30 mm wide and 35 mm long on the right. Owing to a similar vermiform rugose surface texture that matches the mandibular condyle in some specimens of Coronodon (CCNHM 164, 166), this fossa is best interpreted as part of the glenoid fossa rather than the tympanosquamosal recess (see below). The postglenoid process is dorsoventrally shorter in juvenile specimens CCNHM 8722 and ChM PV 4745; it does not curve anteroventrally at its apex in any specimens.
The medial wall of the cranial hiatus is formed by the basioccipital (Figs. 13, 17, 18), and in ChM PV 4745, the lateral surface of that bone is nearly planar (Fig. 18), differing from the dorsolateral swelling in the holotype that, in concert with the spiny process, constricts the cranial hiatus forming a bilobate outline in ventral view (Figs. 4B, 13, 18). Instead, the cranial hiatus of ChM PV 4745 is oval. In ChM PV 4745 the dorsal fissure of the cranial hiatus is widely open and extends anterior to the anterior process of the periotic, whereas in the holotype it terminates at the anterior margin of the pars cochlearis and is developed only as a transversely narrow fissure between the basioccipital and parietal. Dorsal to the cranial hiatus in ChM PV 4745 the medial wall of the alisphenoid is vertical whereas it is ventrolaterally sloping in the holotype (Figs. 17 and 18). In juvenile ChM PV 4745 the alisphenoid is incompletely ossified and forms a deeply triangular notch, and bears a laminated rhomboidal lateral portion that contacts the squamosal, overlaps the tip of the anterior process of the periotic, but does not obscure the parietal in ventral view. In the adult holotype, however, the parietal is not visible, and the lateral wall of the cranial hiatus anteromedial to the periotic fossa instead seems comprised entirely by nodular posterolateral growths of the alisphenoid and a clear alisphenoid-squamosal suture can be traced (Fig. 18C). Under this interpretation, the foramen ovale is nearly completely encircled by the alisphenoid, and it is confluent with a long, anterolaterally (but nearly transversely) oriented sulcus for the mandibular branch of the trigeminal nerve. However, owing to the extreme rugosity of the cranial hiatus, it is possible that some of the bone medial to the anterior half of the periotic fossa may represent the parietal. A transverse suture lateral to the transversely thicked part of the basioccipital, however, suggests that all of this nodular bone is best identified as alisphenoid (Fig. 18C).
The basioccipital crests are more widely set apart in CCNHM 108 than in ChM PV 4745 (Fig. 4); in the juvenile, the crests are only slightly wider than the occipital condyles. The basioccipital crest in ChM PV 4745 has a sharp posteroventral and anteroventral edges and is deeply concave laterally (Fig. 17); in CCNHM 108, the crest is more transversely inflated and smoothly convex, and the lateral surface is planar (Fig. 18). The paroccipital concavity in CCNHM 164 is deeper than in the holotype and bears two deep pits on the left side (Fig. 4C).
In the holotype the left pterygoid is completely missing and parts of the right pterygoid are present (Fig. 4B). The pterygoid is more completely preserved in ChM PV 4745 (Fig. 17). The lateral lamina arises from the region of the foramen pseudovale, which is located just lateral to the squamosal-alisphenoid suture. A narrow rectangular and horizontal band of the alisphenoid is exposed ventrally in the temporal fossa of this specimen. The medial lamina of the pterygoid extends posteriorly toward the basioccipital crest and posterior to the anterior edge of the cranial hiatus. The foramen ovale is incised into the posterior margin of the alisphenoid in ChM PV 4745 (Fig. 17); this margin is irregular and bears pits and posterolaterally directed finger-like nodules of bone within the alisphenoid. The basisphenoid-basioccipital suture is anterodorsally trending and open but partly obscured by the vomer. The pterygoid sinus fossa is deeply concave, smooth, and proportionally small relative to Basilosauridae, being roughly smaller than the cranial hiatus.
The alisphenoid is restricted to dorsoventrally shallow exposure on the cranial wall anterior to the squamosal and ventral to the parietal along and just above the subtemporal crest (Fig. 13). Anteriorly, part of the alisphenoid was ventrally obscured by the medial lamina of the pterygoid within the pterygoid sinus fossa.
The occipital condyles are set out on a more distinct neck in CCNHM 164 than in the holotype specimen, where the articular edges are nearly flush with the posterior surface of the exoccipital (Fig. 7). The occipital condyles of ChM PV 4745 are proportionally much larger than in the holotype (Figs. 14A and 14B), constituting 33% of bizygomatic width v. 24% in CCNHM 108.
Periotic
The periotics of Coronodon havensteini (Figs. 16–18, 19–22; Table 3), as well as Coronodon newtonorum n sp., Coronodon planifrons n. sp., and the unnamed coronodonids ChM PV 5720 and CCNHM 214 share the following unique combination of features, to the exclusion of all other cetaceans: low pars cochlearis (in ventral view) with triangular outline in ventral view, medial apex of which positioned just anterior to fenestra rotunda (Figs. 19A, 19C, 19E); anterior pars cochlearis narrowed into a dorsoventrally shallow cochlear ridge (Figs. 20A, 20C, 20E); bladelike anterior process with sharply pointed anterodorsal angle and sharp anterior crest, but anteroventral angle not developed (Figs. 19–21); medial tubercle present anterior to pars cochlearis (Fig. 21A); anteroposteriorly long, transversely narrow trough-like suprameatal fossa and completely excavated superior ridge; spine-like posterodorsal angle; distally widening posterior bullar facet with flat distal edge (Figs. 19E, 21A); pair of tubercles on body near posteroexternal foramen (Fig. 20F). Some of these features (low triangular pars cochlearis, cochlear ridge, suprameatal fossa developed as long trough) are shared with Kekenodon onamata, and others (bladelike anterior process with spine-like anterodorsal angle, spine-like posterodorsal angle, and trapezoidal posteriorly widening posterior bullar facet with flat posterior end) are further shared with cf. Kekenodon (OU 22294).
Figure 19: Left periotics of Coronodon havensteini.
Periotic of referred juvenile CCNHM 8722 in ventral (A) and dorsal (B) view; periotic of referred juvenile ChM PV 4745 in ventral (C) and dorsal (D) view; periotic of holotype CCNHM 108 in ventral (E) and dorsal (F) view; periotic of referred adult CCNHM 164 in ventral (G) and dorsal (H) view.Figure 20: Left periotics of Coronodon havensteini.
Periotic of referred juvenile CCNHM 8722 in medial (A) and lateral (B) view; periotic of referred juvenile ChM PV 4745 in medial (C) and lateral (D) view; periotic of adult holotype CCNHM 108 in medial (E) and lateral (F) view; periotic of referred adult CCNHM 164 in medial (G) and lateral (H) view.Figure 21: Right periotic of Coronodon havensteini holotype.
(A) Ventral view, (B) medial view, (C) dorsal view, (D) lateral view.Figure 22: Dorsal cochlear structures and internal acoustic meatus of Coronodon spp. in dorsomedial view.
(A) Left periotic of referred juvenile Coronodon havensteini CCNHM 8722, (B) left periotic of referred juvenile Coronodon havensteini (ChM PV 4745), (C) left periotic of adult holotype Coronodon havensteini (CCNHM 108) with labeled inset, (D) right periotic of adult holotype Coronodon newtonorum (ChM PV 2778), (E) right periotic of adult holotype Coronodon planifrons (CCNHM 166).| Measurement | Coronodon havensteini | C. newtonorum | C. planifrons | |||
|---|---|---|---|---|---|---|
| CCNHM 8722 | ChM PV 4745 | CCNHM 108 | CCNHM 164 | ChM PV 2778 | CCNHM 166 | |
| Ant/post length periotic | ? | 86.3 | 91.3/94.6 | 102.6/? | 78.4 | 102.8/93.7 |
| Ant/post length anterior process | 25.4 | 22.5 | 22.7/22 | 25e/? | 25.8 | 21.3/22.9 |
| Transverse width anterior process midpoint | 16.4 | 17.1 | 18.1/15.4 | 23.4/21.9 | 12.8 | 19/20.7 |
| Dorsoventral depth anterior process midpoint | 23.8 | 23.3 | 27.7/28.1 | 33.4/? | 24 | 30.9/32.8 |
| Distance, perilymphatic duct to fenestra rotunda | 7 | 8.3 | 7.1/4.6 | ? | 5.7 | ?/8 |
| Endolymphatic duct to fenestra rotunda | 10.6 | 9.8 | 9.3/9.2 | ? | 8.6 | ?/11.5 |
| Max diameter of perilymphatic duct | 3.4 | 6.1 | 2/2.5 | ? | 3.1 | ?/3 |
| Endolymphatic duct max | 10.1 | 8.5 | 6.4/7.1 | ? | 9.7 | ?/8.6 |
| Endolymphatic duct min | 3.6 | 3 | 2.2/2.3 | ? | 4.1 | 3.4 |
| Fenestra rotunda max | 5.6 | 5.8 | 3.2/3.3 | ? | 5.1 | 5.8/5.6 |
| Fenestra rotunda min | 3.6 | 3.3 | 5.4/5.5 | ? | 3.1 | ?/4 |
| Fenestra ovalis max | 3.9 | 4.6 | 4/3.5 | ? | 4.2 | ?/4.7 |
| Fenestra ovalis min | 2.8 | 3 | 2.8/2.7 | ? | 2.9 | ?/2.7 |
| Length of promontorium (from fen. Rot.) | 25.1 | 20 | 24.5/25.6 | 29e/? | 21.1 | 25.7/24.8 |
| Greatest transverse width of pars coch medial to fen ovalis | 12.6 | 13.7 | 11.4/11.9 | ? | 11.2 | 12.4/15.6 |
| IAM ant/post (including hiatus fall. If confluent) | 13.5 | 12.2 | 25.2/24.1 | ? | 20.1 | 16e/16.6 |
| IAM transverse | 9.1 | 8.2 | 8.3/7.8 | ? | 8 | ?/8 |
| Post process ant/post length | ? | 28.2 | 37.4/42.4 | 51.1/? | 31 | 52.6/38.3 |
| Post process transverse width | ? | 21.3 | 32.9/32.8 | 36.5/35+ | 22.4 | 39.5/36.2 |
| Antpost diameter mallear fossa | 9.5 | 7.5 | 7.7/7.7 | 10.8 | 8.7 | 9.3/9.8 |
| Dorsoventral depth superior process above IAM | 0 | 0 | 9.3/5.1 | ? | 0 | ?/5.6 |
| Superior process depth at endolymphatic duct | 10.9 | 4.1 | 13.6/12.1 | ? | 3.2 | ?/21.2 |
| Transverse width of fossa incudis | 2.5 | 2.7 | 2.5/2.7 | 3e/3.3 | 1.9 | ?/? |
| Distance fenestra ovalis to fenestra rotunda | 5.9 | 5.26 | 7.9/7.4 | ? | 5.3 | 8.5/7.8 |
| Distance apices of anterodorsal and posterodorsal angles | 42.3 | 10.5 | 40.1/35e | ? | 45 | 45.7 |
| Transverse diameter of body lateral to fen ovalis | 15.6 | 16.8 | 22.9/22.8 | 28.5/? | 17 | 23.7/22.5 |
| Transverse diameter of lateral tuberosity lateral to fen ovalis | 20.2 | 18.7 | 19.5/18.8 | 24.9/? | 21.5 | 26.2/24.2 |
Partial or complete periotics are known for all specimens of Coronodon havensteini (Figs. 19–21). The anterior process, body, and pars cochlearis are all approximately the same anteroposterior length, but the periotic becomes transversely inflated, dorsal structures become more elaborated, and the posterior process lengthens and widens during postnatal ontogeny (Figs. 19C, 19E, 19G). For example, the distance from the anterior process to the fenestra ovalis is 44.3 mm in juvenile CCNHM 8722, 40.1mm in juvenile ChM PV 4745, and 44.6 mm in adult CCNHM 108. Unlike the anterior process length, the posterior bullar facet is short in juveniles (27.4 mm in ChM PV 4745) and considerably longer in adults (43.5 mm in CCNHM 108; 50.1 mm in CCNHM 164). Periotics of Coronodon havensteini (and indeed, Coronodon spp.) are highly unusual in lacking a continuous superior ridge (Figs. 20 and 21), possessing dorsoventrally deep anterodorsal and posterodorsal angles (Figs. 20 and 21), an obtuse (~160°–180°) angle between the pars cochlearis and the anterior process (Figs. 19, 21), an elongate pars cochlearis that is dorsoventrally shallow anteriorly, forming a cochlear ridge; and a trough-like suprameatal fossa. The dorsal side of the periotic looks dramatically different in these specimens (Fig. 22), as ossification begins ventrally within Cetacea and progresses dorsally during postnatal growth (Bisconti, 2001). Amongst all Cetacea, these periotics most closely resemble Kekenodon onamata (Corrie & Fordyce, 2022: Fig. 4) and the Eomysticetidae, and to a lesser extent, Aetiocetidae and Mammalodontidae.
The ontogenetically youngest specimen, CCNHM 8722, has the most gracile periotic (Figs. 19A, 19B, 20A, 20B). The posterior process is missing, but it is otherwise well-preserved; it and somewhat larger juvenile ChM PV 4745 (Figs. 19C, 19D, 20C, 20D) have a more gracile, transversely narrow anterior process and a lateral tuberosity that extends laterally beyond the margin of the body. In ChM PV 4745, the body is more inflated and the lateral tuberosity extends only slightly beyond the lateral margin (Fig. 19C). The anteroexternal sulcus is broader and deeper in CCNHM 8722 and ChM PV 4745 than in adult specimens; in CCNHM 108 and 164, the sulcus is more narrowly (and shallowly) incised, where it is pinched between the body and swollen anterior process (Figs. 20B, 20D, 20F, 20H). The anterior process is transversely thicker in each successively (ontogenetically) older specimen (Fig. 19), having a width of 16.4 mm at anteroposterior midpoint in CCNHM 8722, 17.1 mm in ChM PV 4745, 16.5 mm in the holotype, and 23.1 mm in CCNHM 164. The mallear fossa is larger in CCNHM 164 than in the holotype, measuring 10 mm wide v. 7 mm in the holotype; the fossa incudis is deeper and more defined, perhaps a result of the anomalous surficial wear in the holotype periotics.
The anterior incisure is more deeply incised in the juveniles and accommodates a trough for the tensor tympani (Figs. 20A, 20C, 20E, 20G); just medial to this trough is a longitudinal ridge on the anteroventral surface of the pars cochlearis, similar to Kekenodon, and lower than in Basilosauridae and Kekenodon. In CCNHM 108 and 164 the inflation of the anterior process has resulted in ossification that overlaps and fills this trough (Figs. 19–21), obscuring the ridge on the pars cochlearis. The incisure itself is an obtuse angle in all specimens of Coronodon (Fig. 19), but in juvenile specimens CCNHM 8722 and ChM PV 4745 the incisure forms an angle of 143° and 145° (respectively), as opposed to 173° in the holotype. An anterointernal sulcus is present in older specimens; in CCNHM 164 the sulcus is finely incised, runs along the ventral margin, and bifurcates into a dorsal and ventral branch (Fig. 20G). The dorsal part of the anterointernal sulcus runs toward the anterodorsal angle and the ventral branch runs along the ventral margin. The anterodorsal angle is dorsally higher and more acute in the holotype, relatively lower in CCNHM 8722, and slightly higher in ChM PV 4745; it is prominent in CCNHM 164 (Fig. 20). In ChM PV 4745, the anterodorsal angle is positioned further anteriorly than in CCNHM 8722 or 108. In CCNHM 8722, there is a secondary spur just posterior to the dorsal terminus of the anteroexternal sulcus; a pair of foramina is present at the ventral end of the sulcus. In CCNHM 164, two anteroexternal sulci are present: the primary sulcus that runs anterodorsally and a shorter vertically oriented sulcus just posterior. A fissure-like transverse sulcus, not connected to the anteroexternal sulcus, is present posterior to the anteroexternal sulcus at the anterior margin of the suprameatal fossa in both CCNHM 8722 and ChM PV 4745; it crosses the low superior ridge and trends posteromedially into the fossa. In CCNHM 108, this sulcus is only developed medial to the crest and defines a highly rugose and inflated segment of the crest (Fig. 22C, inset). The anteroventral angle of the periotic is more defined and corner-like in CCNHM 164, and it forms a vertical crest along the anterior margin of the anterior process. The anterior process is dorsoventrally deeper in CCNHM 164, being 37.5 mm deep v. 34.2 mm deep in the holotype (Fig. 20). The anterodorsal angle is more greatly developed and lacks a dorsomedial fossa seen in CCNHM 8722, ChM PV 4745, and CCNHM 108 (Fig. 20). Anteriorly within the suprameatal fossa of the juvenile specimens, just anterior to the facial canal, are irregular fissures, corresponding to a 4 × 9 mm region of cancellous or micronodular bone in the holotype.
In both juveniles (CCNHM 8722 and ChM PV 4745) the facial canal opening is oval shaped and dorsomedially oriented (Fig. 22), and lacks an elongated fissure-like hiatus fallopii like the holotype specimen, which measures 9.5 mm in length. The crista transversa is deeply recessed in these specimens so that the facial canal occurs within the meatus (Figs. 22A, 22B), as opposed to the separate canal and meatus in CCNHM 108 (Fig. 22C). The foramen for the superior vestibular area (=foramen singulare of earlier studies) occurs laterally within the meatus in these juveniles. The lateral wall of the meatus extends further dorsally than the medial wall in all specimens. Posterolateral meatal spurs are present in the adult holotype (CCNHM 108; Fig. 20E) and absent in the juveniles (CCNHM 8722, ChM PV 4745), instead bearing a smoothly convex posterolateral meatal rim in medial view (Figs. 20A, 20C).
Juvenile periotics (CCNHM 8722, ChM PV 4745) lack a secondary spur medial and adjacent to the posterodorsal angle, and a longitudinal sulcus is absent in CCNHM 8722 (Figs. 19B, 19C); this sulcus is present anterior to the angle in ChM PV 4745, but does not separate this secondary spur from the posterodorsal angle as in CCNHM 108 (Figs. 19, 22). This secondary spur is conical in CCNHM 108 (Figs. 19F, 22C, inset) and is equivalent to the “pyramidal process” of Marx & Fordyce (2015). The posterodorsal angle is low in CCNHM 8722, somewhat more prominent in ChM PV 4745, and much higher in CCNHM 108; the condition in CCNHM 164 is unclear owing to breakage but appears to have been at least as well-developed as in the holotype (Fig. 20).
The ventral side of the pars cochlearis in the holotype is anomalously polished but well-preserved in CCNHM 8722 and ChM PV 4745; in these specimens, and especially CCNHM 8722, there is a low longitudinal crest immediately medial to the fenestra ovalis (Figs. 19, 21A). Deep promontorial grooves are present in all specimens (Figs. 20A, 20C, 20E, 21B, 22); the dorsal groove is present just medial to the meatus, and the ventral groove is positioned just ventral to the medial edge of the pars cochlearis. In CCNHM 8722, the groove is floored by finely laminated and apparently fibrolamellar bone indicating rapid growth (Fig. 22A).
The lateral surface of the body is not inflated in CCNHM 8722, and bears a smooth and punctate surface texture (Figs. 19A, 20A); the lateral tuberosity is long (21.9 mm from fenestra ovalis, v. 18.3 mm in ChM PV 4745 and 19.5 mm in CCNHM 108) and projects far beyond (7 mm) the lateral margin of the body in ventral view. In ChM PV 4745 it is slightly more inflated and projects only 2 mm beyond the lateral margin (Fig. 19C). In CCNHM 108 and 164 the lateral edge of the lateral tuberosity does not project beyond the lateral edge of the body and instead the body extends 4 and 2 mm (respectively) past the tuberosity (Figs. 19E, 19G). In CCNHM 108 and 164, the lateral surface is swollen and bears a rugose surface texture, especially posteriorly near the posteroexternal foramina.
Only a single posteroexternal foramen is present in CCNHM 8722 and ChM PV 4745 (Figs. 20B, 20D); in CCNHM 108 there are three posteroexternal foramina (Fig. 20F). Though damaged in the more complete left periotic of CCNHM 164, a fragment of the right periotic confirms the presence of three posteroexternal foramina. Only a single posteroexternal foramen is present in Coronodon newtonorum n sp., Coronodon planifrons n. sp., and all other toothed mysticetes.
The aperture for the vestibular aqueduct is wider in CCNHM 8722 (10.7 mm v. 7 mm in CCNHM 108); the other juvenile specimen, ChM PV 4745, has a narrow fissure-like aperture as in the holotype (Fig. 22). A large tubercle is present in CCNHM 8722 posterodorsal to the fenestra rotunda, and bears several short sulci; this tubercle is less prominent in CCNHM 108, 164, and ChM PV 4745.
The posterior process of the periotic is anteroposteriorly short in ChM PV 4745 and bears a nearly diamond-shaped posterior bullar facet, differing from the posteriorly expanding facet in CCNHM 108 and 164 (Fig. 19). It lacks pits or a facet for the posttympanic ridgeseen in CCNHM 108 and 164. In CCNHM 164, the posterior bullar facet (Fig. 19G) differs from the trapezoidal facet in the left periotic of the holotype (Fig. 19E), and instead resembles the slightly diamond shaped facet in the right periotic of the holotype (Fig. 22A); the posterior margin, while being slightly pointed, still exhibits a nearly flat posterior margin and the entire facet widens posteriorly. The posterior process is longer as well, being 52 mm in CCNHM 164 v. 42.6 mm in the holotype and only 28.2 mm in ChM PV 4745. The facet in CCNHM 164 is transversely convex and bears subtle striations, but more obviously developed than in the holotype; in this specimen, the posterior process extends 11 mm past the termination of the facet, forming a posteroventrally facing secondary articular facet for the posttympanic ridge of the squamosal. This means that in late postnatal ontogeny, the posttympanic ridge began to anteriorly overlap the posteriormost end of the posterior process of the periotic—the latter of which appears to have grown posterodorsally. The entire periotic of CCNHM 164 is 102.9 mm v. 94.7 mm in CCNHM 108, and 86.3 mm in ChM PV 4745; as outlined above, the increase in anteroposterior length is driven by the postnatal growth of the posterior process as the anterior process length is similar in all specimens of Coronodon havensteini (Figs. 19A, 19C, 19E, 19G).
Tympanic bulla
A tympanic bulla is preserved in the holotype and both juvenile specimens (Fig. 23; Table 4). Juvenile bullae are similar to those of the adult but are absolutely smaller; CCNHM 8722 is 75 mm long, ChM PV 4745 is 76.7 mm long, and CCNHM 108 is 85 mm long. The bulla of CCNHM 8722 is slightly proportionally narrower (Fig. 23B), the width being 61% of length whereas the width is 66% of the length in CCNHM 108.
Figure 23: Tympanic bullae of Coronodon havensteini.
Right bulla of CCNHM 8722 in medial (A), ventral (B), and dorsal (C) view; right bulla of ChM PV 4745 in medial (D), ventral (E), and dorsal (F) view; left bulla of CCNHM 108 in medial (G), ventral (H), and dorsal (I) view.| Coronodon havensteini | C. newtonorum | |||
|---|---|---|---|---|
| CCNHM 8722 | ChM PV 4745 | CCNHM 108 | ChM PV 2778 | |
| Greatest length bulla | 74.9 | 76.7 | 83.2/85 | 79.9 |
| Max width of bulla at sigmoid process | 46.7 | 50.2 | 50.3/55.6 | 50.1 |
| Greatest depth of involucrum | 33 | 31.7 | 36.5/36.4 | 35.6 |
| Transverse width of medial lobe | 23.3 | 30.1 | 31.8/31.3 | 34 |
| Anteroposterior length of posterior lobe | ? | ? | 50.4/48.4 | 46.5 |
| Dorsoventral depth of bulla at level of sigmoid | ? | ? | 62.9/? | 58.8 |
The medial margin of the involucrum is more concave in CCNHM 8722 than ChM PV 4745, resembling the holotype, and also bears an oblique furrow (Figs. 23C, 23F, 23I). The dorsal margin of the involucrumis posteriorly flat in the holotype (Fig. 23G), and anteriorly sloped; a similar condition is present in ChM PV 4745 (Fig. 23D), and in CCNHM 8722 it is continuously sloping along its length (Fig. 23A). The involucrum bears shallow transverse creases in both juvenile ChM PV 4745 and the adult holotype, but the surface texture is smoother in juvenile CCNHM 8722 (Fig. 23A). Immediately anterior to the inner posterior pedicle there is a small fossa on the dorsal side of the involucrum in CCNHM 8722 as compared to a prominent bulge in the holotype. Both the inner and outer posterior pedicles are more delicate in CCNHM 8722. The medial lobe is proportionally smaller than in the holotype, constituting 50% of transverse width, rather than 60% as in the holotype and juvenile ChM PV 4745 (Figs. 23C, 23F, 23I). All specimens bear a very short median furrow; in CCNHM 8722, the furrow terminates into a low ventral prominence, as in Basilosauridae and Llanocetus. However, in ChM PV 4745, CCNHM 108, and 164, Coronodon newtonorum n sp., Coronodon planifrons n. sp., and virtually all other toothed mysticetes, this ventral convexity is absent and the surface is instead flat to shallowly concave. Scattered pores are present ventrally in all specimens, but the pores are proportionally larger in juveniles CCNHM 8722 and ChM PV 4745 (Figs. 23B, 23E) and present across the entire surface; in the adult holotype, the pores are more frequent medially and only a few exist lateral to the median furrow (Fig. 23H). The nearly complete bulla of Coronodon newtonorum n. sp. (below) has a sigmoid process that is elevated above the outer lip to a similar degree as other stem mysticetes (e.g., Eomysticetidae, Aetiocetidae), indicating that the condition in the holotype specimen is anomalous and likely a result of improper gluing.
Dentition
Juvenile CCNHM 8722 preserves no teeth, but ChM PV 4745 preserves two caniniform teeth and the upper left M1 and M2 (Fig. 24; Table 5). CCNHM 108 preserves the upper P3 and M1/2 and nearly the entire lower dentition (Figs. 25 and 26; Table 5), missing only the incisors, canines, and m4. CCNHM 164 has a more incomplete lower dentition (n = 6; p2-m4), but preserves numerous upper teeth (n = 9, P3-M3). The total dental formula known from all of these specimens is 3.1.4.3/3.1.4.4.
Figure 24: Dentition of referred juvenile Coronodon havensteini, ChM PV 4745.
Upper molars in labial (A), occlusal (B), and lingual (C) view; caniniform tooth in labial (D) and lingual (E) view; caniniform tooth in lingual (F) and labial (G) view.| Catalog # | Tooth type | Side | Upper lower | Number of roots | L base crown | W base crown | L labial | |
|---|---|---|---|---|---|---|---|---|
| C. have. | CCNHM 108 | I2 | Right | Upper | 1 | 17.05 | 13.6 | 31 |
| C. have. | CCNHM 108 | P2 | Right | Upper | 2 | 35.47 | 14.68 | 20 |
| C. have. | CCNHM 108 | P3 | Left | Upper | 2 | 50.89 | 15.55 | 25 |
| C. have. | CCNHM 108 | M2 | Left | Upper | 2 | 51.4 | 14.98 | 24 |
| C. have. | CCNHM 108 | p1 | Left | Lower | 2 | 33.73 | 14.87 | 30 |
| C. have. | CCNHM 108 | p2 | Left | Lower | 2 | 52.13 | 16.27 | 29 |
| C. have. | CCNHM 108 | p3 | Left | Lower | 2 | 58.17 | 20.97 | 30 |
| C. have. | CCNHM 108 | p4 | Left | Lower | 2 | 58.43 | 17.93 | 29 |
| C. have. | CCNHM 108 | m1 | Left | Lower | 2 | 57.71 | 18.15 | 28 |
| C. have. | CCNHM 108 | m2 | Left | Lower | 2 | 52.21 | 17.66 | 29 |
| C. have. | CCNHM 108 | p1 | Right | Lower | 2 | 34.73 | 15.04 | 29 |
| C. have. | CCNHM 108 | p2 | Right | Lower | 2 | 52.3 | 16.63 | 29 |
| C. have. | CCNHM 108 | p3 | Right | Lower | 2 | 55.86 | 17.57 | 28 |
| C. have. | CCNHM 108 | p4 | Right | Lower | 2 | 59.54 | 17.79 | 26 |
| C. have. | CCNHM 108 | m1 | Right | Lower | 2 | 56.38 | 17.91 | 26 |
| C. have. | CCNHM 108 | m2 | Right | Lower | 2 | 53.03 | 17.03 | 28 |
| C. have. | CCNHM 164 | p3 | Left | Upper | 2 | 49.6+ | ? | 40.5+ |
| C. have. | CCNHM 164 | p3 | Right | Upper | 2 | 51.61 | ? | ? |
| C. have. | CCNHM 164 | p4 | Left | Upper | 2 | 51.8 | 15.65 | 39.44 |
| C. have. | CCNHM 164 | m1 | Right | Upper | 2 | ? | 15.5 | 34.31+ |
| C. have. | CCNHM 164 | m2 | Right | Upper | 2 | 52.25 | 13.11 | 34.39 |
| C. have. | CCNHM 164 | m2 | Left | Upper | 2 | ? | 12.7 | 28+ |
| C. have. | CCNHM 164 | m3 | right | Upper | 2 | ? | 13.7 | 40.04 |
| C. have. | CCNHM 164 | i1 | ? | ? | 1 | 12.35 | 9.9 | 17.9+ |
| C. have. | CCNHM 164 | i2 | ? | ? | 1 | 15.19 | 12.76 | 21.6 |
| C. have. | CCNHM 164 | i3 | ? | ? | 1 | 12.24 | 11.61 | 21.61+ |
| C. have. | CCNHM 164 | p2 | Right | Lower | 2 | 34.91+ | 14.2 | 38.49+ |
| C. have. | CCNHM 164 | p3 | Right | Lower | 2 | 53.26+ | 15.61 | 41.57+ |
| C. have. | CCNHM 164 | m1-2 | Right | Lower | 2 | 57.41 | ? | 57.32 |
| C. have. | CCNHM 164 | m2-3 | Right | Lower | 2 | 57.04+ | 18.16 | 48.59+ |
| C. have. | CCNHM 164 | m3 | Left | Lower | 2 | ? | ? | 42.84+ |
| C. have. | CCNHM 164 | m4 | Right | Lower | 2 | 45.5+ | 14.59 | 38.05+ |
| C. plan. | CCNHM 166 | i? | ? | ? | 1 | 18.5 | 13 | 25.1 |
| C. plan. | CCNHM 166 | c1 | ? | ? | 1 | 21.3 | 14e | 27.2 |
| C. plan. | CCNHM 166 | p1/c1 | ? | ? | 1 | 22.6 | 13.8 | 27.3 |
| C. plan. | CCNHM 166 | p3 | Left | Upper | 2 | 51.5+ | 18.2 | 41.7 |
| C. plan. | CCNHM 166 | p3 | Right | Upper | 2 | 51.2 | 17.1 | 36+ |
| C. plan. | CCNHM 166 | p4 | Left | Upper | 2 | 55.6 | 16.3 | 41.5 |
| C. plan. | CCNHM 166 | p4-m1? | ? | ? | 2 | ? | 16+ | 46+ |
| C. plan. | CCNHM 166 | m1 | Right | Upper | 2 | 50+ | 15.9 | 37+ |
| C. plan. | CCNHM 166 | m1 | Left | Upper | 2 | 55.2 | 15.9 | 35+ |
| C. plan. | CCNHM 166 | m2 | Left | Upper | 2 | 44.7 | 13.2 | 27.1+ |
| C. plan. | CCNHM 166 | m3 | Right | Upper | 2 | 37.9 | 13e | 20+ |
| C. plan. | CCNHM 166 | p3 | Right | Lower | 2 | 47.6 | 16.1 | ? |
| C. plan. | CCNHM 166 | m1 | Left | Lower | 2 | 61.5 | 18.4 | 46+ |
| C. plan. | CCNHM 166 | m2 | Left | Lower | 2 | 61.6 | 18.8 | 47+ |
| C. plan. | CCNHM 166 | p3 | Left | Lower | 2 | ? | 15.8 | ? |
Figure 25: Upper dentition of adult Coronodon havensteini, CCNHM 108 and 164.
Abbreviations: li, lingual; la, labial.Figure 26: Lower dentition of adult Coronodon havensteini, CCNHM 108 and 164.
Abbreviations: li, lingual; la, labial.For dental descriptions besides the caniniform teeth, cusps are identified as either the central cusp or those mesial or distal to it, each denticle identified as being first, second, third, and so forth, with respect to their distance from the central cusp. The central cusp is determined by being the largest cusp and is typically at or near the mesiodistal center of the tooth. Each of these denticles, including the central cusp, are united by a crown base. As these teeth slightly resemble a hand, the analogy could be carried out to make the denticles and central cusp the fingers and the crown base the palm. Each of the rows of denticles follow an axis along the mesiodistal length of the tooth and are sloped in labial and lingual view with respect to the crown base. These slopes extend from the crown base to the apex of the central cusp, but are not straight lines. The differences in slopes with respect to the crown base are noted, as is the curvature of that slope, which always tend to be arched (but to lesser and greater degrees). In this way, one might differentiate the rounded profile of coronodonid postcanines from the triangular profile often seen of the postcanines of basilosaurids.
Regarding enamel texture and smaller details, it should be noted that none of the teeth of Coronodon have cingula, yet they all have carinae. The carinae follow mesiodistally along the edge of each denticle and the central cusp, and tend to be more pronounced at the base of each denticle, resulting in the base of each denticle being slightly pinched in, making them all appear a bit “plump”. This coincides with a depression of the crown base in between each denticle that can carry down to the basal-most edge of the crown, forming a shallow trough. These apicobasal crown base troughs usually result in clustering the more mesial or distal denticles on one side, and those denticles closer to the central cusp on the other. A central apicobasal crown base trough typically lies basal to the central cusp itself (possibly making the teeth prone to taphonomic breakage along the central cusp), usually found to be deeper on the lingual than the labial side of the tooth.
The enamel is thinner (between approximately 0.1 to 0.4 mm in thickness, as measured with digital calipers from broken edges of various teeth) than that found in Basilosaurus (typically 0.5 mm (Sahni & Koenigswald, 1997)). The enamel of coronodonids appears to be thin like that seen in modern odontocetes (Loch et al., 2013), though this needs to be evaluated more carefully. This enamel is typically covered with undulating oblong bumps and depressions at a very small scale, much less than a millimeter in size. These bumps and depressions can be found everywhere on the enamel besides polished surfaces of the apices of some cusps and denticles and the surfaces of shear facets. More on enamel thickness and external texture will be included in a forthcoming article on dental features of coronodonids.
Regarding the roots, for those teeth (both upper and lower) for which both roots are preserved, it is readily notable that the mesial root is thicker, straighter, and more vertically oriented than the distal root of the same tooth. Distal roots tend to be tilted more distally and slightly narrower, not only in the thick part nearest the crown base, but the distal roots also taper more than the mesial roots.
Caniniform teeth
All of the caniniform teeth (by definition), have a single, pointed cusp (Figs. 24 and 25), including the single upper left first incisor of the juvenile specimen, ChM PV 4745 (Fig. 24). The caniniform teeth appear to have apicobasal lengths greater than their mesiodistal lengths, with roots approximately twice the length of the apicobasal length of the crown. Subtle carinae can be seen on all cusps of these teeth as well as some minor apicobasal ridges extending from the base of the crown to very near the apices. All of them appear to have had a thicker root at some point, with a layer of cementum that thickened within a centimeter of the crown’s base.
Upper dentition
The third upper left premolar (CCNHM 164.37) exhibits the same palmate cusp structure found in the holotype CCNHM 108 (Fig. 25). The upper right P3 has four denticles mesial to the central cusp, and three distal to the central cusp. The mesial row of denticles get smaller mesially, whereas the distal row of denticles are more subequal in size and do not get as progressively smaller distally. The slope of the mesial row of denticles appears to dip toward the base of the crown more steeply than does the distal row of denticles, though this illusion seems due to the increased number denticles on the mesial row, making it extend further basally, as well as the greater change in size of the denticles along the mesial row as compared to the distal row of denticles.
The upper left P4 (CCNHM 164.3) has four mesial and four distal denticles surrounding the central cusp (Fig. 25). The denticles of the mesial and distal rows get smaller the further away from the central cusp, but they do not seem to do so in an appreciably different degree way. The slopes of the mesial and distal rows of denticles also do not seem to differ from each other. Ultimately, this makes the crown of the P4 more symmetrical, with the roots primarily indicating mesial and distal ends of the tooth.
The upper M1 (CCNHM 108 and CCNHM 164.8) is only known from the right side for CCNHM 164, and several denticles are broken or worn, but it clearly had three mesial and three distal denticles when intact (Fig. 25). The most mesial and most distal denticles are more similar in size to the central cusp than those of either the P3 or P4. The slopes of the mesial and distal denticle rows appear to be less than the same slopes on the P4, but this, too, could be because of the smaller denticle count and greater similarity of denticle size within rows.
CCNHM 108 has an upper molar that is a left M2 and CCNHM 164.39 could either be a distal fragment of the upper right M2 or M3 (Fig. 25). Based on the tendency for the shallow apicobasal groove to lie basal to the central cusp, it seems that the large cusp of CCNHM 164.39 preserved here is the central cusp. Distal to it are four progressively smaller distal denticles. These denticles differ in size more than the distal denticles of the M1 or P4, and are more similar in decreasing proportions like the distal denticles of the right P3 (CCNHM 164.7).
The most complete M3 is from the right (CCNHM 164.6) and is missing much of its mesial edge (only preserving two denticles), though its distal side retains four well-preserved distal denticles (as does the left M3, CCNHM 164.4) (Fig. 25). The apices of the central cusp and denticles of the M3 have a more triangular profile (in labial/lingual view) than do the equivalent denticles of the more mesial teeth (which appear more rounded in profile). This more triangular profile makes the tip of each denticle narrower than their base, which resembles the denticles of Borealodon and Metasqualodon more than the rounded profiles of the denticles of the more mesial teeth of Coronodon.
Four teeth are preserved in juvenile specimen ChM PV 4745, including two loose caniniform teeth likely representing I3 or C1 and C1 or P1, as well as the upper left M1-2, both of which are in situ within the maxilla (Fig. 24). All teeth are hollow with voluminous pulp cavities, only ~2 mm thick dentine, absent or negligible dentine, and relatively short roots, no longer than about 21 mm in the larger caniniform tooth. Both caniniform teeth possess erect subconical crowns with sharp, smooth mesial and distal carinae. The labial enamel is smooth but lingually there are low, parallel apicobasal ridges. The M1-2 are similar to the preserved M2 in the holotype (CCNHM 108) but differ in possessing four mesial and four distal accessory cusps (rather than five of each in CCNHM 108). These molars are only partly erupted, with the mesial and distal edges of the enamel crown base still in the labial part of the alveolus (and obscured by the maxilla) in lateral view. Unlike the anteroposteriorly aligned alveoli of the holotype, the molars are posterolaterally imbricated and overlap by 9.5 mm in ChM PV 4745, with the distal edge of M1 positioned labial to the mesial edge of M2, forming a posterolaterally directed interdental slot like the mandibular postcanine dentition in the holotype (Geisler et al., 2017).
Lower dentition
CCNHM 164.5 is a right lower p2 (Fig. 26), and has a large central cusp surrounded by two much smaller mesial denticles and three distal denticles. The equivalent tooth in the holotype (CCNHM 108) has only one mesial denticle and three distal denticles. The mesial denticles are half the size of the distal denticles. This tooth looks superficially similar to the Nishiyama specimen of Metasqualodon from the Ashiya Group (Okazaki, 1982), except that the Nishiyama tooth lacks mesial denticles altogether and the distal denticle arises off a location closer to the apex of the central cusp. In addition, the lower right p2 of CCNHM 164 has some waviness in the profile of its carinae for the mesial and distal denticle adjacent to the central cusp, as well as the carinae of the central cusp itself.
The right lower third premolar (CCNHM 164.2) (Fig. 26) has three mesial denticles and five distal denticles. The mesial denticles are approximately the same size as the distalmost three denticles of the distal row, with only the distal denticle adjacent to the central cusp being larger than all of the other denticles (mesial and distal). This asymmetry in number and size creates an asymmetry in the central cusp that makes the slope of the mesial denticle row appear steeper than the distal row. The central cusp is also shifted a few millimeters mesially, and its distal edge and carina are apicobasally shorter than its mesial edge.
CCNHM 164.10 is identified as a right lower m1 or m2 (Fig. 26). CCNHM 164.10 has five mesial and five distal denticles. The denticles are approximately the same size and the denticle rows are almost at the same slope. The only minor difference between the two denticle rows is that the mesial row is arranged in a slightly straighter line and the distal row’s profile is more rounded, like an arc (this is especially notable from the lingual view).
CCNHM 164.9, a right lower m3, has four mesial and four distal denticles, though the denticles of the distal row are larger than those of the mesial row. Like CCNHM 164.10, the mesial row of denticles has a steeper and straighter slope and the distal row appears to have a more rounded profile (like an arc), in contrast to the mesial denticle row, which is a bit straighter.
The lower left m3 is represented by CCNHM 164.41 (Fig. 26), though this identification is tentative because this tooth is incomplete. This partial tooth consists of the mesial row of denticles, the mesial root, and the majority of the crown base, with evidence of five mesial denticles aligned at an angle that is steep like in the other lower molars. The preserved part of the labial side of the crown base includes shallow apicobasal grooves indicative of the presence of at least two distal denticles, though there were surely more.
CCNHM 164.4 is the lower right m4 and has four mesial and four distal denticles (Fig. 26). The mesial denticles are approximately the same size as their respective opposite on the distal denticle row, but the mesial row itself is longer and more steeply sloped than the distal row. Like the lower molars mesial to the m4, the mesial denticle row’s profile appears straighter than the slightly more arced profile of the distal denticle row.
Mandible
Mandibles are only preserved in the adult specimens, including the nearly complete left and right mandibles in the holotype and a partial right mandible in CCNHM 164 (Figs. 27–29; Table 6). The posterior half of the right mandible in CCNHM 164 is partially preserved with partial alveoli for p3-m2, a well-preserved coronoid process and mandibular condyle (Figs. 27C, 27E, 28C), though the angular process and entire medial surface is missing ventral to the m4 and coronoid process, so that the morphology of the mandibular foramen is unclear. Like the holotype, the ventral margin of the mandible is roughly straight, and a slight curvature around the p4-m1 may be due to accumulated minor inaccuracies created when gluing many fragments of the mandible together. It is slightly longitudinally sinuous, and lacks the more extreme ventral curvature seen in Coronodon newtonorum n sp.
Figure 27: Mandibles of Coronodon havensteini.
Holotype specimen CCNHM 108 left mandible (A) and right mandible (B) in lateral view, and referred specimen CCNHM 164 right mandible in lateral view (C); holotype specimen CCNHM 108 left mandible in dorsal view (D) and referred specimen CCNHM 164 right mandible in dorsal view (E).Figure 28: Mandibles of Coronodon havensteini.
Holotype specimen CCNHM 108 left mandible (A) and right mandible (B) in medial view; referred specimen CCNHM 164 right mandible (C) in medial view.Figure 29: Mandibular condyles of Coronodon spp. in articular view.
(A) Left mandibular condyle of adult Coronodon havensteini holotype (CCNHM 108), (B) left mandibular condyle of referred adult Coronodon havensteini (CCNHM 164), (C) left mandibular condyle of adult Coronodon planifrons holotype (CCNHM 166).| Coronodon havensteini | Coronodon newtonorum | Coronodon planifrons | ||
|---|---|---|---|---|
| CCNHM 108 | CCNHM 164 | ChM PV 2778 | CCNHM 166 | |
| Mandible length | 848+/830+ | ? | 700e | ? |
| Length lower toothrow | 562/575 | ? | 620e | ? |
| Length mand symphysis | ?/153 | ? | 180 | ? |
| Depth at c1 | 63.0/65.9 | ? | 70.7 | ? |
| Depth at p4 | 72/74.1 | ?/74.9 | 84.6 | 78.9 |
| Depth at m4 | 134/129 | ?/132 | 129.7 | 139 |
| Max depth at coronoid | 248/247 | ?/228 | 260e | 244 |
| Trans width at c1 | 43.4/43.9 | ? | 41 | ? |
| Width at p4 | 50.2/51.3 | 48.2 | 55.2 | 52 |
| Width at m4 | 64.2/61.3 | ? | ? | 55 |
| Condyle width | 54.9/49.1 | ? | 60.5 | 51.6 |
| Condyle depth | 54.3/54.3 | 45.6 | 57.7 | 55.6 |
| Height of coronoid above condyle | ?/110 | 100.5 | 115 | 144 |
| Length of neck (coronoid-condyle gap) | 84/86 | 80 | 70 | 64.5 |
The m4 alveolus is elevated approximately 3–4 cm dorsal to the mandibular condyle in CCNHM 164 (Fig. 27C), somewhat higher than in the holotype (Figs. 27A and 27B), though this part of the mandible is damaged. The coronoid process is complete in the right mandible of the holotype and CCNHM 164 (Figs. 27B and 27C); it is intermediate in morphology between the triangular condition in Basilosauridae and the elongate tongue-shaped process in Aetiocetidae: it is somewhat lobate with convex and equally sloping anterior and posterior margins, whereas in Basilosauridae the posterior margin is nearly vertical. The coronoid is slightly transversely thicker anteriorly and is transversely thickened at the apex. A pre-coronoid trough is present medially along the anterior margin of the coronoid (Figs. 28A and 28B), in line with the m4 and posterior toothrow. In CCNHM 164 there is a low but well-developed tubercle posteromedially along the posterior margin of the coronoid, 35 mm dorsal to the margin of the mandibular foramen.
The mandibular foramen of CCNHM 164 is voluminous and approximately 10 cm deep dorsoventrally; unlike the holotype, the margins are unknown (Fig. 28). This breakage reveals that the mandibular canal is similarly large and continues anteriorly to at least the level of the p4; the walls of the mandible increase in thickness anteriorly. The mandibular condyle (Fig. 29) is separated from the coronoid process by an 8 cm long neck; the condyle is planoconvex in articular view, shallowly excavated medially by the mandibular fossa, and it faces posterodorsally. The articular surface is deeply pitted and rugose.
Atlas
Complete atlases are preserved in ChM PV 4745 and the holotype; a fragmentary atlas is present in CCNHM 164 (Figs. 30–32; Table 7). The atlas of CCNHM 164 is similar in size and proportions to CCNHM 108 but the transverse process is dorsoventrally shallower. The atlas of ChM PV 4745 differs from the holotype in being anteroposteriorly somewhat flatter and having an oval-shaped neural foramen that does not narrow ventrally. Like the holotype and unlike Coronodon planifrons n. sp. (CCNHM 166), a hypapophysis is not developed. ChM PV 4745 possesses the only complete transverse process in Coronodon; it is transversely directed and rectangular in anterior view with a vertical lateral margin, unlike the bifurcated posterolaterally directed condition in Basilosauridae and some stem odontocetes.
Figure 30: Postcranial elements of Coronodon havensteini holotype specimen CCNHM 108.
Figure 31: Postcranial elements of Coronodon havensteini referred specimen CCNHM 164.
Cervical vertebrae shown in anterior and posterior views, and thoracics, lumbars, and caudals shown in anterior view only.Figure 32: Atlas vertebra of Coronodon havensteini referred specimen ChM PV 4745.
Atlas in anterior (A) and posterior (B) view.| Coronodon havensteini | Coronodon planifrons | |||
|---|---|---|---|---|
| ChM PV 4745 | CCNHM 108 | CCNHM 164 | CCNHM 166 | |
| Atlas vertebra max width | 143.4 | 180 | ? | 170+ |
| Atlas vertebra max depth | 111.4 | 139 | ? | 137.3 |
| Atlas ne for max width | 46.2 | 49.7 | ? | 49.6 |
| Atlas ne for max depth | 63 | 66.9 | ? | 64.6 |
| Atlas width condylar facets | 105e | 110e | ? | 128.5 |
| Atlas ant post length | 46.7 | 64.6/62/2 | 60e | 55.7 |
| Axis, max. width | ? | 184 | ? | ? |
| Axis, max. depth | ? | 135.2 | ? | ? |
| Axis, max. length including odontoid | ? | 52.1 | 58.5 | ? |
Axis
A complete axis is preserved in CCNHM 108 and a partial axis is present in CCNHM 164 (Figs. 30–32; Table 7). The more complete axis of CCNHM 108 is noteworthy for exhibiting a transverse foramen. The axis of CCNHM 164 possesses a more projecting odontoid process and larger hypapophysis and a less dorsally arched ventral margin in anterior view. The anterior part of the neural spine is preserved and is massive, stout, and pyramidal; the neural spine is proportionally wide.
C3–C7
Two partial mid-cervicals are present in CCNHM 164, C3 and C4 based on comparison with CCNHM 108 (Figs. 30–32; Table 8). They do not differ from CCNHM 108 except in possessing centra with more rounded ventral margins, whereas the mid-cervical centra in the holotype are all nearly rectangular. The C7 lacks a diapophysis and has a more pointed ventral margin; the centrum slightly longer than C3-4, and it is slightly deeper than T1-2.
| Coronodon havensteini | Coronodon planifrons | ||
|---|---|---|---|
| CCNHM 108 | CCNHM 164 | CCNHM 166 | |
| C3 anterior width | 76 | 73.6 | 66.2 |
| C3 anterior depth | 58.3 | ? | ? |
| C3 length | 25.2 | 25 | 23.1 |
| C4 anterior width | 70.2 | 81.5 | 77.3 |
| C4 anterior depth | 58.9 | 61.1 | 62 |
| C4 length | 22.7 | 24 | 24.1 |
| C5 anterior width | 71.4 | ? | ? |
| C5 anterior depth | 57 | ? | ? |
| C5 length | 25.6 | ? | ? |
| C6 anterior width | 74 | ? | ? |
| C6 anterior depth | 55.1 | ? | ? |
| C6 length | 24.3 | ? | ? |
| C7 anterior width | 74.3 | 72 | 69.1 |
| C7 anterior depth | 55.4 | 63.2 | 62 |
| C7 length | 27.7 | 32.9 | 31.6 |
Thoracic vertebrae
An apparently complete set of nine thoracic vertebrae is preserved in CCNHM 164, whereas in CCNHM 108, only seven are preserved (Figs. 30–32; Table 9). Based on measurements these form a continuous series in CCNHM 164 (T1 to T9). The anteriormost thoracics (T1-2) are dorsoventrally shallow and possess oval centra bearing shallow notochordal pits posteriorly. Pore-like notochordal pits are present anteriorly in T1-7 and small fissure-like pits are present in T8-9. T1-2 bear anterior costal facets at the dorsolateral edge of the centrum but T3 does not. Small posterior costal facets are present in T1-5, whereas in T6-7 they are large and concave, small again in T8, and absent in T9. T3-5 are successively longer than T1-2, and length increases steadily throughout the thoracics; centrum depth increases from T1 to T5, and depth is consistent throughout the remaining thoracics (T6-9). In the posterior thoracics (T6-9) the dorsal edge of the centrum becomes more flattened; all thoracics bear a rounded ventral margin and lack a ventral keel. In T7-9 the costal articulations transition rapidly. T6-7 bear a facet for the tubercle on the pedicle of the vertebra but in T8 the tubercular facet is further ventrally at the base of the pedicle at its junction with the centrum; the capitular facet is located only 15 mm ventrally. In T9 there is only a single capitular facet for a rib lacking a tubercle; it is positioned laterally on a short transverse process positioned at the level of the dorsal half of the centrum.
| Coronodon havensteini | Coronodon planifrons | ||
|---|---|---|---|
| CCNHM 108 | CCNHM 164 | CCNHM 166 | |
| T1 anterior width | 76e | 83.6 | 86.2 |
| T1 anterior depth | 55 | 55.9 | 61.2 |
| T1 length | 40.7 | 40.7 | 40.6 |
| T2 anterior width | 89.2 | 77.5 | 80.2 |
| T2 anterior depth | 54 | 50.9 | 57.1 |
| T2 length | 46.4 | 44.1 | 44.1 |
| T3 anterior width | 76e | 74.4 | ? |
| T3 anterior depth | 49.2 | 53.5 | ? |
| T3 length | 48.6 | 52.1 | ? |
| T4 anterior width | 71.9 | 75.8 | 75.2 |
| T4 anterior depth | 48.3 | 55 | 53.6 |
| T4 length | 52.9 | 55 | 46.6 |
| T5 anterior width | ? | 76 | 80 |
| T5 anterior depth | ? | ? | 55 |
| T5 length | ? | 59.8 | 54.6 |
| T6 anterior width | 71.3 | 80.7 | 79.8 |
| T6 anterior depth | 47.3 | 59 | 58 |
| T6 length | 55e | 62.5 | 58.4 |
| T7 anterior width | 71.6 | 79.1 | 81.6 |
| T7 anterior depth | 48.3 | 58.2 | 61 |
| T7 length | 62.2 | 66.5 | 60.9 |
| T8 anterior width | 79 | 86.4 | 94 |
| T8 anterior depth | 52.3 | 61.9 | 62.3 |
| T8 length | 62.7 | 10.2 | 64.4 |
| T9 anterior width | ? | 86.6 | 95e |
| T9 anterior depth | ? | 32.1 | 65.7 |
Lumbar vertebrae
Lumbar vertebrae are preserved only in CCNHM 164, which preserves three recognized here as LA, LB, and LC (Figs. 30–32; Table 10); these are of nearly identical centrum length but are arrayed in anteroposterior sequence based on increasing centrum width and decreasing neural foramen diameter. LA has a subpentagonal anterior centrum with a flat dorsal edge and somewhat pointed median ventral margin; the ventral surface has a sharp median keel. LA also exhibits a long (but partial) ventrolaterally projecting transverse process, oriented 26° from horizontal. LB has a more circular centrum as well as a sharp ventral keel. LC is quite abraded and weathered but had a circular to oval posterior centrum with an arthritic pathology forming a ventral lip along the ventralmost margin and somewhat on the left side. All three lumbars possess 3–4 mm deep fissure-like notochordal pits.
| Coronodon havensteini, CCNHM 164 | Coronodon planifrons, CCNHM 166 | |
|---|---|---|
| L1 anterior width | ? | |
| L1 anterior depth | 65.9 | |
| L1 length | 77.4 | |
| L2 anterior width | 90 | |
| L2 anterior depth | ? | |
| L2 length | 88.6 | |
| L3 anterior width | 93.2 | |
| L3 anterior depth | 79.8 | |
| L3 length | ? | |
| L4 anterior width | 90e | 94.3 |
| L4 anterior depth | 78.8 | 83.8 |
| L4 length | 95.6 | 94.2 |
| L5 anterior width | 94 | |
| L5 anterior depth | 84.7 | |
| L5 length | 94.8 | |
| L6 anterior width | 94.2 | |
| L6 anterior depth | 86.7 | |
| L6 length | 95.8 | |
| L7 anterior width | 98 | |
| L7 anterior depth | 91 | |
| L7 length | 94.9 | |
| L8 anterior width | 94.5 | 101.2 |
| L8 anterior depth | 89 | 91.1 |
| L8 length | 95.3 | 94.2 |
| L9 anterior width | 90e | 103 |
| L9 anterior depth | 96.8 | 91.4 |
| L9 length | 100.5 | 93.7 |
| L10 anterior width | 100 | |
| L10 anterior depth | 95.2 | |
| L10 length | 92.3 |
Ribs
Several partial ribs are preserved in the holotype (CCNHM 108; Geisler et al., 2017: fig. S3) and rib fragments are preserved in CCNHM 164. See Geisler et al. (2017: supporting information) for a description of the holotype ribs. No ribs are inflated or possess pestle-shaped distal ends as in Basilosauridae (Kellogg, 1936; Buffrenil et al., 1990). Fractures in the ribs of the holotype (CCNHM 108) and referred adult (CCNHM 164) possess a dense cortex but porous center, like Coronodon planifrons n. sp. (below), and therefore differ from the pachyosteosclerotic condition reported in Basilosauridae or Mystacodon (Buffrenil et al., 1990; Muizon et al., 2019).
Scapula
A partial right scapula is preserved in CCNHM 164 (Fig. 23), including the distal end and the inferior border. The scapula appears to have been more strongly fan-shaped relative to the anteroposteriorly narrow scapula of most Basilosauridae (and to a lesser extent, Mystacodon), and widens more abruptly immediately proximal to the glenoid fossa. The inferior border seems straight but it is unclear if a posteroventral hook was present. The glenoid fossa is large and oval in shape, measuring 60 mm wide and 80 mm long; the fossa is shallowly concave and bears a slightly pointed supraglenoid tubercle anteriorly, which extends anteroventrally. The broken base of the coracoid process is present, measuring 18 mm in diameter and is circular in shape. Based on the broken cross-section the anterior border of the scapula was transversely thick, about 3 cm just dorsal to the distal end.
Coronodon newtonorum n. sp. LSID urn:lsid:zoobank.org:act:987DE600-70D1-426B-9F6E-4FE477E3387D
Etymology
Newtonorum, after Claude and Albert Newton, who discovered the holotype specimen, and for the generosity of the Newton family for volunteering at Charleston Museum.
Type specimen
ChM PV 2778, partial skeleton including nearly the entire left side of a cranium, three (incisor, p1, and M1), periotic, bulla, nearly complete left mandible, three vertebrae, and one rib, collected October 1978 by Claude and Albert Newton, Albert Sanders, and Peter Coleman from the Chandler Bridge Formation in the vicinity of North Charleston, Charleston County, South Carolina, USA.
Type locality
The holotype specimen of Coronodon newtonorum n sp. was collected from a manmade exposure of the Chandler Bridge Formation in the vicinity of North Charleston, Charleston County, South Carolina, USA. Detailed locality information on file at ChM.
Horizon and age
Chandler Bridge Formation, late Oligocene (24.7–23.5 Ma).
Diagnosis
Coronodon newtonorum n. sp. is a large species of toothed mysticete (estimated BZW = 40 cm, estimated CBL = 80–90 cm). Coronodon newtonorum n. sp. differs from Coronodon havensteini in possessing a concave-up profile of the alveolar margin of the mandible and maxilla, a smaller P2 with lower accessory cusps, shorter roots, and a shorter crown (70% lower relative to anteroposterior crown length), and absence of maxillary embrasure pits posterior to P2; from both Coronodon havensteini and Coronodon planifrons n. sp. in possessing a ventrally convex ventral margin of the mandible, a mandibular condyle elevated far above the m4 alveolus, and possessing a more dorsoventrally inflated preorbital process of the frontal that is deeper than the postorbital process, possessing a gracile periotic with transversely narrow anterior process, short posterior process, a less inflated lateral surface of the body and a lateral tuberosity that is much longer and laterally prominent in medial view, possessing periotic with proportionally larger spiral cribriform tract and crista transversa recessed shallowly within internal acoustic meatus; from Coronodon planifrons n. sp. in having a ventrolaterally sloping supraorbital process of the frontal, possessing periotic with transversely narrow fissure-like endocranial opening of facial canal (shared with C. havensteini), and suprameatal fossa developed as a narrow trough along its entire length.
Ontogenetic status
The holotype specimen of Coronodon newtonorum n sp. possesses a skull similar in size to the adult holotype of Coronodon havensteini (Fig. 8), fully erupted teeth lacking open pulp cavities and possessing mild tooth wear, at least partly closed exoccipital-squamosal and parietal-squamosal sutures, and obliterated vertebral epiphyseal sutures (two thoracics, one caudal). Fracturing has likely resulted in the loss of the parietal rather than antemortem disarticulation of the skull, as the squamosal-exoccipital suture has a similar degree of mortising as the squamosal-parietal suture. In sum, the holotype of Coronodon newtonorum n sp. is best interpreted as an adult (=Class 5 or 6 of Perrin, 1975). The lack of embrasure pits in Coronodon newtonorum n sp. is likely autapomorphic rather than indicating young ontogenetic status (as in referred Coronodon havensteini juveniles ChM PV 4745 and CCNHM 8722) given that the teeth are fully erupted.
Description
Rostrum
ChM PV 2778 is unique amongst specimens of Coronodon spp. in preserving the maxilla in articulation with the frontal, albeit imperfectly (Fig. 33; Table 2). The frontal seems to be tilted so that the medial part is anteroventrally deflected and the rostrum is deflected medially and to the right side; a corrected reconstruction is shown in Fig. 34. A ventral sliver of the left premaxilla is preserved in articulation with the maxilla, and preserves alveoli for somewhat procumbent I1-3; a similar fragment of the right premaxilla is also preserved. The premaxilla is transversely narrow and bears shallow pits on its lateral surface between alveoli for teeth.
Figure 33: Holotype skull (ChM PV 2778) elements of Coronodon newtonorum.
Antorbital notch in dorsolateral view (A), skull in dorsal view (B), premaxillae and fragment of left maxilla in ventral view (C), skull in ventral view (D), left M1 in labial (E), lingual (F) and occlusal (G) view, vomer in ventral view (H), and skull in lateral view (I).Figure 34: Reconstruction of the holotype skulls of Coronodon havensteini, Coronodon planifrons, and Coronodon newtonorum in lateral view; supplementary reconstruction of alternate mandibular tooth count for Coronodon planifrons.
The maxilla has a straight lateral edge in dorsal view (Fig. 33B); in cross-section it is flat dorsally and dorsoventrally thick (~5 cm medially) but thins laterally and becomes dorsolaterally convex in cross section. Anteriorly the entire surface of the maxilla slopes laterally. The antorbital notch is developed as a shallow inclined groove below the antorbital process and presumably transmitted the facial nerve (Fig. 33A). This groove faces anteroventrally and somewhat laterally. A short, but incomplete zygomatic process of the maxilla extends ventral to the preorbital process of the frontal, but does not underlie the orbit as in archaeocetes.
In concert with specimens of Coronodon havensteini, the preserved lacrimal of Coronodon newtonorum n sp. clarifies the morphology of the lacrimal and its articulations with the maxilla and frontal in Coronodon (Fig. 33A). The antorbital process is developed as a steep face anterior to the lacrimal as in all other specimens of Coronodon spp. (partial in CCNHM 8722 and 164), with a thin flange of maxilla buttressing the anterior margin of the lacrimal in ChM PV 2778. In Coronodon havensteini, the lacrimal is missing, the fossa for the lacrimal is well-preserved in ChM PV 4745 and CCNHM 108 (Figs. 3, 7); the fossa is smooth, dorsoventrally shallow, oval, and anteromedially trending. The maxilla-lacrimal suture is clear despite fracturing in ChM PV 2778 (Fig. 33A) and in concert with specimens of Coronodon havensteini, apparently unfused at all ontogenetic stages. Sutures between the frontal and maxilla are not mortised. The lacrimal occupies a gap between the frontal and maxilla, and extends medially for about 6–8 cm; flexion between the rostrum and frontal likely occurred at the frontal-lacrimal joint.
In lateral view, the alveolar margin of the maxilla is slightly convex ventrally (Fig. 33I), conforming to the curvature of the alveolar margin of the mandible (Fig. 34). Ventrally, alveoli are present for C1, P1-4, and M1-3; only the C1-P1 are single rooted. P2 has closely appressed alveoli for the roots; they are slightly more widely separated in P3, and widely separated in P4-M3. Large diastemata are present between C1-P2; small (~1 cm) diastemata are present between P2-4 (Fig. 33D). P2 and P3 are aligned parallel with the maxillary edge, but all alveoli posterior to this (P4-M3) are rotated with the mesial root alveolus shifted labially and the distal root alveolus shifted lingually so that the mesial edges of the teeth are oriented slightly anteromedially (rather than anteriorly or even anterolaterally to be parallel with the maxillary edge). Further, the overlapping of the alveoli indicates that these teeth would have overlapped with the distal root lying anterolabial to the mesial root lobe of the tooth immediately posterior to it, with posterolaterally oriented interdental slots like the mandibular cheek teeth of Coronodon havensteini. Accessory alveoli for ‘demi-roots’ are not present, unlike Coronodon havensteini.
Embrasure pits are present between C1 and P1 and between P1 and P2, but there is only a shallow embrasure pit posteromedial to P2. Other pits present further posteriorly in Coronodon havensteini (CCNHM 108), such as those medial to the M1 and M2, are not obviously developed in ChM PV 2778 (Fig. 33D). The palate is similar to Coronodon havensteini but appears less excavated medial to the molars; like the holotype of Coronodon havensteini, there appears to have been a broad ventral triangular exposure of the vomer posteriorly. A deeply excavated and medially convex greater palatine sulcus is developed along the medial edge of the maxilla. The lateral edge of the maxilla descends ventrally to form a vertical lip along the labial edge of the teeth.
Frontal
The frontal is similar to Coronodon havensteini and Coronodon planifrons n. sp. in dorsal and ventral view (Figs. 33B, 33D) and shares a similar articulation with the rostral elements. In lateral view, the preorbital process is massive and dorsoventrally thick, and deeper than the postorbital process; this differs from the condition in Coronodon havensteini, where the pre- and postorbital processes are equivalent in depth, and from Coronodon planifrons n. sp. where the postorbital process is deeper. The postorbital process has a rectangular outline in lateral view (Fig. 33I). The preorbital process shares a concavo-convex ball joint with the lacrimal (Fig. 33A). The orbitotemporal crest was positioned posterodorsally with a subvertical posterior surface, like in the Coronodon havensteini holotype (CCNHM 108), though it notably overhangs the temporal fossa more than in CCNHM 108.
Squamosal
The left squamosal is well preserved (Figs. 33B, 33D, 33I), and does not differ much from Coronodon havensteini, but has a lower squamosal prominence and a dorsoventrally deeper postglenoid process. The lateral edge of the zygomatic process is more convex in dorsal and ventral view. The squamosal prominence is positioned further anteromedially than in Coronodon havensteini. The squamosal-parietal suture bears a sharp anterolateral corner in dorsal view (Fig. 33B), so that the anteriormost part of the suture jogs laterally; in Coronodon havensteini this forms a smoothly convex arc.
Periotic
The periotic (Fig. 35; Table 3) is well preserved and similar in size and anteroposterior length to Coronodon havensteini and Coronodon planifrons n. sp., but differs chiefly in being much more gracile in overall proportions and most closely resembles the periotics of juvenile Coronodon havensteini (ChM PV 4745, CCNHM 8722; Figs. 19A–19D). The anterior process is transversely narrow and the body of the periotic is not laterally inflated (Fig. 35B); the distance between the fenestra ovalis and the lateral margin is close to the transverse width of the pars cochlearis (150%), whereas it is slightly thicker (approximately 170% of the pars cochlearis width) in Coronodon havensteini and Coronodon planifrons n. sp. The anterior process is dorsoventrally shallower than other species of Coronodon, with a flat medial margin in ventral view as opposed to the slightly convex margin in other Coronodon spp. (Figs. 35C and 35D). The angle between the anterior process and pars cochlearis in ventral view is 152° (Fig. 35B), similar to juveniles of Coronodon havensteini (143°–145°). The suprameatal fossa is transversely narrow and the superior process is so reduced that the medial wall of the fossa is visible in lateral view.
Figure 35: Holotype right periotic (ChM PV 2778) of Coronodon newtonorum.
Periotic in dorsal (A), ventral (B), lateral (C), and medial (D) view.The mallear fossa is proportionally large and circular. The lateral tuberosity has a sharp transverse crest along the ventral surface and the tuberosity protrudes far beyond the lateral margin of the periotic body, a result of the lack of inflation of the periotic body (Figs. 35A and 35B). Even so, the lateral tuberosity is large as in Coronodon planifrons n. sp., differing strongly from the tubercle-like tuberosity in Coronodon havensteini; large, triangular lateral tuberosities are present in other early diverging Neoceti like Eomysticetidae (Boessenecker & Fordyce, 2015a, 2015b) and odontocetes including Xenorophidae (Geisler, Colbert & Carew, 2014), and the simocetid-like Olympicetus (Racicot et al., 2019), but absent in Basilosauridae and most toothed mysticetes (Mammalodontidae, Aetiocetidae; Fitzgerald, 2010; Marx & Fordyce, 2015). The internal acoustic meatus is distinctive (Fig. 22D); the spiral cribriform tract and facial canal are not aligned as in other Coronodon spp. Instead, the spiral cribriform tract is anterolaterally divergent and forms an obtuse angle medially with the opening of the facial canal.
The posterior process is short, equidimensional, and leaf-shaped (Fig. 35B), similar to juvenile Coronodon havensteini specimen ChM PV 4745 (Fig. 19C); it is approximately as long as the pars cochlearis, whereas it is 150% of pars cochlearis length in the Coronodon havensteini holotype. The posterior bullar facet is more deeply grooved than in the holotype of Coronodon havensteini. The posterior process does not widen posteriorly and lacks the spurs on the posterior margin characteristic of the Coronodon havensteini holotype.
Tympanic bulla
The tympanic bulla (Fig. 36; Table 4) is very similar to Coronodon havensteini and approximately the same size as the adult holotype (CCNHM 108). The bulla differs from Coronodon havensteini in possessing a more deeply excavated concavity on the medial margin of the involucrum in dorsal view (Fig. 36C); in medial view, the involucrum has a more even dorsal margin with a less prominent step (Fig. 36A). Further, in medial view the ventral edge of the involucrum is evenly convex (Fig. 36A) whereas in Coronodon havensteini the margin is straight. The sigmoid process is imperfectly reassembled in the Coronodon havensteini holotype, and ChM PV 2778 clarifies the morphology in Coronodon. The sigmoid process is erect and canted about 20 degrees posterolaterally from the transverse plane, and the tip of the sigmoid is elevated above the level of the inner posterior pedicle (Figs. 36A–36C). This suggests that the unusual position in the Coronodon havensteini holotype is caused by improper gluing, and that the sigmoid process has been artificially rotated dorsally and medially. The conical process is dorsoventrally deep and hemispherical. The medial half of the ventral surface of the bulla is strongly punctate (Fig. 36D), more extremely so than specimens of Coronodon havensteini of any ontogenetic stage (Fig. 23). Like the posterior process of the periotic, the posterior process of the bulla is small, short, and bears a leaf-shaped articular facet (Figs. 36E and 36F).
Figure 36: Holotype tympanic bulla (ChM PV 2778) of Coronodon newtonorum.
Bulla in medial (A), lateral (B), dorsal (C), and ventral (D) view, posterior process of bulla in dorsal (E) and ventral (F) view.Dentition
An isolated incisiform tooth, left p1, and left M1 are preserved in ChM PV 2778 (Figs. 33, 37). The incisiform tooth has a bioeroded, straight root with an oval-cross section, and the crown is only minimally recurved, suggesting a somewhat procumbent tooth perhaps corresponding to the i1 or i2. The small size of the crown resembles incisiform teeth in Coronodon havensteini referred adult specimen CCNHM 164 (Fig. 26). The p1 is similar to that of the Coronodon havensteini holotype but the tooth is slightly smaller and has an apicobasally shorter crown (70% of crown height in CCNHM 108), smaller mesial and distal denticles, and an apicobasally shorter isthmus between the roots, with the roots being slightly more split in ChM PV 2778 (Figs. 37H–37I).
Figure 37: Mandible, dentition, and postcrania of holotype specimen (ChM PV 2778) of Coronodon newtonorum.
Left M1 in labial (A) and lingual (B) view, left mandible in lateral (C), dorsal (D), and medial (E) view, upper left I1 in lingual labial (F) and lingual (G) view, lower left p2 in labial (H) and lingual (I) view; mid-thoracic vertebra in anterior (J) and posterior (K) view; posteriormost thoracic (T9) or anterior lumbar (L1) vertebra in anterior (L) and posterior (M) view; caudal vertebra (Ca 5, 6, or 7) in anterior (N) and posterior (O) view.The M1 is not preserved in the Coronodon havensteini holotype but this position is preserved in ChM PV 2778 and CCNHM 164. The M1 of Coronodon newtonorum n. sp. compares well with the M1 of Coronodon planifrons n. sp. though ithe M1 is higher crowned than in Coronodon planifrons n. sp. (CCNHM 166), and the base of the enamel is more dorsally arched in C. havensteini. The M1 of ChM PV 2778 differs from the M1 of CCNHM 164 in possessing five rather than four distal denticles (Figs. 33E–33G, 36A and 36B); the M2 of CCNHM 108 also has five but the basal denticle is minute. Five distal denticles are present in CCNHM 166. The M1 of ChM PV 2778 possesses four mesial denticles (Figs. 33E–33G, 37A and 37B), unlike the M1 of CCNHM 166 (five mesial denticles) and the M2 of CCNHM 108.
Mandible
The partial left mandible (Fig. 37; Table 6) is missing the angular process, ventral half of the “pan bone” and lateral wall adjacent to the molars. Otherwise, aside from some Osedax bioerosion, the mandible is well-preserved. The mandible is notable for possessing alveoli for eight (p1-m4) rather than seven postcanine teeth (Fig. 37D) as originally identified in Coronodon havensteini (see Revised Tooth Count in Coronodon and Implications for Polydonty in Neoceti, below). The mandible has a similarly shaped coronoid process to Coronodon havensteini, but the mandibular condyle is elevated far above the m4 alveolus (Fig. 37C); in Coronodon havensteini and Coronodon planifrons n. sp., the condyle is at the level of the m4 alveolus. The fracture at the base of the coronoid process in the Coronodon newtonorum n sp. holotype is tight and this is not a result of improper alignment of fragments. The ventral margin is also convex (Figs. 37C, 37E), whereas it is straight in both Coronodon havensteini and Coronodon planifrons n. sp. The mandible of Coronodon newtonorum n. sp. further differs from Coronodon havensteini in possessing a more dorsoventrally tapered anterior tip. The mandibular foramen is partly preserved, and its anterior margin seems to have been entirely posterior to the coronoid apex (Fig. 37E). The coronoid process is subtriangular and separated from the condyle by an 92 mm long neck (Figs. 37C and 37D). Like Coronodon havensteini, the mandibular symphysis is unfused and the symphyseal surface is smooth and flat to slightly undulatory; it is anteroposteriorly short, extending only to the level of the C1 (Fig. 37E). A short, shallow symphyseal furrow is present ventrally, and forms a posterior embayment in the edge of the symphyseal surface, resembling the intramandibular joint in Coronodon havensteini.
Postcrania
Three vertebrae and one rib are preserved in ChM PV 2778 (Fig. 37). The vertebrae include a mid thoracic (Figs. 37J and 37K), a vertebra representing the last thoracic (T9, Figs. 37L and 37M) or first lumbar (L1), and a mid-caudal vertebra (Figs. 37N and 37O), likely Ca5, 6, or 7 (based on comparison with CCNHM 164 and 166). The mid-thoracic vertebra has a deep centrum with a flat dorsal edge, a capitular facet on the dorsolateral edge of the anterior (but not posterior) epiphysis, a robust pedicle, and a swollen transverse process. The T9/L1 has a dorsoventrally shallow and small (compared to posterior lumbars in CCNHM 166) centrum with a truncated dorsal margin (more closely resembling the T9 rather than L1 of CCNHM 166 in this regard), and a dorsoventrally deep, horizontal, and dorsally positioned transverse process. The mid-caudal vertebra is large with a circular anterior epiphysis and slightly transversely narrowed posterior epiphysis, a low neural arch with a small and low neural spine, small, short, and ventrally deflected transverse processes, and large subtriangular haemal facets anteriorly and posteriorly.
Coronodon planifrons n. sp. LSID urn:lsid:zoobank.org:act:445DF386-7800-453F-ACA7-3D3EE8143149
Etymology
Planifrons, after the Latin planus + frons, meaning flat forehead, referring to the horizontal supraorbital processes of the frontal in anterior view.
Type specimen
CCNHM 166, partial skeleton including rostrum fragments, braincase, left and right periotics, 24 teeth, left mandible, four cervical vertebrae, seven thoracic vertebrae, ten lumbar vertebrae, 11 caudal vertebrae, and at least 13 ribs, discovered and collected by Jeremmiah Volcko, Taffie Chapman, and Mark Havenstein, November 2010 from an exposure of the Chandler Bridge Formation in the vicinity of North Charleston, Dorchester County, South Carolina.
Referred specimen
CCNHM 8732, an isolated upper right M3, collected by an unknown collector from the vicinity of Summerville, South Carolina, USA.
Type locality
The type specimen of Coronodon planifrons n. sp. was collected from an exposure of the Chandler Bridge Formation in a drainage ditch in North Charleston, Dorchester County, South Carolina, USA. More detailed locality records available on file at CCNHM.
Horizon and age
Chandler Bridge Formation, likely, but uncertainly from Bed 2, late Oligocene (24.7–23.5 Ma).
Diagnosis
Coronodon planifrons n. sp. is a large toothed mysticete (BZW = 460 mm) differing from Coronodon havensteini and Coronodon newtonorum n. sp. in possessing a horizontal (rather than ventrolaterally sloping) supraorbital process of the frontal (sloping 4°, v. 14°–15° in C. havensteini and 20°–25° in C. newtonorum); possessing a dorsoventrally deep postorbital process that is deeper than the preorbital process (9.5% of postorbital width v. 5% in C. havensteini); possessing a crescentic dorsal extension of the sternomastoid fossa, nearly to the posterior apex of the nuchal crest (fossa in C. havensteini is approximately 1/3 this length); frontals penetrating further posteriorly into the intertemporal region, 13% of postorbital width v. 8% in C. havensteini; zygomatic process dorsoventrally deeper than in C. havensteini, relative to height at vertex of cranium (not possible to evaluate in C. newtonorum n. sp); medially excavated and undercut basioccipital crest that protrudes further ventrally than C. havensteini; longer intertemporal region (distance from anteriormost point of orbitotemporal crest to supraoccipital apex 54% of postorbital width v. 34–36% in C. havensteini); more deeply excavated dorsal condyloid fossae;; lower m3 has six rather than five mesial denticles as in C. havensteini; upper M2 slightly smaller and lower crowned than in C. havensteini; upper M3 dramatically smaller than M2. Coronodon planifrons n.sp. further differs from Coronodon newtonorum n. sp. in possessing a straight ventral margin of the mandible, a mandibular condyle not elevated above the toothrow, and absolutely larger teeth and periotic. Coronodon planifrons further differs from Coronodon havensteini in possessing a larger lateral tuberosity of the periotic, with rectangular outline, and projecting beyond lateral margin of periotic (shared with Coronodon newtonorum n. sp.).
Ontogenetic status
The holotype specimen of Coronodon planifrons is similar in bizygomatic skull width to the holotype of Coronodon havensteini, and additionally possesses fully erupted teeth with closed pulp cavities and severe tooth wear, tall sagittal and nuchal crests, closed parietal-occipital sutures, and epiphyseal fusion throughout the vertebral column. This combination indicates that this specimen is an adult, equivalent to class 5 or 6 of Perrin (1975).
Description
General Remarks on Skull
The holotype (CCNHM 166) preserves a partial, somewhat fractured braincase (Fig. 38; Table 2), right posterior premaxilla, right nasal, fragments of the maxilla, left and right periotics, and numerous teeth. This description will emphasize features differing from Coronodon havensteini and Coronodon newtonorum n. sp.
Figure 38: Holotype skull (CCNHM 166) of Coronodon planifrons.
Right nasal in lateral (A) and medial (B) view, right premaxilla in lateral (C), dorsal (D), and medial (E) view; skull in dorsal (F), ventral (G) and lateral (H) view.Premaxilla
The premaxilla is disarticulated from the maxilla (Fig. 38), permitting description of morphology obscured in the holotype of Coronodon havensteini. It is mostly damaged, but the posterior half of the right premaxilla is well preserved and isolated; it is rod-like, somewhat dorsoventrally deeper than wide, and the dorsal surface steeply slopes medially into the bony nares. The dorsal surface is flattened to slightly convex, becoming more horizontal anterior to the nares. In dorsal view, the entire element is laterally bowed around the nares. There is a longitudinal groove along the ventromedial margin of the premaxilla, likely for a simple premaxilla-vomer articulation.
Several vascular channels ascend from a common sulcus on the lateral side of the bony nares, curving posterodorsally towards the nasal. Four or more grooves and at least three ridges form a deeply mortised but unfused and open frontal-premaxilla articulation; this articular surface is at least 10 cm long and faces dorsomedially. The nasal-premaxilla articulation is similar, at least 9 cm long, 15 mm wide, but bears a deep dorsomedially-facing trough with discontinuous ridges and grooves recessed within.
The ventral margin of the rod-like middle part of the premaxilla is rounded and convex in cross-section. The lateral surface is somewhat flat, smooth, and bears a shallow discontinuous trough; this trough receives the medial edge of the maxilla and, posteriorly, the ascending process of the maxilla. Just lateral to the nasals, the trough bears a median longitudinal ridge measuring 45 × 8 mm. Aside from this, the entire premaxilla-maxilla articulation is developed as a slightly undulating butt joint.
Maxilla
Fragments of the maxilla are preserved (Fig. 38) but are too incomplete to make any meaningful comparisons with Coronodon newtonorum n.sp. or Coronodon havensteini.
Nasal
The right nasal is preserved and similar to the Coronodon havensteini holotype in being anterodorsally flaring, rectangular in dorsal view, and shallowing towards a flat posterior end (Fig. 38). Scattered diploic foramina are present on the posterior half; these are about 1–1.5 mm in diameter and bear short posteriorly directed sulci. The medial surface bears deep longitudinal grooves for the internasal suture—five ridges and grooves, anteroventrally directed, and towards the anterior tip this surface gives way to a flat articular surface without grooves.
The nasal is dorsally sloped laterally with a median ridge, which becomes more prominent anteriorly and gives the nasal a triangular cross section. The ventral surface bears a prominent ventromedial ridge to articulate with the groove on the medial side of the prenarial process of the frontal; the lateral edge of the nasal instead overlaps onto the premaxilla to articulate with its posterodorsal surface. The anteromedial face of the nasal has an anterodorsally curving, anteriorly widening trough for the nasal passage, and appears to have been vertical like the holotype of Coronodon havensteini.
Frontal
Most of the frontal forms the somewhat rectangular supraorbital process; the supraorbital process is approximately horizontal (Figs. 38 and 39; Tables 1 and 2). The frontal bears a narrowly triangular prenarial process to articulate with the premaxilla and nasal; the prenarial process is dorsoventrally deeper than the supraorbital process and bears longitudinal ridges and grooves. Lateral to this the frontal is dorsoventrally thin and excavated into a shallow fossa to receive the ascending process of the maxilla. The preserved parts of this fossa are smooth and lack the deep parallel ridges and grooves that characterize the frontal-premaxilla and frontal-nasal articulations. Based on changes in bone texture and the edges of this fossa, the ascending process of the maxilla was subtriangular with a convex posterolateral margin and covering a region of the frontal approximately 50 mm wide and 50 mm long. The prenarial process is laterally undercut by a groove for the medial edge of the ascending process of the maxilla.
Figure 39: Holotype skull (CCNHM 166) of Coronodon planifrons.
(A) Skull in anterior view, (B) skull in posterior view.The frontonasal sutures occupy approximately ¾ of the anteroposterior length of the supraorbital process (not including the prenarial process) and transition into a slightly rugose bone texture (Fig. 38). This zone of rough texture forms a 90 mm wide parabolic ‘halo’ surrounding the articular grooves for the nasal and premaxilla and terminating nearly at the posterior margin of the supraorbital process. Diploic foramina are present dorsally in two sets; the first are posterolaterally opening, radially oriented, 1 mm wide foramina with 5–40 mm long sulci positioned on the medial 2/3 of the frontal, and the second are a cluster of larger 1–2 mm wide foramina lateral to the posterior tip of the nasals and 40 mm from the midline. There is a fissure at the median frontal suture, suggesting a persistent unfused suture into adulthood, but is more likely the result of breakage during collecting along a zone of weakness (as in Coronodon havensteini, CCNHM 164). The postorbital process is triangular, posterolaterally flaring, and tapers to a point. It is rectangular in lateral view and dorsoventrally thick; the orbit is moderately concave in lateral view. The frontoparietal suture is deeply V-shaped with the frontals penetrating 55 mm posterior to the anterior margin of the temporal fossa. In lateral view, the frontoparietal suture descends posteroventrally.
The orbitotemporal crest forms the posterior edge of the supraorbital process of the frontal, which has a concave posterior margin (Fig. 38). The postorbital process extends far posterolaterally to the anteromedial margin of the temporal fossa. The orbitotemporal crest slightly overhangs the postorbital ridge medially, and the posterior surface of the frontal is approximately vertical, intermediate between the condition in basilosaurids and chaeomysticetes. This surface is concave and slightly excavated and bears a single large laterally opening foramen on the posteromedial surface.
The frontal groove is laterally shallow and triangular in ventral view, rapidly narrowing medially; it bears laterally opening diploic foramina within. The optic canal is posteriorly placed within the supraorbital process and shallow, curving posteromedially (Fig. 38G). Anterior to the optic canal and medial to the preorbital process is a shallow fossa of uncertain homology, and not clearly associated with the maxilla. One large diploic foramen is present at the boundary between the optic canal and this fossa.
Intertemporal constriction, parietal, and vertex
The intertemporal constriction is dorsoventrally deep and transversely narrow but is broken ventrally; the preserved part is acutely triangular in cross-section and narrows dorsally (Fig. 38; Table 2). The sagittal crest is sharp along most of its length and is proportionally longer than in Coronodon havensteini. A single dorsally arched, roughly horizontal to posteroventrally trending sulcus emanates posteriorly from the broken region of the frontoparietal suture and onto the parietals, approximately 4 cm long on right and 7 cm long on left.
Like the Coronodon havensteini holotype, the vertex is at the level of the posterior third of the temporal fossa; though the nuchal crests are broken, the supraoccipital apex was triangular. Breakage artificially makes the occipital shield appear more triangular than it likely was when complete. In dorsal view the anterolateral 75% of the nuchal crest is composed of parietal and the posterior 25% is composed of the occipital. A faint external occipital crest is developed on the dorsal third of the occipital shield (Fig. 39B).
Squamosal
The apex of the zygomatic is more completely preserved in CCNHM 166 (Fig. 38) than in the Coronodon havensteini holotype (Figs. 5–7, 15), and in lateral view it deepens dorsoventrally at mid-length; anterior to this it tapers abruptly into an acutely triangular apex, giving the process an overall ‘spindle’ shape. In dorsal view, the apex curves slightly anteromedially; ventrally there is a poorly defined facet for the jugal along the anterior 30 mm of the zygomatic. The zygomatic process is composed of highly cancellous bone and is likely damaged in all other known specimens of Coronodon spp., including the otherwise well-preserved juvenile ChM PV 4745 and holotype (CCNHM 108) of Coronodon havensteini.
The squamosal prominence is developed as a transversely thickened and blunt knob on the supramastoid crest (Fig. 38F); it is medially situated, emarginates the squamosal fossa in dorsal view, and is dorsally adjacent to the sternomastoid fossa. The sternomastoid fossa is large and rectangular to crescentic in shape, faces posterolaterally, and is approximately 90 mm deep and 80 mm wide. The fossa has a concave posterior margin where it is emarginated by the exoccipital. The fossa is smooth anteriorly but deeply pitted close to the exoccipital; dorsomedially the fossa ascends as a trough along the lateral edge of the nuchal crest, differentiating it from Coronodon havensteini (Fig. 15). Ventrally the fossa continues onto the lateral surface of the posterior meatal crest.
The postglenoid process is tongue-shaped and transversely narrow, and laterally is anteroposteriorly thickened at its ventral apex (Fig. 38H). The anterior meatal crest is short but sharp and leads to the broken base of the spiny process, which bears a pit for the sigmoid process of the bulla. The glenoid fossa is developed as a pair of shallow fossae separated by a low convexity; the lateral fossa bears a cluster of vascular foramina, and the medial fossa bears cancellous bone. The medial fossa is bordered by a sharp ridge that transitions anteriorly into the falciform process.
The periotic fossa is solid, smooth, and transversely bowl-shaped—but developed as an anteroposteriorly oriented trough with a slight reniform outline, being medially concave and conforming to the shape of the lateral surface of the periotic (Fig. 38G). A low tubercle is present on the dorsal side of the periotic fossa, corresponding to a gap on the superior process of the periotic between the anterodorsal and posterodorsal angles. Dorsal to this, the medial wall of the squamosal is flat with faint dorsoventrally oriented striations of presumed vascular origin, perhaps corresponding to a rete.
As in Coronodon havensteini, there is a gap between the anterior process of the periotic and squamosal, and the periotic only seems to tightly articulate with the periotic fossa posteriorly and at the lateral tuberosity. The periotic fossa is divided into three cavities by a transverse ridges; the anterior ridge separates the articular surface for the anterior process from that of the body of the periotic. The posterior ridge is present at the level of the spiny process and separates the middle cavity for the body of the periotic from a posterior cavity for the posterior process of the periotic. The anterior ridge is low in juveniles (CCNHM 8722, ChM PV 4745) and the holotype (CCNHM 108) and high in old adult CCNHM 164. The division of the posterior process is much larger in adult specimens (CCNHM 108, 164). The skull is amastoid, and the posttympanic ridge is formed from cancellous bone and separates the posterior process of the periotic from the lateral edge of the skull by approximately 35 mm. The posttympanic ridge abuts the truncated margin of the posterior process of the periotic; on the left side, the posttympanic ridge is partly fused to the pathological posterior process of the periotic.
Exoccipital and basioccipital
The exoccipital is anteroposteriorly thick ventrally and shares a closed suture with the squamosal (Fig. 38). Laterally both bones are composed of porous, cancellous bone. The paroccipital process bears a circular to oval paroccipital concavity; it is deeper and circular on the right side, and shallow and oval on the left. The paroccipital concavity is posterior to the medial edge of the posterior process of the periotic. Medially, the anterior face of the exoccipital is smooth and bears a trough for the jugular notch.
Posteriorly the exoccipital is dorsoventrally low and projects ventrolaterally; the posterior surface is smooth. The occipital condyles are set out on a short neck, projecting somewhat further than in Coronodon havensteini; each is nearly rectangular, perhaps a consequence of incompleteness. The foramen magnum is dorsoventrally deep, transversely narrow, and oval shaped. Deep dorsal condyloid fossae are preserved on the right side and the ventral condyloid fossa is deeper, and positioned lateral to the ventral third of the condyle.
The basioccipital crest is large, transversely wide, and composed of cancellous bone. The medial side is slightly more excavated than in the holotype of Coronodon havensteini. There is a continuous sharp crest along the ventral margin, which is anteriorly contiguous with the pharyngeal crest. The medial part of the basioccipital is ventrally smooth; a long sulcus separates the crest from the medial trough for the vomer. The posteromedial part of the right basioccipital crest edge forms a spur; this is instead rounded on the left side.
Periotic
The periotic of Coronodon planifrons n. sp. (Fig. 40; Table 3) differs from Coronodon havensteini in possessing a more transversely inflated anterior process with a more elongated anterodorsal angle, a better defined anterior bullar facet, a flatter posterior bullar facet, a more shallowly excavated suprameatal fossa, a roofed over hiatus fallopii, and a larger, longer lateral tuberosity that projects beyond the lateral margin of the body.
Figure 40: Holotype periotics and bulla (CCNHM 166) of Coronodon planifrons.
Left and right periotic in ventral (A, B) views, dorsal (C, D) views, medial (E, F) views, and lateral (G, H) views. Posterior process of right tympanic bulla in dorsal (I) and ventral (J) views.The anterior process is grossly inflated transversely, as is the body, so that there is a deep crease separating the anterior process and lateral tuberosity. A lozenge-shaped tubercle is present anteromedial to the pars cochlearis on the right but not the left periotic. The tubercle may be homologous to the incisural flange (sensu Boessenecker & Fordyce, 2015b) and is demarcated by a short anterointernal sulcus that bifurcates closer to the anteroventral angle. The anterior incisure is a deep groove between the pars cochlearis and anterior process; more broadly, the angle (in ventral view) between the anterior process and pars cochlearis at the incisure is 173° in the left periotic and 179° in the right periotic.
The lateral tuberosity is large (relative to C. havensteini and other toothed mysticetes) and bears a pointed tip and a chisel-shaped apex in ventral view; there is a prominent continuous ridge forming the anterior margin of the mallear fossa that is laterally contiguous with the posterior edge of the lateral tuberosity. In adult specimens of C. havensteini, the lateral edge of the periotic body extends beyond the lateral tuberosity in ventral view. In the non-inflated bodies of juvenile periotics of C. havensteini (CCNHM 8722, ChM PV 4745) and the gracile adult holotype of C. newtonorum n. sp., the lateral tuberosity projects beyond the lateral edge of the body. In C. planifrons n. sp., the lateral tuberosity projects beyond the lateral tuberosity despite similar transverse inflation of the periotic body late in ontogeny like C. havensteini (CCNHM 108, 164). Anterior to the mallear fossa is a small broken nodule of bone where the accessory ossicle of the tympanic bulla was partially fused to the anterior process of the periotic and broken; the fracture is clearer in the right periotic. This structure is worn in the holotype of Coronodon havensteini, but appears to have been partly fused as well.
The endocranial opening of the facial canal is partly subdivided, forming a hiatus fallopii in the right periotic; in the left periotic, this structure is instead developed into a long fissure as in the C. havensteini holotype. The aperture for the cochlear aqueduct is elevated further dorsally (and further separated from the fenestra rotunda) than in C. havensteini. In the C. havensteini holotype, the suprameatal fossa has a deep anteroposterior trough within its posterior half; this is absent in C. planifrons n. sp., and instead the suprameatal fossa is bowl-shaped. In C. havensteini this trough bifurcates the posterodorsal angle, forming a more medial tuberosity referred to as the pyramidal process sensu stricto by Marx & Fordyce (2015). This sulcus is absent in C. planifrons n sp. (and C. newtonorum n. sp.) and the pyramidal process and the posterodorsal angle are essentially the same structure.
The lateral surface of the periotic is more rugose and cancellous than in the C. havensteini holotype and C. newtonorum n. sp. Posterolaterally, paired rugose tubercles are developed anteroventrally to the posteroexternal foramen. The posteroexternal foramen is actually a cluster of three foramina just lateral to the stylomastoid fossa, like the C. havensteini and C. newtonorum holotypes; in juvenile Coronodon havensteini (ChM PV 4745, CCNHM 8722) as well as unnamed coronodonid ChM PV 5720, there is a single foramen, like other stem mysticetes.
The posterior process has a flatter and less transversely convex posterior bullar facet compared to C. havensteini; the medial half is partly concave. The posterior process is also dorsoventrally thicker in C. planifrons n.sp. The posterior process lacks the conspicuous posterior spurs on the posterior margin, as in the periotic of the Coronodon havensteini holotype, instead having a smooth subrectangular margin as in C. newtonorum n. sp. and C. havensteini juvenile specimen ChM PV 4745. Unlike the C. havensteini holotype, there is a groove separating the epitympanic hiatus from the posterior process.
The left periotic has a fused posterior process of the bulla, periotic, and posttympanic ridge of the squamosal. The combined process is massively inflated, bluntly conical in shape, and appears to be composed nearly entirely of cancellous bone. Owing to this unusual bone texture (typically dense bone in most cetaceans except for some extant mysticetes) and lack of fusion of the posterior processes in non-chaeomysticetes, this fusion is best interpreted as a pathology. A thin bulla-periotic suture is visible just dorsal to the facial sulcus, but is difficult to trace on the cancellous external (posterior) surface of the compound process. Articulation of the left periotic with the skull is difficult, and breakage suggests that the compound posterior process was fused anterolaterally with the posttympanic ridge.
Tympanic bulla
The posterior process of the right tympanic bulla is isolated and well preserved (Figs. 40I and 40J). It is quite dense with some cancellous bone developed laterally, and is triangular in shape with a flat articular facet and transversely convex ventral surface. The ventral surface bears fine sulci but is otherwise smooth; the articular facet for the periotic bears shallow longitudinal grooves, corresponding to the ridges on the posterior bullar facet of the periotic. Near the posterior pedicle there is a broken oval-shaped ridge that is excavated by a fossa, dividing it into the outer and inner posterior pedicles; the fossa represents an excavation by part of the peribullary sinus.
Dentition
The teeth of Coronodon planifrons (CCNHM 166, and referred molar CCNHM 8732) have no cingula and all have carinae, just like C. newtonorum and C. havensteini (Fig. 41; Table 5). The carinae are virtually identical in size and have a similar effect on denticle shape and notch formation as seen in C. newtonorum and C. havensteini. Likewise, the depressions on the crown base between denticles form shallow troughs, as is seen in C. newtonorum and C. havensteini. The enamel is similarly thin (ranging from 0.2 to 0.4 mm in thickness, measured with a digital caliper from broken edges of various teeth) in C. planifrons n. sp. and, like C. havensteini, is covered in undulating oblong bumps and depressions less than a millimeter in size (this enamel texture will be described further in a forthcoming study of enamel in coronodonids). Root morphology of C. planifrons n. sp. is also essentially identical to C. newtonorum and C. havensteini, the mesial root is thicker, straighter, and more vertically oriented than the distal root of the same tooth. The total dental formula known from all of these specimens is 3.1.4.3/3.1.4.4.
Figure 41: Holotype dentition (CCNHM 166) of Coronodon planifrons.
Abbreviations: li, lingual; la, labial.Caniniform teeth
The caniniform teeth of CCNHM 166 all have a carina and very shallow apicobasal ridges and grooves along the surface, just like in C. newtonorum and C. havensteini. Like C. newtonorum and C. havensteini, the crowns of these teeth have a greater apicobasal height than their mesiodistal length. But the mesiodistal lengths of the caniniform teeth of CCNHM 166 are greater than that of C. havensteini (CCNHM 164).
Upper dentition
The upper left third premolar (CCNHM 166.45) is partly broken, missing most of the distal denticles besides the one adjacent to the central cusp. This first distal denticle is almost as large as the central cusp, and is slightly larger than the first mesial denticle. The mesial denticle row retains four large denticles and a fifth that is negligible in size and lacks a point. Though the distal denticle row is mostly missing and therefore not comparable, the mesial denticle row exhibits a similar straight and steeply sloped arch as seen in other premolars of Coronodon spp.
Both left (CCNHM 166.29) and right (CCNHM 166.48) upper fourth premolars are preserved. The right P4 (CCNHM 166.48) lacks the distal denticle row, but the left P4 (CCNHM 166.29) retains all of the denticles, including four mesial and four distal denticles. The distal denticles are slightly larger than their respective counterparts on the mesial denticle row, though the two denticle rows themselves appear to be similarly arched and equally sloped.
Both left (CCNHM 166.49) and right (CCNHM 166.34) upper first molars are preserved. The left M1 (CCNHM 166.49) has all of its cusps preserved, but the right M1 (CCNHM 166.34) is missing its mesial denticle row completely. The mesial denticles are mostly equal in size to their respective distal denticles, with five on each denticle row. This is unlike the M1 of C. newtonorum, which has only four mesial and five distal denticles (Fig. 33). Of the denticles of CCNHM 166.34, the distalmost and mesialmost denticles are small and lack a point; they are borderline denticles but have the pinched edge of their adjacent denticle (like those described above for other Coronodon teeth). The mesial and distal denticle rows are both similarly arched, yet the mesial denticle row appears to extend more basally than the distal denticle row. Even though the mesial denticles are missing on the right M1, the crown base is preserved on the labial side, indicating that it, too, had a more basally-extended mesial side.
The second upper molar is represented by CCNHM 166.50, which exhibits an extreme amount of wear and damage to the lingual side of the mesial denticles and central cusp. This makes it challenging, but not impossible, to recognize its four mesial and four distal denticles. The four mesial denticles are of similar size to their respective distal denticles, but the mesial denticle row is more steeply sloped and straighter than the more arched and shallowly sloped distal denticle row. The mesial denticle row extends further basally than the distal denticle row does, as in the first molar.
CCNHM 166.51 is a right upper third molar that has partial damage to its central cusp and the mesial denticle just adjacent to it. It has three mesial denticles and four distal denticles. The central cusp is broken, but from what remains of it, it was most likely slightly larger than its adjacent denticles, like in other cheek teeth. The mesialmost denticle is also broken, so it is unclear whether it was of similar size or smaller than the first distal denticle. The second and third mesial denticles are smaller than their respective distal denticles, hinting that the first mesial denticle was probably smaller than the first distal denticle as well. Both denticle rows appear to be similarly arched and sloped. CCNHM 8732 is an isolated right upper third molar that is missing much of the root and mesial cusps but preserves a complete, low principal cusp slightly larger in size to the apicalmost distal accessory cusp; there are a total of three preserved distal cusps, and a fourth may have been present.
Lower dentition
The second premolar, possibly a lower (CCNHM 166.44), has a large central cusp and one mesial denticle preserved. There may have been a distal denticle or an additional mesial denticle, but the specimen is incomplete. The mesial denticle is curved toward the central cusp and is 1/3 the size of it, and are similar in overall proportions to the p2 in C. havensteini (CCNHM 164 and CCNHM 108) and C. newtonorum n. sp., in which the first distal denticle is approximately half the size of the central cusp. The mesial denticle of the first premolar of the holotype of C. havensteini is much smaller than in the second premolar, approximately 1/4 or 1/5 the size of the central cusp.
CCNHM 166.47 (left) and CCNHM 166.32 (right) are lower third premolars. CCNHM 166.47 has only three cusps preserved: the central cusp and the mesial and distal denticles on either side of it. CCNHM 166.32 has one mesial denticle preserved (although there were certainly more), a central cusp, and five distal denticles. For both specimens, the first mesial denticle is slightly smaller than the first distal denticle, making the apicobasal height of the distal carina of the central cusp a bit shorter than the mesial carina of the central cusp. This is the same pattern found in the lower p3 denticles adjacent to the central cusp of C. havensteini (CCNHM 164). The mesial carina of the central cusp is not completely smooth, but has some jagged edges forming pseudoserrations (Fig. 42) like that seen in lower right p2 of C. havensteini (CCNHM 164). These pseudoserrations are undulating labiolingual deviations of the mesiodistal arc of the carina, leading to a jagged profile of the denticle that is macroscopically visible. This is a form of true ziphodonty, as has been seen on the serrations of the carinae in other aquatic amniotes (Young et al., 2013).
Figure 42: Pseudoserrations in the p2 of Coronodon havensteini, CCNHM 164.
The lower fourth premolar is preserved as CCNHM 166.46, which is only the distal half of the tooth, including a central cusp, five denticles, and the distal root. It is unclear whether this is the left or right, though the curvature of the crown seems to indicate it is a left. The distalmost denticle is extremely small, sitting at the base of the fourth distal denticle. The crown base is smaller in this tooth, with no enamel basal to the fourth and fifth distal denticles.
The left first molar is CCNHM 166.30 and has preserved evidence of five mesial denticles (though the two closest to the central cusp are broken/worn away) and five distal denticles. The mesial denticle row extends further basally than does the distal row and has a steeper and more extreme slope than the arched distal denticle row. The central cusp and all preserved denticles exhibit the same sort of kink in the carina that forms a pseudoserration on their mesial and distal sides.
CCNHM 166.27 is the lower left m2, which has six mesial and five distal denticles. The mesialmost denticle is extremely small and lacks a proper point, but instead resembles the bulge of the crown base. Like the first molar, the mesial denticle row is more steeply inclined and less arced than the distal denticle row. The central cusp and all preserved denticles retain the same pseudoserrations as the first molar.
The lower left m3 is represented by CCNHM 166.28, and it also has six mesial and five distal denticles. Likewise, the mesialmost denticle is extremely small and lacks a point, like that seen in the m2. Also like the first molar, the mesial denticle row is more steeply inclined and less arced than the distal denticle row. Like the first and second molars, the central cusp and denticles have carinae with pseudoserrations on their mesial and distal sides.
CCNHM 166.33 is the lower left m4. The m4 has six mesial and four distal denticles. This tooth exhibits the most extreme form of the steeply sloped mesial denticle row that extends further basally than the distal denticle row. The mesial half of the tooth is longer than the distal half, including the root. The central cusp has a pseuodserration on the mesial side, but not the distal side, whereas the two distal denticles closest to the central cusp have prominent single pseudoserrations on their distal sides.
Mandible
The posterior half of the left mandible is well preserved (Fig. 43; Table 6) and includes complete alveoli for m2-4 and partial alveoli for m1 and p4. Anteriorly the mandible has a rectangular outline with parallel ventral and dorsal margins; the ventral margin is straight to slightly concave along the preserved length of the mandible. The M2-4 are positioned posteriorly along the inclined part of the toothrow, each more dorsal than the prior tooth, with M4 positioned on the anterior margin of the coronoid process (alveolar margin of this tooth 1/3 of the distance from the condyle to the coronoid apex). All molars are double-rooted and the M2-3 have small alveoli for a labially positioned demi-root. The alveolar margins are similar in height for M2-3 but the labial margin is raised dorsally about 2 cm relative to the lingual margin at the level of M4. Posterior to the M4, there is a shallow longitudinal furrow positioned medially along the anterior edge of the coronoid process. The posterior margin of the coronoid process is damaged but appears to have been dorsally rounded with a straight, inclined anterior margin and a near vertical posterior margin; this shape is intermediate between the triangular coronoid of basilosaurid archaeocetes and the elongate tongue-shaped coronoid of later diverging aetiocetid mysticetes. The coronoid process shape is similar to Coronodon havensteini and has a more straight anterior margin than Coronodon newtonorum n. sp. The coronoid process generally compares well with that of Janjucetus hunderi, Mammalodon colliveri, and Llanocetus denticrenatus but is less lobate and less posteriorly directed. Posteriorly, the base of the coronoid widens and descends posteroventrally towards the mandibular neck.
Figure 43: Holotype mandible (CCNHM 166) of Coronodon planifrons.
Mandible in lateral (A), medial (B), and dorsal (C) view.About 3–5 cm anterior to the condyle there is a 12 mm long, 8–9 mm wide, dorsally facing foramen that perforates the neck; it has smooth, round margins. It is likely pathological or congenital in origin (a similar fenestra is present in the posterior mandible of Tohoraata raekohao; Boessenecker & Fordyce, 2015a). A similar, but smaller anteroposteriorly directed foramen is also present in the same location on the neck of the right holotype mandible of Coronodon havensteini (CCNHM 108) but is absent in the left. The condyle is relatively small, dorsoventrally shallow, and bears a triangular articular surface. The surface is pathological, bearing deep pits and transverse sulci and rows of deep foramina separated by smooth compact bone; this rugose surface texture is also present anterodorsally in the secondary glenoid fossa of the squamosal, also observed in Coronodon havensteini (CCNHM 164; see above). The medial side of the condyle is deeply excavated by the mandibular fossa. The condyle faces posteriorly but bears a horizontal transverse ‘corner’ in medial/lateral view. The mandibular fossa is large and the cavernous mandibular canal dominates the entire preserved section of mandible, becoming somewhat narrower with thicker walls anteriorly at the level of the M2. The angular process is missing, but preserved bone indicates that the posteroventral margin was slightly concave below the condyle. Laterally the coronoid process bears a shallow but large masseteric fossa, which is most deeply excavated anterodorsally where it defines a robust ridge along the anterior margin of the coronoid process. A horizontal, longitudinal, and broadly transversely convex ridge at the level of the condyle defines the ventral margin of the fossa. A shallow oval fossa on the medial surface of the coronoid process, measuring approximately 50 × 50 mm, is present posteroventral to the M4 and is positioned anterodorsally to the mandibular fossa and foramen; this is likely the insertion for the temporalis.
Cervical vertebrae
The atlas (Fig. 44; Table 7) is well preserved but lightly bioeroded in places; it is missing the apices of the transverse processes. The atlas is anteroposteriorly flattened and dorsoventrally deep relative to basilosaurids and has a nearly circular outline in anterior view. The atlas bears several pathologies. The left ventrolateral margin is swollen 8–10 mm more than the right. There is a low convex bulge on the left condylar facet near the dorsal margin, corresponding to a pit in the same location on the left occipital condyle. Lastly, the anterior part of the lamina, anterior to the right transverse foramen, is completely resorbed; on the right side it is dorsoventrally thicker, albeit bioeroded. The condylar facets are shallowly concave, dorsoventrally deep, and separated by a shallow median furrow ventrally. The hypapophysis is low and robust, ventrally positioned, and posteroventrally directed. In lateral view, the centrum is approximately rectangular. The transverse process is anteroposteriorly flattened, posterolaterally directed, and dorsoventrally deep (~45–50% of atlas depth). There is no evidence of a foramen penetrating the process, though the lateral edge is missing. The neural arch is robust and dorsoventrally deeper anteriorly. It bears a low, pyramidal neural spine. The neural foramen is teardrop shaped and widens slightly dorsally. The axial facets are flat, lunate in shape, and expand dorsally; they are separated by a 30 × 30 mm circular, posterodorsally facing odontoid fossa. The transverse foramen in the neural arch is approximately 10 mm in diameter and transversely oriented.
Figure 44: Holotype vertebrae (CCNHM 166) of Coronodon planifrons.
Cervical vertebrae shown in anterior and posterior views, and thoracics, lumbars, and caudals shown in anterior view only.One isolated cervical vertebra represents C4 based on comparison with the Coronodon havensteini holotype (Table 8). A less complete C3 centrum fragment is also present, being slightly thinner than C4. The C4 centrum is anteroposteriorly flattened (23 mm in length) and subcircular to oval in shape; at the center of the anterior face of the centrum is a shallow fossa. A large lateral vertebral foramen is developed and incompletely encircled by bone. It is oval, dorsomedially sloping in anterior view, and measures 4.5 cm wide and 2 cm deep. Ventral to this foramen is a robust parapophysis that projects ventrolaterally and expands into a subrectangular end with three apices: a dorsally pointing apex that is the remnant of the lateral branch to the diapophysis, a ventrolateral apex that points posteriorly, and a ventromedial tubercle. The diapophysis is small, triangular, and ventrolaterally projecting; the pedicle is rectangular and anteroposteriorly flattened. The pre- and postzygapophyses are aligned, near vertical, and anteroposteriorly short. The lamina is short and surrounds the oval neural foramen. The lamina is delicate and culminates in a small, 2 cm high neural spine that is triangular in lateral view.
A partial C7 bears an oval centrum 3 cm in length and exhibits large and deeply excavated notochordal pits. A minute hypapophysis is present and parapophyses are absent; a small costal tubercle is present just below the flattened and dorsoventrally deep (1/2 of centrum depth) transverse process.
Thoracic vertebrae
Both T1 and T2 are preserved (Fig. 44; Table 9), and quite similar in morphology. They possess longer centra than C7 (40 and 45 mm, respectively) that are oval, slightly flatter dorsally, and bear costal facets at the lateral apex of the centrum; the anterior facets are slightly more strongly defined. The pedicle is elevated and directed dorsolaterally and anteriorly; the transverse process is positioned anterior to the centrum and widens distally. The transverse process bears a tubercular facet ventrally and a short shelf-like prezygapophysis dorsally.
Based on centrum lengths, only T3 is absent; T4 has a centrum that is slightly deeper and flatter dorsally than T2. Costal facets are only present posteriorly. There is a subtle, shelf-like prezygapophysis, and an anteroposteriorly long, posteriorly shifted postzygapophysis. The neural spine has a wide base and is anteroposteriorly long and bears a vertical groove posteriorly at the midline. The neural foramen is subtriangular. Only a fragmentary centrum of T5 is preserved.
Four additional posterior thoracic vertebrae are present and constitute a continuous series, likely corresponding to T6-T9 based on measurements and comparisons with Coronodon havensteini (CCNHM 164). These vertebrae are roughly similar to T4 but possess successively longer, deeper, and wider centra and anteroposteriorly longer laminae; T6 possesses a wider neural spine base. Posterior costal facets become larger further posteriorly within these vertebrae.
Lumbar vertebrae
Ten lumbar vertebrae are preserved (Fig. 44; Table 10) and presumed to represent L1-10 based on the ancestral lumbar count of 10 for Neoceti (Buchholtz & Gee, 2017). None preserve spines or complete arches, and only two preserve partial transverse processes. The centra become larger in all dimensions (length, width, depth) from anterior to posterior and maintain similar proportions, with L10 being the largest. The centra are circular throughout most of the series (e.g., L3-L10) but the L1 is still slightly shallower than wide like the posterior thoracics. The ventral side of L1 is transversely convex, but in L3-L10 there is a well-defined median ventral keel, a purported synapomorphy of Neoceti (Davydenko, Mörs & Gol’Din, 2021). The pedicles are transversely narrow and become even more closely positioned to the midline posteriorly (e.g., L6-L10). The transverse process slopes ventrolaterally in L4 and L8 (22°), but is closer to horizontal in L10 (14°); in no case do the transverse processes slope as extremely ventrally as in Basilosauridae (e.g., 30° in Dorudon atrox).
Caudal vertebrae
Nine nearly complete and two partial caudal vertebrae are preserved (Fig. 44; Table 11), including Ca1-4, Ca6, Ca8, two posterior caudals (CaC and CaD) and fragments of two or possibly three additional posterior caudals. The anterior caudals (Ca1-3) are similar to L10 in size and centrum proportions but possess wider-set posterior haemal facets, weak anterior haemal facets, and paired longitudinal dorsolateral ridges medial to the transverse process. In Ca2 there is a ventrolateral ridge present. The transverse process in Ca2 is short (6 cm long), triangular, and positioned anteriorly with a straight, transverse anterior margin. All anterior and mid caudal vertebrae (Ca1-8) possess a vertical fissure-like notochordal pit.
| Measurement | Coronodon planifrons, CCNHM 166 |
|---|---|
| Ca1 anterior width | 101.5 |
| Ca1 anterior depth | 94.8 |
| Ca1 length | 90.3 |
| Ca2 anterior width | 95.6 |
| Ca2 anterior depth | 95.5 |
| Ca2 length | 89.3 |
| Ca3 anterior width | 103.4 |
| Ca3 anterior depth | 96.2 |
| Ca3 length | 88.7 |
| Ca4 anterior width | 100.4 |
| Ca4 anterior depth | 97.4 |
| Ca4 length | 86.3 |
| Ca5 anterior width | 98.9 |
| Ca5 anterior depth | 98.2 |
| Ca5 length | 83.6 |
| Ca6 anterior width | 95.2 |
| Ca6 anterior depth | 98.9 |
| Ca6 length | 82.6 |
| Ca8 anterior width | 94.5 |
| Ca8 anterior depth | 98 |
| Ca8 length | 76.3 |
| CaC anterior width | 73.4 |
| CaC anterior depth | 78.1 |
| CaC length | 47.1 |
| CaD anterior width | 64.3 |
| CaD anterior depth | 59 |
| CaD length | 34 |
The mid-caudals (Ca4-8) are of similar height but possess successively shorter transverse processes, shorter centra, dorsoventrally flattened neural arches with smaller canal-like neural foramina, larger haemal facets raised on inflated tubercles, and more dorsally positioned dorsolateral ridges. In Ca6 and 8, the anterior part of the neural arch bears paired tubercles aside a narrow 10–12 mm wide neural foramen instead of prezygapophyses. The transverse process in Ca5 appears bifurcated, apparently pierced by the vertebrarterial foramen, and the transverse process is 1–2 cm long, In Ca6 it is triangular, anteriorly shifted with a dorsoventrally deep tubercle present anteriorly at its base. In Ca8, the transverse process is reduced to a low ridge with a ventrally directed tubercle. In Ca5-8, the process is slightly narrower than deep.
The posterior caudals are represented by CaC, a circular anteroposteriorly flattened vertebra, and CaD, a wide and slightly rectangular terminal caudal vertebra. CaC is pierced by vertical vertebrarterial canals that are positioned laterally. Ventrally, a deep transverse sulcus emanates from these canals and continues ventrally; it is contiguous with a deep longitudinal trough at the ventral midline. Dorsally a short sulcus is present with an additional transverse sulcus that connects to a minute neural foramen. A small pit is present laterally. CaD is similar but lacks a neural foramen entirely, instead possessing a continuous transverse sulcus and ventral parasagittal fissures emanating dorsally onto the anterior side from the ventral opening of the vertebrarterial canal.
Ribs
Fourteen complete and partial ribs are preserved in CCNHM 166 (Fig. 45), including the left rib 1 and several other positions throughout the series. Left rib 1 is the shortest and most highly curved rib; dorsally it is dorsoventrally deep with a small anteroposteriorly flattened tubercle set far (4 cm) from the similarly small and triangular capitulum. The tubercle bears a posteromedially facing articular facet; the rib shaft is anteroposteriorly flattened and gradually tapers distally. The dorsal ¼ of the rib shaft has a longitudinal furrow anteriorly. This rib articulates well with the first thoracic vertebra and C7.
Figure 45: Holotype ribs (CCNHM 166) of Coronodon planifrons in anterior view.
Mid-thoracic ribs have a more proximally positioned tubercle, a short neck, and a more robust shaft that is anteroposteriorly thicker; the shaft is less transversely bowed. One proximal fragment of a mid-thoracic rib has a possibly pathological pit distally but is too fragmentary to evaluate. Posterior thoracic ribs are straighter and longer than the anterior ribs, and possess tubercles positioned close to the capitulum; the capitulum increases in diameter posteriorly throughout the rib series. One of the posteriormost ribs, likely the eight rib, has a large hemispherical capitulum, a reduced tubercle positioned on the posterior face and not dorsally elevated; it has a nearly round cross-section, and bears a shallow longitudinal furrow dorsally on the anterior surface of the shaft. A circular anteromedial facet on the capitulum may represent an articular pathology. Ribs of Coronodon planifrons n. sp. taper distally (lacking the pestle-shaped distal ends of Basilosauridae) have porous centers and dense cortex based on fractured cross-sections, but are not pachyosteosclerotic as reported for Basilosauridae and Mystacodon (Buffrenil et al., 1990; Muizon et al., 2019).
Coronodon sp.
Referred specimens
ChM GPV 2029 (also bearing number ChM PV 9162), lower left P3 or P4 and associated fragment of a second molariform tooth, collected from the vicinity of Chandler Bridge Creek in Ladson, SC, by S. Deal and J. Chapman fall 1974; ChM PV 9161, partial lower left m4 or perhaps upper left M3, collector, locality and collection date unknown; ChM PV 9163, upper posterior molariform tooth, likely P4 or M1, collector, locality and collection date unknown; ChM PV 9177, partial lower right molar, collector, locality and collection date unknown; ChM PV 9584, associated caniniform tooth and posterior molariform tooth (P4 or M1), likely but uncertainly from the Chandler Bridge excavation site in Bed 3 of the Chandler Bridge Formation, unknown collector; CCNHM 556 a partial lower right molariform, likely P3-M2, collected by C. Kaufman from the bank of the Ashley River in August 2015; CCNHM 1839, upper left posterior molariform tooth (likely M1 or M2), collected from the Edisto River by J. Kiser in July 2016; CCNHM 8729, caniniform tooth, perhaps lower right I3 or C1 or upper left I3 or C1, collector, locality and collection date unknown; CCNHM 8739, upper left M2 or M3, collector, locality and collection date unknown. CCNHM 8731, isolated C1 or P1, collector, locality and collection date unknown.
Remarks
These teeth all conform to the range of variation seen in Coronodon spp. (Fig. 46), but generally lack stratigraphic context, collector data, or locality data; all of these owing to poor record keeping. One of these, ChM PV 9163 (GPV 2029), was collected in 1974, representing one of the earliest discoveries of Coronodon. Another specimen that lacks data was stored with the rest of the collection of cetaceans from the Chandler Bridge excavation (Sanders, 1980), most of which were derived from Bed 3 of the Chandler Bridge Formation. Though some specimens such as CCNHM 8732 must represent the small posteriormost molar and resemble Coronodon planifrons, the lack of stratigraphic context suggests identification to only the genus level at present. Others, such as CCNHM 1830, also resemble the M2 or M1 of Coronodon planifrons. The somewhat broad and low crowns of ChM PV 2029 and PV 5854 resemble the M1 of Coronodon newtonorum n. sp. As is clear from the sample available for Coronodon havensteini, there is a degree of variation in cusp count and dimensions in the molariform teeth, precluding ready identification past the genus level. At present these teeth seem smaller than those of the larger coronodonid taxon represented by ChM PV 5720 and CCNHM 214, but detailed comparisons may be warranted once this larger taxon is described. In the absence of stratigraphic context, locality data is usually helpful in permitting provisional assignment to stratum; for example, most specimens found in Charleston area river bottoms, riverbanks, and spoil piles are likely derived from the Ashley Formation, whereas fossils in shallowly incised streams further inland produce specimens more clearly from the overlying Chandler Bridge Formation. This rough approximation is not possible here, however, since no data was recorded for most of these specimens. ChM PV 2029, however, was collected from a stream with known exposures of the Chandler Bridge Formation, and likely represents Coronodon planifrons or Coronodon newtonorum n. sp.—but lacks clear diagnostic features of either species. This specimen does possess some mesial pseudoserrations on the principal cusp; such pseudoserrations are more prevalent in the unnamed taxon represented by ChM PV 5720, but do occur in some specimens of Coronodon. Isolated coronodonid teeth are rare, and unfortunately some of the data and paperwork associated with several of these isolated teeth from The Charleston Museum were misplaced after the passing of Albert Sanders (M. Gibson, personal communication, 2020), former Curator of Natural History. Likewise, many ‘minor’ specimens at CCNHM were acquired without collector or locality data prior to 2015 (S. J. Boessenecker, 2022, personal communication). Curiously, isolated discoveries of the highly distinctive periotics or tympanic bullae have not yet been made.
Figure 46: Isolated teeth of Coronodon from the Charleston embayment.
Upper left molar ChM PV 9162 in labial (A) and lingual (B) view; associated caniniform and upper left molar of ChM PV 9584 (Coronodon sp.) in labial (C, E) and lingual (D, F) view; isolated right upper third molar CCNHM 8732 (Coronodon planifrons) in labial (G) and lingual (H) view, upper left molar CCNHM 1839 (Coronodon sp.) in labial (I) and lingual (J) view; isolated lower left premolar ChM PV 9163/GPV 2029 (Coronodon sp.) in lingual (K) and labial (L) view, partial lower postcanine CCNHM 556 (Coronodon sp.) in lingual (M) and labial (N) view, lower molar fragment ChM PV 9163 (Coronodon sp.) (O, P), isolated P1 or C1 CCNHM 8729 in labial (R) and lingual (Q) view, isolated upper molar (M2-3) or lower M3 ChM PV 9161 (Coronodon sp.) (S, T), isolated caniniform CCNHM 8729 (Coronodon sp.) in labial (U) and lingual (V) view, isolated posterior left upper molar (M2-3) CCNHM 8730 (Coronodon sp.) in labial (W) and lingual (X) view, isolated lower left molar ChM PV 9177 in labial (Y) and lingual (Z) view.Results of Phylogenetic Analysis
The equal weights (EW) phylogenetic analysis recovered 13,836 most parsimonious trees, each 13,974 steps in length. Additional trees were found but not saved because the allocated memory was exceeded, and based on the ratio of trees to be swapped to trees saved, the actual number of most parsimonious trees is considerably higher. Fortunately, the strict consensus of these 13,836 trees is identical to a consensus obtained by the driven search (Fig. 47, Datas S3 and S4), thus we are reasonably confident that the strict consensus summarizes the common topologies among the total population of trees of this length. As is typically the case, the implied weighting (IW) analysis recovered far fewer trees: 15 trees with a fit of 1110.70330 (Data S3).
Figure 47: Results of phylogenetic analyses with equal weights (EW).
Strict consensus of 13,836 shortest trees (each 13,974 steps in length) obtained from an analysis where all characters have equal weights, divided into three parts (A, B, C). Numbers next to nodes indicate bootstrap support values; unnumbered nodes indicate support values <50%.Both phylogenetic analyses (EW and IW) supported monophyly of Coronodonidae and the genus Coronodon (Figs. 47, 48). Bootstrap support for Coronodonidae, which includes the undescribed taxon ChM PV5720, is quite high at 93% (EW) or 80% (IW). Although the Family Coronodonidae is named in the present study, OTUs consisting of individual specimens of coronodonids have been included in phylogenetic studies for more than 20 years, and they have always formed a clade (e.g., Geisler & Sanders, 2003; Fitzgerald, 2006; Boessenecker & Fordyce, 2015a, 2015c, 2017). Support for Coronodon is somewhat lower than that for Coronodonidae, but still fairly high in one analysis, 82% (EW), but not the other, >50% (IW). Species of Coronodon from the Chandler Bridge Formation (i.e., C. newtonorum n. sp. and C. planifrons n. sp.) are sister-groups in both analyses, with high to moderate bootstrap support (86% EW or 53% IW). Coronodonidae is diagnosed by at least three synapomorphies, including overlapping cheek teeth (character 295: state 1 > state 0); tooth enamel lacking longitudinal fluting (308:1>2), and cheek teeth possessing wide and low crowns (309:1>2). These, and an additional 10 synapomorphies, were found on the IW trees, with the differences between the two analyses related to whether Borealodon (IW) or Metasqualodon (EW) is the sister-group to Coronodonidae. The former is coded for many more characters than the latter, and as a result, in the EW analysis it is unclear if many of these synapomorphies diagnose Coronodonidae or Coronodonidae + Metasqualodon. These additional synapomorphies include: frontal/maxilla contact loose (45:0>1), lacrimal/frontal contact unsutured (59:0>1), nasal edges converge anteriorly (62:1>0), anterior ends of nasals flare dorsally (65:0>1), high sagittal crest (97:1>0), facial and vestibulocochlear canals subequal (197:0>1), fenestrae rotunda and ovalis partially occur at same level (215:0>1), accessory promontorial groove (225:0>1), sharp involucral ridge of bulla (242:1>0), and medial lobe of bulla forms sharp posterior corner (246:1>0). The genus Coronodon is characterized by three synapomorphies in the IW and EW analyses, including posterior end of premaxilla faces anteromedially (14:0>2), premaxilla/maxilla contact unsutured (52:1>2), and endocranial foramina on periotic aligned (160:0>1). The clade of C. planifrons n. sp. and C. newtonorum n. sp. is supported by the presence of a conical lateral tuberosity of the periotic (158:0>1) and an oval fenestra rotunda (206:1>0) (both IW and EW analyses).
Figure 48: Results of phylogenetic analyses with implied weights (IW).
Results of phylogenetic analyses with implied weights (IW). Strict consensus of 15 best fit trees (each with a fit of 1110.70330) obtained from an analysis using implied weighting and the constant k = 3, divided into three parts (A, B, C). Numbers next to nodes indicate bootstrap support values; unnumbered nodes indicate support values <50%.Somewhat surprisingly, both analyses supported the poorly known Metasqualodon and Borealodon as sequential sister taxa to Coronodonidae, although as mentioned above, the sequence differs in the IW and EW trees. The broader clade of Metasqualodon, Borealodon, and Coronodonidae, as well as a sister-group relationship between Coronodonidae and Borealodon (IW) or Metasqualodon (EW) are not well supported (<50% bootstrap). Previously, Borealodon was positioned as the sister-group to a clade including Aetiocetidae and Chaeomysticeti, diverging off of the mysticete stem one node higher than Mammalodontidae (Shipps, Peredo & Pyenson, 2019), whereas Metasqualodon was positioned as the second lineage to diverge off the mysticete stem, one node higher than Coronodon (Geisler et al., 2017). The close relationship between Metasqualodon and Coronodonidae is diagnosed by two features: thick lateral edge of maxilla (16:1 or 2>0) and basal cusps on mesial side of cheekteeth point mesially (318:0>1). Alternatively, a sister-group relationship of Borealodon and Coronodonidae is supported by just a single synapomorphy: more than five cusps on cheekteeth (306:1>0). The clade all of three is supported by one (IW) or three (EW) synapomorphies, with none shared between the two analyses. The sole synapomorphy from the IW analysis is having, on average, 4.5 to 5 distal cusps on premolars (307:1>0) whereas the synapomorphies from the EW analysis include: nasal terminates at posterior half of supraorbital process of frontal (64:1>2), dorsal and posterior margins of periotic meet at right angle (188:0>1), and sharp crest between stylomastoid and suprameatal fossae (217:0>1).
Several other aspects of the strict consensus trees from both analyses are similar. Like Corrie & Fordyce (2022), we found Kekenodon to be the sister-group to a clade that includes odontocetes, mysticetes, and the putative mysticetes Mystacodon and Coronodonidae. If we apply a crown-based definition for Neoceti, as advocated by Fordyce (2009), then Kekenodon would be excluded from Neoceti. Synapomorphies of the clade of Neoceti and Kekenodon, but excluding basilosaurids, shared by the IW and EW analyses are: premaxilla terminates over anterior half of supraorbital process of frontal (8:0>1), mallear fossa of periotic medial to lateral tuberosity (180:0>1), anteromedial corner of pars cochlearis is rounded (184:0>1), medial lobe of bulla terminates as a blunt corner (246:0>1), roots of double-rooted teeth partially merged (304:0>1), upper cheekteeth lack a lingual cingulum (311:0>1), lower molars lack reentrant grooves (314:0>1), and lower molars bear accessory cusps on mesial carina (315:0>1).
Odontoceti is monophyletic in both analyses, as is the recently named Kinetomenta (Aetiocetidae + Chaeomysticeti; Gatesy et al., 2022) within Mysticeti. However, application of the phylogenetic definition of Gatesy et al. (2022) for Kinetomenta to our EW trees would exclude Niparajacetus from this clade, and would shift some key features of this clade (e.g., loose mandibular symphysis, laterally bowed mandibles) to more basal nodes. Otherwise, all other aetiocetids form a clade in the IW trees (Fig. 48), and all aetiocetids but Morawanacetus and Kaaucetus form a clade in the EW trees. These latter two taxa vary in their positions among the shortest trees, resulting in Aetiocetidae collapsing into a polytomy at the Kinetomenta node (Fig. 47). Llanocetus is closely related to the unnamed taxon represented by ZMT-62 (Fordyce, 1989) in both analyses, similar to some of the implied weighting analyses of Geisler et al. (2017), and Chaeomysticeti is monophyletic, a result consistent with nearly all phylogenetic studies that include fossil mysticetes (Deméré et al., 2008; Marx & Fordyce, 2015; Boessenecker & Fordyce, 2017; Geisler et al., 2017; Peredo et al., 2018; Muizon et al., 2019; Bisconti, Munsterman & Post, 2019).
Unlike Peredo et al. (2018), we found Maiabalaena and Sitsqwayk to be within Eomysticetidae. Those authors placed these genera as the sole members of a lineage diverging just crownward to Aetiocetidae but immediately before the Eomysticetidae. In our EW trees Maiabalaena is one of five lineages forming a polytomy at the base of Eomysticetidae and Sitsqwayk is the sister-group to Eomysticetus, whereas in our IW trees, Maiabalaena is the sister-group to Yamatocetus and Sitsqwayk is the most basal eomysticetid. Support for Eomysticetidae, including Maiabalaena and Sitsqwayk, is moderate to high, with a bootstrap value of 81% (EW) or 65% (IW) and a total of seven supporting synapomorphies common to both analyses, including supramastoid crest terminating posterior to the temporal fossa (123:0>2), margins of zygomatic process parallel in dorsal view (130:0>1), presence of a secondary squamosal fossa (132:0>1), squamosal prominence forms a large cylindrical tubercle (135:1>2), presence of a ventral fossa on apex of zygomatic process (136:0>1), squamosal medially bowed in dorsal view (138:0>1), and sharp involucral ridge of bulla (241:1>0). Differences in tree topology can be accounted for by a number of mis-codings for Maiabalaena and Sitsqwayk in the matrices of Peredo & Uhen (2016) and Peredo et al. (2018) that likely pulled these taxa further stemward. Examples include miscoding Maiabalaena nesbittae for possessing a ‘peaked’ vertex in lateral view (character 2 of Peredo, Peredo & Pyenson, 2018a, coded for state 0 instead of 1); an occipital lacking a ‘trefoil’ shape (109:1 instead of 0); a short squamosal fossa that is less than 3/4 of the width of the temporal fossa (139:2 instead of 1). In each case Maiabalaena does not differ from other Eomysticetidae. Further errors include codings made for anatomical structures or bones missing or otherwise too poorly preserved in the holotype of Sitsqwayk cornishorum to code for, including a premaxilla not overhanging the maxilla on the rostrum (6:0 instead of ?); a rostral portion of the premaxilla that is transversely convex (12:0 instead of ?); an antorbital process of the maxilla extending anterior to the antorbital notch (23:1 instead of ?), rostrum with regular lateral curvature of the maxilla (41:0 instead of ?), anterior edge of the narial fossa present in the posterior 3/4 of the rostrum (47:0 instead of ?); premaxilla-maxilla suture on rostrum firmly ankylosed (51:1 instead of ?), embrasure pits on palate absent (52:1 instead of ?), parallel edges of nasals anterior to preorbital process (58:1 instead of ?). None of these features are preserved in Sitsqwayk (Peredo & Uhen, 2016: figs. 2 and 3), with the exception of the premaxilla-maxilla suture (char. 51); however, in this case, the preservation is likely too poor to evaluate and should be coded as ‘?’ as well. We also highlight that many of the synapomorphies listed in the diagnosis of Maiabalaena are either incorrect (e.g., the nuchal crest is actually higher than the vertex rather than lower) or are incorrectly diagnosed and the correct condition is an eomysticetid synapomorphy (Maiabalaena lacks a supramastoid crest and the zygomatic process is dorsally convex in cross-section along its length, like other Eomysticetidae). Additionally, many, if not all, of the proposed synapomorphies of the Sitsqwayk + Maiabalena clade (Peredo, Peredo & Pyenson, 2018a: e2) are either also miscoded, coded differently in each (or missing in one) of these taxa (e.g., char. 265), and/or the opposite character state is listed in the text (p. e2) vs. the actual coding in their matrix (e.g., chars. 11, 57, 69, 85, 86, 217, 268, 297, 329). Many of these features are coded the same as other Eomysticetidae in our matrix, and some characters vary somewhat within Eomysticetidae (e.g., char. 297).
As such, although Maiabalaena is an important taxon for understanding the early evolution of Mysticeti, the insights it provides are largely aligned with those outlined in previous studies of eomysticetids (e.g., Sanders & Barnes, 2002a, 2002b; Boessenecker & Fordyce, 2015b, 2015c). The purported toothlessness and absence of baleen in Maiabalaena has been challenged (Ekdale & Deméré, 2022; Gatesy et al., 2022), and recognition of its phylogenetic placement within Eomysticetidae suggests it is no more relevant to discussions of the origin of baleen and loss of teeth than other eomysticetids with better rostral and mandibular preservation that include evidence of vestigial dentition and palatal vasculature best interpreted as associated with baleen (e.g., Tokarahia, Waharoa, Yamatocetus, Boessenecker & Fordyce, 2015a, 2015b, 2015c; Okazaki, 2012).
Despite the many similarities among the trees from our EW and IW analyses, there are important differences, particularly with respect to the position of Coronodonidae. Although most previous studies (e.g., Geisler & Sanders, 2003; Fitzgerald, 2010; Boessenecker & Fordyce, 2017; Geisler et al., 2017; Fordyce & Marx, 2018; Peredo & Pyenson, 2018) have found Coronodonidae to be the most basal lineage within Mysticeti, our EW analyses placed Coronodonidae as the second or third most basal mysticete lineage, more apical than a clade of Mystacodon + Llanocetus + ZMT 62, and in some trees, more apical than Mammalodontidae too (Fig. 47). There have been a few studies that placed Coronodonidae, or equivalent taxa, in a more apical position (e.g., Marx & Fordyce, 2015; Lambert et al., 2017; Peredo, Peredo & Pyenson, 2018a; Muizon et al., 2019) but the phylogeny supported by our EW analyses is unique. By contrast, our IW trees exclude Coronodonidae from Mysticeti and a crown-based definition for Neoceti (Fig. 48). The same is true for the putative mysticete Mystacodon, which is in an even more basal position. Corrie & Fordyce (2022) also recovered Coronodon and Mystacodon as outside Mysticeti and Neoceti, although the exact relationships among these and other taxa differ. They found that Mystacodon and Coronodon were members of a toothed “mysticete” clade outside of Neoceti or that Mystacodon was more basal than Coronodon, depending on whether they used implied weighting in their cladistic analyses. In our IW trees, support for excluding Coronodon from Mysticeti and Neoceti is low, both relevant nodes have bootstrap values <50%.
Lambert et al. (2017) described the toothed mysticete Mystacodon selenensis; their phylogenetic analysis recovered it as the most basal mysticete, and this is aligned with it being the oldest mysticete, at 36.4 Ma. This basal position was later corroborated by Muizon et al. (2019) but contradicted by Fordyce & Marx (2018), who found Coronodon as the most basal mysticete. Our EW trees recovered a sister-group relationship between Mystacodon and Llanocetus, the second oldest mysticete, although bootstrap support is <50% (Fig. 47). A close relationship between these taxa was first found by Fordyce & Marx (2018), and then later in the implied weighting analysis of Corrie & Fordyce (2022). Intriguingly, when Mystacodon is positioned outside of Mysticeti and Neoceti, as occurs in our IW trees, then a clade of Southern Ocean mysticetes emerges including Llanocetus, ZMT-62, and mammalodontids (Fig. 48). Synapomorphies of this Southern Ocean clade include: rostrum has a gradually sloping profile anterior to nares (49:0>1); presence of channel for the lacrimal canal (60:1>0); orbital margin deeply notched in dorsal view (73:0>1); and, on average, 4.5 to 5 distal cusps on premolars (307:1>0). The characters that support the conflicting clades in our EW and IW analyses are covered in more detail in the “Discussion”.
Finally, we also tested our assignment of individual specimens to Coronodon havensteini by replacing our composite, species-level OTU with the four specimens we assigned to this species: CCNHM 108 (the holotype), CCNHM 164, CCNHM 8722, and ChM PV4745. EW and IW analyses for this modified matrix produced trees (Fig. 49) that were quite similar to those obtained when a composite OTU for C. havensteini was used; specifically, the IW analysis yielded 15 trees, each with a fit of 1110.91468 (Data S3), and the EW analysis yielded in excess of 10,000 most parsimonious trees (Data S5), each 13,984 steps in length. Each analysis (i.e., the EW and IW) supported coronodonid monophyly, monophyly of Coronodon, and a sister-group relationship between C. planifrons and C. newtonorum (Fig. 49). Two of the three specimens we are referring to C. havensteini do form a clade with the holotype (i.e., CCNHM 164 and ChM PV4745) but CCNHM 8722 is placed as the sister-group to the clade of C. planifrons + C. newtonorum. This result is discussed below in “Taxonomic unity of Coronodon specimens from the Ashley Formation”.
Figure 49: Relationships within Coronodonidae when specimens in that family are coded separately in the phylogenetic analysis.
(A) Portion of the strict consensus tree when all characters are weighted equally. A total of 10,000 trees were found, each 13,984 steps in length, (B) Portion of the strict consensus tree when analysis used implied weighting and the constant k set to 3. A total of 15 best fit trees were found, each with a fit of 1110.91468. Complete trees can be viewed in Data S3 and S5.Discussion
Toothed mysticete diversity in the Western North Atlantic
Toothed mysticetes from the Oligocene of Charleston have been informally recognized since the 1990s (Barnes & Sanders, 1996a, 1996b), and isolated, but previously unpublished teeth have been collected as early as the initial excavation of the Chandler Bridge Formation in 1970–1972 (e.g., ChM PV 2029). The morphology of the “archaeomysticetes” has finally been illuminated with the publication of Coronodon havensteini, Coronodon newtonorum n. sp., and Coronodon planifrons (Geisler et al., 2017; this study). Two of these (Coronodon newtonorum n. sp., Coronodon planifrons) are the first toothed mysticetes from the Chandler Bridge Formation. In addition to Coronodon spp. there is at least one other poorly known coronodonid represented by CCNHM 8745, and a second, much larger Basilosaurus-sized genus of Coronodonidae represented by ChM PV 5720 and CCNHM 214 from the Chandler Bridge Formation. Altogether, this sample suggests a total of five species of toothed mysticetes from the Oligocene of the western North Atlantic. In addition, the Ashley and Chandler Bridge formations have also produced purportedly toothless eomysticetids Micromysticetus and Eomysticetus. Eomysticetids, and potentially five species of toothed mysticetes constitute the entire mysticete assemblage, whereas the odontocete assemblage is substantially more diverse. Odontocetes from these strata include 9–10 species of Xenorophidae (five of which are named; Kellogg, 1923; Whitmore & Sanders, 1977; Geisler, Colbert & Carew, 2014; Churchill et al., 2016; Boessenecker, Ahmed & Geisler, 2017), Agorophius (Godfrey et al., 2016), at least two species of Ankylorhiza (Boessenecker et al., 2020), Ediscetus and several other waipatiid-grade odontocetes (Albright, Sanders & Geisler, 2018; R. W. Boessenecker, 2021, personal observation), and several other taxa (Geisler & Sanders, 2003; Albright et al., 2019). With the exception of Ankylorhiza (body length 4.8 m), no Oligocene odontocetes approached Coronodon in body length. Coronodon was originally interpreted as an ecologically flexible taxon capable of dental filtration and raptorial feeding, similar to extant leopard seals (Hocking, Evans & Fitzgerald, 2013; Geisler et al., 2017). Such a capability may have supported a degree of niche differentiation and eased competition with early giant dolphins like Ankylorhiza. However, subsequent studies have called into question the filter feeding adaptations of Coronodon based on a single metric (Hocking et al., 2017).
Regardless of the feeding morphology of Coronodon, the fossil record of toothed mysticetes in the North Atlantic contrasts strongly with that of the North Pacific, where smaller aetiocetid whales are numerically common and surprisingly diverse (Barnes et al., 1995; Hernández-Cisneros & Velez-Juarbe, 2021). Aetiocetid whales are typically small (BZW = 22–32 cm; estimated body length 2–4 m) with some exceptions (e.g., 65 cm BZW, 8 m body length; Tsai & Ando, 2016) relative to Coronodon (BZW = 46 cm, estimated body length 5 m). While the feeding morphology of aetiocetids is hotly contested, at least some have been interpreted as raptorial (e.g., Fucaia buelli; Marx, Tsai & Fordyce, 2015), benthic suction feeders (Marx et al., 2016), and “protobaleen”-bearing filter feeders capable of raptorial fish eating (Aetiocetus; Deméré et al., 2008; Deméré & Berta, 2008). The excessive diversity of aetiocetids is not recognized in any single stratum, where typically 2–3 species may be present; however, the bulk assemblage from the North Pacific preserves 21 species (Hernández-Cisneros & Velez-Juarbe, 2021). Few species are documented in coeval strata at different localities, raising the possibility that local mysticete diversity in Oligocene North Pacific marine basins was equivalent to the toothed mysticete diversity in the Charleston embayment (n = 2–3) and that extreme North Pacific richness is exaggerated by pooling of distant localities. Regardless, virtually all aetiocetid taxa are based on single type specimens and frequently lack overlapping parts, raising the possibility of diversity inflation through taxonomic splitting. Further study of ontogenetic and individual variation within toothed mysticetes, such as in this study, is clearly warranted, given the possibility of taxonomic synonyms and rare examples of identification of referred specimens in the past study of Aetiocetidae.
Taxonomic unity of Coronodon specimens from the Ashley Formation
Specimens of Coronodon from the Ashley Formation seem to share common morphological features and lack the autapomorphies of Coronodon newtonorum n. sp. and Coronodon planifrons, and are best interpreted as representing a single species, Coronodon havensteini. Features uniting specimens from the Ashley Formation include preorbital and postorbital processes of approximately equal depth, upper molars of similar anteroposterior length, and a ventrolaterally sloping posterior edge of the supraorbital process of the frontal (Table 1). These specimens (where preserved) have a straight lateral edge of the maxilla, differing from the concave-up margin (in lateral view) in Coronodon newtonorum n. sp. While CCNHM 164 does not preserve a complete enough rostrum to evaluate this feature, the m4 alveolus is not elevated so high above the mandibular condyle and the ventral margin of the mandible is nearly straight, differing from Coronodon newtonorum n. sp. These specimens all possess a sternomastoid fossa that does not extend far up the lateral side of the nuchal crest, differing from Coronodon planifrons. The lateral tuberosity of the periotic is generally short in Coronodon havensteini, measuring up to 18–20 mm in length in CCNHM 108, 8722, and ChM PV 4745 (ChM PV 4745 is an exception, measuring 24.5 mm), and in adults the lateral tuberosity does not extend beyond the lateral margin of the swollen periotic.
Our analyses that treated each specimen of C. havensteini as a separate OTU allowed us to test our referral of three specimens to this species (Fig. 49). Our EW and IW analyses (Fig. 49) support referral of CCNHM 164 and ChM PV 4745 to C. havensteini, although bootstrap support for the C. havensteini clade was low (<50%). CCNHM 8722, which we also refer to C. havensteini, was placed in both analyses as next to the clade of C. planifrons n. sp. + C. newtonorum n. sp., although also with a bootstrap value <50%. We interpret these results as indicating that support for referring this specimen to C. havensteini is weak, but we suggest its unexpected position in the phylogeny is a consequence of its young ontogenetic stage. Only one character diagnoses the clade of C. havensteini minus CCNHM 8722: a rounded anterior margin of the bulla in medial view (245:0>1); however, this feature is unknown “?” in CCNHM 8722. There are two putative synapomorphies of the clade of CCNHM 8722, C. planifrons n. sp., and C. newtonorum n. sp.: conical and laterally projecting lateral tuberosity of the periotic (158:0>1) and tympanic bulla that is narrow relative to its length (253:0>1). The first feature is shared with ChM PV4745 and appears to correlate with age, and the second feature appears likely to adjust with growth (Fig. 23; see Ontogeny in Coronodon havensteini).
Because there are two distinct species of Coronodon in the Chandler Bridge Formation, it is possible that a second species of Coronodon or Coronodonidae may occur in the Ashley Formation, possibly represented by CCNHM 8745. However, given the available sample, differences between specimens from the Ashley Formation seem minor and best attributed to individual variation within Coronodon havensteini. The lack of obvious synapomorphies uniting all specimens of Coronodon havensteini, in concert with CCNHM 8722 forming a sister taxon relationship with Coronodon planifrons n. sp. and Coronodon newtonorum n. sp. (Fig. 49), may suggest that Coronodon havensteini is directly ancestral to both species of Coronodon from the younger Chandler Bridge Formation. An alternative hypothesis might suggest that CCNHM 8722 is a separate species from Coronodon havensteini, but all of its features that resemble Coronodon newtonorum n. sp. and Coronodon planifrons n. sp. and differ from other specimens of Coronodon havensteini are juvenile features that are modified through ontogeny. A detailed description of the as yet unnamed coronodonid represented by ChM PV 5720 may better polarize characters within the family and yield additional autapomorphies of C. havensteini.
Ontogeny in Coronodon havensteini
A number of cranial features in Coronodon havensteini change during postnatal ontogeny; most of these changes relate to the proportions of the rostrum, intertemporal region, squamosal, periotic, bulla, and eruption of the dentition. Many minor changes are mentioned in the description, including for example the dorsoventrally deeper maxilla in juveniles, and are not discussed further.
Juvenile specimens of Coronodon havensteini possess relatively short intertemporal constrictions, constituting 33.6% of bizygomatic width in CCNHM 8722 and 29.2% in ChM PV 4745. In comparison, the intertemporal region of the adult holotype is 57.9% of bizygomatic width. In this case, the juvenile condition is not the plesiomorphic condition, and the adult condition instead converges on the archaic long intertemporal region of basilosaurids. In comparison, juvenile basilosaurids possess elongated intertemporal regions early in ontogeny and the frontals subsequently widen (e.g., Uhen, 2004a; Fahlke, 2012).
Several changes are evident in the taxonomically and phylogenetically informative periotic. Most obvious is the transverse inflation of the anterior process and body of the periotic; in this regard, there is a clear increase in the transverse thickness of the anterior process and the body. As a result, the lateral tuberosity extends beyond the body in juveniles and the body extends beyond the lateral tuberosity in adults; likewise, the inflation becomes so great that in an old adult of Coronodon havensteini (CCNHM 164) a deep crease forms between the body and the anterior process. The posterior process increases in length during postnatal ontogeny, being shortest in ChM PV 4745 and increasingly longer in CCNHM 108 and 164; this parallels the growth of the posterior process in Crown Mysticeti (Bisconti, 2001).
The tympanic bulla of Coronodon havensteini is not fully developed at birth, as the youngest specimen (CCNHM 8722) possesses a bulla that is 74.9 mm long; the bulla in the slightly older juvenile (ChM PV 4745) is slightly larger (76.7 mm), and the adult holotype has a bulla measuring 83.2–85 mm in length. This postnatal increase in bulla size parallels that of the basilosaurid archaeocete Dorudon atrox (Uhen, 2004a: appendix IVB) as well as the eomysticetid whale Waharoa ruwhenua (Boessenecker & Fordyce, 2015c). In contrast, the bulla (excluding the posterior process) of odontocetes is already full size at birth and does not grow postnatally (Buffrenil, Dabin & Zylberberg, 2004; Lancaster et al., 2015).
Maxillary postcanine teeth are less emergent in juveniles, with the base of the molar crowns at least 6 mm below the lateral edge of the maxilla in ChM PV 4745. In the Coronodon havensteini holotype the crowns are highly emergent, the enamel base of which is 13.6–17 mm ventral to the edge of the maxilla. Accordingly, the embrasure pits of juvenile specimens CCNHM 8722 and ChM PV 4745 are more poorly developed and restricted anteriorly. This indicates that the embrasure pits in Coronodon are only resorbed after the crowns have erupted more extensively. Coronodon differs from basilosaurid whales and most other toothed mysticetes in the extreme degree of tooth eruption.
The upper cheek teeth of adult Coronodon havensteini are aligned anteroposteriorly, unlike the p3-m4 in the lower dentition that are posterolabially slanted and overlap one another (Geisler et al., 2017). However, the upper M1 and M2 in juvenile Coronodon havensteini (e.g., ChM PV 4745) overlap by 18 mm (measured obliquely along axis of interdental notch). This overlap appears to be a result of the large size of the teeth erupting in an absolutely small juvenile maxilla; as the maxilla increases in length later in growth the overlap is lost by adulthood. A similar situation is evident in juvenile Basilosauridae (Uhen, 2004a: figs. 12, 14). Curiously, in the adult holotype of Coronodon newtonorum n. sp. the upper cheek teeth overlap with the posterior root of each cheek tooth lying labial to the anterior root of the tooth just posterior to it. Given the overlapping teeth in juvenile Coronodon havensteini, it is likely that dental overlap is neotenically retained in Coronodon newtonorum n. sp.
Some features notably do not change during postnatal ontogeny. For example, the proportional length of the rostrum is static in all specimens, with the maxilla measuring approximately 85–105% of bizygomatic width. Juvenile specimen ChM PV 4745 has minimum rostral length of 104% of bizygomatic width, whereas the holotype has a proportionally shorter rostrum measuring 85% of bizygomatic width. In comparison, that value is 96% in juvenile CCNHM 8722 and 105% in adult CCNHM 164, which we interpret to be individual non-ontogenetic variation. Thus, the shape of the palate is approximately the same at all ontogenetic stages and it does not appear that the rostrum proportionally lengthened (or shortened) during ontogeny. This differs from the postnatal lengthening of the rostrum in odontocetes and later diverging mysticetes (e.g., Boessenecker & Fordyce, 2015c), and implies that rostrum proportions are critical to the feeding ecology of Coronodon. Unfortunately, little attention has been paid to ontogenetic changes in the skulls of Basilosauridae, and further study is needed for comparison with the rapidly improving fossil record of early mysticetes and odontocetes.
Revised tooth count in Coronodon and implications for polydonty in Neoceti
Geisler et al. (2017) stated that Coronodon havensteini had eleven upper and lower teeth with all teeth, or their corresponding alveoli, preserved in the holotype skull. This interpretation was consistent with its fairly basal position in mysticete phylogeny, and implies that it diverged before polydonty evolved among stem mysticetes. However, further study of the holotype (CCNHM 108), in combination with the new specimens described in the present article, indicates that C. havensteini had four lower molars (a total of twelve lower teeth), and that the mandibles are missing the anterior tips and the i1 alveoli. Several lines of evidence support this, including (1) new observations from the Coronodon havensteini holotype, (2) mandibular evidence from Coronodon newtonorum n. sp., (3) the lack of wear on the last lower molars in new specimens of Coronodon (indicating the lack of an antagonistic tooth), and (4) supplementary observations on the mandible of ChM PV 5720, referred to the unnamed sister taxon of Coronodon. We inferred a fourth lower molar rather than a fifth lower premolar owing to the historical identification of the upper teeth by Geisler et al. (2017) to which the lower teeth occlude with, and the identification by Barnes & Sanders (1996b) of the last mandibular tooth in these specimens as an m4. Owing to the trend towards postcanine homodonty and similarity of the p4 with the molars, it is unclear if a posterior premolar was instead duplicated. Premolars and molars within mammals are defined on possessing and lacking deciduous precursors (respectively); because deciduous teeth are unknown in Coronodon and early Neoceti are widely thought to be monophyodont (Uhen & Gingerich, 2001), dental locus replication must be inferred based on permanent tooth morphology only Although archaeocetes have a clear morphological discontinuity at the premolar/molar boundary, Coronodon displays gradual changes throughout its postcanine series. Thus, any proposed premolar-molar boundary will be tentative. However, evidence supporting our identification of a duplicated molar locus stems from the increasing anteroposterior length of the premolars from p1 to p4 and P1 to P4; such changes parallel other cetacea with the primitive tooth count for mammals (e.g., Basilosauridae) and contrast with near identical morphology of the m1-m3 (also similar to one another in the dentition of Basilosauridae). More discoveries of early Neoceti highlighing variations in tooth count are needed to further elucidate the regions within the dentition where incipient polydonty evolved in early Neoceti.
When fitting the Coronodon havensteini holotype mandibles into “occlusion” with the embrasure pits in the skull, it is not possible to articulate the dentition so that the anteriormost lower incisor lies mesial (anterior) to the anteriormost upper incisor, as is the conserved occlusal relationship in mammals; when attempted, there is a 3 cm gap between the mandibular condyle and the glenoid fossa. When the mandible is in articulation with the squamosal, the posteriormost lower molar lies just distal (posterior) to the upper M3—but the anteriormost mandibular tooth, identified as the i1 by Geisler et al. (2017), instead is positioned mesial to the upper I2. This suggests that an additional tooth was present, that the i1 of Geisler et al. (2017) is actually the i2, and that there were four lower molars instead of three. In addition, the spacing in the embrasure pits does not align under the interpretation of Geisler et al. (2017), either the anterior lower teeth fit in their corresponding embrasure pits or the posterior teeth fit, but not both. If the mandible is shifted posteriorly one tooth position, then the entire series of embrasure pits in the skull match the apices of the lower dentition. Furthermore, our new position resolves some observations that we had assumed were the result of taphonomic distortion. In our original occlusal interpretation (i.e., Geisler et al., 2017) it appeared that the coronoid process might contact the supraorbital process and M3 might impact the mandible. Shifting the mandible posteriorly, relative to the cranium, solves both of these problems; for the M3, the mandibular body shallows anteriorly so that there is now ample space for this tooth during occlusion, and shifting the coronoid process posteriorly provides plenty of clearance between it and the supraorbital process of the frontal. Finally, our new interpretation results in the upper and lower teeth of the holotype (CCNHM 108) being more similar in morphology. This is most evident among the anterior premolars, where the toothrow transitions from caniniform anterior teeth to multi-cusped posterior teeth. Under the arrangement suggested by Geisler et al. (2017), the “P2” had two mesial and four distal denticles. By contrast their “p2” had three mesial and five distal denticles, and the “P2” was about 60% the length of the “p2”. Our new arrangement results in the upper and lower second premolars being nearly identical in length and much more similar in morphology. Both teeth have three distal denticles, and the P2 has two mesial denticles whereas the p2 has one mesial denticle. On both teeth the mesial denticles are much smaller than the distal denticles.
Having a different number of upper and lower molars means that the m4 would not occlude with another tooth. The new specimen of C. havensteini (CCNHM 164) and the holotype of C. planifrons n. sp. (CCNHM 166) both preserve the m4, and occlusal shear facets are absent on the m4 of each specimen (but present on other lower premolars and molars). The right and left mandibular bodies of the holotype of C. havensteini were missing their anterior tips. What was preserved indicated only a single alveolus, and the anterior ends of the mandibular bodies were reconstructed with only 11 lower teeth. It is possible that the C. havensteini had only two lower molars, but we suspect that more of the mandible was missing than we originally realized and that there were in fact twelve lower teeth on each side. The complete left mandible of Coronodon newtonorum n. sp. confirms this and possesses alveoli for twelve mandibular teeth. Though the mandible of the unnamed large toothed mysticete ChM PV 5720 has a damaged anterior end, there is a partial alveolus for a procumbent long-rooted first incisor (though difficult to observe), in addition to eleven better preserved alveoli for the i2-m4. In sum, Coronodon havensteini, Coronodon newtonorum n. sp., and ChM PV 5720 all possessed eleven upper and twelve lower teeth, accommodated by differing numbers of molars: three upper molars and four lower molars. These observations on ChM PV 2776 and 5720 were published in early conference abstracts by Barnes & Sanders (1996a, 1996b), and we should have given this possibility more consideration prior to our original description (i.e., Geisler et al., 2017).
When superimposed onto a line drawing of the mandible of Coronodon havensteini, the posteriormost molar in Coronodon planifrons—identified conservatively in the description above as the m4—lies entirely posterior to the m4 of Coronodon havensteini, and the m3 of Coronodon planifrons is in the same position as the m4 in Coronodon havensteini. Two possibilities exist: first, and most conservatively, is that the entire toothrow of Coronodon planifrons is simply shifted posteriorly along the mandible. The second, and more speculative possibility, is that Coronodon planifrons possessed an additional molar (m5), relative to other species of Coronodon. Testing this hypothesis will require the discovery of a complete mandible (or dentition) of Coronodon planifrons.
Palatal foramina in Coronodon
Extant mysticetes are most notable for their unique filter feeding structure, baleen, a series of keratinous plates that attach to the palate. Because baleen is a soft tissue and only rarely fossilizes (Gioncada et al., 2016), its presence in extinct mysticetes has generally been inferred from toothlessness or the presence of extensive vascular channels in the maxilla (Deméré et al., 2008). These channels, the lateral palatal foramina and associated sulci, have been proposed as osteological correlates for baleen (Deméré et al., 2008), and more recently, CT study has confirmed their homology with that in extant mysticetes as the foramina in both descend from the superior alveolar canal (Ekdale & Deméré, 2022). The co-existence of teeth and lateral palatal foramina in several species of Aetiocetidae led to the hypothesis that teeth and baleen existed simultaneously and that the teeth to baleen transition was stepwise in nature (Deméré et al., 2008; Gatesy et al., 2022). Challenges to this hypothesis include the suggestion that such vascular structures may have instead been related to thickened gums (Marx et al., 2016; Fordyce & Marx, 2018) and that tooth wear is suggestive of alternative feeding modes like benthic suction feeding (Marx et al., 2016) or perhaps raptorial feeding (Marx & Fordyce, 2015)—though we note that this evidence does not actually preclude filter feeding as some extant marine mammals switch between raptorial and filter feeding modes (Hocking, Evans & Fitzgerald, 2013). Eomysticetidae possess lateral palatal foramina (Okazaki, 2012; Boessenecker & Fordyce, 2015a) and although the first eomysticetids were interpreted as toothless (Sanders & Barnes, 2002b), subsequent discoveries including possible alveoli in Yamatocetus and Waharoa and a partial possible tooth matching the alveolar dimensions in Tokarahia (Okazaki, 2012; Boessenecker & Fordyce, 2015a, 2015c) suggest the coexistence of baleen and vestigial, non-functional teeth. The eomysticetid (see below) Maiabalaena nesbittae was proposed to be a toothless early non-eomysticetid chaeomysticete lacking lateral palatal foramina, therefore indicating that early mysticetes went through a toothless suction feeding stage where they also lacked baleen (Peredo, Peredo & Pyenson, 2018a), and appearing to further diminish the stepwise evolution hypothesis of Deméré et al. (2008). However, the palate of Maiabalaena is quite poorly preserved and the margins fractured (R. W. Boessenecker, 2012, personal observation; Ekdale & Deméré, 2022; Gatesy et al., 2022), and as noted by Ekdale & Deméré (2022) and Gatesy et al. (2022), Peredo et al. (2018) described structures in their supplementary description consistent with poorly preserved lateral palatal foramina and possible alveoli. As this specimen is now robustly identified as an eomysticetid, its relevance is less significant than other better preserved eomysticetids described previously (Okazaki, 2012; Boessenecker & Fordyce, 2015a, 2015c) and even if properly interpreted, autapomorphic tooth loss is in Maiabalaena is more likely than re-evolution of teeth (Gatesy et al., 2022) in other Eomysticetidae under either the phylogenetic hypothesis of Peredo et al. (2018) or the phylogeny of this study. Most recently, the proposed existence of lateral palatal foramina in extant non-cetacean artiodactyls like hippos (which lack baleen) suggests that these structures cannot be reliably used to infer the presence of baleen in fossil mysticetes (Peredo, Pyenson & Uhen, 2022). However, questions of homology arise, as no canals reconstructed by Peredo, Pyenson & Uhen (2022) connect to the tooth roots, suggesting that the structures do not connect to the superior alveolar canal and are misinterpreted.
Several lateral palatal foramina were noted in the original supplementary description of Coronodon havensteini (Geisler et al., 2017: supporting information), and we have identified several additional foramina here. By inspecting CT data of the holotype skull, we were able to trace all but one (Foramen 6) of the foramina into canals that course posterodorsolaterally towards the tooth roots. Although the interior of the maxilla is poorly preserved around the roots, and thus these canals cannot be traced to an intact superior alveolar canal, their orientation is strongly suggestive that these are branches off of the superior alveolar canal, rather than branches of the greater palatine sulcus or canal, which is positioned medially in Coronodon and most, if not all other mammals. Did Coronodon havensteini possess baleen? Relative to skull size, the palatal foramina are certainly smaller and less numerous than in Aetiocetus weltoni (Deméré et al., 2008), and far smaller and less numerous than those of extant mysticetes. Thus, like Geisler et al. (2017), we do not consider this sufficient evidence for the presence of baleen, although certainly noteworthy. Coronodon havensteini was proposed to have thickened gingiva gingiva based on the emergent teeth and the presence of extensive dental erosion on the labial side of the upper teeth, and it was suggested that the gingiva adjacent to potential filter-feeding slots framed by baleen could have later evolved into baleen (Geisler et al., 2017:2039). Given the presence of these palatal foramina in Coronodon, it is possible that some rudimentary form of baleen was present in Coronodon, although we still consider this unlikely. It is possible that rudimentary gingival projections may have aided in dental filtration and co-opted for baleen in later mysticetes, or possible that these foramina are associated with thickened gingiva. More completely preserved specimens of Coronodon are needed to test these hypotheses, though we note that distinguishing between thickened gums (e.g., Marx et al., 2016; Fordyce & Marx, 2018) and baleen from skeletal evidence alone may not be possible. Further study of the feeding morphology and behavior of Coronodon is currently underway and incorporates data from many of the new specimens reported herein.
Mandibular kinesis in Coronodon
The mandible of Coronodon resembles that of Basilosauridae in many respects. Despite many plesiomorphic basilosaurid-like features (e.g., mandibular body that deepens posteriorly, embrasure pits, large plate-like and subtriangular coronoid process), however, Coronodon possesses an anteroposteriorly short and unfused mandibular symphysis that has only a low-relief articular surface. This differs from planar, rugose articular surface seen in archaeocetes and most terrestrial mammals (e.g., Coronodon havensteini, CCNHM 108; Coronodon newtonorum n. sp., ChM PV 2778). A condition similar to basilosaurids is also present in Mystacodon selenensis (Muizon et al., 2019), and inferred for Janjucetus and Mammalodon based on isolated mammalodontid mandibles (Fitzgerald, 2010, 2012). The symphyseal morphology of Coronodon instead shares a lack of a tightly interdigitating symphyseal suture more similar to that of the north Pacific toothed mysticetes, the Aetiocetidae, as well as Chaeomysticeti, and the lack of a tight articular suture indicates flexibility at the intramandibular joint. Coronodon seems to lack a symphyseal groove, present in the Aetiocetidae + Chaeomysticeti clade, which was recently named the Kinetomenta by Gatesy et al. (2022). A shallow furrow is present ventrally in the holotypes of Coronodon havensteini (CCNHM 108) and Coronodon newtonorum n. sp. (ChM PV 2778), and this may be the homolog of the more deeply incised groove in the Kinetomenta. This groove appears to be an ontogenetic remnant of the groove for the Meckel’s cartilage (Mead & Fordyce, 2009) which persists into early postnatal ontogeny in some early chaeomysticetes (Boessenecker & Fordyce, 2015c). Coronodon differs from Aetiocetidae and all other Kinetomenta by possessing a flattened symphyseal surface. If some intramandibular motion was permitted, it likely was much less than that occurring in extant mysticetes.
Intramandibular kinesis is generally interpreted as an adaptation for filter feeding in mysticetes (Lambertsen, Ulrich & Straley, 1995; Deméré et al., 2008; Gatesy et al., 2022). It would permit longitudinal rotation of the mandible as well as slight lateral abduction of the tips of the mandibles, both motions of which serve to increase the volume of the oral cavity during feeding in extant mysticetes (Lambertsen, Ulrich & Straley, 1995; Goldbogen, Pyenson & Shadwick, 2007; Potvin, Goldbogen & Shadwick, 2009). Accordingly, loss of a firm mandibular joint in Coronodon, though not initially cited, may further support the filter feeding interpretation for Coronodon (Geisler et al., 2017; Gatesy et al., 2022). Like the Aetiocetidae, Coronodon possesses straight mandibles that are not laterally bowed like those of Chaeomysticeti, which suggests only an incipient increase in oral volume during filter feeding. Other hypotheses that have not yet been proposed might be worth exploring; for example, mandibular fusion vs. the retention of a suture (analogous to basilosaurids) in terrestrial and aquatic Carnivora is related to bilateral biting (fusion) or unilateral chewing and gnawing of even harder food items (Scapino, 1981; Scott, Hogue & Ravosa, 2012; Tseng, Grohé & Flynn, 2016). Complete loss of mandibular articulation is rare in mammals and aside from cetaceans, seems to occur only in anteaters (Ferreira-Cardoso et al., 2020). It is perhaps not a coincidence that anteaters, like baleen whales, have also lost their dentition (Ferreira-Cardoso, Delsuc & Hautier, 2019). A loose mandibular symphysis in Coronodon seems best associated with filter feeding (e.g., Gatesy et al., 2022), though incipient kinesis (e.g., firm, rather than rigid) may have accommodated a powerful bite as in some extant mammalian carnivores employing a unilateral bite (Scapino, 1981).
In addition to intramandibular kinesis, the glenoid fossa of the referred adult skull of Coronodon havensteini (CCNHM 164) and the holotype of Coronodon planifrons (CCNHM 166) both possess a bilobate glenoid fossa. In these specimens, a secondary fossa with a deeply pitted rugose texture matching the rugose texture of the mandibular condyles of all known specimens of Coronodon is present dorsomedially to the glenoid fossa proper. This secondary fossa suggests the presence of an unusual articulation, and perhaps indicates a movable craniomandibular joint permitting the longitudinal rotation and/or medial adduction of the posterior mandible by a few centimeters as in extant mysticetes (Lambertsen, Ulrich & Straley, 1995). This secondary glenoid fossa was not observed in the Coronodon havensteini holotype as this surface is completely smooth. Because the secondary fossa in CCNHM 164 and 166 bears similarly rugose, somewhat vermiform pattern of ridges matching the surface texture of the mandibular condyle, the secondary glenoid fossa cannot be dismissed as a sinus (such as the tympanosquamosal recess of odontocetes; Fraser & Purves, 1960). The presence of a clear concave glenoid fossa likely indicates the existence of a synovial joint in Coronodon, as inferred for Eomysticetidae (Boessenecker & Fordyce, 2015a).
Rostral kinesis in Coronodon
Extant mysticetes possess sutures between the rostral elements and between the rostrum and frontal that are completely or partially open (Bouetel, 2005). Most Chaeomysticeti possess a completely unfused premaxilla-maxilla suture and slight mortising (reciprocal ridges and grooves) of the premaxilla-frontal and maxilla-frontal joints, the latter of which is confined to the ascending process of the maxilla. Eomysticetids possess an intermediate morphology with premaxillae that are somewhat firmly articulated with the prenarial process of the frontal and share a firm sutured joint with the lateral edge of the nasal, but lack any sutural grooves or rough articular surfaces for the maxilla-frontal suture, suggesting that the maxilla was movable (Boessenecker & Fordyce, 2015c). Toothed mysticetes such as the Aetiocetidae, Mammalodontidae, and Llanocetus have typically been inferred to have firm (akinetic) rostral and rostro-frontal sutures (Fitzgerald, 2010). Among these, Mammalodon exhibits some postmortem splaying of the maxilla and premaxilla, revealing an open suture and only a lightly mortised maxilla-frontal and premaxilla-frontal joint, generally resembling chaeomysticetes. Advanced dental wear in the Mammalodon holotype indicates that rostral disarticulation this cannot simply be dismissed as representing an early ontogenetic stage prior to suture closure. Kinetic rostra have not been reported in the Aetiocetidae, though many of these are collected and physically prepared from highly indurated concretions and observation of features of kinetic rostra would require acid preparation or CT imaging. Some toothed mysticetes exhibit rostral sutures that are clearly firmly closed, including Llanocetus denticrenatus, Mystacodon selenensis, Janjucetus hunderi, and Fucaia goedertorum, all of which possess a closed maxilla-premaxilla and/or maxilla-frontal suture. For example, in Fucaia goedertorum, loss of parts of the ascending process of the maxilla reveals a lightly rugose frontomaxillary sutural surface (R. W. Boessenecker, 2022, personal observation), differing from the planar surface of the frontal in Coronodon. Regardless, limits of study imposed by preservation and preparation methods suggest that the assumption of akinetic rostra in most toothed mysticetes has not been substantiated by careful observation.
Coronodon spp., on the other hand, possess a premaxilla-maxilla suture with only faint topography that instead is developed more like a planar ‘butt joint’. The maxilla-frontal articulation bears no sutural ridges or grooves. In contrast, the frontal bears deep grooves and ridges for an anteroposteriorly short premaxilla-frontal and nasofrontal articulation, and the premaxilla also articulates with a similar surface on the ventrolateral edge of the nasal. This condition is similar to that of the Eomysticetidae, with immobile premaxillae buttressed by short triangular prenarial processes (longer in Eomysticetidae) of the frontal that underlie the posteriormost premaxilla, accompanied by apparently mobile maxillae.
These sutures suggest a greater degree of kinesis in the rostrum of Coronodon than other toothed mysticetes, and differs strongly from the rigid rostra of basilosaurids. Rostral kinesis has received relatively little attention in the highly contested debates over the origin of filter feeding in stem mysticetes (e.g., Boessenecker & Fordyce, 2015c). Kinesis is poorly understood even in extant mysticetes, but is hypothesized to permit some flexibility of the rostrum during bulk filter feeding (Bouetel, 2005). It is unknown whether rostral kinesis is simply passive during filter feeding (e.g., accommodating hydrodynamic forces imposed upon the rostrum and palate during filter feeding) or actively controlled; it is hard to imagine the latter scenario, given the lack of muscles that insert onto the rostrum in extant mysticetes (Schulte, 1916). Several possibilities could explain rostral kinesis in Coronodon. Kinesis could passively permit slight deformation of the rostrum by hydrodynamic forces during filter feeding; active movement of the maxilla could further permit adjustment to the alignment of upper and lower teeth to control the dental filtering process. It is also possible that this loss of a firm articulation may be non-functional, paralleling the loss of a median premaxillary articulation and development of the mesorostral groove in Neoceti. If a looser premaxilla-maxilla articulation parallels the mesorostral groove, perhaps this open suture might rather represent an exaptation in later filter feeding mysticetes.
Tympanoperiotic fusion in Coronodon planifrons
Fusion of the posterior processes into a compound process is a key character in mysticete phylogeny, at present considered to diagnose a somewhat more exclusive clade of Chaeomysticeti excluding the Eomysticetidae and other archaic chaeomysticetes like Horopeta, Toipahautea, and Whakakai (Boessenecker & Fordyce, 2015c; Tsai & Fordyce, 2015, 2016, 2018) but including Mauicetus (Tsai & Fordyce, 2015; Marx & Fordyce, 2015). The derived condition also characterizes all extant species of mysticetes. Initially, a fused and long posterior process was considered a mysticete synapomorphy, prior to the discovery of toothed mysticetes and eomysticetids with unfused posterior processes (e.g., Geisler & Sanders, 2003).
The holotype of Coronodon planifrons is distinctive in possessing fused posterior processes of the bulla and periotic, but only on the left side. Owing to the asymmetry of this structure in the Coronodon planifrons holotype (CCNHM 166) and absence of fusion in any other toothed mysticetes, this condition is best interpreted as pathologic. However, if the Coronodon planifrons holotype had been discovered prior to Coronodon havensteini, and only with the fused periotic, such a condition could be misinterpreted as indicating a more crownward position of Coronodon along the mysticete stem. More practically, the asymmetrical morphology of the periotics of Coronodon planifrons indicates that the periotic morphology of stem mysticete taxa known by only a left or right periotic from a single specimen (e.g., Fucaia buelli, Mammalodon colliveri, Salishicetus meadi, Tohoraata raekohao) should be interpreted carefully.
Postcranial morphology and locomotor adaptations in Coronodon
The holotype skeleton of Coronodon havensteini possesses a complete set of cervical vertebrae and a nearly complete thoracic series. Extensive postcrania in the newly referred skeleton of C. havensteini CCNHM 164 and the holotype of Coronodon planifrons (CCNHM 166) reveal much of the remaining postcranial morphology, vertebral count, and locomotor adaptations in the earliest diverging toothed mysticetes (Fig. 50; Tables 7–11).
Figure 50: Composite skeletal reconstruction and composite vertebral profile of Coronodon.
(A) Composite skeletal reconstruction of Coronodon, (B) composite vertebral profile of Coronodon. Skull and cervical vertebrae after Coronodon havensteini holotype CCNHM 108, thoracic vertebrae and scapula after referred Coronodon havensteini specimen CCNHM 164, and lumbocaudal vertebrae after Coronodon planifrons holotype CCNHM 166. Measurements for cervical and thoracic vertebrae for vertebral profile derived from Coronodon havensteini holotype (CCNHM 108), and measurements for lumbar and caudal vertebrae derived from Coronodon planifrons holotype (CCNHM 166). Vertebral profile diagram after Buchholtz (2001) and Boessenecker et al. (2020).No single specimen of Coronodon possesses a complete series of thoracic and lumbar vertebrae, however, the Coronodon planifrons holotype (CCNHM 166) preserves the posteriormost thoracics and a complete set of lumbars. Referred Coronodon havensteini specimen CCNHM 164 preserves three isolated lumbars and nine thoracics (Tables 7–11). Centrum measurements of the thoracic vertebrae indicate that these constitute a continuous series from the T1 through the T9; the T9, critically, matches the posteriormost thoracic vertebra and presumed T9 in CCNHM 166 (C. planifrons n. sp.). Based on these two specimens, a count of 9 thoracics is most likely for the genus, though a count of 10 may be possible. CCNHM 166 (C. planifrons n. sp.) preserves 10 lumbar vertebrae, and a jump in measurements between the anteriormost (L1) and the next preserved vertebra may suggest that L2 is missing, and that a total of 11 lumbar vertebrae were present; a count of ten is conservatively estimated. Nine caudal vertebrae (and additional fragments) are preserved, and the total likely exceeded 20 caudals in CCNHM 166 (C. planifrons n. sp.), consistent with the primitive number of 21 caudals for Neoceti reconstructed by Buchholtz & Gee (2017). Comparison of the holotype vertebrae with those of CCNHM 164 and C. planifrons n. sp. (CCNHM 166) and measurements of the holotype vertebrae indicate that only T5 and T9 are missing. Initially, Geisler et al. (2017) assumed a higher thoracic count for basal Neoceti (e.g., Buchholtz & Gee, 2017), and under the assumption that the thoracic series was too incomplete to identify further, only identified T1-2.
Like other toothed mysticetes and chaeomysticetes, the vertebral column of Coronodon includes relatively flattened disk-like cervical vertebrae, thoracic and lumbar vertebrae with wide centra, and gradually increasing length, depth, and width in the lumbar series peaking around the lumbocaudal boundary (Fig. 50B). Centrum length peaks in the mid-lumbars, whereas centrum height peaks in the anterior caudals; this suggests incipient development of a stiffened tail stock (Fig. 50B). However, the caudals are all relatively wide, suggesting that, like early odontocetes (Albertocetus, Ankylorhiza; Boessenecker, Ahmed & Geisler, 2017; Boessenecker et al., 2020), Coronodon did not possess a transversely narrowed caudal peduncle and that this feature evolved independently within Odontoceti and Mysticeti (Boessenecker et al., 2020). The posteriormost caudal, Ca D, of CCNHM 166 (C. planifrons n. sp.) is rectangular and somewhat dorsoventrally shallower than wide (Figs. 44, 50B), indicating the presence of a caudal fluke like Basilosauridae and all other Neoceti for which caudal vertebrae are known (Uhen, 2004a; Gingerich, Antar & Zalmout, 2019).
Gradual changes in vertebral dimensions and the lack of clear regionalization of the vertebral column (Tables 8–11, Fig. 50B) indicates that Coronodon can be assigned to “Pattern 1” swimmers, like basilosaurid whales and other mysticetes (Buchholtz, 2001). Though of similar size, the giant dolphin Ankylorhiza (from the same Oligocene strata as Coronodon spp.) was a “Pattern 2” swimmer (similar to Ziphiidae and the beluga, Delphinapterus; Boessenecker et al., 2020) and was apparently a somewhat more efficient swimmer than Coronodon (E. Buchholtz, 2022, personal communication). The vertebral profile of Coronodon (Fig. 50B) is relatively similar to the small basilosaurid Zygorhiza as well as the toothed mysticete Aetiocetus cotylalveus (Buchholtz, 2001). If Coronodon was an apex predator as proposed by Hocking et al. (2017), it possessed no postcranial specializations for it, like the earlier apex predator Basilosaurus (Fahlke, 2012; Voss et al., 2019) and was also a pattern 1 swimmer (Buchholtz, 2001). In contrast, the contemporary odontocete Ankylorhiza was an apex predator and pattern 2 swimmer (Boessenecker et al., 2020).
Body length and skull proportions of Coronodon
Estimation of body length using the equations of Pyenson & Sponberg (2011) resulted in a length of 4.22 m using the bizygomatic skull width and 4.41 m using the partial least squares method. Estimation of the body length of Coronodon used the skull length of CCNHM 108 (=99 cm; C. havensteini), the cumulative cervical length of CCNHM 108 (=24 cm; C. havensteini), cumulative thoracic length of CCNHM 164 (=53 cm; C. havensteini), the cumulative lumbar length of CCNHM 166 (=91 cm; C. planifrons n. sp.), and the cumulative caudal length of CCNHM 166 with missing vertebrae estimated (=140 cm; C. planifrons n. sp.) to fill the ancestral count of 13 anterior caudals and nine fluke caudals from Buchholtz & Gee (2017), for a skeletal length of 4.08 m. To estimate the length of the vertebral column constituted by cartilaginous intervertebral disks, we applied the average disk:vertebra length ratio of 24:100 in Delphinus delphis from Long et al. (1997) to the average vertebra length within each region and multiplied by the vertebra count from each region, resulting in a total additional length of 76.8 cm. Altogether, this yields a body length of 4.8–5.0 m, depending upon the exact count of caudal vertebrae.
Pyenson & Sponberg (2011) did not indicate the unit of skull measurements to be entered into their equations, and if millimeters rather than centimeters are used, the bizygomatic width equation provides a much smaller body length estimate of 3.55 m for Coronodon havensteini. We are confident that the correct units are centimeters; we plugged in bizygomatic width (in cm) into their equations and were able to replicate values they provided in their Table 4 for several taxa. For further comparability/repeatability, it would be ideal if the dataset used for the analysis by Pyenson & Sponberg (2011) were published. It is important to determine if the difference between the lengths calculated from BZW and PLS equations, and the length estimated from the vertebral column, which we consider to be more reliable, are just expected errors or a sign that fundamental proportions between the skull and the vertebral column have changed over time, and cannot be easily inferred from equations derived from extant taxa only.
Geisler et al. (2017) predicted that if Coronodon engaged in dental filtration, then the relative skull length, and size of the oral cavity in particular, would increase at the origin of Mysticeti. Subsequent study of Mystacodon by Muizon et al. (2019) suggests such an initial increase of oral volume, through a larger palate. Extant mysticetes have enormous heads, which allow for a larger oral cavity and greater efficiency for filter-feeding, and thus there is clear functional link between behavior and relative head size. Physeter macrocephalus may be an exception as it is a suction feeder, though its massive head size is likely driven by sexual selection and intraspecific combat (Carrier, Deban & Otterstrom, 2002). Using our length estimate from the preserved vertebral columns of Coronodon spp., we estimate that the skull of Coronodon comprised approximately 20% of body length. This is much greater than the relative skull size in basilosaurids (Uhen, 2004a; Muizon et al., 2019) and at first glance would appear to support the prediction of Geisler et al. (2017). However, protocetids have a skull that comprises a much greater proportion of the body length, as compared to basilosaurids, and also have fewer lumbar vertebrae (Gingerich et al., 2009; Uhen, 2014). In addition, the basal odontocete Ankylorhiza (Boessenecker et al., 2020) has a head that also comprises about 20% of body length. Thus to clarify the evolution of head length, relative to body length, across the archaeocete to neocete transition will require a better understanding of the relationships of stem neocetes to basilosaurids and other members of Pelagiceti, as well as how proportions are influenced by changes in length of the rostrum, vertebral count, and vertebral length.
Do Neoceti and Mysticeti include Coronodonidae and Mystacodon?
As described in Results of Phylogenetic Analysis, our EW analysis placed Coronodonidae and Mystacodon within Mysticeti (Fig. 47). Character support for these “traditional” placements have been thoroughly discussed in previous studies (Geisler et al., 2017; Muizon et al., 2019), but with our unconventional IW trees in hand (Fig. 48), we can reexamine some of these characters, explore characters that support exclusion of Coronodon and Mystacodon from Mysticeti, and compare the degree that these osteological characters support or contradict alternative placements of these genera (Fig. 51). Our EW trees support Coronodonidae and Mystacodon as mysticetes, and there are seven characters that are synapomorphies of Mysticeti that have a shorter length as compared to the IW trees, including maxilla/premaxilla suture marked by a deep groove (52:0>1; 1 step shorter), supraorbital process of frontal narrows laterally (78:1>0; 1 to 3 steps shorter), orbitotemporal crest extends onto frontals (98:0>1; 2 to 3 steps shorter), paroccipital process swollen with pit for stylohyoid (112:0>2; 2 to 4 steps shorter), triangular supraoccipital (114:0>1; 2 to 3 steps shorter), bulbous basioccipital crest (153:0>1; 1 step shorter), and, on average, 4.5 to 5 distal cusps on premolars (307:1>0; 2 steps shorter). Somewhat surprisingly, a traditional mysticete synapomorphy, the antorbital process (Barnes, 1990; Sanders & Barnes, 2002b), is the same length on the EW and IW trees. We coded the antorbital process as present in the early odontocete Olympicetus, mainly based on CCNHM 1000 (Racicot et al., 2019). As a result, in the EW trees, where coronodonids are mysticetes, its presence in Olympicetus is optimized as convergent (two steps), whereas in the IW trees, where coronodonids are outside of Mysticeti, this character state is a synapomorphy of all neocetes and its absence in most odontocetes is considered a reversal (also two steps).
Figure 51: Morphology of Coronodon and character evidence for key clades across the archaeocete-neocete transition.
Character states in Coronodon supporting the Equal Weights (EW) phylogeny shown in black boxes; states supporting the Implied Weighting (IW) phylogeny shown in white boxes; states supporting both shown in grey. Colored circles show character states supporting each node on the EW and IW trees. X indicates the absence of a synapomorphic character state in Coronodon, chiefly synapomorphies for Neoceti B.A Neoceti that excludes Coronodonidae, Mystacodon, Borealodon, and Metasqualodon, as was found in our IW trees, is diagnosed by eight synapomorphies. Six of the characters that include these synapomorphies also require less steps (i.e., support) than the traditional, and more inclusive, concept for Neoceti, including parietals are wider than long in dorsal view (Fig. 51; 93:0>2; 1 step shorter), fenestra ovalis within anterior 2/3rds of promontorium (177:1 to 2 steps shorter), posterior bullar facet of periotic lacks longitudinal grooves (183:1>0; 1 step shorter), largest tooth is of medium size (320:2 or 3>1; 5 to 6 steps shorter), neural canal of C3–C7 has a flat ventral margin (339:0>1; 1 step shorter), and elevated transverse process of C7 (340:0>1; 2 steps shorter). For each of these characters, coronodonids share the same morphology as basilosaurids but differ from the morphology in many basal odontocetes. Thus, repositioning coronodonids outside of crown Neoceti requires less steps. The largest decrease in length occurs in the character that codes for tooth size relative to bizygomatic width (Fig. 51; character 320). Under the IW trees, there is a clear trend for decreasing tooth size among stem odontocetes and stem mysticetes; the first reduction occurs at Neoceti and then a second reduction at the base of Mysticeti. This trend co-occurs with a more complicated pattern of tooth simplification and reduction in heterodonty in cetaceans (Gatesy et al., 2013; Peredo, Peredo & Pyenson, 2018a), as seen in the differences between the teeth of basilosaurids and early Neoceti like Aetiocetus cotylalveus or Echovenator sandersi. A reduction in tooth size is consistent with the hypothesis that suction feeding evolved before the origin of Neoceti (Johnston & Berta, 2011); once this behavior developed, teeth played less of a role in prey capture.
Another clade in the IW trees includes coronodonids and neocetes, but excludes Mystacodon, Kekenodon, and basilosaurids. This result conflicts with previous studies that supported a sister-group relationship between Mystacodon and Llanocetus (Fordyce & Marx, 2018), other studies that placed Mystacodon as the most basal mysticete (Lambert et al., 2017; Peredo et al., 2018), and our EW trees. There are three characters that diagnose the clade that excludes Mystacodon and also require less steps, as compared to the EW trees (Fig. 51): maxilla and premaxilla lack a scalloped edge (55:0>1; 1 step shorter), small anterior teeth (299:0>1; 1 step shorter), and scapular blade rapidly widens (350:0>1; 1 step shorter). One new character in the present study, the size of the anterior teeth relative to bizygomatic width, mirrors the previously discussed decrease in largest tooth size (character 319). This is interesting because whereas reductions in the largest tooth size among basal neocetes likely reflect a reduction in mastication and prey processing (Gatesy et al., 2013), a reduction in the anterior teeth is important evidence for a decreased reliance on macroraptorial feeding (Werth, 2000). The holotype skull (CCNHM 108) of Coronodon havensteini preserves a single lower canine but none of the incisors. The discovery of referred specimen CCNHM 164 reveals that the incisors and canines are all surprisingly small. The anteroposterior diameter of these teeth is only 3–4% of bizygomatic width, comparing well with odontocetes. In contrast, basilosaurid and protocetid archaeocetes possess anterior teeth (i3/I3 or c/C) with an anteroposterior crown length between 6% and 10% of bizygomatic width. Mystacodon selenensis has an upper I3 and C that are 8–9% of the bizygomatic width, similar in size to these teeth in basilosaurids. Muizon et al. (2019) calculated the sum of the mesiodistal lengths of the anterior dentition for Mystacodon and three cetaceans straddling the archaeocete/neocete transition, and they too found Mystacodon to have larger anterior teeth than Coronodon. However, when calculated in this way, the anterior teeth of Mystacodon are more intermediate in size between those of archaeocetes and Coronodon, rather than being within the archaeocete range of variation. Finally, the distal end of the scapula of Mystacodon is quite narrow, resembling those of archaeocetes and likely convergent with the morphology of balaenids (Benke, 1993). By contrast, a specimen of Coronodon havensteini (CCNHM 164) has a partial scapula, which, although incomplete, clearly had a blade that rapidly increased in width. Scapulae of basal neocetes are very rare, and it is important that additional specimens are found to test whether an abruptly widening scapular blade is characteristic of all neocetes.
Although not differing in length among trees from our two hypotheses, two other characters that are optimized as synapomorphies of the clade that excludes Mystacodon merit discussion. The first is the occurrence of 12 mandibular teeth (Fig. 51; 293:4>5) and the second is the presence of seven upper postcanine teeth (300:0>1). Having 12 mandibular teeth is an instance of polydonty, where there are more teeth than the highly conserved tooth limit, at least within Eutheria, of 11 teeth per dental quadrant. Polydonty was proposed, with caveats, as a potential synapomorphy for the Neoceti by Fordyce & Muizon (2001). All extant odontocetes are polydont or evolved from a polydont ancestor, and the embryonic dentition of most extant mysticetes is polydont (Karlsen, 1962; Thewissen et al., 2017; Lanzetti, Berta & Ekdale, 2020). However, as noted by Fordyce & Muizon (2001), some stem mysticetes and stem odontocetes possess a tooth count identical with basilosaurid whales (ten uppers and eleven lowers), such as the mysticetes Janjucetus and Mystacodon, and the odontocete Simocetus (Fordyce, 2002; Fitzgerald, 2006, 2010; Muizon et al., 2019). Aetiocetid mysticetes, including Aetiocetus spp., Salishicetus meadi, and Morawanocetus yabukii, the mammalodontid toothed mysticete Mammalodon colliveri as well as early odontocetes including most Xenorophidae (except the toothless Inermorostrum), Ankylorhiza, and Waipatia have minimal (8–9 postcanines) to moderate (10–15 + postcanines) polydonty, perhaps suggesting that the common ancestor of mysticetes and odontocetes had the developmental capacity for polydonty. Our IW trees support a small degree of polydonty (i.e., 12 mandibular teeth) as a neocete synapomorphy and that the presence of only 11 teeth in Simocetus is considered a reversal to the primitive condition. The morphology in coronodonids is based on the holotype of C. havensteini, which as explained above is now interpreted to have four lower molars and 12 lower teeth in total. Under our EW trees, the occurrence of 12 mandibular teeth in coronodonids is best interpreted as convergent with the presence of 12 or more teeth in odontocetes, such as Echovenator sandersi, but homologous to the presence of 12 or more teeth in most toothed mysticetes, such as Mammalodon and Aetiocetus. For the second character, the number of upper postcanine teeth (character 300), the primitive condition for our EW and IW trees is six postcanine teeth, consistent with this feature being a synapomorphy of Pelagiceti (Martinez-Caceres, Lambert & Muizon, 2017). The optimization on our trees is driven by the inclusion of the basilosaurids Zygorhiza, Dorudon, and Basilosaurus, which lack M3 and only have four premolars and two molars. More basal cetaceans, such as pakicetids and protocetids, have three upper molars and seven postcanine teeth (Hulbert et al., 1998; Cooper, Thewissen & Hussain, 2009). Thus, the optimization of seven postcanine teeth at the node that excludes Mystacodon raises two important questions: is the presence of seven postcanine teeth the plesiomorphic state, with the loss of the M3 as a synapomorphy of Basilosauridae, or are last molars in coronodonids not homologous to M3 of protocetids? If the answer to the latter question is yes, then the occurrence of seven postcanine teeth in early neocetes could mark concurrent polydonty in the upper and lower dentition; the lowers going from 11 to 12 teeth and the uppers from 10 to 11 (6 to 7 postcanines).
Returning to the question that headed this section: “Do Neoceti and Mysticeti include Coronodonidae and Mystacodon?”, we tentatively suggest that that the answer to both is yes, based on our EW analyses. This is the traditional view of these taxa, and although implied weighting can be more efficient in yielding the shortest trees (Goloboff et al., 2008), it can become problematic when different partitions have very different amounts of missing data, such as a morphological partition with many fossils as compared to a molecular partition where fossils cannot be coded (Goloboff, 2014). Although there are some analytical techniques that address these complexities (Goloboff, 2014), they often require substantially more computing time and additional assumptions of weights among partitions, which can be difficult to justify. In the present study, one of the characters that supports the IW over the EW trees is a rapidly widening scapular blade; Mystacodon has a narrow blade, like archaeocetes. However, most extinct mysticetes are not represented by scapulae, and thus the homoplasy of this character is likely undercounted, as compared to many cranial characters, which are better represented in the fossil record. This undercounted homoplasy likely leads to higher weights in the implied weighting analysis. That said bootstrap values for the conflicting nodes between the EW and IW trees are poorly supported, and we can easily envision that one or two new fossil species, as well as careful evaluation of known characters, could more clearly tip the balance in favor of one topology over the other. Such examples might include the description of OU 22294, cf. Kekenodon (e.g., Clementz et al., 2014: fig. 1).
We encourage future studies to include more protocetids into this dataset, which should help polarize characters and ensure that the root is accurately identified. In addition, we encourage more detailed study of two character-rich regions. The first is the anterior edge of the orbit, including the antorbital process, antorbital notch, zygomatic process of the maxilla, posteriormost dentition (in some taxa), lacrimal, and lacrimal foramina and/or canals. This region of the skull is quite different in odontocetes and mysticetes, and both are also unique as compared to basilosaurids. Careful comparison of basal odontocetes and mysticetes would help ensure that characters are coded consistently and that individual changes are not “upweighted” by the inclusion of logically separate, but clearly related and non-independent morphological characters. Another likely fruitful approach would be direct comparisons of the teeth of basal mysticetes and odontocetes, with an eye to improving existing characters and developing new ones. One challenge in this undertaking would be the basic homology statements needed to code characters, including whether the teeth in basal neocetes are homologous to the deciduous or adult teeth of archaeocetes (e.g., Uhen & Gingerich, 2001; Geisler et al., 2017) as well as the homology of teeth among taxa with very different tooth counts.
Synapomorphies for Neoceti revisited
Neoceti is the taxon that refers to the crown group including Odontoceti and Mysticeti (Fordyce, 2009). It is equivalent to Autoceta, an older, rarely used, and imprecisely defined name with a similar taxonomic composition (Geisler & Sanders, 2003; Fordyce, 2009). The phylogeny and origin of the clade Neoceti has come into focus in recent years with many studies reporting ever-more plesiomorphic stem mysticete and stem odontocete fossils, resulting in continual reevaluation of character transformations across the archaeocete-neocete transition (Fordyce, 2002; Geisler & Sanders, 2003; Fitzgerald, 2006, 2010; Uhen, 2008; Sanders & Geisler, 2015; Geisler, Colbert & Carew, 2014; Geisler et al., 2017; Lambert et al., 2017; Velez-Juarbe, 2017; Fordyce & Marx, 2018; Corrie & Fordyce, 2022). Although many synapomorphies proposed in the 1990s, prior to the detailed study of Oligocene stem odontocetes and mysticetes, have been challenged or refuted through the discovery of plesiomorphic fossils (Geisler & Sanders, 2003), a few reliable synapomorphies have remained. However, what are, or are not, neocete synapomorphies should be revisited given the recent redescription of Kekenodon onamata, which Corrie & Fordyce (2022) placed as the sister-group to Neoceti, as well as the possibility that several taxa traditionally considered toothed mysticetes might instead fall outside Neoceti (i.e., Mammalodontidae, Llanocetus, Mystacodon, and Coronodon).
One character frequently cited as a neocete synapomorphy, an immobile elbow joint (Barnes, 1990; Muizon et al., 2019; Lambert et al., 2017; Boessenecker et al., 2020), results from separate, flat facets for the radius and ulna on the distal humerus. Sanders & Geisler (2015) suggested the archaic odontocete Mirocetus had a mobile elbow joint, but the holotype and only known skeleton is not well preserved, and the second author of that study now believes the morphology of this taxon is better considered uncertain. While such a joint is unknown in Coronodon, it is clearly immobile in the basilosaurid-like toothed mysticete Mystacodon (Muizon et al., 2019). This character was included in the present study (character 359), and it is a synapomorphy of Neoceti or Kekenodon + Neoceti on the EW trees or is a synapomorphy of one of two clades of the IW trees (Mystacodon + Coronodonidae + Neoceti or this clade + Kekenodon). Determining which inference is correct will require the elbow of Kekenodon, or a close relative, to be described.
Geisler & Sanders (2003) stated that a posterior position of the ascending process of the premaxilla, specifically one where this bone terminates in line with the orbit, is a synapomorphy of Neoceti. In most mammals the premaxilla typically terminates on the rostrum between the nasal and maxilla, but in cetaceans, the nasals migrate posteriorly along with the bony nares (Churchill et al., 2018; Roston & Roth, 2019; Coombs et al., 2022). In protocetids, the premaxilla typically terminates around the middle of the rostrum, whereas in basilosaurids, it extends further posteriorly along the posterior quarter of the rostrum (Geisler, Sanders & Luo, 2005). In the EW and IW trees of the present study, premaxillae terminating in line with the orbits (Fig. 51; character 8) is not a synapomorphy of Neoceti, but instead diagnoses a larger clade that includes Kekenodon, Coronodon, and Neoceti. However, it should be noted the terminal ends of the premaxillae are not preserved in the holotype skull of Kekenodon onamata, and this inference is based on an interpretation of the sutural surfaces on the frontal (Corrie & Fordyce, 2022). If this inference is incorrect, then premaxillae reaching the level of the orbits would still be a neocete synapomorphy on the EW trees.
Loss of the sagittal crest was identified by Martinez-Caceres, Lambert & Muizon (2017) as a possible synapomorphy of Neoceti. However, this was a result of the limited sample of mysticete and odontocete OTUs, which did not include Coronodon or Mystacodon, both of which have a sagittal crest. Tall basilosaurid-like sagittal crests are not yet known in Odontoceti, though the xenorophids Albertocetus and Xenorophus (CCNHM 104, 168, 1077, ChM PV 4823) possess low sagittal crests and an ovoid cross-section of the intertemporal constriction (Boessenecker, Ahmed & Geisler, 2017; R. W. Boessenecker and J. H. Geisler, 2021, unpublished data). We also evaluated this character in the present study (character 97) and found multiple, equally parsimonious states for Neoceti, reflecting the variability of this trait in stem odontocetes and mysticetes.
Extant mysticetes and odontocetes are distinctive in possessing a loss of the bony articulation of the premaxillae dorsally and anterior to the bony nares, forming an open mesorostral groove floored by the vomer. In protocetids and basilosaurids the medial surface of the premaxilla is flat and articulates directly with the opposite premaxilla along a planar joint. Based on this distribution, Fordyce & Muizon (2001) suggested that a continuous mesorostral groove was a neocete synapomorphy, a result corroborated by Fitzgerald (2010). In Coronodon and other toothed mysticetes like Mystacodon, Janjucetus and Aetiocetus, the premaxillae are separate along most of their length, but articulate anteriorly; this articulation is quite reduced relative to basilosaurids. Unpublished skulls of Xenorophus possess a thin ascending flange of the premaxilla that slightly roofs over the mesorostral groove, and although the premaxillae nearly contact, there is no articular surface (R. W. Boessenecker, 2021, personal observation). In Echovenator, the ascending premaxillae completely roof over the canal and may be fused dorsally at the anterior tip (Churchill et al., 2016), though fracturing may obscure these details. The extent of a mesorostral groove was included in the present study (character 13), and it was not consistently optimized as a synapomorphy of Neoceti or adjacent node on our EW or IW trees, presumably because of anterior articulation of the premaxillae in several stem odontocetes and mysticetes.
A posterodorsally facing occipital shield, along with a supraoccipital apex shifted anteriorly relative to the occipital condyles, was proposed as a neocete synapomorphy by Martinez-Caceres, Lambert & Muizon (2017). In contrast, the occipital shield faces posteroventrally in protocetids and posteriorly in most basilosaurids. In early odontocetes (Xenorophidae, Agorophius, Simocetus, Ashleycetus, Mirocetus, Ankylorhiza) and all toothed mysticetes (Llanocetus, Mystacodon, Mammalodontidae, Aetiocetidae, and Coronodon) the occipital shield is subvertical and faces at least somewhat posterodorsally. Martinez-Caceres, Lambert & Muizon (2017) also proposed that a transverse constriction of the occipital shield at mid-depth is a synapomorphy of Neoceti. In general, most Neoceti possess a rectangular, semicircular, or triangular occipital shield when viewed in posterodorsal view. However, some basilosaurids also possess a rectangular occipital shield that is not constricted, including Basilosaurus cetoides, Cynthiacetus spp., and some specimens of Dorudon atrox (Kellogg, 1936; Uhen, 2005; Martinez-Caceres, Lambert & Muizon, 2017). Neither character was included in the present study, but a related one, the anteriormost extent of the supraoccipital (character 107) was. A supraoccipital that is extended anteriorly will also face posterodorsally, one not extended will be vertical, and one extended posteriorly will face posteroventrally. In our EW trees, a supraoccipital that extends anteriorly beyond the level of the posterior margin of the temporal fossae is a possible synapomorphy of Mysticeti, not Neoceti. By contrast, on our IW trees, it is equally parsimonious that this state is a synapomorphy of Neoceti or for it to have evolved convergently in coronodonids, some basal odontocetes, and other toothed mysticetes.
Possession of three or more dorsal infraorbital foramina was proposed by Barnes (1990) as a synapomorphy of Neoceti and later confirmed by the phylogenetic analysis of Geisler & Sanders (2003) and Fitzgerald (2010). Like terrestrial mammals, protocetids and earlier cetaceans only have a single dorsal infraorbital foramen. By contrast, some basilosaurids possess two (e.g., some specimens of Zygorhiza), and all mysticetes and odontocetes possess three or more (Geisler & Sanders, 2003), though their position and size vary considerably. This character was included in the present study (character 36) and is important for resolving the origin of Neoceti. On our EW trees, three dorsal infraorbital foramina diagnoses Neoceti or the clade of Neoceti + Kekenodon. Multiple equally parsimonious optimizations occur on our IW trees for this character, and it is difficult to summarize them. Two or three infraorbital foramina is a synapomorphy for Kekenodon + Coronodonidae + Mystacodon + Neoceti; and some optimizations have three foramina evolving on multiple occasions (e.g., Chaeomysticeti, Coronodonidae and kin, and Odontoceti). Fordyce & Muizon (2001) suggested that an antorbital notch incised into the maxilla, which transmits the facial nerve, was a synapomorphy of Neoceti. In basilosaurids, there is a shallow furrow that faces ventrally, although this furrow is more a consequence of the zygomatic arch extending laterally beyond the alveolar portion of the maxilla, as is common to many mammals. In Coronodon, most other toothed mysticetes, and some stem odontocetes (e.g., Simocetus), the antorbital notch is a deeply incised, laterally facing furrow that curves anteriorly to become more dorsally or anteriorly facing. In Xenorophidae the antorbital notch is vertical and forms a transversely broad embayment lacking a clear groove; in most later diverging stem odontocetes (Squalodon, Prosqualodon, “Waipatiidae”) and crown Odontoceti the notch is clearly incised and vertically oriented, forming a gap between the base of the rostrum and the antorbital process. In other stem odontocetes like Ankylorhiza and Agorophius, an intermediate condition is present where the notch is dorsolaterally facing and developed between the preorbital process of the frontal and the maxilla. An antorbital notch was also evaluated in the present study (Fig. 51; character 22) and it diagnoses one of two clades on our EW trees (Neoceti or Neoceti + Kekenodon) or one of two clades on our IW trees (Neoceti + Mystacodon or Neoceti + Mystacodon + Kekenodon). The discovery and description of a more complete skull of Kekenodon, or a close relative, with this region preserved (e.g., OU 22294, cf. Kekenodon; Clementz et al., 2014: fig. 1) will allow the node that this synapomorphy applies to be determined.
Fordyce & Muizon (2001) listed loss of the exposure of the posterior/mastoid process of the periotic on the external skull wall as a synapomorphy for Neoceti, which was later supported by the phylogenetic analysis of Fitzgerald (2010). In all basal Neoceti and all odontocetes, the posttympanic ridge of the squamosal obscures the posterior process from the lateral edge of the skull. This includes all specimens of coronodonids examined in the present study. In basilosaurids, and especially protocetids, the posterior process is exposed laterally (Luo & Gingerich, 1999). Reversals to this plesiomorphic condition occur in cetotheriid baleen whales (Bouetel & de Muizon, 2006) and most, if not all, extant mysticetes (Luo & Gingerich, 1999), though the posterior process of the bulla and periotic are fused within Crown Mysticeti and the degree of exposure of the periotic is uncertain and possibly completely obscured by the posterior process of the bulla in many taxa. The derived ‘amastoid’ condition appears in at least one unpublished kekenodontid whale (Fordyce, 2004), although the condition of this character is unknown for Kekenodon onamata (Corrie & Fordyce, 2022). Thus, although this feature (Fig. 51; character 194) supports a clade of neocetes to the exclusion of basilosaurids and other archaeocetes, it diagnoses one of two clades on our EW trees (Neoceti or Neoceti + Kekenodon) or one of two clades on our IW trees (Neoceti + Mystacodon or Neoceti + Mystacodon + Kekenodon).
Extant cetaceans are highly distinctive in possessing a monophyodont dentition (Fordyce & Muizon, 2001; Uhen & Gingerich, 2001); odontocetes only possess a single set of teeth, and extant mysticetes develop and resorb a single set during fetal development (Karlsen, 1962; Thewissen et al., 2017; Lanzetti, Berta & Ekdale, 2020). This is a difficult character to evaluate as a sample of juveniles of the same species is needed to confirm the presence of deciduous and permanent teeth (Uhen, 2004a, 2004b); at present, few, if any, stem odontocetes or mysticetes are known from such samples. Coronodon havensteini is now known from four individuals, the largest available sample of any toothed mysticete species. At present, ChM PV 4745 possesses teeth that are as large as those of the holotype skull of Coronodon havensteini, indicating ontogenetically early eruption of permanent teeth as in some extant odontocetes (Perrin, 1975). However, it is possible that deciduous teeth could have been present and shed in utero as in extant pinnipeds (Scheffer & Kraus, 1964), given that ChM PV 4745 is a relatively large juvenile. At present it is unclear whether cetaceans have lost the permanent teeth and retained the deciduous teeth or vice versa, or even some combination of both sets (Fordyce, 1982; Uhen & Gingerich, 2001). Similarly, the holotype specimen of the small basilosaurid archaeocete Chrysocetus healeyorum, hypothesized as one of the most crownward basilosaurids, possesses relatively large (presumed) permanent teeth despite being a subadult, suggesting that monophyodonty may have evolved prior to the origin of Neoceti and within the Basilosauridae (Uhen & Gingerich, 2001), given that Zygorhiza and Dorudon both preserve juveniles with a mix of deciduous and permanent teeth (Kellogg, 1936; Uhen, 2004a, 2004b). However, the premolars in Coronodon more closely resemble the deciduous teeth of basilosaurids in having relatively smooth enamel and accessory cusps that are nearly as large as the primary cusp, suggesting the evolution of monophyodonty through the loss of the permanent dentition (Geisler et al., 2017). Evaluation of these varying hypotheses will require the discovery of ontogenetically younger neonatal toothed mysticetes and stem odontocetes, dental histology, or geochemical/isotopic study of early neocete teeth. Although this character was not included in the current data matrix, we infer that it is a synapomorphy of one of two clades on our EW trees (Neoceti or Neoceti + Kekenodon) or one of four clades on our IW trees (Neoceti, Neoceti + Coronodon, Neoceti + Coronodon + Mystacodon, Neoceti + Coronodon + Mystacodon + Kekenodon).
Embrasure pits are deep recesses formed through the remodeling of bone in the rostrum and mandible that prevent the apices of the opposing dentition from impacting bone during occlusion of large teeth, as is characteristic of archaeocetes (Uhen, 2004a). Loss of these embrasure pits was proposed as a synapomorphy of Neoceti by Fordyce & Muizon (2001). However, Coronodon havensteini possesses embrasure pits along the entire upper toothrow and the anterior half of the lower toothrow; likewise, embrasure pits are present in the toothed mysticete Mystacodon as well as stem odontocetes including adult Xenorophidae and the anterior dentition of Ankylorhiza (Geisler, Colbert & Carew, 2014; Muizon et al., 2019; Boessenecker et al., 2020). Some embrasure pits are present on the posterior palate in Crown Odontoceti, including pomatodelphine dolphins (Allen, 1921) and some sperm whales (Kellogg, 1965; Lambert, Bianucci & Muizon, 2016). Loss of the embrasure pits anterior to p1/P1 (Fig. 51; character 53) is a synapomorphy of Neoceti or Mysticeti on our IW trees, where Coronodon and Mystacodon are excluded from both groups. On the EW trees, the presence of these anterior pits in those taxa, as well as in some basal odontocetes, leads to multiple, equally parsimonious optimizations for the basal nodes straddling the archaeocete/neocete transition. By contrast, the loss of the posterior embrasure pits is either a synapomorphy of Neoceti or the clade of Neoceti + Kekenodon (both EW and IW trees). This optimization occurs, in part, because Metasqualodon lacks posterior embrasure pits, and we consistently recovered it as closely related to Coronodonidae. Thus, under the EW trees, the presence of the posterior embrasure pits is best interpreted as a reversal to the primitive condition.
Postcranial morphology is typically underreported for early Neoceti, especially in comparison to the level of attention given to that of basilosaurids (Boessenecker et al., 2020). Surprisingly, a possible postcranial synapomorphy of Neoceti was recently proposed by Davydenko, Mörs & Gol’Din (2021): a ventral median keel on the lumbar vertebrae. This condition seems to characterize all modern cetaceans we examined and differs from the ventrally rounded condition in the lumbar vertebrae of Basilosauridae. We found that a ventral median keel was present on all preserved lumbar vertebrae of Coronodon, and further found that such keels are present in other toothed mysticetes such as Aetiocetus cotylalveus and Fucaia goedertorum, the eomysticetids Eomysticetus, Maiabalaena, Micromysticetus, and many stem odontocetes including Xenorophus, Albertocetus, Ankylorhiza, and waipatiid-grade dolphins (R. W. Boessenecker, 2022, personal observation). We included a new phylogenetic character for this feature in our phylogenetic matrix (Fig. 51; character 344), and it is indeed a synapomorphy of Neoceti or Neoceti + Kekenodon on our EW trees or a synapomorphy of one of three, nested clades on our IW trees (Neoceti + Coronodon, Neoceti + Coronodon + Mystacodon, Neoceti + Coronodon + Mystacodon + Kekenodon). Gatesy et al. (2013) proposed loss of external hindlimbs as another neocete synapomorphy, based largely on the morphology seen in extant cetaceans. Basilosaurids retain small, but partially functional hindlimbs (Gingerich, Smith & Simons, 1990), but it is unclear if the absence of similar lower limb bones among stem odontocetes and mysticetes is due to true absence or non-preservation. Although not included in the present study, we can infer that this feature could still be a synapomorphy of Neoceti, Neoceti + Kekenodon, or one of the same clades mentioned above for the ventral keel character. However, the innominate of Mystacodon selenensis (Lambert et al., 2017; Muizon et al., 2019) is very similar to Basilosaurus (which possessed an external hindlimb; Gingerich, Smith & Simons, 1990), raising the strong possibility that external hindlimbs were lost convergently in Odontoceti and Mysticeti. Widespread postcranial convergence has already been demonstrated in the forelimb and vertebral column of Odontoceti and Mysticeti (Boessenecker et al., 2020). Inomminata are mostly known for archaeocetes and mysticetes (e.g., Gol’din, 2014) and as of yet unknown for stem odontocetes; discovery of early odontocete innominata or more distal elements in early mysticetes is needed to further test these hypotheses.
Like Corrie & Fordyce (2022), we found a clade comprised of various toothed mysticetes (including Coronodon and Mystacodon), Chaeomysticeti, all odontocetes, but excluding Kekenodon (Figs. 47–49, 51). This clade is diagnosed by four unambiguous synapomorphies in our EW trees, including supraorbital process of frontal is as long as wide (79:0>1), anterolateral sulcus of periotic (181:0>1), upper premolars entirely lack a third root (304:0>1), central cusp of cheekteeth subequal in size to accessory cusps (312:0>1), and lower molars and premolars are subequal in height (313:0>1). Only the first of these synapomorphies was supported by the IW analysis, and instead this analysis diagnosed this clade with three other features: supraorbital process widens laterally (78:1>0), nasals bones level with anteriormost margin of supraorbital process (80:0>1), and upper cheekteeth lack ectocingula (310:0>1). To our knowledge, none of these characters has ever been suggested as a neocete synapomorphy, despite the fact that prior to the redescription and phylogenetic analysis of Kekenodon, this clade had the same content as Neoceti. The next node towards the base of our cladograms (both EW and IW trees) includes Kekenodon and all taxa traditionally considered neocetes, but excludes basilosaurids. The characters that diagnose this node in the EW and IW analyses are: premaxilla terminates over anterior half of supraorbital process of frontal (8:0>1), mallear fossa of periotic medial to lateral tuberosity (180:0>1), anteromedial corner of pars cochlearis is rounded (184:0>1), medial lobe of bulla terminates as a blunt corner (246:0>1), roots of double-rooted teeth partially merged (304:0>1), upper cheekteeth lack a lingual cingulum (311:0>1), lower molars lack reentrant grooves (314:0>1), and lower molars bear accessory cusps on mesial carina (315:0>1). Only one of these, a rounded anteromedial corner of pars cochlearis (184:0>1), was listed as a neocete synapomorphy in a previous study (Fitzgerald, 2010). The presence of this morphology in Kekenodon onamata shifts this feature to the next more inclusive clade (Fig. 51).
Conclusions
The initial discovery and description of the toothed mysticete whale Coronodon havensteini focused only on the holotype specimen, with an emphasis on its feeding morphology and adaptations. New specimens from the Ashley Formation (Rupelian, early Oligocene) expand the hypodigm of Coronodon havensteini, permitting the first evaluation of ontogenetic changes within a toothed mysticete. Chief among these are the continued postnatal eruption of the long roots of the cheek teeth, loss of juvenile, upper, postcanine overlap in adults, increase in size of the bulla, lengthening of the intertemporal constriction and sagittal crest, inflation of the anterior process and body of the periotic, and lengthening of the posterior process of the periotic. Additional specimens represent the first records of the genus from the overlying Chandler Bridge Formation (Chattian, late Oligocene) and further represent two new presumed sympatric species named herein: Coronodon newtonorum n. sp., characterized by a concave up alveolar profile and a periotic resembling juveniles of Coronodon havensteini, and Coronodon planifrons, characterized by a horizontal supraorbital process of the frontal and small upper molars. This large collection of new specimens permits naming and diagnosing the family Coronodonidae as well as providing a new generic diagnosis for Coronodon. New specimens and observations of the dentition of Coronodon indicate the development of incipient polydonty, with the addition of at least one mandibular (and possibly a maxillary) tooth relative to basilosaurid whales. Disarticulated rostra of Coronodon havensteini and Coronodon planifrons reveal a lightly or loosely articulated maxilla-premaxilla suture on the rostrum and a loose maxillofrontal suture, suggesting early evolution of rostral kinesis, paralleling a loose mandibular symphysis. Newly referred specimens of Coronodon spp. preserve much of the vertebral column, indicating a vertebral formula of C7/T9/L10/L20+, presence of a caudal fluke, and a body length of about 4.9–5 m. Phylogenetic analyses revealed widely different topologies of Cetacea, with analyses under equal weighting highlighting placement of Coronodon as the second earliest diverging lineage of Mysticeti (diverging just after Mystacodon) and implied weighting analyses placing Coronodon, Mystacodon, and Kekenodon just outside Neoceti, but more crownward than Basilosauridae. Traditional synapomorphies supporting Coronodon within Mysticeti (and Neoceti) generally require fewer steps than the alternative topology from the implied weighting analysis. Regardless, these differing results prompted a preliminary review of synapomorphies of Neoceti and their presence (or absence) in Coronodon and other early presumptive Neoceti. Future studies of the late Paleogene radiation of early Mysticeti, early Neoceti, and Pelagiceti will require greater taxon and character sampling, with matrices including more archaeocete (e.g., Basilosauridae, Protocetidae, Remingtonocetidae) and odontocete taxa.