Telomerase activity, relative telomere length, and longevity in alfalfa (Medicago sativa L.)
- Published
- Accepted
- Received
- Academic Editor
- Muhammad Zia-Ul-Haq
- Subject Areas
- Agricultural Science, Developmental Biology, Molecular Biology, Plant Science
- Keywords
- Alfalfa, Telomerase activity, Relative telomere length, Longevity, Aging
- Copyright
- © 2022 A et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Telomerase activity, relative telomere length, and longevity in alfalfa (Medicago sativa L.) PeerJ 10:e14102 https://doi.org/10.7717/peerj.14102
Abstract
Background
Medicago sativa L. ‘Qingshui’ is a valuable rhizomatous forage germplasm resource. We previously crossed Qingshui with the high-yielding Medicago sativa L. ‘WL168’ and obtained novel rhizomatous hybrid strains (RSA-01, RSA-02, and RSA-03). Telomere dynamics are more accurate predictors of survival and mortality than chronological age. Based on telomere analyses, we aimed to identify alfalfa varieties with increased stamina and longevity for the establishment of artificial grazing grasslands.
Methods
In this study, we performed longitudinal analysis of telomerase activity and relative telomere length in five alfalfa varieties (Qingshui, WL168, RSA-01, RSA-02, and RSA-03) at the age of 1 year and 5 years to examine the relationship among telomerase activity, rate of change in relative telomere length, and longevity. We further aimed to evaluate the longevity of the examined varieties. Telomerase activity and relative telomere length were measured using enzyme-linked immunosorbent assay and real-time polymerase chain reaction, respectively.
Results
We observed significant differences in telomerase activity between plants aged 1 year and those aged 5 years in all varieties except WL168, and the rate of change in telomerase activity does not differ reliably with age. As telomerase activity and relative telomere length are complex phenomena, further studies examining the molecular mechanisms of telomere-related proteins are needed. Relative telomere lengths of Qingshui, WL168, RSA-01, RSA-02, and RSA-03 in plants aged 5 years were higher than those aged 1 year by 11.41, 11.24, 9.21, 10.23, and 11.41, respectively. Relative telomere length of alfalfa tended to increase with age. Accordingly, alfalfa varieties can be classified according to rate of change in relative telomere length as long-lived (Qingshui, WL168, and RSA-03), medium-lived (RSA-02) and short-lived (RSA-01). The differences in relative telomere length distances of Qingshui, WL168, RSA-01, RSA-02, and RSA-03 between plants aged 1 and 5 years were 10.40, 13.02, 12.22, 11.22, and 13.25, respectively. The largest difference in relative telomere length was found between Qingshui and RSA-02 at 2.20. Our findings demonstrated that relative telomere length in alfalfa is influenced by genetic variation and age, with age exerting a greater effect.
Introduction
The development of artificial grazing grassland provides ecological protection and improves the productivity of grass and livestock farming (Moinardeau et al., 2020). Alfalfa (Medicago sativa L.) is an important forage legume predominantly grown in monoculture. Recently, alfalfa has been extensively used in the development of grazing-type artificial grasses, which requires varieties that are long-lived, provide stable yields, and have advantageous nutritional characteristics (Moinardeau et al., 2020; Mikhalev & Silantyeva, 2020). Currently, due to the influence of monoculture, relatively short-lived and medium-lived varieties have been selected over long-lived varieties (Cui et al., 2021). Medicago sativa L. ‘Qingshui’ is a high-quality, rhizome-rooted, legume suitable for grazing-type agriculture (Chen & Shi, 2015) that is water-retaining and resistant to drought, cold temperatures, and trampling but has a relatively low yield (A et al., 2021).
The cross breeding in alfalfa can combine the superior genes of both parents to produce progenies that surpass the characteristics of the parents, thereby rapidly creating new germplasm with superior performance (Suchowilska et al., 2020). We previously crossed Qingshui with the high-yielding Medicago sativa L. ‘WL168,’ leading to the establishment of novel rhizomatous hybrid strains (RSA-01, RSA-02, and RSA-03; Table 1 summarizes the characteristics of each variety) (A et al., 2021). Previous studies have reported improved production performance of parental Qingshui by crossing with WL168 (Table 2 presents the basic data) (A et al., 2021). The classification of life types of herbaceous plants is traditionally determined by the number of years required by the aboveground reproductive branches to complete their development cycle or the number of years that below-ground parts survive (García, Picó & Ehrlén, 2008). In recent years, there has been increasing interest in classifying lifespan using telomere dynamics (Eastwood et al., 2019; Adwan et al., 2019).
The lifespan of an organism is determined by the interaction of genes and environment, including development and aging (Adwan et al., 2019). Several studies have demonstrated that the rate of change in telomere length can predict life expectancy more accurately than chronological age (Marioni et al., 2016). Telomeres are specialized structures at the physical ends of eukaryotic chromosomes that are necessary for chromosome stability (Lee & Cho, 2019; Choi et al., 2021). Telomeric repeats comprise tandem arrays of G-rich repeats and sequence-specific DNA binding proteins of varying length. Approximately all plant telomeres have shown the presence of the heptanucleotide repeat (TTTAGGG)n (Lee & Cho, 2019; Choi et al., 2021; Sun, 2019; Liang et al., 2015), with the exception of a group belonging to the monocot order, Asparagales (Sykorova et al., 2003). Telomeres protect the chromosome ends from DNA degradation, end-to-end fusions, rearrangements, and chromosome loss (Victorelli & Passos, 2017). Further, they play essential roles in various cellular processes, including DNA replication, cell cycle progression, meiosis, and mitosis (Watson & Riha, 2011). Previous studies have demonstrated that chromosome ends lose short stretches of DNA during each cell division owing to the unidirectional nature of DNA replication and requirement for a primer to initiate synthesis (Marioni et al., 2016; Lee & Cho, 2019; Choi et al., 2021). This phenomenon causes telomeres to become progressively shorter after each cell division. In general, the activity of telomerase or other less common telomere lengthening mechanisms can compensate for telomere shortening (Watson & Riha, 2011). Telomerase can use its own RNA sequence as a template to reverse transcribe telomeric DNA sequences, thus compensating for the shortening of telomeric DNA fragments during cell division (Lee & Cho, 2019; Choi et al., 2021). In the absence of telomerase activity, telomere length progressively shortens with each cell division, resulting in chromosomal instability and diminished cell viability, which occur because cells approach their maximum replicative capacity (Song et al., 2009), thereby limiting the number of cell divisions that normal cells can ultimately undergo (Flanary & Kletetschka, 2005). The DNA end-replication problem along with tissue-limited expression of telomerase led to the telomere hypothesis of aging, in which limits on cellular proliferation are genetically determined by the telomere length of a cell (Flanary & Kletetschka, 2005; Nelson, Beilstein & Shippen, 2014). Significant telomere shortening can trigger apoptosis, contributing to the aging process and ultimately death at an organism level. For this reason, experts have described telomere length as a “biological clock” that controls lifespan (Marioni et al., 2016). Moreover, highly significant correlations between telomere length and age have been observed in humans (Frenck, Blackburn & Shannon, 1998), chiropteran (Ineson et al., 2020), birds (Eastwood et al., 2019), arboreal plants (Song et al., 2009; Liu et al., 2007; Aronen & Ryynänen, 2011), and herbaceous plants (Sun, 2019; Liang et al., 2015; Sykorova et al., 2003).
| Category | Characteristics | |
|---|---|---|
| Parental materials | Qingshui | The slender and stiff stalk, horizontal and sloping stem, lax plants; shorter plant height and relatively low leaf volume; horizontal or oblique rhizomatous roots; Qingshui is the first rhizome-rooted alfalfa variety approved and registered in China. The National Grass Variety Validation Committee validation registration number is 412 (A et al., 2021). |
| WL168 | The thicker stalk, erect stem; higher absolute plant height; more horizontal roots; high-yield and excellent-quality; long-term planting and adaptable variety; the first-choice variety in the drier, semi-arid and cold regions of Northwest China (A et al., 2021; Hao et al., 2019). | |
| Hybrid materials | RSA-01 | The erect stem; angle between stem and branch and ground of 70–80°; higher absolute plant height and larger leaf volume (A et al., 2021). |
| RSA-02 | The semi-horizontal stem, angle between stem and branch and ground of 30–69°; higher absolute plant height and larger leaf volume (A et al., 2021). | |
| RSA-03 | The horizontal stem, angle between stem and branch and ground of less than 30°; higher absolute plant height and larger leaf volume (A et al., 2021). | |
| Name | Germination rate/% | Plant height/cm | Biomass/t/hm2 | Crude protein/% | Ether extract/% |
|---|---|---|---|---|---|
| Qingshui | 87.77 | 61.12 | 56.39 | 17.61 | 2.58 |
| WL168 | 95.30 | 69.33 | 71.73 | 20.36 | 2.64 |
| RSA-01 | 86.17 | 63.22 | 53.87 | 19.86 | 2.43 |
| RSA-02 | 87.26 | 64.47 | 58.60 | 19.08 | 2.82 |
| RSA-03 | 87.11 | 68.94 | 70.33 | 18.79 | 2.69 |
Current research into the effect of telomere length on longevity and aging is predominantly focused on animal studies and human medicine (Lyons & Bartolomucci, 2020; Starnino et al., 2021). Similar plant studies are mainly conducted on trees (Flanary & Kletetschka, 2005; Nelson, Beilstein & Shippen, 2014) and herbs (Sun, 2019; Liang et al., 2015). However, extremely limited studies have been conducted on alfalfa. Cross breeding is an important method of creating varieties with different longevity. In the present study, the hybrid strains (RSA-01, RSA-02, and RSA-03) were obtained by crossing Qingshui with a single group of WL168 plants. In plant samples with known ages, telomerase activity and relative telomere length were measured using enzyme-linked immunosorbent assay (ELISA) and quantitative real-time polymerase chain reaction (qPCR), respectively. This study aimed to select alfalfa varieties with increased stamina and longevity for the establishment of artificial grazing grasslands using analysis of telomere dynamics. We measured telomerase activity and relative telomere length in five alfalfa varieties at 1 and 5 years of age to examine the relationship among rate of change in telomerase activity, telomere length, and longevity. This allowed the characterization of the lifespan of the five alfalfa varieties (Qingshui, WL168, RSA-01, RSA-02, and RSA-03).
Materials & Methods
Plant material
The breeding strains (RSA-01, RSA-02, and RSA-03) of the hybrid progenies of rhizome-rooted Medicago sativa L. ‘Qingshui’ (A et al., 2021) and creep-rooted Medicago sativa L. ‘WL168’ (Hao et al., 2019) were used in this study. WL168 was purchased from Beijing Zhengdao Ecological Technology Co., Ltd., and other varieties were provided by the College of Grass Industry of Gansu Agricultural University.
Development of RSA-01, RSA-02, and RSA-03 selective breeding strains
In 2002, wild alfalfa was found in shrub grassland in Qingshui County, Gansu. Individual plants were excavated and transplanted at the experimental base of Gansu Agricultural University for ramet propagation and domestication. In 2005, rhizomatous plants with erect stems, large leaves, and multiple tall branches were selected and crossed artificially with creep-rooted WL168. Hybrid progeny selection was performed by considering rhizome roots and increased aboveground biomass as desired traits. Hybrid seeds were harvested from the autumn ramets in 2005. The group selection method was used to select strains with target traits, giving rise to the simultaneous establishment of the RSA-01, RSA-02, and RSA-03 strains from 2006 to 2013. Basic characteristics and yield performances for each strain are presented in Tables 1 and 2, respectively (A et al., 2021).
Growth conditions and treatments
All experiments were performed in July and August 2021 at the experimental base of Gansu Agricultural University (34°05′N, 105°41′E, 1,525 m altitude), Lanzhou, northwestern China. Plants aged 1 and 5 years were used as experimental subjects as optimal growth for alfalfa is considered to be 5 years in semi-arid areas (Cai et al., 2020; Zhou et al., 2019). Seeds were sown on April 25, 2016, and July 10, 2020, under a field environment. A total of five experimental groups (three hybrid strains and two parental varieties) with four replicates were grown for each treatment in a randomized complete block design. The plot area was 3 m × 5 m with a row spacing of 30 cm. Seeds were sown in a strip pattern at a density of 1 g/m2. Timely field management was performed throughout the experimental period, which included irrigation, manual weeding, and pest control.
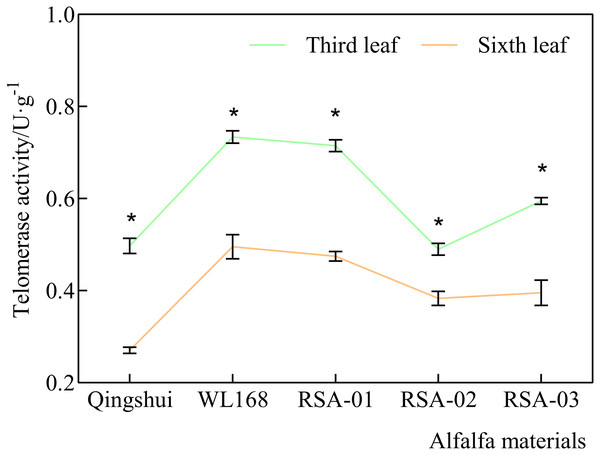
Our previous preliminary experiment comparing the middle leaflet between the third and sixth leaf demonstrated that telomerase activity was significantly higher in the third leaf than in the sixth leaf. The third leaf was therefore selected as the sampling point in the present experiment (Fig. 1). In this experiment, sampling was performed under sunny conditions on July 20, 2021, during the early blooming phase at the second cutting. Eight leaves were randomly selected at the third leaf position of each branch for each variety. The middle leaflets of each leaf were immediately frozen in liquid nitrogen and stored at −80 °C. Analyses of telomere length and activity were performed using four biological replicates.
Figure 1: Comparison of telomerase activity between the third leaf and the sixth leaf.
The green line indicates telomerase activity in third leaf, and the orange line indicates telomerase activity in sixth leaf. * indicates significant differences at the level of 0.05 between the same material at third leaf and sixth leaf.Measurement indexes
Determination of telomerase activity
Each leaf tissue sample was cut to obtain a weight of approximately 0.2 g and ground into powder with liquid nitrogen using 1.8 mL of phosphate-buffered saline (PBS) homogenate. These samples were transferred to 2.5 mL centrifuge tubes and were centrifuged at 3,000–5,000 rpm for 10–15 min, and supernatants were extracted. PBS homogenate was prepared using 0.27 g of KH2PO4, 1.42 g of Na2HPO4, 8 g of NaCl, and 0.2 g of KCl dissolved in approximately 800 mL of deionized water before adding concentrated hydrochloric acid to adjust the pH at 7.2–7.4. The obtained solution was then diluted to a volume of 1 L.
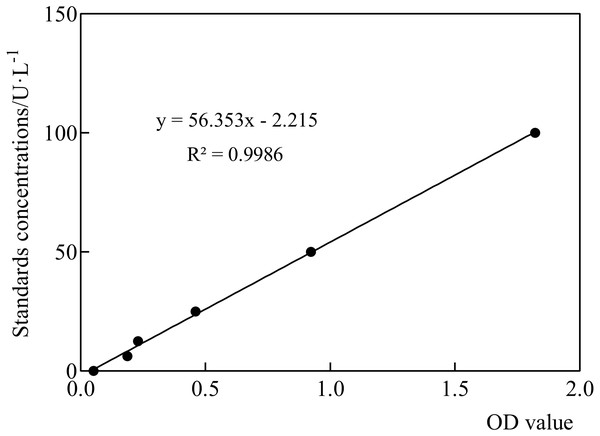
ELISA-based measurement of telomerase activity was performed using the TRAPEZE ELISA Telomerase Detection Kit (Intergen) (Mahboobeh et al., 2021). The sample supernatant, standard, and horseradish peroxidase-labeled detection antibody were added sequentially to the prepacked microtiter wells with the plant telomere antibody. After incubation in a warm bath, the stop solution changed the color from blue to yellow, and the intensity of the color was measured at 450 nm using a spectrophotometer. The concentration of plant telomerase in the sample was calculated using the standard curve (Fig. 2). The standard curve was constructed by plotting the average optical density obtained for six standard concentrations on the vertical (Y) axis, which was used to determine the concentration of an unknown sample. The standard curve equation for telomerase activity was y = 56.353x − 2.215 (R2 = 0.9986), indicating good feasibility and linearity. Color intensity was positively correlated with plant telomerase concentration.
Figure 2: Standard product linear regression curve.
The standard curve was generated by plotting the average O.D. obtained for six standard concentrations on the vertical (Y) axis, which is used to determine the amount in an unknown sample.DNA extraction
Hi-DNA secure Plant Kit (TIANGEN Biotech, Co., Ltd, Beijing, China) was used to extract DNA from alfalfa leaf samples for PCR, according to the manufacturer’s instructions. DNA quality was evaluated via agarose gel electrophoresis. DNA purity (A260/A280 ratio) was determined using a NanoDrop 2000 microspectrophotometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). The Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA) was used to accurately measure DNA concentration and integrity.
Determination of relative telomere length
Telomere qPCR was performed as described in the study by Lin and Hudon (Lin et al., 2019; Hudon et al., 2021) with the following modifications. For each sample well, real-time kinetic qPCR was used to determine Ct, i.e., the fractional cycle number at which the accumulating fluorescence of the well crosses a set threshold, which is several standard deviations above the baseline fluorescence. The qPCR method is used to measure the relative telomere length and not the absolute telomere length. The relative telomere length is believed to reflect the actual differences in telomere length among individuals. The amount of the gene of interest (T) was measured and compared with that of a single copy gene (S), which was assumed to be constant. Experiments were performed using separate 96-well plates. The single copy gene used in our study was β-actin, which is an important cytoskeletal protein. Relative telomere length is expressed relative to the internal single gene control (β-actin) measured from the same sample of DNA. Primer sequences for genes used in this study, including primers for β-actin and the telomere sequence, are shown in Table 3 (Chen et al., 2020; D’Anderson et al., 2021). qPCR was performed using the CFX96 Real-Time System (Bio-Rad, Hercules, CA, USA). The T/S ratio reflects the length differences in telomeric DNA relative to the constant β-actin amplicon and was calculated using the following formula: telomere length =2(−ΔCt) where ΔC, expressed as the amount of telomere hexameric repeats and termed as relative telomere length.
| Oligo name | Oligo sequence (5′-3′) |
|---|---|
| β-actin Forward | CAAAAGATGGCAGATGCTGAGGA |
| β-actin Reverse | CATGACACCAGTATGACGAGGTCG |
| Telomere Forward | CCCCGGTTTTGGGTTTTGGGTTTTGGGTTTTGGGT |
| Telomere Reverse | GGGGCCCTAATCCCTAATCCCTAATCCCTAATCCCT |
The crossing point of a sample depends on the initial DNA concentration. Similar to previous studies, the DNA concentration used in the present study was 30 ng/µl, and the number of cycles used was 30 for telomere repeats and β-actin gene each.
Statistical analyses
Data from four independent biological replicates were analyzed using the SPSS20.0 software. GraphPad Prism 8 was used for mapping. Data are presented in tables and bar graphs as the mean ± standard error. Statistical analyses were assessed using one-way analysis of variance followed by Duncan’s multiple range test (P < 0.05). Comparison between the mean values was performed using least square difference test at a 5% probability level.
Results
Analysis of telomerase activity in alfalfa varieties of different ages
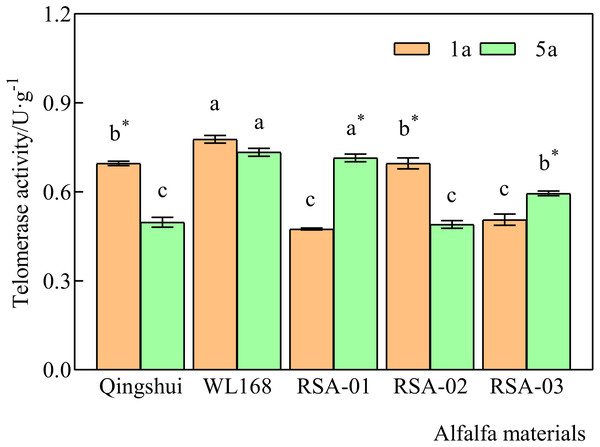
Telomerase activity at 1 and 5 years of age was significantly higher in WL168 than in Qingshui by 11.71% and 47.49%, respectively (Fig. 3) (P < 0.05). Compared with the parental varieties, telomerase activity at 1 year of age was significantly lower in RSA-01 and RSA-03 than in Qingshui by 31.81% and 27.32%, respectively. Telomerase activity at 1 year of age in RSA-01, RSA-02, and RSA-03 was significantly lower than that in WL168 by 38.95%, 10.48%, and 34.94%, respectively. At 5 years of age, telomerase activity in RSA-01 and RSA-03 was significantly higher than that in Qingshui by 43.73% and 19.54%, respectively. Further, telomerase activity in RSA-02 and RSA-03 was significantly lower than that in WL168 by 33.22% and 18.95%, respectively. These results indicate that hybrid strains with rich telomerase activity can be generated by crossing Qingshui with WL168.
Figure 3: Telomerase activity of alfalfa varieties at 1 and 5 years of age.
Lowercase letters indicate significant differences between varieties at the level of 0.05. An asterisk (*) indicates significant differences at the level of 0.05 between the same variety at age 1 and 5 years.Telomerase activity differed significantly between 1-year-old and 5-year-old plants in all varieties except WL168 (Fig. 3). It was observed that 5-year-old Qingshui and RSA-02 had significantly lower activity than 1-year-old plants by 28.53% and 29.62%, whereas 5-year-old RSA-01 and RSA-03 showed significantly higher telomerase activity than 1-year-old plants by 50.60% and 17.53%, respectively. The above longitudinal (1- and 5-year-old) analyses of telomerase activity demonstrated that the rate of change in telomerase activity in alfalfa varieties does not reliably differ with plant age.
Testing of DNA yield and DNA quality
DNA extraction was performed, and the electrophoresis strip was clear, indicating high-yield and integrity (Fig. 4). DNA purity was determined, and the ratio of A260/A280 in all varieties was between 1.8–2.0, indicating high DNA purity in the samples.
Figure 4: Electropherogram.
M, marker; 1–5, Qingshui, WL168, RSA-01, RSA-02, and RSA-03 at 1-year-old; 6–10, Qingshui, WL168, RSA-01, RSA-02, and RSA-03 at 5-year-old.Analysis of relative telomere lengths in alfalfa varieties of different ages
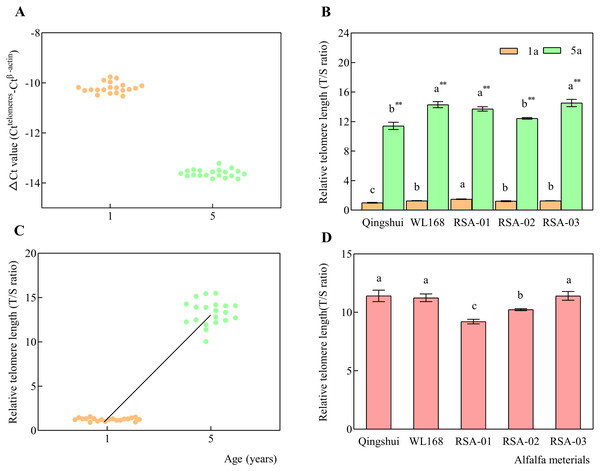
ΔCt was calculated for each sample, which is expressed as the Ct value of the interest gene (telomere) minus that of the reference gene (β-actin) for each group. As Ct values for β-actin were larger for the target gene, ΔCt values were negative for all samples, as shown in the distribution of each point in Fig. 5A. Our results found that ΔCt values for each alfalfa variety had distinct distribution ranges of −10.53 to −9.61 for 1-year-old samples and −13.84 to −13.22 for 5-year-old samples. The T/S ratio (2−ΔΔCt), i.e., relative telomere length, was calculated by subtracting the mean ΔCt of the control group from that of each treatment group.
Figure 5: Relative telomere lengths of alfalfa varieties at 1 and 5 years of age.
Real-time kinetic quantitative PCR was used to measure relative telomere length. (A) ΔCt values (Ct(telomeres)-Ct( β-actin)) for alfalfa varieties at 1 and 5 years of age. (B) Relative telomere lengths (T/S ratio) in alfalfa varieties at 1 and 5 years of age. (C) Relative telomere length distributions at 1 and 5 years of age. (D) Relative telomere length extension in 5-year-old samples compared with 1-year-old samples. Lowercase letters indicate significant differences between varieties at the level of 0.05. Asterisk (**) indicate significant differences at the level of 0.01 between the same variety at age 1 and 5 years.Qingshui at the age of 1 year was found to have the shortest relative telomere length; therefore, it was selected as the control group (Fig. 5B). The T/S ratio was calculated for each sample at 1 and 5 years of age. The relative telomere length (T/S ratio) of WL168 was significantly higher than that of Qingshui at 1 and 5 years of age. RSA-01 and RSA-03 had significantly higher relative telomere length than Qingshui at 1 and 5 years of age. RSA-01 was significantly higher than WL168 at 1 years of age, while RSA-02 was significantly lower than WL168 at 5 years of age.
The mean relative telomere length of Qingshui, WL168, RSA-01, RSA-02, and RSA-03 significantly increased from 1.00, 1.27, 1.49, 1.22, and 1.27 at 1 year of age to 11.41, 14.30, 13.71, 12.44, and 14.54 at 5 years of age, respectively (P < 0.01; Fig. 5B), indicating that relative telomere length increased with age (Fig. 5C). The relative telomere lengths (T/S ratio) of Qingshui, WL168, RSA-01, RSA-02, and RSA-03 were higher at 5 years of age than at 1 year of age by 11.41, 11.24, 9.21, 10.23, and 11.41, respectively (Fig. 5D). Accordingly, the lifespan of each variety can be classified as follows: Qingshui, WL168, and RSA-03 are relatively long-lived alfalfa varieties; RSA-02 is a relatively medium-lived alfalfa variety; and RSA-01 is a relatively short-lived alfalfa variety.
We then examined the role of age and genetic variation in influencing telomere length in alfalfa. Differences in the distances of relative telomere length in Qingshui, WL168, RSA-01, RSA-02, and RSA-03 between plants aged 1 and 5 years were 10.40, 13.02, 12.22, 11.22, and 13.25, respectively. The relative telomere length in the five varieties ranged from 9.21–11.41. The smallest difference in relative telomere length was observed between Qingshui and WL168 at 0.01, indicating higher similarity in longevity. The largest difference in relative telomere length was found between Qingshui and RSA-02 at 2.20, indicating a greater difference in longevity. Our analysis demonstrated that relative telomere length is influenced by genetic variation and age in alfalfa, with age exerting a greater influence.
Discussion
Telomerase activity has been observed in several plant tissues, including somatic and germ cells, and both herbaceous and woody plants (Watson & Riha, 2011). Liang et al. reported higher telomerase activity in ginseng leaves than in stems (Liang et al., 2015); however, Fitzgerald et al. (1999) were unable to detect telomerase activity in the shoot apices of Leguminosae soybean. We compared telomerase activity in the middle leaflets between the third and sixth leaves in alfalfa and found high telomerase activity in the third leaves. Therefore, the third leaf was selected as the sampling site in the present study. Our results are consistent with those of the study by Mu et al. (2014). As hybrid strains developed from the cross breeding of Qingshui and WL168, strains with comparable telomerase activity, have been shown to possess differing telomerase activities, it is evident that telomerase activity is controlled by genetic characteristics, and the external environmental factors (season, year) have a certain impact on it. To fully understand telomerase activity, we need to conduct in-depth research at multiple levels in the future. Flanary & Kletetschka (2005) reported cyclical variations in telomerase activity with age in all parts (needles, roots, and cores) of bristlecone pine, Pinus longaeva. Previous studies have shown that telomerase activity is season-specific in ginkgo (Song et al., 2009), ash, and willow (Mu et al., 2014), and trees at various ages react differently to seasonal changes. We found that telomerase activity in alfalfa did not vary regularly in plants at 1 and 5 years of age. Notably, telomerase activity is associated with cell proliferation. In perennials, meristems proliferate throughout the plant’s life cycle and have robust DNA repair and genome maintenance mechanisms (Watson & Riha, 2011). Alfalfa is usually harvested 3–4 times in a year. During the process of nutritional growth, leaves are renewed several times in a year, which requires the leaf cells to divide rapidly, leading to relatively large changes in telomerase activity. Longevity may be due to of the high telomerase activity that can slow or prevent telomere attrition and subsequent entry of cells into senescence (Watson & Riha, 2011; Flanary & Kletetschka, 2005). Accordingly, the relationship between telomerase activity and plant development is complex and may be influenced by species, strain, environmental stress, and stem cell activity.
The correlation between telomere length and tree age has been frequently studied. Telomere length is predominantly measured for the determination of tree age and longevity in plants. Analysis of dynamic changes in somatic telomere length revealed that ginkgo shows age-specific telomere length (i.e., telomere length increases with age) (Song et al., 2009), corroborating the results of previous studies on P. longaeva (Flanary & Kletetschka, 2005), ginseng (Liang et al., 2015), ginkgo biloba trees (Liu et al., 2007), and Scots pine (Aronen & Ryynänen, 2011). We found that alfalfa telomere length increased with age over a 5-year period, thus confirming the results of the abovementioned study. In contrast, progressive shortening of telomere length is observed in animal somatic cells with aging, which is also observed in barley (Kilian, Stiff & Kleinhofs, 1995), almond (D’Anderson et al., 2021), and Elymus sibiricus L. (Sun, 2019). These results support the dependency of cellular senescence on elapsed cell divisions, raising the question of whether telomere shortening also contributes to organismal aging. Furthermore, telomere length is reported to be stable in the leaves of 4-week-old to 6-month-old tomato plants (Broun, Ganal & Tanksley, 1992), and no change was observed in telomere length with increased plant age over a 5-year period in apples (Malus domestica) and Prunus yedoensis (Moriguchi et al., 2007). In this study, the relative telomere length of WL168 was significantly higher than that of Qingshui in different age groups, and the telomere length of the hybrid progeny varied from that of the parental plants, showing richness in variety. Diversity in telomere length is probably influenced by various factors, including genetics, epigenetics, and environmental factors (Nelson, Beilstein & Shippen, 2014; Kilian, Stiff & Kleinhofs, 1995; Mu et al., 2015). Previous studies have shown that the rate of change in telomere length can predict life expectancy more accurately than chronological age (Eastwood et al., 2019; Adwan et al., 2019; Marioni et al., 2016). Several cross-sectional and longitudinal studies have been conducted on different organisms with various maximum lifespans to investigate the relationship between chronological age and telomere shortening (Chen et al., 2011; Haussmann et al., 2003). Vera et al. (2012) demonstrated that mice with higher rates of telomere shortening lived shorter lives, whereas those with lower rates of telomere shortening lived longer lives. In the present study, compared with 1-year-old plants, the relative telomere length in 5-year-old plants was increased by 11.41, 11.24, 9.21, 10.23, and 11.41 in Qingshui, WL168, RSA-01, RSA-02, and RSA-03 varieties, respectively. In contrast to animals, plants have an extremely basic structure during embryogenesis. In alfalfa, vegetative organs such as stems and leaves are predominantly harvested, whereas the vegetative meristem can regenerate into a new organism. One of the most common explanations for changes in telomere length is that various stressors, such as oxidative stress, accelerate telomere loss (Adwan et al., 2019; Von, 2002; Schrumpfová, Fojtová & Fajkus, 2019). Numerous studies have demonstrated that trees that tend to grow slowly with age do not appear to succumb to cellular senescence and could better adapt to biotic or abiotic (ultraviolet, oxidative) stress (Song et al., 2009; Lanner, 2002). The average telomere length of long-lived trees can be effectively maintained over several years of nutritional growth, with ecological and evolutionary advantages. Numerous structural and physiological features observed in long-lived trees are suggested to play roles in increasing longevity, including continuous retention of stem cell-like meristematic cells and specialized vascular and hormonal control systems (Lanner, 2002). The results of the present study in alfalfa support this conclusion. There are reportedly no significant differences in telomere length between female and male leaf tissues in mature ginkgo trees, and telomere length is significantly higher in both ancient and mature trees compared with seedlings (Liu et al., 2007). These results are similar to those of the present study in which age was found to exert a greater effect on longevity than genetic variation. Although we can advance these different hypotheses based on the measured results, other possible explanations should be considered. Further studies are required to understand the reason for the existence of such phenomena in plants. It is apparent that the relationship between telomere length and life history is different in plants compared with other organisms, such as animals. In mammals and other eukaryotic systems, shorter telomeres are generally associated with aging, in part because of the association between telomere shortening and cellular senescence (Urquidi, Tarin & Goodison, 2000). This is obviously not the case for the studied perennial plant species (Giraudeau et al., 2019; Choi et al., 2021), and research is needed from the perspective of alfalfa telomere-related genes and their mechanisms of regulating longevity in the future.
Telomere length is primarily maintained in species-specific equilibrium through competition between telomerase-mediated lengthening and loss of terminal DNA during telomere replication (Watson & Riha, 2011). Telomere length shortens after each cell division due to telomere replication (Kilian, Stiff & Kleinhofs, 1995; Riha et al., 1998). Optimal telomere length is essentially regulated and maintained by telomerase activity, with lagged increase or decrease in telomerase activity depending on telomere length (Moriguchi et al., 2007). However, the relationship between telomerase activity and telomere length is complex (Song et al., 2009) and is influenced by various factors, including species and environmental stress (Mu et al., 2015; Joeng et al., 2005). The results of previous studies support the hypothesis that telomere repair mechanisms are equally likely to become active or inactive when telomeres are at or near their optimal length, resulting in telomere splitting. When the maximum telomere length is achieved, telomeres are shortened due to aberrant end replication, thus impairing telomerase function (Song et al., 2009; Schrumpfová, Fojtová & Fajkus, 2019). In the present study, WL168 and Qingshui with comparable telomerase activity and relative telomere length were crossed, giving rise to progeny with differing telomerase activity. Telomere length was higher in all 5-year-old alfalfa plants compared with 1-year-old plants, whereas there was no regularity in telomerase activity between different age groups. The results of the present study indicate that the relationship between telomerase activity and telomere length is complex. Moreover, genetic analysis of different maize self-pollinating lines has shown that telomere length is under strict genetic control (Burr et al., 1992). Proteins that bind to telomere repeats have also been identified in plants and may be involved in maintaining telomere length along with regulating other processes (Schrumpfová, Fojtová & Fajkus, 2019; Zentgraf, Hinderhofer & Kolb, 2000; Regad, Lebas & Lescure, 1994).
Conclusion
The relative telomere length of alfalfa leaves was a trend toward increased in 5 years; however, telomerase activity does not reliably differ at 1 and 5 years of age. As telomere length and telomerase activity were found to be complex, further studies examining the molecular mechanisms of telomere-related proteins are needed. Accordingly, alfalfa varieties can be classified according to rate of change in telomere length as long-lived (Qingshui, WL168, and RSA-03), medium-lived (RSA-02) and short-lived (RSA-01). Relative telomere length in alfalfa was influenced by genetic variation and age, with age exerting a greater influence.