Genome-wide identification and expression analysis of ethylene responsive factor family transcription factors in Juglans regia
- Published
- Accepted
- Received
- Academic Editor
- Mahmood-ur-Rahman Ansari
- Subject Areas
- Agricultural Science, Bioinformatics, Genomics, Molecular Biology, Plant Science
- Keywords
- Juglans regia, Ethylene response factor, Bioinformatics, Expression analysis
- Copyright
- © 2021 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Genome-wide identification and expression analysis of ethylene responsive factor family transcription factors in Juglans regia. PeerJ 9:e12429 https://doi.org/10.7717/peerj.12429
Abstract
Background
Walnut is an important economic tree species with prominent economic value and ecological functions. However, in recent years, walnuts have become susceptible to drought stress, resulting in a decline in comprehensive benefits. Therefore, it is necessary to identify the regulatory molecular mechanism associated with walnut response to drought. In many plants, ethylene responsive factor (ERF) gene family plays important roles in response to biotic and abiotic stress, especial drought. Therefore, the identification and characterisation of walnut ERF genes will benefit walnut with regard to the clarification of drought response mechanism as well as the management, production, and quality of plantations.
Methods
‘ERF’ was compared against the walnut transcriptome, and the JrERFs with a complete open reading frame (ORF) were identified by ORF Finder. The molecular weights, amino acid residues, and theoretical isoelectric point (pI) were predicted by ExPASy. The distribution of JrERFs in chromosome locations was determined based on walnut genome data from NCBI. The intron-exon structures and conserved domains were analysed using Gene Structure Display Server 2.0 and CD-Search, accordingly. Multi-sequence alignment and a phylogenetic tree were constructed by ClustalX2.1 and MEGA7, respectively. The conserved motifs were acquired using MEME. Total RNA was isolated using the cetyltrimethylammonium ammonium bromide (CTAB) method (Yang et al., 2018). Gene expression was determined by using real-time quantitative polymerase chain reaction (qRT-PCR) analysis and calculated according to the 2−ΔΔCT method (Livak & Schmittgen, 2001).
Results
A total of 44 JrERFs were identified from the walnut transcriptome, whose ORFs were 450–1,239 bp in length. The molecular weights of the JrERF proteins (consisting 149–412 amino acids) were 16.81–43.71 kDa, with pI ranging from 4.8 (JrERF11) to 9.89 (JrERF03). The JrERFs can be divided into six groups (B1–B6), and among the groups, B6 contained the most number of members. Each JrERF contained 1–6 motifs and each motif comprised 9–50 amino acids. Among the motifs, motif1, motif2, and motif3 were the most abundant. More than 40% of JrERFs were up-regulated continuously when subjected to ethephon (ETH), PEG6000, and PEG6000+ETH treatments. Of all the JrERFs, JrERF11 showed the highest expression. Therefore, we conclude that walnut ERF genes are highly conserved and involved in the regulation of drought response in the presence of ETH. JrERFs are possibly important candidate genes for molecular breeding; hence, the findings of this study provides the theoretical basis for further investigation of ERF genes in walnut and other species.
Introduction
Juglans regia is an economic tree species and is distributed widely all over the world (Abdallah et al., 2015). In China, walnut has become an important woody oil tree species for Poverty Alleviation and Rural Revitalization (Yang et al., 2019). However, like other plants, the growth and development of walnut is restricted by biotic (such as pests, diseases) and abiotic factors (such as moisture, temperature, and light). These factors cause a sharp reduction in the yield and quality of walnut. When exposed to external stimuli, plants mobilise protective mechanisms to reduce damage through many pathways, including releasing stress signals, adapting to stress stimuli, activating a series of molecular pathways, regulating related gene expression, and physiological responses (Hilker & Schmülling, 2019). For example, WRKY, NAC, MYB, AP2/ERF, and bZIP transcription factor (TF) gene families in Arabidopsis thaliana were highly enriched and involved in regulating the expression of 56% of common genes in response to drought and cold stresses (Sharma et al., 2018). GmWRKY54 transgenic soybean improved drought tolerance through abscisic acid (ABA) and Ca2+ signalling pathways (Wei et al., 2019). To enhance tolerance to high temperature stress, the expression of walnut JrGRAS2 stimulates the transcription activity of heat shock proteins (Yang et al., 2018). Therefore, the identification of important TF families can reveal the stress adaptation mechanism of walnut. It will provide a theoretical basis for adversity-related molecular breeding.
TFs are the key molecules for the regulation of gene expression and exist in all organisms. APETALA2/ethylene responsive factor (AP2/ERF) is one of the TF families in plants. After the first AP2/ERF family TF was isolated and identified from A. thaliana in 1994 (Jofuku et al., 1994), it had been reported widely in various plants, such as Ananas comosus (Zhang et al., 2021), Betula platyphylla (Lv et al., 2020), and Raphanus sativus (Karanja et al., 2019). According to the type and number of AP2/ERF conserved domain, AP2/ERF TFs can be divided into the following subfamilies: APETALA2 (AP2), related to ABI3/VP1 (RAV), dehydration-responsive element binding protein (DREB), ERF, and Soloist (Sakuma et al., 2002). AP2 subfamily, containing two highly conservative AP2/ERF domains, is involved in cell growth and differentiation (Luo et al., 2020). RAV subfamily, including one AP2/ERF and one B3 domains, is involved in plant flowering and stress response (Zhao et al., 2017). DREB subfamily has an AP2/ERF domain, whose amino acid 14 and 19 are valine and glutamic acid, respectively. DREB has been found to be positive response genes in low temperature, drought, and ABA signalling pathways (Sarkar et al., 2019). The ERF subfamily has only one AP2/ERF domain, whose amino acid 14 and 19 are alanine and aspartic acid, respectively, which are the sites that distinguish the subfamily of ERF from DREB. ERF subfamily relates with various stimuli, such as hormones, low temperature, drought, salt, and pathogens (Lv et al., 2020). Soloist is an orphan of the AP2/ERF family with only one AP2/ERF domain, whose amino acid motif and gene structure are significantly different from those of other AP2/ERF subfamilies. Soloist is mainly involved in the response to low temperature and the associated signal transduction pathways (Wang et al., 2018).
Among the subfamilies of AP2/ERF, ERF has received the greatest attention because of its broad response to various stresses. For instance, plants with Arabidopsis ERF96 overexpression displayed enhanced resistance to necrotrophic pathogens, which included the fungus Botrytis cinerea and the bacterium Pectobacterium carotovorum (Fröschel et al., 2019). Ectopic expression of Phaseolus vulgaris ERF35 in tobacco promoted salt stress tolerance (Kavas et al., 2020). Apple ERF38 played positive role in drought tolerance relating to anthocyanin biosynthesis (An et al., 2020). Solanum lycopersicum ERFB3 could respond to cold, heat, and flooding and plays a role in the layout of stress symptoms under cold stress (Klay et al., 2014). Especially in the regulation of drought tolerance, ERF has attracted much attention. For example, the overexpression of barley HvSHN1 in transgenic tobacco improved drought tolerance without compromising growth (Djemal & Khoudi, 2021). In GT31, a sugarcane variety with no tolerance to drought, Saccharum spontaneum SsDREB1L showed higher expression level after re-watering, indicating that SsDREB1L may facilitate plant recovery from drought stress (Li et al., 2021). Tobacco ERF172 improves plant drought tolerance partly by regulating CAT-mediated H2O2 homeostasis (Zhao et al., 2020). However, in woody plants, there are little or no reports on the identification of ERF gene family, especially in walnut, where there is no report of ERF in stress response. Therefore, in this study, the walnut ERF TFs were identified and the basic bioinformation, conserved motifs, and evolutionary relationship were analysed. Meanwhile, considering that ERFs are involved in the ethylene (ETH) signalling pathway (Gu et al., 2017; Kazan, 2015; Xie et al., 2019), three stresses (PEG6000, ethephon, and PEG6000+ethephon) were used to assess the potential transcription activity of the selected JrERFs. The results of this study will provide profound platform for subsequent investigation of JrERFs response to stress.
Materials & methods
Plant materials and treatments
New branches were obtained from 6-year-old ‘Xiangling’ walnut (a phenotype of J. regia planted widely in China) and inserted into a mixture of turf peat and sand (2:1 v/v) in plastic pots and grown in a greenhouse (22 ± 2 °C, relative humidity 70 ± 5%, illumination cycle 14 h light/10 h dark) for 2 years. Considering that PEG6000 is a common reagent for drought simulation stress test (Abdel-Ghany et al., 2020; Ahmad et al., 2020), ERF is involved in ethylene signal pathway (Gu et al., 2017; Kazan, 2015; Xie et al., 2019), and ethephon (ETH) is an ethylene donor (Kim et al., 2018; Li & Tran, 2017), the 2-year-old seedlings were treated with 15% (w/v) PEG6000, 100 μmol/L ETH, 15% (w/v) PEG6000 plus 100 μmol/L ETH (PEG6000+ETH). Next, the leaves were collected at 0 (control), 6, 24, and 72 h, and stored at −80 °C. A fresh water-only control was conducted in parallel. RNA was isolated from all the samples. Each treatment consisted of six seedlings.
Identification, chromosomal location, and gene structure of JrERFs
To identify and analyse all the members of ERF gene family in walnut, the Arabidopsis’s genome sequences of ERF family members were downloaded from TAIR (https://www.arabidopsis.org/) and used for homology search in walnut transcriptome, and several walnut ERF TFs were obtained. ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) was used to find the open reading frame (ORF). Basic biological information, including amino acid number, theoretical isoelectric point (pI), and molecular weight were predicted by ExPASy (https://web.expasy.org/protparam/). The chromosomal location information of 44 JrERFs in the walnut genome (Juglans microcarpa × J. regia) were obtained from NCBI (https://www.ncbi.nlm.nih.gov/genome/?term=txid2249226[orgn) (Zhu et al., 2019). The genomic DNA sequence of JrERFs were obtained from NCBI (https://www.ncbi.nlm.nih.gov/) through Gene ID (Table 1), and the gene structure map of the exon-intron of JrERFs were determined by Gene Structure Display Server 2.0 (GSDS 2.0: http://gsds.gao-lab.org/) (Hu et al., 2015).
| Gene names | Transcriptome number | GeneBank accession number | Gene ID | Chromosome site | ORF length (bp) | Number of aa | MV (kDa) |
pI |
|---|---|---|---|---|---|---|---|---|
| JrERF01 | comp22717_c0 | MZ688063 | LOC109004979 | chr1S | 1,143 | 380 | 42.47 | 5.02 |
| JrERF02 | comp2477_c0 | MZ688064 | LOC108983929 | chr1D | 735 | 244 | 27.29 | 6.04 |
| JrERF03 | comp25333_c0 | MZ688065 | LOC108986419 | chr1D | 687 | 228 | 25.83 | 9.89 |
| JrERF04 | comp18721_c0 | MZ688066 | LOC108989254 | chr1D | 564 | 187 | 20.98 | 8.76 |
| JrERF05 | comp9598_c0 | MZ688067 | LOC108988992 | chr1D | 795 | 264 | 30.03 | 8.97 |
| JrERF06 | comp22552_c1 | MZ688068 | LOC109007057 | chr1D | 753 | 250 | 27.86 | 5.42 |
| JrERF07 | comp8892_c0 | MZ688069 | LOC109004887 | chr1D | 723 | 240 | 25.84 | 4.98 |
| JrERF08 | comp44754_c0 | MZ688070 | LOC108998815 | chr2S | 597 | 198 | 22.37 | 5.12 |
| JrERF09 | comp8896_c0 | MZ688071 | LOC108998823 | chr2S | 537 | 178 | 19.68 | 5.29 |
| JrERF10 | comp13191_c0 | MZ688072 | LOC108984080 | chr2S | 513 | 170 | 18.34 | 5.26 |
| JrERF11 | comp10222_c0 | MZ688073 | LOC108991055 | chr2S | 714 | 237 | 25.61 | 4.80 |
| JrERF12 | comp22357_c0 | MZ688074 | LOC108995850 | chr2S | 678 | 225 | 24.82 | 9.04 |
| JrERF13 | comp24921_c1 | MZ688075 | LOC108993210 | chr2S | 717 | 238 | 25.85 | 4.99 |
| JrERF14 | comp15973_c0 | MZ688076 | LOC109020121 | chr2S | 501 | 166 | 18.25 | 9.78 |
| JrERF15 | comp16155_c0 | MZ688077 | LOC108980339 | chr2D | 1,185 | 394 | 42.83 | 6.20 |
| JrERF16 | comp23695_c0 | MZ688078 | LOC109013239 | chr2D | 1,161 | 386 | 42.8 | 5.77 |
| JrERF17 | comp12993_c0 | MZ688079 | LOC108986643 | chr2D | 678 | 225 | 24.31 | 9.27 |
| JrERF18 | comp27438_c0 | MZ688080 | LOC108993692 | chr2D | 522 | 173 | 18.95 | 6.97 |
| JrERF19 | comp9852_c0 | MZ688081 | LOC109020467 | chr2D | 615 | 204 | 22.52 | 4.94 |
| JrERF20 | comp18782_c0 | MZ688082 | LOC108995608 | chr2D | 636 | 211 | 23.61 | 6.98 |
| JrERF21 | comp55856_c0 | MZ688083 | LOC108995945 | chr2D | 510 | 169 | 18.85 | 9.48 |
| JrERF22 | comp22253_c0 | MZ688084 | LOC108992157 | chr3S | 675 | 224 | 24.53 | 5.47 |
| JrERF23 | comp26055_c0 | MZ688085 | LOC108981535 | chr3S | 1,158 | 385 | 42.45 | 7.14 |
| JrERF24 | comp10353_c0 | MZ688086 | LOC108982941 | chr3S | 762 | 253 | 28.61 | 5.38 |
| JrERF25 | comp26003_c0 | MZ688087 | LOC108997399 | chr3D | 681 | 226 | 24.95 | 6.10 |
| JrERF26 | comp25379_c1 | MZ688088 | LOC108984027 | chr3D | 1,107 | 368 | 40.07 | 6.01 |
| JrERF27 | comp18282_c0 | MZ688089 | LOC109002490 | chr4S | 708 | 235 | 26.17 | 7.6 |
| JrERF28 | comp29196_c0 | MZ688090 | LOC108994146 | chr4D | 963 | 320 | 35.96 | 5.08 |
| JrERF29 | comp40170_c0 | MZ688091 | LOC108994146 | chr4D | 1,002 | 333 | 37.79 | 5.79 |
| JrERF30 | comp10012_c0 | MZ688092 | LOC108992195 | chr4D | 615 | 204 | 22.86 | 8.75 |
| JrERF31 | comp14427_c0 | MZ688093 | LOC108984175 | chr5D | 489 | 162 | 17.47 | 9.83 |
| JrERF32 | comp9632_c0 | MZ688094 | LOC108984188 | chr5D | 732 | 243 | 26.35 | 9.71 |
| JrERF33 | comp26703_c0 | MZ688095 | LOC109010126 | chr6S | 939 | 312 | 34.25 | 6.75 |
| JrERF34 | comp23499_c0 | MZ688096 | LOC108997304 | chr6S | 981 | 326 | 37.09 | 5.09 |
| JrERF35 | comp53300_c0 | MZ688097 | LOC108990846 | chr6S | 702 | 233 | 25.21 | 5.46 |
| JrERF36 | comp20406_c0 | MZ688098 | LOC108994010 | chr6S | 714 | 237 | 26.08 | 8.80 |
| JrERF37 | comp21129_c0 | MZ688099 | LOC109005134 | chr6D | 870 | 289 | 31.16 | 8.18 |
| JrERF38 | comp18221_c1 | MZ688100 | LOC109005151 | chr6D | 990 | 329 | 36.52 | 7.10 |
| JrERF39 | comp23713_c0 | MZ688101 | LOC108989912 | chr6D | 762 | 253 | 27.99 | 6.96 |
| JrERF40 | comp29151_c0 | MZ688102 | LOC109013315 | chr6D | 654 | 217 | 23.51 | 7.02 |
| JrERF41 | comp22664_c0 | MZ688103 | LOC108981529 | chr8S | 1,239 | 412 | 43.71 | 7.04 |
| JrERF42 | comp28472_c0 | MZ688104 | LOC108990403 | chr8S | 450 | 149 | 16.81 | 8.92 |
| JrERF43 | comp17398_c0 | MZ688105 | LOC109021567 | chr8S | 999 | 332 | 36.36 | 6.27 |
| JrERF44 | comp21821_c0 | MZ688106 | LOC108994552 | chr8D | 1,011 | 336 | 37.48 | 5.36 |
Note:
Amino acid, aa; Molecular weight, MV; Theoretical isoelectric point, pI.
Multiple sequence alignment, conserved domain, and phylogenetic analysis of JrERFs
CD-Search (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi), Pfam (http://pfam.xfam.org/), and SMART (http://smart.embl-heidelberg.de/) were used to analyse the conserved domains of JrERFs. Multi-sequence alignment was applied using ClustalX2.1. MEME online tools (http://alternate.meme-suite.org/) were adopted to uncover the conservative motifs. The setting parameters were as follows: the number of motifs was 20, any number repetition was allowed, motif width was from 6 to 50. We downloaded 63 A. thaliana ERF proteins from TAIR, 37 P. trichocarpa ERFs from Joint Genome Institute P. trichocarpa version 1.1 database (https://mycocosm.jgi.doe.gov/Poptr1_1/Poptr1_1.home.html). The ERF proteins from A. thaliana, P. trichocarpa, and J. regia were used to construct a neighbour-joining tree for evolutionary analysis with a bootstrap replicate value of 1,000 using MEGA7. The phylogenetic tree was modified using iTOL (https://itol.embl.de/) (Letunic & Bork, 2021).
Expression analysis of JrERFs by real-time quantitative polymerase chain reaction (qRT-PCR) (cetyltrimethylammonium ammonium bromide)
The total RNA of each sample was isolated using the CTAB method (Yang et al., 2018) and digested with DNase (Takara, Dalian, China). Next, 0.5 μg RNA of each sample was reverse transcribed into cDNA using PrimeScript™ RT reagent Kit (CWBIO, Beijing, China). The cDNA was diluted 10-fold by sterile water and used as the template of qRT-PCR. The reaction mixture (20 μL) contained 10 μL of SYBR Green Real-time PCR Master Mix (CWBIO, Beijing, China), 0.5 μM of each forward and reverse primer, and 2 μL cDNA template (equivalent to 100 ng of total RNA). qRT-PCR was performed in StepOne™ Real-Time PCR System produced by Applied Biosystems. The amplification was achieved according to the following parameters: 94 °C for 30 s, followed by 44 cycles at 94 °C for 12 s, 60 °C for 30 s, 72 °C for 40 s, and at 81 °C for 1 s. The internal reference gene is walnut 18S rRNA (HE574850) (Xu et al., 2012), and the primers are shown in Table S1. The relative expression levels were calculated based on the threshold cycle using the 2−ΔΔCT method (Livak & Schmittgen, 2001).
Results
Sequence characteristics and chromosomal locations of JrERFs
In summary, a total of 44 JrERFs were identified from walnut transcriptome. The ORFs of JrERFs were between 450 bp (JrERF42) and 1,239 bp (JrERF41), consisting 149–412 amino acids. The molecular weight of the proteins ranged from 16.81 kDa (JrERF42) to 43.71 kDa (JrERF41), and the pI ranged from 4.80 (JrERF11) to 9.89 (JrERF03) (Table 1).
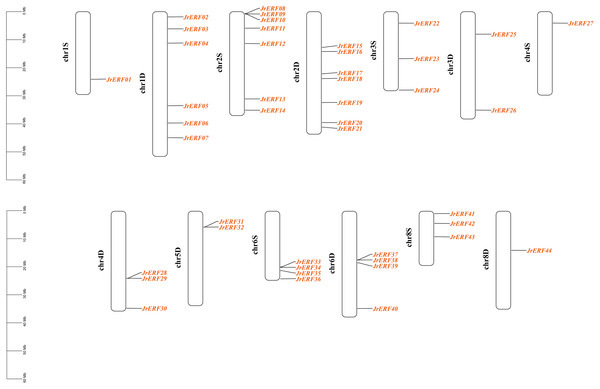
These 44 JrERFs were unevenly located on 13 chromosomes (chr1S, chr1D, chr2S, chr2D, chr3S, chr3D, chr4S, chr4D, chr5D, chr6S, chr6D, chr8S, chr8D) of J. regia. The chromosomes 1D, 2S, and 2D covered the most number of JrERFs (6, 7, and 7 genes, respectively), while the chromosomes 1S, 4S and 8D included the least number of JrERFs (1 gene, accordingly). The chromosomes 5S, 7S, and 7D contained no JrERFs (Fig. 1).
Figure 1: Distribution of the JrERFs on chromosomes of J. regia.
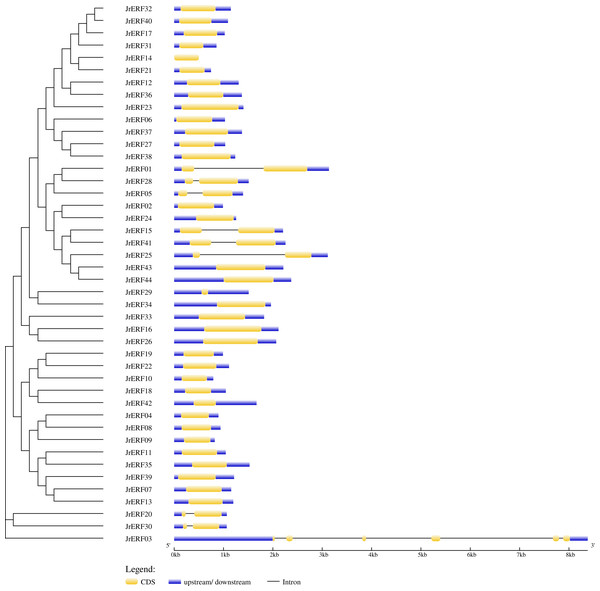
The chromosome number is shown on the left side of each chromosome, D: Dominant; S: Subdominant.The intron and exon structure of JrERFs were analysed by comparing the genomic DNA sequence of JrERFs. The results showed that the gene structures of nine JrERFs (JrERF01, JrERF03, JrERF05, JrERF15, JrERF20, JrERF25, JrERF28, JrERF30, JrERF41) were destroyed by introns, and the other 35 JrERFs contained no intron and their structures are relatively stable (Fig. 2).
Figure 2: Gene structure map of JrERFs.
The vertical phylogenetic tree and gene structure of JrERFs was constructed by GSDS online software. Yellow boxes indicate exons; blue boxes indicate upstream or downstream; black lines indicate introns.The conserved domains of JrERFs
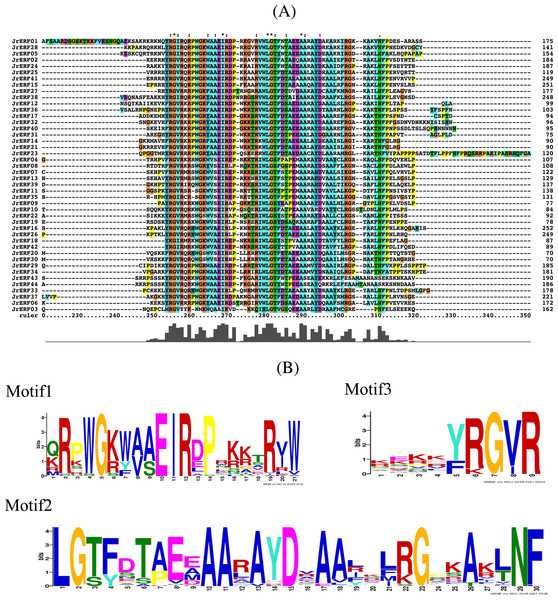
Conserved domain analysis showed that there was a common AP2 conserved domain with different degrees of insertion or deletion in the JrERF proteins (Fig. 3A; Fig. S1). MEME and Tbtools (Chen et al., 2020) were used to construct the conserved domain sequence and sequence logo, and the result suggested that most JrERFs contain conserved motifs. Three motifs (motif1, motif2, and motif3) existed in most of the sequences. We found that motif1–3 may be part of the AP2 domain; sequence similarity was 100% at sites 5, 10, 11, and 12 in motif1, sites 1, 2, 10, 11, 15, 23, and 29 in motif2, and sites 7 and 9 in motif3 (Fig. 3B).
Figure 3: Multiple sequence alignment of the JrERFs proteins.
(A) Multiple sequence alignment of the AP2s of JrERF proteins. ‘:’ means the mutation at that position is a conservative mutation; ‘.’ means a semi-conservative mutation; ‘*’ indicates that the sequence is consistent at that site. (B) The seqlogos of motif1, motif2 and motif3.The conservative motif of JrERFs
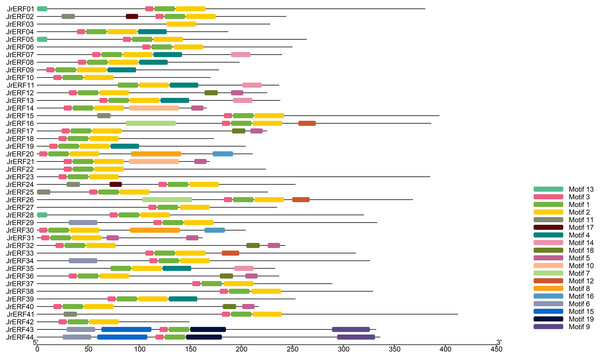
We used MEME to analyse the motifs in the 44 JrERFs and downloaded the basic information (width and best possible match sequence) (Table 2). The results showed that each motif contained 9–50 amino acids, and each sequence contains 1–6 motifs. Among 44 amino acid sequences, JrERF43 and JrERF44 had six conserved motifs (motif6, motif15, motif3, motif1, motif19, motif9), while JrERF03 had only one (motif2). JrERF12, 17, 32, 36, and 40 had five (motif3, motif1, motif2, motif18, motif5). In addition, the most frequent motifs of JrERFs are motif1 (QRPWGKWAAEIRDPRKKTRVW), motif2 (LGTFDTAEEAARAYDRAALKLRGPKAKLNF), and motif3 (KEKKYRGVR). They represented the AP2 domain and are widely present in the 44 JrERFs (Fig. 4).
Figure 4: Distribution of the conserved motifs in JrERF proteins.
The different colours represent different motifs (motif1—motif19).| motif | Width | Best possible match |
|---|---|---|
| motif1 | 21 | QRPWGKWAAEIRDPRKKTRVW |
| motif2 | 30 | LGTFDTAEEAARAYDRAALKLRGPKAKLNF |
| motif3 | 9 | KEKKYRGVR |
| motif4 | 29 | PELVESLPRPASSSPRDIQAAAAKAAAMK |
| motif5 | 13 | RRPLPFDLNLPPP |
| motif6 | 29 | KMRVVRIIVSDPYATDSSSSEDDSEKCVK |
| motif7 | 50 | TPMFSEGFSSQNQMGFEQPGPJGLNQLTPSQILQIQAQIQLQKQNQQRQQ |
| motif8 | 50 | KLRKCCKDPYPSLTCLRLDAENSHIGVWQKRAGQRSDSNWIMRIPLGKKN |
| motif9 | 38 | FFDDFEVCGTEDGGGNELPDWDFADICDDFGWMNEPLN |
| motif10 | 50 | LVITPPVLSGGAGACELFGWRPKPECFSGAGNPPPVRSEYKGYKMENVDV |
| motif11 | 14 | MSIMVSALTHVVSG |
| motif12 | 18 | DYKPLHSSVDAKLQAICQ |
| motif13 | 11 | MCGGAIISDFI |
| motif14 | 20 | MPNLLVDMAEGMLVSPPRIN |
| motif15 | 50 | KRLVREIHJPLVKQPPPKLLQSESSCQDSNNGGRTPKVIEAEKKRVLAKT |
| motif16 | 21 | MDEEERIALQMIEELLNWNCP |
| motif17 | 13 | CPVCNIDGCLGCN |
| motif18 | 14 | GCHSDSDSSSVVDD |
| motif19 | 36 | LGTFNTPEEASEAYZKKRLEFEAAMAANANSEKSKN |
The evolutionary relationship of JrERFs
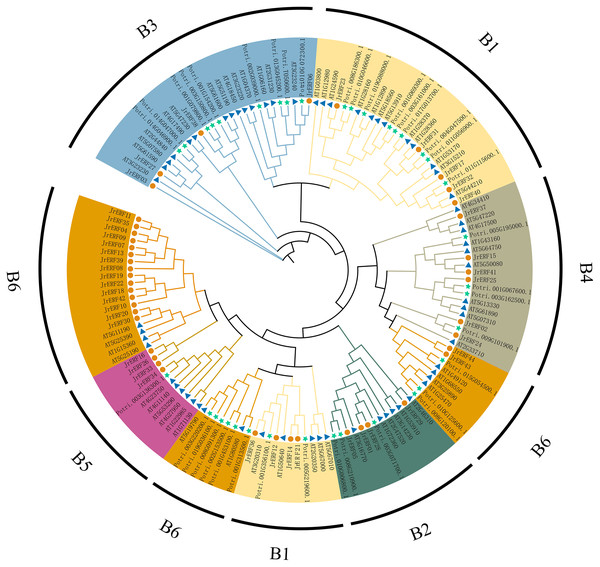
To analyse the evolutionary relationships of JrERFs, 44 JrERFs, 63 A. thaliana ERFs, and 37 P. trichocarpa ERFs were aligned for the construction of the phylogenetic tree. The results showed that JrERFs can be classified into six groups (B1–B6). Group B6 contained the highest number of JrERF proteins (17 members); groups B2, B3, and B5 had only four members they respectively include JrERF01, JrERF05, JrERF28, JrERF29; JrERF03, JrERF06, JrERF27, JrERF38; JrERF16, JrERF26, JrERF33, JrERF34; groups B4 contained six members (JrERF02, JrERF15, JrERF24, JrERF25, JrERF37, JrERF41); and the remaining nine JrERF proteins were in group B1 (Fig. 5).
Figure 5: Phylogenetic relationship of ERF proteins from J. regia, A. thaliana and P. trichocarpa.
B1–B6 means six groups of JrERFs, respectively, which are displayed in different colours. A total of 63 Arabidopsis ERFs are represented by blue triangles, 44 walnut ERFs are represented by vermilion circles, 37 P. trichocarpa ERFs are represented by green five-pointed stars.Expression of JrERFs in response to drought stress
To explore the potential function of JrERFs in response to drought stress, the expression of all the JrERFs were analysed under PEG6000, ETH, and PEG6000+ETH treatments (Figs. 6–8).
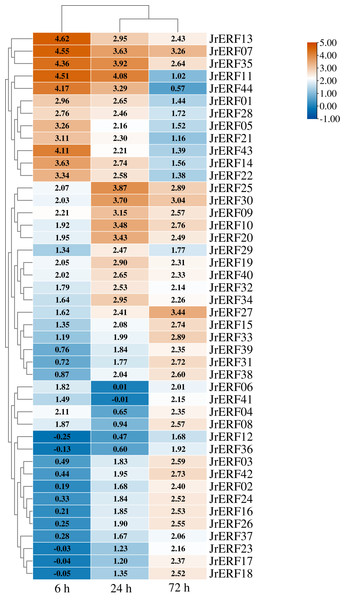
Figure 6: The relative expression of the 44 JrERFs under PEG6000 stress.
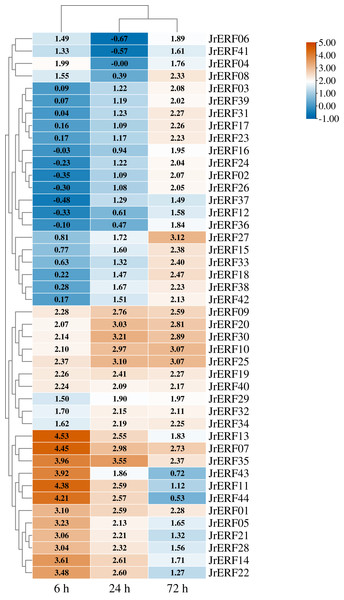
The expression is relative to the expression of the internal reference gene and at 0 h.Figure 7: The relative expression of the 44 JrERFs under ethephon (ETH) stress.
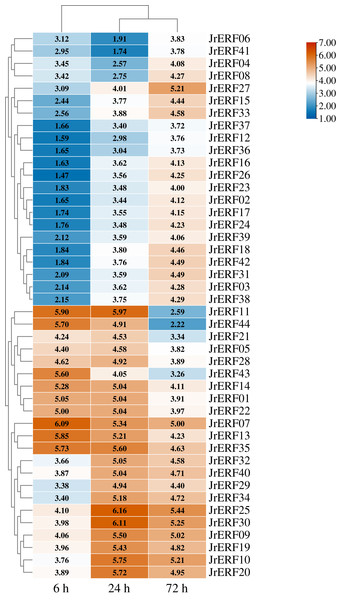
The expression is relative to the expression of the internal reference gene and at 0 h.Figure 8: The relative expression of the 44 JrERFs under PEG6000+ETH stress.
The expression is relative to the expression of the internal reference gene and at 0 h.Under PEG6000 stress
The expression levels of 44 JrERFs under PEG6000 stress showed four different trends. (i) The expression of 18 JrERFs (JrERF02, JrERF03, JrERF12, JrERF15, JrERF16, JrERF17, JrERF18, JrERF23, JrERF24, JrERF26, JrERF27, JrERF31, JrERF33, JrERF36, JrERF37, JrERF38, JrERF39, JrERF42) showed a continuous increasing trend at 6–72 h; the expression level of JrERF27 at 72 h was 2.05-fold higher than that of JrERF12. (ii) The expression levels of 12 JrERFs (JrERF01, JrERF05, JrERF07, JrERF11, JrERF13, JrERF14, JrERF21, JrERF22, JrERF28, JrERF35, JrERF43, JrERF44) showed a continuous decreasing trend at 6–72 h; JrERF11 had the highest expression at 6 h, which was 1.21-fold higher than the average expression level of the other genes in the group. (iii) The expression levels of 10 JrERFs (JrERF09, JrERF10, JrERF19, JrERF20, JrERF25, JrERF29, JrERF30, JrERF32, JrERF40, JrERF34) increased at 6–24 h and decreased at 24–72 h. The average expression value of this group was 3.11 at 24 h. (iv) The relative expression levels of four JrERFs (JrERF04, JrERF06, JrERF08, JrERF41) decreased at 6–24 h and increased at 24–72 h. JrERF04 showed the highest expression at 6 h, which was 1.41-fold higher than that of JrERF41 (Fig. 6).
Under ETH stress
Four different expression trends were observed when the 44 JrERFs were subjected to ETH treatment. (i) The expression levels of 21 JrERFs (JrERF02, JrERF03, JrERF10, JrERF12, JrERF15, JrERF16, JrERF17, JrERF18, JrERF23, JrERF24, JrERF26, JrERF27, JrERF29, JrERF31, JrERF33, JrERF34, JrERF36, JrERF37, JrERF38, JrERF39, JrERF42) showed a continuous increasing trend at 6–72 h, and the expression level of JrERF27 at 72 h was 2.09-fold of JrERF37. JrERF10 and JrERF27 maintained high expression after 72 h. (ii) The relative expression of 12 JrERFs (JrERF01, JrERF05, JrERF07, JrERF11, JrERF13, JrERF14, JrERF21, JrERF22, JrERF27, JrERF35, JrERF43, JrERF44) showed a continuous declining trend at 6–72 h, and the average expression level was 3.75 at 6 h. (iii) The expression levels of six JrERFs (JrERF09, JrERF19, JrERF20, JrERF25, JrERF30, JrERF32) increased at 6–24 h and decreased at 24–72 h. The expression level of JrERF20 at 6 h was 1.22-fold higher than that of JrERF32. (iv) The relative expression of five JrERFs (JrERF04, JrERF06, JrERF08, JrERF40, JrERF41) decreased at 6–24 h and increased at 24–72 h. At 6 h, JrERF40 showed the highest expression, which was 1.41-fold higher than the average expression level of the other genes in the group (Fig. 7).
Under PEG6000+ETH stress
JrERFs displayed four different expression trends under PEG6000+ETH stress. (i) The expression levels of 18 JrERFs (JrERF02, JrERF03, JrERF12, JrERF15, JrERF16, JrERF17, JrERF18, JrERF23, JrERF24, JrERF26, JrERF27, JrERF31, JrERF33, JrERF36, JrERF37, JrERF38, JrERF39, JrERF42) showed a continuous increase at 6–72 h; JrERF27 and JrERF33 maintained high expression after 72 h, and the expression level of JrERF27 at 72 h was 1.40-fold higher than that of JrERF37. (ii) The relative expression of seven JrERFs (JrERF01, JrERF07, JrERF13, JrERF14, JrERF35, JrERF43, JrERF44) showed a continuous decreasing trend at 6–72 h, and the average expression value was 5.61 at 6 h in this group. (iii) The expression levels of 15 JrERFs (JrERF05, JrERF09, JrERF10, JrERF11, JrERF19, JrERF20, JrERF21, JrERF22, JrERF25, JrERF28, JrERF29, JrERF30, JrERF32, JrERF34, JrERF40) increased at 6–24 h and decreased within 24–72 h, and the average expression level was 5.33 at 24 h. (iv) The expression levels of four JrERFs (JrERF04, JrERF06, JrERF08, JrERF41) showed a decreasing trend at 6–24 h and increasing trend at 24–72 h, and their expression levels were higher than 3.50 at 72 h (Fig. 8).
These results showed that the expression of 44 JrERFs in the presence of ETH was higher than that observed with PEG6000 stress alone. It is worth mentioning that the expression levels of seven JrERFs (JrERF01, JrERF07, JrERF13, JrERF14, JrERF35, JrERF43, JrERF44) exceeded 5.00 at 6 h under the PEG6000+ETH stress, with JrERF07 showing the highest expression (6.09). These findings indicate that in the presence of ETH, the relative expression of JrERFs to drought stress is enhanced, and some JrERFs have potential drought resistance function.
Discussion
Walnut is an important economic species, and like other plants, its growth and development are restricted by adverse environmental conditions (Zhang et al., 2020b). There are little or no reports on ERF, a big subfamily of AP2/ERF playing important roles in stress response, in walnut tree. To increase the yield and quality of walnuts as well as ensure farmers’ economic income and stable development of walnut industry under adverse conditions, it is necessary to elucidate the molecular mechanism associated with adversity adaptation in walnut. Therefore, in this study, a total of 44 JrERFs that may have potential functions in drought stress response were obtained from walnut transcriptome and divided into six groups (Fig. 5). This classification was consistent with previous evolutionary analyses of Apium graveolens (Li et al., 2019a), Zay mays (Hao et al., 2020), and Dimocarpus longan (Zhang et al., 2020a). The various characteristics including ORF length, pI, amino acid number, and molecular weight of the walnut ERF family had a large span (Table 1). The phenomenon is similar to the sequence characteristics of Arabidopsis and P. trichocarpa (Zhuang et al., 2008), indicating that the ERF family of walnut has a certain similarity in sequence characteristics with other species.
Multiple alignment result showed that the 44 JrERFs are highly conserved with AP2 domain (Fig. 3A; Fig. S1), and this was consistent with the conserved region of AtERFs (Fig. S2). This result further confirmed that ERF TFs have highly conserved structures in procession of species evolutionary (Nakano et al., 2006). Gene structure analysis showed that 79.55 % of the JrERFs had no intron, implying that most JrERFs have relatively stable gene structure (Fig. 2), which was similar to that observed with AP2/ERF genes from tartary buckwheat (Fagopyum tataricum) (Liu et al., 2019). Most JrERFs contained motif1, motif2, and motif3, which were related to AP2 domain (Fig. 3B). Motif1–motif19 were distributed in the 44 JrERFs at different degrees (Fig. 4), which was also similar to what was observed with ERF TFs from F. tataricum and Medicago sativa (Jin et al., 2019; Liu et al., 2019). Because different motifs and the number of motifs are related to functions (Tripathi et al., 2020), our results suggest that JrERFs may play different roles in plant stress response or other biological functions.
Considering that drought has a great impact on the walnut industry (Knipfer et al., 2018), the responses of the identified 44 JrERFs to PEG6000 were analysed in order to uncover the functions of walnut AP2/ERF family in response to drought stress. The results showed that most of the JrERFs were induced by PEG6000 (Fig. 6). The expression of genes in response to different stresses can often effectively predict their potential functions. For example, it has been reported that the expression levels of various AP2/ERF TFs (Bra-ERF036, Bra-ERF069a, and Bra-ERF104a) from Brassica oleracea are rapidly up-regulated by drought stress, which confirms their important roles in tolerance to abiotic stress (Li et al., 2017). IbRAP2-12, a member of sweet potato ERF family, was rapidly enhanced by drought stress and played a crucial role in enhancing plant tolerance to drought stress (Li et al., 2019b). The expression of JrWRKY2 and JrWRKY7 from walnut was enhanced by PEG6000 treatment, and both were further confirmed to be positive TFs in response to drought stress (Yang et al., 2017). According to these findings, we speculate that JrERFs can respond to drought stress to varying degrees and some members may play positive roles in the regulation of drought tolerance.
Ethylene, a plant growth regulator, participates in multiple plant stress response, such as drought, low temperature, salinity, and mechanical damage (Bleecker & Kende, 2000; Johnson & Ecker, 1998; Mizoi, Shinozaki & Yamaguchi-Shinozaki, 2012). In drought response, ethylene plays a positive role in enhancing drought resistance (Wan et al., 2011). ERFs are associated with ethylene response, and most ERFs can be regulated by ethylene (Müller & Munné-Bosch, 2015; Xie et al., 2019). For instance, the OsDERF1 from rice can be activated by ethylene to improve tolerance to drought stress (Wan et al., 2011). Therefore, in order to verify that the response of JrERFs under drought is related to the ethylene signal pathway, walnuts were treated with ETH and PEG6000+ETH, and the expression levels of JrERFs were analysed. Furthermore, the expression levels of JrERFs under the two treatments were compared. The results showed that most JrERFs can be up-regulated by ETH (Fig. 7), suggesting that ETH has regulatory effect on the expression of JrERFs. Moreover, under PEG6000+ETH, the expression levels of 44 JrERFs were obviously higher than those observed under PEG6000 alone (Fig. 8). These results are similar to other reports. For instance, the expression level of ZmEREB180 was significantly improved with increase in the level of ethylene, which benefited the waterlogging tolerance of plant, indicating that the drought response role of ZmEREB180 was mediated by ethylene (Yu et al., 2019). Arabidopsis RAP2.2 regulated the ability of plants to resist hypoxia through ethylene-controlled signal transduction pathways (Hinz et al., 2010). In soybean, ethylene treatment significantly enhanced the expression of GmERF3, and the overexpression of GmERF3 in tobacco improved the salt and drought tolerance of transgenic lines (Zhang et al., 2009). Therefore, we can conclude that the ethylene signalling pathway is involved in the response of JrERFs to drought stress.
In addition, among the 44 JrERFs, JrERF11 showed the highest expression. The expression of JrERF11 reached the peak at 24 h (5.97) under PEG6000+ETH stress, which was 1.41-fold higher than that of other JrERFs at 24 h. JrERF11 contains no intron and is evolutionarily close to PbERF027 from Pyrus brestschneideri (Fig. S3). PbERF027 is involved in the regulation of genes related to plant hormones (Pei et al., 2016). Moreover, the expression of NnERF026, another homologue from Nelumbo nucifera (Fig. S3), could be induced by drought stress and may function positively in drought stress (Cao et al., 2021). Based on these findings, it can be speculated that JrERF11 is a potential useful gene for drought tolerance and its function should be further investigated.
Conclusions
In this study, a total of 44 JrERFs were identified from J. regia and their basic biological information, chromosome locations, gene structure, conserved motifs, phylogenetic relationship were analysed. We found that the JrERFs were highly conserved and belonged to six groups. More than 40% of the JrERFs can be induced by drought stress in the presence of ETH, implying that JrERFs can respond to drought stress through the ethylene signalling pathway. JrERF11 is the most prominently expressed gene and worthy of further study. The result of our study can provide solid foundation for further investigation of JrERFs under multiple abiotic stresses and for exploring the molecular mechanism underlying abiotic stress responses in walnut and other woody plants.
Supplemental Information
Multiple sequence alignment of the AP2s of 44 JrERFs proteins and 63 A. thaliana ERF proteins.
Phylogenetic tree analysis of JrERF11 protein and the homologous from other species.
Tc: Theobroma cacao; Hu: Herrania umbratica; Dz: Durio zibethinus; Pa: populus alba; Jc: Jatropha curcas; Pb: Pyrus brestschneideri; Zj: Ziziphus jujuba; Jr: Juglans regia; Cc: Citrus clementina; Nn: Nelumbo nucifera.