Effects of dietary fennel (Foeniculum vulgare Mill.) seed powder supplementation on growth performance, nutrient digestibility, small intestinal morphology, and carcass traits of broilers
- Published
- Accepted
- Received
- Academic Editor
- Korakot Nganvongpanit
- Subject Areas
- Food Science and Technology, Zoology
- Keywords
- Fennel seed powder, Growth performance, Apparent metabolic rate, Carcass traits, Intestinal morphology
- Copyright
- © 2021 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. Effects of dietary fennel (Foeniculum vulgare Mill.) seed powder supplementation on growth performance, nutrient digestibility, small intestinal morphology, and carcass traits of broilers. PeerJ 9:e10308 https://doi.org/10.7717/peerj.10308
Abstract
Background
With the increasing demands in livestock and poultry breeding and the growing number of food-borne diseases, it is necessary to practice food safety and develop strategies to produce healthy livestock. Fennel (Foeniculum vulgare Mill.) has been used as an additive in poultry production by some researchers, but there are few studies on the systemic beneficial effects of dietary fennel seed powder supplementation on broilers. Therefore, this study aimed to investigate the effect of dietary fennel seed powder supplementation on feed intake, the apparent metabolic rate of nutrients, intestinal morphology, and carcass traits in Cobb broilers.
Methods
A single-factor experimental design was used. In total, 160 1-day-old Cobb broiler chicks were randomly assigned to four treatments, with four replicates each (n = 10/replicate). Broilers in the control (CN) group were fed a basal diet without fennel seed powder, and broilers in the treatment groups were fed a basal diet supplemented with 0.15% (LF), 0.30% (MF), or 0.45% (HF) fennel seed powder, respectively. Feeding trials lasted for 42 days under the conditions of ad libitum access to feed and water, and 24-h illumination. During the third and sixth weeks, digestive and metabolic assays were carried out. When the broilers were 42 days old, one chicken with a weight close to the average was selected from each repetition, euthanized by an intravenous injection of 5% sodium pentobarbital, and carcass traits were measured and intestinal samples were collected for morphological assessment.
Results
There was no significant difference in growth performance of broilers (P > 0.05). The breast muscle percentage, fat width and fat width index, breast muscle area, and breast muscle area index of broilers in the LF group were higher than those in other groups (P < 0.05). Jejunum weight and length were higher in MF than in CN and LF broilers (P < 0.05). Additionally, duodenal villi height, ileal villi height, and ileal wall thickness were higher in MF than in CN broilers (P < 0.05). There were no significant differences in nutrient utilization among all groups (P > 0.05), except that the ash apparent metabolic rate in MF broilers at 21 days of age was higher than that in LF broilers (P < 0.05). In conclusion, dietary supplementation with a moderate concentration of fennel affects carcass performance, and intestinal morphology, and promotes the growth and development of broilers.
Introduction
With the rapid development of livestock and poultry breeding, the number of reported cases of food-associated infections and the occurrence of drug residues, including antibiotics, synthetic drugs, steroids, and other feed additives in meats, eggs, and milk have increased rapidly. Thus, it is very important to focus on food safety from both the consumers’ and the food industries’ perspectives (Kăcániová et al., 2019). The European Union has banned the use of most growth-promoting antibiotics in feed additives due to issues such as the development of cross-resistance against pathogens and residue accumulation in tissues (Ertas et al., 2005; Olgun & Yıldız, 2014; Kăcániová et al., 2019). Accordingly, the search for safe and low-toxicity feed additives has gained increased research interest. Plants, especially herbs, have been used for medicinal purposes for centuries, and some have played a significant role in the maintenance of animal health (AI-Kassie, 2008). Several studies have been conducted on herbal additives owing to their many advantages, including low cost, easy availability, and lack of residual effects, and because of the absence of concerns regarding the use of antibiotics (Saeedi et al., 2017). Supplementation of livestock and poultry feed with plants containing bioactive components has produced encouraging results. These additives can promote growth and performance, and improve feed efficiency, nutrient digestion, antioxidant status, immunological indices, and poultry health (Dhama et al., 2015; Alagawany et al., 2019).
Fennel (Foeniculum vulgare Mill.) is a common herbal medicine and a popular aromatic plant that belongs to the family Apiaceae and is related to cumin, dill, caraway, and anise, which all bear aromatic fruits that are commonly called seeds (Dhayalan, Jegadeeshwari & Gandhi, 2015). Fennel’s seed is commonly used as a natural remedy against digestive disorders such as dyspepsia, bloating, and flatulence, and possesses analgesic, antipyretic, and antioxidant properties (El-Deek, Attia & Hannfy, 2003; Choi & Hwang, 2004; Guimarăes et al., 2011). It is also used to flavor foods, liqueurs and in the perfumery industry (Choi & Hwang, 2004). Essential oils derived from fennel have antioxidant, antimicrobial, and hepatoprotective activities. They are composed of several monoterpenes and phenylpropanoids, where trans-anethole, estragole, fenchone and limonene exist as main constituents. trans-anethole, often the most prevalent constituents, counts for the anise taste, fenchone provides the bitterness, and estragole (methylchavicol) the sweetness (Ruberto et al., 2000; El-Deek, Attia & Hannfy, 2003; Oktay, Gülçin & Küfrevioğlu, 2003; Choi & Hwang, 2004; Gharaghani, Shariatmadari & Karimi Torshizi, 2013).
The use of fennel in poultry diets improves appetite, digestion, nutrient absorption, and immunity, and does not produce drug residues. Mohammed & Abbas (2009) demonstrated that broilers fed diets containing various levels of fennel seed showed improved weight gain and feed efficiency. Similarly, Abou-Elkhair, Selim & Hussein (2018) reported that laying hens fed a diet supplemented with fennel, black cumin tended to have a higher body weight gain than control diet. El-Deek, Attia & Hannfy (2003) reported significantly improved color intensity when a mixture of 0.1% of fennel plus anise was supplemented to broiler diets. Therefore, in this study, we hypothesized that fennel seed powder (FSP) would improve growth performance, carcass performance, nutrient utilization, and intestinal morphology in broilers. To test this hypothesis, we investigated the effects of dietary supplementation with different concentrations of FSP on these features.
Materials and Methods
Ethics statement
The study protocol was reviewed and approved by the Animal Welfare Ethics Committee of Gansu Agricultural University (Approval No. DK-005). And the animal procedures used in this study strictly abide by the Administrative Measures of Gansu Province on Experimental Animals (2005–2012).
Experimental animals and design
In total, 160 1-day-old healthy, male Cobb broiler chicks were obtained from Baoji Dacheng Poultry Co., Ltd. (Shanxi, China). The chicks were randomly assigned to four treatments, with four replicates each (n = 10/replicate). A single-factor experimental design was used. The treatments were as follows: different basal diets were fed during the initial (0–21 days) and finisher (21–42 days) stages (Table 1), and broilers in the control (CN) group were fed a basal diet without FSP, whereas those in the treatment groups were fed a basal diet supplemented with 0.15% (LF), 0.30% (MF), or 0.45% (HF) FSP. Fennel seeds were purchased from the local market (Gansu, China). The basal diets, based on corn and soybean meal, were formulated according to the nutritional requirements of broilers in China (2004) (Table 1).
| Items | Initial (1–21 days) | Finisher (22–42 days) |
|---|---|---|
| Ingredients (%) | ||
| Corn | 46.84 | 55.91 |
| Bran | 6.24 | 1.12 |
| Soybean meal | 31.05 | 28.00 |
| Cottonseed meal | 6.41 | 6.35 |
| Rapeseed meal | 5.60 | 5.20 |
| Calcium phosphate | 1.32 | 1.17 |
| Limestone powder | 1.22 | 1.10 |
| Methionine | 0.15 | 0.07 |
| Salt | 0.27 | 0.18 |
| 50% Choline chloride | 0.40 | 0.40 |
| Additive premix1 | 0.50 | 0.50 |
| Total | 100 | 100 |
| Nutrient composition2 | ||
| Metabolizable energy (kcal/kg) | 12.54 | 12.96 |
| Crude protein (%) | 21.49 | 20.00 |
| Crude fiber (%) | 3.65 | 4.24 |
| Ether extract (%) | 3.42 | 3.12 |
| Lysine (%) | 1.00 | 1.07 |
| Methionine (%) | 0.45 | 0.40 |
| Calcium (%) | 1.19 | 0.90 |
| Available phosphorus (%) | 0.50 | 0.41 |
| Sodium (%) | 0.20 | 0.30 |
| Chloride (%) | 0.18 | 0.15 |
Notes:
Each chick was numbered according to the treatment. In the first week of the experiment, Multi-Vitamins (Shanghai Neotrieon Boi-Tech Co., Ltd, Shanghai China) were added in the drinking water to relieve stress. The chicks used a vacuum drinker in the first 3 weeks, and in the fourth week, the vacuum drinker was replaced with a nipple drinker. Feed and water were provided ad libitum. The chicks were housed under a combination of natural and artificial auxiliary light, and the illumination time varied depending on the weather and temperature. The temperature and humidity were adjusted to the needs in different growth stages based on daily monitoring of the behavior of the chickens. The room temperatures for broiler were 34–32, 32–28, 28–26, 26–24, 24–22, and 24–22 °C for the first, second, third, fourth, fifth, and sixth week, respectively. Chicks were vaccinated against Newcastle disease (Yangling Green Bio-engineering Co., Ltd, Shanxi, China) at 7 days and 21 days of age, and against mucosal sac disease (Yangling Green Bio-engineering Co., Ltd, Shanxi, China) at 15 days and 28 days of age.
Measurements
Growth performance measurements
Body weight (BW) and feed intake were measured for each replication group each week. Then, the growth performance of broilers was calculated by the average daily feed intake (ADFI), average daily gain (ADG) and ratio of feed to gain (F/G).
Nutrient digestibility measurements
Digestive and metabolic assays were carried out from days 18 to 21 and days 39 to 42; starting from 7:00 am, feces were collected. Fecal samples from the same repeat group were mixed, and a certain fraction of the pooled sample was placed in an aluminum box, sprayed with 10 mL of 10% sulfuric acid solution per 100 g of sample, and dried in an oven at 65 °C. In addition, during days 18 to 21 and days 39 to 42, 20–30 g dietary samples were collected daily, mixed, and transported to the laboratory. Dietary and fecal samples were pulverized using a 0.45-mm sieve for nutrient composition analysis of the following feed constituents according to guidelines of the Association of Official Analytical Chemists methods (AOAC, 2002): analytical crude protein (CP; method 990.03), crude ash (Ash; method 942.05), dry matter (DM; method 930.15), and organic matter (OM).
Carcass and intestinal measurements
At 42 days of age, one chick that had a body weight close to the average was selected from each replicate group. All chicks were treated humanely and with efforts to minimize suffering. To induce loss of consciousness and death with a minimum of pain and distress, chicks were euthanized by an intravenous injection of 5% sodium pentobarbital (Yin et al., 2018). No criteria were established for euthanizing animals prior to the planned end of the experiment, euthanasia was carried out only to alleviate suffering. Immediately following euthanasia, the abdominal cavity was opened, the pancreas, liver and small intestines were removed, and to determine carcass characteristics. The carcass weight, half-eviscerated weight, eviscerated weight, abdominal fat weight, breast muscle weight, breast muscle area, fat width, and leg muscle weight were recorded, and percentages were calculated (Attia et al., 2015). Simultaneously, the small intestines were segmented and measured. The length and weight of the duodenum, jejunum, ileum, and cecum were measured, and the intestinal index was calculated for the same (Ding & Zhang, 2009). The following formulas were used:
Carcass percentage (%) = carcass weight/live weight × 100;
Half-eviscerated percentage (%) = half-eviscerated weight/live weight × 100;
Eviscerated percentage (%) = eviscerated weight/live weight × 100;
Abdominal fat percentage (%) = abdominal fat weight/eviscerated weight × 100;
Breast muscle percentage (%) = breast muscle weight/eviscerated weight × 100;
Leg muscle percentage = leg muscle weight/eviscerated weight × 100;
Fat width index = fat width (mm)/live weight (kg);
Breast muscle area index = breast muscle area (cm2)/live weight (kg);
Intestinal weight index = intestinal weight (g)/live weight (kg);
Intestinal length index = intestinal length (cm)/live weight (kg);
The remaining broiler chickens that survived in the slaughter experiment were sold to local farms and farmers at the end of the experiment to achieve a certain economic value.
Intestinal histology morphology measurement
Tissue blocks of approximately one cm2 were collected from the middle part of the duodenum, jejunum, and ileum and rinsed gently with physiological saline and then, were fixed in paraformaldehyde solution. The intestine tissue was transversely trimmed, and the sections of five microns were stained with hematoxylin and eosin. Sectioned for morphological analysis using a DP71 microscope (Olympus Corporation, Ltd, Tokyo, Japan) equipped with a camera. Image-Pro Express 6.0 image processing software (Media Cybernetics, Inc., Rockville, MD, USA) was used to measure villus height (from the tip of the villus to the villus-crypt junction), crypt depth (from villus-crypt junction to the base of the crypt), and intestinal wallthickness of the duodenum, jejunum, and ileum. Four representative visual fields were selected in each section, and the above features were measured thrice in each visual field, and the results were averaged (Liu et al., 2020; Zhang et al., 2020).
Evaluation of meat quality indicators
The pressure loss method (Farouk, Wieliczko & Merts, 2004) was used to measure the water loss rate. Approximately 1.5 g of each breast and leg muscle samples devoid of fat, tendons, and sarcolemma were compressed to a 5 cm × 5 cm × 1 cm thin piece of meat along the vertical direction of the muscle fibers. Weight before and after compression were determined.
The tenderness of breast muscle was measured using TA-XTM tenderness tester (Bosin Industrial Development Co., Ltd., Shanghai, China).
Within 1 h after slaughter, breast muscle samples of approximately 3 cm width and 2 cm thickness were weighed (W1). Then, the meat samples were placed inside a plastic bag without contacting the wall of the bag. The bags were sealed and stored at 4 °C for 24 h, and then weighed (W2).
Breast muscle drip loss (%) = W2/W1 × 100.
Within 1 h after slaughter, approximately 100 g of breast muscle was collected and weighed (W1). The samples were placed in a steamer for 30 min, moved to a cool place, and weighed again after 20 min (W2).
Cooked meat rate (%) = W2/W1 × 100.
To measure pH, breast muscle and leg muscle samples were placed in glass dishes, and an acidity meter was inserted directly into the meat at three different time points (15 min, 1 h, and 24 h).
Statistical analysis
Data were analyzed by one-way ANOVA (SPSS 19.0, IBM, Chicago, IL, USA) using the following equation:
Xij = μ + αi + eij,
where Xij is the observation of the dependent variable (i, 1–4, j, 1–4), μ is the population mean, αi is the ith treatment effect, and eij is the random error associated with the observation. Significance was defined as P ≤ 0.05, and a tendency as 0.05 < P ≤ 0.20, using Tukey’s multiple comparison test.
Results
Effect of FSP on growth performance of broilers
Table 2 shows the effects of the supplementation of FSP in broiler diets on growth performance. Based on the obtained results, the addition of different levels of FSP in the diet of broilers had no significant differences in BW, ADG, ADFI and F/G.
Effect of FSP on apparent nutrient digestibility in broilers
Table 3 presents the nutrient digestibility affected by the additive of FSP in broiler diets. The results indicate that the apparent digestibility of DM, OM, and CP in broilers were not significantly different among the different levels of FSP supplementation (P > 0.05), except for the apparent digestibility of Ash in LF broilers was significantly lower than that in MF broilers (P < 0.05).
| Items | CN1 | LF | MF | HF | P value2 |
|---|---|---|---|---|---|
| 21 days | |||||
| DM | 72.96 ± 2.94 | 71.15 ± 3.25 | 74.49 ± 3.58 | 70.3 ± 1.67 | 0.238 |
| OM | 75.29 ± 2.68 | 74.11 ± 3.10 | 76.63 ± 3.27 | 72.68 ± 1.40 | 0.254 |
| CP | 63.24 ± 7.42 | 67.22 ± 3.30 | 68.02 ± 4.10 | 64.3 ± 4.10 | 0.710 |
| Ash | 38.56 ± 6.87ab | 27.46 ± 5.71b | 42.79 ± 8.40a | 35.04 ± 5.84ab | 0.044 |
| 42 days | |||||
| DM | 65.95 ± 3.22 | 68.92 ± 2.25 | 68.78 ± 1.15 | 70.61 ± 5.35 | 0.315 |
| OM | 68.90 ± 3.08 | 71.36 ± 2.27 | 71.71 ± 1.10 | 73.13 ± 5.02 | 0.346 |
| CP | 48.76 ± 2.76 | 51.91 ± 9.02 | 48.56 ± 7.02 | 53.55 ± 9.76 | 0.749 |
| Ash | 19.36 ± 6.96 | 30.17 ± 10.92 | 22.17 ± 3.55 | 30.51 ± 11.47 | 0.233 |
Effect of FSP on carcass traits of broilers
The results of carcass traits are shown in Table 4.The breast muscle percentage was significantly affected by levels of FSP (P < 0.05),and decreased with increasing FSP. Supplemented with LF group FSP had significantly increased fat width and the fat width index than in the other three groups (P < 0.05). The breast muscle area was higher in LF than in CN and HF broilers (P < 0.05), and breast muscle area index was higher in LF than in HF broilers (P < 0.05).
| Parameter1 | CN2 | LF | MF | HF | P value3 | |
|---|---|---|---|---|---|---|
| live weight (kg) | 2.08 ± 0.12 | 2.17 ± 0.15 | 2.18 ± 0.16 | 2.13 ± 0.15 | 0.769 | |
| CW (kg) | 1.94 ± 0.14 | 2.04 ± 0.16 | 2.03 ± 0.16 | 2.01 ± 0.14 | 0.796 | |
| CP (%) | 93.27± 2.32 | 94.07 ± 1.93 | 93.32 ± 2.32 | 94.76 ± 0.38 | 0.980 | |
| HEW (kg) | 1.80 ± 0.12 | 1.89 ± 0.05 | 1.88 ± 0.95 | 1.85 ± 0.12 | 0.568 | |
| HEP (%) | 86.59 ± 2.33 | 87.68 ± 1.32 | 86.41 ± 1.48 | 86.84 ± 1.85 | 0.899 | |
| EW(kg) | 1.56 ± 0.10 | 1.65 ± 0.07 | 1.64 ± 0.06 | 1.60 ± 0.08 | 0.431 | |
| EP (%) | 75.19 ± 2.37 | 76.36 ± 1.53 | 75.62 ± 3.87 | 75.09 ± 1.36 | 0.983 | |
| AFW (g) | 23.05 ± 9.83 | 17.98 ± 6.43 | 21.13 ± 7.17 | 24.76 ± 4.17 | 0.596 | |
| AFP (%) | 1.48 ± 0.67 | 1.09 ± 0.38 | 1.29 ± 0.45 | 1.54 ± 0.24 | 0.518 | |
| BMW (kg) | 0.54 ± 0.04 | 0.62 ± 0.07 | 0.54 ± 0.08 | 0.52 ± 0.04 | 0.141 | |
| BMP (%) | 34.33 ± 1.28ab | 37.77 ± 2.23a | 32.97 ± 3.85b | 32.65 ± 0.99b | 0.045 | |
| LMW (kg) | 0.25 ± 0.03 | 0.26 ± 0.02 | 0.22 ± 0.03 | 0.26 ± 0.04 | 0.306 | |
| LMP (%) | 16.24 ± 1.38ab | 15.83 ± 0.70ab | 13.58 ± 1.20b | 16.47 ± 1.66a | 0.040 | |
| Fat width (mm) | 0.72 ± 0.02b | 0.93 ± 0.04a | 0.71 ± 0.01b | 0.74 ± 0.01b | 0.022 | |
| FWI (mm/kg) | 0.35 ± 0.025b | 0.43 ± 0.025a | 0.33 ± 0.025b | 0.35 ± 0.02b | 0.011 | |
| BMA (cm2) | 65.13 ± 1.38a | 65.75 ± 3.38a | 59.88 ± 4.50ab | 56.38 ± 4.99b | 0.013 | |
| BMAI (cm2/kg) | 31.39 ± 1.98ab | 30.39 ± 1.56a | 27.49 ± 1.82ab | 26.35 ± 0.73b | 0.002 | |
Notes:
Effect of FSP on digestive organs, intestinal development, and morphology of broilers
Jejunum length and weight were significantly higher in the MF group than in the other groups (P < 0.05), and did not significantly differ among the other groups (P > 0.05) (Table 5).
| Parameter | CN1 | LF | MF | HF | P value2 |
|---|---|---|---|---|---|
| Stomach weight | |||||
| Muscular stomach | 15.74 ± 1.16 | 16.67 ± 2.37 | 18.26 ± 2.05 | 18.57 ± 3.25 | 0.315 |
| Glandular stomach | 5.46 ± 0.48 | 5.98 ± 0.96 | 6.03 ± 0.68 | 5.45 ± 0.82 | 0.565 |
| Intestinal segment weight | |||||
| Duodenum | 10.88 ± 2.04 | 10.21 ± 1.45 | 12.36 ± 0.71 | 10.00 ± 1.59 | 0.175 |
| Jejunum | 18.54 ± 1.31b | 20.50 ± 1.67b | 23.67 ± 2.26a | 18.27 ± 1.54b | 0.030 |
| Ileum | 14.92 ± 1.16 | 15.67 ± 0.61 | 18.05 ± 2.75 | 15.70 ± 1.64 | 0.113 |
| Cecum | 15.25 ± 1.18 | 14.50 ± 1.64 | 15.50 ± 1.42 | 15.00 ± 0.81 | 0.733 |
| Intestinal segment length | |||||
| Duodenum | 12.25 ± 0.91 | 11.25 ± 0.92 | 13.00 ± 1.73 | 10.75 ± 0.35 | 0.369 |
| Jejunum | 50.75 ± 3.32b | 52.50 ± 4.87b | 62.25 ± 4.09a | 57.02 ± 4.45ab | 0.010 |
| Ileum | 52.50 ± 4.03 | 50.00 ± 3.71 | 54.75 ± 3.25 | 54.50 ± 2.69 | 0.236 |
| Cecum | 15.25 ± 0.89 | 14.50 ± 1.32 | 15.50 ± 1.45 | 15.00 ± 1.24 | 0.708 |
Table 6 shows the impact of different funnel supplementation on intestinal weight and length index. The highest intestinal weight index of duodenum, jejunum and ileum recorded in the MF group (P > 0.05). The highest intestinal length index of jejunum, ileum and total intestinal were observed in the MF group (P > 0.05).These results reflect the positive effect of feeding the broilers with a diet supplementation 0.3% FSP on the intestinal development.
| Parameter | CN1 | LF | MF | HF | P value2 |
|---|---|---|---|---|---|
| Intestinal weight index | |||||
| Duodenum | 5.25 ± 1.29a | 4.70 ± 1.12b | 5.70 ± 1.12a | 4.67 ± 0.92b | 0.035 |
| Jejunum | 8.94 ± 0.82b | 9.51 ± 1.26b | 10.84 ± 0.47a | 8.55 ± 0.25b | 0.018 |
| Ileum | 7.19 ± 0.74b | 7.25 ± 0.38b | 8.25 ± 0.92a | 7.37 ± 0.95ab | 0.033 |
| Cecum | 7.35 ± 0.67 | 6.71 ± 0.82 | 7.10 ± 0.24 | 7.04 ± 0.62 | 0.567 |
| Intestinal length index | |||||
| Duodenum | 5.90 ± 0.45 | 5.20 ± 0.47 | 6.00 ± 0.97 | 5.04 ± 0.29 | 0.101 |
| Jejunum | 24.45 ± 1.92b | 24.28 ± 2.36b | 28.76 ± 3.97a | 26.70 ± 1.49ab | 0.098 |
| Ileum | 25.32 ± 2.7ab | 23.13 ± 2.06b | 25.15 ± 1.23a | 25.56 ± 1.50ab | 0.016 |
| Cecum | 7.36 ± 0.76 | 6.70 ± 0.64 | 7.10 ± 0.17 | 7.02 ± 0.49 | 0.458 |
| Total intestinal | 146.00 ± 5.25b | 142.37 ± 16.56b | 167.50 ± 9.24a | 153.00 ± 14.76ab | 0.047 |
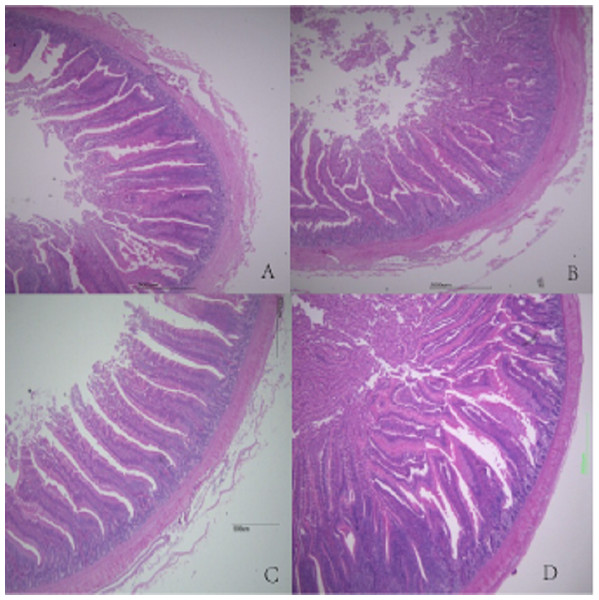
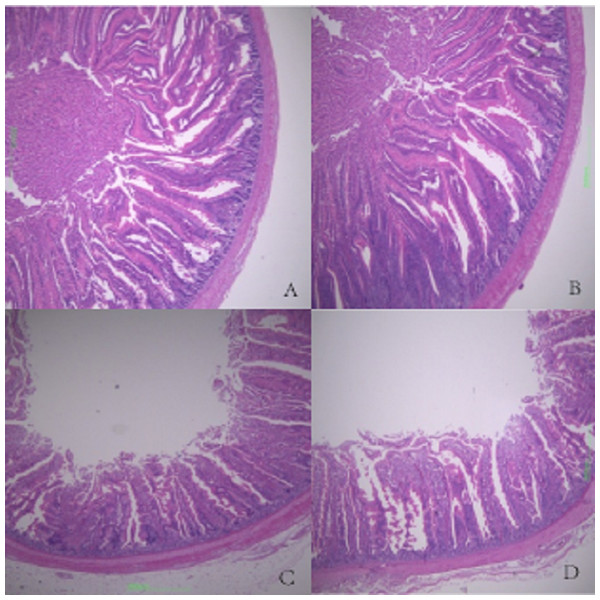
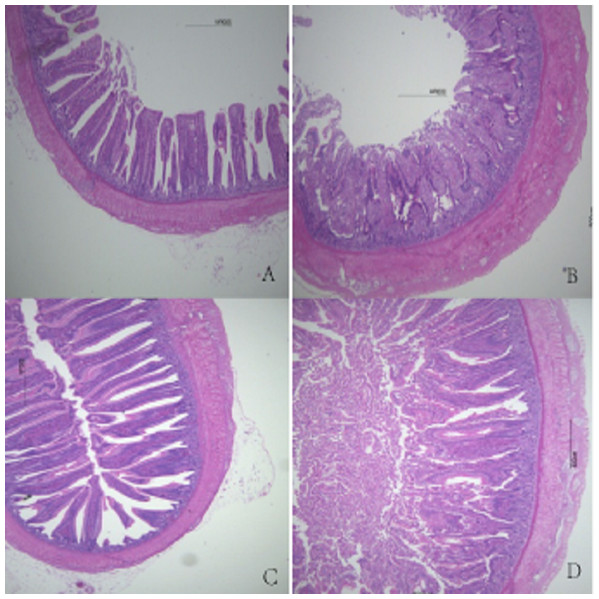
Duodenal villus height, ileal wall thickness, and ileal villus height were significantly higher in MF broilers than in the other groups (P < 0.05). There were no significant differences in the other features evaluated among the other groups (P > 0.05). There was a gradual increase in intestinal wall thickness, crypt depth, and villus height in duodenum, jejunum, and ileum with increasing FSP level (P > 0.05), except for HF group (Table 7). Histological figures of duodenum, jejunum and ileum are shown in Figs. 1–3.
| Parameter | CN1 | LF | MF | HF | P value2 |
|---|---|---|---|---|---|
| Intestinal wall thickness | |||||
| Duodenum | 238.22 ± 29.89 | 260.52 ± 33.05 | 280.06 ± 44.23 | 278.55 ± 36.19 | 0.364 |
| Jejunum | 252.56 ± 53.91 | 262.73 ± 33.94 | 309.35 ± 48.08 | 284.26 ± 43.05 | 0.341 |
| Ileum | 335.25 ± 47.55b | 410.41 ± 75.83ab | 453.74 ±55.53a | 420.22 ± 58.66ab | 0.089 |
| Crypt depth | |||||
| Duodenum | 209.83 ± 41.45 | 226.87 ± 29.76 | 263.72 ± 40.33 | 245.52 ± 35.37 | 0.245 |
| Jejunum | 202.35 ± 38.91 | 225.50 ± 28.87 | 240.15 ± 34.28 | 226.12 ± 24.67 | 0.448 |
| Ileum | 281.52 ± 50.17 | 296.08 ± 68.33 | 320.36 ± 47.14 | 306.01 ± 52.67 | 0.789 |
| Villus height | |||||
| Duodenum | 1314.93 ± 266.30b | 1378.09± 294.33ab | 1507.44 ± 280.85a | 1477.66 ± 363.67ab | 0.332 |
| Jejunum | 1366.21 ± 193.91 | 1391.82 ± 141.86 | 1530.84 ± 236.66 | 1438.63 ± 110.52 | 0.588 |
| Ileum | 1128.76 ± 159.24b | 1211.87 ± 210.67ab | 1492.33 ± 220.92a | 1369.88 ± 98.82ab | 0.058 |
Figure 1: Histological figures of duodenum.
(A) Basal diet without FSP; (B) 0.15% FSP; (C) 0.5% FSP; (D) 0.45% FSP.Figure 2: Histological figures of jejunum.
(A) Basal diet without FSP; (B) 0.15% FSP; (C) 0.5% FSP; (D) 0.45% FSP.Figure 3: Histological figures of ileum.
(A) Basal diet without FSP; (B) 0.15% FSP; (C) 0.5% FSP; (D) 0.45% FSP.Effect of FSP on broiler meat quality
There were no significant differences in the meat quality parameters measured among any of the groups (P > 0.05). The water loss rate in breast muscle increased with increasing level of FSP in the LF and MF groups; however, it decreased again in the HF group. The water loss rate in leg muscle decreased with increasing levels of FSP in the LF and MF groups; however, it increased in the HF group. Breast muscle drip loss and water loss rate were similar in all groups. Breast muscle tenderness and the cooked meat rate were lower in all experimental groups than in the control group. The pH of breast and leg muscles was higher in all experimental groups than in the control group (Table 8).
| Parameter | CN1 | LF | MF | HF | P value | |
|---|---|---|---|---|---|---|
| Water loss rate (%) | ||||||
| breast muscle | 19.62 ± 4.24 | 22.87 ± 5.87 | 23.72 ± 6.71 | 19.56 ± 3.90 | 0.588 | |
| leg muscle | 25.12 ± 3.72 | 23.23 ± 5.24 | 20.99 ± 2.72 | 24.17 ± 3.97 | 0.416 | |
| Breast muscle drip loss (%) | 12.61 ± 0.43 | 12.63 ± 0.51 | 12.65 ± 0.53 | 12.57 ± 0.53 | 0.996 | |
| Breast muscle tenderness (kg·f) | 3.78 ± 0.88 | 3.09 ± 0.87 | 3.34 ± 0.47 | 2.49 ± 0.57 | 0.138 | |
| Cooked meat rate (%) | 69.50 ± 1.48 | 68.36 ± 1.66 | 68.18 ± 0.99 | 69.17 ± 1.10 | 0.470 | |
| pH45min | ||||||
| breast muscle | 5.84 ± 0.54 | 6.36 ± 0.39 | 6.00 ± 0.32 | 6.035 ± 0.23 | 0.323 | |
| leg muscle | 5.64 ± 0.42 | 6.16 ± 0.39 | 5.82 ± 0.21 | 6.05 ± 0.06 | 0.131 | |
| Abdominal fat percentage (%) | 1.47 ± 0.44 | 1.23 ± 0.36 | 1.28 ± 0.21 | 1.54 ± 0.21 | 0.521 | |
Note:
Discussion
Mohammed & Abbas (2009) reported that the addition of fennel at 1, 2, or 3 g/kg to the feed resulted in significant improvements in body weight and feed efficiency in chickens. Fennel has a specific flavor and can be used as a natural flavoring agent to improve the palatability of feeds, thereby increasing food intake and promoting nutrient absorption in animals (Gende et al., 2009; Wu et al., 2019). In this study, we did not observe significant effects of fennel on weight gain, feed intake, and feed conversion efficiency, the best BW, ADG and ADFI were obtained when broilers were supplemented with 0.15% of FSP In line with our findings, Safaei-Cherehh et al. (2018) observed that diet supplementation with fennel extract lead to feed intake restriction (FIR) of 4.66–7.63%, and corresponding weight reduction (WR) of 4.07–7.33%. AL-Sagan et al. (2020) reported that the addition of 1.6 and 3.2% FSP to the feed did not affect broiler body weight gain, feed intake under normal temperatures, while, under chronic heat stress, results showed significant alterations in broiler performance, production. Moreover, our findings were in agreement with those of (El-Deek, Attia & Hannfy, 2003; Gharaghani, Shariatmadari & Karimi Torshizi, 2013; Abou-Elkhair, Selim & Hussein, 2018). The contrasting effects of fennel on growth performance in various studies could be attributed to differences temperature of room, type of basal diet, concentration of fennel, or treatment methods. Due to the small number of experimental samples and limited levels of FSP supplementation in this study, the effect of FSP on growth performance in broilers needs to be studied further.
In 21-day-old broilers, the digestibility of DM, OM, CP, and Ash was the highest for 0.30% FSP supplementation and decreased at higher and lower concentrations. The results were in line with those reported by Cheng & Xu (2019). This suggested that supplementation of FSP at a medium concentration can improve nutrient metabolism. However, at 42 days, the apparent digestibility of DM, OM, CP, and Ash tended to increase in broilers that were fed 0.45% FSP, indicating a dose-dependent effect of FSP with the increase of broiler age. Lack of research on the effect of fennel on nutrient utilization in broiler chickens, but studies on anise and cumin related to fennel have been reported. Ertas et al. (2005) demonstrated that adding an essential oil mix derived from oregano, clove, and anise to animal feed positively affected digestibility of nutrient. The effect of fennel on improving nutrient utilization was found to be closely related to its role in stimulating appetite, gastric juice secretion, and intestinal peristalsis, and it was not as significant as that of essential oil in broilers, which could be attributed to the fact that essential oil is richer in aromatic components and thus has a more pronounced characteristic flavor. Further, improvement of the nutrient utilization rate in broilers is closely related to improvement of intestinal development. Fennel may stimulate digestive enzyme secretion and activity and is a potential effector of microbial communities (Dorman & Deans, 2000; Platel & Srinivasan, 2000; Williams & Losa, 2001; Burt, 2004), which can further improve nutrient metabolism. Amad et al. (2011) showed that adding 150, 750, or 1,500 mg/kg mixed essential oils of anise and thyme to the feed significantly improved the apparent CP utilization rate in broiler chickens in a dose-dependent manner. Cho, Kim & Kim (2014) reported that adding star anise and thyme essential oils to the feed significantly improved the DM utilization rate and gross energy in broilers. Thus, we speculate that when fennel is combined with other dietary supplements, an additive or synergistic effect may be achieved.
Carcass characteristic is an important economic index in broiler production, and a valuable parameter to measure the net meat production capacity of broilers and to evaluate the meat production performance and feeding effect in animals. Carcass and eviscerated percentages are the main indices to measure the meat production performance of livestock and poultry. It is generally considered that the carcass percentage is above 80%, the eviscerated percentage is above 60%, and the meat performance is good (Li et al., 2019). In this study, the carcass percentage of broiler chickens was 93.86%, and the eviscerated percentage was 75.57%, indicating that the performance of meat was better. Dietary FSP supplementation did not seem to substantially affect carcass characteristics, except for fat width, breast muscle percentage, breast muscle area and fat width index, breast muscle area index were significantly higher in the 0.15% supplementation group. The results of carcass characteristics in this study were in line with those obtained by AL-Sagan et al. (2020), who reported that the supplementation of 1.6% and 3.2% FSP did not show differences for carcass characteristics. Similar results were also reported by Mohammed & Abbas (2009) and El-Deek, Attia & Hannfy (2003).
In this study, the weight and length of the jejunum, the jejunal and ileal weight indices, and the ileal and total intestinal length indices were significantly higher in the 0.30% FSP supplementation group than in the other groups. This suggested that FSP promotes intestinal growth and development in broilers, which could be attributed to the improvement of the intestinal environment by the FSP. Nutrient digestion, absorption, and utilization mainly occur in the small intestine. Good intestinal morphology is essential for digestive function and to promote body growth and development. Intestinal villus height and crypt depth reflect the degree of development and function of the intestinal epithelium (Hampson, 1986). With increasing intestinal villus height, digestion and absorption improve, the incidence of diarrhea decreases, and growth and development accelerate. Shallower crypts indicate an increased rate of cell maturation and increased secretion (Beers-Schreurs et al., 1998). Reisinger et al. (2011) showed that dietary supplementation of essential oils of star anise, oregano, and citrus fruit significantly increased the recess depth and villus height in the ilea of broilers. Hong et al. (2012) found that adding star anise and oregano to the feed significantly increased duodenal villus height in broilers. In our study, when 0.30% FSP was added, duodenal villus height, ileal wall thickness, and ileal villus height were significantly increased. With increasing FSP supplementation level, the wall thickness and villus height in the jejunum and ileum gradually increased. There was no significant difference in crypt depth among the groups. This indicated that FSP supplementation promoted intestinal growth and development and improved intestinal morphology in broilers. Anise has been reported to improve the intestinal microbiota structure (Charal, 2014; Wang et al., 2015), and a similar effect may account for the effects of fennel on intestinal development and morphology, that is, certain active ingredients or unknown factors in fennel promote the growth of beneficial intestinal microorganisms in broilers. A healthy intestinal micro-ecosystem promotes normal intestinal tract development and ensures the integrity of the intestinal morphological structure. However, fennel showed different effects on different intestinal segments in broilers, which could be attributed to differences in the morphology and structure of each intestinal segment or the incomplete utilization of fennel components. This further affected the growth performance of broilers. Further research is needed to back up these findings.
The water-holding capacity of meat tissue is the ability of muscle tissue to retain water, and it is an important index to evaluate the quality of poultry meat. At present, water loss rate, drip loss and cooked meat rate are used to evaluate (Yue et al., 2015). In the present investigation, there is no differences were observed in the meat quality, the FSP-supplemented groups reduced breast muscle tenderness, which seemed to improve the edible quality. The pH of high-quality chicken meat is 6.0–6.5 (Hu, Zhu & Li, 2005). Except for the low pH in leg muscles in the MF group, the pH in LF and HF groups were in the normal range and higher than that in the control group. Too high a pH is harmful to the conversion of normal muscle to edible meat, and too low a pH will cause meat deterioration. Meat pH is related to the water-holding capacity (Brewer, 2004; Jung et al., 2010), meat with a high pH value has a higher water-holding capacity (Hu, Zhu & Li, 2005), which affects the texture, juiciness, and flavor of meat (AL-Sagan et al., 2020).
Conclusions
In conclusion, dietary supplementation of FSP, resulted in a beneficial impact on broiler digestion and absorption abilities, carcass traits and intestinal histological, especially at a concentration of 0.30%, which improved intestinal morphological development and thus promoting healthy and efficient development in Cobb broiler chickens. But more investigation is needed to determine the optimum concentration to be added to the poultry diets.