SKP1-like protein, CrSKP1-e, interacts with pollen-specific F-box proteins and assembles into SCF-type E3 complex in ‘Wuzishatangju’ (Citrus reticulata Blanco) pollen
- Published
- Accepted
- Received
- Academic Editor
- Julin Maloof
- Subject Areas
- Agricultural Science, Biotechnology, Molecular Biology, Plant Science
- Keywords
- Citrus retuculata Blanco, Self-incompatibility, SCF complex, F-box, SKP1-like, Cullin1
- Copyright
- © 2020 Ren et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. SKP1-like protein, CrSKP1-e, interacts with pollen-specific F-box proteins and assembles into SCF-type E3 complex in ‘Wuzishatangju’ (Citrus reticulata Blanco) pollen. PeerJ 8:e10578 https://doi.org/10.7717/peerj.10578
Abstract
S-ribonuclease (S-RNase)-based self-incompatibility (SI) mechanisms have been extensively studied in Solanaceae, Rosaceae and Plantaginaceae. S-RNase-based SI is controlled by two closely related genes, S-RNase and S-locus F-box (SLF), located at a polymorphic S-locus. In the SI system, the SCF-type (SKP1-CUL1-F-box-RBX1) complex functions as an E3 ubiquitin ligase complex for ubiquitination of non-self S-RNase. Pummelo (Citrus grandis) and several mandarin cultivars are suggested to utilize an S-RNase-based SI system. However, the molecular mechanism of the non-S-factors involved in the SI reaction is not straightforward in Citrus. To investigate the SCF-type E3 complex responsible for the SI reaction in mandarin, SLF, SKP1-like and CUL1 candidates potentially involved in the SI reaction of ‘Wuzishatangju’ (Citrus reticulata Blanco) were identified based on the genome-wide identification and expression analyses. Sixteen pollen-specific F-box genes (CrFBX1-CrFBX16), one pollen-specific SKP1-like gene (CrSKP1-e) and two CUL1 genes (CrCUL1A and CrCUL1B) were identified and cloned from ‘Wuzishatangju’. Yeast two-hybrid (Y2H) and in vitro binding assays showed that five CrFBX proteins could bind to CrSKP1-e, which is an ortholog of SSK1 (SLF-interacting-SKP1-like), a non-S-factor responsible for the SI reaction. Luciferase complementation imaging (LCI) and in vitro binding assays also showed that CrSKP1-e interacts with the N-terminal region of both CrCUL1A and CrCUL1B. These results indicate that CrSKP1-e may serve as a functional member of the SCF-type E3 ubiquitin ligase complex in ‘Wuzishatangju’.
Introduction
Self-incompatibility (SI) promotes genetic diversity and inhibits inbreeding depression by recognizing self (or genetically related) pollen (Franklin-Tong, 2008). In S-RNase-based SI system, the recognition specificity is regulated by a highly polymorphic locus, named S-RNase and multiple SLFs. The S-RNase is abundant in style cells, and then secretes into the stylar canal to infiltrate into the pollen tube, where it produces cytotoxin in an S-haplotype independent manner (Luu et al., 2000; McClure et al., 1990). SLFs are specifically expressed in pollen and serve as pollen determinant in SI system (Kubo et al., 2010). The mechanisms of S-RNase-based SI reaction have been extensively investigated in Rosaceae, Solanaceae and Plantaginaceae, and some species of Rutaceae and Rubiaceae (Nowak et al., 2011; Zhang et al., 2018; Liang et al., 2020).
‘Self-recognition’ and ‘non-self-recognition’ systems can be used to understand SI mechanism (Fujii, Kubo & Takayama, 2016). Pollen-specific SFB (S-haplotype specific F-box) is a single S pollen gene which specifically links with S-RNase (Ushijima et al., 2003). In the system, non-self S-RNase is inactivated by a ‘general inhibitor’, an F-box protein encoded by SLFL (S-locus F-box like) which is also linked to S-RNase. The activity of self S-RNase is specifically protected by SFB, leading to RNA degradation of self-pollen tube and growth inhibition (Ushijima et al., 2004). In a ‘non-self-recognition’ system, the pollen determinants are regulated by multiple S-locus F-box genes, termed as SLF in Solanaceae and Plantaginaceae and SFBB (S-haplotype specific F-box brothers) in Maloideae. In general, multiple types of SLF/SFBB with intrahaplotypic diversity are linked with S-RNase within a haplotype. Each type of SLF/SFBB can recognize and degrade a subset of non-self S-RNase proteins via the ubiquitination pathway (Kakui et al., 2011; Kubo et al., 2010).
In S-RNase-based reaction, the F-box protein acts as an acceptor of the canonical SCF complex (E3 ubiquitin ligase), and coordinates ubiquitination of non-self S-RNase with degradation by 26S proteasome (Hua & Kao, 2006; Entani et al., 2014). The role of S-locus F-box gene in SI has been confirmed by genotype-dependent transformation in vivo, where the F-box targets non-self S-RNase and degrades it (Sijacic et al., 2004). In Rosaceae, pollen-part mutant of S-locus which confers the self-compatibility reaction by encoding a non-functional F-box protein can be used to identify pollen factor (Hauck et al., 2006; Ushijima et al., 2004). However, no typical amino acid patterns for S-locus F-box proteins have been reported. In addition to F-box domain, S-locus F-box proteins also contain a FBA domain at the C-terminal region. In Arabidopsis, 92 non-S-locus F-box containing a FBA domain called SLF-like (SFL) was identified (Wang et al., 2004). In apple, all SFBBs contain FBA domain in the C-terminus (Minamikawa et al., 2010). Therefore, FBA domain can be used as a trait to characterize S-locus F-box candidates. Additionally, S-locus F-box genes show pollen-specific expression (Chen et al., 2018a; Zhao et al., 2002). SFBBs commonly cluster with the S-RNase in apple genome by S-locus deciphering (Minamikawa et al., 2010). Intra- (Inter-) haplotype sequence divergences of S-locus F-box proteins are considered to be an important characteristic to recognize various allelic polymorphic S-RNase proteins (Fujii, Kubo & Takayama, 2016; Kakui et al., 2011). So far, the S-locus F-box candidate genes have not been elucidated in ‘Wuzishatangju’.
Ubiquitin-dependent proteolysis contributes to detoxification caused by SCF-type E3 ligase in S-RNase-based SI reaction. Ubiquitin targeting is achieved by an ATP-dependent consecutive reaction of a ubiquitin activating enzyme (E1), ubiquitin conjugating enzymes (E2) and ubiquitin ligase (E3). At the end of the cascade, the E3 ligase confers specificity for substrate recognition and brings the target to E2 for ubiquitin conjunction (Schwechheimer, 2018). For the canonical SCF-type E3 ligase complex, the CUL1 protein acts as a scaffold that adopts a stalk-like structure to interact with an F-box protein through the SKP1 protein adaptor in N-terminal region and binds RBX1 on the other end. F-box subunit recognizes the protein substrate via the C-terminal region which exhibits high diversity (Ning et al., 2016; Zimmerman, Schulman & Zheng, 2010). The SKP1-like gene involved in S-RNase-based SI reaction has been identified. A pollen specific AhSSK1 encoding an SKP1-like protein was first identified in Antirrhinum hispanicum, and its interaction with S-locus F-box protein was confirmed by Y2H and GST-pull down assays (Huang et al., 2006). Down-regulation of the PhSSK1 expression in Petunia hybrida had a negative effect on fertility of cross-compatible pollen (Zhao et al., 2010). In Petunia inflata, 17 S-locus F-box proteins are assembled into SCF complex with PiSSK1, PiCUL1-P, and PiRBX, indicating that PiSSK1 is responsible for specific function in SI reaction (Li et al., 2016). This hypothesis was confirmed in a PiSSK1 knockout setting where PiSSK1 deletion caused incompatibility of mutational pollen in otherwise-compatible pistils. This also revealed the essential role of SKP1-like gene in SI reaction (Sun & Kao, 2017). In Rosaceae, many SKP1-like gene such as PavSSK1 from Prunus avium (Matsumoto et al., 2012), PbSSK1 and PbSSK2 from Pyrus bretschneideri and MdSSK1 from Malus domestica (Xu et al., 2013; Yuan et al., 2014) were found to be involved in SI response. In Citrus, an SKP1-like gene involved in flower development of pummelo (C. grandis), CgSKP1, was identified from ‘Shatian’ cultivar and it was highly expressed in leaf and flower (Chai et al., 2010). In our previous work, we isolated full-length cDNA and DNA sequences of CrWSKP1 from ‘Wuzishatangju’ (Miao et al., 2015). However, we did not clarify whether these genes are involved in SI response of Citrus.
CUL1 protein has been identified from several plant species. In P. inflata, PiCUL1-C and PiCUL1-G proteins were obtained and the PiCUL1-G was proposed to assemble into a novel SCF complex with PiSLF and PiSPB1 proteins (Hua & Kao, 2006). SpCUL1 was proposed to be the determinant of interspecies unilateral incompatibility in Solanum pennellii. Down-regulation of SpCUL1 expression impaired the fertility of pollination in wild SI cultivar (Li & Chetelat, 2010; Li & Chetelat, 2014). PiCUL1-P, which is the ortholog of SpCUL1 formed a complex with PiSSK1 and PiRBX1 when a GFP-fused S2-SLF1 protein was co-immunoprecipitated with pollen extracts in P. inflata (Li et al., 2014). Knockdown of PhCUL1-P compromised fertility of cross-compatible pollination in P. hybrida (Kubo et al., 2016). Both PavCul1A and PavCul1B protein physically interact with PavSSK1 in P. avium (Matsumoto & Tao, 2016; Matsumoto & Tao, 2019). A PbCUL1 protein has been characterized in P. bretschneideri (Xu et al., 2013). However, whether CUL1 gene (s) is involved in SI reaction in Citrus remains to be investigated.
The SI reaction in Citrus was considered to be S-RNase-based SI type (Zhang et al., 2018; Liang et al., 2020). S-RNase-mediated SI evolved only once before the split of Asteridae (e.g., Solanaceae) and Rosidae (e.g., Rosaceae and Rutaceae). Therefore, S-RNase is proposed to be a putative pistil S-determinant (Igic & Kohn, 2001; Vieira, Fonseca & Vieira, 2008; Zhang et al., 2018). A T2-type RNase gene, CgSL2, was constitutively expressed and associated with ovary senescence in ‘Zigui shatian’ pummelo (Chai et al., 2011). Another S-like RNase gene which shared high sequence identity with CgSL2 did not show tissue-specific in ‘Wuzishatangju’ (C. reticulata) (Miao et al., 2011). In ‘Shatian’ pummelo (C. grandis), CgRNS3 possessed several common characteristics of the pistil determinant of SI and was specifically expressed in pistil (Liang et al., 2017), however, it was not anchored into the S-locus (Liang et al., 2020). Pistil-specific CtRNS3 from Citrus tamurana showed S1-genotype-dependent in different cultivars, implying that CtRNS3 may serve as the S 1 -gene (Honsho et al., 2019). Liang et al. (2020) studied the S-locus in pummelo and concluded that S-RNase and SLF mediate SI reaction in Citrus.
‘Wuzishatangju’ (C. reticulata Blanco) is a natural mutant. Cytological studies show that gametophytic SI causes seedlessness in ‘Wuzishatangju’ by inhibiting fertilization in the ovary (Ye et al., 2009). Several genes related to SI of ‘Wuzishatangju’ have been obtained by suppression-subtractive hybridization (SSH) cDNA library and RNA-Seq technology. However, it is still not known which factor regulates SI reaction in ‘Wuzishatangju’ (Miao et al., 2013; Ma et al., 2017). In this study, 16 pollen-specific F-box genes (CrFBX1-CrFBX16), one SKP1-like gene (CrSKP1-e) and two CUL1 genes (CrCUL1A and CrCUL1B) were cloned from ‘Wuzishatangju’ by genome-wide analyses. Among CrFBX genes, 13 CrFBXs (CrFBX1-CrFBX12 and CrFBX14) were homologous with SFBB and SLFL, 10 CrFBXs (CrFBX1-CrFBX10) were located into S-locus. CrSKP1-e and CrCUL1A were predominately found in pollen of ‘Wuzishatangju’ while CrCUL1B was least abundant in pollen. Yeast two-hybrid, in vitro binding and LCI assays showed that CrSKP1-e links with a subset of CrFBX proteins and binds to both CrCUL1A and CrCUL1B. These results suggested that CrSKP1-e acts as an adaptor in the assembly of SCF-type E3 ligase in ‘Wuzishatangju’.
Materials and Methods
Plant materials
‘Wuzishatangju’ (self-incompatible) (C. reticulata) and ‘Chuntianju’ (self-compatible) (C. reticulata) mandarins were planted in an orchard at South China Agricultural University (Guangzhou, China). Young leaves, petals, filaments, stigmas, styles and ovaries were collected, immediately frozen in liquid nitrogen and stored at −80 °C for later use. Buds were collected one day before anthesis and anthers were dried in an oven at 28 °C for 48 h. Pollen grains were gathered by filtering with 75 µm stainless sieve after desiccation and then stored at −80 °C. ‘Chuntianju’ pollen was used to clone CrFBX7.
Identification of F-box, SKP1-like and CUL1 proteins
F-box, SKP1-like and Cullin (CUL) family proteins were retrieved using in silico method based on the Hidden Markov Model (HMM) profile of F-box domain seed (PF00646), SKP1 seed (PF01466) and CUL seed (PF00888), respectively, obtained from the Pfam database (http://pfam.xfam.org/) as a query to search the predicted C. clementina proteome (https://phytozome.jgi.doe.gov/pz/portal.html) using HMMER software package 3.0 (http://hmmer.org/download.html) with E ≤1, (Finn, Jody & Eddy, 2011). The MAFFT program was used for multiple sequence alignments based on the amino acid sequences, while the MEGA software (Version 7.0) with 1,000 replicated bootstrap tests were used for phylogenetic analyses (Kumar, Stecher & Tamura, 2016; Yamada, Tomii & Katoh, 2016).
Expression analyses of candidate genes
Total RNA was isolated and digested with DNase I using Plant RNA Kit (Huayueyang, Beijing, China). Total RNA (0.4–0.5 µg) was used to synthesize the first-strand cDNA with oligo (dT) primer according to the manufacturer’s instructions using a RevertAid First Strand cDNA Synthesis Kit (ThermoFisher, USA). The expression patterns of CrFBXs, CrSKP1-e, CrCUL1A and CrCUL1B were analyzed with quantitative real-time PCR (qRT-PCR) and normalized by the expression level of Actin gene (Actin_F: CATCCCTCAGCACCTTCC and Actin_R: CCAACCTTAGCACTTCTCC) (Zhou et al., 2010). qRT-PCR was conducted in ABI 7500 real-time PCR System (Applied Biosystems, CA, USA) using the SYBR qPCR Mix (Vazyme, Nanjing, China). The 20 µL reaction mixture contained about 1 µL template cDNA, 0.2 µM of each forward and reverse gene-specific primers and 10 µL SYBR. The relative expression levels were calculated using the 2−ΔΔCT formula (Livak & Schmittgen, 2001). For qRT-PCR, samples were collected during three consecutive days. Samples from each day served as a biological repeat.
The full-length cDNA of pollen-specific CrFBXs, CrSKP1-e, CrCUL1A and CrCUL1B were cloned from ‘Wuzishatangju’ using gene-specific primer pairs based on C. clementina genome reference; transcriptome datasets (https://figshare.com/articles/Citrus_reticulata_Blanco-Unigene_5-3_fa/12198627) (Ma et al., 2017) and resequencing data (https://figshare.com/articles/wuzi_rmdup_bam/11880303). CrFBX7 was cloned from ‘Chuntianju’ pollen. Fragments were purified and cloned into the pEASY-Blunt vector (Transgen, Beijing, China) for sequencing. All the primer pairs are presented in Table S1.
Yeast two-hybrid (Y2H) analysis
Full-length CDS of CrFBXx (x represents the number of CrFBX, the full-length CDS of CrFBX7 was cloned from ‘Chuntianju’) was cloned into pGBKT7 vector (Clontech, USA), which was respectively digested by Eco R I and Bam H I enzymes, to express fusion proteins with GAL4 binding domain (BD). CrSKP1-e was cloned into the pGADT7 vector (Clontech, USA) to produce fusion proteins with the GAL4 activation domain (AD). Different combinations with BD and AD vectors were co-transformed into Y2HGold strain (Clontech, USA) and incubated with SD/-Leu/-Trp at 30 °C for 4 d. Three clones were respectively dotted on SD/-Leu/-Trp/-Ade/-His medium containing 200 ng/mL Aureobasidin A (AbA) (TaKaRa, Japan) and 40 µg/mL X- α-gal (TaKaRa, Japan) and then cultivated at 30 °C for 5 d.
Luciferase complementation imaging (LCI) assay
CrCUL1A and CrCUL1B proteins were truncated at the N-terminal region (CrCUL1A-N, 1-415; CrCUL1B-N, 1-415, respectively). All fragments were cloned into pCAMBIA-nLuc vector and CrSKP1-e was cloned into a pCAMBIA-cLuc vector (Chen et al., 2008). The constructed plasmids were individually transformed into Agrobacterium GV3101 strains and incubated at 28 °C for 2-3 d on the YEP (yeast extract peptone) medium supplemented with 25 µg/mL rifampicin (Rif) and 50 µg/mL kanamycin (Kan). A single colony was incubated at 200 rpm and 28 °C for 8-10 h in YEP liquid medium containing 25 mg/L Rif and 50 mg/L Kan. The presence of the corresponding plasmid was verified by PCR. Then 30 µL of positive Agrobacterium suspension was inoculated in 15 mL YEP liquid medium and incubated until OD600 reached 0.6–0.8. After centrifugation for 5 min at 6,000 rpm and 25 °C, the medium was discarded. The pellet was resuspended and the OD600 adjusted to 0.2 with MAA buffer (10 mM MES, 10 mM MgCl2, 0.1 mM acetosyringone, pH5.6) for infiltration of 3–4 weeks old Nicotiana benthamiana (16-h day/8-h night, 25 °C). For the co-infiltration assay, the suspension was mixed with an equal volume of Agrobacterium strains containing the recombinant plasmid. After 3 d, the Luciferase Assay Substrate (Promega, USA) was infiltrated into the leaf and the reaction imaging was captured by a low-light cooled charge-coupled device (CCD) imaging system (Bio-Rad, USA). For luciferase activity, 0.05 g sample was ground in liquid nitrogen and 500 µL tissue lysis reagent (Promega, USA) was added and centrifuged at 4 °C. The luminescence signal was detected using the chemiluminescence analysis system (Thermo Scientific, USA) after mixing 20 µL supernatant with 100 µL Luciferase Assay Substrate (Promega, USA). All assays were replicated thrice.
In vitro binding assay
For CrSKP1-e and CrFBX proteins binding assay, the full-length of CrFBX2 and CrFBX7 (derived from ‘Chuntianju’) and CrFBX9, CrFBX13 and CrFBX15 were fused with MBP (maltose-binding protein) tag and cloned into pET-28a vector. CrSKP1-e was cloned into pGEX-4T-2. All the constructs and negative control (pET28a-MBP and pGEX-4T-2) were transformed into BL21 (DE3) strains to express MBP, MBP-CrFBX2, MBP-CrFBX7, MBP-CrFBX9, MBP-CrFBX13, MBP-CrFBX15, GST (glutathione S-transferase) and GST-CrSKP1-e proteins. For CrSKP1-e and CrCUL1 binding assay, the full length of CrSKP1-e was cloned into pET28b and the N-terminal regions of CrCUL1A (1-415) and CrCUL1B (1-415) proteins were respectively cloned into pGEX-4T-2. All the constructs and negative control (pGEX-4T-2) were transformed into Rosetta (DE3) to express His-CrSKP1-e, GST-CrCUL1A-N and GST-CrCUL1B-N proteins. For protein accumulation, three clones were incubated in the LB (Lysogeny broth) medium containing 100 mg/L ampicillin at 37 °C for 6 h. The culture was then diluted (about 1:100) into a fresh LB medium for further incubation until the OD600 was 0.6–0.8. Approximately 0.2 mM isopropyl- β-D-thiogalactosidase (final concentration) was added into the culture and incubated at 28 °C under shaking condition (200 rpm) for 5–6 h to induce the recombinant protein accumulation. For His-CrSKP1-e protein purification, the bacteria culture was sonicated and purified with Ni-NTA His Bind resin (TransGen, China) according to the manufacturer’s instructions and then exchanged with PBS (phosphate-buffered saline) buffer (140 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4, pH7.4–7.5) using an Amicon Ultra-15 (Millipore, 10K) The MBP fused proteins were sonicated and purified using Dextrin Beads (SMART-lifesciences, Changzhou, China) according to the manufacturer’s instructions. Protein concentration was measured using Bradford Protein Assay Kit (TaKaRa, Japan).
For binding assays, bacteria suspension expressing GST and GST fusion proteins were sonicated in PBS buffer and the supernatant was reacted with 40 µL of 50% slurry of Glutathione Sepharose 4B (GE Healthcare, USA). The mixture was incubated at 4 °C under soft shaking condition for 2 h. About 30 µg His-CrSKP5, MBP, MBP-CrFBX2, MBP-CrFBX7, MBP-CrFBX9, MBP-CrFBX13 and MBP-CrFBX15 were respectively incubated with protein-bound Glutathione Sepharose 4B at 4 °C under soft shaking conditions overnight. The beads were washed five times with 10 × PBS buffer. The protein-bound beads were boiled and separated with SDS-PAGE (sodium dodecyl sulfate-polyacrylamide gel electrophoresis). The His-CrSKP1-e was detected with an anti-His monoclonal antibody and the MBP-fused proteins were detected with an anti-MBP polyclonal antibody (Yeasen, China).
Results
Identification of pollen-specific F-box genes
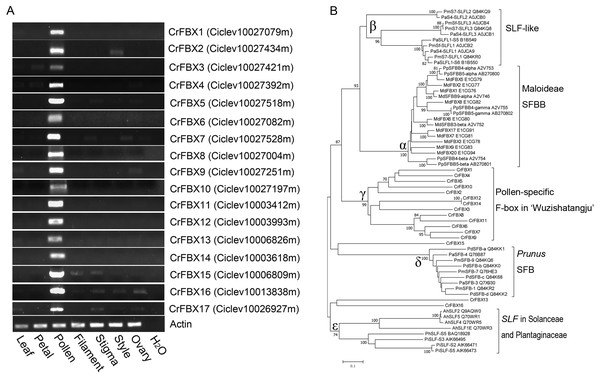
An HMMER search was conducted for genome-wide investigation of the number and domain organization of the F-box proteins in C. clementina (v1.0). A total of 298 non-redundant F-box proteins and 46 F-box associated (FBA) subfamily F-box proteins were identified (Fig. S1). SLFs and SLFLs genes are significantly expressed in mature pollen and contain a typical FBA domain in the C-terminal region. Therefore, semi-quantitative RT-PCR was performed to investigate the tissue-specific expression of the 46 FBA subfamily genes in different tissues (leaf, petal, filament, pollen, stigma, style and ovary) of ‘Wuzishatangju’ (Fig. 1A and Figs. S2A, S2B). In total, 17 F-box genes (termed CrFBX1-CrFBX17) were significantly expressed in pollen. Full-length coding sequences of all the CrFBX genes except CrFBX17 (Ciclev10026927m) were cloned from the pollen cDNA pool of ‘Wuzishatangju’ and aligned with homologous transcripts of C. clementina. The identity ranged from 80% to 100% (Table S2 ). However, a 1-base pair (bp) deletion in the 3′-terminus of CrFBX7 in ‘Wuzishatangju’ was found (Fig. S3). To verify the CrFBX7 gene in C. reticulata, the full-length CDS sequence of CrFBX7 from self-compatible ‘Chuntianju’ (C. reticulata) was obtained and used for further analyses (Fig. S3).
Figure 1: Expression analyses and phylogenetic relationships of CrFBX genes from ‘Wuzishatangju’.
(A) PCR analyses of the expression pattern of the 17 CrFBX genes, using the expression of the actin gene as the reference. Expression was assessed in the leaf, petal, pollen, filament, style and ovary. (B) Phylogenetic relationships between CrFBX and SLF/SFBB/SLFL/SFB proteins. The numbers of each interior branch are the percentage of bootstrap values. The numbers after each SLF/SFBB/SLFL/SFB proteins are the UniProt accession numbers. Md-, Malus ×domestica; Pm-, Prunus mume; Pd-, Prunus dulcis; Pa-, Prunus avium; Cr-, Citrus reticulata; Pi-, Petunia inflata; Ah-, Antirrhinum hispanicum.The phylogenetic relationships of pollen-specific F-box proteins with SFBBs (SFBs), SLFs and SLFLs, derived from Rosaceae, Solanaceae and Plantaginaceae were explored. Full-length amino acid sequences of pollen-specific CrFBX proteins, SLFs from Petunia and Antirrhinum, SFBs and SLFLs from Prunus and SFBBs from Maloideae were aligned using the MAFFT program and a phylogenetic tree was constructed (Fig. 1B). The topological structure included clades α (SFBB), β (SLFL), γ (CrFBX), δ (SFB) and ε (SLF). All clades showed stronger bootstrap values (≥92%). The pollen factor (SFB) from Prunus failed to cluster with SFBB, SLFL and SLF clades but formed a single cluster. This was consistent with the finding that SFB and SFBB/SLFL/SLF originated from distinct ancestral genes, whereas the functions of SFBB and SLFL in the S-RNase-based SI system in flowering plant derived from a common origin (Akagi et al., 2016). Besides, 13 pollen-specific CrFBX proteins were clustered into the γ clade with a bootstrap value of 99%, which were different from those of SFBB, SLFL, SFB and SLF (Fig. 1B). CrFBX13, CrFBX16 and CrFBX15 were not homologous with the S-locus F-box proteins. To characterize SLF genes in ‘Wuzishatangju’, CrFBX1-CrFBX16 were mapped into the C. clementina genome. The results showed that 10 CrFBX (CrFBX1-CrFBX10) was located in the S-locus (Fig. S4) (Liang et al., 2020). These results suggested that the S-locus, which is similar to the apple or pummelo, is also existent in the ‘Wuzishatangju’ genome.
Pollen-specific expression analyses of SKP1-like genes
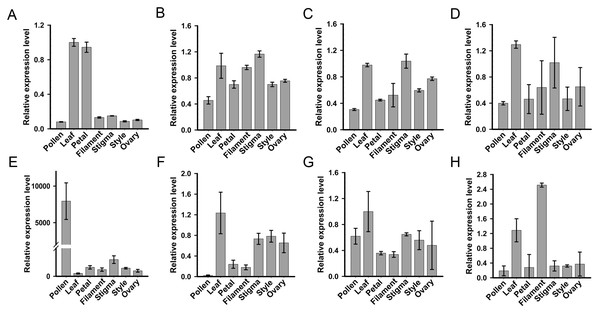
For systematic identification of the SKP1 family protein(s), which potentially serve as adaptors for assembling the SCF complex in C. reticulata, we conducted an HMMER search for available pollen and pistil transcriptome datasets of ‘Wuzishatangju’ (Ma et al., 2017) using the Hidden Markov Model (HMM) profile of the SKP1 family protein as a query. Eight independent genes (termed CrSKP1-a to CrSKP1-h) were characterized (Fig. S5B). Expression profiles of all the SKP1 family genes in C. reticulata were analyzed with qRT-PCR (Fig. 2). Among these SKP1 candidate genes, only CrSKP1-e showed higher pollen-specific expression patterns in ‘Wuzishatangju’ (Fig. 2E). In addition, while CrSKP1-f was less expressed in pollen (Fig. 2F), CrSKP1-a was highly expressed in the leaf and the petal (Fig. 2A). CrSKP1-h was also highly expressed in the leaf and the filament (Fig. 2H). However, CrSKP1-b, CrSKP1-c, CrSKP1-d and CrSKP1-g showed constitutive expression patterns in all ‘Wuzishatangju’ tissues (Figs. 2B, 2C, 2D, 2G).
Figure 2: Expression analyses of SKP1 family genes in different tissues.
Quantitative RT-PCR analysis of the eight SKP1 family genes, using the Hidden Markov Model (HMM) profile of the SKP1 family protein as a query. (A) CrSKP1-a; (B) CrSKP1-b; (C) CrSKP1-c; (D) CrSKP1-d; (E) CrSKP1-e; (F) CrSKP1-f; (G) CrSKP1-g and (H) CrSKP1-h.The HMM profile of SKP1 was used to search the C. clementina genome for the comprehensive identification of the SKP1 genes. A total of 13 non-redundant SKP1 genes were characterized (Figs. S5A, S5B). For phylogenetic analyses, an unrooted Neighbor-Joining (NJ) tree was constructed based on the multiple sequence alignments. Three groups (group I, group II and group III with 88%, 89% and 100% bootstrap values, respectively) were distinctly clustered (Fig. S5A). The transcripts and expression patterns of SKP1 genes were investigated in C. reticulata. One gene was not detected in pollen (Fig. S5C) and three genes were an unbiased expression in C. reticulata pollen (Figs. S5D–S5F). The results from expression analyses showed that the CrSKP1-e gene was the candidate gene involved in the SI reaction in ‘Wuzishatangju’.
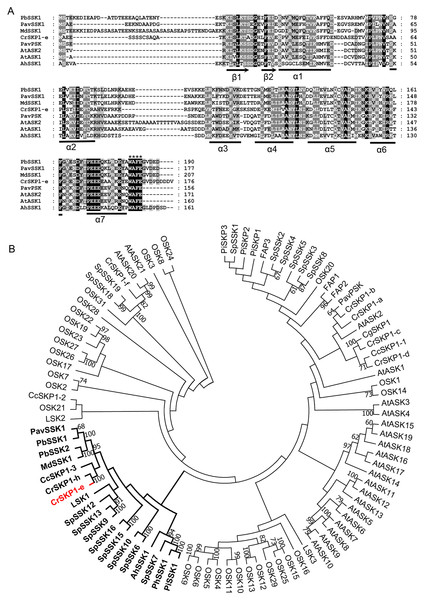
Figure 3: Multiple sequence alignment and phylogenetic analyses of pollen-specific CrSKP1-e.
(A) CrSKP1-e protein consisted of β1 and β2 sheets in the N-terminus, which connects the F-box domain and the seven helices (α1- α7) binding the CUL1 protein in the C-terminus. (B) An unrooted neighbor-joining (NJ) tree of CrSKP1-e and 84 SKP1-like proteins in other plants. The CrSKP1-e protein was well clustered with the SSK1 protein involved in S-RNase-based SI reaction in Rosaceae, Antirrhinum and Solanaceae, but was not the orthologs from rice (OSK1-OSK31) and Arabidopsis (AtSK1-AtSK21). All the accession numbers are presented in Table S3.Cloning and phylogenetic analyses of CrSKP1-e
The full-length coding sequence of pollen-specific CrSKP1-e was cloned from ‘Wuzishatangju’. CrSKP1-e encoded 176 residues and was identical with the reference transcript of Ciclev10006034m in C. clementina (Figs. 3A, Figs. S5B). However, CrSKP1-e was different from CrWSKP1 obtained from the suppression subtractive hybridization (SSH) library of ‘Wuzishatangju’ (Miao et al., 2015). The CrSKP1-e protein consisted of β1 and β2 sheets in the N-terminus, which connects the F-box domain and the seven helices (α1- α7) binding the CUL1 protein in the C-terminus (Fig. 3A). Compared with a typical SKP1 protein, the CrSKP1-e protein not only contained the conserved terminal residues ‘WAFE’ found in most SKP1 family proteins, but also had the unique C-terminal tail ‘GVDPDDDDV’ (Fig. 3A). The unique residue tail following the ‘WAFE’ domain was characterized in the SKP1-like protein, which was exclusively involved in S-RNase-based the SI reaction in Rosaceae (e.g., PbSSK1, PavSSK1 and MdSSK1) and Antirrhinum (e.g., AhSSK1) (Fig. 3A). Phylogenetic analyses were conducted based on the amino acid residues of the 84 SKP1-like proteins (Table S3). The CrSKP1-e protein was well clustered with the SSK1 protein involved in the S-RNase-based SI reaction in Rosaceae, Antirrhinum and Solanaceae (Fig. 3B). Furthermore, the CrSKP1-e protein was not the orthologs from rice (OSK1-OSK31) and Arabidopsis (AtSK1-AtSK21) (Fig. 3B). A relatively lower expression level of CrSKP1-h was detected in the pollen, although it was homologous with CrSKP1-e (Fig. 2H). This indicated that CrSKP1-h might not be a candidate gene involved in the SI reaction in ‘Wuzishatangju’. These results suggested that CrSKP1-e potentially serves as a link for assembling the SCF-type E3 complex involved in the SI response of ‘Wuzishatangju’.
Identification and cloning of CUL1 genes
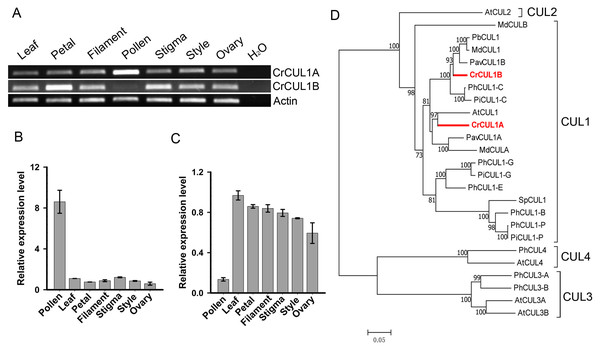
To characterize the CUL1 (Cullin1) protein(s) that potentially serve as scaffolds for the assembly of the SCF complex in ‘Wuzishatangju’, a genome-wide analysis of the CUL family sequences in C. clementina was conducted. Sixteen CUL-related proteins were identified (Table S4). To investigate the CUL1 homologs, an NJ tree was generated to assess the phylogenetic relationships between the candidate CUL proteins and the known AtCUL1-AtCUL4 proteins from A. thaliana. Three genes (Ciclev10018125m, Ciclev10019010m and Ciclev10004406m) belonging to the AtCUL1 subgroup were obtained (Fig. S6). No expression of Ciclev10018125m was detected in all the tissues tested (data not shown). The full-length coding sequences of the two CUL1 genes termed CrCUL1A and CrCUL1B were cloned from ‘Wuzishatangju’. Compared with C. clementina, the coding sequence of CrCUL1A and Ciclev10019010m from ‘Wuzishatangju’ shared identical sequence while the two single-nucleotide polymorphisms (SNP) sites were detected between CrCUL1B and Ciclev10004406m (Figs. S7A, S7B). CrCUL1A showed preferential expression in mature pollen compared to the other tissues (Figs. 4A, 4B). On the contrary, CrCUL1B showed unbiased expression in all tissues except for the lower expression level detected in pollen (Figs. 4A, 4C). Phylogenetic analyses indicated that CrCUL1A and CrCUL1B belonged to Rosaceae-related CUL1 groups. However, CrCUL1A and CrCUL1B failed to cluster with PiCUL1-P, which is considered as an essential component of the SCF complex for the non-self-recognition system in Petunia (Fig. 4D). These results suggested that CrCUL1A and CrCUL1B potentially interact independently or jointly with the non-S-locus ortholog, CrSKP1-e, and form an SCF complex involved in the SI reaction in C. reticulata.
Figure 4: Expression analyses and phylogenetic relationships of CrCUL1A and CrCUL1B in C. reticulata.
(A) Semi-quantitative PCR analysis of the expression patterns of CrCUL1A and CrCUL1B genes, using the expression of the actin gene as the reference. Quantitative RT-PCR analyses of (B) CrCUL1A and (C) CrCUL1B. (D) Unrooted NJ tree of the deduced Cullin (CUL) proteins constructed using MAGA7 software. The numbers of each interior branch are the percentage of bootstrap values. At-, A. thaliana; Md-, M. domestica; Pb-, P. bretschneideri; Pav-, P. avium; Ph-, P. hybrida; Pi-, P. infilata; Cr-, C. reticulata; Sp-, S. pennellii. The GenBank accession numbers are presented in Table S3.Interaction of CrSKP1-e with CrFBX proteins using Y2H and in vitro binding assays
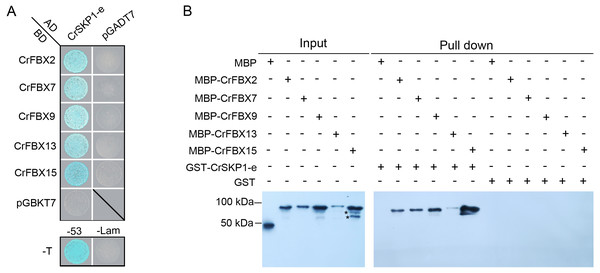
The interactions of pollen-specific CrFBX1-CrFBX16 with CrSKP1-e were investigated using the Y2H and in vitro binding assays. The growth of Y2HGold strains showed that the CrSKP1-e protein could interact with CrFBX2, CrFBX7, CrFBX9, CrFBX13 and CrFBX15 proteins (Fig. 5A). However, based on the phylogenetic analyses and genomic physical location results, CrFBX13 and CrFBX15 were not the S-locus genes. GST (negative control) and GST-CrSKP1-e were reacted with Glutathione Sepharose 4B. The GST-bound beads were then incubated with MBP (negative control) and MBP-fused proteins. The results showed that CrFBX2, CrFBX7, CrFBX9, CrFBX13 and CrFBX15 were bound to CrSKP1-e (Fig. 5B). Taken together, these results suggested that CrSKP1-e protein could interact with a cluster of F-box proteins, including some non-S-locus F-box proteins.
Figure 5: Interaction of CrSKP1-e and CrFBX proteins.
(A) The interaction of CrSKP1-e and CrFBX proteins was evaluated using the Y2H assay. A combination of BD-CrFBXx (x means the number of CrFBX proteins) vectors and AD-CrSKP1-e vectors were co-transformed into Y2HGold strain and selected on SD/-L/-T medium (Fig. S8). The positive transformants were inoculated on a selective medium SD/-A/-H/-L/-T containing X- α-Gal and AbA for growth. pGADT7-T and pGBKT7-Lam were used as negative controls. pGADT7-T and pGBKT7-53 were used as positive controls. (B) The interaction of CrSKP1-e and CrFBX proteins was evaluated using the in vitro binding assay. GST (negative control) and GST-CrSKP1-e were reacted with Glutathione Sepharose 4B, and then GST-bound beads were incubated with MBP (negative control) and MBP-fused proteins. The MBP signal was detected using western blotting. A asterisk (*) indicates the non-specific proteins.Interaction of CrSKP1-e with CrCUL1A and CrCUL1B proteins
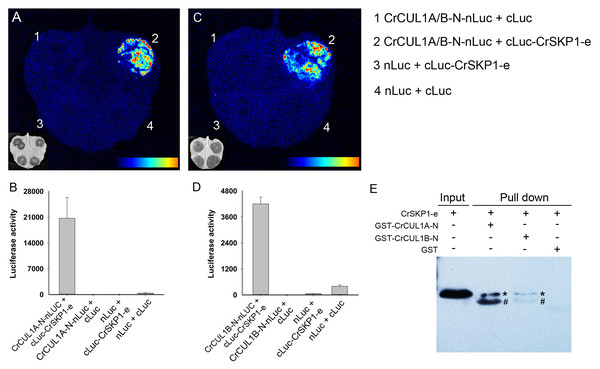
To examine the potential interaction between CrSKP1-e and CUL1 proteins, the LCI assay was conducted. As shown in Fig. 6, co-expression of CrSKP1-e (cLuc-CrSKP1-e) and the N-terminal regions of CrCUL1A (CrCUL1A-N-nLuc) in N. benthamiana leaves resulted in strong luciferase activities (Figs. 6A, 6B). Similarly, luciferase activity was also strongly detected during the co-expression of CrSKP1-e (cLuc-CrSKP1-e) and the N-terminal regions of CrCUL1B (CrCUL1B-N-nLuc) (Figs. 6C, 6D). In vitro binding assay indicated that His-CrSKP1-e reacted with GST-CrCUL1A-N and GST-CrCUL1B-N (Fig. 6E). These results suggested that the CrSKP1-e protein could interact with the N-terminal regions of CrCUL1A and CrCUL1B protein.
Figure 6: Interaction assay of CrSKP1-e and CrCUL1 proteins.
Luciferase imaging (A) and luciferase activity (B) during co-expression of CrSKP1-e and the N-terminal region of CrCUL1A (CrCUL1A-N). Luciferase imaging (C) and luciferase activity (D) during co-expression of CrSKP1-e with the N-terminal region of CrCUL1B (CrCUL1B-N). (E) In vitro binding assay for CrSKP1-e and CrCUL1 proteins. As asterisk (*) indicated CrSKP1-e protein, a number sign (#) indicated non-specific proteins.Discussion
Identification of the S-locus F-box genes in C. reticulata
The F-box family proteins are identified by the existence of the 60 conserved residue regions in the N-terminal region called the F-box domain, which acts as a receptor to bind to the SKP subunit (Qiao et al., 2004; Williams et al., 2014; Zhao et al., 2002). The number of F-box genes vary widely between species. In plants, at least 692, 337, 779 and 285 F-box genes have been characterized in Arabidopsis, poplar, rice and chickpea, respectively (Xu et al., 2009; Gupta et al., 2015). In this study, 298 non-redundant F-box superfamily proteins were obtained from C. clementina, which represents about 1.2% of the annotated proteins in the C. clementina (v1.0) genome. In addition to the F-box domain, several F-box proteins such as Kelch, Leucine-rich repeat, FBD, PP2 motif and FBA domains, were predicted to contain a C-terminal region. This indicates that the F-box proteins are responsible for the significant function during growth and development, disease resistance and reproduction (Fig. S1) (Gagne et al., 2002). Most of the F-box proteins contain the C-terminal protein-protein interaction domains. In clementine mandarin, the most abundant F-box type is the FBA subfamily (46 proteins). Some members of the FBA subfamily act as pollen determinants to recognize S-RNase or as ‘inhibitors’ (SLFLs from Prunus) to inactivate self-S-RNase during S-RNase-based SI response in Rosaceae, Solanaceae and Plantaginaceae. In Citrus, information about the genetic linkage between SI and the S-genotype has been elucidated in pummelo (Liang et al., 2020). SI reaction in mandarin is suggested to be an S-RNase-mediated system (Liang et al., 2020). In the present study, 16 F-box genes derived from the FBA subfamily were obtained based on the C. clementina genome and ‘Wuzishatangju’ pollen transcript data. These were specifically expressed in the ‘Wuzishatangju’ pollen. Based on the phylogenetic analyses, not all the pollen-specific F-box genes were orthologous to SFB genes in Prunus; and 13 genes were clustered with SFBB/SLFL (Fig. 1B). Physical organization analysis suggested that CrFBX1-CrFBX10 genes were located in the S-locus in Citrus, indicating that intrahaplotypic diversity of CrFBX potentially causes the polymorphism of pollen determinants involved in S-RNase recognition. Furthermore, CrFBX7 had a 1-bp deletion at the 3′-terminus in ‘Wuzishatangju’, which is predicted to trigger translation termination failure. However, it is not clear whether non-functional CrFBX7 is involved in the SI reaction of ‘Wuzishatangju’.
Identification of SKP1-like and CUL1 genes
The SKP1 protein acts as an adaptor to bind the F-box and CUL1 protein for assembling of the canonical SCF-type E3 ligase complex, which mediates the loading of ubiquitin to various substrates. In Arabidopsis, SKP1-like protein 13 regulates seed germination and seedling growth (Rao et al., 2018). Pollen-specific SKP1-like proteins are essential for pollen tube elongation in lily (Chang et al., 2009). SKP1 is also involved in salt and drought tolerance in soybean (Chen et al., 2018b). In this study, the genome-wide identification of SKP1 family genes was conducted to identify candidate non-S factors based on the C. clementina genome, which are potentially involved in the SI reaction in ‘Wuzishatangju’. The results from the expression analyses indicated that the CrSKP1-e gene was significantly up-regulated in pollen compared to the other tissues of ‘Wuzishatangju’. The expression pattern of CrSKP1-e in ‘Wuzishatangju’ was consistent with that of MdSSK1 in M. domestica, PbSSK1 and PbSSK2 in P. bretschneideri, PhSSK1 in P. hybrida and PavSSK1 in P. avium (Zhao et al., 2010; Matsumoto et al., 2012; Xu et al., 2013; Yuan et al., 2014). These results suggest that CrSKP1-e genes are mainly responsible for pollen function. In addition, the CrSKP1-e protein had a conserved C-terminal region, a motif ‘GVDPDDDDV’ following the conventional ‘WAFE’ motif, which is found in most SKP1 family proteins. Interestingly, this unique tail in the C-terminal region of the SKP1-like genes involved in the S-RNase-based SI reaction is always present. For instance, this tail is ‘GVDED’ in Rosaceae. However, it is not invariable in Solanaceae and Plantaginaceae but for the ‘D’ in the last position, which is conserved (Aguiar et al., 2015). Phylogenetic relationships suggested that CrSKP1-e clustered with PavSSK1, MdSSK1, PbSSK1 and PbSSK2. These results imply that CrSKP1-e, homologous to SSK1, is also present in ‘Wuzishatangju’.
Different CUL1 homologs, such as PiCUL1-G and PiCUL1-C proteins in P. inflata, Cullin1-like protein in Antirrhinum and CUL1 orthologs in Rosaceae, were proposed to be one of the members of the SCF complex in the SI reaction (Hua & Kao, 2006; Qiao et al., 2004; Xu et al., 2013). In P. inflata, though PiCUL1-G, SBP1 and SLF were known to be the novel E3 ligase complex mediating S-RNase ubiquitination, PiCUL-P, another CUL1 homolog protein, was proved to be a component of the canonical SCF ligase complex involved in non-self-recognition in the SI reaction (Kubo et al., 2016). In P. avium, PavCUL1A and PavCUL1B were considered to be components of a functional SCFSFB complex (Matsumoto et al., 2012). In this study, the Cullin family proteins were first identified from C. clementina. The three CUL proteins were closely related to the AtCUL1 protein (Fig. S6). Only the CUL1 subfamily proteins serve as scaffolds for assembling the SCF complex. CrCUL1A was significantly accumulated in ‘Wuzishatangju’ pollen and strongly interacted with CrSKP1-e. These results suggest that CrCUL1A potentially functions as a scaffold for the SCF complex in pollen. However, the CrCUL1B gene was less expressed in ‘Wuzishatangju’ pollen. Further studies are necessary to confirm whether CrCUL1B is a redundant or an alternative protein for assembling the SCF complex involved in the SI system.
Identification of putative SCF-type E3 complex in ‘Wuzishatangju’ pollen
The interactions between F-box and SKP1 proteins have been extensively explored. In Arabidopsis, 92 AtSLF-S2-related proteins were identified; most of them interacted with one or more SKP1 proteins in the yeast system (Wang et al., 2004). To investigate the SCF complex involved in the SI reaction in P. inflata, 17 SLF proteins and an SLF-like protein were co-immunoprecipitated with PiSSK1:FLAG:GFP (Li et al., 2016). In apple, MdSSK1 interacted with 4 MdSFBB and 6 MdSFBBL proteins in the yeast system (Yuan et al., 2014). In the present study, yeast system interaction assays revealed that CrSKP1-e protein interacted with 4 CrFBX proteins (CrFBX2, CrFBX9, CrFBX13 and CrFBX15) in ‘Wuzishatangju’ and at least one CrFBX protein (CrFBX7) in ‘Chuntianju’. The LCI and in vitro assays indicated that CrSKP1-e binds to the N-terminal region of both the CrCUL1A and CrCUL1B proteins. The CrSKP1-e protein could act as an adaptor that links CrFBX and CrCUL1 for assembling of the SCF complex in ‘Wuzishatangju’. Interestingly, CrSKP1-e not only interacted with the S-locus CrFBX proteins (CrFBX2, CrFBX7 and CrFBX9) in mandarin but also bound to the non-S-locus CrFBX proteins (CrFBX13, CrFBX15). These results suggest that CrSKP1-e potentially functions as an adaptor in the SI reaction. However, further studies are needed to elucidate the function of CrSKP1-e genes in the SI reaction of ‘Wuzishatangju’.
Conclusions
In summary, the genome-wide characterization of the 298 F-box family proteins was performed using the in silico method based on the C. clementina reference genome, which supported in-depth identification of the S-locus F-box genes. Ten pollen-specific CrFBX genes homologous to SFBB/SLFL are mapped into the S-locus. CrSKP1-e connects the S-locus and non-S-locus CrFBX proteins to the two CrCUL1 proteins (CrCUL1A and CrCUL1B) for SCF complex assembling in ‘Wuzishatangju’ pollen.
Supplemental Information
Identification of F-box family proteins in C. clementina
(A) A total of 12 additional C-terminal domains were obtained by searching Pfam/SMART website and NCBI conserved domain database; (B) 12 groups (group A-group L) were tentatively classified according to the common node. A total of 46 non-redundant FBA subfamily proteins are identified (45 proteins are cluster into group B and one cluster into group E); (C) The majority of FBA subfamily (30 genes) are lack of intron and 14 genes have one intron, only two predicted genes (Ciclev10011950m.g, Ciclev10013681m.g) have two introns.
Expression analyses of FBA subfamily genes.
Expression patterns of FBA subfamily genes were performed in different tissues (leaf, petal, filament, pollen, stigma, style and ovary) of ‘Wuzishatangju’ by RT-PCR analyses.
Expression analyses of FBA subfamily genes.
Verification of expression patterns of CrFBX1- CrFBX 17 in different tissues of ‘Wuzishattangju’ by qRT-PCR analyses.
Sequence alignment of CrFBX7 gene between ‘Wuzishatangju’ and ‘Chuntianju’
A 1-bp deletion (red star) in 3′-termini of CrFBX7 in ‘Wuzishatangju’ is predicted to cause failure of translation termination. CrFBX7 derived from ‘Chuntianju’ encodes normal F-box protein.
The schematic of S-locus in Citrus
(A) The schematic was described according to the published genome (https://www.citrusgenomedb.org/) and the S-RNase was marked red, Sm -RNase (red words) is identified in C. clementina and C. sinensis; (B) Cglg001950 is identified as S6 -RNase and co-separated with the S6 genotype of F1 hybrids (Liang et al., 2020).
Characterization of SKP1 family genes in C. clementina
(A) Phylogenomic analyses and structure of SKP1 family genes in the reference genome of C. clementina, the SKP1 family genes were clustered into group I-group Ⅲ; (B) Orthologs of CrSKP1 in C. clementina. CcSKP1-1 to CcSKP1-5 were not identified in the transcriptome dataset of C. reticulata. CcSKP1-4 was just annotated as a fragmentary coding sequence without the AUG in C. clementina (presented by asterisk); (C) The expression profile of CrSKP1-e and CcSKP1-3 in different tissues of C. reticulata. But the CcSKP1-3 was not detected; (D) The expression profile of CcSKP1-5 in C. reticulata; (E) The expression profile of CcSKP1-2 in C. reticulata. (F) The expression profile of CcSKP1-1 in C. reticulata.
Identification of Cullin (CUL) family proteins in C. clementina
The orthologs of AtCUL1 were bold. The UniProt accessions followed with corresponding proteins.
Sequence alignment of CUL1 genes
Identity of CrCUL1A between C. reticulata and C. clementina Ciclev10019010m and Ciclev10004406m are peptide accession numbers of C. clementina which is downloaded from JGI database (https://phytozome.jgi.doe.gov). The sequence alignment was conducted by DNAMAN software.
Sequence alignment of CUL1 genes
Identity of CrCUL1B between C. reticulata and C. clementina. Ciclev10019010m and Ciclev10004406m are peptide accession numbers of C. clementina which is downloaded from JGI database (https://phytozome.jgi.doe.gov). The sequence alignment was conducted by DNAMAN software.