AgGMP encoding GDP-D-mannose pyrophosphorylase from celery enhanced the accumulation of ascorbic acid and resistance to drought stress in Arabidopsis
- Published
- Accepted
- Received
- Academic Editor
- Genlou Sun
- Subject Areas
- Genetics, Molecular Biology, Plant Science, Infectious Diseases
- Keywords
- Apium graveolens, Arabidopsis thaliana, Ascorbic acid, GDP-D-mannose pyrophosphorylase, Drought stress, Gene expression
- Copyright
- © 2022 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. AgGMP encoding GDP-D-mannose pyrophosphorylase from celery enhanced the accumulation of ascorbic acid and resistance to drought stress in Arabidopsis. PeerJ 10:e12976 https://doi.org/10.7717/peerj.12976
Abstract
Ascorbic acid (AsA) is an important nutrient in celery, the conversion of D-mannose-1-P to GDP-D-mannose catalyzed by GDP-D-mannose pyrophosphorylase (GMPase) represents the first committed step in the biosynthesis of AsA. To clarify the function of the AgGMP gene of celery, the AgGMP gene was cloned from celery cv. ‘Jinnan Shiqin’ . It contains an open reading frame (ORF) with the length of 1,086 bp, encoding 361 amino acids. AgGMP protein was highly conserved among different plant species. Phylogenetic analysis demonstrated that the GMP proteins from celery and carrot belonged to the same branch. AgGMP protein was mainly composed of three α-helixes and certain random coils. No signal peptide was found in the AgGMP protein. The subcellular localization indicated that the AgGMP protein was located in the cytoplasm. The relative expression levels of AgGMP in ‘Jinnan Shiqin’ were significantly up-regulated at 2 h and 4 h under drought stress treatments. AsA contents in transgenic Arabidopsis lines hosting AgGMP gene were higher than that in wild type plants, and the root lengths were also longer in the MS medium containing 300 mM mannitol. The present study provides useful evidence for the functional involvement of AgGMP in regulating AsA accumulation and response to drought stress in celery.
Introduction
GDP-D-mannose pyrophosphorylase (GMPase) is a rate-limiting enzyme in the L-galactose pathway, a key biosynthetic pathway for L-ascorbic acid (AsA) in plants. In this pathway, the initial step is the formation of GDP-D-mannose, which is catalyzed by GMPase (Wang et al., 2011). AsA is a water-soluble antioxygenic organic micromolecule widely found in plants. It’s essential for cardiovascular function, immune cell development, connective tissue health, and iron utilization. As a key enzyme in AsA biosynthesis, GMPase in higher plants plays important roles in removing reactive oxygen species (ROS) generated by adverse environmental conditions (Xue et al., 2018), such as high temperature, low temperature (Li et al., 2018a; Liu et al., 2019; Huang et al., 2016), and salt stress (Zhang et al., 2012). In addition, the resistance of abiotic stress in plants could be improved through modulating GMP gene expression to increase the AsA content. Until now, GMP genes have been isolated from a number of higher plants, including Arabidopsis thaliana (Wang, Guo & Tang, 2011), Camellia sinensis (Xiao et al., 2015), Solanum esculentum (Wang et al., 2011), and Dendrobium huoshanense (He, Yu & Tei, 2017).
Plant resistance against abiotic stress was associated with the changes of antioxidant defense system, which consisted of enzymatic and non-enzymatic antioxidants (Garg, Varshney & Jain, 2014; Hasanuzzaman et al., 2017; Li et al., 2018a; Liu et al., 2021). AsA belongs to non-enzymatic antioxidants (Liu et al., 2019), drought stress affects AsA content by controlling the activities of enzymes involved in AsA metabolism (e.g. GMP, GalDH, APX, DHAR, MDHAR) (Huang et al., 2016; Liu et al., 2019). AsA can directly remove ROS (reactive oxygen species) produced by stress, and also indirectly remove H2O2 through the AsA-GSH cycle to protect tissues from harmful oxidative products and to keep certain enzymes in their required reduced forms (Liebler, Kling & Reed, 1986; Padh, 1990; Barth, Gouzd & Steele, 2009), as well as improve the ability of plants to resist abiotic stress.
Under low and high temperature stresses, the relative expression of SlGMP, GMPase activity, contents of AsA and dehydroascorbic acid (DHA) were increased, and the content of malondialdehyde (MDA) was decreased in transgenic tomato plants (Wang et al., 2011). The overexpression of SlGMP also delayed the senescence of potato (Lin et al., 2011). OsGMP gene affected AsA synthesis and GMPase activity in rice (Oryza sativa), and overexpression of OsGMP reduced the inhibitory effect of NH4+ on root growth in A. thaliana (Li et al., 2010). The OsGMP gene also was reported to play a key role in the rice during its nutritional and reproductive stages under salt stress (Kempinski, Haffar & Carina, 2011; Qin et al., 2016a, 2016b). The GmGMP1 gene can be induced to express under high temperature, low temperature, drought and salt stresses. The overexpression of GmGMP1 gene in A. thaliana and soybean (Glycine Max) increases GMPase activity and AsA content, and enhances the ability for eliminating ROS (Xue et al., 2018). The tobacco (Nicotiana Tabacum) harboring PpGMP showed stronger resistance to salt or drought stresses by increasing the AsA content (Ai, Liao & Li, 2016). AtGMP positively regulates the synthesis of AsA. AsA content in transgenic lettuce (Lactuca sativa) overexpressing AtGMP gene increased to 2.5-fold of that in control (Wang, Guo & Tang, 2011).
Celery (Apium graveolens) growth is influenced by multiple environmental factors. In previous studies, it is reported that GMP gene participated in plant response to stress. We speculated that AgGMP may be involved in celery resisting abiotic stress through modulating the AsA accumulation. However, the characteristics and transcription regulation mechanism of AgGMP under drought stress in celery remain unclear. In this study, AgGMP, a gene encoding GDP-D-mannose pyrophosphorylase, was cloned from celery, and then its expression patterns were detected in celery under abiotic stress treatments. The transgenic A. thaliana plants overexpressed AgGMP were obtained to examine the AsA level and compared their root growth with wild-type under drought stress. This study further clarified the roles of AgGMP in abiotic stress, and provided a theoretical basis for stress response of celery.
Materials and Methods
Plant materials, growth conditions and stress treatments
Celery cv. ‘Jinnan Shiqin’, A. thaliana ecotype Columbia (WT), and transgenic A. thaliana were grown in pots within a soil/vermiculite mixture in phytotron at the Key Laboratory of Crop Genetics and Germplasm Enhancement, Nanjing Agricultural University (32°03′N, 118°84′E). The phytotron program was 25 °C/18 °C (day/night) for 16 h/8 h. The light intensity was 300 μmol m−2 s−1 at daytime, with relative humidity of 75%. Two-month-old (day after germinating) ‘Jinnan Shiqin’ plants were grouped and treated with 4 °C, 38 °C, 200 g·L−1 PEG 6000 and 200 mM NaCl, respectively. The leaf blades of celery with the longest petiole were collected at 0, 1, 2, 4, 8, and 24 h after treatments. All the samples were frozen in liquid nitrogen immediately and then stored at −80 °C for RNA extraction. WT and AgGMP-OE lines were grown on Murashige and Skoog (MS) medium with or without 300 mM mannitol (control). Each experiment was performed with three biological replicates.
Total RNA extraction, cDNA synthesis and AsA content determination
Total RNA of celery and A. thaliana were extracted using the total RNA extraction Kit (Tiangen, Beijing, China) according to the manufacturer’s instructions. cDNA was obtained by Prime Script RT reagent Kit (TaKaRa, Dalian, China) based on the operation instruction. The content of AsA was determined using UPLC (ultra performance liquid chromatography) system according to the method described as previous study (Liu et al., 2019).
Bioinformatics analysis
The sequences of GMP proteins from other species were downloaded from the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). Primer Premier 6.0 was utilized to design primers. Nucleotide and encoded amino acid sequence of AgGMP gene was analyzed using BioXM software. MEGA7.0 software was used to construct the phylogenetic tree. The alignment of amino acid sequences of GMPs from celery and other plants were carried out by DNAMAN 8.0 software. SOPMA software (http://pbil.ibcp.fr/) was used to predict the secondary structure of GMP protein. The protein tertiary structural model was established using CPH models 3.2 Server (http://www.cbs.dtu.dk/services/CPHmodels/). Signal peptide was predicted by Signal P software.
Isolation of the AgGMP gene, overexpression vector construction and A. thaliana transformation
The putative AgGMP gene sequence was retrieved from celery genome and transcriptome database (Feng et al., 2018a; Li et al., 2020c). The full lengths ORF (open reading frame) of AgGMP was amplified with special primers (AgGMP-7736-F: 5′-TTTACAATTACCATGGGATCCATGAAGGCTCTTATTCTTGTTGGA-3′; AgGMP-7736-R: 5′-ACCGATGATACGAACGAGCTCTCACATCACAATCTCTGGCTTCAA-3′). The PCR product was cloned into the pCAMBIA1301 and then sequenced (Genscript, Nanjing, China). The recombinant plasmid (35S: AgGMP) was introduced into the Agrobacterium tumefaciens strain GV3101 via electroporation method. The floral-dip method was used for Agrobacterium-mediated transformation of A. thaliana (Zhang, Henriques & Lin, 2006). Transgenic A. thaliana were initially screened on MS medium containing hygromycin (40 mg/L), and then further confirmed by β-glucuronidase (GUS) assay, PCR amplification and sequencing.
Subcellular localization
The ORF of AgGMP without stop codon was amplified using specific primers (AgGMP-PA7-F: 5′-CACCATCACCATCACGCCATGATGAAGGCTCTTATTCTT GTTGGA-3′ and AgGMP-PA7-R: 5′-CACTAGTACGTCGACCATGGCCATCACAATCTCTGGCTTCAA-3′). The amplification product was ligated into pA7 vector via Nco I site. The control vector (pA7-GFP) and recombinant vector (AgGMP-GFP) were bombarded into onion epidermal cells using the biolistic bombardment Biolistic PDS-1000 (Bio-Rad, Hercules, CA, USA). After 18 h of dark growth on MS solid medium, the GFP fluorescence of samples were observed using a laser confocal microscope LSM780 (Zeiss, Oberkochen, Germany).
Real-time quantitative PCR analysis
Real-time quantitative PCR (RT-qPCR) was conducted to detect the expression level of AgGMP. Premier 6.0 software was used to designed primers (AgGMP-qF: 5′-TGCTGGAATCTACCTGCTGAACC-3′, AgGMP-qR: 5′-TGCTGGAATCTACCTGCTGAACC-3′). The SYBR Premix Ex Taq (TaKaRa, Dalian, China) and Bio-Rad IQ5 real-time PCR System (Bio-Rad, Hercules, CA, USA) were used for RT-qPCR reaction. AgActin gene was used as internal standard (Li et al., 2016). Each reaction set three biological replicates. The relative expression data of AgGMP were analyzed using 2−ΔΔCt method (Pfaffl, 2001).
Statistical analysis
All data in the text were obtained from the average of three biological repeats. Data significant difference was analyzed using SPSS 24.0 by one way ANOVA at a 0.05 level.
Results
Analysis of AgGMP sequence
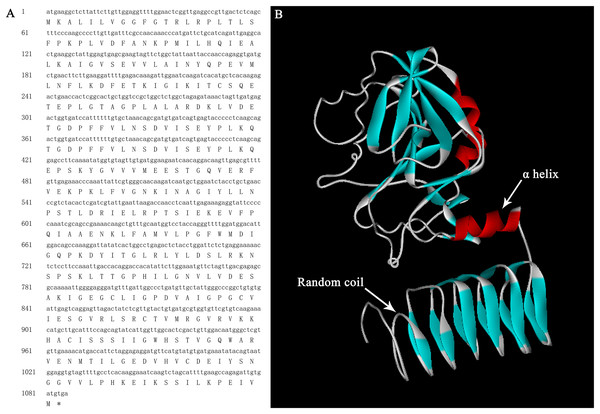
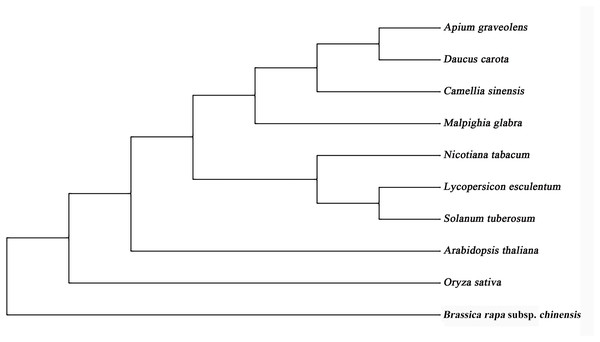
The full lengths cDNA sequence of AgGMP gene was obtained from ‘Jinnan Shiqin’ (GenBank No. OL757646), which contained an open reading frame (ORF) with the length of 1,086 bp, encoding 361 amino acids (Fig. 1A). The secondary structure was mainly composed of 30.77% α-helixes, 29.09% extended strand, 9.07% β-turn and 37.67% random coils. Prediction results indicated that the protein tertiary structure of AgGMP protein was mainly composed of three α-helixes and certain random coils (Fig. 1B). AgGMP protein has no signal peptide according to the prediction by Signal P software. The amino acid sequence of AgGMP were aligned with homologous sequences from Daucus carota (carrot, AQM57027.1), Lycopersicon esculentum (tomato, accession ID DQ449030), Solanum tuberosum (potato, NP_001275205.1), Camellia sinensis (tea plant, AGI78460.1), Arabidopsis thaliana (AT4G30570), Oryza sativa (rice, LOC4327472), Malpighia glabra (acerola, ABB53473.1), Brassica rapa subsp. chinensis (non-heading Chinese cabbage, AET14212.1), Nicotiana tabacum (tobacco, BAB62108.1) (Fig. 2). GMP proteins were highly conserved (90.80% of consistency) among different species. Phylogenetic analysis demonstrated that AgGMP protein had the closest evolutionary relationship with carrot (Fig. 3).
Figure 1: Bioinformatics analysis of AgGMP.
(A) Nucleotide and encoded amino acid sequence of AgGMP gene. (B) The tertiary structural model of AgGMP protein.Figure 2: The alignment of amino acid sequences of GMPs from celery and other plant species.
Figure 3: Phylogenetic tree of GMP proteins from celery and other plant species.
Expression profiles of AgGMP under abiotic stress in celery
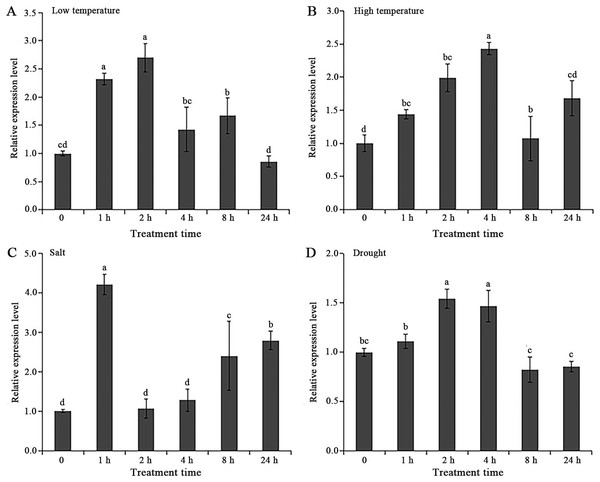
As shown in Fig. 4, the relative expression levels of AgGMP gene in ‘Jinnan Shiqin’ were up-regulated significantly under low temperature, high temperature, salt and drought treatments. The relative expression levels of AgGMP gene were obviously increased at 1 h after low temperature, high temperature and salt treatments, respectively (Figs. 4A–4C). In salt treatment, the relative expression levels of AgGMP gene were remarkably elevated at 8 h and 24 h than that at 0 h, which were 2.52 and 2.79-folds of that at 0 h, respectively. The highest expression of AgGMP gene were occurred at 2 h, 4 h, 1 h and 2 h of low temperature, high temperature, salt and drought treatments, which were 2.70, 2.43, 1.54, and 4.2-folds of the 0 h, respectively. Under drought stress, the expression profiles of AgGMP exhibited an increase at 1 h, 2 h, 4 h, which were 1.11, 1.54 and 1.47, and then decreased at 8 h, 24 h, which were 0.82 and 0.85, respectively (Fig. 4D). The results indicated that AgGMP involved in the response to abiotic stress in celery.
Figure 4: Expression analysis of AgGMP in celery under abiotic stress treatments.
(A) Low temperature. (B) High temperature. (C) Salt. (D) Drought. Data are expressed as the means ± standard deviation (SD) of three replicates. Different letters indicate significant difference at 0.05 level.Subcellular localization analysis of AgGMP
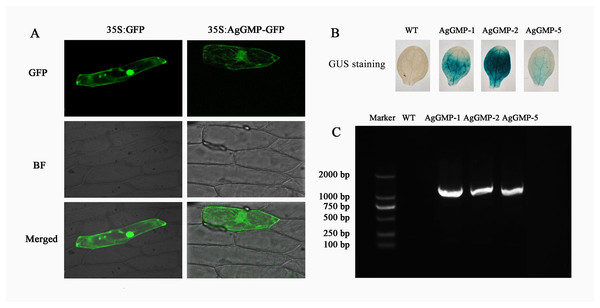
The vector AgGMP-GFP and pA7-GFP were transferred into the onion epidermis cell to detect the subcellular localization of AgGMP. The onion epidermal cell with AgGMP-GFP and pA7-GFP displayed bright fluorescence throughout the entire cell, suggesting that the AgGMP protein was located in cytoplasm (Fig. 5A).
Figure 5: Subcellular localization of AgGMP protein and identification of transgenic A. thaliana lines.
(A) Subcellular localization of AgGMP protein. (B) GUS staining of leaves from transgenic A. thaliana and WT. (C) Identification of transgenic A. thaliana using PCR amplification.Identification of transgenic A. thaliana
To investigate the function of AgGMP gene, transgenic A. thaliana lines were generated via Agrobacterium-mediated transformation. The transgenic A. thaliana lines (AgGMP-1, AgGMP-2, and AgGMP-5) were screened on the MS medium containing hygromycin. The leaves of transgenic A. thaliana lines were immersed in X-gluc (5-bromo-4-chloro-3-indolyl-β-D-glucuronic acid) and appeared blue (Fig. 5B). About 1,000 bp PCR products were observed only in the transgenic lines, AgGMP-1, AgGMP-2 and AgGMP-5, based on the PCR amplification (Fig. 5C). The results indicated that AgGMP was successfully transferred into A. thaliana, three OE lines (AgGMP-1, AgGMP-2 and AgGMP-5) harboring AgGMP gene were obtained.
Overexpression of AgGMP up-regulated the AsA content in Arabidopsis
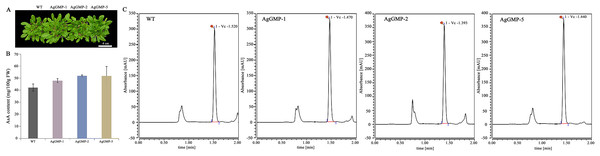
There are no obvious differences observed in phenotype among three OE lines and WT plants (Fig. 6A). According to the AsA content determination, the transgenic A. thaliana lines contained higher AsA accumulation compared with WT (Figs. 6B and 6C). The AsA contents in WT, AgGMP-1, AgGMP-2 and AgGMP-5 lines were 42.25, 48.04, 51.98, and 52.73 mg/100g FW (fresh weight), respectively. The results indicated that overexpression of AgGMP up-regulated the AsA level in transgenic A. thaliana.
Figure 6: AsA contents of WT and transgenic A. thaliana.
(A) Phenotype of WT and transgenic A. thaliana. Scale bars = 3.5 cm. (B) AsA content. (C) UPLC chromatogram. Data are expressed as the means ± standard deviation (SD) of three replicates.The change of seedling root lengths in A. thaliana under 300 mM mannitol treatment
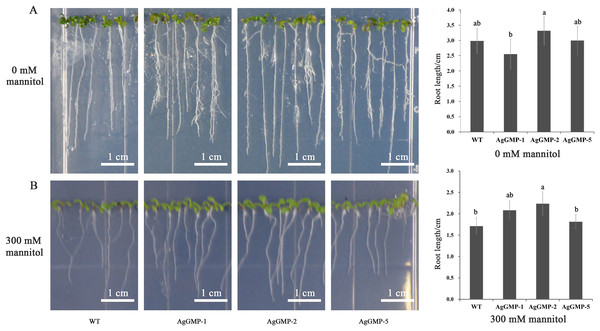
The seeds of three A. thaliana OE lines and WT were germinated and grown on MS medium containing different mannitol concentrations. The root lengths of 7-day-old (day after germinating) A. thaliana plants were measured. On MS medium without mannitol, no significant difference in root lengths was observed between WT and transgenic A. thaliana lines hosting AgGMP gene. The root lengths of WT, AgGMP-1, AgGMP-2, and AgGMP-5 lines, were 2.98, 2.55, 3.31 and 3.00 cm, respectively (Fig. 7A). Whereas, the roots lengths were inhibited in WT and three transgenic A. thaliana lines treated with 300 mM mannitol, which were 1.71, 2.08, 2.23, and 1.81 cm, respectively (Fig. 7B). On MS medium without mannitol, the root lengths of A. thaliana were longer, more lateral roots and root hairs, as well as better root growth. The root lengths of transgenic plants were longer than that of WT on MS medium containing 300 mM mannitol. In particular, the root lengths of AgGMP-2 were significantly increased compared with WT.
Figure 7: Root growth of WT and transgenic A. thaliana lines subjected to mannitol application.
(A) Root lengths of transgenic A. thaliana and WT on MS medium without mannitol application. Scale bars = 1.5 cm. (B) Root lengths of transgenic A. thaliana and WT on MS medium with 300 mM mannitol application. Data are expressed as the means ± standard deviation (SD) of three replicates. Different letters indicate significant difference at 0.05 level.Discussion
Celery is one of important leafy vegetables with rich nutrients (Kooti & Daraei, 2017; Li et al., 2018b), such as anthocyanin (Feng et al., 2018b, 2021), apigenin (Tan et al., 2017; Yan et al., 2019; Wang et al., 2021), carotenoids (Li et al., 2019; Yin et al., 2020; Ding et al., 2021), AsA (Liu et al., 2021), and dietary fiber (Duan et al., 2020). AsA is not only is one of important nutrients, but also a key mediator that triggers plant response to various abiotic stress (Barth, Gouzd & Steele, 2009). AsA accumulation is affected by abiotic stress, such as salt (Zhang et al., 2012), low temperature, high temperature (Wang et al., 2011), drought stress (Padh, 1990), light (Li et al., 2020a), and a variety of nutrient elements (Li et al., 2020b), which is speculated to accomplished by increasing the activity of related enzymes involved in the AsA biosynthesis pathway in plants.
In plants, there are four pathways for AsA biosynthesis, including L-galactose pathway, myo-inositol pathway, L-gulose pathway, and D-galacturonate pathway. The GMP gene, encoding GMPase, plays an essential role in the L-galactose pathway. GMPase catalyzes the initial steps of AsA biosynthesis to form GDP-D-mannose, the precursor of AsA. Thus, GMP gene could affect AsA content by controlling the activities of GMPase. Our present result indicated that AgGMP involved in the response to abiotic stress in celery. The identified function of AgGMP is similar to that of soybean GmGMP1, GMP genes expression were both significantly induced by heat, cold and salt stresses. Under abiotic stress treatments, the expression level of GmGMP1 peak at 1 h and then decreased (Xue et al., 2018). Under drought stress, the expression levels of the AgAPX1 and AgGMP of celery were also similar, which significantly higher than that of the control at 2 h, and peaked at 4 h followed by a decrease (Liu et al., 2019).
It is acknowledged that drought is an important factor affecting agricultural production, improving plant drought resistance also is an ongoing hot topic. Considering evidences have suggested the relationship between AsA and stress resistance (Wang et al., 2011; Zhang et al., 2012). One of the consequences of abiotic stress is that it triggers an oxidative burst due to formation of reactive oxygen species. AsA could directly remove ROS produced by stress, and also indirectly remove H2O2 through the AsA-GSH cycle to protect tissues from harmful oxidative products, as well as keep certain enzymes in their required reduced forms. GMPase is a key rate limiting enzymes in AsA biosynthesis. Declined expression of GMP gene, encoded GMPase, usually was followed by a decreased resistance to drought stress in plants. Overexpression of the GMP gene of soybean enhanced the plant drought resistance in transgenic plants (Xue et al., 2018). In this study, we found that the relative expression levels of AgGMP gene were up-regulated significantly under low temperature, high temperature, salt and drought treatments, which reached the peak at 2 h, 4 h, 1 h, and 2 h, respectively. The transcript of AgGMP in celery were induced by drought stress, the expression of AgGMP in celery was significantly up-regulated of AgGMP under drought stress at 2 h and reach peak at 4 h and then declined.
To further investigate the function of AgGMP gene in response to drought stress, the AsA content and root lengths of three A. thaliana OE lines and WT were measured. Compare with the WT, overexpression of AgGMP up-regulated the AsA content in three transgenic A. thaliana lines, the root lengths were also longer when subjected to 300 mM mannitol. It is possible that overexpression of AgGMP can improve the activity of GMPase that involved in AsA biosynthesis pathway in A. thaliana, and increase the AsA accumulation to neutralize part of the stress effects. AsA could remove ROS and H2O2 produced by drought stress, protect tissues from harmful oxidative products and keep certain enzymes in their required reduced forms (Wang et al., 2011; Zhang et al., 2012). The three transgenic A. thaliana lines showed reduced root damage caused by mannitol and presented greater tolerance to drought stress. We speculated that AgGMP was participated in the process of celery resisting drought stress and overexpression of AgGMP can induce an increased resistance to drought stress in transgenic plants by enhancing the accumulation of AsA.
Conclusion
GMPase was a rate-limiting enzyme in the L-galactose pathway, a key biosynthetic pathway for L-ascorbic acid (AsA) in plants. Here, the gene AgGMP encoding the AgGMPase was identified and characterized. The AgGMP gene contained an ORF of 1,086 bp, encoding 361 amino acids. Sequence alignment suggested that AgGMP protein was highly conserved among different plant species. The transcript of AgGMP was induced by abiotic stress in celery, the heterologous overexpression of AgGMP in A. thaliana proved the role of AgGMP in regulating AsA accumulation and modifying drought stress resistance. These findings suggested that AgGMP acted a regulator in AsA accumulation and response to abiotic stress in celery. In the future, we hope to achieve its homologous over-expression through transgenic technology and knock it out using the CRISPR/Cas9 method in celery.