Soil health and nutrient density: preliminary comparison of regenerative and conventional farming
- Published
- Accepted
- Received
- Academic Editor
- Maria Luisa Fernandez-Marcos
- Subject Areas
- Agricultural Science, Food Science and Technology, Soil Science
- Keywords
- Soil health, Nutrient density, Agriculture, Regenerative agriculture
- Copyright
- © 2022 Montgomery et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2022. Soil health and nutrient density: preliminary comparison of regenerative and conventional farming. PeerJ 10:e12848 https://doi.org/10.7717/peerj.12848
Abstract
Several independent comparisons indicate regenerative farming practices enhance the nutritional profiles of crops and livestock. Measurements from paired farms across the United States indicate differences in soil health and crop nutrient density between fields worked with conventional (synthetically-fertilized and herbicide-treated) or regenerative practices for 5 to 10 years. Specifically, regenerative farms that combined no-till, cover crops, and diverse rotations—a system known as Conservation Agriculture—produced crops with higher soil organic matter levels, soil health scores, and levels of certain vitamins, minerals, and phytochemicals. In addition, crops from two regenerative no-till vegetable farms, one in California and the other in Connecticut, had higher levels of phytochemicals than values reported previously from New York supermarkets. Moreover, a comparison of wheat from adjacent regenerative and conventional no-till fields in northern Oregon found a higher density of mineral micronutrients in the regenerative crop. Finally, a comparison of the unsaturated fatty acid profile of beef and pork raised on one of the regenerative farms to a regional health-promoting brand and conventional meat from local supermarkets, found higher levels of omega-3 fats and a more health-beneficial ratio of omega-6 to omega-3 fats. Despite small sample sizes, all three crop comparisons show differences in micronutrient and phytochemical concentrations that suggest soil health is an under appreciated influence on nutrient density, particularly for phytochemicals not conventionally considered nutrients but nonetheless relevant to chronic disease prevention. Likewise, regenerative grazing practices produced meat with a better fatty acid profile than conventional and regional health-promoting brands. Together these comparisons offer preliminary support for the conclusion that regenerative soil-building farming practices can enhance the nutritional profile of conventionally grown plant and animal foods.
Introduction
Reported declines in the nutrient density of crops (Mayer, 1997; Davis, Epp & Riordan, 2004; White & Broadley, 2005; Ekholm et al., 2007; Davis, 2009) are typically attributed to crop breeders having focused almost exclusively on increasing yields (Morris & Sands, 2006; Marles, 2017). However, studies demonstrating that fertilization regimes and soil life affect mineral uptake by crops (e.g., Lambert, Baker & Cole, 1979; Marschner & Dell, 1994; Miller, 2000; Jansa, Wiemken & Frossard, 2006; Ryan et al., 2008; White & Broadley, 2009; Zhang et al., 2012; Lehmann et al., 2014; Adak et al., 2016; Konecny et al., 2019) suggest that conventional farming practices of intensive tillage, nitrogen fertilization, and synthetic pesticide applications may have contributed to declining nutrient density through disrupting crop symbioses with soil life (Montgomery & Biklé, 2016, 2022). While a number of previous assessments compared differences in the nutritional quality of foods grown with conventional and organic production practices (Svec, Thoroughgood & Mok, 1976; Smith, 1993; Worthington, 2001; Brandt et al., 2011; Hunter et al., 2011; Smith-Spangler et al., 2012; Baranski et al., 2014), few have considered directly the influence of soil health—as reflected in soil organic matter and soil life—on nutrient density (see Hepperly, Omondi & Seidel (2018) for a notable exception).
Although proponents of farming practices that rebuild soil organic matter and soil health (which we collectively term “regenerative”) contend that such practices result in more nutrient-dense food, such claims remain little tested. Here we compare the effect of regenerative farming on soil health and crop nutrient density from a cohort of paired farm trials across the United States. Along with evidence from several other paired farm and plot studies this comparison indicates that regenerative agricultural practices employing no-till, cover crops, and diverse crop rotations enhance soil health and the micronutrient and phytochemical density of various crops. We also compare the fatty-acid profile of beef and pork raised on one of the regenerative farms to a regional health-promoting brand and conventionally raised meat purchased at a local grocery store. Our results suggest that farming practices that affect soil organic matter and microbial communities are under-appreciated influences on crop nutrient density, particularly for micronutrients and phytochemicals relevant to plant health and chronic disease prevention in humans. These preliminary results point to soil health as a more pertinent metric for assessing the impact of farming practices on the nutrient composition of crops than the usual distinction of organic and conventional practices (Montgomery & Biklé, 2021).
Paired Farms
Methods
We tested the influence of soil health, as measured by soil organic matter content and soil health scores (Haney et al., 2018), on the nutrient density of crops by measuring both on eight pairs of regenerative and conventional farms across the United States, in North Carolina, Pennsylvania, Ohio, Iowa, Tennessee, Kansas, North Dakota, and Montana (Fig. 1). Each regenerative farm was paired with a proximal conventionally-managed farm with the same soil type and planted with a stand of the same crop variety as their conventional neighbor (peas, sorghum, corn, or soybeans). In addition, we compared measures of soil health and nutrient density on the same variety of cabbage grown on a regenerative no-till vegetable farm in California paired with a field at the start of being converted to tillage-based organic practices, and thus with soil still representative of a conventional farm. We also measured soil health on another regenerative farm in Connecticut and a neighboring conventional farm but failed to secure a same-cultivar comparison crop. Regenerative farms were selected from a cohort of farmers whose practices had combined no-till, cover crops, and diverse rotations (Montgomery, 2017) in a system known as Conservation Agriculture (Kassam, Friedrich & Derpsch, 2019). At the time of our survey these regenerative farmers had fully employed such systems for 5 to 10 years, while some had used no-till methods or cover crops for longer.
Figure 1: Locations of paired farm trials.
While soil organic matter is a common soil-health metric, it does not account for microbial abundance and activity, which is known to affect nutrient cycling. So in addition to measuring soil organic matter, in each of the sampled fields on the paired farms we also used a test (Haney et al., 2018) that combines water extractable organic carbon and nitrogen content with a measure of microbial respiration (24-h CO2 release) to gauge microbial activity, assess the amount of food readily available to soil microbes, and determine an overall soil health score for the soil. At each set of farms we collected a topsoil sample aggregated from the upper eight inches collected at locations across each field in which tested crops were grown. Soil samples were then sent to Ward Laboratories (www.wardlab.com) for analyses. This gave us a total of ten comparisons of soil health metrics (soil organic matter and Haney test scores) and nine paired observations on crop nutrient density.
Soil organic matter levels were measured through loss on ignition. To determine soil health scores each soil sample was dried at 50 °C and ground to pass a 2 mm sieve. Two 4 g samples were place in 50 ml Erlenmeyer flasks and one 40 g sample was placed in a 50 ml plastic beaker. The larger (40 g) sample was wetted through capillary action with 20 ml of DI water and analyzed after a 24-h incubation test at 24 °C using an infrared gas analyzer for CO2-C. The two 4 g samples were respectively extracted with 40 ml of DI water and 40 ml of H3A organic acid extract, shaken for 10 min, centrifuged for 5 min, and filtered through Whatman 2 V filter paper. The extracts were analyzed for NO3-N and NH4-N on a Lachat 8,000 flow injection analyzer, and the water extract was analyzed on a Teledyne-Tekmar Torch C:N analyzer for water-extractable organic C and total N. Haney soil health scores (SHS) were then calculated as
(1) where CO2C is the one day CO2−C release (mg/kg soil), WEOC is active organic C (mg/kg soil), and WEON is the active organic soil N (mg/kg soil) (Haney et al., 2018).
Upon harvest, each of the regenerative farmers sent a sample of both their harvested crop and the corresponding crop from their conventional neighbor’s farm to the Linus Pauling Institute at Oregon State University for measurement of certain vitamins (B, C, E, and K), minerals (Al, Ca, Cu, Fe, K, Mg, Mn, Na, P, and Zn), and phytochemicals (total phenols, total phytosterols, and total carotenoids). This gave us nine comparisons of crops from paired regenerative and conventional farms (as a same-cultivar crop comparison was not available for the Connecticut farms).
Crop samples were shipped immediately after harvest in insulated bags containing ice blocks and were either processed immediately or briefly stored at −20 °C until processed. All samples were ground into powders in a stainless steel blender containing liquid nitrogen to minimize nutrient degradation. Powdered samples were then stored at −80 °C until analyzed. Vitamins, minerals, and phytochemicals were analyzed according to published methods (see below). Samples were kept cold during sample preparation by keeping the sample container immersed in liquid nitrogen during the weighing step. Vitamins were measured by HPLC with amperometric detection for vitamins E (Podda et al., 1996) and C (Frei, England & Ames, 1989), and by mass spectrometry for vitamins K (Farley et al., 2014) and B (Cellar et al., 2016). Minerals were analyzed in the Soil Health laboratory at Oregon State University by ICP OES following microwave digestion in nitric acid (da Silva et al., 2020). Phytochemicals were measured by UV-Vis spectrophotometry for total phenolics (Durst & Weaver, 2013), total phytosterols (Larissa et al., 2013), and total carotenoids (Durst & Weaver, 2013).
Paired farm results
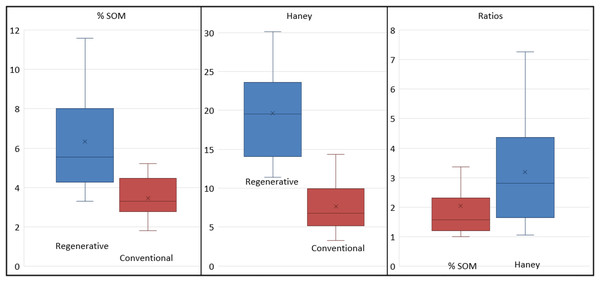
Across the board, the soil on the regenerative farms had more soil organic matter (two tailed T-test, p = 0.0087) and higher soil health scores (two tailed T-test, p = 0.000033) (Table 1). Specifically, regenerative farms had 3% to 12% soil organic matter (mean = 6.3%), whereas those on conventional farms had 2% to 5% (mean = 3.5%) (Fig. 2). Soil health scores for regenerative farms ranged from 11 to 30 (mean = 20). Those for the conventional farms ranged from 3 to 14 (mean = 8). In terms of individual farm pairs, the regenerative farms were consistently higher, with up to 5 times more soil organic matter (mean = 2.0), and up to 7 times higher soil health scores (mean = 3.3) (Fig. 3). The two no-till vegetable farms had the highest soil organic matter ratios (between 2:1 and 3:1) and soil health scores. The eight paired conventional and regenerative farms had soil organic matter ratios between 1:1 and 2:1.
| Location (Crop) | SOM | Haney | SOM | Haney | SOM Ratio | Haney ratio |
|---|---|---|---|---|---|---|
| (R) | (R) | (C) | (C) | (R/C) | (R/C) | |
| Lebanon, CT (-) | 11.1 | 23.9 | 3.3 | 3.3 | 3.4 | 7.3 |
| Lewisburg, PA (corn) | 3.3 | 23.3 | 3.3 | 5.8 | 1.0 | 4.0 |
| Hickory, NC (corn) | 7.0 | 18.2 | 5.2 | 3.4 | 1.3 | 5.4 |
| Manchester, TN (soy) | 6.3 | 23.5 | 3.2 | 6.2 | 2.0 | 3.8 |
| Shenandoah, IA (corn) | 5.7 | 12.8 | 4.6 | 7.2 | 1.2 | 1.8 |
| Waverly, KS (soy) | 5.4 | 14.5 | 3.7 | 13.3 | 1.5 | 1.1 |
| Holton, KS (sorghum) | 4.6 | 17.7 | 4.4 | 14.3 | 1.1 | 1.2 |
| Bismarck, ND (peas) | 5.0 | 20.8 | 3.0 | 8.8 | 1.7 | 2.4 |
| Ekalaka, MT (peas) | 3.3 | 11.4 | 1.8 | 6.3 | 1.8 | 1.8 |
| Sebastopol, CA (cabbage) | 11.6 | 30.1 | 2.1 | 8.1 | 5.5 | 3.7 |
Notes:
Soil health metrics for paired regenerative (R) and conventional (C) farms.
SOM = % soil organic matter.
Haney = Haney soil test soil health score.
Figure 2: Soil health metrics for regenerative and conventional farms.
Distributions of soil health metrics for regenerative (blue) and conventional (red) farms for (left) % soil organic matter, (middle) Haney test scores, and (right) ratios of paired regenerative and conventional farm values for % soil organic matter (red) and Haney test scores (blue).Figure 3: Plots of (upper) soil organic matter and (lower) Haney test soil score for regenerative farms vs their paired conventional farm.
Dotted lines represent 1:1 correspondence, dashed lines represent other indicated ratios.Given our minimal sample size, and therefore lack of statistical power for individual crops tested, in the discussion below we note only differences greater than 10% in measured values of vitamins, minerals, and phytochemicals. Results varied substantially for individual nutrients and farm pairs, but averaged across all nine farm pairings the regenerative farm crops had 34% more vitamin K (10% more to 57% more), 15% more vitamin E (11% less to 70% more), 14% more vitamin B1 (17% less to 2 times more), and 17% more vitamin B2 (17% less to 3 times more) (Table 2). The crops from the regenerative farms also had 15% more total carotenoids (6% less to 48% more), 20% more total phenolics (14% less to more than twice as many), and 22% more total phytosterols (25% less to more than 2 times more). In addition, regeneratively grown crops had 11% more calcium (1% less to 43% more), 16% more phosphorus (10% less to twice as much), and 27% more copper (16% less to twice as much). Cabbage from the regenerative farm had 20%, 41%, and 70% more vitamins C, K, and E, respectively, as well as more than twice the phenolics and phytosterols, and 48% more carotenoids than cabbage from the conventional field. Corn, soy, and sorghum grown under regenerative practices respectively had 17%, 22%, and 23% more zinc. In addition, peas and sorghum grown using regenerative practices had more vitamins, and regenerative soy and sorghum had more copper. However, averaged across all crops the regenerative ones also had less vitamin B6 and manganese, and regenerative soy had less vitamin C and several B vitamins (B1, B3, and B6). Overall, more substantial differences were observed in cabbage, peas, and sorghum than in corn and soybeans. Absolute values for each crop and nutrient pairing are given in Supplemental Materials (File S1).
| Nutrient | All crops | Cabbage | Peas | Soy | Corn | Sorghum |
|---|---|---|---|---|---|---|
| Vitamin K | 1.34 | 1.41 | 1.57 | 1.10 | - | 1.38 |
| Vitamin E | 1.15 | 1.70 | 1.20 | 1.14 | 0.89 | 1.19 |
| Vitamin C | 1.03 | 1.20 | 1.81 | 0.53 | - | - |
| Vitamin B1 | 1.14 | 2.00 | 1.05 | 0.83 | 0.93 | 1.45 |
| Vitamin B2 | 1.17 | 1.00 | 1.00 | 0.83 | 0.93 | 3.00 |
| Vitamin B3 | 1.08 | 0.80 | 0.91 | 0.89 | 1.14 | 1.20 |
| Vitamin B5 | 1.04 | 0.67 | 0.88 | 0.97 | 1.19 | 1.13 |
| Vitamin B6 | 0.83 | - | 1.33 | 0.50 | 1.00 | infinite |
| Total Phenolics | 1.20 | 2.23 | 1.26 | 0.99 | 0.86 | 1.58 |
| Total Phytosterols | 1.22 | 2.13 | 1.00 | 1.06 | 1.15 | 0.75 |
| Total Carotenoids | 1.15 | 1.48 | 1.94 | - | 1.08 | 1.24 |
| Al | 1.04 | 0.88 | 0.97 | 0.98 | 0.98 | 1.47 |
| Ca | 1.11 | 1.03 | 0.99 | 1.13 | 1.01 | 1.43 |
| Cu | 1.27 | - | 0.84 | 1.33 | - | 2.00 |
| Fe | 0.90 | 0.07 | 0.87 | 1.07 | 1.11 | 1.01 |
| K | 1.06 | 1.17 | 0.97 | 1.08 | 0.97 | 1.22 |
| Mg | 1.00 | 1.06 | 0.82 | 1.06 | 1.02 | 1.10 |
| Mn | 0.76 | - | 0.80 | 0.61 | - | 0.96 |
| Na | 1.15 | 0.48 | 1.30 | 1.47 | 1.73 | 1.25 |
| P | 1.16 | 2.03 | 1.19 | 1.07 | 0.90 | 1.29 |
| Zn | 0.99 | 0.79 | 0.74 | 1.22 | 1.17 | 1.23 |
Note:
Average ratio of concentrations of individual nutrients for paired regenerative and conventional farms. Values with more than 10% difference shown in bold (increase) or italics (decrease).
No-till Vegetable Farms
Methods
We further compared the nutrient density of cabbage from the California regenerative no-till vegetable farm to that of the same variety of cabbage grown on another (second) field on the same nearby farm from the comparison discussed above, this one a fully certified organic field subject to frequent mechanical tillage. We also measured soil health metrics on this tilled organic-certified field to compare with those from the regenerative farm and the just converted to organic field still representative of conventional practices. In addition, we measured the nutrient density of carrots and spinach on both the California regenerative farm and the regenerative, no-till vegetable farm in Connecticut. Both of these regenerative no-till vegetable farms used hand sowing or hand transplants of vegetable starts and used no herbicides or pesticides. The methods employed on the Connecticut farm are as described by O’Hara (2020). We also compared aspects of the nutritional profile of cabbage, carrots, and spinach grown on these regenerative no-till vegetable farms with values from the USDA nutritional database [USDA SR28] and from previously published analyses of these same crops conventionally grown and purchased from New York supermarkets (Chun et al., 2005).
No-till vegetable results
On the California organic farm the organic-certified field had higher soil organic matter (2.9%) and Haney test score (9.2) than the “conventional” field only recently converted to organic tillage (the one reported in Table 1). Still, the soil on the California regenerative farm had almost 4 times the soil organic matter and a soil health score 3 times higher than either of these two fields at the tilled organic farm. Likewise, the regenerative Connecticut farm had 3 times the organic matter and 7 times the soil health score than the neighboring conventional farm (Table 1).
Cabbage grown on the regenerative California farm had 46% more vitamin K, 31% more vitamin E, and 33% more vitamin B1, 60% more vitamin B3, and 23% more vitamin B5 than cabbage from the regularly tilled organic field. The regenerative cabbage also had 41% more calcium, 22% more potassium and less than a third of the sodium, 35% more carotenoids, and 74% more phytosterols. Absolute values are reported in supplemental materials (File S1).
Relative to standard reference values in the USDA nutrient database (USDA SR28, No. 11749, cabbage, common (Danish, domestic, and pointed types), freshly harvest, raw) the regenerative cabbage had 50% more zinc and magnesium, and just a fifth of the sodium that one would expect to find in a conventionally grown cabbage. While the USDA database does not (yet) include phenolics, a 2005 study published in the Journal of the Science of Food and Agriculture reported total phenolic contents for fruits and vegetables purchased from New York supermarkets, including values for cabbage, spinach, and carrots (Chun et al., 2005). The regenerative cabbage we sampled had almost two and a half times more. In addition, spinach from both the Connecticut and California regenerative farms had about 4 times the total phenolics, and carrots from these farms had 60% to 70% more total phenolics than reported values from samples obtained at New York supermarkets (Chun et al., 2005).
Wheat Tests
Wheat comparison methods
An additional comparison examined the effect of regenerative practices on the mineral density of wheat from two adjacent no-tilled fields in northern Oregon that had undergone different weed control and crop rotation treatments. On one field the farmer followed the region’s conventional fallow-winter wheat rotation with regular applications of herbicide (glyphosate). In contrast, the regenerative field was planted in a diverse cocktail of cover crops between crops of spring barley and winter wheat.
For two years running the farmer planted both no-tilled fields with the same variety of wheat, fertilized with either compost or a “starter blend” of chemical fertilizers. To test for differences in mineral levels in the two crops we had the farmer send us samples of the second-year wheat crop from both fields. We ground up wheat grains from each field in a heavy-duty blender thoroughly cleaned with de-ionized water between sample preparations. Three acid-digested replicates of each sample were then analyzed in a mass spectrometer in the Trace Element Laboratory at the University of Washington’s College of the Environment to determine elemental concentrations of B, Na, Mg, K, Ca, Mn, Fe, Ni, Cu, Zn, Mo, and Cd.
Wheat comparison results
The farmer reported that both fields produced a harvest of 5 metric tons per ha (75 bushels an acre), and thus that the cover crops worked to suppress weeds without reducing the harvest. The cover cropped wheat samples had higher mineral density, with significantly greater amounts (p < 0.05) of boron (41%), magnesium (29%), calcium (48%), zinc (56%), and molybdenum (4 times more), and at lower statistical significance (p < 0.10) more potassium (26%), and manganese (35%), and just two-thirds as much nickel (Table 3). In all, more mineral micronutrients were getting into the wheat harvested from the no-till, no-herbicide, cover-cropped plot.
| Element | CC mean (ppm) | ±Sd | NC mean (ppm) | ±Sd | CC/NC | p value |
|---|---|---|---|---|---|---|
| B | 0.90 | 0.03 | 0.64 | 0.10 | 1.41 | 0.017 |
| Na | 13.12 | 0.69 | 13.20 | 3.13 | 0.99 | 0.064 |
| Mg | 1439 | 54 | 1112 | 181 | 1.29 | 0.040 |
| K | 7219 | 197 | 5750 | 1002 | 1.26 | 0.067 |
| Ca | 32.50 | 2.26 | 21.92 | 3.91 | 1.48 | 0.051 |
| Mn | 50.96 | 3.84 | 37.66 | 9.57 | 1.35 | 0.089 |
| Fe | 40.78 | 1.19 | 34.10 | 5.92 | 1.20 | 0.128 |
| Ni | 0.20 | 0.01 | 0.30 | 0.06 | 0.67 | 0.055 |
| Cu | 2.56 | 0.16 | 2.17 | 0.40 | 1.18 | 0.202 |
| Zn | 18.99 | 0.59 | 12.21 | 3.10 | 1.56 | 0.021 |
| Mo | 0.220 | 0.060 | 0.053 | 0.010 | 4.15 | 0.011 |
| Cd | 0.220 | 0.000 | 0.023 | 0.010 | 0.87 | 0.373 |
Notes:
Mineral density of wheat from cover cropped (CC) and chemical/fallow (NC) plots.
Standard deviations are for three replicates of each sample.
For CC/NC ratios, bold indicates significant at p = 0.05, and italics indicate significant at p = 0.10 (two tailed T-tests).
Fatty Acid Profiles
Fatty acid testing methods
Prior studies have shown that the diet of cows affects the fatty acid profile of their meat (Wood & Enser, 1997; Razminowicz, Kreuzer & Scheeder, 2006; Scollan et al., 2006; Lorenzen et al., 2007; De la Fuente et al., 2009; Daley et al., 2010; French et al., 2000; Duckett et al., 2013), particularly the amount of conjugated linoleic acid (CLA) as well as the amounts of omega-3 and omega-6 fats. We compared the amount of these fatty acids in beef and pork raised on one of the regenerative farms in our paired farm comparison (Bismarck, ND) with the fatty acid profiles of the same cuts (boneless ribeye and pork chops) of both conventional meat and meat marketed as a healthier alternative purchased at a local supermarket.
Samples were sent to the Iowa State University Department of Animal Science testing lab where samples were prepared for fatty acid analysis by trimming external fat to prepare 2 g of muscle tissue that was ground up and homogenized prior to analysis for total omega 3 and 6 content as well the essential omega-3 alpha-linolenic acid (ALA), the long-chain omega-3 fats eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), docosahexaenoic acid (DHA), and CLA (for beef). Lipids were extracted from each sample following Folch, Lees & Stanley (1957) and the extracted lipid was then esterified using the acetyl chloride/methanol method, and processed following methods described by Pogge, Lonergan & Hansen (2014). Fatty acid profiles were then analyzed using gas chromatography following methods described by Richter, Drewnoski & Hansen (2012).
Samples of each type of meat represent three different styles of animal husbandry, as described below. The regenerative beef was 100% grass-fed and grass-finished, raised and grazed on restored native prairie, and on regeneratively grown cover crop forages and bale grazed over the winter (Brown, 2018). Cows from the regional health promoting brand comparison were also grass-fed and finished but not on regeneratively managed land. The conventional beef was finished in a feedlot with an industry standard diet of corn and soybean meal, silage, and other grains grown on conventional farms. The regenerative pork was raised on pasture after weaning and also offered a mix of regeneratively raised ground grains, typically oats, peas, barley and flax. The regional health promoting (organic) brand raises pigs in large barns with access to the outdoors on a diet of non-GMO corn and soybean meal. The conventional pork came from a brand that raises pigs in confinement for their entire lives on a diet of conventionally grown GMO corn and soybeans.
Fatty acid testing results
The beef from the regenerative farm had 3 times more omega-3 fats, and more than 6 times more of the essential omega-3, alpha linolenic acid (ALA), than the conventional beef (Table 4). The regenerative beef also had more than half more to almost three quarters more of the trio of long-chain omega-3s (EPA, DPA, and DHA), as well as two-thirds of the omega-6 fats, making for an omega-6 to omega-3 ratio one fifth as large as for conventional beef (1.3:1 vs 6.2:1). Values for the regional brand of grass-fed beef were intermediate. The pork from the regenerative farm had more than 9 times as many omega-3s, and 3 times the omega-6s, making for an omega-6 to omega-3 ratio one-third as large as for the conventional pork (Table 5). The regenerative pork had more than 11 times as much ALA, almost 2 times as much EPA, 3 times as much DPA, and more than 4 times as much DHA than the conventional pork.
| Fatty acid | Regen. | Regional | Conv. | Ratio |
|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (g/100 g) | (Regen/Conv) | |
| Conjugated linoleic (CLA) | 0.0208 | 0.0142 | 0.0067 | 3.1 |
| Alpha linolenic (ALA) | 0.0622 | 0.0369 | 0.0099 | 6.3 |
| Eicosapentaenoic (EPA) | 0.0120 | 0.0112 | 0.0078 | 1.5 |
| Docosapentaenoic (DPA) | 0.0262 | 0.0184 | 0.0166 | 1.6 |
| Docosahexaenoic (DHA) | 0.0026 | 0.0014 | 0.0015 | 1.7 |
| Total omega-3 | 0.1056 | 0.0693 | 0.0358 | 2.9 |
| Total omega-6 | 0.1416 | 0.1508 | 0.2216 | 0.6 |
| Omega-6/Omega-3 | 1.3140 | 2.1777 | 6.1933 | 0.2 |
Notes:
Regen = regenerative (100% grass fed), Regional = regional health promoting brand (outdoor, non-GMO), Conv. = conventional (confined, grain-fed).
Values are per 100 g of homogenized meat.
| Fatty acid | Regen. | Regional | Conv. | Ratio |
|---|---|---|---|---|
| (g/100 g) | (g/100 g) | (g/100 g) | (Regen/Conv) | |
| Conventional | ||||
| Alpha linolenic (ALA) | 0.1537 | 0.0717 | 0.0136 | 11.3 |
| Eicosapentaenoic (EPA) | 0.0021 | 0.0015 | 0.0011 | 1.9 |
| Docosapentaenoic (DPA) | 0.0197 | 0.0126 | 0.0062 | 3.2 |
| Docosahexaenoic (DHA) | 0.0054 | 0.0021 | 0.0012 | 4.5 |
| Total omega-3 | 0.2131 | 0.0982 | 0.0229 | 9.3 |
| Total omega-6 | 1.6964 | 1.0804 | 0.5605 | 3.0 |
| Omega-6/Omega-3 | 7.9610 | 11.0060 | 24.4306 | 0.3 |
Notes:
Regen = regenerative, Regional = regional health promoting brand (outdoor, non-GMO), Conv. = conventional (confined, grain-fed).
Values are per 100 g of homogenized meat.
Discussion
The differences described above might be considered to represent an upper range for what may be rapidly attainable through soil-building practices as the regenerative farmers were selected based on their success in developing and applying such methods. Nonetheless, the consistently greater micronutrient content of regeneratively grown foods points to the potential for soil-building farming practices to enhance the nutritional profile of crops and livestock.
Soil
As each of the regenerative farms had been conventionally farmed and had soil organic matter content similar to its paired conventional farm before conversion to regenerative practices, our analyses show that such practices can increase topsoil organic matter and enhance soil health after less than a decade of fully adopting regenerative practices based on Conservation Agriculture methods combining no-till, cover crops, and diverse rotations. Moreover, the roughly doubled soil organic matter measured on average for the regenerative farms is large enough to substantially contribute toward reversing the roughly 50% historical decline in soil organic matter reported previously as typical for American cropland in general (Baumhardt, Stewart & Sainju, 2015). In addition, the consistently higher soil health scores on regenerative farms indicate the potential for greater nutrient cycling compared to conventionally managed fields.
The soils on the two small no-till vegetable farms had between 2 and 3 times as much topsoil organic matter as conventional farms, whereas the larger no-till row crop farms in the national comparison had between 1 and 2 times as much soil organic matter (Fig. 3). This difference suggests the potential to more rapidly increase soil organic matter on small-scale vegetable farms than on larger, more grain-oriented farms. Moreover, the very high soil organic matter content of the small no-till vegetable farms suggests the potential to increase levels above the range typical for native soils.
However, a 2014 review of previous studies (Powlson et al., 2014) concluded that despite no-till practices tending to increase soil organic matter in surficial soil horizons compensating losses deeper in the soil profile limit the net increase. That study, however, looked at no-till only, which by itself has limited potential to increase soil organic matter. Notably, over the two decades from 1993 to 2013 the combination of all three practices (no-till, cover crops, and diverse rotations) at the North Dakota regenerative farm increased topsoil (A horizon) depths from 8 cm to 36 cm (Brown, 2018). Hence, further evaluation of the net increase in soil organic matter integrated over the full soil profile on regenerative farms is warranted to assess the potential for different systems of regenerative farming to build and retain soil organic matter.
Crops
While our sample sizes are small, all three lines of investigation discussed above (paired farms, no-till vegetable comparisons, and wheat field trials) find that relative to conventional farming, regenerative practices yield crops with higher levels of key phytochemicals, vitamins, and/or minerals relevant to human health. Despite substantial variability between crops and specific farm pairings our wheat comparison revealed that conventionally grown grain had more Cd, Ni, and Na, elements detrimental to human health, whereas regeneratively grown grain had more micronutrients beneficial to human health. Overall, our results are consistent with those reported by Hepperly, Omondi & Seidel (2018) for the effect of building soil organic matter on the mineral content of crops. It appears that healthier soil with more soil organic matter and the life it sustains can enhance the nutrient-density of crops, increasing levels of micronutrients and phytochemicals through, we suspect, influencing nutrient cycling as well as biochemical signaling involved in phytochemical production. These results parallel those obtained from biofortification of mineral density through use of micronutrient fertilizers and growth-promoting bacterial inoculation advocated as ways to help address mineral micronutrient deficiencies in the human diet (Rana et al., 2012; Bouis & Saltzman, 2017; de Valença et al., 2017).
Our results for wheat in particular demonstrate the potential for rapid increases in mineral micronutrient density comparable in magnitude to reported historical nutrient declines (e.g., Davis, Epp & Riordan, 2004; Garvin, Welch & Finley, 2006; Fan et al., 2008; Murphy, Reeves & Jones, 2008; Davis, 2009). As the mineral composition of the soil itself could not change this much over two years without corresponding chemical inputs, the observed changes in mineral uptake by the cover cropped wheat indicate that something else was going on. What could change that fast? Soil biology and its influence on micronutrient uptake (Montgomery & Biklé, 2016).
Considered all together, our preliminary analyses show that regenerative farming methods affect both soil organic matter and nutrient density. Notably, the roughly doubled soil organic matter measured on average for the regenerative farms is the inverse of the typical decline reported for North American cropland (Baumhardt, Stewart & Sainju, 2015).
Fatty acids
Key aspects of the fatty acid profile of regeneratively grazed meat were substantially better than for either grass-fed or conventionally raised examples. While a large body of research has shown that a grass vs grain fed diet affects the fatty acid composition of meat (Wood & Enser, 1997; Razminowicz, Kreuzer & Scheeder, 2006; Scollan et al., 2006; Lorenzen et al., 2007; De la Fuente et al., 2009; Daley et al., 2010; French et al., 2000; Duckett et al., 2013), the observation that the regeneratively raised meat in our comparison also had higher levels of omega-3s than the other grass-fed, outdoor-raised comparison suggests that the greater soil health on the regenerative farm further enhanced the fatty acid profile over what would be expected from the grass-fed vs grain-fed distinction.
A number of studies have ascribed health benefits to greater consumption of both CLA and omega-3s. Dietary intake of CLA has been linked to quelling inflammation and reducing risk of various chronic diseases (Viladomiu, Hontecillas & Bassaganya-Riera, 2016; den Hartigh, 2019). And while human beings evolved on diets with roughly equal amounts of omega-6 and omega-3 fats (Simopoulos, 2006), a substantial body of evidence implicates a high omega-6 to omega-3 ratio (such as the 10:1 to 20:1 ratio typical of a modern Western diet) as contributing to inflammation that underlies obesity and chronic diseases like cardiovascular and irritable bowel disease, rheumatoid arthritis, and neurodegenerative diseases such as Alzheimer’s (Patterson et al., 2012). Increased dietary omega-3 consumption (or supplementation) also has been shown or proposed to reduce the effects or risk of Crohn’s disease (Belluzzi, 1996; Calder, 2008), arthritis (James & Cleland, 1997; Curtis et al., 2000; Adam et al., 2003; Calder, 2017), asthma (Brigham et al., 2019), diabetes (Raheja et al., 1993), breast cancer (Grammatikos et al., 1994; Maillard et al., 2002; Abdelmagid et al., 2016) and obesity (Buckley & Howe, 2009; Simopoulos, 2016).
Conclusions
The mechanisms and relationships through which regenerative farming practices influence the nutrient density of food, and thereby potentially human health, deserve greater attention from both agronomists and nutritionists alike. Relative to conventional farming, regenerative practices based on Conservation Agriculture produced crops with higher levels of phytochemicals, vitamins, and minerals, although which ones and by how much varied among farm pairings. Most notably, soil health appears to influence phytochemical levels in crops, indicating that regenerative farming systems can enhance dietary levels of compounds known to reduce risk of various chronic diseases (e.g., Weisberger, 1991; Alloway, 2009; Kang et al., 2011; Wang et al., 2011, 2014; Del Rio et al., 2013; Zanotti et al., 2015). Given the complexity of soil ecology, the human microbiome, and other important factors, it will prove challenging to more rigorously link soil health and human health. Still, our preliminary comparisons suggest the potential for regenerative agricultural practices that build soil health to enhance the nutritional profile of crops and livestock, and thereby influence human health and risk of chronic diseases.
Supplemental Information
Crop data from paired farm comparisons.
CAB = cabbage CAR = carrots PEA = peas SOY = soy SPI = spinach COR = corn RGN = Regenerative farm sample ALT = Conventional farm sample