aaquetzalli is required for epithelial cell polarity and neural tissue formation in Drosophila
- Published
- Accepted
- Received
- Academic Editor
- Shree Ram Singh
- Subject Areas
- Developmental Biology, Genetics
- Keywords
- Epithelia, Cell polarity, Ectoderm, Embryonic developent, Neurulation, Drosophila
- Copyright
- © 2018 Mendoza-Ortíz et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. aaquetzalli is required for epithelial cell polarity and neural tissue formation in Drosophila. PeerJ 6:e5042 https://doi.org/10.7717/peerj.5042
Abstract
Morphogenetic movements during embryogenesis require dynamic changes in epithelial cell polarity and cytoskeletal reorganization. Such changes involve, among others, rearrangements of cell-cell contacts and protein traffic. In Drosophila melanogaster, neuroblast delamination during early neurogenesis is a well-characterized process requiring a polarized neuroepithelium, regulated by the Notch signaling pathway. Maintenance of epithelial cell polarity ensues proper Notch pathway activation during neurogenesis. We characterize here aaquetzalli (aqz), a gene whose mutations affect cell polarity and nervous system specification. The aqz locus encodes a protein that harbors a domain with significant homology to a proline-rich conserved domain of nuclear receptor co-activators. aqz expression occurs at all stages of the fly life cycle, and is dynamic. aqz mutants are lethal, showing a disruption of cell polarity during embryonic ventral neuroepithelium differentiation resulting in loss of epithelial integrity and mislocalization of membrane proteins (shown by mislocalization of Crumbs, DE-Cadherin, and Delta). As a consequence, aqz mutant embryos with compromised apical-basal cell polarity develop spotty changes of neuronal and epithelial numbers of cells.
Introduction
Cell polarity is a crucial crossroads for epithelial development and function. Epithelial polarization generates and maintains differentiation of apical and basolateral membrane domains. In fruit fly embryos, cellularization and cell polarity come first and preempt gastrulation and further development (Campos-Ortega & Hartenstein, 1997). Cellular blastoderm fly embryos exhibit cell polarization (Nusslein-Volhard & Wieschaus, 1980). For this, both membrane-attached and cytosolic cytoskeletal proteins are required. Failure of proper polarization leads to early death with a very early phenotype during cellularization and/or germband extension (Pai et al., 1996; Peifer & Wieschaus, 1990; Tepass et al., 1996).
Once attained, cellular polarization must be regulated and maintained (Campos-Ortega & Hartenstein, 1997). A well-studied case is neurulation in the fly. The embryonic tissue that gives rise to both fly neural and skin precursors is the ventral ectoderm, or neuroectoderm (Campos-Ortega & Hartenstein, 1997; Campos-Ortega & Knust, 1990). Early positional cues establish the neuroectodermal region in the procephalon and the ventrolateral ectoderm (Egger, Chell & Brand, 2008; Technau, Berger & Urbach, 2006). Within this epithelial sheet, initially equivalent groups of cells, the proneural groups, form, evidenced by expression of marker genes, like the basic-helix-loop-helix (bHLH) achaete-scute complex and molecules of the Notch (N) signaling pathway. Among these cells, Notch signaling limits the number of neural precursors that delaminate and form neuroblasts (Bertrand, Castro & Guillemot, 2002; Campos-Ortega & Knust, 1990; Cornell & Ohlen, 2000; Hartenstein et al., 1992; Portin, 2002; Skeath et al., 1992; Technau, Berger & Urbach, 2006; Zhao, Wheeler & Skeath, 2007). From each individual proneural cluster, normally just one cell differentiates as a neuroblast (Cabrera, 1990; Fortini, 2009; Fortini & Artavanis-Tsakonas, 1993; Schrons, Knust & Campos-Ortega, 1992). Neuroblasts migrate and divide to give rise to ganglion mother cells, and ultimately, to neurons and glia (Karcavich, 2005; Zhong & Chia, 2008). Non-selected cells receiving the Delta signal remain in the ectoderm and become epidermoblasts, differentiating into skin cells (Hartenstein & Wodarz, 2013; Portin, 2002). The process necessitates maintenance of polarity and changes in cell shape (Harris & Tepass, 2008; Harris, 2012; Wang et al., 2004).
Recent studies show that cell polarity regulatory proteins (CPRPs), such as Cdc42, Par complex components, and WASP proteins establish apical-basal cell polarity (Bayraktar, Zygmunt & Carthew, 2006; Harris & Tepass, 2008; Krahn & Wodarz, 2009; Tepass, 2012). Epithelial defects like loss of cell dissociation and apical-basal polarization affect adherens junctions (AJ), and the way in which Dl and N interact (Buszczak et al., 2007). Membrane polarization and Dl activation are critical events during early neurogenesis (Bayraktar, Zygmunt & Carthew, 2006; Fehon et al., 1991; Harris & Tepass, 2010; Kooh, Fehon & Muskavitch, 1993; Liu et al., 2012; Weinmaster & Fischer, 2011). Disrupted CPRPs alter epithelial integrity and cause abnormalities in neuroblast proliferation and morphogenetic movements during early neurogenesis. This may result in nervous system hyperplasia paralleled by loss of epidermis, a typical “neurogenic” phenotype (Bayraktar, Zygmunt & Carthew, 2006; Harris & Tepass, 2008; Harris & Tepass, 2010; Krahn & Wodarz, 2009; Wang et al., 2004; Weinmaster & Fischer, 2011).
Here we characterize the aaquetzalli (aqz) gene, which encodes several alternatively spliced transcripts expressed throughout the life cycle. aqz, meaning ‘fan’ in nahuatl, and the name refers to the abnormal embryonic cuticular phenotypes of mutant embryos. Mutations in aqz are lethal, with an extended phenocritical period. Previous studies suggest that aqz is required pleiotropically during Drosophila embryogenesis: aqz mutant germline clones die with cuticular holes (Perrimon et al., 1996), and aqz RNAi injected in developing embryos show axon guidance and synaptogenesis defects (Ivanov et al., 2004). These mutant phenotypes are in ectodermally derived cells (epidermis and nervous tissue), despite affectation in all cells in the first case, and generalized injection of RNAi in developing embryos in the second case. Therefore, these experiments support the tenet that aqz is required in ectodermal tissues.
In order to fully characterize the locus, we generated and identified new mutant aqz alleles. These alleles show new, more generalized zygotic neural and epidermal mutant phenotypes similar to N pathway mutants (“neurogenic” phenotypes). aqz is expressed early in the ectoderm and ectodermally derived tissues. Our findings describe and establish an early role for aqz in ectoderm differentiation as a modulator of cell polarity, promoting apical polarization to maintain epithelial integrity. aqz modulates Dl membrane localization during neural lateral inhibition.
Materials & Methods
Fly strains, genetics and husbandry
Flies were housed in standard conditions (25 °C, 12:12 light:dark cycles and 50% humidity) in standard food vials (unrefined sugar-baker’s yeast-agar-gelatin-water). Oregon R and yw were used as control stocks. aqz2, aqz6, and aqz7 are lethal insertion alleles. aqz2 is Bloomington stock #14691 (y1 w67c23(y1 w67c23; P{y[+mDint2] w[BR.E.BR]=SUPor-P}KG08159 ry506); aqz6 is Bloomington stock # 20315 (y1 w67c23; P{EPgy2}CG9821EY11495). aqz7 was a lethal insertion line that is now lost (former Bloomington stock #1492). aqzGFP is also a transposon insertion, and was stock #CB03335 from the former FlyTrap Stock Collection at Yale University, USA. It is a lethal insertion that generates chimeric proteins GFP::Aqz. New aqz alleles generated: aqz1, aqz3 and aqz5, are excision events. aqz3 is an excision event from the now lost aqz7 insertion. aqz1 and aqz5 are excisions from aqz2. All excision alleles are lethal.
aqzrescue was generated by transgenesis using P[acman] BAC #CH322-25015 (Venken et al., 2009). CH322-25015 has approximately a 19 Kb fly genomic fragment from 4631615 to 4650999 on chromosome three, including the complete aqz locus (CG9821), CG9836 or IscU, iron sulfur cluster assembly enzyme (Tian et al., 2014), where the known mutants alleles die between late larval life and pupariation, CG8369 (an undescribed Kazal domain containing protein), CG9837 and CG8359, or hng2, hinge2, plus a lncRNA: CR43130, that partially overlaps CG9821, but in the opposite orientation. The whole genomic rescue construct was inserted on 2R by recombineering at 53B2 (using Bloomington line #9736). We also used three different deficiencies that uncover the aqz locus to perform complementation tests: Bloomington stock # 7629 (w1118; Df(3R)Exel6150, P{XP-U}Exel6150/TM6B, Tb1); Bloomington stock # 24982 (w1118; Df(3R)BSC478/TM6C, Sb1 cu1), and Bloomington stock #25010 (w1118; Df(3R)BSC506/TM6C, Sb1 cu1).
in situ hybridization
Embryo in situ hybridization was done following (Tautz & Pfeifle, 1989), with few modifications for fluorescence detection. Briefly, whole embryos were fixed with formaldehyde, and hybridized with digoxigenin RNA probes overnight at 55°C in hybridization buffer. aqz 5′ RNA probes (derived from clone LD47990, which corresponds to the 5′ end of aqz, sense and anti-sense) were synthesized using Roche’s digoxigenin RNA labeling kit following the manufacturer’s protocol (Roche, Basel, Switzerland). The fixed and hybridized embryos were incubated with polymerized HRP-conjugated anti-digoxigenin antibody at a 1:2000 dilution for 1 hr 45 min at room temperature, washed and incubated with 1:10 diluted Tiramidine-CY3 for 45 min, using the tyramidine derivative system (TSA; Perkin Elmer, Waltham, MA, USA), and mounted in Vectashield (Vector Laboratories, Burlingame, CA, USA).
Northern blot analysis
Total RNA was isolated from 8–12 hr old embryos, 2nd and 3rd instars larvae, pupae, and adults with Trizol, according to the manufacturer’s instructions (Gibco/BRL, Waltham, MA, USA). Northern blotting was done according to standard procedures (Sambrook, Fritsch & Maniatis, 1989). Approximately 3–4 micrograms of total RNA was loaded per lane. DNA probes were from cDNA clone LD47990 (Stapleton et al., 2002), for the aqz 5′ region, which is common to all aqz transcripts, and clone LD02090, which labels the 3′ end of the gene not present in aqz RA, but present in aqz RB, RC, and RD. The probes were labeled with a-32P deoxycytidine 5′-triphoshpate, following Feinberg & Vogelstein (1984). Both cDNA clones were purchased from the Berkeley Drosophila Genome Project clone collection.
Antibody generation
A rabbit polyclonal antiserum was generated against the KSIRLKKGAC peptide, representing the Aqz amino terminus. Immunoglobulins G (IgGs) were affinity purified from immune serum (New England Peptide, MA, USA). This antibody failed to detect Aqz proteins in Western blots, but detectd Aqz in tissue sections.
Western blot
Embryos were homogenized and immunoblotted following (Wodarz, 2008). Proteins were separated on 10% denaturing acrylamide gels (SDS-PAGE), transferred to nitrocellulose membranes and incubated with rabbit anti-GFP (1:5000, SC-8334, Santa Cruz Biotechnology, USA) as primary antibody. We pre-absorbed anti-GFP before use by incubating diluted primary antibody with wild type fixed embryos in incubation solution overnight. We used Alkaline Phosphatase (AP)-conjugated secondary antibodies (goat anti-rabbit 1:1000 from Zymed; Zymed, South San Francisco, CA, USA). Secondary antibody was incubated for 1 h at room temperature. We used the NBT/BCIP substrate solution (Roche Diagnostics, Basel, Switzerland) for AP detection.
Immunohistochemistry
We followed the embryo staining protocol in (Karr & Alberts, 1986), except for aqzGFP embryos. The aqzGFP embryos were washed in methanol only once, and rehydrated immediately. For primary antibodies, we used rat anti-Deadpan (1:2, gift of Cheng-Yu Lee), rabbit polyclonal anti-Aqz (1:500 this work), and rabbit anti-GFP (1:100, SC-8334, Santa Cruz Biotechnology, pre-absorbed with wild type embryos in incubation solution overnight before use). Polyclonal rat anti-Elav (7E8A10, 1:250), polyclonal rat anti-DE-cad (DCAD2, 1:10), mouse monoclonal anti-FASIII (7G10, 1:100), mouse monoclonal anti-EVE (3C10, 1:10), mouse monoclonal anti-Coracle (C615.16, 1:250), mouse monoclonal anti-22C10 (1:200), mouse monoclonal anti-BP102 (1:200), mouse monoclonal anti-Repo (8D12, 1:200), mouse monoclonal anti-Dl extracellular domain (C594.9B, 1:100), mouse monoclonal anti-Crumbs (Cq4, 1:100), and mouse monoclonal anti-Dlg (4F3, 1:3) were from the Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA. Primaries were incubated with fixed embryos overnight at 4 °C. Embryos were then washed 4 times for 20 min each using 0.3% Triton X-100 in PBS. Anti-Deadpan, anti-Aqz, anti-Eve, anti-DE-cadherin, anti-GFP and anti-Delta antibody signals were amplified using biotin-conjugated secondary antibodies in combination with the Vector Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) according to the manufacturer’s instructions. Fluorescent-coupled (1:1000, Zymed, South San Francisco, CA, USA) and biotin-conjugated secondary antibodies (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) were incubated for 2 h at room temperature. For embryos incubated with biotin-conjugated antibodies, we used the dye-labeled tyramidine derivative system (TSA) for signal amplification. After washing 4 times with 0.3% Triton X-100 in PBS, fluorescently labeled embryos were mounted in Vectashield.
Cuticle preparations
We followed the protocol described by Nusslein-Volhard & Wieschaus (1980). Embryos and 1st instar larvae were collected 24 h after egg laying, dechorionated in 50% bleach for 5 min, rinsed in distilled water and placed in an Eppendorf tube containing 50% heptane and 50% methanol, and shaken vigorously for 2 min. The upper phase was removed and both embryos and 1st instar larvae were washed with 100% methanol three times. Embryos and 1st instar larvae were rehydrated with PBT (PBS + 0.3% Triton X-100). Once rehydrated, they were carefully dropped onto a glass slide and covered with mounting medium (Hoyer’s Medium or PVA) and coverslipped. They were incubated at least one day in a hot plate. Cuticles were visualized using dark field microscopy.
Embryonic lethality
To assess embryonic lethality, we separated and collected aqzGFP homozygote eggs from 24 h egg-laying plates under a dissecting microscope equipped with a blue light source and a GFP filter. Homozygote mutant embryos fluoresce with GFP, and can be distinguished from control (non-fluorescent) or heterozygous (weakly fluorescent) eggs. These were placed on fresh egg-laying plates alongside wild type, control Oregon R eggs from the same day, and incubated a further 72 h. At the end, non-hatching, dead embryos were counted.
DNA sequencing
Automated sequencing was performed using an ABI 310 sequencer. We sequenced fully cDNAs SD19655, LD74990, RE74095, LD18978 and LD02060. For aqz1, we selected homozygous mutant embryos by lack of GFP expression present in the balancer chromosome, and made a crude homogenate from them, to use for PCR amplification. We PCR-amplified using primers AqzA (TACACCACCGTCTATTGCG) and AqzB (CCCCATTGTGTCAGATTTCTT), amplifying the aqz promoter region for aqz1. We cloned the ensuing PCR products in pGEMT Easy vector (Promega, Madison, WI, USA). The PCR product inserts were sequenced fully. We sequenced three independent mutant clones, and all confirmed the mutation in the promoter region of aqz1.
Genetic assays
Crosses or stocks were let to lay eggs in agar plates, and eggs laid were collected, classified, and counted. We selected stage 14–15 embryos (late neurogenesis). Embryos were fixed and stained using anti-Elav (NS) and anti-Coracle (epidermis), to score neurogenic phenotypes. The percentage of embryos with wild type (WT, no defects in the NS and epidermis) or “neurogenic” phenotype was calculated by using the following formula: % of WT or neurogenic embryos = WT or neurogenic embryos/total of homozygotes embryos counted*100. Fisher’s exact test was used to assess significance of the differences indicated. We scored at least 400 embryos for every experiment.
aqz rescue experiments
To rescue aqz in an endogenous genomic context that includes the whole locus and alternatively spliced transcripts, we used a genomic BAC (#CH322-25015) that has a genomic insert of 19, 384 bp in the attB-P[acman]-CmR-BW vector, roughly centered on CG9821 encompassing the whole locus, but including four other neighboring genes (CG9836, CG8369, CG9837, and CG8359, plus a lncRNA: CR43130, that partially overlaps the aqz sequence in the opposite orientation). These other loci, excepted aqz, have no known lethal alleles. Of these, only IscU (CG9836) and hng2 (CG8359), besides aqz, are expressed significantly during embryogenesis (there is mid- to late embryonic expression of CG9837) (Dos Santos et al., 2015). Mutant alleles or insertions in these loci (stocks # 22145, 34193, 31835, 14691) behave differently from aqzGFP, as they fully complement the Df(3R)Exel6150 that is embryonic lethal over aqzGFP, and not surprisingly fully complement aqzGFP as well (Table S1). We inserted this genomic construct, thereafter called aqzRESCUE construct, by standard methods at an attB acceptor site at 53B2, as stated above. This insertion has no phenotype in a wild type background. We then constructed stocks aqzRESCUE; aqzmutant and controls that were doubly balanced with the T(2:3)CyO-TM3,Ser,GFP fused chromosome two and three balancers (Bloomington stock #5703). Homozygous mutants either with one or two copies of the aqzRESCUE construct were assessed for rescue both by staining embryos as above for neurogenic phenotypes, and for adult viability. For adult viability, homozygous mutant embryos and controls with and without rescue construct present were collected from egg lays, allowed to develop, and emerging adults were scored. Fisher’s exact test was used to assess significance of the differences.
Image acquisition and processing
We studied embryos using light, fluorescence, and laser scanning confocal microscopy (LSM). Cuticles and immunofluorescence of whole embryos were imaged using a digital camera (CoolSnap; Photometrics, Tuscon, AZ, USA) on a Nikon E600 Eclipse microscope with Plan-Fluor 20X/0.50 NA and a Nikon Super High Pressure Mercury Lamp. Confocal sections of embryos were made in a Zeiss LSM 510 Meta with Plan-Neofluar 25x/0.8 NA and a Plan-FLUAR 100X/1.45 NA objectives (Carl Zeiss, Oberkochen, Germany), or a Zeiss LSM 780 with Plan-APOCHROMAT 25X/0.8 NA and Plan-APOCHROMAT 63X/1.40 NA objectives (Carl Zeiss, Oberkochen, Germany) at 18 °C. For cuticle and fluorescence image processing, we use iVision 4.0.12 software (BioVision Technologies, Milpitas, CA, USA). Confocal sections were processed using the Image Browser software (Carl Zeiss, Oberkochen, Germany) used to generate image stacks. All captured images were processed using Adobe Photoshop software.
Ribosome footprinting, mRNA-seq, sequence visualization and alignment
We used raw data generated and publish by (Dunn et al., 2013) available in NCBI’s Gene Expression Omnibus (Edgar, Domrachev & Lash, 2002) under GEO series accession number GSE49197. We used the Integrated Genome Browser (IGB) software for visualization and exploration of the aqz genome locus and corresponding annotations (Nicol et al., 2009).
Results
The aqz locus
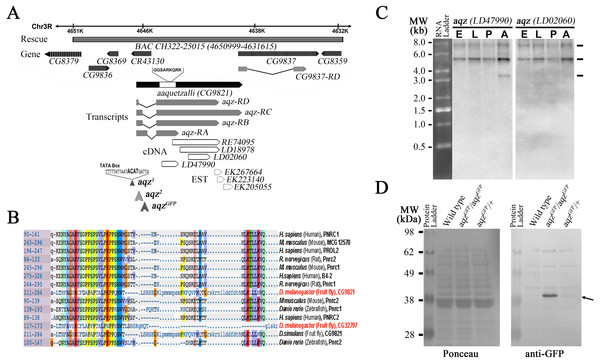
The isolated aqz transposon insertions map to a locus (CG9821 in Flybase) at 85B2 on the right arm of the third chromosome. This locus has alternative splicing at the end of the first exon and different 3′ ends, as judged from ESTs from the locus, and gives rise to at least three different transcripts differing in size and composition (Figs. 1A, 1C (Dos Santos et al., 2015)). The CG9821 locus spans over 7Kb, partially overlapping a non-coding RNA gene at its 5′ end (CR43130). It also overlaps CG9837 at its 3′ end (at least 89 bp of the 3′ end of CG9821 overlap the CG9837 5′ end). CG9837 is transcribed in the same direction as CG9821. The 5′ end of CG9821 has a proline-rich domain found in nuclear receptor co-activators (PNRC-domain proteins), present in invertebrate and vertebrate proteins (Fig. 1B).
Figure 1: The aaquetzalli (aqz) locus codes for at least three alternatively spliced transcripts.
(A) aqz encompasses a little above 7 Kb, from 4647000 to 4638000 at the base of chromosome 3R at 85B2; in the figure the genes are oriented such that the 5′ end of aqz is to the left. Besides aqz (solid black arrow), other genes mapping in the region are shown as grey-hashed arrows. aqz codes for four predicted different mRNA species, each with two exons (grey boxes) and one intron (black line). Several cDNAs and ESTs (white boxes with black borders, and white boxes with black dotted lines, respectively) map in the locus. Underneath the depicted chromosome line a grey bar signals the extent of the genomic rescue construct employed (BAC CH322-25015), inserted in the second chromosome at 53B2 (Bloomington line #9736). The location of two transposon insertion mutants, one in the promoter region (aqz2), and one in the intron (aqzGFP), are shown. aqzGFP is a fly trap line (#CB03335), coding for a predicted fusion protein, where the GFP moiety is fused to the 5′ end of Aqz. aqz1 has a four base pairs insertion, depicted in bold, in the promoter region of aqz between the predicted TATA box and initiation of transcription. aqz1 was isolated as an imprecise excision of the aqz2 transposon. Nearly all isolated aqz alleles are lethal. (B) Aqz has a conserved domain at the 5′ end of the protein. The homology is for a proline-rich conserved domain of nuclear receptor co-activators (PNRC-domain proteins) in single letter amino acid code, present in both invertebrate and vertebrate proteins. Numbers to the left indicate position of the domain within the proteins. (C) Northern blot using two different aqz cDNAs as probes reveal three different transcripts of 7.53, 5.0 3 and 3.16 Kb. The smallest band (3.16 Kb) is only present in adult flies, and is only seen using a 5′ cDNA (LD47990), present in all aqz isoforms. The same blot that was used with the first probe, was photographed, then stripped, and re-probed with the a more 3′ cDNA (LD02060), that recognizes aqz RB, RC, and RD, but not aqz RA. n > 10. (D) Aqz mutant and control Western blot using an antibody against GFP recognizes a band at approximately 40 kD (right black arrow) in aqzGFP homozygotes, and weakly, in aqzGFP heterozygotes. All other weak bands are present in all lanes, including the control, wild type lane, and are, consequently, not specific.aqz cDNAs and proteins
We identified and sequenced cDNAs and genomic fragments from the aqz locus. There is a wealth of ESTs isolated from the CG9821 region, consistent with widespread expression. We sequenced fully several of these ESTs and pieced them together with other sequences available. The two exons composing the transcribed part of the locus vary in size, due to alternative splicing in the first exon and different endings of the second exon (Fig. 1A).
The Aqz protein harbor a domain with significant homology to a proline-rich conserved domain of nuclear receptor co-activators (PNRC-domain proteins; Fig. 1B). This conserved domain, a proline rich, SH3 protein-protein interaction domain thought to mediate union to several nuclear receptors in vertebrates, is present in both vertebrate and invertebrate proteins, and is 60 aa long. Within it, a YAG motif besides several proline residues and two more carboxy-terminus-located small regions of homology constitute the conserved core of the domain. Vertebrate proteins are thought to interact with nonsense-mediated RNA decay proteins (Lai et al., 2012), as well as with a variety of nuclear receptors. Knock out mice for PNRC2 are viable and fertile, but males exhibit a lean phenotype due to higher energy expenditure (Zhou et al., 2008). Whether fly proteins also exhibit similar binding and functional roles is as yet unclear. Drosophila melanogaster, among sequenced Drosophila species, possesses a second uncharacterized protein with this motif: CG32797 (Fig. 1B), mutations in which may have similar phenotypes to those encountered in mice PNCR2 knockouts. It remains to be seen whether any Aqz mutant has higher energy expenditure, or similar phenotypes to the PNCR2 knockouts.
The theoretically reconstructed cDNAs are somewhat at variance from data published for CG9821 and CG9837 (St Pierre et al., 2014). cDNAs predicted for CG9821 are shorter than the ones we pieced together, but the annotations do not take into consideration several ESTs located towards the 3′ end of CG9821, also reported, that partially overlap other identified ESTs for CG9821 and CG9837 (Fig. 1A; Fig. S1), and the RNAseq data posted from the modEncode project, which shows that the two annotated loci overlap (Celniker et al., 2009). There are four predicted different isoforms for CG9821 mRNAs, all with the same coding sequence, two of which are very similar in size (aqz-RD and aqz-RB), varying only in the size of the first exon (cDNAs of 4950 and 4719 nucleotides long, respectively). The size of the shortest predicted cDNA isoform (aqz-RA) is just 2357 nucleotides long. The longest predicted cDNA (aqz-RC) is approximately 6145 nucleotides long and it overlaps at least 89 bp with the CG9827 locus (Fig. 1A).
In order to contrast these reconstructions and annotations from ESTs and gene-prediction software (Celniker et al., 2009), we performed Northern blots. We identified in Northern blots with 5′ probes at least three differently sized mRNAs that correspond roughly in size to the ones we reconstructed, two of which are expressed throughout the fly life cycle (Fig. 1C). This last agrees well with published data showing aqz expression at all life cycle stages and in multiple tissues including the brain, ovary, imaginal discs, and larval salivary glands (Jagadeeshan & Singh, 2005; St Pierre et al., 2014).
We observe a darker band at slightly over 5 Kb that may correspond to the two 4.9 and 4.7 bands annotated for CG9821. We observe a fainter larger band at 7.5 Kb; this last may correspond to the longest reconstructed cDNA spanning CG9821 and overlapping CG9837. We also observe a smaller band at 3.1 Kb with a 5′ probe; the closest annotated CG9821 mRNA is 2.35 Kb long. This smaller mRNA species is only seen in adult flies with a 5′ probe, and not with a more 3′ probe, showing that the small cDNA comes from the 5′ end of the locus, as predicted for the 2.35 Kb species. These cDNAs share the same 5′ end harboring the PNRC domain, but have different 3′ extensions (Fig. 1C). These extensions are mostly predicted as non-coding (St Pierre et al., 2014). Since the 5′ coding sequence is common for the cDNAs, we generated an antibody against an Aqz amino terminal peptide fragment. Unfortunately, we were not able to obtain a clear and specific signal using this antibody in Western blots.
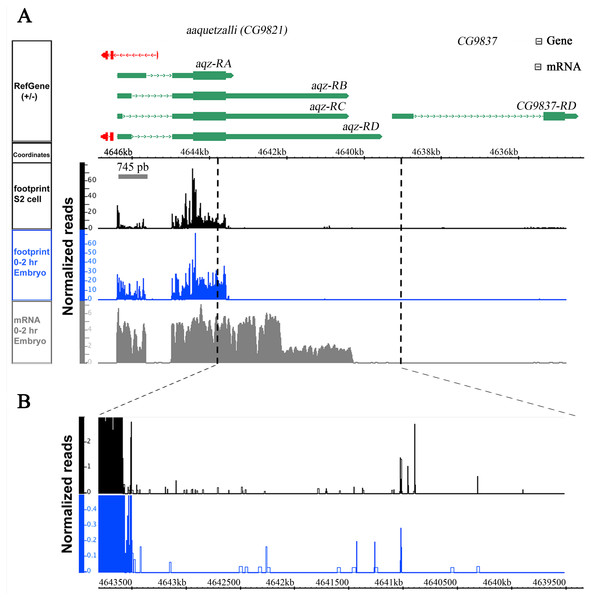
We also analyzed ribosome occupancy data published for the locus, using data from a modified protocol of ribosome profiling assay. This assay has helped reveal that stop codon read-through plays an important regulatory role in N-terminally and C-terminally protein extension during mRNA translation (Dunn et al., 2013). We performed an aqz mRNAs ribosome footprint analysis using RNA abundance and ribosome density data generated and published (Dunn et al., 2013), available at NCBI GEO (http://www.ncbi.nlm.nih.gov/geo/), and viewed the display alignments using the Integrated Genome Browser 8.1 (IGB) sequence viewer software (Fig. 2). The analysis reveals that the first exon is transcribed as well as infrequent 3′ extensions in the second exon, beyond the predicted stop codon. These data are consistent with our Northern results (Fig. 1C).
Figure 2: aqz has alterative splicing.
aqz mRNAs first exon is translated, and some have also 3′ stop codon read-through in the second exon, judging from ribosome footprinting data. The aqz locus (CG9821) together with the annotated predicted mRNAs are depicted on top (green) with dotted lines depicting introns, and red lines depicting other transcripts. The neighboring CG9837 locus, overlapping CG9821 at the 5′ end, is also depicted in green. The ribosome footprinting data is depicted below taken from S2 cells (black), 0–2 hrs. embryos (blue) and mRNA-seq (gray) in reads per million. Inset shows a magnification of the Aqz C-terminal region where protein extension may occur.aqz alleles
We identified and generated new aqz alleles. Two previously described mutant alleles appear to be weak loss-of-function alleles (named here aqz2 (also known as P14691), an insertion in the promoter, and aqz7 (also known as P1492), an insertion in an intron, now lost Perrimon et al., 1996; Fig. 1A). We generated imprecise excision lines. Mutant embryos have zygotic embryonic mutant phenotypes affecting the central NS and the cuticle (Fig. S2). The RNAi study referred to above also pointed to an aqz requirement in axon guidance and synaptogenesis (Ivanov et al., 2004).
The new alleles generated by imprecise excisions are lethal, and from these we characterized aqz1 further. Molecular analysis of aqz1 revealed a 4 bp insertion in the promoter region of CG9821 (sequence inserted: ACAT, 189 bp 5′ from the transcription start site, and 171 bp 5′ from the parental P transposon insertion), probably due to the imprecise P-element excision event (Fig. 1A). This 4 bp insertion is not present in control strains, in other aqz mutants, or in revertants from the same P-element excision experiment.
We also identified another lethal allele, aqzGFP, from a collection of gene traps in the former Yale Gene Trap stock center (Kelso et al., 2004). This allele, aqzGFP, has an insertion in the first intron/first exon (depending on the splice variant), coding for GFP, theoretically giving rise to a chimeric protein made of GFP fused to the Aqz amino terminus (GFP::Aqz). As shown in Fig. 1D, we detected only one band of approximately 40 kDa in Western blots from aqzGFP homozygous mutant embryos using an anti-GFP antibody. This band must contain GFP (of approximately 26.9 kDa), and a fragment of the Aqz protein product (of approximately 13 kDa).
In order to genetically characterize the aqz locus, we conducted complementation tests of lethal mutant alleles. The aqz1 and aqz2 alleles complement each other and three different deficiencies uncovering the locus. These mutant alleles also complement the aqzGFP allele. In contrast, aqzGFP does not complement the same three different deficiencies that uncover the locus, effectively mapping aqzGFP to the common interval uncovered by them at 85B, where the aqz locus, and the insertion points of aqzGFP and the other aqzinsertions, lie (for example, aqz2 and aqzGFP lie only 322 bp apart within the promoter/first exon/intron of the locus), yet they complement (Fig. 1A; Table 1). The fact that aqz1 complements the three deficiencies used (that do not complement aqzGFP) and aqzGFP could also be explained saying that the lethality of aqz1 maps elsewhere, despite the molecular lesion and similar mutant phenotypes. All aqz mutants, despite their origin, have the same mutant phenotypes. In all, a fraction of mutant embryos have central NS defects and holes in the cuticle (Figs. 3A–3F, Figs. S2 and S3A–S3F). As aqzGFP behaves genetically as a loss-of-function allele (does not complement all the deficiencies uncovering the locus tested with similar phenotypes and theoretically all Aqz protein isoforms are affected), we mainly focused our studies on aqzGFP.
| Allele | aqz1 | aqz2 | aqzGFP | Df(3R)Exel6150 | Df(3R)BSC478 | Df(3R)BSC506 |
|---|---|---|---|---|---|---|
| aqz1 | HL | + | + | + | + | + |
| aqz2 | HV | + | + | + | + | |
| aqzGFP | HL | L | L | L | ||
| Df(3R)Exel6150 | HL | L | L | |||
| Df(3R)BSC478 | HL | L | ||||
| Df(3R)BSC506 | HL |
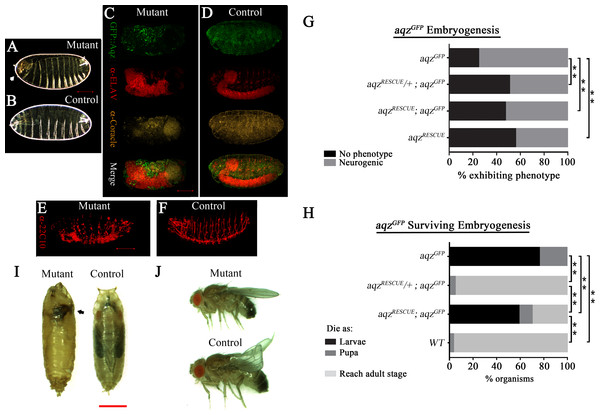
Figure 3: aqzGFP mutant phenotypes.
(A) and (B) show mutant (aqzGFP∕aqzGFP) and wild type cuticles, respectively, the former one with an anterior hole and head involution defects (white arrow). (C) and (E) show mutant embryos stained with neuronal and epithelial markers: in (C) anti-Elav for neuronal bodies and anti-Coracle (Cora) for epithelial cells, while in (E) 22C10 for axons and some neuronal bodies. (D) and (F) show the same stainings using wild type control embryos. Scale bar is 100 µm. (G) Shows the percentages of embryos with neural phenotypes in aqzGFP∕aqzGFP embryos, with one or two copies of a genomic rescue construct. One copy of the rescue construct rescues significantly the mutant phenotype, whereas two copies effects a significant, but smaller, rescue, pointing to some gain-of-function effects with two rescue constructs. Two copies of the rescue construct in a wild type background have neurogenic phenotypes in over 40% of embryos, phenotypes similar to aqz mutants. In (G) and (H) aqzGFP is homozygous mutant, aqzRECUE∕+ is one copy of the rescue construct, and aqzrecue indicates two copies of the rescue construct. (H) Extended phenocritical period of aqzGFP∕aqzGFP mutants. The surviving aqzGFP∕aqzGFP mutant embryos die majorly as larvae, with approximately 20% reaching pupariation. No adults are ever observed. The aqzGFP∕aqzGFP lethality is rescued to wild type levels with one copy of the aqz genomic rescue construct, but two copies of the rescue construct, while having a significant rescue effect, are less effective, pointing to some gain-of-function effects with two rescue constructs. (I) aqzGFP∕aqzGFP mutant pupae die early, with necrosis (black arrow), compared to a control pupae. Scale bar is 1 mm. (J) Rescued aqzGFP∕aqzGFP mutant fly, compared to sibling control fly (has curved wings as marker). In (A–H), ** denotes significant differences. In (A–H), for all embryo experiments, representative ones are shown, and n examined is over 200 per condition.We used a genomic construct encompassing the aqz locus, and performed rescue experiments for aqzGFP and aqz1 (Figs. 1A, 3G–3J–Figs. S3G–S3I). Rescue experiments show that aqzGFP behaves as a loss-of-function allele, as a significant proportion of mutant embryos survive embryogenesis and larval stages, and go on to emerge as viable adults (fertile males and unfertile females) using one copy of the rescue construct (Figs. 3H–3J). All the foregoing data, taken as a whole and in conjunction with published data, establishes the CG9821 transcription unit as being the aqz locus.
Gain of function phenotypes
Having two copies of the rescue construct did not increase embryonic rescue; rather, it proved deleterious for larvae, as many died as larvae, and, as a consequence, significantly fewer reach pupation, and adulthood (Fig. 3H). Two copies of the rescue construct in a wild type background yielded a fraction of embryos with neurogenic defects (Fig. 3G), showing that loss and gain of function conditions give similar results. These results suggest that Aqz isoforms abundance and proportions are critical for normal development. Consistently, aqz1 mutants show no adult rescue; rather, if done with one or two rescue constructs, results show a significant increment in the frequency of mutant phenotypes. This is consistent with at least a partial gain-of-function nature for aqz1, as the small Aqz protein is still present in aqz1 (Figs. S3G–S3H). Together with the rescue results of aqzGFP and overexpression, this leads support to the notion that the ratio and abundance of different Aqz protein isoforms is critical. We focused our studies on the embryonic stages, where defects are first observed.
aqz embryonic expression
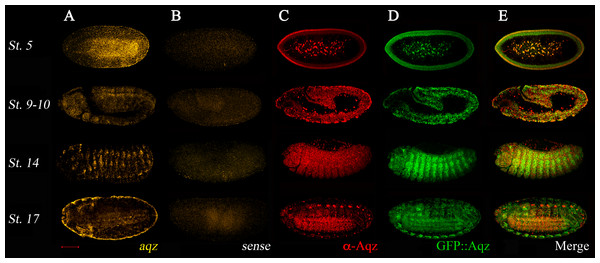
aqz embryonic expression is dynamic (Fig. 4). Expression is largely coincidental using a 5′ in situ probe from the locus, the aqzGFP GFP expression in heterozygotes, and the anti-Aqz antibody, meaning that transcription and translation are not differentially regulated. This coincidental expression of GFP::Aqz with the anti-Aqz antibody staining in aqzGFP heterozygotes can be used as a marker for Aqz expression (Fig. 4). aqzGFP homozygotes have a clumped, abnormal accumulation of GFP::Aqz, in many cases appearing nuclear. This altered expression serves as a marker for homozygous mutant embryos. During early embryogenesis in the syncytial blastoderm (st. 5 in Fig. 4), expression is seen in the peripheral cytoplasm and centrally located nuclei of future vitellophages. In later stages, during germband extension/retraction and dorsal closure stages, the ectoderm is heavily labeled, particularly the neuroectoderm. There is also mesodermal Aqz labeling (st. 9–10 and 14 in Fig. 4). During germband retraction, the peripheral nervous system (PNS) and ectoderm are labeled, and some amnioserosa cells nuclei (st. 14 in Fig. 4). As the NS condenses and embryogenesis finishes, the NS expresses aqz again, whereas the epidermal tissue expression diminishes (st. 17 in Fig. 4). This expression pattern is consistent with aqz requirements in ectoderm and ectodermally derived embryonic tissues.
Figure 4: Aqz mRNAs and proteins are expressed throughout embryogenesis in the ectoderm and in ectodermally derived structures, and have maternal contribution.
(A) Anti-sense aqz in situ hybridization results at different embryonic stages of development; (B) shows results with the sense control probe (both derived from aqz cDNA LD47990). Embryos in (C) are stained with the anti-Aqz antibody, (1:100), whereas (D) shows expression of chimeric GFP::Aqz proteins using an anti GFP antibody (1:100) from the same embryos, which are heterozygous for aqzGFP, and thus have expression of both GFP::Aqz and native Aqz protein. As shown in (E), results from both are largely coincidental except some PNS cells and some amnioserosa cells, only labeled with the anti-Aqz antibody. In (A–E), anterior is left and dorsal is up. All views are lateral views except the lower row, where ventrolateral views are shown to evidence the developing central nervous system. Stage 5 early syncytial embryo, stage 9–10 germband extension/retraction stages, stage 14 dorsal closure, and stage 17, end of embryogenesis. Scale bar is 100 µm.aqz ectodermally-derived mutant phenotypes
Two previous genetic screens identified aqz during embryogenesis. Mutant aqz germline clones generated dead embryos with anterior and dorsal holes (Perrimon et al., 1996). RNAi injection in developing wild type embryos lead to underdeveloped commissures in the ventral nerve cord, resulting in widening of the distance between longitudinal connectives of the ventral nerve cord commissures (VNC), and severe hypoplasia of the PNS (Ivanov et al., 2004). In both cases, end-of-embryogenesis phenotypes were evaluated and not quantitated. Despite possible caveats (e.g., multiple mutant hits on the chromosome arm homozygosed for germline clones generation, and putative specificity problems of RNAi, etc.) mutant phenotypes strongly implied multiple aqz requirements in embryonic ectodermally derived tissues.
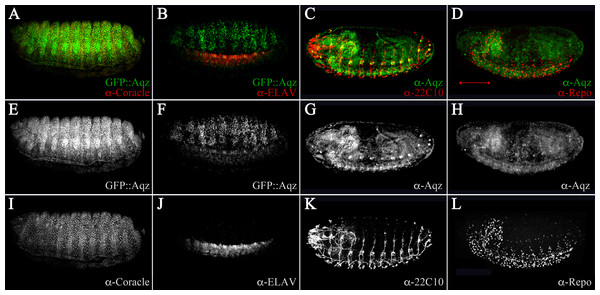
We used 22C10 and BP102 antibodies to examine nervous system commissures and the PNS, and anti-Elav plus anti-coracle antibodies to examine post mitotic neurons and epidermis, respectively. In late stage wild type embryos, there is co-expression of Coracle and Aqz proteins in the epidermis, especially in the anterior compartment of every segment (Fig. 5A). In contrast, there is no overlap with Elav staining (Fig. 5B), but there is a clear overlap in some somas of the PNS throughout the body (Fig. 5C). Finally, there is no overlap with Repo protein in glial cells (Fig. 5D). We examined embryonic defects in aqzGFP, aqz1, and other aqz mutant alleles. We wanted to know whether results reported for embryonic RNAi injections and germline clones would be similar. We corroborated and extended the aqz mutant cuticles and the disarray of the NS findings reported previously (Ivanov et al., 2004; Perrimon et al., 1996). We first quantified the number of aqzGFP mutant homozygotes that die during embryogenesis. We separated and cultured homozygous mutant aqzGFP embryos alongside wild type Oregon R control embryos, and found that 65.5% (n = 417) die as embryos, before hatching, whereas only 20.8% Oregon R embryos fail to hatch (n = 173). All aqzGFP mutant larvae fail to reach adulthood. A percentage of aqzGFP mutant embryos exhibit lack of or abnormal commissures and a strong reduction of the PNS, and sport dorsal, lateral and/or ventral holes in the cuticle (Figs. 3A–3F). aqz1 mutant embryos show the same phenotypes (Figs. S3A–S3F). All other alleles examined showed similar mutant phenotypes (Fig. S2). Mutant embryos also exhibit neurogenic-like phenotypes and cell polarity defects, consistent with Aqz being required in the developing ectoderm (see below).
Figure 5: Aqz expressed in ectodermally derived tissue.
(A) shows an anti-GFP staining for GFP::Aqz expression together with an epidermal marker, anti-Cora, showing some coincidental expression (yellow). For all immune-stainings in the figure, the panels marked underneath the top A–D labeled E–F show the GFP::Aqz channel separate, and the G–H show the anti-Aqz. The bottom row of panels marked with I, J, K and L depict the anti-coracle (I), anti-Elav (J), anti-22C10 (K), and anti-repo (L) channels, respectively. (B) shows anti-GFP for GFP::Aqz and anti-Elav, to mark neuronal cells in the developing central NS. (C) Using monoclonal 22C10, there is coincidental expression with anti-Aqz antibody, showing that some neurons in the PNS are positive for both Aqz and 22C10 (yellow). (D) In contrast no glial cells (marked by anti-Repo antibody) are coincidental with anti-Aqz antibody staining, showing that glial cells do not express Aqz. Scale bar is 100 µm.Our expression results, the germline clones, and the RNAseq data from modEncode all suggest an aqz maternal contribution partly fulfilling early Aqz requirements. These data firmly establish an aqz requirement for ectoderm development and differentiation. Since these are the first aqz mutant phenotypes, we focused on these embryonic ectodermal phenotypes, as they may help explain later ones.
aqz NS phenotypes
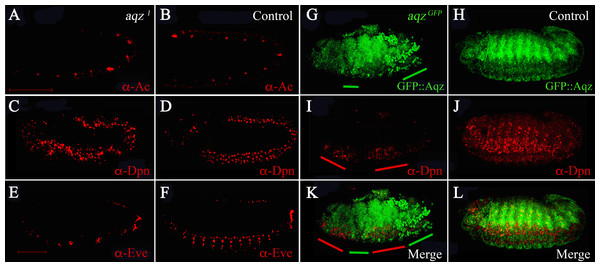
We wanted to study when are NS defects first seen in aqz mutants. To study this, we stained mutant embryos at different stages with different markers of the differentiating NS (Fig. 6). aqz1 mutant embryos does not seem to have a different number of Achaete-positive staining cells (Figs. 6A compared to 6B). Later, during germband extension/retraction, aqz1 mutant embryos have a non-significant, but higher number of Deadpan-staining cells, that labels delaminating neuroblasts (Figs. 6C compared to 6D). aqz1 heterozygotes have 121 ± 15.4 Deadpan-positive cells per embryo (n = 12), while aqz1 mutant homozygotes have 157 ± 22 Deadpan–positive cells per embryos (n = 16). aqzGFP mutant embryos, also stained with Deadpan antibodies, show irregular staining. Some embryonic areas show increased density of Deadpan positive cells, whereas other areas show a dearth of labeled cells (Figs. 6I compared to 6J). Cells are irregularly spaced, with some areas of the embryo showing ectopic stained cells (Figs. 6I compared to 6K, 6L). In a complementary fashion, in the mutant, clumped Aqz protein accumulation (in this instance the chimeric GFP::Aqz) occurs where there is little or no Deadpan staining (Figs. 6G compared to 6I). A similar phenotype is seen in aqz1: Deadpan positive cells are irregularly spaced (compare Figs. 6C with 6D). Aqz expression increases early before neuroblast delamination, and decreases after the neuroblasts have delaminated, and start dividing (Fig. 4). In aqz1, ganglion mother cells are much reduced and irregularly spaced, as labeled by Anti-Eve antibodies (Figs. 6E compared to 6F). This may mean that some mutant neuroblasts fail to properly differentiate to ganglion mother cells, or differentiate later, a rationale that may explain the tendency of an increased number of neuroblasts.
Figure 6: aqz NS defects are detected from the onset of NS formation.
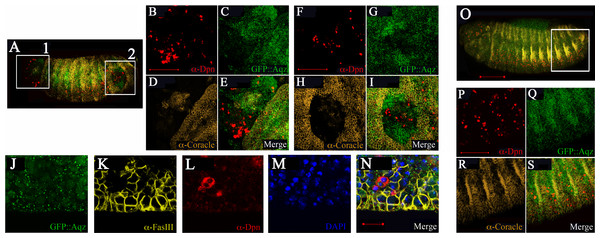
(A, C, E and G) are aqz homozygous mutant embryos stained with markers for the NS. (B, D, F and H) are corresponding heterozygous embryos (controls). All views are lateral with anterior to the left, dorsal up. A and B show anti-Achaete staining marking proneural cell during germband extension/retration. C and D show deadpan marked neuroblasts during germband extension/retraction and condensation of the NS. Notice irregular appearance of neuroblasts in C compared with D (control). E and F show expression of Evenskipped (Eve), marking ganglion mother cells, showing a disruption in the mutant (compare E to F) at this later stage of development (stage 15). G and H mark expression of GFP::Aqz. Green lines underneath G mark parts of the embryo with abnormal GFP::Aqz expression, compared to H, where the GFP::Aqz signal is distributed homogeneously. I marks neuroblasts. They also show a clumped and irregular distribution (compare to J which shows a regular array of neuroblasts). Red lines mark areas with abundant neuroblasts in I. K and L show the merged images. Scale bar is 100 µm.By stage 16, Aqz is expressed in the epidermis (Fig. 5A). Using epidermal markers, some parts of the ectoderm show lack of staining (Figs. 7A–7I). In these areas, there are more Deadpan-positive cells, suggesting ectopic neuroblasts have formed at the expense of epidermal precursors (Figs. 7A–7I compared to 7O–7S). This could explain the lack of cuticle in some parts, and as a consequence, the cuticular holes found in dead embryos, strongly suggesting a neurogenic phenotype.
Figure 7: Mutations in Aqz lead to irregular distribution of epithelial tissue and NS.
A and O show lateral views of embryos (anterior to the left and dorsal up) stained for neuroblasts (Deadpan antibodies, red), epithelia (Coracle antibodies, yellow), also showing expression of Aqz (green). Scale bar is 100 µm. In A, two distinct regions showing irregular distribution of labels: A1 and A2, are enclosed in white rectangles and enlarged to the right. For comparison, a similar control area is shown in O, the heterozygous control embryo. B, F and P show enlarged anti-Deadpan staining (red); C, G and Q show enlarged GFP::Aqz expression, D, H and R show enlarged anti-Coracle expression, whilst E, I and S show merged images, respectively. Scale bar is 50 µm. J–N show another example of lack of epithelial cells using a different epidermal marker (anti-FasIII, K) and an irregular distribution of neuroblasts (anti-Deadpan, L) in aqzGFP mutant embryos (GFP::Aqz, J). Nuclei are labeled by DAPI (M). N show the respective merged image.Aqz, Notch pathway and epithelial polarity
The Notch pathway regulates neuroblast and epidermoblast numbers and specification via lateral inhibition during ectoderm differentiation (Campos-Ortega & Knust, 1990; Hartenstein et al., 1992). Aqz mutants show perturbed neurogenesis and epidermogenesis, with phenotypes akin to Notch mutants, i.e., neurogenic phenotypes.
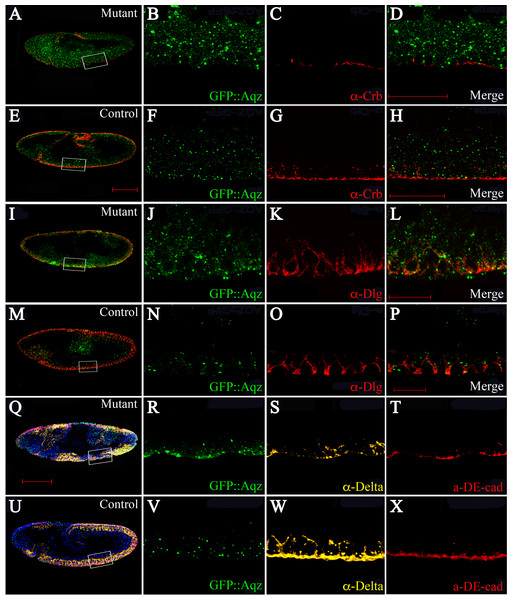
How can Aqz affect Notch signaling? One possibility is that Aqz alters epithelial cell polarity, which is known to affect the apical localization of Dl and consequently, its interaction with the Notch receptor (Krahn & Wodarz, 2009). In order to study epithelial polarity, we used antibodies against different known epithelial polarity markers in aqzGFP embryos. We used anti-Crumbs antibodies (anti-Crb) as an apical domain marker (Figs. 8A–8H), and anti-Discs large antibodies (anti-Dlg) as a basolateral marker (Figs. 8I–8P). Results show that the apical domain is compromised, showing reduced Crumbs staining, whereas the basolateral domain is expanded (Discs large staining is augmented).
Figure 8: The aqz mutant neuroepthelium has polarity defects.
A, E, I, M, Q, and U are lateral views of embryos undergoing dorsal closure, with anterior to the left and dorsal up. Bars are 100 µm. A–D, I–L, and Q–T are aqzGFP mutant embryos, whereas E–H, M–P, and U–X are aqzGFP heterozygtes as controls. B, F, J, N, R, and V show GFP::Aqz expression, C and G anti-Crumbs (Crb) (red), K and O anti-Discs large (Dlg) (red), and S and W anti-Delta (Dl) (yellow). D, H, L, and P show respective merged images. Scale bar is 50 µm. T and X show anti-DE-cadherin (DE-Cad) (red). Note that homozygous mutant GFP::Aqz expression appears more clumped compared to the heterozygous controls, as stated before, and aids in identification of mutant embryos.We then asked whether adherens junctions and Delta expression are altered. We used DE-cadherin antibodies as an adherens junction marker. Adherens junctions are also compromised, since DE-cadherin shows significantly reduced and patchy staining in aqzGFP homozygotes, and so does Dl expression, which is also reduced and patchy (Figs. 8Q–8X).
Altogether, the altered epithelial polarity and consequent reduced Dl expression, may explain the neurogenic phenotypes associated with aqz mutants, and the ectodermally derived aqz phenotypes. This implies cell polarization defects starting at neurulation being the ultimate cause of aqz mutant defects and lethality, and provide an explanation for aqz involvement in ectodermally derived tissues. It would be interesting to investigate whether other epithelia at other stages also bear similar defects.
Discussion
aaquetzalli is a complex locus encompassing at least three different mRNAs and three different protein products. They code for proteins with a conserved domain, the PNRC, of which D. melanogaster harbors two genes. Vertebrate genes with such a domain function in nonsense-mediated RNA decay proteins, and interact with nuclear receptors of varied function. What does Aqz do? Having a second gene with the same conserved domain might mean they are redundant, or partially redundant, and that some functions might be masqued by the activity of the other, even if they diverged somewhat and acquired new functions. Coding for multiple proteins also means that unless we mutate all forms (like in aqzGFP), we might not get a ‘pure’ loss-of-function phenotype, and instead have complicated alleles that may be at the same time loss and gain of function. It also leads to complex complementation patterns and phenotypes. Regardless, we observe that most of the phenotypes are consistent, and, in embryos, affect the ectoderm and ectodermally derived tissues.
Why is the phenotype partial in embryos? We know penetrance is not 100% (not all mutant embryos die). We reason that besides the second PNRC-containing gene alluded to above, there is maternal contribution, that may obscure early mutant phenotypes. In aqzGFP adults are never seen, with death occurring during a prolonged phenocritical period from embryogenesis to pupariation. From this, we sought to study the earliest defects, in order to gain insight into the causes originating the mutant phenotypes. We found that these phenotypes start very early, around cellularization and cell polarization.
We also show here that Aqz is expressed throughout the life cycle, and is pleiotropic. Yet we find it works in cell polarity early in development, setting the stage for organ differentiation in ectodermally derived tissues, like the epidermis and the NS. We also show that the common thread for these defects is cell polarization, in particular, loss or reduction of the apical domain. This, in turn, leads to compromised Dl and adherens junctions (AJ) localization and abundance (evidenced by Dl and E-Cad staining, respectively), and a neurogenic-like phenotype.
Other genes, besides Notch genes, have neurogenic phenotypes. Some have cell polarity defects, like the cell polarity regulatory proteins (CPRPs), that promote distribution of cellular components (i.e., E-cadherin, a component of AJ, and Dl) in asymmetric patterns resulting in polarized cells (Sasaki et al., 2007). Abnormal conditions in these genes, by way of cell polarity defects, may lead to the defects seen in aqz mutants. The way in which they cause these defects is currently unknown, but would be interesting to see whether they interact with Aqz in flies, or an Aqz-like vertebrate protein, as Aqz behaves much like them.
Conclusions
In this paper we have studied the embryonic epithelial defects of aqz mutants, specially in the embryonic ectoderm, and ectodermally derived tissues. We have found that there is a defective neural/epithelial development and differentiation, and that these defects could be ascribed to faulty epithelial polarization. It remains to be seen whether aqz, being transcribed at all stages of the life cycle, is similarly required for other epithelial polarization processes, like in the compound eye or in the wing epithelia. It also remains to be seen whether the aqz maternal contribution is requied earlier in development, and whether the other PNRC-domain-containing protein in the fly genome indeed is partially redundant with Aqz function.
Supplemental Information
Df(3R)Exel6150 and aqzGFP genetically complement stocks with insertions on aqz neighboring genes
Df(3R)Exel6150 and aqzGFP are homozygous lethal, but fully complement insertions in CG9836, CR43130, CG9837 and CG8359. + indicates complementation and ND indicates not determined. The crosses between Df(3R)Exel6150 and mutant alleles produced adults with and without balancers (for PEPIscUG18758(CG9836) = 70 with and 41 without balancers, for PSUPor-PKG08159ry506(CR43130) = 18 with and 82 without balancers and for MiMICCG9837MI01411(CG9837) = 49 with and 81 without balancers). Also the crosses between aqzGFP and mutant alleles produced adults with and without balancers (for PEPIscUG18758(CG9836) = 105 with and 77 without balancers, for PSUPor-PKG08159ry506(CR43130) = 106 with and 64 without balancers, for MiMICCG9837MI01411(CG9837) = 35 with and 53 without balancers and PEPgy2hng2EY18943(CG8359) = 23 with and 50 without balancers).
aqz locus cDNAs partially sequenced
We sequenced both the 5′and 3′ends of SD19655 and RE74095 to help us piece together the extent of the transcripts of the locus.
Other aqz mutant alleles have ectoderm-derived mutant phenotypes
(A) shows cuticular phenotypes; mutant embryos have holes in various positions (dorsal, lateral, anterior), compared to a wild type cuticle (bottom left). (B) shows nervous system staining using the Elav staining, where clear deformities of the central nervous system are shown, compared to the wild type (bottom panel). (C) shows anti-22C10 staining marking neuronal somas and the peripheral nervous system. aqz mutant embryos have irregularities in the disposition of the peripheral nervous system, as well as abnormal regions of low and high density of peripheral nervous system cells, compared to wild type (bottom panel). (D) shows anti-BP102 staining showing abnormalities in aqz mutant connectives in the ventral nervous chord in late embryos (wild type control, bottom panel). All embryos are shown with anterior to the left and dorsal up.
aqz1 mutant phenotypes
(A) aqz1 mutants have a cuticular phenotype, similar to aqzGFP, but at a lower penetrance. aqz1 cuticles have holes (arrows), compared to (B), a wild type cuticle. Mutant aqz1 embryos have nervous system phenotypes similar to aqzGFP as (C) shows a disorganized and deformed nervous system stained with anti-Elav (top panel), and also shows abnormal epithelial tissue, as marked by anti-Coracle staining (middle panel). Lower panel shows a merged image. (D) shows a similarly stained control embryo. (E) shows an aqz1 mutant embryo displaying abnormal peripheral nervous system, as evidenced by anti-22C10 staining, compared to (F), a wild type control embryo. In all panels, embryos are shown with dorsal up and anterior left. Scale bar are 100 µm. (F) shows a quantification of aqz1 nervous system phenotypes, akin to neurogenic phenotypes, in roughly a third of mutant embryos. Two copies of a genomic rescue construct significantly increase this percentage: over 60% embryos with the rescue constructs exhibit a neurogenic phenotype. (H) The majority of aqz1 embryos that survive embryogenesis die as pupae, with a few (less than 10%) dying as larvae. Adding two aqz genomic rescue constructs significantly increases the number of larvae (over 50%) that do not reach pupation. In (G) and (H) adding wild type copies of aqz increases the aqz1 mutant phenotypes, similar to the effect of two rescue wild type copies of aqz in aqzGFP homozygotes. (I) shows two aqz1 mutant pupae with necrotic patches (black arrows), compared to a wild type control pupa (right). Scale bar 1 mm. Anterior is up.
aqz1 has mutant phenotypes in epidermis and nervous system
A and C show mutant embryos, while B and D show similarly stained control embryos (heterozygotes). A and B show anti-Deadpan for neuroblasts, and anti-Fascilin III to mark the epithelium. Note disorganization in the mutant embryos. Scale bar is 100 µm. C and D show anti-BP102 staining, showing abnormalities in mutant connectives in the ventral nervous chord. All embryos are oriented dorsal up and anterior left.
Raw, original blots for Figure 1
In (A) is the RNA gel electrophoresis used for the Northern blot shown in Figure 1C. After separating the Drosophila purified RNA, the blot was cut in three pieces (molecular weight markers, and two four lane sections for hybridization) to then probe with the radioactive labelled RNA probes. (B) shows the full Ponceau stained transfer of the electrophoretically separated Drosophila proteins, and in the right the full blot developed with anti-GFP antibodies, and alkaline phosphatase NBT/NCIP substrate.
Deadpan staining quantification
Raw data for Deadpan quantification.