The preclinical pharmacological study on HX0969W, a novel water-soluble pro-drug of propofol, in rats
- Published
- Accepted
- Received
- Academic Editor
- Zunnan Huang
- Subject Areas
- Anesthesiology and Pain Management, Pharmacology
- Keywords
- Intravenous anesthetics, Water-soluble propofol, Pharmacodynamics, Pharmacokinetics, HX0969W
- Copyright
- © 2020 Zhang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2020. The preclinical pharmacological study on HX0969W, a novel water-soluble pro-drug of propofol, in rats. PeerJ 8:e8922 https://doi.org/10.7717/peerj.8922
Abstract
Background
Propofol is the most widely used intravenous sedative-hypnotic anesthetic in clinical practice. However, many serious side effects have been related to its lipid emulsion formulation. The pro-drug design approach was used to develop the water-soluble propofol, which could effectively resolve the limitations associated with the lipid emulsion formulation. Thus, the new water-soluble pro-drug of propofol, HX0969W, was designed and synthesized. The objective of this study was to conduct preclinical pharmacological studies on this novel water-soluble pro-drug of propofol.
Methods
The assessment of the loss of the righting reflex (LoRR) was used for the pharmacodynamic study, and liquid chromatography-tandem mass spectrometry and high-performance liquid chromatography- fluorescence were used for the pharmacokinetic study.
Results
The potency of HX0969W (ED50 [95% CI], 46.49 [43.89–49.29] mg/kg) was similar to that of fospropofol disodium (43.66 [43.57–43.75] mg/kg), but was lower than that of propofol (4.82 [4.8–14.82] mg/kg). Administered with a dose of 2-fold ED50, propofol required a shorter time to cause LoRR than that of HX0969W and fospropofol. However, the LoRR duration was significantly longer in response to the administration of HX0969W and fospropofol disodium than that caused by propofol. In the pharmacokinetic study, the Cmax of fospropofol was higher than that of HX0969W. HX0969W had a shorter mean residual time and a rapid clearance rate than that of fospropofol disodium. There was no significant difference between the Tmax of the propofol whether it was released by HX0969W or fospropofol disodium; the Cmax of propofol released by HX0969W was similar to that of propofol, which was higher than the propofol released by fospropofol disodium.
Introduction
General anesthesia is a combination of drugs, including sedative-hypnotic agents, analgesics, or muscle relaxants, which put the patients in a sleep-like state before surgery or other medical examinations. Propofol is the most widely used intravenous sedative-hypnotic anesthetic in clinical practice (Hemphill et al., 2019). However, many serious side effects have been related to its lipid emulsion formations, such as emulsion instability, injection pain, hyperlipidemia, infection, fat metabolism disorder, and propofol-related infusion syndrome (Diaz et al., 2014; Lee et al., 2014; Mirrakhimov et al., 2015; Pestana, Garcia-de Lorenzo & Madero, 1996; Prankerd & Stella, 1990; Singh, Jindal & Singh, 2011; Wachowski et al., 1999; Wolf et al., 2001; Zhou et al., 2015). The pro-drug design approach was widely used to develop the novel water-soluble propofol, which could effectively avoid the limitations associated with its lipid emulsion formulation (Feng et al., 2017).
Fospropofol disodium is the first water-soluble pro-drug of propofol that has been approved by the U.S. Food and Drug Administration to be administered as a bolus injection for adult patients undergoing diagnostic or therapeutic procedures (Moore, Walker & MacLaren, 2009; Telletxea et al., 2012). Although it is confirmed that formaldehyde, which is one of the metabolites of fospropofol disodium, does not accumulate after a single administration (Garnock-Jones & Scott, 2010; Kumpulainen et al., 2008), it is still considered a potentially risk for systemic toxicity and has limited the clinical indication of fospropofol disodium for continuous infusion. A new water-soluble pro-drug of propofol was designed and synthesized in our laboratory (patent number, WO2011160268; denoted as HX0969W), which was metabolized to propofol, γ-hydroxybutyrate (GHB), and phosphate in vivo. GHB was found to be further converted into carbon dioxide and water (Lang et al., 2014; Rousseau et al., 2012). In contrast to fospropofol disodium, HX0969W cloud produce an effective sedative-hypnotic effect with lesser potential risks of systemic toxicity.
In the present study, we evaluated the median effective dose (ED50) of HX0969W, fospropofol disodium, and propofol for the loss of righting reflex (LoRR) in rats using the up and down method. Then, we measured the onset time and duration for HX0969W, fospropofol disodium and propofol after single intravenous injections. Furthermore, the pharmacokinetic parameters of the drugs were assessed in rats.
Material and Methods
Materials
HX0969W and fospropofol disodium were synthesized at Yichang Humanwell Pharmaceutical Co., Ltd. (Yichang, China). Propofol was purchased from AstraZeneca (Shanghai, China). 7-Hydroxycoumarin, thymol, and ammonium acetate were purchased from Sigma-Aldrich Co., Ltd. (MO, USA). Acetonitrile and methanol were obtained from ROE Scientific Inc. (DE, USA). Ultrapure water was produced using Milli-Q® integral water purification system (Merck Millipore, Germany).
Experimental animals
Adult Sprague-Dawley rats (age: 8–10 weeks, body weight: 220–350 g) were purchased from Dossy Biological Technology Co., Ltd. (Chengdu, China) and housed in polypropylene cages (less than 5 animals per cage) at the Experimental Animal Center of Sichuan University (Chengdu, China) at an ambient temperature of 25 ± 1 ° C, a controlled humidity of 50%–70%, and a 12-h light-dark cycle (7 a.m. to 7 p.m.). The rats were provided with a radiation-sterilized commercial diet and filtered water ad libitum. The rats were subjected to fasting for 12 h with uncontrolled water supply prior to initiation of dosing; food was supplied immediately after dosing. The experimental interventions and sample collections in this study were accomplished through the tail vein after venipuncture (Terumo® intravenous catheter; 24G, Tokyo, Japan). Thus, no anesthetic procedure was used in this study. At the end of experiments, all rats were euthanized via overdose of pentobarbital sodium. All experiments were performed with the approval of the Committee of Scientific Research and the Institutional Animal Experimental Ethics Committee of West China Hospital, Sichuan University, Chengdu, China (2015015A).
Measurement of the hypnotic median effective dose
The ED50 values of HX0969W, fospropofol disodium, and propofol were determined by the up and down method (Dixon, 1991; Glen & Hunter, 1984; Kilpatrick et al., 2007). The tail vein was cannulated with a Terumo® intravenous catheter (24G; Tokyo, Japan) for drug administration. After administered the drug, the rats were assessed one by one in separate box for the LoRR effect (once the rat lay prone and no reaction to mild stimuli, the rat was gently changed to the supine position recognized as LoRR), and were provided with warm blanket and oxygen inhalation to reduce external stimulation. If the duration of LoRR after drug administration was more than 30 s, the next rat was administered with a lower dose. On the contrary, if the duration of LoRR was less than 30 s (none-LoRR), a higher dose was injected in the next rat. Recording the LoRR to none-LoRR as a cross, the measurement of the ED50 value was terminated once more than five crosses occurred. An accurate ED50 value with 95% confidence intervals (CI) was calculated using standard computing equations (Dixon, 1991; Glen & Hunter, 1984; Kilpatrick et al., 2007).
Pharmacodynamic study in rats
Thirty adult Sprague-Dawley rats were randomly divided into three groups to evaluate the efficacy of HX0969W, fospropofol disodium, and propofol (n = 10 in each group, 5 males and 5 females). The drugs were administered via tail vein through the Terumo® intravenous catheter (24G; Tokyo, Japan) at equivalent doses (2-fold dose of ED50 for LoRR in rats) at an injection speed of 0.25 mL/s. Then, each rat was placed in a separate box with a warm blanket and oxygen inhalation. For the pharmacodynamic study, the time to LoRR (onset time) and time to recovery from LoRR (duration) were recorded. All rats were observed closely for any mortality, behavioural changes, and clinical symptoms of toxicity.
Pharmacokinetic study in rats
Before the experiment, the rats were subjected to fasting for 12 h with uncontrolled water supply; food was supplied immediately after drug administration. The tail vein was cannulated with a Terumo® intravenous catheter (24G; Tokyo, Japan) for drug administration and sample collection. HX0969W, fospropofol disodium, and propofol were injected at their equivalent doses in the rats through their tail veins (n = 10 in each group, 5 males and 5 females). The blood samples from the rats were collected at different times, which were determined based on the results of the preliminary experiments. For HX0969W and fospropofol disodium, 50 µL blood samples were collected at 1, 2, 3, 4, 5, 7, 10, 15, 20, 30, 45, 60, 90, 120, 180, and 240 min after drug administration. For propofol, 50 µL blood samples were collected at 0.5, 1, 2, 3, 5, 7, 10, 15, 20, 30, 45, 60, 90, 120, and 180 min after drug administration.
Sample preparation and quantitative method
Blood samples from the HX0969W and fospropofol disodium groups were deproteinized by methanol solution with 7-Hydroxycoumarin (internal standard, IS). The mixture was centrifuged for 10 min at 25,000 × g at 4 °C after vortexing. The supernatant was then subjected to determination and quantification by liquid chromatography-tandem mass spectrometry (LC-MS/MS). The LC-MS/MS analysis system consisted of an Agilent 6460 triple quadrupole mass spectrometer (Agilent Technologies, CA, USA) with an electrospray ionization source. Chromatographic separation was performed using a Zorbax eclipse plus C8 column (100 mm × 2.1 mm, 3 µm) at 12 °C with 5 mM ammonium acetate in deionized water and acetonitrile at a volume ratio of 65: 35 for HX0969W and 70: 30 for fospropofol disodium, at a flow rate of 0.3 mL/min. The mass spectrometry conditions were as follows: negative ionization mode; the sheath gas flow rate, 5.0 l/min; sheath gas heater temperature, 350 ° C; nebulizer pressure, 30 psi; capillary voltage, 4,500 V; m/z 343.0 →177.0 for HX0969W, m/z 287.00 →177.00 for fospropofol disodium, and m/z 160.7 →89.0 for IS.
For propofol, the samples were deproteinized with a methanol solution with thymol (IS). The mixture was centrifuged for 10 min at 25,000 × g at 4 °C after vortexing. The supernatant was then subjected to determination and quantification by high-performance liquid chromatography- fluorescence. The analysis system consisted of an Agilent Zorbax XDB (Agilent Technologies, CA, USA) equipped with a C18 column (150 × 4.6 mm, 5 µm) and a fluorescence detector. The liquid chromatography conditions were as follows: mobile phase solvent, acetonitrile and water at the volume ratio of 40: 60 at a flow rate of 1.2 mL/min; fluorescence detector, the wavelength of excitation and emission at 276 and 310 nm, respectively.
The method validation results were detailed in the supplementary material includes the following parameters: specificity, linearity, lower limit of quantitation (LLOQ), precision, accuracy, matrix effect, extraction recovery, stability and dilution integrity.
Pharmacokinetic analysis
Noncompartmental pharmacokinetic methods were used for pharmacokinetic analysis of HX0969W, fospropofol disodium, and propofol using the Phoenix Winnonlin® software (version 6.3, NJ, USA). The pharmacokinetic parameters used in this study were as follows: maximum concentration (Cmax) and the time to acquire (Tmax), area under curve (AUC) from zero to the last time point (AUC0−t), mean residual time (MRT), half-time (t1∕2), and clearance (CL).
Data analysis
The statistical analyses were performed using SPSS (version 21, IL, USA). The ED50, presented as mean and 95% CI, was calculated using the up and down method. The Kruskal–Wallis test followed by the Mann–Whitney U test was used for the data that did not fit normality, and the one-way ANOVA followed by Tukey’s test was used for the data that did not fit normality and equality of variance. The level of statistical significance was set at p < 0.05.
Results
Measurement of the median effective dose for the sedative-hypnotic effect
Following a single intravenous administration of the drug, each rat was placed in a separate box to record the time to LoRR and time to recovery from LoRR. Then, the median effective dose to achieve the sedative-hypnotic effect (Table 1) was calculated using the up and down method (Dixon, 1991; Glen & Hunter, 1984; Kilpatrick et al., 2007). The potency of HX0969W in rats (ED50 [95% CI], 46.49 [43.89–49.29] mg/kg) was found to be similar to that of fospropofol disodium (ED50 [95% CI], 43.66 [43.57–43.75] mg/kg), but lower than that of propofol (ED50 [95% CI], 4.82 [4.81–4.82] mg/kg). Due to the pro-drug design, HX0969W and fospropofol disodium are metabolized to propofol, which produces the sedative-hypnotic effect. Thus, the dosages of HX0969W and fospropofol used for general anesthesia were higher than those of propofol.
| Substance | Dose (mg/Kg) | X | Loss of the righting reflex (±) | t | C | M | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HX0969W | 59.17 | 1.772 | + | 1 | 1.772 | 3.140 | ||||||||||||||||||||
| 53.25 | 1.726 | + | + | – | + | 4 | 6.904 | 11.916 | ||||||||||||||||||
| 47.93 | 1.681 | + | + | – | + | – | – | – | 7 | 11.767 | 19.780 | |||||||||||||||
| 43.13 | 1.635 | – | + | – | + | + | – | 6 | 9.810 | 26.682 | ||||||||||||||||
| 38.82 | 1.589 | – | – | – | 3 | 4.767 | 7.575 | |||||||||||||||||||
| ED50= lg−1 (ΣC/ Σt) = 46.49 mg/Kg (95%CI [43.894–49.293] mg/Kg) | ||||||||||||||||||||||||||
| Fospropofol | 61.80 | 1.791 | 0 | 0 | 0 | |||||||||||||||||||||
| disodium | 55.62 | 1.745 | 0 | 0 | 0 | |||||||||||||||||||||
| 50.06 | 1.700 | + | + | 2 | 3.400 | 5.780 | ||||||||||||||||||||
| 45.05 | 1.654 | + | – | + | + | + | + | + | + | 8 | 13.232 | 21.886 | ||||||||||||||
| 40.55 | 1.607 | – | – | – | – | – | – | – | 7 | 11.249 | 18.077 | |||||||||||||||
| ED50= lg−1 (ΣC/ Σt) = 43.66 mg/Kg (95%CI [43.567–43.752] mg/Kg) | ||||||||||||||||||||||||||
| Propofol | 6.00 | 0.778 | + | + | 2 | 1.566 | 1.211 | |||||||||||||||||||
| 5.04 | 0.702 | – | + | + | + | + | – | + | + | + | 8 | 5.616 | 3.942 | |||||||||||||
| 4.23 | 0.626 | – | – | – | – | – | – | – | 6 | 3.756 | 2.351 | |||||||||||||||
| 3.56 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| 2.99 | 0 | 0 | 0 | 0 | ||||||||||||||||||||||
| ED50= lg−1 (ΣC/ Σt) = 4.82 mg/Kg (95%CI [4.818–4.821] mg/Kg) | ||||||||||||||||||||||||||
Notes:
1, X = lg(dose); t = sum total of rat; C = X × t; M =X2 × t. 2, + at with loss of the righting reflex; −: rat without loss of the righting reflex. 3,3, 95%CI = lg 1 (lgED 50 ± 1.96 slgED 50); SlgED 50 = [ΣM − (ΣC)2∕Σt]∕(Σt(Σt − 1))1∕2.
Pharmacodynamic study in rats
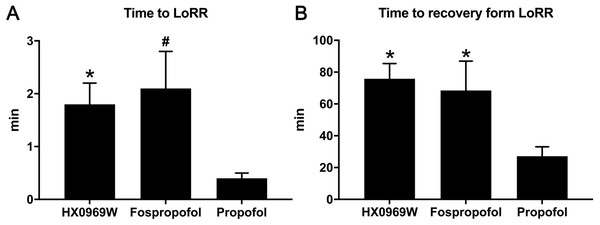
After administering a 2-fold ED50 dose for LoRR, the time to LoRR (onset time) and time to recovery from LoRR (duration) were measured in the rats. No mortality or significant clinical symptoms of toxicity were observed in these rats. As for the onset time (Fig. 1A), propofol (0.4 ± 0.1 min) required a shorter time to cause LoRR than that required for HX0969W (1.8 ± 0.4 min, p = 0.017) and fospropofol disodium (2.1 ± 0.7 min, p = 0.006). However, the duration of LoRR for HX0969W (75.8 ± 9.6 min, p = 0.036) and fospropofol disodium (68.5 ± 18.4 min, p = 0.041) were significantly longer than that of propofol (27.1 ± 6.0 min, Fig. 1B).
Figure 1: The onset time (A) and duration (B) of hypnotic behavior observation after intravenous administration of 2-fold ED50 drugs (n = 10 in each group).
Data are expressed as mean ± SD.∗p < 0.05; # p < 0.01.Pharmacokinetic study of HX0969W and fospropofol disodium in rats
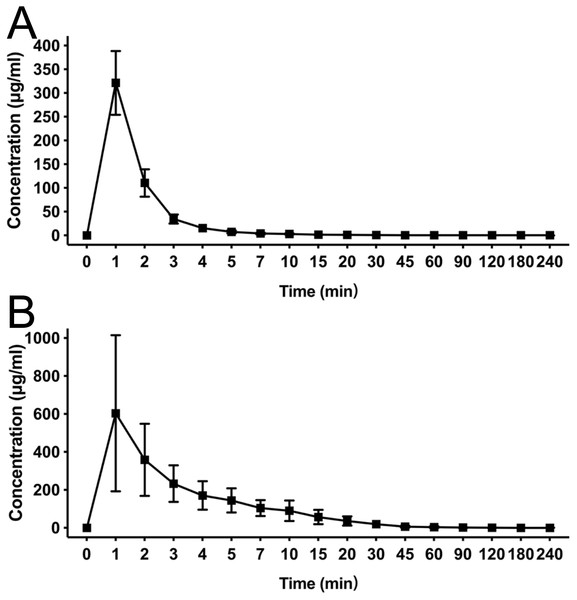
After a single intravenous injection, there was no significant difference in the action onset time and duration between HX0969W and fospropofol disodium. The efficacy of a drug is closely related to its pharmacokinetic features. Therefore, we measured the concentration–time profiles of HX0969W and fospropofol disodium in rats. The concentration–time curves are presented in Fig. 2, and the calculated pharmacokinetic parameters are shown in Table 2. Following the administration of equivalent doses (92.98 mg/kg for HX0969W, 87.32 mg/kg for fospropofol disodium), the maximal concentration of fospropofol disodium (603.49 ± 411.29 µg/mL) was found to be higher than that of HX0969W (321.30 ± 67.22 µg/mL). In rat plasma, HX0969W had a shorter mean residual time (3.67 ± 1.71 min) and a rapid clearance rate (89.97 ± 15.94 ml/min/kg) than those of fospropofol disodium (13.15 ± 5.45 min and 31.12 ± 19.09 ml/min/kg, respectively). The computed AUC value of HX0969W from zero to the last time point was 1053.78 ± 214.37 min* µg/ml, which was less than that of fospropofol disodium (3804.92 ± 2091.75 min* µg/ml). Thus, we considered that HX0969W, after a bolus injection in rats, had a faster metabolism in vivo as compared to fospropofol disodium. The pharmacokinetic parameters of HX0969W and fospropofol disodium could not explain the lack of significant differences in the onset time and duration. Therefore, we further evaluated the concentration of propofol released by HX0969W and fospropofol disodium in rats.
Figure 2: Mean concentration-time profiles of HX0969W (A) and fospropofol disodium (B) in plasma after intravenous administration with of 2-fold ED50 in rats (n = 10 in each group).
Data are expressed as mean ± SD.| Parameters | HX0969W | fospropofol |
|---|---|---|
| Cmax (μg/ml) | 321.3 ± 67.22 | 603.49 ± 411.29 |
| t1∕2(min) | 71.79 ± 59.52 | 49.29 ± 40.31 |
| AUC0∼t (min* μg/ml) | 1053.78 ± 214.37 | 3804.92 ± 2091.75 |
| MRT (min) | 3.67 ± 1.71 | 13.15 ± 5.45 |
| CL (ml/min/kg) | 89.97 ± 15.94 | 31.12 ± 19.09 |
Notes:
- AUC
-
area under the curve]
- MRT
-
mean residual time
- CL
-
clearance
Pharmacokinetic study of propofol in rats
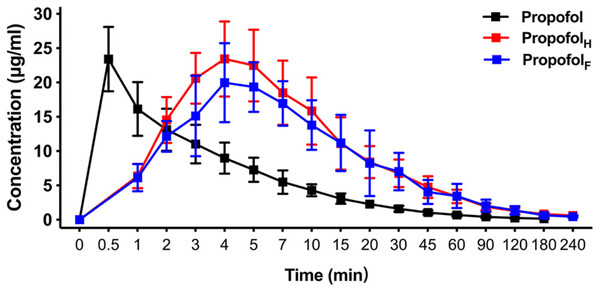
Using an equivalent dose of HX0969W, fospropofol disodium and the parent drug propofol, the concentration–time curves are presented in Fig. 3, and the calculated pharmacokinetic parameters are shown in Table 3. The Tmax of propofol released by HX0969W (propofolH, 4.0 ± 0.47 min) or fospropofol disodium (propofolF, 4.5 ± 1.18 min) in plasma had no significant difference, which was longer than that for propofol (0.5 min). However, the Cmax of propofolH (24.26 ± 5.14 µg/ml) was similar to the parent drug propofol (23.4 ± 4.69 µg/ml), which was higher than that for propofolF (21.91 ± 4.98 µg/ml). The AUC, MRT, and CL of propofolF (740.5 ± 186.33 min* µg/ml, 52.6 ± 2.90 min, and 54.67 ± 13.83 ml/min/kg, respectively) were similar to those of propofolH (704.53 ± 226.25 min* µg/ml, 50.92 ± 4.40 min, and 68.12 ± 22.84 ml/min/kg, respectively), but were higher than those of propofol (219.23 ± 42.21 min* µg/ml, 29.45 ± 4.63 min, and 43.28 ± 8.46 ml/min/kg, respectively). In this pharmacokinetic study, the longer onset time and duration of HX0969W and fospropofol disodium, than those of the parent drug propofol, were due to their pharmacokinetic features, which included a larger AUC and a longer MRT. Furthermore, the onset time and duration of HX0969W and fospropofol disodium, which showed no significant difference, could be related to the undifferentiated pharmacokinetic features of propofolH and propofolF, including Tmax, Cmax, AUC, MRT and CL.
Figure 3: Mean concentration-time profiles of propofol in plasma after intravenous administration of HX0969W, fospropofol disodium, and propofol at dose of 2-fold ED50 in rats (n = 10 in each group).
Data are expressed as mean ± SD. PropofolH, propofol released by HX0969W; PropofolF, propofol released by fospropofol disodium.| Parameters | Propofol | PropofolH | PropofolF |
|---|---|---|---|
| Cmax (μg/ml) | 23.4 ± 4.69 | 24.26 ± 5.14 | 21.91 ± 4.98 |
| Tmax (min) | 0.5 | 4.0 ± 0.47 | 4.5 ± 1.18 |
| t1∕2(min) | 54.24 ± 16.18 | 88.62 ± 77.35 | 78.97 ± 29.05 |
| AUC0∼t (min*μg/ml) | 219.23 ± 42.21 | 740.5 ± 186.33 | 704.53 ± 226.25 |
| MRT (min) | 29.45 ± 4.63 | 52.6 ± 2.90 | 50.92 ± 4.40 |
| CL (ml/min/kg) | 43.28 ± 8.46 | 54.67 ± 13.83 | 68.12 ± 22.84 |
Discussion
In this study, we have demonstrated that HX0969W requires a longer time to induce the sedative-hypnotic effect, but with longer duration than propofol in rats. HX0969W had a large Cmax, a short MRT, and a rapid CL than fospropofol disodium in rats. The Tmax of propofol released by HX0969W or fospropofol disodium showed no significant difference, and the Cmax of propofol released by HX0969W was similar to that of propofol, which was higher than that of the propofol released by fospropofol disodium.
The main objective of this study was to develop a new water-soluble pro-drug of propofol with non-toxic metabolites. In the pharmacodynamic study, the potency of HX0969W was found to be similar to that of fospropofol disodium, but lower than that of the parent drug propofol. Similar to fospropofol disodium, the onset time and duration of HX0969W after single intravenous administration, were longer than those of propofol. Thus, HX0969W is the new water-soluble pro-drug of propofol that can be used for induction and maintenance of general anesthesia. In contrast to fospropofol disodium, which is metabolized into a toxic metabolite, HX0969W was metabolized to propofol, GHB, and phosphate in vivo; GHB is further converted into carbon dioxide and water (Lang et al., 2014; Rousseau et al., 2012). Previous studies have demonstrated that GHB is mainly used as a sedative drug for children suffering from burns or for the treatment of alcohol and opioid addiction (Brambilla et al., 2012; Gallimberti et al., 1993; Rousseau et al., 2012). However, it was reported that GHB produced an obvious hypnotic effect after oral administration at least 800 mg/kg (Lettieri & Fung, 1979). In our published study, HX0969W produced a sedative-hypnotic effect after oral administration with 193.08 mg/kg in rats (Wang et al., 2015), which was far less than 800 mg/kg. Meanwhile, there is no data to show if there is any synergy between the effects of propofol and GHB. Therefore, the LoRR caused by HX0969W administration was mainly caused by the propofol released by HX0969W. As compared to fospropofol disodium, HX0969W cloud be an effective sedative-hypnotic agent with lesser potential risks of systemic toxicity.
In the open-label, single-arm, phase 3 clinical trial (Gan et al., 2010), five (4.1%) patients administered with fospropofol experienced the adverse events: hypoxemia (n = 1, 0.8%), hypotension (n = 4, 3.3%), bradycardia (n = 1, 0.8%). No patient experienced apnea during the procedure. In a randomized, double-blind, phase 3 study (Cohen et al., 2010), hypotension occurred in two patients (1.3%) in the fospropofol group, and one patient (0.6%) experienced hypoxemia that resolved after repeated verbal stimulation. Undergoing flexible bronchoscopy (Silvestri et al., 2009), the most common cardiopulmonary adverse events were hypoxemia (n = 36, 14.3%) and hypotension (n = 8, 3.2%). In another clinical trial (Cohen, 2008), four patients in fospropofol group experienced sedation-related adverse events: mild hypotension (n = 2, 2%) and hypoxemia (n = 2, 2%). Only one patient of hypoxemia required the airway assistance (verbal stimulation). Thus, fospropofol showed lower incidences of hypotension, respiratory depression, apnea, and loss of airway patency because of its slower onset of action (Mahajan, Mahajan & Kaushal, 2012). On the basis of the findings in this study, there is no significant difference between HX0969W and fospropofol disodium in pharmacodynamic and pharmacokinetic studies. Therefore, it would be reasonable to consider the possibility that HX0969W could reduce the cardiopulmonary side effects by a slow-released propofol as fospropofol disodium. In the subsequent experiments, we will focus on evaluating the safety of HX0969W and propofol in the cardiovascular and respiratory systems.
Propofol has been the most widely used intravenous anesthetic in clinical practices due to its rapid onset and recovery from sedation (Hemphill et al., 2019). However, the pharmacokinetic features of HX0969W, including a longer MRT and t1∕2, and a larger AUC, have superseded the advantages of propofol. Therefore, HX0969W is more appropriate for patients undergoing longstanding surgeries or a long-term sedation in intensive care unit, and its use can avoid the side effects of propofol by its lipid emulsion preparation. Meanwhile, HX0969W generated a hypnotic effect with rapid onset and shorter duration than fospropofol disodium and propofol after oral administration in rats (Wang et al., 2015). Therefore, there might be a new clinical indication for HX0969W for use in patients undergoing pre-operative sedation, transitory diagnostic or therapeutic procedures by oral administration. Above all, our results show that HX0969W can avoid the disadvantages associated with the lipid emulsion formulation of propofol. Thus, HX0969W is more suitable for long-term sedation and pre-operative preparation for children who do not cooperate with venipuncture.
Conclusions
With the equivalent dose, HX0969W and fospropofol disodium had a longer time to cause LoRR with longer duration than propofol in rats. In pharmacokinetic study, the Cmax of fospropofol disodium was higher than HX0969W. HX0969W had a shorter MRT and a rapid CL than fospropofol disodium. Due to the pro-drug design, HX0969W and fospropofol disodium are metabolized to propofol, which produces the sedative-hypnotic effect. The Tmax of propofol released by HX0969W and fospropofol disodium had no significant difference, which was longer than that for propofol. The Cmax of propofol released by HX0969W was similar to the parent drug propofol, which was higher than that for that of propofol released by fospropofol disodium.