A multi-class classification model for supporting the diagnosis of type II diabetes mellitus
- Published
- Accepted
- Received
- Academic Editor
- Antonio Palazón-Bru
- Subject Areas
- Diabetes and Endocrinology, Public Health, Computational Science
- Keywords
- Diagnosis, Machine-learning techniques, Predictive models, Type 2 diabetes mellitus
- Copyright
- © 2020 Kuo et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. A multi-class classification model for supporting the diagnosis of type II diabetes mellitus. PeerJ 8:e9920 https://doi.org/10.7717/peerj.9920
Abstract
Background
Numerous studies have utilized machine-learning techniques to predict the early onset of type 2 diabetes mellitus. However, fewer studies have been conducted to predict an appropriate diagnosis code for the type 2 diabetes mellitus condition. Further, ensemble techniques such as bagging and boosting have likewise been utilized to an even lesser extent. The present study aims to identify appropriate diagnosis codes for type 2 diabetes mellitus patients by means of building a multi-class prediction model which is both parsimonious and possessing minimum features. In addition, the importance of features for predicting diagnose code is provided.
Methods
This study included 149 patients who have contracted type 2 diabetes mellitus. The sample was collected from a large hospital in Taiwan from November, 2017 to May, 2018. Machine learning algorithms including instance-based, decision trees, deep neural network, and ensemble algorithms were all used to build the predictive models utilized in this study. Average accuracy, area under receiver operating characteristic curve, Matthew correlation coefficient, macro-precision, recall, weighted average of precision and recall, and model process time were subsequently used to assess the performance of the built models. Information gain and gain ratio were used in order to demonstrate feature importance.
Results
The results showed that most algorithms, except for deep neural network, performed well in terms of all performance indices regardless of either the training or testing dataset that were used. Ten features and their importance to determine the diagnosis code of type 2 diabetes mellitus were identified. Our proposed predictive model can be further developed into a clinical diagnosis support system or integrated into existing healthcare information systems. Both methods of application can effectively support physicians whenever they are diagnosing type 2 diabetes mellitus patients in order to foster better patient-care planning.
Introduction
Diabetes mellitus (DM), as defined by the American Diabetes Association (2010), refers to a group of metabolic disorders primarily induced by impaired insulin secretion and/or action. Insulin deficiency and increased insulin resistance may lead to an elevated blood glucose level and impaired metabolism of carbohydrates, fat, and protein (American Diabetes Association, 2010). DM is one of the most prevalent endocrine disorders, influencing more than 200 million people universally (Kavakiotis et al., 2017). DM has therefore become a global public health challenge, and it is a key health concern worldwide. DM is expected to increase dramatically, and it could potentially be the seventh-leading reason of death in 2030 (World Health Organization, 2016). In terms of health-related issues, DM can lead to other serious medical complications such as chronic kidney disease, acute kidney injury, cardiovascular disease, ischemic heart disease, stroke or even to death (World Health Organization, 2016). The direct and indirect estimated total cost of diabetes management in the U.S. in 2012 was $245 billion and increased to $327 billion in 2017 (Centers for Diseases Control & Prevention, 2017). The burden of DM is rapidly increasing on a global basis and has become a major public health concern. On the other hand, despite the possibly-related complications, DM can be appropriately managed with a comprehensive care plan, such as with a reasonable lifestyle change and significant medication control (American Diabetes Association, 2015).
There are two prevalent types of DM, including type 1 diabetes and type 2 diabetes (T2DM), according to the etio-pathology of the disorder (Maniruzzaman et al., 2017). T2DM, accounting for 90% of DM patients, is the most common form of diabetes (Maniruzzaman et al., 2017). Several risk factors which include smoking, overweight and obesity, physical inactivity, high blood pressure, high cholesterol, and high blood glucose levels were reported to be associated with T2DM (Centers for Diseases Control & Prevention, 2017). However, the links between T2DM and some risk factors still remain unclear (Eckel et al., 2011). Currently, the diagnosis of T2DM can be based on elevated Hemoglobin A1c, high fasting or random plasma glucose, and a clinical manifestation of increased urinary frequency (polyuria), thirst (polydipsia), and hunger (polyphagia) (American Diabetes Association, 2010). However, it has been estimated that nearly 7.2 million people (23.8% of diabetes patients) remain undiagnosed in the United States (Centers for Diseases Control & Prevention, 2017). Hence, there is a rising need for related research to early identify and to confirm T2DM diagnosis more efficiently and accurately in clinical settings (Kagawa et al., 2017).
Information technologies such as machine-learning techniques have become a vital instrument in determining T2DM diagnosis and affecting management for health care providers and patients (Rigla et al., 2017). Numerous studies have utilized machine-learning techniques to predict the onset of T2DM. While previous DM prediction studies have shown a potential for detecting the onset of T2DM (Alghamdi et al., 2017; Anderson et al., 2015; Esteban et al., 2017; Kagawa et al., 2017; Maniruzzaman et al., 2017; Nilashi et al., 2017; Pei et al., 2019; Talaei-Khoei & Wilson, 2018; Upadhyaya et al., 2017; Wu et al., 2018), no studies, to our knowledge, have been aimed at predicting a suitable diagnosis code for T2DM patients. Further, ensemble machine-learning techniques such as bagging and boosting approaches are less utilized in these studies (Esteban et al., 2017). Most importantly, less multi-class studies, to our knowledge, have been conducted (Esteban et al., 2017). Therefore, the intended purpose of this study is to leverage routinely available clinical data in order to establish a multi-class predictive model based on bagging and boosting machine-learning techniques useful to identify Asian T2DM patients with a corresponding diagnosis code. The major contribution of our proposed predictive model is its ability to identify a corresponding ICD-10-CM code, not just to identify the onset of T2DM. The correct identification of ICD-10-CM code for T2DM can help physicians and patients form a proper patient-care plan, thus improving the conditions of T2DM patients while reducing the associated heavy financial burden caused by T2DM.
The remainder of this article is organized as follows: In section 2, we briefly introduce artificial neural networks, decision trees, ensemble models, and support vector machine. In section 3, we present the review of T2DM related studies that used machine-learning techniques. In section 4, we explain the methodology used for data collection, preparation, and analysis in this study. In section 5, we present the results and in section 6, we discuss the findings of this study. Finally, in section 7, we summarize and conclude this study.
Machine Learning Algorithms
Artificial neural network
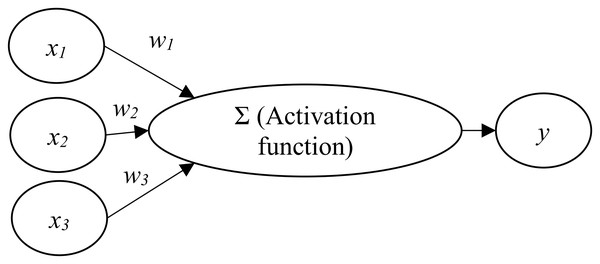
An artificial neural network (ANN) involves the development of models that enable computers to learn in ways similar to the human brain (Ciaburro & Venkateswaran, 2017; Larrañaga et al., 2019). An ANN is usually organized in layers which comprise a number of interconnected and weighted nodes (or neurons) (Clark, 2013; Lantz, 2015). To constitute an ANN, at least three layers, including an input layer, a hidden layer, and an output layer, should be included (Lewis, 2016).
Figure 1 shows the relations between input nodes (xi) and the output node (y). Each of the input nodes is weighted (wi) based on its importance (Beysolow, 2017). The input nodes are then summed and passed on according to the activation function (Clark, 2013; Lantz, 2015). An activation is the mechanism by which the artificial neuron handles incoming information and disseminates it all over the network (Lewis, 2016).
Figure 1: Artificial neural network architecture.
Decision trees
Decision trees utilize a tree structure to model the associations found among features and the possible outcomes (Provost & Fawcett, 2013). As Fig. 2 shows, a decision to be considered starts at the root node (Faul, 2020), and a decision is made based on the questions of whether the value is higher or lower than a threshold (Brownlee, 2017). These decisions then split the data across branches indicating likely outcomes of a decision (Clark, 2013). If a final decision can be reached, the tree is terminated by terminal nodes (Faul, 2020). There are many implementations of decision trees, one of the most famous is the C5.0 algorithm, an improvement of C4.5 algorithm (Quinlan, 1996), and has become a de-facto standard to create decision trees.
Figure 2: Decision trees.
Ensemble model (bagging and boosting)
The technique of merging and managing the predictions of multiple models is known as an ensemble approach (Lantz, 2015). More specifically, ensemble methods are hinged on the notion that by merging multiple weaker learners, a stronger learner is generated (Clark, 2013). Bagging and boosting are widespread accepted ensemble methods currently.
Bagging
One of the ensemble approaches to receive widely acknowledgement adopted a technique named bootstrap aggregating or bagging, to generate a number of datasets for training by bootstrap sampling from the primitive training dataset (Lantz, 2015). These data are then utilized to create a set of models with each incorporating only one classifier. Averaging (for numeric prediction) or voting (for classification) are used to determine the model’s terminal predictions (Beysolow, 2017; Clark, 2013). Among many bagging classifiers, random forest, a combination of several decision trees (Beysolow, 2017), merges the basic rules of bagging with random feature selection to increase additional variety to the building of the models. After the ensembles of trees is created, the model utilizes a vote to merge the tree’s predictions (Beysolow, 2017).
Boosting
Another ensemble-based method is known as boosting since it is a method of boosting weak learners to become strong learners (Beysolow, 2017; Brownlee, 2017; Faul, 2020). In boosting, each new tree is a fit on an adjusted version of the primitive dataset. Different from bagging, boosting resampled datasets are constructed to generate complementary learners, and boosting gives each learner’s vote a weight based on its past performance (Lantz, 2015).
Among the many boosting classifiers, eXtreme gradient boosting (Chen & Guestrin, 2016) is one of the most popular applications of gradient boosting concept. This classifier is basically designed to enhance the performance and speed of a machine learning model. What makes eXtreme gradient boosting peculiar is that it utilizes a more regularized model formalization to regulate over-fitting, which thus gives it better performance (Lantz, 2015).
Support vector machine
A support vector machine (SVM), an instance-based algorithm, tries to maximize the margin between two classes by using kernel function (Marsland, 2015). In other words, SVM creates a boundary called a hyperplane (Beysolow, 2017) and tries to search for the maximum margin hyperplane (Brownlee, 2017), which breaks the space to create the best homogenous partitions on two different classes (see Fig. 3). The support vectors are the points from each class that are the nearest to the maximum margin hyperplane, which is a key feature of SVMs (Lantz, 2015). SVMs can be utilized along with almost any type of learning task, including numeric prediction and classification (Kuhn & Johnson, 2013).
Figure 3: Margin hyperplane, support vector, and convex hull.
Materials and Methods
Data
Diagnosing T2DM depends primarily on laboratory test results (American Diabetes Association, 2010), we therefore required a collection of those data from T2DM patients. A plausible T2DM patient list was first obtained, containing patients who had visited an endocrinologist (one of our authors) between November, 2017 and May, 2018 at a large hospital in southern Taiwan. The Institutional Review Board of E-Da Hospital approved our study protocol and waived informed consent regarding this study (EMRP-107-048). In consideration of the features to be included, we elected to adopt 10 common features based on our review of prior studies related to DM prediction models (Anderson et al., 2015; Pei et al., 2019; Talaei-Khoei & Wilson, 2018; Wu et al., 2018). These readily available features can be drawn directly or indirectly from the content of Electronic Medical Records. By doing so, the predictive model we proposed can be adopted by most hospitals since these selected features are already stored in existing databases.
The 10 health-related features can be primarily classified into two categories: demographic data and laboratory test results. Demographic data included age, gender, smoking status, and BMI which were reported to be associated with the onset of T2DM (Yuan et al., 2018). On the other hand, laboratory data are comprised of total cholesterol, triglyceride, glucose (AC), Hemoglobin A1c, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol which were indicators of impaired metabolic function pre-disposing DM (Guasch-Ferré et al., 2016).
Eligibility criteria for the study were that a patient must (1) be diagnosed through an international classification of diseases, tenth revision, clinical modification (ICD-10-CM) starting with E11, and (2) no missing data in total cholesterol, triglyceride, glucose (AC), Hemoglobin A1c, high-density lipoprotein cholesterol, and low-density lipoprotein cholesterol was evident. Initially, a total of 10,527 plausible T2DM patient information were obtained and duplicated patient listings were first removed. Patients with missing laboratory test results were then removed. Since there may be many ICD-10-CM codes utilized for diagnosing T2DM, we limited our predicted classes to the first five digits of the ICD-10-CM code; and as such, these five-digit codes must be among the top ICD-10-CM codes appearing in our collected data. Finally, 149 eligible records, including E1121 (T2DM with diabetic nephropathy, n = 45), E1143 (T2DM with diabetic autonomic [poly]neuropathy, n = 88), and E1165 (T2DM with hyperglycemia, n = 16), without missing values were collected.
Our inclusion of these 10 features primarily differs from prior T2DM-related studies in that we only included demographic data and laboratory test results, while prior T2DM studies included a wider variety of data. In words, we aimed to build a parsimonious predictive model possessing minimum features. Table 4 shows the detailed operational definition of features used in our study.
| Features/Target class | Measurement | Definition | References | |
|---|---|---|---|---|
| Target class | Diagnosis of T2DM | Discrete | The probability of four kinds of T2DM diagnosis: E1121, E1143, and E1165 | NA |
| Features | Gender | Discrete | Gender of the patients, Male or Female. | Anderson et al. (2015), Pei et al. (2019), Wu et al. (2018) |
| Age | Continuous | Age (in years) during out-patient services | Anderson et al. (2015), Pei et al. (2019), Talaei-Khoei & Wilson (2018) | |
| Smoking status | Discrete | Yes, quit, or no | ||
| BMI | Continuous | Body mass index | Anderson et al. (2015), Pei et al. (2019), Wu et al. (2018) | |
| Total Cholesterol | Continuous | The level of total cholesterol during out-patient services | ||
| Triglyceride | Continuous | The level of triglyceride during out-patient services | Anderson et al. (2015), Talaei-Khoei & Wilson (2018) | |
| Glucose (AC) | Continuous | The level of glucose (AC) during out-patient services | Anderson et al. (2015) | |
| Hemoglobin A1c | Continuous | The level of Hemoglobin A1c during out-patient services | Anderson et al. (2015), Kagawa et al. (2017), Talaei-Khoei & Wilson (2018) | |
| High density lipoprotein cholesterol | Continuous | The level of high-density lipoprotein cholesterol during out-patient services | Anderson et al. (2015), Talaei-Khoei & Wilson (2018) | |
| Low density lipoprotein cholesterol | Continuous | The level of low-density lipoprotein cholesterol during out-patient services | Anderson et al. (2015) | |
Experimental setup
To predict a diagnosis code for the T2DM patient, we adopted R 4.0.0 software (R Core Team, 2020) for purposes of data analysis. Since our data is non-linear, machine-learning techniques are well-suited for predicting the ICD-10-CM code of T2DM. Based on the methodological gaps found in our review of T2DM related studies, we decided to choose five machine-learning algorithms including instance-based (Support vector machine), decision trees (C5.0), deep neural network, and ensemble (Random forest and eXtreme gradient boosting) as primary learners in our study.
We used the mlr 2.17.1 package (Bischl et al., 2016) to automatically tune the optimal model parameters for these four learners aiming to obtain a better level of predictive performance. The R packages used for machine-learning algorithms and their respective optimal model parameters are shown in Table 5. Further, since our predicted class is imbalanced, we utilized a synthetic minority over-sampling technique provided by UBL package (Branco, Ribeiro & Torgo, 2016) in order to improve the model performance.
| Method | Parameters | Best parameter setting | R packages |
|---|---|---|---|
| Support vector machine | sigma | 0.664667494 | kernlab 0.9-29 |
| C | 11.07262251 | ||
| C5.0 | winnow | FALSE | C50 0.1.3 |
| trials | 43 | ||
| Deep neural network | hidden | 200 | h2o 3.30.0.1 |
| input_dropout_ratio | 0 | ||
| activation | Maxout | ||
| eXtreme gradient boosting | nrounds | 154 | xgboost 1.0.0.2 |
| max_depth | 10 | ||
| eta | 0.745922343 | ||
| gamma | 3.194824195 | ||
| colsample_bytree | 0.945590117 | ||
| min_child_weight | 3.35705624 | ||
| subsample | 0.802348509 | ||
| Random Forest | mtry | 2 | randomForest 4.6-14 |
We adopted: (1) 10-fold cross-validation; (2) leave-one-subject-out; and (3) holdout approaches to assess the performance of the five learners. The 10-fold cross-validation approach randomly splits the dataset into 10 subsets with roughly similar sizes, among which nine subsets are used for constructing the model and the remaining one subset is utilized for testing the model (Provost & Fawcett, 2013). Leave-one-subject-out cross-validation is a special case of k-fold cross-validation since k is the number of samples while holdout simply splits data into training samples for building the predictive model and testing samples for estimating model performance (Kuhn & Johnson, 2013).
Performance metrics
To better evaluate the performance of a multi-class setting, we employed average accuracy, area under receiver operating characteristic (AUC), Matthew correlation coefficient (MCC), and the macro-averaging of precision, recall, and F1 score (weighted average of precision and recall) according to the suggestions taken from the literature (Sokolova & Lapalme, 2009). These metrics were measured based on a confusion matrix (see Table 6).
| Predicted class | |||
|---|---|---|---|
| Positive | Negative | ||
| Actual class | Positive | True positive (TP) | False negative (FN) |
| Negative | False positive (FP) | True negative (TN) | |
The average accuracy, MCC, micro- and macro-averaging precision, recall, and F1 score were then acquired using the formulae located in Table 7.
| Metric | Formula |
|---|---|
| Average accuracy | |
| Matthew correlation coefficient | |
| PrecisionM | |
| RecallM | |
| F1 scoreM |
Note:
l denotes class levels, M denotes macro-averaging metrics, TP means true positive, FP denotes false positive, FN means false negative, and TN denotes true negative.
Regarding the interpretation of these metrics, the average accuracy, AUC, MCC, macro-averaging and micro-averaging precision, recall, and F1 score value between 0 and 1, with values approaching 1, imply better performance.
Results
Data profiles
Table 8 demonstrates the descriptive statistics for T2DM patients. Among these figures, the proportion of the male sample is higher than that of female, aged 21–91 years, and most samples did not smoke, or had quit smoking, at the time of survey administration. Furthermore, the average BMI of samples belonging to the “obesity” level, and the average levels of glucose (AC) and Hemoglobin A1c are higher than the normal values. On average, other laboratory test results fall inside the normal range.
| Feature | Range | Summary statistics |
|---|---|---|
| Gender | Male/Female | Male: 86, Female: 63 |
| Age | 21~91 | M = 61.27, SD = 13.70 |
| Smoking status | No/Quit/Yes | No = 123, Quit = 10, Yes = 16 |
| BMI | 15.49~44.05 | M = 26.63, SD = 4.77 |
| Total cholesterol | 77~311 | M = 151.98, SD = 34.38 |
| Triglyceride | 37~546 | M = 136.64, SD = 93.30 |
| Glucose (AC) | 68~346 | M = 146.58, SD = 51.72 |
| Hemoglobin A1c | 5.1~11.6 | M = 7.46, SD = 1.21 |
| High density lipoprotein cholesterol | 16~98 | M = 47.44, SD = 14.87 |
| Low density lipoprotein cholesterol | 29~152 | M = 71.42, SD = 25.66 |
Note:
M denotes mean and SD means standard deviation.
Model performance
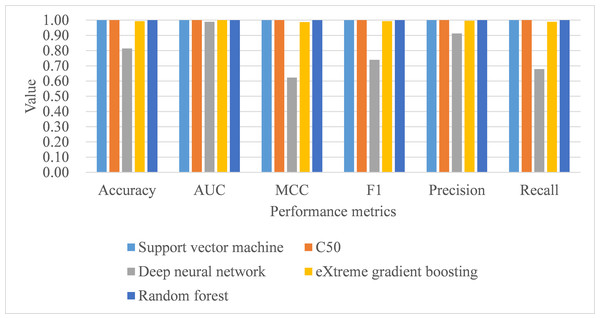
Under 10-fold cross-validation, the performance of support vector machine ranked the highest in accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall metrics with training samples (see Table 9). This was followed by random forest, C5.0, deep neural network, and eXtreme gradient boosting. Further, the process time for training the support vector machine was also the shortest compared to the remaining algorithms. When comparing the performance of the five trained models in the test samples, support vector machine, C5.0, and random forest perfectly achieved one in accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall metrics (see Table 9; Fig. 4). eXtreme gradient boosting learner also achieved higher than 0.9 in all metrics. Deep neural network however performed poorer than the other four learners in all metrics. We then compared the model performance by use of the Stuart–Maxwell test which is better suited for multi-class classification models than McNemar test (Maxwell, 1970; McNemar, 1947; Stuart, 1955). Since support vector machine, C5.0, and random forest perfectly predicted ICD-10-CM codes used for T2DM, we only statistically compared the performance of deep neural network and eXtreme gradient boosting learners. The Stuart–Maxwell tests demonstrated significant results for both deep neural network (p < 0.001) and eXtreme gradient boosting (p = 0.002), thus indicating significant difference disagreement between these two algorithms and the observed data.
| Sample | Learner | Accuracy (SD) | AUC (SD) | MCC (SD) | Macro | Process time | Stuart–Maxwell test | ||
|---|---|---|---|---|---|---|---|---|---|
| F1 (SD) | Precision (SD) | Recall (SD) | |||||||
| Train | SVM | 0.998 (0.006) | 1.000 (0.000) | 0.995 (0.011) | 0.994 (0.012) | 0.997 (0.008) | 0.991 (0.015) | 2.22 | |
| C5.0 | 0.984 (0.015) | 0.999 (0.001) | 0.969 (0.031) | 0.981 (0.020) | 0.987 (0.015) | 0.975 (0.026) | 6.74 | ||
| DNN | 0.947 (0.019) | 0.985 (0.016) | 0.896 (0.033) | 0.935 (0.027) | 0.956 (0.031) | 0.922 (0.028) | 13.56 | ||
| XGB | 0.943 (0.021) | 0.992 (0.008) | 0.885 (0.044) | 0.918 (0.050) | 0.946 (0.036) | 0.894 (0.058) | 7.86 | ||
| RF | 0.986 (0.010) | 1.000 (0.000) | 0.972 (0.017) | 0.985 (0.011) | 0.992 (0.006) | 0.978 (0.016) | 4.59 | ||
| Test | SVM | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| C5.0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| DNN | 0.855 | 0.985 | 0.730 | 0.678 | 0.876 | 0.684 | χ2(3) = 253.20, p < 0.001 | ||
| XGB | 0.989 | 1.000 | 0.979 | 0.985 | 0.992 | 0.978 | χ2(2) = 13.00, p = 0.002 | ||
| RF | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
Note:
AUC, area under receiver operating characteristic; SD, standard deviation; MCC, Matthew correlation coefficient; SVM, support vector machine; DNN, deep neural network; XGB, eXtreme gradient boosting; RF, random forest, the second is used to measure process time.
Figure 4: Model performance of test dataset—10-fold cross-validation.
AUC, area under receiver operating characteristic curve; MCC, Matthew correlation coefficient.Under leave-one-subject-out cross-validation, both support vector machine and random forest performed better than the remaining classifiers, with training samples, in terms of all metrics, including process time (see Table 10). As for the model performance of testing samples, support vector machine, C5.0, and random forest perfectly achieved one in accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall metrics (see Table 10; Fig. 5). Deep neural network and eXtreme gradient boosting still did not perform as well as the remaining classifiers. Stuart–Maxwell tests were then conducted for deep neural network and eXtreme gradient boosting. And, the results revealed that deep neural network still showed significant difference with the observed data (p < 0.001) while eXtreme gradient boosting showed insignificant difference with the observed data (p = 0.06).
| Sample | Learner | Accuracy (SD) | AUC (SD) | MCC (SD) | Macro | Process time | Stuart–Maxwell test | ||
|---|---|---|---|---|---|---|---|---|---|
| F1 (SD) | Precision (SD) | Recall (SD) | |||||||
| Train | SVM | 0.999 (0.000) | 1.000 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 280.67 | |
| C5.0 | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 879.37 | ||
| DNN | 0.984 (0.004) | 0.998 (0.001) | 0.968 (0.008) | 0.981 (0.005) | 0.983 (0.004) | 0.979 (0.005) | 2145.94 | ||
| XGB | 0.992 (0.002) | 0.999 (0.000) | 0.985 (0.005) | 0.990 (0.004) | 0.994 (0.003) | 0.986 (0.005) | 1028.34 | ||
| RF | 0.999 (0.000) | 1.000 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 0.999 (0.000) | 639.22 | ||
| Test | SVM | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| C5.0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| DNN | 0.893 | 0.996 | 0.802 | 0.797 | 0.902 | 0.781 | χ2(3) = 87.45, p < 0.001 | ||
| XGB | 0.993 | 0.999 | 0.985 | 0.989 | 0.994 | 0.985 | χ2(2) = 5.67, p = 0.06 | ||
| RF | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
Note:
AUC, area under receiver operating characteristic, SD, standard deviation, MCC, Matthew correlation coefficient, SVM, support vector machine, DNN, deep neural network, XGB, eXtreme gradient boosting, RF, random forest, the second is used to measure process time.
Figure 5: Model performance of test dataset—Leave-one-subject-out cross-validation.
AUC, area under receiver operating characteristic curve; MCC, Matthew correlation coefficient.Under hold-out cross-validation, support vector machine still performed better than the remaining classifiers, with training samples, in terms of all metrics, including process time (see Table 11; Fig. 6). Deep neural network and eXtreme gradient boosting still did not perform as well as the remaining classifiers. The Stuart–Maxwell tests demonstrated significant results for both deep neural network (p < 0.001) and eXtreme gradient boosting (p = 0.018), indicating significant difference disagreement between these two algorithms and the observed data.
Figure 6: Model performance of test dataset—Holdout cross-validation.
AUC, area under receiver operating characteristic curve; MCC, Matthew correlation coefficient.| Sample | Method | Accuracy | AUC | MCC | Macro | Process time | Stuart–Maxwell test | ||
|---|---|---|---|---|---|---|---|---|---|
| F1 | Precision | Recall | |||||||
| Train | SVM | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 0.23 | |
| C5.0 | 0.950 | 0.996 | 0.903 | 0.933 | 0.948 | 0.920 | 0.59 | ||
| DNN | 0.970 | 0.997 | 0.940 | 0.954 | 0.970 | 0.939 | 1.59 | ||
| XGB | 0.886 | 0.974 | 0.775 | 0.809 | 0.869 | 0.770 | 0.75 | ||
| RF | 0.978 | 1.000 | 0.957 | 0.980 | 0.989 | 0.972 | 0.39 | ||
| Test | SVM | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | ||
| C5.0 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
| DNN | 0.814 | 0.989 | 0.623 | 0.739 | 0.913 | 0.676 | χ2(3) = 205.04, p < 0.001 | ||
| XGB | 0.993 | 1.000 | 0.987 | 0.993 | 0.996 | 0.989 | χ2(2) = 8.00, p = 0.018 | ||
| RF | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | 1.000 | |||
Note:
AUC, area under receiver operating characteristic; SD, standard deviation; MCC, Matthew correlation coefficient; SVM, support vector machine; DNN, deep neural network; XGB, eXtreme gradient boosting; RF, random forest; the second is used to measure process time.
Feature importance
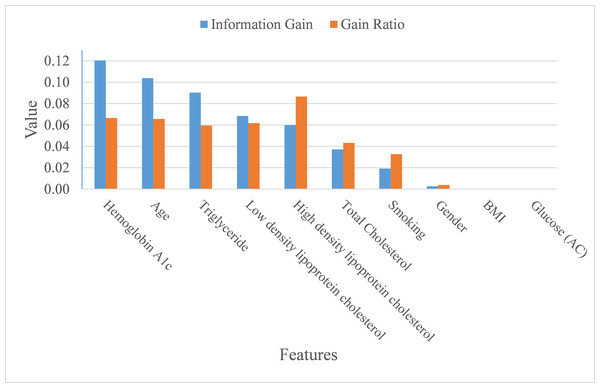
In addition to making a comparison of the performance for the four prediction models, we also ranked the feature importance based on information gain and gain ratio (see Fig. 7). Information gain can be biased if features have a large number of possible outcomes, which may be corrected by gain ratio criteria (Kuhn & Johnson, 2013). From the perspective of information gain, or precisely how much a feature improves entropy (a measure of disorder), Hemoglobin A1c, age, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and total cholesterol ranked as the top six most important features for predicting ICD-10-CM code. After correcting for possible bias, High-density lipoprotein cholesterol, Hemoglobin A1c, age, low-density lipoprotein cholesterol, triglyceride, and total cholesterol ranked as the top six important features. The greatest difference in the rankings, based upon information gain and gain ratio, is high-density lipoprotein cholesterol, ranked 5th by information gain, but ranked 1st by gain ratio. Further, BMI and glucose did not contribute anything to the class prediction of ICD-10-CM code for T2DM.
Figure 7: Importance of features.
Discussion
As mentioned at the beginning of our study, T2DM should be considered as a catastrophic threat to public health that is accompanied by huge financial and personal costs following the onset of T2DM. Therefore, obtaining the means of how to correctly diagnose T2DM patients in order to foster appropriate medical care for T2DM patients is inevitable and of great importance to the health-care profession. This study aimed to build an appropriate model for predicting ICD-10-CM code by utilizing bagging and boosting ensemble techniques for Asian T2DM patients. Our proposed model, based on support vector machine, performed well in terms of average accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall. Based on information gain and gain ratio, our study also distinguished and ranked the top eight variables, including Hemoglobin A1c, age, triglyceride, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and total Cholesterol, along with the habit of smoking, to predict ICD-10-CM codes for T2DM patients.
Although the performance metrics are not entirely consistent among T2DM-related studies that used machine-learning technique, it is still worthwhile to make a comparison between the current study and those studies with available performance metrics (see Table 12). Support vector machine was the best classifier in our study with accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall metrics all equal to one. Prior studies utilized support vector machine also performed well but only in some metrics. For example, the study of Pei et al. (2019) achieved 0.908 accuracy rate for diabetes classification with non-invasive and easily gathered features. Talaei-Khoei & Wilson (2018) used machine-learning techniques to identify people at risk of developing T2DM and found the MCC metric of support vector machine was 0.922. Kagawa et al. (2017) combined expert knowledge and machine-learning approaches to determine whether a patient has T2DM. Among the five classifiers adopted, support vector machine achieved 0.909 in recall metric.
| Algorithms | Study | Accuracy | AUC | MCC | Precision | Recall | F1 score |
|---|---|---|---|---|---|---|---|
| Support vector machine | This study | 1 | 1 | 1 | 1 | 1 | 1 |
| Pei et al. (2019) | 0.908 | 0.763 | NA | 0.903 | 0.908 | 0.905 | |
| Talaei-Khoei & Wilson (2018) | NA | 0.831 | 0.922 | NA | 0.683 | NA | |
| Kagawa et al. (2017) | NA | NA | NA | 0.8 | 0.909 | NA | |
| Neural network | This study | 0.788 | 0.986 | 0.566 | 0.910 | 0.620 | 0.684 |
| Talaei-Khoei & Wilson (2018) | NA | 0.663 | 0.007 | NA | 0.41 | NA | |
| Nilashi et al. (2017) | 0.923 | NA | NA | NA | NA | NA | |
| Esteban et al. (2017) | NA | NA | NA | 0.930 | 0.960 | 0.940 | |
| Random forest | This study | 1 | 1 | 1 | 1 | 1 | 1 |
| Alghamdi et al. (2017) | 0.840 | NA | NA | 0.844 | 0.994 | 0.913 |
Note:
AUC, area under receiver operating characteristic; MCC, Matthew correlation coefficient; NA, not available.
Regarding studies that adopted neural network classifier, the study of Nilashi et al. (2017) achieved 0.923 accuracy rate while the study of Esteban et al. (2017) achieved 0.93 and 0.96 for precision and recall metrics, respectively. Finally, random forest also perfectly predicted ICD-10-CM code in our study for accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall metrics. Alghamdi et al. (2017) adopted random forest to predict T2DM and achieved 0.844 and 0.994 for precision and recall, respectively. The reason that random forest performed quite well in our study may be due to the fact that random forest averages over multiple predictions to reduce the variance in the predictions (Provost & Fawcett, 2013).
Several interesting points can be derived from our findings as a whole. First, as suggested by prior literature (Lantz, 2015), our proposed predictive model implementing ensemble method (i.e., random forest and eXtreme gradient boosting) has performed, despite not being the best, satisfactorily with average accuracy, AUC, MCC, macro-averaging F1 score, macro-averaging precision, and macro-averaging recall being higher than 0.97 for all metrics among three resampling strategies. Future research may prove to implement these techniques that will lead to improved model predictive power.
Second, by using Asian samples, the findings determined in our study can be further compared with prior similar studies, and attention can be placed on the differences. For example, the link between obesity and T2DM remains uncertain (Eckel et al., 2011), BMI ranked the ninth important feature for predicting T2DM diagnosis in terms of both information gain and gain ratio. Future research can further explore why and how this difference comes to exist between eastern and western population samples.
Third, differing from most prior studies, our proposed models aimed to predict a multi-class classification task, which may provide more accurate predictions over and above binary classification tasking (Zhou, Tam & Fujita, 2016) since there may be numerous features that specifically identify a certain category. It is therefore of practical significance to apply a multi-class classification approach useful to predict ICD-10-CM code for T2DM patients.
Finally, our predictive model can be further developed into a clinical diagnosis support system, or even better when integrated into existing healthcare information systems aiming to support physicians, when diagnosing T2DM patients. By means of such a support system/function, physicians can better diagnose and foster medical care plans for T2DM patients to follow. The ability to predict disease sub-categories may assist and further remind physicians to early detect and manage possible complications in the earliest stages of disease onset.
One of the most important limitations found in our study is that we utilized only three ICD-10-CM codes pertinent to T2DM for predictive purposes. There are in fact many ICD-10-CM codes available for T2DM diagnosis and care; so, it is possible for future research to increase the number of ICD-10-CM codes in the predicted class in order to broaden diagnostic applications. In order to ensure as complete a data set as possible in building our model, we were required to remove those samples with missing data which resulted in only useable 149 samples extant. Future studies may choose to increase the sample size in order to enhance external the generalizability of the findings.
Conclusions
Our study adopted machine-learning techniques using 10 features adapted from Electronic Medical Records for identifying diagnosis code for T2DM patients. By adopting 10-fold, leave-one-subject-out, and holdout resampling strategy, support vector machine and random forest showed the best classification metrics in identifying an ICD-10-CM code for the test samples. These results demonstrated that our established model successfully achieved predictive and wholly appropriate ICD-10-CM code for T2DM patients to use. The implementation of our established predictive model in conjunction with using machine-learning algorithms along with data from Electronic Medical Records enables an in-depth exploration toward supporting diagnosis of T2DM patients. This approach may be easily applied within healthcare facilities which have implemented complete electronic medical record-keeping.