Identification of key genes and pathways associated with cholangiocarcinoma development based on weighted gene correlation network analysis
- Published
- Accepted
- Received
- Academic Editor
- Yezaz Ghouri
- Subject Areas
- Bioinformatics, Gastroenterology and Hepatology, Oncology
- Keywords
- Cholangiocarcinoma, Prognosis, Weighted gene correlation network analysis, Progression, Cho
- Copyright
- © 2019 Liu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. Identification of key genes and pathways associated with cholangiocarcinoma development based on weighted gene correlation network analysis. PeerJ 7:e7968 https://doi.org/10.7717/peerj.7968
Abstract
Background
As the most frequently occurred tumor in biliary tract, cholangiocarcinoma (CCA) is mainly characterized by its late diagnosis and poor outcome. It is therefore urgent to identify specific genes and pathways associated with its progression and prognosis.
Materials and Methods
The differentially expressed genes in The Cancer Genome Atlas were analyzed to build the co-expression network by Weighted gene co-expression network analysis (WGCNA). Gene ontology (GO) as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were conducted for the selected genes. Module–clinical trait relationships were analyzed to explore the association with clinicopathological parameters. Log-rank tests and cox regression were used to identify the prognosis-related genes.
Results
The most related modules with CCA development were tan module containing 181 genes and salmon module with 148 genes. GO analysis suggested enrichment terms of digestion, hormone transport and secretion, epithelial cell proliferation, signal release, fibroblast activation, response to acid chemical, wnt, Nicotinamide adenine dinucleotide phosphate metabolism. KEGG analysis demonstrated 15 significantly altered pathways including glutathione metabolism, wnt, central carbon metabolism, mTOR, pancreatic secretion, protein digestion, axon guidance, retinol metabolism, insulin secretion, salivary secretion, fat digestion. Key genes of SOX2, KIT, PRSS56, WNT9A, SLC4A4, PRRG4, PANX2, PIR, RASSF8, MFSD4A, INS, RNF39, IL1R2, CST1, and PPP3CA might be potential prognostic markers for CCA, of which RNF39 and PRSS56 also showed significant correlation with clinical stage.
Discussion
Differentially expressed genes and key modules contributing to CCA development were identified by WGCNA. Our results offer novel insights into the characteristics in the etiology, prognosis, and treatment of CCA.
Introduction
Cholangiocarcinoma (CCA), characterized by cholangiocyte differentiation, represents a type of aggressive tumor which derive form lesions in different parts of the biliary tree (Bergquist & Von Seth, 2015). As the most frequently occurred biliary tumor and the second after liver cancer, CCA is mainly characterized by its late diagnosis and poor outcome (Blechacz, 2017). Currently, it is estimated that overall 5-year survival rate of CCA is less than 10% and only approximately one third of the CCA patients are suitable for curative treatment at the time of diagnosis (Doherty, Nambudiri & Palmer, 2017). Therefore, it is urgently required to identify specific genes and pathways associated with its initiation, progression, and prognosis.
The common category of CCA are on basis of different locations of intrahepatic, perihilar, or distal CCA (Oliveira et al., 2017). Most patients are diagnosed with non-resectable status and suffer an extremely poor prognosis (Rizvi & Gores, 2017). A number of diseases have been reported to be related with the development of CCA including viral hepatitis B and C, choledocholithiasis, obesity, exposure to toxic agents, liver cirrhosis, diabetes mellitus, congenital hepatic fibrosis, and primary sclerosing cholangitis (Erichsen et al., 2009). Every year, a larger number of cases occur in Southeast Asia, in which hepatobiliary flukes Opisthorchis viverrini infection or Clonorchis sinensis infection were considered to be implicated in the development of CCA (Razumilava & Gores, 2014). Interleukin-6, a pro-inflammatory cytokine associated with downstream activation of oncogenic pathways, has been linked with CCA development (Park et al., 1999). Frequent mutations of oncogenes such as KRAS, as well as cancer suppressor genes of TP53 and SMAD4 were identified by next generation sequencing in CCA (Chan-on et al., 2013). In addition, research from several case-controlled studies has demonstrated multiple genetic polymorphisms that might be implicated in CCA carcinogenesis (Bridgewater et al., 2014).
Although a number of genes and mechanisms have been proved to be closely implicated in the development of CCA, the comprehensive picture of the whole genes and regulations of CCA is still unclear. In recent years, bioinformatic methods become increasingly effective in exploration and analysis of multiple genes or proteins of complicated diseases. Weighted gene co-expression network analysis (WGCNA), a new gene co-expression analysis method, has been successfully used to screen biomarkers and pathways that could be applied in susceptibility genes, diagnose and treatment of cancer. In this study, WGCNA was conducted to analyze data of The Cancer Genome Atlas (TCGA) data repository of CCA to screen modules and core genes in pathogenesis, progression, and survival of CCA.
Materials and Methods
Publically available data sets
RNA expression as well as clinical parameters of CCA patients were obtained from TCGA database (cancergenome.nih.gov). The level of gene expression was tested as Transcripts Per Kilobase of exon model per Million mapped reads. Clinical characteristics had the sample type, histology grade, recurrence, histologic grade, and prognosis. Each sample must had complete pathology stage and histology records. If the expression of genes showed limited variation, we regraded them as noise and discard these ones because the results of these genes might come from systematic error and have limited significance.
Construction of co-expression network of genes
In this study, we adopted WGCNA method to build a co-expression network for certain genes using R language (Langfelder & Horvath, 2008). We used WGCNA method to calculate power number in order to construct modules through co-expression. WGCNA method was also performed for construction of the co-expression network and extraction of the genetic information in the most relevant module. “Heatmap tool” package of R software was selected to analyze the correlation degree among modules. As a representative of the gene expression profiles of a module, module eigengene (ME) was used to evaluate the relationship between module and overall survival.
Identification of clinical traits-related modules
We then performed Pearson’s correlation examination to explore the relation of MEs with clinical traits of histologic grade, recurrence, pathologic M, pathologic N, pathologic T. A P-value less than 0.05 indicated statistical significance. The two most significant modules are selected as hub modules.
GO and KEGG analysis
In the present study, we adopted the clusterprofiler package of R language to investigate the possible biological procedures and signaling pathways of selected genes in different module. Gene ontology (GO) analysis (Ashburner et al., 2000) as well as Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis were conducted (Kanehisa et al., 2004), which enable computational prediction of higher-level complexity of cellular processes and organism behavior from genomic information.
Prognostic value of genes of hub modules
Prognosis investigation was conducted using the “survival” package within R software. Cox regression test was used to calculate the hazard ratio and its 95% confidence interval while Kaplan–Meier method was adopted to draw survival curve. The increased and decreased expression level of each gene in the candidate module was decided according to median value.
Screening for candidate hub genes
We chose genes associated with prognosis as candidate genes. Next, we analyzed the association between candidate genes and stage parameter. The genes associated with both overall survival and clinical stage were selected as hub genes.
Results
Gene expression profile of CCA
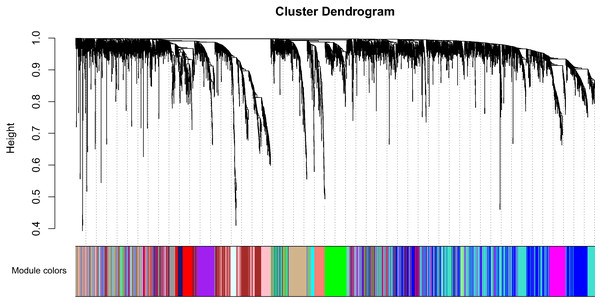
Clinical characteristics and RNA expression value for 33 CCA individuals were downloaded at TCGA. Altogether 6,417 relatively more variant genes on the basis of median absolute deviation value were considered in our subsequent investigaiton. When soft thresholding power β came to 7, the connectivity amone genes accord with a scale-free network distribution (Fig. S1). A total of 21 modules were screened using hierarchical clustering along with Dynamic branch Cutting by WGCNA analysis. And each different color represent a sole module. Interaction relationship analysis of co-expression genes was shown in Fig. 1. The gene counts within modules were from 37 to 909. In this study, the gray module collected a series of genes which did not belong to any module.
Figure 1: Interaction relationship analysis of co-expression genes in cholangiocarcinoma.
Association of module with clinical traits
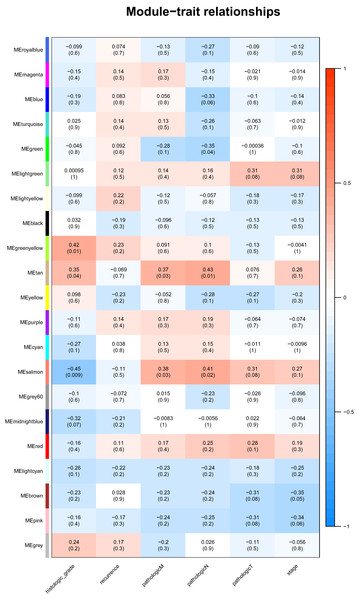
Identification of genes and modules which associate with clinical characteristic would greatly help the understanding the underlying mechanisms of certain traits. In our analysis, the clinical parameters of CCA of histologic grade, recurrence, pathologic M, pathologic N, pathologic T were selected in the module–trait relationship analysis. As was suggested in Fig. 2, the two relatively most significant modules of tan and salmon were selected as hub modules because their significant relation with clinical parameters including grade and T, N, M of CCA.
Figure 2: The module–clinical trait relationships of genes involved in clinicopathological parameters of cholangiocarcinoma patients.
Enrichment investigation of the hub modules
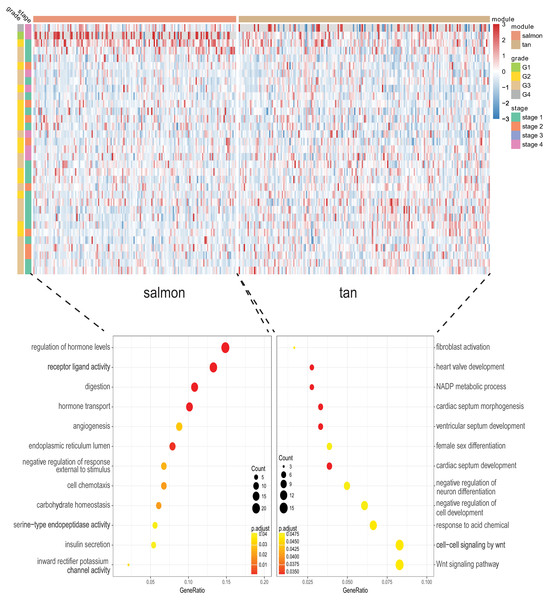
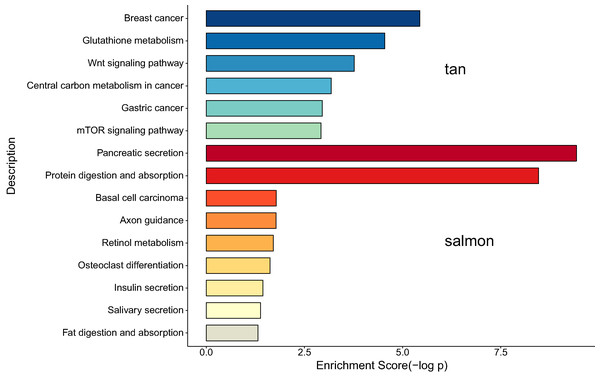
We next proceeded GO and KEGG investigation of the genes within tan module and salmon module. Altogether 50 terms showed significant difference in GO enrichment analysis (Table S1) and the most important terms were summarized in Table 1. As was illustrated in Fig. 3, the salmon module in GO analysis was related with digestion, hormone transport, hormone secretion, epithelial cell proliferation, regulation of hormone levels, signal release while the tan module was associated with fibroblast activation, response to acid chemical, wnt signaling pathway, cell-cell signaling by wnt, Nicotinamide adenine dinucleotide phosphate (NADP) metabolic process, negative regulation of neuron differentiation, negative regulation of cell development. According to the KEGG analysis, 15 pathways were significantly altered in hub modules including glutathione metabolism, wnt signaling pathway, central carbon metabolism in cancer, mTOR signal, pancreas secretion, protein digestion or absorption, axon guidance, retinol metabolism, insulin secretion, salivary secretion, and fat digestion and absorption (Fig. 4; Table 2).
Figure 3: Gene Ontology analysis for genes in the hub modules of tan module and salmon module in cholangiocarcinoma.
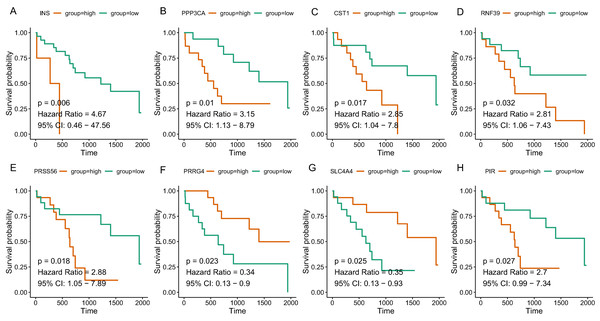
Figure 4: The correlation between the expression levels of key genes of hub modules and the survival of CAA patients.
| Module | ID | Description | GeneRatio | P-value | Count |
|---|---|---|---|---|---|
| tan | GO:0003179 | Heart valve morphogenesis | 5/181 | 3.36E-05 | 5 |
| tan | GO:0006739 | NADP metabolic process | 5/181 | 3.36E-05 | 5 |
| tan | GO:0003170 | Heart valve development | 5/181 | 5.67E-05 | 5 |
| tan | GO:0003281 | Ventricular septum development | 6/181 | 5.83E-05 | 6 |
| tan | GO:0060411 | Cardiac septum morphogenesis | 6/181 | 6.92E-05 | 6 |
| tan | GO:0003279 | Cardiac septum development | 7/181 | 7.37E-05 | 7 |
| tan | GO:0072537 | Fibroblast activation | 3/181 | 0.00013 | 3 |
| tan | GO:0001101 | Response to acid chemical | 12/181 | 0.00015 | 12 |
| tan | GO:0016055 | Wnt signaling pathway | 15/181 | 0.00016 | 15 |
| tan | GO:0198738 | Cell-cell signaling by wnt | 15/181 | 0.00017 | 15 |
| salmon | GO:0007586 | Digestion | 16/148 | 1.02E-12 | 16 |
| salmon | GO:0010817 | Regulation of hormone levels | 22/148 | 1.53E-10 | 22 |
| salmon | GO:0009914 | Hormone transport | 15/148 | 7.10E-08 | 15 |
| salmon | GO:0046879 | Hormone secretion | 14/148 | 2.85E-07 | 14 |
| salmon | GO:0023061 | Signal release | 16/148 | 6.22E-07 | 16 |
| salmon | GO:0030072 | Peptide hormone secretion | 12/148 | 1.16E-06 | 12 |
| salmon | GO:0046883 | Regulation of hormone secretion | 12/148 | 1.84E-06 | 12 |
| salmon | GO:0050679 | Positive regulation of epithelial cell proliferation | 10/148 | 2.07E-06 | 10 |
| salmon | GO:0090276 | Regulation of peptide hormone secretion | 10/148 | 8.73E-06 | 10 |
| salmon | GO:0050673 | Epithelial cell proliferation | 13/148 | 1.32E-05 | 13 |
| Module | ID | Description | GeneRatio | P-value | Count |
|---|---|---|---|---|---|
| tan | hsa05224 | Breast cancer | 10/81 | 3.65E-06 | 10 |
| tan | hsa00480 | Glutathione metabolism | 6/81 | 2.84E-05 | 6 |
| tan | hsa04310 | Wnt signaling pathway | 8/81 | 0.00017 | 8 |
| tan | hsa05230 | Central carbon metabolism in cancer | 5/81 | 0.00066 | 5 |
| tan | hsa05226 | Gastric cancer | 7/81 | 0.00111 | 7 |
| tan | hsa04150 | mTOR signaling pathway | 7/81 | 0.0012 | 7 |
| salmon | hsa04972 | Pancreatic secretion | 11/64 | 3.74E-10 | 11 |
| salmon | hsa04974 | Protein digestion and absorption | 10/64 | 3.45E-09 | 10 |
| salmon | hsa05217 | Basal cell carcinoma | 3/64 | 0.01654 | 3 |
| salmon | hsa04360 | Axon guidance | 5/64 | 0.0167 | 5 |
| salmon | hsa00830 | Retinol metabolism | 3/64 | 0.01948 | 3 |
| salmon | hsa04380 | Osteoclast differentiation | 4/64 | 0.02372 | 4 |
| salmon | hsa04911 | Insulin secretion | 3/64 | 0.03605 | 3 |
| salmon | hsa04970 | Salivary secretion | 3/64 | 0.04162 | 3 |
| salmon | hsa04975 | Fat digestion and absorption | 2/64 | 0.04786 | 2 |
Prognosis analysis
Since the tan and salmon module were selected from as the hub modules, we are interested in whether the genes with in the modules might predict the survival of CCA patients. Of the 358 genes, a total of 15 genes (SOX2, KIT, PRSS56, WNT9A, SLC4A4, PRRG4, PANX2, PIR, RASSF8, MFSD4A, INS, RNF39, IL1R2, CST1, and PPP3CA) demonstrated significant association with the prognosis of CCA (Table 3). The survival curve for the top four genes in salmon module (INS: HR = 4.670, P = 0.006; RNF39: HR = 2.811, P = 0.032; CST1: HR = 2.846, P = 0.017; PPP3CA: HR = 3.151, P = 0.010) and the top four gene in tan module (PRSS56: HR = 2.876, P = 0.018); SLC4A4: HR = 0.352, P = 0.025; PRRG4: HR = 0.339, P = 0.023; PIR: HR = 2.701, P = 0.027) were illustrated in Fig. 5. In addition, key genes of DNA repair and immune regulation were analyzed in relation to CCA prognosis, the results of which suggested that DNA repair gene XPC (HR = 0.369, P = 0.048) and immune regulator CD28 (HR = 0.364, P = 0.045) were related with favorable survival of CCA (Table S2). We also analyzed the prognosis of genes more frequently implicated in CCA including KRAS, TP53, BAP1. The mutation frequency of KRAS, TP53, BAP1 were 5.71%(2), 8.57%(3), 5.71%(2) out of 33 CCA patients. And no significant association with prognosis was observed.
Figure 5: The correlation between the expression levels of key genes of hub modules and the survival of CAA patients.
(A) INS; (B) PPP3CA; (C) CST1; (D) RNF39; (E) PRSS56; (F) PRRG4; (G) SLC4A4; (H) PIR.| Gene | Full name | Module | HR | 95% CI | P-value |
|---|---|---|---|---|---|
| SOX2 | SRY-box 2 | tan | 0.382 | [0.145–1.002] | 0.043 |
| KIT | KIT proto-oncogene receptor tyrosine kinase | tan | 0.341 | [0.132–0.883] | 0.048 |
| PRSS56 | Serine protease 56 | tan | 2.876 | [1.048–7.892] | 0.018 |
| WNT9A | Wnt family member 9A | tan | 0.389 | [0.144–1.050] | 0.041 |
| SLC4A4 | Solute carrier family 4 member 4 | tan | 0.352 | [0.133–0.931] | 0.025 |
| PRRG4 | Proline rich and Gla domain 4 | tan | 0.339 | [0.127–0.900] | 0.023 |
| PANX2 | Pannexin 2 | tan | 2.600 | [0.963–7.022] | 0.037 |
| PIR | Pirin | tan | 2.701 | [0.994–7.337] | 0.027 |
| RASSF8 | Ras association domain family member 8 | tan | 0.396 | [0.147–1.065] | 0.040 |
| MFSD4A | Major facilitator superfamily domain containing 4A | salmon | 0.371 | [0.143–0.962] | 0.043 |
| INS | Insulin | salmon | 4.670 | [0.458–47.564] | 0.006 |
| RNF39 | Ring finger protein 39 | salmon | 2.811 | [1.063–7.434] | 0.032 |
| IL1R2 | Interleukin 1 receptor type 2 | salmon | 0.379 | [0.144–0.997] | 0.041 |
| CST1 | Cystatin SN | salmon | 2.846 | [1.039–7.797] | 0.017 |
| PPP3CA | Protein phosphatase 3 catalytic subunit alpha | salmon | 3.151 | [1.129–8.793] | 0.010 |
Identification of hub genes associated with both stage and survival
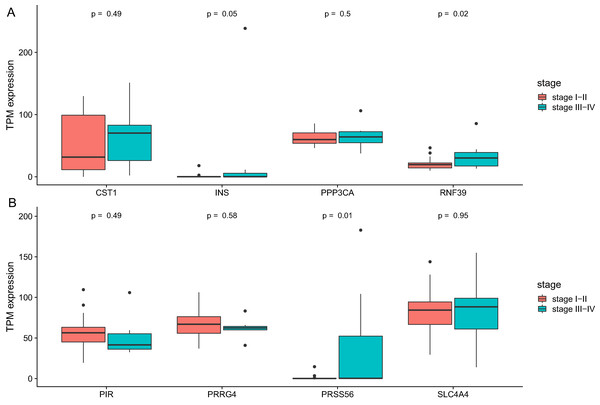
We then analyze the association between clinical stage and gene which showed significant relation with prognosis. As was shown in Fig. 6, two genes (RNF39 and PRSS56) showed difference between two groups (Table 4).
Figure 6: The differential expression of potential hub genes in different stages of CCA patients.
(A) Differential expression of INS, PPP3CA, CST1 and RNF39; (B) Differential expression of PRSS56, PRRG4, SLC4A4 and PIR.| Gene symbol | Gene title | Position | OR | P |
|---|---|---|---|---|
| MFSD4A | Major facilitator superfamily domain containing 4A | 1q32.1 | 1.036 (0.988–1.093) | 0.155 |
| SOX2 | SRY-Box 2 | 3q26.33 | 1.037 (0.986–1.115) | 0.184 |
| INS | Insulin | 11p15.5 | 1.125 (0.996–1.320) | 0.059 |
| KIT | KIT proto-oncogene receptor tyrosine kinase | 4q12 | 1.033 (0.991–1.091) | 0.146 |
| PRSS56 | Serine protease 56 | 2q37.1 | 1.162 (1.002–1.431) | 0.013 |
| RNF39 | Ring finger protein 39 | 6p22.1 | 1.069 (1.006–1.167) | 0.019 |
| WNT9A | Wnt family member 9A | 1q42.13 | 0.965 (0.904–1.018) | 0.235 |
| SLC4A4 | Solute carrier family 4 member 4 | 4q13.3 | 1.001 (0.973–1.029) | 0.945 |
| IL1R2 | Interleukin 1 receptor type 2 | 2q11.2 | 1.027 (0.993–1.068) | 0.131 |
| CST1 | Cystatin SN | 20p11.21 | 1.007 (0.988–1.026) | 0.490 |
| PRRG4 | Proline rich and Gla domain 4 | 11p13 | 0.983 (0.922–1.041) | 0.574 |
| PANX2 | Pannexin 2 | 22q13.33 | 0.987 (0.940–1.024) | 0.517 |
| PIR | Pirin | Xp22.2 | 0.983 (0.930–1.027) | 0.489 |
| RASSF8 | Serine protease 8 | 12p12.1 | 0.942 (0.863–1.002) | 0.094 |
| PPP3CA | Protein phosphatase 3 catalytic subunit alpha | 4q24 | 1.021 (0.960–1.086) | 0.492 |
Note:
The bold values indicate significant association between gene expression and clinical stage of CCA patients.
Discussion
Although surgical therapy and liver transplantation might be possible options for certain patients who suffer CCA, the 5-year survival ratio of CCA remain extremely low. Comprehensive exploration of the genetic and epigenetic profiles as well as their complicated interactions with environment would greatly help the treatment and survival for CCA patients. Until now, our understanding of the complex mechanisms of CCA is still limited. One study using CCA data also analyzed the genes implicated in CCA (Tian et al., 2019). They dissected the genome network by other bioinformatic tool to explore the protein–protein interaction. In this study, we provided our own gene lists with significance by WGCNA. Besides, we adopted KEGG analysis to explore the biological functions for the tumor progression. A differentially expression analysis followed by WGCNA to identify genes and pathways related were performed to the clinical parameters and prognosis of CCA. In addition, enrichment analysis of the genes in core modules suggested significant involvement of pathways such as glutathione metabolism, wnt signaling, mTOR signaling, pancreatic secretion, protein digestion and absorption, insulin secretion, and salivary secretion.
In this present study, RNA sequencing data for CCA samples from TCGA were systematically analyzed. According to the WGCNA analysis of most variant genes, altogether 21 modules were identified and the two most significant modules of tan and salmon were selected as hub modules. GO analysis of salmon module demonstrated enrichment in digestion, hormone transport, hormone secretion, epithelial cell proliferation, regulation of hormone levels, signal release. It can be concluded from our analysis that abnormal hormone regulation might be closely implicated in CCA progression. Previously, autocrine parathyroid hormone-like hormone was indicated to promote intrahepatic CCA cell growth through enhanced ERK/JNK-ATF2-cyclinD1 signaling (Tang et al., 2017). There were also reports of CCA patients with aberrant levels of parathyroid hormone-related protein (Matsumoto et al., 2014; Ozawa et al., 2017). From this point of view, future investigations concerning the synthesis, transport and secretion of relevant hormones might provide novel insight into CCA development.
In addition, the tan module was associated with fibroblast activation, response to acid chemical, wnt signaling pathway, NADP metabolic process, negative modulation of neuron differentiation, negative modulation of cell development. Wnt signaling pathway has been proved to be involved in the growth and progression of various types of cancer (Clevers & Nusse, 2012). Previously, enhanced wnt signaling has been found to be a characteristic of CCA (Boulter et al., 2015) which related with metastasis or chemoresistance (Wang et al., 2015). Besides, lncRNA PCAT1 regulated the development of extrahepatic CCA progression by Wnt and β-catenin signaling pathway (Zhang et al., 2017b). It is worth noting that response to acid chemical was also a hallmark in CCA development according to our study. Indeed, glycocholic acid and taurochenodeoxycholic acid were found to be potential phenotypic biomarkers in CCA (Song et al., 2018). Conjugated bile acids were suggested to promote invasive growth of CCA cells via activation of sphingosine 1-phosphate receptor 2 (Liu et al., 2014). The role of other biological processes such as NADP metabolic process and neuron differentiation required further studies to elucidate.
According to the KEGG analysis, 15 pathways were significantly altered in hub modules including glutathione metabolism, wnt signaling pathway, central carbon metabolism in cancer, mTOR signaling pathway, pancreatic secretion, protein digestion and absorption, axon guidance, retinol metabolism, insulin secretion, salivary secretion, and fat digestion and absorption. Some of the identified pathways have been suggested to play critical role in CCA progression and therapy. In intrahepatic cholangiocarcinoma (ICC) mouse model, investigators proved that mTOR kinase inhibitors inducing strong ICC cell apoptosis and may be beneficial for the treatment of ICC (Zhang et al., 2017a). It has been reported that the contact inhibition of CCA cells could be overcome by c‑Myc via the mTOR pathway (Luo et al., 2017). The identified significant pathways of secretion and absorbance of multiple digestive juice remind us the importance of digestive balance in the development of CCA, which required further investigations to follow. The significant altered pathways of insulin secretion, salivary secretion, and fat digestion and absorption confirmed the key role of digestive regulation in CCA, as was indicated by a number of previous investigations (Chaiyarit et al., 2011; Chen et al., 2013; Rizvi et al., 2014).
The 5-year survival rate of CCA currently remains unsatisfactory despite recent improvements in treatments of surgical resection and chemotherapy (Squadroni et al., 2017). The selected genes hold great possibilities to become potential biomarkers for the prediction of survival of CCA patients. We eventually screened 15 prognosis-related genes in CAA after analyzing the hub modules of tan and salmon. Increased expression of SOX2 in CCA cells lead to cell proliferation, decreased cell apoptosis, and increased cell invasion and metastasis (Sun et al., 2014). Besides, SOX2 expression correlated with aggressive behavior and worse overall survival in ICC (Gu & Jang, 2014). Among the identified prognosis-related genes, RNF39 and PRSS56 also showed significant correlation with clinical stage. Until now, little is known about the functions of these two genes in cancer. RNF39 encoded a member of ring finger proteins, which was studied in Behcets disease, a chronic inflammatory autoimmune disease (Kurata et al., 2010). In addition, RNF39 protein was suggested to participate in the replication process according to both studies on HIV-1 individuals and cell-lines (Lin et al., 2014). PRSS56 has been established as an effective marker of adult neurogenesis in the brain of mouse (Jourdon et al., 2016). Variants in PRSS56 may lead to primary angle-closure glaucoma and high hyperopia (Jiang et al., 2013). Whether RNF39 and PRSS56 could be a robust biomarker for CCA and their underlying mechanisms still required further studies to explore. In addition, DNA repair gene XPC and immune regulator CD28 were related with favorable survival of CCA, which indicate probable implication of DNA repair and immune response in CCA development. Because no distant metastasis information was available in TCGA data of CCA, we did not perform association analysis of genes with CCA metastasis, which require future studies to clarify.
Conclusion
In summary, differentially expressed genes and key modules contributing to progression of CCA were identified by means of WGCNA. Pathways including glutathione metabolism, wnt signaling, central carbon metabolism in cancer, mTOR signaling, pancreatic secretion, protein and fat digestion, insulin secretion, and salivary secretion might be closely related with CCA development. Key genes such as RNF39 and PRSS56 might be potential prognostic markers for CCA. The results offer novel insights into the understanding of the development and prognosis of CAA.