An earliest Triassic age for Tasmaniolimulus and comments on synchrotron tomography of Gondwanan horseshoe crabs
- Published
- Accepted
- Received
- Academic Editor
- Brandon Hedrick
- Subject Areas
- Biodiversity, Evolutionary Studies, Paleontology, Zoology
- Keywords
- Euchelicerate, Xiphosurida, Austrolimulidae, Australia, Synchrotron radiation X-ray tomography
- Copyright
- © 2022 Bicknell et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. An earliest Triassic age for Tasmaniolimulus and comments on synchrotron tomography of Gondwanan horseshoe crabs. PeerJ 10:e13326 https://doi.org/10.7717/peerj.13326
Abstract
Constraining the timing of morphological innovations within xiphosurid evolution is central for understanding when and how such a long-lived group exploited vacant ecological niches over the majority of the Phanerozoic. To expand the knowledge on the evolution of select xiphosurid forms, we reconsider the four Australian taxa: Austrolimulus fletcheri, Dubbolimulus peetae, Tasmaniolimulus patersoni, and Victalimulus mcqueeni. In revisiting these taxa, we determine that, contrary to previous suggestion, T. patersoni arose after the Permian and the origin of over-developed genal spine structures within Austrolimulidae is exclusive to the Triassic. To increase the availability of morphological data pertaining to these unique forms, we also examined the holotypes of the four xiphosurids using synchrotron radiation X-ray tomography (SRXT). Such non-destructive, in situ imaging of palaeontological specimens can aid in the identification of novel morphological data by obviating the need for potentially extensive preparation of fossils from the surrounding rock matrix. This is particularly important for rare and/or delicate holotypes. Here, SRXT was used to emphasize A. fletcheri and T. patersoni cardiac lobe morphologies and illustrate aspects of the V. mcqueeni thoracetronic doublure, appendage impressions, and moveable spine notches. Unfortunately, the strongly compacted D. peetae precluded the identification of any internal structures, but appendage impressions were observed. The application of computational fluid dynamics to high-resolution 3D reconstructions are proposed to understand the hydrodynamic properties of divergent genal spine morphologies of austrolimulid xiphosurids.
Introduction
The increasing availability of three-dimensional (3D) imaging techniques in the preceding two decades has revolutionised the acquisition of morphological data from both biological (Hita Garcia et al., 2017; Parapar et al., 2017; Landschoff et al., 2018; Marcondes Machado, Passos & Giribet, 2019; Raymond et al., 2019) and palaeontological specimens (Sutton, 2008; Pardo & Anderson, 2016; Liu, Rühr & Wesener, 2017; Liu et al., 2019; Forel, Poulet-Crovisier & Korat, 2021). Traditional lab-based micro-computed tomography (CT), along with more sophisticated synchrotron radiation X-ray tomography (SRXT) and neutron micro-tomography (NCT) have permitted non-destructive visualisation of previously unknown and inaccessible morphological features for taxa across all of Metazoa (Donoghue et al., 2006; Tafforeau et al., 2006; Sutton, 2008; Metscher, 2009; Motchurova-Dekova & Harper, 2010; Faulwetter et al., 2013; Faulwetter et al., 2014; Herrera et al., 2020; Snyder et al., 2020). This precludes the need for physical dissection and/or preparation of specimens, which is relevant when describing structures from rare or fragile material (e.g., Metscher, 2009; Haszprunar et al., 2011; Deans et al., 2012; Beutel et al., 2019; Willsch et al., 2020; MacDougall et al., 2021; Stillwell et al., 2020). In palaeontology, 3D data has been used widely in the visualisation of fossils preserved in amber (Lak et al., 2008; Perrichot et al., 2008; Riedel et al., 2012; Xing et al., 2016a; Xing et al., 2016b; Xing et al., 2018; Daza et al., 2020; Bolet et al., 2021) and also in the examination of fossils that are still surrounded in their original rock matrix (Moreau et al., 2014; Schwarzhans et al., 2018; Reid et al., 2019; Mayr et al., 2020).
Research into fossil arthropods has benefitted greatly from the availability of non-destructive 3D imaging techniques (Deans et al., 2012; Liu et al., 2016; Liu et al., 2020; Hegna, Martin & Darroch, 2017; Wesener, 2019; Zhai et al., 2019a; Zhai et al., 2019b; Liu et al., 2020), particularly the diverse array of insects preserved within resins (Tafforeau et al., 2006; Lak et al., 2008; Pohl et al., 2010; Henderickx, Tafforeau & Soriano, 2012; Riedel et al., 2012). In stark contrast, extinct members of Xiphosurida (i.e., horseshoe crabs) have received comparatively limited 3D examination. The anatomy of two extant xiphosurids, the American horseshoe crab—Limulus polyphemus (Linnaeus, 1758)—and the mangrove horseshoe crab—Carcinoscorpius rotundicauda (Latreille, 1802)—has been documented using micro-CT (Göpel & Wirkner, 2015; Bicknell et al., 2018a; Bicknell et al., 2018b; Bicknell et al., 2021b; Bicknell, Melzer & Schmidt, 2021). Magnetic resonance imaging has also been used in studies of the Japanese horseshoe crab—Tachypleus tridentatus (Leach, 1819) (Kutara, Une & Fujita, 2019; Yuen, Kwok & Kim, 2019). However, as Bicknell & Pates (2020) highlighted, there are over 80 extinct xiphosurids that have not been documented or rendered in 3D and most 3D data collected from fossil xiphosurids have been surface scans (Schimpf et al., 2017), with other applications including stereo imaging (Haug et al., 2012; Haug & Rötzer, 2018; Haug & Haug, 2020). A recent study combined CT and computed laminography (Zuber et al., 2017) to image Limulitella Størmer, 1952 from the Winterswijk quarry complex, Middle Triassic (Anisian) Vossenfeld Formation, Netherlands (Klompmaker & Fraaije, 2011; Klein, 2012; Sander et al., 2016; Zuber et al., 2017). These techniques revealed morphological information that was not visible due to the compression and preservation of the specimen. However, no other fossil xiphosurids have been examined using comparable methods. Here we address this lack of data by presenting the first application of SRXT to holotypes of four Australian xiphosurids. In doing so, we also reconsider the temporal range of these four taxa. This revision uncovers a younger age for one genus, pushing the record of Austrolimulidae in Australia to the Triassic.
Methods
We examined the four species of Xiphosurida known from Australia using SRXT: Austrolimulus fletcheri Riek, 1955 from the Hawkesbury Sandstone (Middle Triassic, Anisian), New South Wales (NSW); Dubbolimulus peetae Pickett, 1984 from the Napperby Formation (Middle Triassic, Anisian), NSW; Tasmaniolimulus patersoni Bicknell, 2019 from the Jackey Shale (Early Triassic, Induan), Tasmania; and Victalimulus mcqueeni Riek & Gill, 1971 from Koonwarra Fossil Bed (Early Cretaceous, Aptian), Victoria. All four species fall within the xiphosurid groups Limulidae and Austrolimulidae (Bicknell, 2019; Bicknell et al., 2021a; Lamsdell, 2021). Given advances in the stratigraphic literature since the initial descriptions of these four forms, we conducted a literature review and present a thorough geological contextualisation for each taxon.
Non-destructive X-ray microtomographic measurements were conducted using the Imaging and Medical Beamline at the Australian Nuclear Science and Technology Organisation’s (ANSTO) Australian Synchrotron, Clayton, Victoria, Australia.
A monochromatic beam energy of 70 keV was used for Dubbolimulus peetae and Victalimulus mcqueeni, with a sample-to-detector distance of 500 mm. X-rays were converted to visible photons and detected using the “Ruby detector”, a 20 µm thick Gadox/CsI(Tl)/CdWO4 scintillator screen coupled with a PCO.edge sCMOS camera (16-bit, 2,560 × 2,160 pixels) and a Nikon Makro Planar 50 mm lens to achieve a pixel size of 24.8 × 24.8 µm. A total of 1800 equal angle shadow-radiographs were obtained (i.e., one radiograph every 0.10°) with an exposure length of 0.070 s each as the samples were continuously rotated 180° about their vertical axes. Due to the restricted beam height and field-of-view, this radiograph capture procedure was repeated after lowering the specimen with respect to the beam after a full rotation. This produced a series of overlapping vertical radiographs capturing the full height of each specimen. These were then stitched together into a single set of radiographs prior to reconstruction into 3D volumes. For V. mcqueeni the reconstructed data was binned to voxels of 49.6 µm for visualisation. Tasmaniolimulus patersoni and Austrolimulus fletcheri were similarly scanned with a pixel size of 40.29 × 40.29 µm. An incident monochromatic beam energy of 80 keV was used for T. patersoni and a broad range of higher energy X-rays (pink beam, peak energy of 220 keV) was used for A. fletcheri due to the high attenuation of available monochromatic X-rays.
The raw 16-bit radiographs were normalised relative to the beam calibration files, stitched using the in-house software IMBL Stitch, and reconstructed with CSIRO’s X-TRACT (Gureyev et al., 2011) software available on Australian Synchrotron Computing Infrastructure (ASCI). The filtered-back projection reconstruction method was used to form a 16-bit, 3D volume image of the sample.
The reconstructed slices for each fossil were imported into Mimics version 23.0 (Materialise, Leuven, Belgium) and digitally prepared. Any artefacts in the tomographic slices were removed using the ‘Segmenting’ tool and the remaining components (fossil and matrix) were segmented out and converted to .STL files in Mimics, and imported into Geomagic Studio (3D Systems, North Carolina, USA) to be smoothed. The smoothed .STL files were used to generate 3D PDFs using Terta4D (Adobe Systems; see Figs. S1–S4 found at 10.17605/OSF.IO/AT528). Lighting used in the 3D PDFs was Computer-Aided Design optimised to showcase features prominently and without shadowing. Raw radiograph data associated with this research has been uploaded to MorphoSource (https://www.morphosource.org/projects/000380648). Photographs of each specimen were taken under LED lighting either by the authors or by collection managers for overall comparison to the 3D reconstructions. A note here must be made to the use of stereo-photographs. This imaging technique has effectively been used to illustrate fossil arthropods (Haug et al., 2009; Haug, Martin & Haug, 2015; Haug, Müller & Haug, 2019; Haug, 2020) and particularly fossil xiphosurids (Haug et al., 2012; Haug & Rötzer, 2018; Haug & Haug, 2020). This has been especially informative when specimens are dorsoventrally compressed and may have revealed more structures than the LED lighting photography conducted here. However, as the focus of this research was on the synchrotron scanning and digitisation of the holotypes, we did not apply this method here. Nonetheless, future work on fossil xiphosurid anatomy should consider gathering stereo images for comparative purposes.
Three-dimensional models can also be produced using photogrammetry. This method is particularly useful for illustrating overall specimen morphology and models are cost-effective to produce (Falkingham, 2012; Cunningham, 2021). However, photogrammetry cannot be used to gather data on internal structures—one of the main focuses here. As such, we did not explore the application here. Regardless, photogrammetry should be considered for future research interested in overall 3D morphology of horseshoe crabs.
Geological context
The oldest Australian xiphosurid, Tasmaniolimulus patersoni, was found in the Jackey Shale of the Upper Parmeener Supergroup, Tasmania (Bicknell, 2019). This formation is largely composed of cross-bedded quartz and feldspathic sandstones, laminated dark grey shales and thin coal lenses (Pike, 1973). Stratigraphically, the fossil was located near the very top of the formation, ∼3 m below the base of the overlying Ross Formation, exposed alongside a cliff on the Poatina Highway (41°48′05″S, 146°53′06″E; Ewington, Clarke & Banks, 1989; Bicknell, 2019). Based on the lithology, the unit likely represents deposition of lake and river sediments in a non-marine swamp with limited coastal influence (Banks, 1973; Ewington, Clarke & Banks, 1989). While the Jackey Shale at the stratigraphic level of the collection locality lacks age-diagnostic fossils, palynomorphs from other, temporally contiguous sites can be assigned to the Protohaploxypinus microcorpus Zone, equivalent to upper APP6 (see Price, 1997) and restricted to the Griesbachian substage, early Induan (Early Triassic) based on previous studies in the Sydney Basin (Laurie et al., 2016; Mays et al., 2020). This contradicts previous interpretations of latest Permian that used now outdated chronostratigraphic ages for this palynomorph zone. An Early Triassic age is further supported by the vertebrate fauna and macro- and microflora of the Protohaploxypinus samoilovichii Zone from the overlying Ross Formation which pertains to the younger Smithian substage of the Olenekian (Early Triassic; Forsyth, 1984). The presence of abundant latest Permian macroflora at stratigraphic levels below the level of T. patersoni in the Jackey Shale does suggest that, at least at some locations, the formation does extend into the latest Permian (Ewington, Clarke & Banks, 1989). Nonetheless, given the high stratigraphic position of T. patersoni, it appears more likely that this specimen is of Early Triassic age.
Slightly younger is Dubbolimulus peetae, which was collected from the Napperby Formation (previously the “Ballimore Formation”) of the Gunnedah Basin in central New South Wales (Pickett, 1984). The only known specimen, with an associated counterpart, was found just south of Western Plains Zoo, Dubbo (at approximately 32°17′30.8″S 148°34′35.8″E). The Napperby Formation consists of white, fine–medium grain, quartz-rich, ferruginous sandstone with occasional cross bedding. Thin horizons of grey to red-brown shale and minor conglomerate lenses are interbedded with this sandstone. The stratigraphic horizon within which the specimen was found is a red-brown, slightly micaceous shale. This lithology indicates a high-energy braided river system or lacustrine deposit (Tadros, 1993), possibly part of the same Triassic delta system that continues into the Sydney Basin to the east. The finer grained shale horizons likely represent lower-energy conditions which presumably occurred in quiet, cut-off river channels or small ponds. The possible presence of acritarchs (McMinn, unpublished data, 1982; Early Permian-Early Jurassic palynology of DM Mirrie DDH 1, northwest of Dunedoo. Geological Survey of New South Wales, Report GS1982/289) suggest the unit may have experienced a slight coastal influence occasionally. A diverse macroflora assemblage has been described from both the fossil site itself (Pickett, 1984) and a nearby locality (Holmes, 1982) which broadly correlate to the Dicroidium zuberi Zone (Helby, 1973; Helby, Morgan & Partridge, 1987; Retallack, 1977; Retallack, 1980; Helby, Morgan & Partridge, 1987) of the Anisian (earliest Middle Triassic) in the Sydney Basin. Palynomorphs from core within the Dubbo area, at Mirrie DOH I (McMinn, unpublished data, 1982; Early Permian-Early Jurassic palynology of DM Mirrie DDH 1, northwest of Dunedoo. Geological Survey of New South Wales, Report GS1982/289) and Pibbon DOH 1 (McMinn, unpublished data, 1984; Palynology of DM Pibbon DDH 1, Goulburn River-Binnaway area. Geological Survey of New South Wales, Report 84/4, GS1984/052), support this age interpretation with placement in the Aratrisporites parvispinosus Zone which correlates to the middle to upper Dicroidium zuberi Zone (Young & Laurie, 1966). A middle D. zuberi Zone stratigraphic position, which indicates an earliest Anisian age, is most likely given palynomorphs from other locations in the Gunnedah Basin, which suggest an age range between the upper Aratrisporites tenuispinosus Zone and lower Aratrisporites parvispinosus Zone.
Of a similar age is Austrolimulus fletcheri, from Beacon Hill Quarry, near the suburb of Brookvale, Sydney, New South Wales (Riek, 1955). The exact co-ordinates of the original collection site are unknown, but are considered to be 33°45′11.2″S, 151°15′55.5″E; the location of the original quarry. The specimen originates from a 8 m thick shale lens in the Hawkesbury Sandstone. This lens mostly consists of numerous thin, recessive, grey-red mudrock laminations with little bioturbation (Webby, 1970) and small amounts of rippling (Herbert, 1983). Overall, the Hawkesbury Sandstone was likely formed in a vast coastal floodplain made up of high energy braided rivers, scour channels, lakes, and sand dunes (Conaghan, 1980 and references therein). Shale lenses, like those at the A. fletcheri site, likely represent lower-energy regimes consisting of shallow water bodies disconnected from a main river channel as isolated shallow pools of water (Herbert, 1980; Herbert, 1997; Rust & Jones, 1987). None of the diverse fossil fauna and flora found at Brookvale (see Bicknell & Smith, 2021 for a recent overview) are diagnostic for relative age estimation. However, the Hawkesbury Sandstone is well constrained within the Aratrisporites parvispinosus Zone and upper Dicroidium zuberi Zone based on palynomorphs and macroflora (Helby, 1973; Retallack, 1977; Retallack, 1980; Helby, Morgan & Partridge, 1987). Similar to the Napperby Formation, this places it within the Anisian (earliest Middle Triassic), likely the earliest Anisian. Recent high-precision U-Pb CA-TIMS obtained from the Garie Formation, which underlies the Newport Formation and succeeding Hawkesbury Sandstone, is dated to the latest Olenekian (248.23 ± 0.13 Ma and 247.87 ± 0.11 Ma; Metcalfe et al., 2015). This further supports an Anisian age for the Hawkesbury Sandstone as there is an unconformity in the Sydney Basin between Newport Formation and Hawkesbury Sandstone (Helby, 1973; Herbert, 1980).
Victalimulus mcqueeni from Koonwarra Fossil Bed of the Strzelecki Group (Riek & Gill, 1971), is the youngest xiphosurid known from Australia. A single partial specimen was found at a road cutting along the South Gippsland Highway, approximately 2.4 km east of Koonwarra (38°33′48.9″S 145°57′33.9″E). The unit at this location consists of a thick (∼7–8 m) lower and upper feldspathic sandstone bracketing a grey-green, fossiliferous mudstone (Waldman, 1971; Jell & Roberts, 1986). The mudstone is made up of extremely fine alternating layers of a clay- and silt-dominated matrix. A freshwater lacustrine environment was originally suggested for the Koonwarra Fossil Bed, with the finely laminated mudstones representing a rhythmic varve formed under freezing conditions (Waldman, 1971; Waldman, 1973; Waldman, 1984). However, the highly diverse fossil fauna and flora (see overview in Poropat et al., 2018), instead suggests a cold, but not freezing, swamp or a lacustrine environment with seasonal flooding causing overbank-type deposits (Douglas & Williams, 1982; Jell & Roberts, 1986). Presence of the palynomorphs Clavatipollenite hughesii Couper, 1957 and Foraminisporis asymmetricus Dettmann, 1963 from the Koonwarra Fossil Bed, and absence of other palynomorphs from younger zones, indicate an age within the Upper Cyclosporites hughesii subzone (Jell & Roberts, 1986; Seegets-Villiers & Wagstaff, 2016; Korasidis & Wagstaff, 2020; Wagstaff et al., 2020). This places the unit entirely within the Aptian Stage (Early Cretaceous). Fission track dating of volcanoclastic sediments in the Koonwarra Fossil Beds suggests an age of 118 ± 5–115 ± 6 Ma, which correlates to the mid-Aptian (Gleadow & Duddy, 1980; Lindsay, 1982).
Results
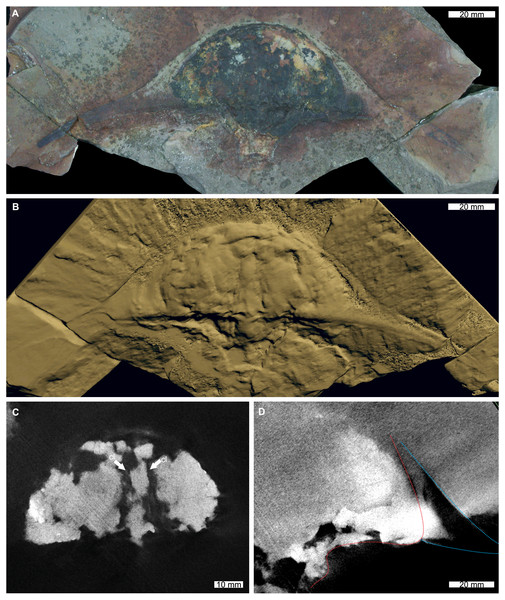
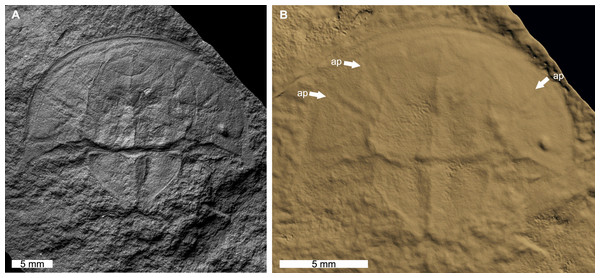
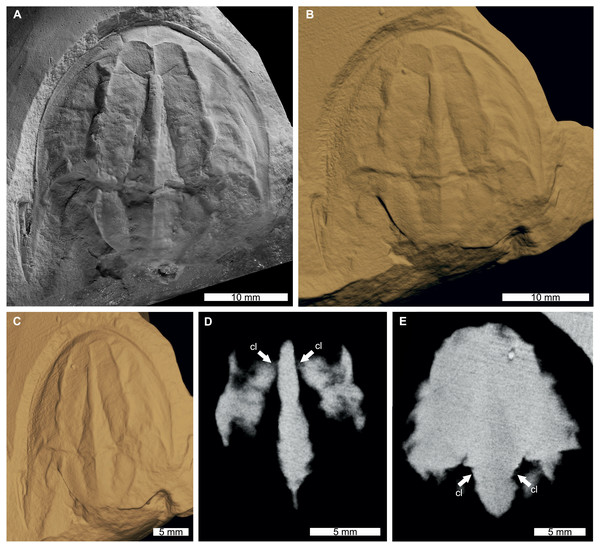
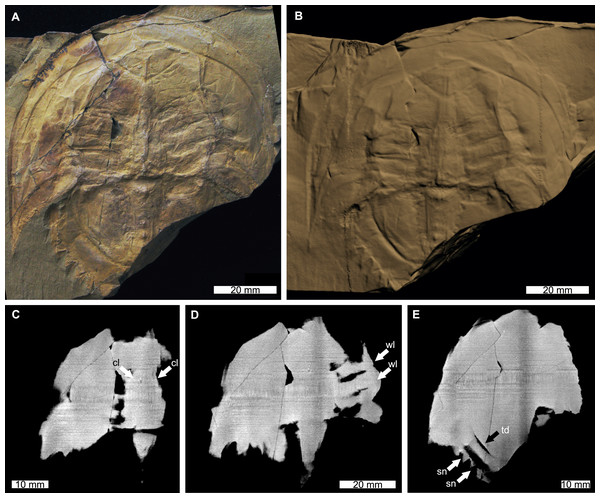
The reconstructed tomographic volumes emphasized morphological information that was less evident under visible wavelengths. The density of the matrix surrounding Austrolimulus fletcheri precluded the unambiguous identification of many internal structures (Fig. 1). However, the cardiac lobe can be more readily distinguished in the reconstructed volume and more depth is observed than exposed on the dorsal surface of the fossil (Fig. 1C). Furthermore, the composition of the genal spines is less dense than the prosoma, suggesting a limited portion of the spine was less sclerotised (Fig. 1D). Dubbolimulus peetae shows no evidence of preserved internal structures. The limited record of anatomical features reflects the strong dorsoventral compression of the specimen (Fig. 2). However, examination of the surface reconstruction reveals impression of the walking legs. These structures are also observed under LED light (Fig. 2A). The cardiac lobe of Tasmaniolimulus patersoni is the most prominent feature visible in the reconstruction (Fig. 3). This structure is observed at different slices in the reconstruction, illustrating the pronounced nature of the cardiac lobe. Finally, the reconstruction of Victalimulus mcqueeni reveals the most anatomical data of the four specimens. There is clear evidence for the thoracetronic doublure, fixed spines, moveable spine notches, and appendage impressions, as noted by Riek & Gill (1971) (Fig. 4). The cardiac lobe is not as pronounced as in A. fletcheri and T. patersoni, reflecting the more compressed nature of V. mcqueeni.
Figure 1: Austrolimulus fletcheri from the Hawkesbury Sandstone (Middle Triassic, Anisian). AM F38275 counterpart of holotype.
(A) Specimen under LED light. (B) 3D reconstruction of specimen, see Fig. S1. (C) X-ray tomographic slice showing pronounced cardiac lobe (white arrows). (D) X-ray tomographic slice showing difference in density between prosoma (red dotted line) and hypertrophied genal spine (blue lines). Abbreviation: cl, cardiac lobe. Image credit: (A) Joshua White. 3D PDF found at 10.17605/OSF.IO/AT528. Raw reconstructed slices found at 10.17602/M2/M380652.Figure 2: Dubbolimulus peetae from the Napperby Formation (Middle Triassic, Anisian). MMF 27693, holotype.
(A) Specimen under LED light. (B) 3D reconstruction of specimen showing appendage impressions (white arrows), see Fig. S2. Abbreviation: ap, appendage impression. Image credit: (A) David Barnes. Image in (A) reproduced from Bicknell & Pates (2020) under a CC BY 4.0 license. 3D PDF found at 10.17605/OSF.IO/AT528. Raw reconstructed slices found at 10.17602/M2/M396665.Figure 3: Tasmaniolimulus patersoni from the Jackey Shale (Early Triassic, Induan). UTGD 123979, holotype.
(A) Specimen under LED light. (B, C) 3D reconstruction of specimen, see Fig. S3. (B) Dorsal view. (C) Oblique view. (D, E) X-ray tomographic slices showing pronounced cardiac lobe (white arrows). (A) Coated in ammonium chloride sublimate and image converted to greyscale. Abbreviation: cl, cardiac lobe. Image credit: (A) Russell Bicknell. 3D PDF found at 10.17605/OSF.IO/AT528. Raw reconstructed slices found at 10.17602/M2/M396670.Figure 4: Victalimulus mcqueeni from the Koonwarra Fossil Bed (Early Cretaceous, Aptian). NMV P22410B, holotype.
(A) Specimen under LED light. (B) 3D reconstruction of specimen, see Fig. S4. (C) X-ray tomographic slice showing cardiac lobe (white arrows). (D) X-ray tomographic slice showing walking leg impressions (white arrows). (E) X-ray tomographic slice showing fixed spines and moveable spine notches (white arrows) and thoracetronic doublure (black arrow). Abbreviations: cl, cardiac lobe; sn, spine notches; td, thoracetronic doublure; wl, walking leg impression. Image credit: (A) Frank Holmes. Image in (A) reproduced from Bicknell & Pates (2020) under a CC BY 4.0 license. 3D PDF found at 10.17605/OSF.IO/AT528. Raw reconstructed slices found at 10.17602/M2/M392556.Discussion
Age of Tasmaniolimulus patersoni
The revised earliest Triassic age of Tasmaniolimulus patersoni has important implications for the timing of morphological innovation within Austrolimulidae. Tasmaniolimulus patersoni was originally considered to be of latest Permian age (Ewington, Clarke & Banks, 1989; Lerner, Lucas & Lockley, 2017; Bicknell, 2019; Lamsdell, 2020). This age indicated that the first appearance of hypertrophied genal spines in Austrolimulidae was before the end-Permian extinction (Bicknell, Naugolnykh & Brougham, 2020). However, the revised date shifts the first appearance of this trait to the earliest Triassic. Furthermore, T. patersoni is now either the oldest Triassic austrolimulid, or contemporaneous with Vaderlimulus tricki Lerner, Lucas & Lockley, 2017 and Psammolimulus gottingensis Lange, 1923—taxa that all have overdeveloped genal spine morphologies (Meischner, 1962; Lerner, Lucas & Lockley, 2017; Bicknell, Hecker & Heyng, 2021).
Comments on application of synchrotron tomography to the study of fossil xiphosurids
The SRXT examination of the Australian xiphosurid fossils did not reveal extensive novel anatomy, nor traces of soft tissues. The aforementioned specimens were preserved primarily in sand- and siltstones which limits the preservation potential of fine, delicate structures. This is in contrast to the tomographic and laminographic reconstructions of the xiphosurid described by Zuber et al. (2017) and which was preserved in fine grained, Muschelkalk-type limestones. These sediments tend to preserve soft-bodied anatomical details in exceptional detail (Vía, De Villata & Esteban Cerdá, 1977; Briggs & Gall, 1990; Cartañài Martí, 1994; Klug, Hagdorn & Montenari, 2005). Nonetheless, non-destructive three-dimensional imaging using SRXT will likely continue to play a role in anatomical studies of fossil xiphosurids, following the rapid adoption of this imaging modality across palaeontology. Furthermore, NCT is being used more commonly in palaeontology, owing to the ability of neutrons to penetrate through typically radiopaque minerals such as iron pyrite, a high sensitivity to hydrogenous material, and thus to residual organic remains, (Gee et al., 2019; Gee, Bevitt & Reisz, 2019; Na et al., 2021; Smith et al., 2021; Bazanna et al., 2021), and to increasing availability of high-quality neutron imaging facilities at nuclear research reactors and spallation neutron sources around the world (see https://www.isnr.de/index.php/facilities/user-facilities). Finally, techniques that can more readily distinguish areas with very small differences in radiopacity, such as phase-contrast enhanced imaging, show promise for more detailed examination of muscles and other internal structures in suitably well-preserved specimens. Any, or all of these approaches could be applied to the study of specimens of Mesolimulus walchi (Desmarest, 1822) from the Nusplingen Lithographic Limestone (Upper Jurassic, Kimmeridgian), Germany, that have muscle traces preserved under the prosoma (Briggs et al., 2005). Muscle traces have also been described from specimens of Euproops danae from the Upper Pennsylvanian (Virgilian) Lawrence Formation, Kansas (Feldman et al., 1993; Babcock & Merriam, 2000; Bicknell et al., 2022b). Further examination of the Lawrence Formation specimens would determine if the muscles exhibit moldic preservation—as is common for Mazon Creek fossils (Clements, Purnell & Gabbott, 2019; Bicknell et al., 2021c)—or if there are additional, unexpressed anatomical features. The collection of novel soft anatomy from these and other fossil xiphosurids are vitally important in presenting and revising hypotheses regarding homology with extant xiphosurids (sensu Briggs et al., 2005; Bicknell et al., 2022b) and resolving conflicts between phylogenetic hypotheses (e.g., Ballesteros & Sharma, 2019; Bicknell, Lustri & Brougham, 2019; Bicknell, Naugolnykh & Brougham, 2020; Lamsdell, 2020). More broadly, this same approach can be applied to the as-of-yet unnamed xiphosuran specimens from the Fezouata Shale Konservat-Lagerstätte (Lower Ordovician, Morocco; Van Roy et al., 2010), as previous micro-CT imagery of material from this deposits has yielded useful results and allowed for specimens to be differentiated in 3D (Kouraiss et al., 2019).
Three-dimensional reconstructions are increasingly used in computational fluid dynamics (CFD) to study the hydrodynamic properties of extinct aquatic taxa (Rahman et al., 2015a; Darroch et al., 2017; Rahman, 2017; Gibson et al., 2019; Ferrón et al., 2020; Hebdon, Ritterbush & Choi, 2020; Gibson et al., 2021; Song et al., 2021). The majority of CFD studies have focused on enigmatic Ediacaran taxa (Rahman et al., 2015a; Rahman, 2017; Gibson et al., 2019), echinoderms (Rahman et al., 2015b; Rahman et al., 2020; Waters et al., 2017), ammonoids (Hebdon, Ritterbush & Choi, 2020), and vertebrate groups (Dec, 2019; Troelsen et al., 2019; Ferrón et al., 2020; Ferrón et al., 2021). While fossil arthropods have received comparatively less attention than the aforementioned groups (e.g., Pates et al., 2021; Song et al., 2021), CFD studies have modelled lift and drag experienced by modern xiphosurids (Bicknell & Pates, 2019; Davis, Hoover & Miller, 2019). Extending CFD studies to fossil xiphosurids will facilitate comparative studies of the hydrodynamic properties of the carapaces of extinct members of the clade, in addition to elucidating the effects of bizarre morphologies, such as the hypertrophied genal spines, on fluid flow. Such spines have been hypothesised to represent an adaptation to movement through unidirectional fluid flow in primarily freshwater or marginal marine environments (Lamsdell, 2016; Lamsdell, 2021; Bicknell & Pates, 2019; Bicknell & Shcherbakov, 2021; Bicknell et al., 2022a); CFD provides the most compelling method for evaluating the likelihood of this hypothesis. Due to compression of the fossils (consider Dubbolimulus peetae) CFD models of compressed xiphosurids would need to be retro-deformed, likely using modern forms as a proxy for inflation, to account for taphonomic alteration. However, there are specimens, such as Crenatolimulus paluxyensis Feldmann et al., 2011 and Tachypleus decheni (Zincken, 1862), that have maintained their three-dimensionality (Bicknell et al., 2021a). Such specimens may be ideal for scanning and immediate CFD analysis.
Palaeontological and biological collections house a wealth of specimens with academic and historic value. Digitisation of holotype specimens is a salient direction for recording and transferring fundamental anatomical information. These records are traditionally conducted by taking photographs or making line drawings. However, two-dimensional data and views cannot (by definition) display all characteristics needed for modern taxonomic and phylogenetic studies (Mathys et al., 2015; Bicknell et al., 2018a). As such, researchers often need to visit collections to examine specimens in person. This process can be prohibitive for logistic, cost, and policy reasons, to name a few. This complication can be circumvented by producing scans of taxonomically important and unique specimens. Such data is becoming a means of transferring important anatomical data to researchers across the globe and provide interested individuals with another medium with which to examine unique material (Hühne, 2018; Shi, Westeen & Rabosky, 2018; Kouraiss et al., 2019).
Conclusion
Reconsidering the four Australian xiphosurids here, we have highlighted the rise of Austrolimulidae in the Gondwanan record began just after the end-Permian extinction. This timing also suggests that, globally, the development of hypertrophied spines within non-belinurid xiphosurids began after the end-Permian. We demonstrate that limited novel anatomical data were obtained for Austrolimulus fletcheri, Dubbolimulus peetae, Tasmaniolimulus patersoni, and Victalimulus mcqueeni using SRXT, reflecting the preservation of these fossils in sand- and siltstones. Future directions include examining similar fossils with NCT, an additional method that achieves an alternative and complementary contrast to X-ray CT, and may resolve features that conventional lab-based- and synchrotron X-rays are unable to reveal. Future applications of these scan data include informing reconstructions needed for computational fluid dynamic analyses; a direction that may uncover the morpho-functional use of overdeveloped spines common to Australian xiphosurids.