The newly described Araguaian river dolphins, Inia araguaiaensis (Cetartiodactyla, Iniidae), produce a diverse repertoire of acoustic signals
- Published
- Accepted
- Received
- Academic Editor
- Jose Maria Cardoso da Silva
- Subject Areas
- Animal Behavior, Biodiversity, Ecology, Zoology, Freshwater Biology
- Keywords
- Boto, Behavior, Acoustic communication, Mother-calf pairs, Non-linear phenomena
- Copyright
- © 2019 Melo-Santos et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2019. The newly described Araguaian river dolphins, Inia araguaiaensis (Cetartiodactyla, Iniidae), produce a diverse repertoire of acoustic signals. PeerJ 7:e6670 https://doi.org/10.7717/peerj.6670
Abstract
The recent discovery of the Araguaian river dolphin (Inia araguaiaensis) highlights how little we know about the diversity and biology of river dolphins. In this study, we described the acoustic repertoire of this newly discovered species in concert with their behaviour. We analysed frequency contours of 727 signals (sampled at 10 ms temporal resolution). These contours were analyzed using an adaptive resonance theory neural network combined with dynamic time-warping (ARTwarp). Using a critical similarity value of 96%, frequency contours were categorized into 237 sound-types. The most common types were emitted when calves were present suggesting a key role in mother-calf communication. Our findings show that the acoustic repertoire of river dolphins is far from simple. Furthermore, the calls described here are similar in acoustic structure to those produced by social delphinids, such as orcas and pilot whales. Uncovering the context in which these signals are produced may help understand the social structure of this species and contribute to our understanding of the evolution of acoustic communication in whales.
Introduction
Compared to their marine counterparts, sounds produced by river dolphins and their role in communication remain understudied. Mizue, Nishiwaki & Takemura (1971) recorded captive Ganges River dolphins (Platanista gangetica) and reported three types of communication sounds: bursts, whistles, twittering and “stratiform” sounds. Other than visualizing spectrograms, no acoustic information was extracted from these sounds. More recently, studies have prioritized the characterization of echolocation (Jensen et al., 2013; Sugimatsu et al., 2017), thus the role of other Ganges dolphin’s sounds remain poorly understood to this date. In the Chinese river dolphin, the baiji (Lipotes vexillifer), whistles are thought to be the only communicative sounds produced by the species (Wang, Wenxiang & Zhifan, 1995; Wang, Wang & Akamatsu, 1999; Wang et al., 2006; Xiao & Jing, 1989). The Baiji whistles are lower in frequency (5–6 kHz), less modulated, and produced more infrequently than delphinid whistles (Wang, Wenxiang & Zhifan, 1995; Wang, Wang & Akamatsu, 1999; Wang et al., 2006; Xiao & Jing, 1989). The social sounds of the Franciscana (Pontoporia blainvillei) also involved the emission of whistles (1.6–94.6 kHz) as well as burst pulses (Cremer et al., 2017).
Like in all riverine dolphins, the acoustic repertoire of the Amazonian Inia, or “boto”, is thought to be restricted to a few types of sounds (Podos, Da Silva & Rossi-Santos, 2002). However, studies of free-ranging and captive botos suggest otherwise. Throughout its distribution several studies have described a variety of sounds including burst-pulsed sounds, jaw-snaps, low-frequency sounds, pulsed sounds, echolocation clicks, and whistles (Amorim et al., 2016; Caldwell, Caldwell & Evans, 1966; Diazgranados & Trujillo, 2002; May-Collado & Wartzok, 2007; Ding, Würsig & Evans, 1995; Ding, Würsig & Leatherwoods, 2001; Kamminga et al., 1993; Podos, Da Silva & Rossi-Santos, 2002; Penner & Murchison, 1970). Ding, Würsig & Leatherwoods (2001) also described the emission of low-frequency whistles (up to 5 kHz) for Peruvian botos. However, this discovery was disputed (Podos, Da Silva & Rossi-Santos, 2002) due to the presence of sympatric tucuxi dolphins (Sotalia fluviatilis) known to emit whistles. Later, May-Collado & Wartzok (2007) confirmed that botos do emit whistles, but at much higher frequencies (up to 48 kHz) than previously thought. These high frequency whistles were recorded from botos at the Yasuni and Napo rivers in Ecuador. Today, there is a consensus that, while botos do emit whistles, these sounds are emitted rarely (Diazgranados & Trujillo, 2002; May-Collado & Wartzok, 2007; Ding, Würsig & Leatherwoods, 2001) and likely play a different social role as the one described for delphinids (May-Collado & Wartzok, 2007). Podos, Da Silva & Rossi-Santos (2002) found that the acoustic repertoire of Amazonian botos consisted primarily of pulsed calls with a low emission rate. However, these results were likely limited by the sampling rate of the recorders used by the authors. Amorim et al. (2016) studied the same population using a broadband frequency recording system and described a high emission of a variety of pulsed calls. Botos sounds are relatively more studied than those of other river dolphin species, however much remains to be researched as most of the previous studies were preliminary or localized. While the acoustic behaviour of botos is better documented than those of other river dolphin species, we still do not know their full repertoire nor the role of these sounds in their daily lives.
Botos are evolutionary relics found exclusively in the Amazon, Orinoco, and Tocantins River Basins of South America (Best & Da Silva, 1989; Best & Da Silva, 1993; Hrbek et al., 2014; May-Collado & Agnarsson, 2011; Pilleri & Gihr, 1977; Dos Santos et al., 2012; Dos Santos et al., 2014. Like the franciscana dolphin (Pontoporia blainvillei), the baiji (Lipotes vexillifer), and the Ganges and Indus river dolphins (Platanista spp.), botos have flexible necks and backbones, a low and large-based dorsal fin, and a slender rostrum (Best & Da Silva, 1989; Best & Da Silva, 1993; Brownell & Herald, 1972; Cassens et al., 2000; Crespo, Harris & González, 1998; Da Silva, 2009; Hamilton et al., 2001; Leatherwoods & Reeves, 1994; Reeves & Martin, 2009; Zhou, 2009). Botos have a preference for habitats with slow currents and high prey concentration such as bays, confluences, small streams, and channels and island margins (Gomez-Salazar et al., 2012; Gomez-Salazar, Trujillo & Whitehead, 2012; Martin & Da Silva, 2004; Martin, Silva & Salmon, 2004; Pavanato et al., 2016). However, residency patterns vary within locations from long-term residency to occasional visitors (Martin & Da Silva, 2004). Although botos are traditionally considered solitary, with long-term social interactions restricted to mothers and their calves, large aggregations have been documented during foraging and mating events (Best & Da Silva, 1989; Best & Da Silva, 1993; Martin, Da Silva & Rothery, 2008). A major constraint in studying river dolphins is that they do not typically perform conspicuous surface displays, making it especially difficult to identify individuals in the field (Best & Da Silva, 1989; Best & Da Silva, 1993; Brownell & Herald, 1972; Cassens et al., 2000; Crespo, Harris & González, 1998; Da Silva, 2009; Hamilton et al., 2001; Leatherwoods & Reeves, 1994; Reeves & Martin, 2009; Zhou, 2009). To overcome these challenges we aimed to study the acoustic repertoire of free-ranging Arauguaian botos (Hrbek et al., 2014) that regularly visit a fish market in Mocajuba in Northern Brazil (Dos Santos et al., 2014; Rodrigues et al., 2019). This semi-controlled setting gave us the unique opportunity to combine acoustic technology with underwater behavioural observations. Our goal was to document the diversity of the acoustic signals produced by these dolphins and the behavioural context in which these signals are produced. As Inia acoustic repertoire has been underestimated in the literature (e.g., Podos, Da Silva & Rossi-Santos, 2002) we expect to find greater diversity of acoustic signals than what was described so far.
Material & Methods
Study area
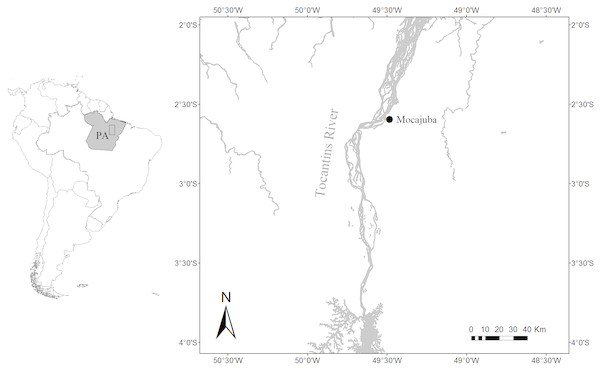
This study took place along the Tocantins River in the town of Mocajuba in Pará State, Brazil (Fig. 1). The Tocantins River is classified as a clearwater river, it has a small floodplain that crosses through narrow valley. There are large sandbanks in the river’s main channel where herbaceous vegetation may occur, in addition to floating vegetation and submerged aquatic macrophytes where there is light penetration (Junk et al., 2011). At its lower reaches, water cycles are very dynamic with the greatest rainfall from November to April, the highest waters in March, and lowest waters in September (Ribeiro, Petrere & Juras, 1995). There is also a daily cycle of tide pulses (Goulding et al., 2003; Ribeiro, Petrere & Juras, 1995). Mocajuba has a fish market that serves as the main place to acquire fish products for the city and the nearby riverside communities. The wastes of the market and the provision of fish by locals attracts botos to the pier. This set-up together with low turbidity waters during the dry season allows rare proximity to botos, enabling us to identify individuals and observe their behaviour in detail.
Figure 1: Location of the study area.
Location of the Mocajuba fish market at the margins of the Tocantins River.Data collection
Acoustic and behavioural data were collected in visits that ranged from 3 to 15 days during October to December 2013, March 2014, June 2015, July, September and December 2016. The presence of botos at the market depends on the market opening hours, which is the time when the animals are fed (Dos Santos et al., 2014). Therefore, our observations took place in the morning. Behavioural observations were collected in a continuous all-events sampling (Mann, 1999). For each session, we collected the following data: number of individuals present, age class (adult, juvenile, calf), and sex (based on the presence of mammary slits). In addition, animals were identified based on natural marks on the dorsal and ventral parts of the body, given that the botos in the market frequently swim upside down (Dos Santos et al., 2014). Photographs of their bodies were taken with a Nikon 3200 SLR Camera (Nikon Corp., Tokyo, Japan) and a 70 × 300 mm zoom lens (Nikon Corp., Tokyo, Japan). Underwater video was collected with a GoPro Hero 4 (GoPro Inc., San Mateo, CA, USA) held by hand. Notes and drawings of the marks and their locations were also taken if we were unable to take pictures. We held permits to perform this study issued by SISBio (number 52892) from the Brazilian Mistry of Environment.
Sound recordings were taken continuously in synchrony with behavioural observations. We used three recording systems along the study: (1) an Aquarian hydrophone (AFAB Enterprises, Anacortes, Washington, WA, USA) connected to a Tascam DR1 digital recorder (22 kHz sampling rate), (2) a CR1 hydrophone (Cetacean Research Technology, Seattle, WA, USA) connected to a pre-amplifier and a Tascam DR-44WL (96 kHz sampling rate) and (3) a Soundtrap (Ocean Instruments, New Zeland, 576 kHz, sampling rate). Given that Inia social sounds are mostly below 48 kHz (May-Collado & Wartzok, 2007) and that the signals found in this study rarely go above 10 kHz (see Results below), we do not believe that the use of different recording systems had an impact in our results. Moreover, the analysis we used here controls for the possible interferences of technical artefacts on sound categorization (see below).
Data analysis
All recorded signals were inspected using a spectrogram analysis in Raven Pro 1.5 (Cornell Laboratory of Ornithology, New York, NY, USA). Only whistles and pulsed calls with high signal to noise ratio were selected for further analysis. Following this, we extracted the contours from the fundamental frequency of the lowest visible element of the selected sounds using a MATLAB routine called Beluga (https://synergy.st-andrews.ac.uk/soundanalysis/). We then used an adaptive resonance theory neural network combined with dynamic time-warping to group the contours into distinct categories (ARTwarp; (Deecke & Janik, 2006)). This analysis classifies frequency contours according to a critical similarity value or “vigilance” (Deecke & Janik, 2006). In order to account for minimal differences in our classifications we used a high vigilance of 96%. Unlike other methods used to categorize sounds, ARTwarp uses the whole signal while running classifications. In addition, the analysis also allows contours to be shrunk or stretched up to a factor of three, ensuring maximum overlap in the frequency domain when sounds are being compared. This feature may increase the chances of sounds being classified into biologically significant categories (Deecke & Janik, 2006), as patterns of frequency modulation are often more relevant for animal auditory perception than patterns of duration. To reduce the effect of ambient noise and possible technical artefacts from the different recording systems, we re-sampled all frequency contours at 10ms. The analysis was conducted on a MATLAB-based (version 2015b, The Mathworks, Inc.) routine called ARTwarp. Using a rarefaction curve (Magurran, 2004), we evaluated how much of the acoustic repertoire was registered during our sampling period. Using a Whittaker diagram (Magurran, 2004), we assessed the occurrence of the signals recorded as part of these animal’s repertoire. Analyses were conducted in R (version 3.5.1) (R Core Team, 2018).
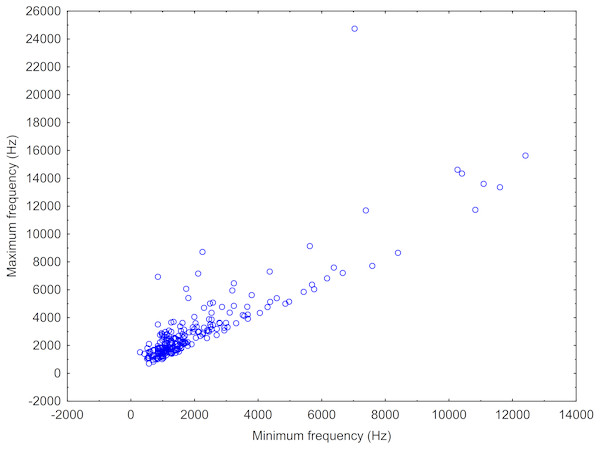
After this, we made a scatterplot of the minimum versus maximum frequency of the sound types (or “neurons”) resulting from the ARTwarp analysis to estimate the frequency bandwidth of vocalization of Araguaian botos. In addition to the ARTwarp analysis we took information on other characteristics of boto sounds: duration—short (<200ms) versus long (>200ms) signals, and the presence of nonlinear phenomena: (a) subharmonics (signals with additional spectral components in the harmonic stack, generally in multiples of or 1/3 of the fundamental frequency) and (b) biphonation (signals with the presence of two independent fundamental frequencies) (Tokuda et al., 2002; Wilden et al., 1998).
Results
Botos were observed on each of 32 days of data collection effort, resulting in 15.57 h of acoustic recordings. Observed groups ranged between three to 12 individuals. Nine dolphins repeatedly visited the market, allowing us to observe them multiple times. These included five adult females, one adult male, one juvenile female, one female calf and one male calf. The only two behaviours observed during acoustic recordings were socialization and feeding (Fig. 2). Social interactions consisted of animals having physical contact with one another and swimming alongside each other. Occasionally animals would bite the neck of another when waiting to be fed. While we did not test for associations between individuals, the most stable associations appeared to be between mothers and their calves. Feeding behaviour consisted of animals soliciting food with their open-mouthed head out of the water or poking humans with their snout. Furthermore, with the help of underwater cameras we were able to match some of the observations to the vocalizing animals (see below).
Figure 2: The two behaviours observed during this study.
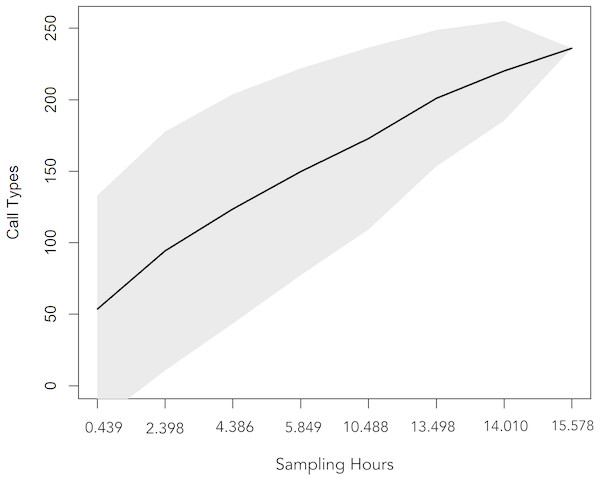
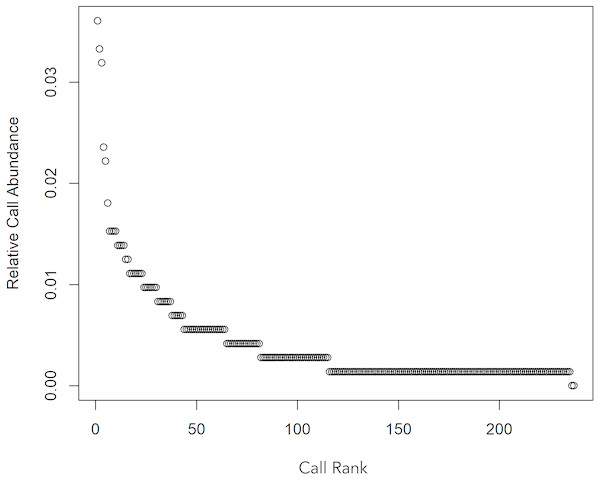
The two behaviours observed during this study were socialization and feeding. (A) and (B): botos engaged in social activity, slow-swimming and physical contact, (C): boto feeding on a fish and (D): waiting to be fed. Photos (A), (B) and (C) by Gabriel Melo-Santos and (D) by Luiza Pereira.A total 727 of good quality acoustic signals primarily ranging between 1–10 kHz (Figs. 3 and 4) were used for the ARTwarp analysis, resulting in 237 sound-types. However, the rarefaction curve indicates that our sample was not sufficient to capture most of the acoustic repertoire of these animals (Fig. 5). While there is a great diversity of signals, these botos do seem to produce some signals more abundantly than others (Fig. 6).
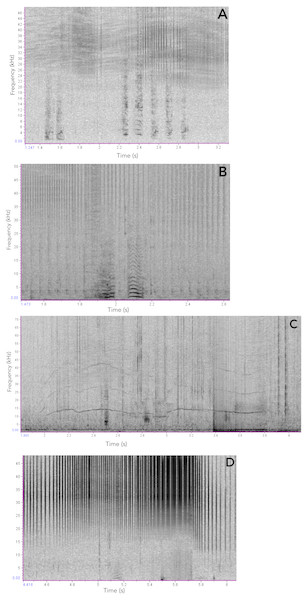
Figure 3: Sound-types produced by Araguaian River dolphins.
Frequency contours of the sound-types (neurons) resulting from the ARTwarp analysis for Araguaian botos from the Mocajuba market, Tocantins River.Figure 4: Scatterplot showing the maximum vs minimum frequencies of the neurons resulting from the ARTwarp analysis.
Figure 5: Rarefaction curve showing the cumulative number of sound-types detected with increasing sampling time (hours of recordings analysed).
The curve suggests that 15.57 hours of acoustic sampling is not enough to capture most of the acoustic repertoire of the Araguaian river dolphin.Figure 6: Whittaker diagram displaying the occurrence of sounds emitted by Araguaian botos with most sounds produced rarely.
Figure 7: Examples of Araguaian botos acoustic signals recorded during our study.
(A) short two-component calls; (B) short calls with subharmonics. (C), narrow-banded frequency modulated whistle and the longest sound registered in this study; (D) Short call with no non-linear phenomena.In addition to ARTwarp we also characterized the acoustic signals based on duration and presence of non-linear phenomena. Based on these criteria, botos sounds can be classified into: long-two-component calls, long calls with subharmonics, short calls with biphonation (short-two-component calls), short calls without non-linear phenomena, short-calls with subharmonics, and tonal sounds (Fig. 7, Table 1). The long-calls (n = 13; classified into 11 sound-types) and tonal sounds (whistles) (n = 21; classified into 18 sound-types) were rarely produced, while the short-two-component calls were the most commonly produced (n = 538). Interestingly, 74% (n = 538; classified into 184 sound-types) of these were short two-component calls. Underwater video of bubble emission from the blowhole indicate that calves produced short-two- component calls followed by physical contact with their mothers (Fig. 8; Audio S1–S4).
| Vocalization type | Number of sounds produced |
|---|---|
| Long two-component calls—pulsed calls longer than 0.200s, with biphonation as the most marking characteristic, may exhibit subharmonics | 12 |
| Long calls with subharmonics—calls longer than 0.200s, may exhibit biphonation | 1 |
| Short two-component calls—pulsed calls shorter than 0.200s, with biphonation as the most marking characteristic, may exhibit subharmonics | 538 |
| Short calls—signals shorter than 0.200s with no non-linear phenomena | 53 |
| Short calls with subharmonics—calls shorter than 0.200s, may exhibit biphonation | 102 |
| Whistles—tonal narrow-banded signals longer than 0.100s, may exhibit harmonics | 21 |
| Total | 727 |
Discussion
Our results show that the Araguaian river dolphin has a more diverse acoustic repertoire than previously documented for the genus Inia (Amorim et al., 2016; Caldwell, Caldwell & Evans, 1966; Diazgranados & Trujillo, 2002; May-Collado & Wartzok, 2007; Ding, Würsig & Evans, 1995; Ding, Würsig & Leatherwoods, 2001; Kamminga et al., 1993; Podos, Da Silva & Rossi-Santos, 2002). Early studies described members of the genus Inia as silent animals (Podos, Da Silva & Rossi-Santos, 2002) or with a limited acoustic repertoire (Amorim et al., 2016; Caldwell, Caldwell & Evans, 1966; Diazgranados & Trujillo, 2002; May-Collado & Wartzok, 2007; Ding, Würsig & Evans, 1995; Ding, Würsig & Leatherwoods, 2001; Kamminga et al., 1993; Podos, Da Silva & Rossi-Santos, 2002). In contrast, we show that the acoustic repertoire of Inia dolphins is diverse and likely as complex as the acoustic repertoire of delphinids.
As described for other Inia species, the Araguaina dolphins recorded in our study rarely emit whistles. These findings agree with previous descriptions of whistle emission from Peruvian (Ding, Würsig & Leatherwoods, 2001) and Ecuadorian botos (May-Collado & Wartzok, 2007) and other river dolphins like the Franciscana and the baiji (Cremer et al., 2017; Wang, Wang & Akamatsu, 1999; Wang et al., 2006). In these studies, the function of emitted whistles was unclear. May-Collado & Wartzok (2007) found that botos emitted whistles in a different social context than in delphinids, keeping distance between each other, rather than promoting social interactions as in marine dolphins.
Among pulsed calls, the short-two-component call was the most commonly produced sound. These calls were emitted in what appear to be mother-calf interactions. Our video footage and some underwater follows show bubbles emanating from calves’ blowholes while they emitted these calls as they approached their mothers after a short separation (see Videoes S1 and S2 in DOI: 10.6084/m9.figshare.7992212). Bubble streams are often used as a cue to identify vocalizing animals (Bebus & Herzing, 2015; Fripp, 2005; Jones, 2014) and in this case the bubble stream revealed that the calves were producing the calls and did so in a repetitive fashion. These vocal patterns are similar to what has been described for calves of bottlenose dolphins, which use signature whistle as contact calls, where calves increase whistle emission as they approach their mothers (Smolker, Mann & Smuts, 1993). Given the strength of mother-calf associations in botos (Best & Da Silva, 1989; Best & Da Silva, 1993) and the characteristics of their habitat, a shared signal that enhances mother-calf recognition may be key as they move through murky waters and complex underwater vegetation. The complex structure of botos’ habitat might also have led to evolution towards signals with short duration, longer signals might suffer interference of echoes caused by obstacles (sandbanks, underwater vegetation, riverbed, even the water surface). Notwithstanding, social signals produced by Inia sister taxa Pontoporia who also evolved in riverine environments are short as well (Cremer et al., 2017). Meanwhile, the frequency bandwidth of Araguaian river dolphins vocalizations are intermediate when compared to delphinids and baleen whales (Au, 2000; Boisseau, 2005; Clark, 1994; Clark, 1995; Lammers, Au & Herzing, 2003; May-Collado & Wartzok, 2009; Tyack, 2000). Araguaian botos’ social sounds are lower in frequency than those of delphinids, though not as low as baleen whale calls. Sounds with lower frequencies should travel greater distances and due to larger wavelength would be able to deviate from possible obstacles in between vocalizing animals (e.g., submerged vegetation, rocks). We hypothesize that because groups o Inia are not as cohesive as delphinids, sounds emitted at intermediate frequency range would be more efficient for communication in a complex habitat as rivers. However, further studies are necessary to test this hypothesis.
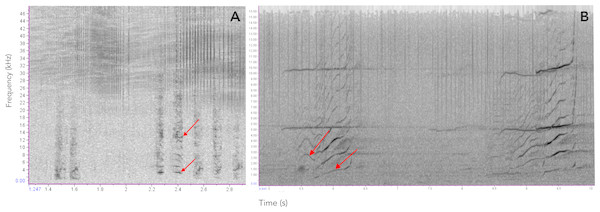
Several species of toothed whales emit calls of similar acoustic nature as the ones described here for botos (Filatova et al., 2012; Fitch, Neubauer & Herzel, 2002; Ford, 1989; Deecke, Ford & Spong, 1999; Deecke et al., 2010; Deecke et al., 2011; Garland, Castellote & Berchok, 2015; Marcoux, Auger-Méthé & Humphries, 2012; Miller & Bain, 2000; Papale et al., 2015; Pérez et al., 2017; Sjare & Smith, 1986; Vergara & Barrett-Lennard, 2008; Vergara, Michaud & Barrett-Lennard, 2010; Yurk et al., 2002; Zwamborn & Whitehead, 2017. For example, the calls of orcas (Orcinus orca) and pilot whales (Globicephala spp.) have been shown to contain non-linear features suggesting they may carry information on group identity and maintaining social cohesion (Deecke et al., 2010; Pérez et al., 2017; Yurk et al., 2002; Zwamborn & Whitehead, 2017) (see Fig. 9 for an example). Similarly, Marcoux, Auger-Méthé & Humphries (2012) show evidence that narwhal (Monodon Monoceros) calls might be related to specific groups or individuals. Non-linear calls have also been reported to convey individuals’ identity and/or emotional state (Fitch, Neubauer & Herzel, 2002; Papale et al., 2015). Given these similarities we propose these two-component signals could have evolved early in the evolutionary history of toothed whales as social contact signals, likely for mother-calf interactions and later in the lineage leading to delphinids it evolved into a group recognition signal.
We recommend that future studies analyse recordings of non-habituated botos, to verify possible differences in the acoustic behaviour of human-habituated and non-human habituated animals. Nevertheless, the botos recorded in this study are free-ranging animals that interact with other members of their population when not in the Mocajuba market, and therefore their sounds are likely representative of their species.
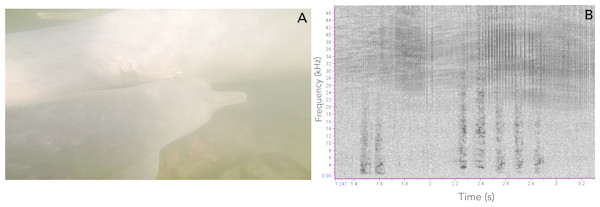
Figure 8: Vocalizations of an Araguaian boto calf.
(A) Vocalizing calf as indicated by the bubbles blowing from its blowhole and associated short-two-component call, taken from video footage by Paulo Castro. (B) Spectrogram of calls produced by a calf of Araguaian boto, followed by a bubble stream.Figure 9: Comparison of sounds produced by Araguaian botos and orcas.
Spectrograms comparing the acoustic structure of two-component pulsed calls produced by (A) Araguaian botos and (B) orcas. The two components of both calls are indicatd by red arrows. Calls from orcas were downloaded from NOOA free database on https://www.nefsc.noaa.gov/psb/acoustics/sounds.html.Conclusions
We show that the acoustic repertoire of botos is diverse and includes a wealth of signal types. The Araguaian river dolphins studied at Mocajuba fish market produce a diverse acoustic repertoire, as we found 237 sound-types, mostly pulsed calls, and our analysis indicate that there is more to discover. Notwithstanding, these sounds are mostly complex in structure presenting nonlinear phenomena. The animals we studied are habituated to humans, which provided a unique opportunity to shed light on the acoustic and social behaviour of this understudied species. Under relatively controlled conditions we identified more than half of the studied animals and recorded their acoustic and underwater behaviour. When possible, we matched recordings with video footage of calves as they reunited with their mothers. During these reunions calves appeared to use the two-component calls as contact calls, nevertheless further investigation is needed to understand the importance of these calls for mother-calf interactions. Given that Araguaian river dolphin pulsed calls are similar in acoustic structure to those of delphinids, we propose that these signals could have evolved early in the evolutionary history of toothed whales as social calls, likely as mother-calf contact calls, and that later in the lineage leading to dolphins its function evolved to group/family call recognition, though this needs to be tested in future studies. Furthermore, studies in areas where dolphins are not human-habituated should be conducted in order to verify possible differences in the acoustic behaviour of Araguaian botos with and without direct interactions with humans.