The ΦBT1 large serine recombinase catalyzes DNA integration at pseudo-attB sites in the genus Nocardia
- Published
- Accepted
- Received
- Academic Editor
- Mario Alberto Flores-Valdez
- Subject Areas
- Genetics, Microbiology, Molecular Biology
- Keywords
- ΦBT1, Serine recombinase, Nocardia, Streptomyces, DNA integration
- Copyright
- © 2018 Herisse et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. The ΦBT1 large serine recombinase catalyzes DNA integration at pseudo-attB sites in the genus Nocardia. PeerJ 6:e4784 https://doi.org/10.7717/peerj.4784
Abstract
Plasmid vectors based on bacteriophage integrases are important tools in molecular microbiology for the introduction of foreign DNA, especially into bacterial species where other systems for genetic manipulation are limited. Site specific integrases catalyze recombination between phage and bacterial attachment sites (attP and attB, respectively) and the best studied integrases in the actinomycetes are the serine integrases from the Streptomyces bacteriophages ΦC31 and ΦBT1. As this reaction is unidirectional and highly stable, vectors containing phage integrase systems have been used in a number of genetic engineering applications. Plasmids bearing the ΦBT1 integrase have been used to introduce DNA into Streptomyces and Amycolatopsis strains; however, they have not been widely studied in other actinobacterial genera. Here, we show that vectors based on ΦBT1 integrase can stably integrate into the chromosomes of a range of Nocardia species, and that this integration occurs despite the absence of canonical attB sites in these genomes. Furthermore, we show that a ΦBT1 integrase-based vector can insert at multiple pseudo-attB sites within a single strain and we determine the sequence of a pseudo-attB motif. These data suggest that ΦBT1 integrase-based vectors can be used to readily and semi-randomly introduce foreign DNA into the genomes of a range of Nocardia species. However, the precise site of insertion will likely require empirical determination in each species to avoid unexpected off-target effects.
Introduction
The actinomycetes comprise diverse bacterial genera, ranging from those well known for their prolific production of secondary metabolites (Streptomyces spp.) to those that contain some of world’s most successful pathogens (Mycobacterium spp.). Nocardia species are actinomycetes that have features in common with pathogens and secondary metabolite producers. For example, several species of Nocardia (i.e., Nocardia asteroides, Nocardia brasiliensis) are opportunistic human and animal pathogens, causing lung, skin and brain infections, predominantly in immunocompromised hosts, while others are reservoirs of bioactive natural products (Hoshino et al., 2011; Tanaka et al., 1997) and are capable of degrading or converting widespread environmental pollutants (Hahn et al., 2013; Santillan et al., 2016). These beneficial properties and their potential biotechnical applications have attracted a great deal of interest in the genus Nocardia. However, genetic tools to manipulate Nocardia spp. are limited in comparison to better studied actinomycetes, such as Streptomyces spp.
Serine integrases are widely used tools in the molecular biology of a range of species, especially actinomycetes. These integrase proteins (Int) are phage-encoded enzymes that mediate the site-specific recombination of DNA molecules between two sites (known as “attachment sites,” or att-sites) on the phage (attP) and host genome (attB) (Rutherford & Van Duyne, 2014). As excision of the integrated DNA requires a separate protein (recombination directionality factor), the integration reaction is both highly directional and stable (Fogg et al., 2014). It is these two key features that have made these enzymes such attractive tools for the modification of bacterial and mammalian genomes (Stark, 2017).
Perhaps the best studied serine integrases are those derived from Streptomyces bacteriophages, including ΦC31 and ΦBT1. Vectors based on the integrase (int) from ΦC31 and ΦBT1 have been utilized to insert DNA into a range of genomes in both prokaryotes (including bacteria and archaea) and eukaryotes (yeast, plant and mammalian cells) (Baltz, 2012). The attP and attB sites for members of the serine recombinase family are often <50 bp in length and contain imperfect inverted repeat sequences that surround a core dinucleotide, known as the crossover site (Smith & Thorpe, 2002). In Streptomyces coelicolor, both ΦC31 Int and ΦBT1 Int mediate DNA insertion into separate genes (SCO3798, a pirin homologue and SCO4848, a putative membrane protein, respectively), inactivating their targets (Gregory, Till & Smith, 2003; Kuhstoss, Richardson & Rao, 1991). While ΦC31 attB sites are highly similar in a number of Streptomyces species, non-specific integration in sequences that have partial identity to attB (pseudo-attB sites) has also been reported, although this appears to occur relatively infrequently (Baltz, 2012; Combes et al., 2002). While vectors utilizing ΦBT1 Int have also been shown to integrate in a range of Streptomyces species and A. mediterranei, their usage outside these organisms has been limited (Gregory, Till & Smith, 2003; Li et al., 2017). Furthermore, the extent to which ΦBT1 Int can catalyze integration at pseudo-attB sites is unknown.
Here, we show that ΦBT1 Int can be used to integrate foreign DNA into a range of Nocardia species and that these insertions occur despite the absence of a canonical attB site. We show that these pseudo-attB sites are unique to each species, that certain sites are preferred within certain species and that minimal homology to the canonical attB is required for DNA insertion. Furthermore, using this range of unique pseudo-attB sites we identify a pseudo-attB motif and delineate the nucleotides important for DNA integration.
Methods
Microbial culture
Nocardia strains were routinely cultured in Brain Heart Infusion (BHI) broth or on BHI agar plates (Difco). E. coli strains were cultured in Luria broth or on Luria agar plates. Streptomyces species were cultured on MS agar for conjugation and isolation of transconjugants (Kieser et al., 2000). All strains used in this study are listed in Table S1. The plasmid pRT801, an E. coli-Streptomyces integrating vector containing the BT1 int gene and attP site, aac3(iv) (encoding apramycin resistance) and a Streptomyces oriT for conjugative transfer, was used in this study and has been previously published (Gregory, Till & Smith, 2003).
DNA extraction procedures
Genomic DNA was extracted from Nocardia strains according to a modified DNA isolation method based on two previously published protocols (Belisle & Sonnenberg, 1998) (Gonzalez-y-Merchand et al., 1996). Briefly, a 10 ml culture of cells was grown in BHI supplemented with 2% glycine for four days at 30 °C with shaking at 200 rpm. Cells were pelleted and resuspended in 2 ml Tris-HCl pH 8.0 and mixed with an equal volume of 1:1 chloroform: methanol for 10 mins with gentle shaking. Cells were pelleted at 8,000×g, 10 min, then both the organic and aqueous phases were removed, leaving only the cells. Cells were dried to remove all traces of chloroform and methanol and were resuspended in 475 μl of TE buffer, followed by the addition of lysozyme at a final concentration of 5 mg/ml. Cells were incubated for 2 h at 37 °C and then pelleted at 8,000×g, 10 min and the supernatant removed. The cell pellet was then resuspended in 500 μl lysis buffer (6 M guanidine HCl, 10 mM EDTA, 1 mM β-mercaptoethanol, 2% Tween 20, 1% IGEPAL CA630, 1% Triton X-100) and the cells were frozen in a dry ice/ethanol bath, followed by thawing at 60 °C. This was repeated three times, then the cell suspension was extracted twice with chloroform. The supernatant was removed to a separate tube and the DNA was precipitated using ethanol. DNA was resuspended in 50 μl of 10 mM Tris-HCl and stored at −20 °C.
Plasmid DNA was extracted from E. coli DH10B cells containing pRT801 using a plasmid mini-prep kit (Favorgen Biotech, Ping-Tung, Taiwan).
Transformation of Nocardia strains
Nocardia strains were transformed according to the method of Ishikawa et al. (2006) (Ishikawa et al., 2006). In brief, Nocardia strains were grown in 100 ml BHI containing 2% glycine for four days at 30 °C prior to transformation. Cells were harvested and washed twice with 50 ml ice-cold water. The cells were then resuspended in 500 μl of ice-cold 10% glycerol and 50 μl was used per transformation and transferred to a chilled electroporation cuvette (2 mm gap). Approximately ∼1 μg of DNA was added and cells were pulsed at 2.4 kV using an electroporator (Electro Cell Porator 600; BTX Inc., Holliston, MA, USA). To each transformation, 900 μl of BHI broth was added and the cells were incubated for 2 h at 37 °C. N. terpenica, N. uniformis and N. brasiliensis transformations were plated onto BHI plates containing 50 μg/ml of apramycin, while N. arthritidis transformations were plated onto BHI plates containing 100 μg/ml apramycin. All plates were incubated for 2–3 days at 30 °C. For each Nocardia strain analyzed for pRT801 insertions sites, transformants were selected from independent transformation reactions to avoid detecting sibling colonies. As such, transformants were picked from 14 independent transformation reactions for N. terpenica; six independent transformations for both N. brasiliensis and N. uniformis; and five independent transformations for N. arthritidis.
Conjugation of plasmids into Streptomyces strains
DNA was transferred from E. coli ET12567 (pUZ8002) to Streptomyces species by conjugation according to a previously published protocol (Kieser et al., 2000).
Confirmation of pRT801 integration in Streptomyces strains
Insertion of pRT801 in S. coelicolor and S. lividans was determined by PCR using the following primers: ScBT1-F-CCCAATACGAAGGAGACGAT; ScBT1-R-GCTCATTCACAACGACAACG. Insertion of pRT801 in S. albus was also determined by PCR, but due to differences upstream of the attB site in S. albus, a different forward primer (SaBT1-F-GGGGTGGGGTTCTTCTCAC) was used in conjunction with the ScBT1-R primer.
DNA sequencing and analysis
Complete sequencing of the N. terpenica genome was performed with a combination of PacBio SMRT and Illumina sequence data. N. terpenica DNA was extracted as outlined above and prepared for sequencing on the PacBio RSII using the Template Prep Kit 1.0 (PacBio, Menlo Park, CA, USA). Genomic DNA was size selected after adapter ligation using a BluePippin system (Sage Biosciences, Beverly, MA, USA) with a 10 kb cutoff. Adapter-ligated, circularized DNA was loaded onto a single SMRT cell at 0.2 nM and sequence data were captured with a 6 h movie time. To complete the genome sequence of N. terpenica, PacBio sequencing data was assembled using HGAP3, as implemented in the SMRT Portal (PacBio, Menlo Park, CA, USA), resulting in a single contig. This contig was polished three times using Quiver (PacBio, Menlo Park, CA, USA) before final indel error correction with Illumina reads using Snippy v3.2 (https://github.com/tseemann/snippy).
For all Illumina sequencing, DNA libraries were created using the Nextera XT DNA preparation kit (Illumina, San Diego, CA, USA) and whole genome sequencing was performed on the NextSeq platform (Illumina, San Diego, CA, USA) with 2 × 150 bp paired-end chemistry. A sequencing depth of >50× was targeted for each sample. Samples with Illumina only data were assembled with SPAdes (v 3.10.1) (Bankevich et al., 2012) and annotated with Prokka v 1.12 (Seemann, 2014). Sequencing reads are available under BioProject ID PRJNA433999. Table S2 lists each read set deposited in SRA, along with species name and corresponding attB site, as listed in Fig. S1 and Table S3 of the manuscript. The sequence of pRT801 has been deposited in GenBank under accession number MH192349.
Insertion sites of pRT801 were manually inspected and identified using Geneious v9.1.6. Motif analysis was performed using MEME (www.memesuite.org, Bailey & Elkan (1994)). Rarefaction analysis was performed in R v3.3.2 (R Development Core Team, 2016) to estimate the total number of pseudo-attB sites present in the N. terpenica genome. To identify sites with homology to the predicted pseudo-attB motif in Nocardia genomes, FIMO was used (http://meme-suite.org/tools/fimo, Grant, Bailey & Noble (2011)) with a threshold value of 1e−08.
Construction of rpl-based phylogenetic tree
Sequences of rpl genes (rplB, rplJ, rplM, rplS and rplT) were extracted from 68 Nocardia genome sequences present in GenBank, as well as the four sequenced Nocardia genomes from this study, using the Perl script gene-puller.pl (https://github.com/kwongj/gene-puller). The rpl sequences were manually curated to a consistent length and then concatenated to create a single 2,352 bp pseudo-sequence for each strain and aligned using Clustal W 2.1 (Larkin et al., 2007). A maximum likelihood phylogenetic tree was inferred with IQ-Tree under a GTR+F+I+G4 nucleotide substitution model using the alignment of concatenated sequences mentioned above. Branch support for the phylogeny was assessed with 1,000 bootstrap replicates (Hoang et al., 2018; Nguyen et al., 2015).
Results
Sequencing of Nocardia terpenica AUSMDU00012715
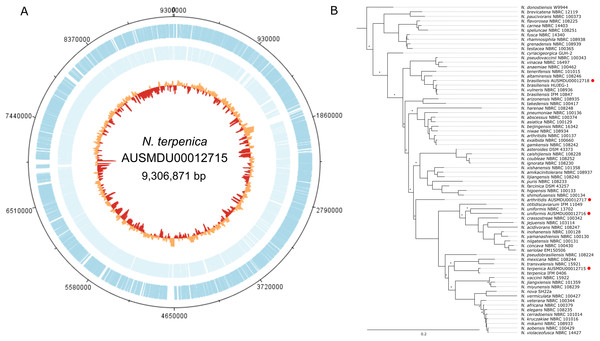
As part of a separate natural product discovery project we sought to assess the genetic tractability of a human pathogenic Nocardia sp (strain ID: AUSMDU00012715). To first establish the species identity of the strain and provide a solid reference for subsequent molecular investigations, we subjected this isolate to combined PacBio SMRT and Illumina sequencing to produce a single 9,306,871 bp circular chromosome with 8,241 predicted CDS and 70 tRNA regions (Fig. 1). No plasmids were detected. Subsequent phylogenetic analysis by comparing five rpl gene sequences from this Nocardia isolate and 68 other Nocardia species (including three others sequenced as part of this study; Tables S1 and S3) showed that this isolate was most closely related to a previously sequenced N. terpenica isolate (14 pairwise SNP differences among a total of 950 potential variable positions), suggesting that it was a member of the species N. terpenica (Fig. 1).
Figure 1: Genome and phylogenetic position of Nocardia terpenica AUSMD00012715.
(A) Genome map of N. terpenica 03-11436. Outer and inner blue and light blue lines represent CDS present on the forward and reverse strand, respectively. The innermost circle is a GC plot, showing variance in GC content across the genome. (B) Concatenated ribosomal gene based phylogenetic tree showing the position of N. terpenica 03-11436 among 71 other sequenced Nocardia strains (including other sequenced as part of this study, marked by red circles). A maximum likelihood phylogeny was inferred under a GTR+F+I+G4 model of nucleotide substitution, using as input an alignment of 2,352 bp concatenated nucleotide sequences for five ribosomal protein genes from 72 Nocardia. Asterisks indicate nodes with <70% bootstrap support (1,000 replicates).pRT801 integrates into the Nocardia terpenica genome
To assess the potential of N. terpenica AUSMDU00012715to accept foreign DNA, we began by transforming this isolate using electroporation with a range of plasmids that have been successfully used in Nocardia and other Actinobacteria. These plasmids included a range of replicating as well as integrating vectors. The replicative plasmids pNV19 and pNV119 (Chiba et al., 2007) were competently hosted by N. terpenica AUSMDU00012715, as was the ΦBT1 Int expressing vector pRT801 (Gregory, Till & Smith, 2003). However, transformation with other integrating vectors, including those utilizing the ΦC31 and ΦL5 integrases failed to produce colonies on selective media.
Following the isolation of potential pRT801 transformants on apramycin-containing media, we screened colonies by PCR for the aac(3)iv gene to confirm the presence of pRT801. As a ΦBT1 integrase system has not previously been reported to function in Nocardia species, we were unsure where pRT801 may have inserted in the genome of N. terpenica transformants. We sequenced the genomes of three randomly selected clones to rapidly identify insertion sites in these transformants. Surprisingly, an analysis of these genomes showed that pRT801 was inserted at a different chromosome location in each transformant, and not at a single unique attB site, as observed in Streptomyces species (Gregory, Till & Smith, 2003). Furthermore, these insertion sites were spread across the genome and had only limited homology to the canonical 73 bp attB site for ΦBT1 (Gregory, Till & Smith, 2003), the previously described minimal 36 bp attB site (Zhang et al., 2008), or even to the reported 9 bp common attP-attB sequence (Gregory, Till & Smith, 2003). An in silico scan of the N. terpenica genome based on these sequenced transformants did not find the canonical S. coelicolor ΦBT1 attB site within the genome, suggesting that all insertions in the N. terpenica genome were at pseudo-attB sites.
All pRT801 insertion sites in N. terpenica occur at pseudo-attB sites
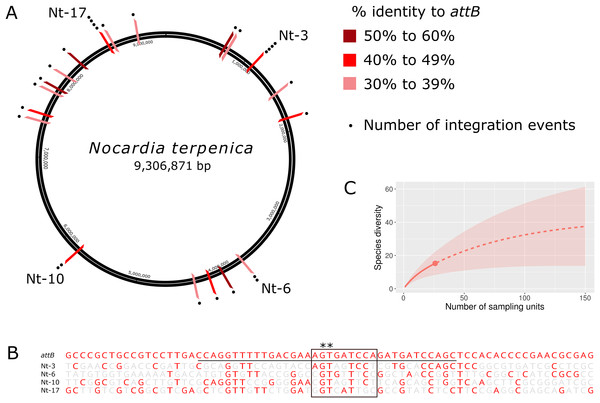
Although insertion into pseudo-attB sites has previously been observed in some actinomycetes following transformation with ΦC31 Int-based vectors (Combes et al., 2002; Matsushima & Baltz, 1996; Murry et al., 2005), the likelihood of insertion at these sites is up to 300 times lower than a true attB site (Combes et al., 2002). Furthermore, pseudo-attB sites have not been reported for ΦBT1 Int-based vectors. To characterize the extent and nature of these variant integration sites, we sequenced and analyzed the genomes of a further 24 N. terpenica pRT801 transformants. Analysis of this set of 27 transformants identified at least 19 individual pseudo-attB sites within the N. terpenica genome (Fig. 2A). Of the 27 identified pseudo-attB sites, two sites had four integration events each (Nt-3 and Nt-17), while a further two sites had two insertions each (Nt-6 and Nt-10) and the remaining 15 different sites had only a single integration event. The identified sites that had multiple integration events occurred in independent pRT801 transformations, meaning that these were not siblings, but rather that some pseudo-attB sites are preferred over others in the N. terpenica genome.
Figure 2: Pseudo-attB insertion sites in N. terpenica AUSMD00012715.
(A) Locations of individual pRT801 insertion sites mapped onto the N. terpenica AUSMDU00012715 genome. Percentage similarity to the minimal attB site of S. coelicolor is indicated by varying shades of red. (B) Comparison of the most common N. terpenica pseudo-attB sites with the 73b canonical attB sequence from S. coelicolor (a comparison of all sites can be found in Fig. S1). Red nucleotides indicate identity to the canonical attB sequence. The common attB–attP core is boxed and the minimal attB sequence is underlined. The core GT dinucleotide is denoted by asterisks. (C) Rarefaction analysis showing number of predicted sites in the N. terpenica AUSMDU00012715 genome.Analysis of the 27 insertion sites confirmed our initial observations and showed that these pseudo-attB sites were scattered across the chromosome (Fig. 2A), with insertions in a range of genes and non-coding regions (Table 1). Furthermore, we observed that even though recombination target site precision was 100%, with no deletions or insertions, these sites had only limited homology to the canonical ΦBT1 attB site (Gregory, Till & Smith, 2003) (Fig. 2B). Indeed, a comparison between all N. terpenica pRT801 insertion sites and the previously described ΦBT1 attB site showed that the only sequence that was completely conserved was a core GT dinucleotide (Fig. 2B and Fig. S1). A comparison of the N. terpenica pseudo-attB sites with the canonical 73 bp attB site revealed that nucleotide identity among these sites was between 27% and 48% (Table 1). Comparison with the minimal 36 bp attB site increased the percentage identity somewhat; however, it was still below 60% for all analyzed sites (Table 1). This suggests that, even though these pseudo-attB sites differ from the reported S. coelicolor attB by greater than 40% (a minimum of 14 out of 36 non-matching nucleotides), ΦBT1 Int is still capable of recognizing these sites and integrating donor DNA. We also noticed that the insertion sites that had more than one integration event were not the sites with the highest levels of similarity to the canonical 73 bp attB-site or even the 36 bp minimal attB site (Table 1). It appeared that greater nucleotide identity over the minimal attB region correlated with greater identity over the whole attB sequence, but that this did not necessarily correlate with greater identity over the attB-attP site. These observations indicate that the “site-specific” nature of ΦBT1 integration events are not dependent on strict adherence to the canonical attB DNA sequence, but rather are tolerant to alterations within this sequence and suggest that DNA topological factors (spacing between certain nucleotides) may also be important.
| Insert name | Identity to attB (%)a | Identity to minimal attB (%)b | Identity with attB–attP sitec | Insertion events | Genome position | Encoded protein |
|---|---|---|---|---|---|---|
| Nt-1 | 26 (36%) | 19 (52%) | 5 | 1 | 731398 | MsrR family regulator |
| Nt-2 | 20 (27%) | 14 (38%) | 4 | 1 | 751814 | Disulfide bond forming protein |
| Nt-3 | 26 (36%) | 17 (47%) | 6 | 4 | 1066609 | Polyketide synthase |
| Nt-4 | 28 (38%) | 19 (52%) | 6 | 1 | 1505104 | Carboxymethylenebutenolidase |
| Nt-5 | 30 (41%) | 17 (47%) | 3 | 1 | 1826014 | Non-coding region |
| Nt-6 | 22 (30%) | 13 (36%) | 5 | 2 | 3715745 | Non-coding region |
| Nt-7 | 34 (46%) | 20 (55%) | 5 | 1 | 3939350 | FtsX-like permease |
| Nt-8 | 27 (37%) | 15 (41%) | 6 | 1 | 4126594 | Hypothetical protein |
| Nt-9 | 25 (34%) | 13 (36%) | 3 | 1 | 4286641 | Hypothetical protein |
| Nt-10 | 25 (34%) | 17 (47%) | 4 | 2 | 5761121 | Hypothetical protein |
| Nt-11 | 20 (27%) | 14 (38%) | 4 | 1 | 7436287 | FadD15 fatty acid ligase |
| Nt-12 | 26 (36%) | 16 (44%) | 3 | 1 | 7530197 | Hypothetical protein |
| Nt-13 | 25 (34%) | 13 (36%) | 3 | 1 | 7795264 | Putative deoxyribonuclease YcfH |
| Nt-14 | 25 (34%) | 19 (52%) | 5 | 1 | 7823861 | Non-coding region |
| Nt-15 | 25 (34%) | 12 (33%) | 4 | 1 | 8033744 | Telomeric repeat binding factor 2 |
| Nt-16 | 35 (48%) | 21 (58%) | 6 | 1 | 8246408 | MNT1/THI5-like protein |
| Nt-17 | 31 (42%) | 16 (44%) | 4 | 4 | 8620438 | Non-ribosomal peptide synthetase |
| Nt-18 | 22 (30%) | 14 (38%) | 5 | 1 | 8670073 | Hypothetical protein |
| Nt-19 | 23 (31%) | 14 (38%) | 4 | 1 | 8971553 | Phosphomevalonate kinase |
Notes:
Due to the diversity of the pseudo-attB sites identified in N. terpenica and the fact that some sites were identified more frequently than others, we sought to predict how many possible pseudo-attB sites were present in the genome. Rarefaction analysis was performed, which projected a total of 40 insertion sites in the N. terpenica genome (Fig. 2C), based on those already sequenced. This analysis suggests that there are many more potential insertion sites in the N. terpenica genome.
Identification of pseudo-attB sites in other Nocardia species
To confirm whether this phenomenon of pseudo-attB site insertion was seen in other Nocardia species, we transformed pRT801 into three other clinical Nocardia isolates. As for N. terpenica, apramycin resistant colonies of N. brasiliensis, N. arthritidis and N. uniformis were obtained and the presence of aac(3)iv in each transformant was confirmed by PCR. The genomes of several transformants from each species were sequenced, which showed, similarly to N. terpenica, that pRT801 had inserted at pseudo-attB sites in all three organisms. Furthermore, none of the pseudo-attB insertion sites in N. uniformis, N. arthritidis or N. brasiliensis were the same as those seen in N. terpenica and none of the insertion sites were conserved across the different species.
Of the 12 sequenced N. brasiliensis AUSMDU00012716 isolates, nine different insertion sites were identified (Table S3), which ranged in identity to the canonical attB from 30% to 46% and from 33% to 52% when compared to the minimal attB site (Table S3). Likewise, a similar situation was seen for the nine sequenced N. arthritidis AUSMDU00012717 isolates, where five unique insertion sites were identified. Pseudo-attB sites found in this strain had between 34% and 42% identity to the canonical attB site, which increased to 41–58% when only the minimal attB site was investigated (Table S3). The range of identity to both the full-length attB and minimal attB from both of these strains is similar to that seen for N. terpenica insertion sites. The number of single insertion sites identified in these strains (Table S3; Fig. S1) suggests that further pseudo-attB sites remain to be identified. Again, as for N. terpenica, we did not find a correlation between sites with the highest identity to attB and an increased number of insertions. Interestingly, although the identity between the 9 bp attB-attP overlap site ranged from 4 to 6 bp, it appears that a minimum 2 bp overlap at this site (Nb-6 (Fig. S1), where only the essential GT dinucleotide was conserved) is all that is required for insertion.
Analysis of the 14 sequenced N. uniformis AUSMDU00012718 transformants showed a slightly different pattern, where only two unique insertion sites were identified. The preferred site, found in 13 of the 14 transformants, which we have labelled Nu-1, had 35 identical nucleotides (48%) to the canonical attB sequence and 22 nucleotides (61%) that were identical to the minimal ΦBT1 attB sequence (Table S3; Fig. S1). The other pseudo-attB site in this species (named Nu-2) was more dissimilar to both the canonical and minimal attB sites than Nu-1 (28/73 (38%) nucleotides and 17/36 identical nucleotides (47%), respectively). It is possible that other pseudo-attB sites exist in this strain; however, their usage is likely to be rare. Nu-1 had the highest identity to attB seen in this study, perhaps explaining why Nu-1 appears to be the preferred insertion site in N. uniformis. However, given that the level of identity between Nu-1 and the minimal attB is still only 61% (14 of 36 non-identical nucleotides), this suggests that increasing identity of pseudo-attB with the canonical attB results in preferential insertion into these sites, although this is not absolute. As for N. terpenica, we observed that insertion at each target site occurred with 100% precision, and confirmed the formation of pseudo-attL and pseudo-attR sites at both the left and right hand ends of each insertion, indicating that these insertions occurred at genuine pseudo-attB sites and were not the result of DNA repair mechanisms (Fig. S2).
Integration of pRT801 plasmid in the genome of Streptomyces species
Non-specific integration at pseudo-attB sites has previously been reported in Streptomyces species for ΦC31 Int (Baltz, 2012; Combes et al., 2002). Sequencing of our Nocardia transformants allowed us to investigate individual insertion sites without any a priori knowledge of their location. Previous studies using ΦBT1 Int in actinomycetes have determined insertion only by PCR around the known attB site (Gregory, Till & Smith, 2003; Li et al., 2017), meaning that insertions at pseudo-attB sites would have been classed as “non-integrants” and most likely ignored. We sought to determine whether ΦBT1 pseudo-attB sites exist in various Streptomyces species and if these sites are utilized in strains with canonical attB sites.
To do this, we first investigated the homology between attB sites from 22 Streptomyces genomes available in Genbank. We chose species that had previously been shown to be competent for the insertion of plasmid vectors based on ΦBT1 Int (Baltz, 2012; Gregory, Till & Smith, 2003). A comparison of these attB sites showed that they range from 74% to 100% identity with S. coelicolor attB (Table S4; Fig. S3). Variations in these sequences exist within the minimal attB region, as well as within the essential 9 bp core site, confirming our Nocardia findings in which it appears that only partial identity between attP and attB at this crossover site is sufficient for integration.
To investigate whether pseudo-attB insertion sites could be identified in Streptomyces species, we introduced pRT801 into S. coelicolor, S. lividans, S. albus and S. cinnamonensis by conjugation. As the genome sequences for S. coelicolor, S. lividans and S. albus are available, we designed PCR primers surrounding the attB insertion sites in these genomes, where a PCR product would confirm insertion of pRT801 at the canonical attB site. As the S. albus ΦBT1 attB site has 82% DNA identity to attB from S. coelicolor, we hypothesized that off-target insertions may be more likely in S. albus. A total of 50 S. coelicolor, S. lividans and S. albus transconjugant colonies were screened by PCR and all were positive for insertion of pRT801 into the canonical attB site (data not shown), indicating that if pseudo-attB site insertions do occur in these species, they are rare events. To confirm that integration had occurred in a single copy and that there were not alterations to the insertion site, we sequenced the genomes of a S. coelicolor and a S. albus transconjugant. As the genome sequence for S. cinnamonensis was unavailable, we initially sequenced four apramycin resistant transconjugants to identify the attB site in this species. Integration occurred at the same site in the four sequenced strains and alignment of the S. cinnamonensis ΦBT1 minimal attB with that from S. coelicolor showed 91% identity, with a 100% overlap in the 9 bp core sequence (Fig. 3). This suggests that while ΦBT1 Int can insert DNA at the same genomic region in these various Streptomyces strains, Int has a certain level of tolerance to mutations within attB and also suggests that sequence length and topological factors may be more important than nucleotide identity for DNA integration.
Figure 3: Comparison of attB sites in Streptomyces species.
Comparison of S. coelicolor ΦBT1 attB with those from S. albus J1074 (obtained from GenBank), and Streptomyces strains sequenced as part of this study (S. albus J1074 (TS), S. coelicolor (TS) and S. cinnamonensis). The core GT site is in marked with asterisks, the minimal attB region is underlined and the 9 bp overlap region is marked with a box. Red nucleotides are those that differ from the canonical attB sequence.Delineating the ΦBT1 pseudo-attB site motif
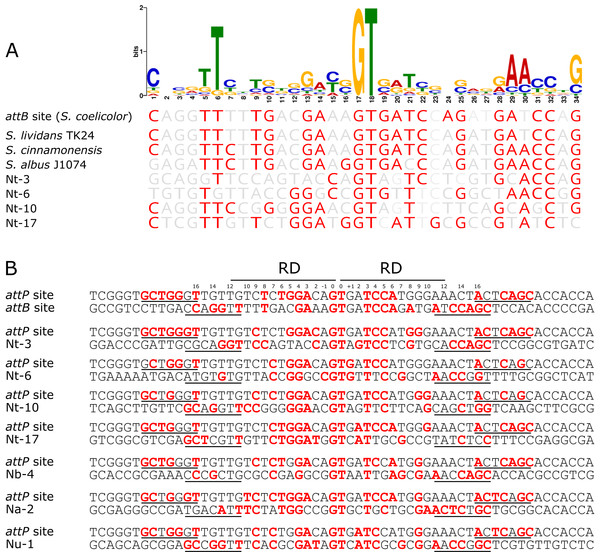
To determine if there was a conserved DNA signature to each of the pseudo-attB sites, motif analysis using MEME was performed. The 36 bp minimal attB regions from all unique sites of the pRT801-containing Nocardia transformants (36 sites total) were analyzed. The only motif found to be completely conserved across all sequences was the crossover site, or core GT dinucleotide (Fig. 4). However, a 34 bp motif, centered on the core GT dinucleotide, was identified and appears to contain a weakly conserved inverted repeat sequence (Fig. 4A). MEME analysis showed the importance of an almost invariant T at position six of the motif, as well as highly conserved nucleotides at positions 1, 5, 29, 30 and 34 of the motif (Fig. 4A). These conserved positions include TTC at positions 5–7, which is mirrored by GAA at positions 28–30 and both are the same distance from the core GT dinucleotide (11 bases distal on either side) (Fig. 4A). These positions lie within the putative zinc ribbon binding domain region of ΦBT1 attB (Rutherford et al., 2013) and a number of corresponding bases in attP can be matched within each half site (Fig. 4B), showing the bases sufficient for recombination between attP and pseudo-attB sites. When examining the presence of the conserved motif nucleotides within the Streptomyces attB and Nocardia pseudo-attB sites, there is partial conservation of the motif within every site (Fig. 4A and Fig. S4). The sequences containing the closest match to the pseudo-attB motif are those from the Streptomycetes, perhaps explaining why ΦBT1 integrase-based insertions at pseudo-attB sites are not seen in these strains. Furthermore, these data suggest that the presence of an imperfect inverted repeat of approximately the same length as the minimal attB sequence, surrounding a GT dinucleotide, is sufficient for ΦBT1 Int catalyzed DNA integration (Fig. 4).
Figure 4: The pseudo-attB motif in Nocardia species and comparison of pseudo-attB sites with attP.
(A) Alignment of attB sites with the pseudo-attB motif identified from Nocardia pseudo-attB insertion sites. Red nucleotides are those that are identical to the most common nucleotide at each position within the predicted motif. (B) Alignment of ΦBT1 attP and attB sites. The canonical attP–attB sites from ΦBT1 and S. coelicolor, respectively, are shown at the top, followed by an alignment of attP with the most common N. terpenica pseudo-attB sites, as well as those pseudo-attB sites that were most commonly seen from the other Nocardia species investigated in this study. The four predicted ZD binding motifs for ΦBT1 Int on each attP and pseudo-attB half site are underlined, with the predicted RD binding site shown at the top. Residues that are identical in three of the four ZD motifs within each alignment are shown in red. Residues that are identical at three out of four positions within −/+10 of the RD binding region are also shown in red. The central GT dinucleotide is in red and labelled as position 0.To confirm our motif prediction, we performed a search for the pseudo-attB motif in the N. terpenica AUSMDU00012715 genome using FIMO (Grant, Bailey & Noble, 2011). A total of 52 sites with significant homology to the combined Nocardia pseudo-attB motif were identified at the relatively strict cut-off of 1e−08, which is in agreement with the potential number of sites estimated from our rarefaction analysis (Fig. 2C). A comparison of these sites identified by FIMO with those identified from the sequenced transformants found four matching sites. This suggests that there may still be additional refinements that can be made to the motif to further match previously identified pseudo-attB sites.
Discussion
As serine recombinase integration systems lead to unidirectional and highly stable integration of foreign DNA, these genetic tools have been widely used in both prokaryotes and eukaryotes for genetic engineering (Stark, 2017). ΦBT1 Int has been shown to integrate DNA site-specifically into a single site within SCO4848 (encoding a membrane protein) in the S. coelicolor genome and the corresponding attB-site has been mapped (Gregory, Till & Smith, 2003). ΦBT1 Int has also been shown to integrate DNA into an orthologous gene in other Streptomyces species, such as S. albus and S. lividans (Gregory, Till & Smith, 2003). However, we have shown here that the nucleotide sequences of the attB-sites in these strains are not identical to the S. coelicolor attB sequence, revealing that the “site-specific” nature of insertion is tolerant to alterations at various positions within attB. Here we have shown that ΦBT1 Int can mediate DNA insertion into the genomes of several Nocardia species and that insertion occurs at pseudo-attB sites that have as little as 27% identity to the canonical attB site. Furthermore, our use of whole genome sequencing to rapidly identify and confirm the insertion site sequences meant that we were able to avoid the tedious digestion, relegation and selection of colonies required for plasmid rescue followed by Sanger sequencing of insertion sites. We propose that for similar studies looking at pseudo-insertion sites of integrases, sequencing costs are no longer prohibitive and allow for the use of whole genome sequencing to investigate such phenomena.
The integration sites for integrases from ΦC31 and ΦBT1 have been well characterized in Streptomyces species. Previous studies have shown that ΦC31 Int can catalyze DNA insertion at non-attB sequences in S. coelicolor in the absence of attB (Combes et al., 2002) and that pseudo-attB sites also exist in the human genome (Thyagarajan et al., 2001). However, pseudo-attB insertion sites for ΦBT1 Int have not been previously identified. Our data here provide the first description of ΦBT1 Int insertion of foreign DNA at pseudo-attB sites and show that insertions can occur at multiple pseudo-attB sites within each Nocardia genome. We have also shown that the essential requirement for insertion appears to be the core GT dinucleotide surrounded by an imperfect inverted repeat sequence (Fig. 4). A previous study has shown that matching core dinucleotides between attP and attB of ΦBT1 are essential for recombination (Zhang et al., 2010). However, our data suggests that multiple substitutions within the minimal 36 bp attB site also do not impact on the ability for recombination to occur, suggesting that the requirements for integration in terms of sequence homology are relatively relaxed, provided the core GT dinucleotide is present.
A comparison of attB and Nocardia pseudo-attB sites revealed DNA identities in the range of 27–46% (20 to 34 nucleotides) (Table 1 and Table S3). This low level of identity (at most no better than one in two bases) is also reflected in identity with the minimal attB site, which is at similarly low levels. However, this lack of identity does not seem to inhibit integration, as some of these pseudo-attB sites were identified in multiple transformants from independent transformation reactions. Interestingly, it was not always the sites with the highest identity to S. coelicolor attB that were seen most often, but those integration sites that were seen most often do appear to have good conservation of bases that are involved in binding of the zinc ribbon domain (ZD) of ΦBT1 Int and of nucleotides that are putatively involved in binding of the recombinase domain (Fig. 4B).
Indeed, based on our deduced motif, certain nucleotides appear to be more important than others for DNA integration, which has also been noted with other large serine recombinases (Rutherford & Van Duyne, 2014). A previous study on the ΦC31 integrase showed that certain nucleotides within the minimal ΦC31-attB sequence are crucial for substrate recognition and interactions between the integrase and its attachment site (i.e., nucleotides −/+15 and −/+16), but also for the efficacy of recombination (i.e., nucleotides −/+2) (Gupta, Till & Smith, 2007). Interestingly, we found that across all the pseudo-attB sites, nucleotides at positions 1 and 34 (−/+16 from the core GT) are relatively well conserved, as are the nucleotides at positions 5 and 30 and 6 and 29 (−/+11 and −/+12, respectively) (Fig. 4A), which lie within the putative ZD binding region of ΦBT1 attB (Rutherford et al., 2013). Indeed, conservation of these sites aligns with the known mutation-sensitive bases present in the attB sites of other large serine recombinases, such as ΦC31 Int, which occur at similar locations from the core dinucleotide and appear to lie within the ZD and RD binding regions of these integrases (Rutherford et al., 2013; Smith, 2015). On the other hand, nucleotides at positions 11 and 24 (−/+6), 8 and 27 (−/+9) and 2 and 33 (−/+15) appear to have a random distribution and despite these sites being part of the putative RD and ZD binding regions, ΦBT1 Int binding appears to be relatively insensitive to substitutions at these positions. Furthermore, nucleotides at 16 and 19 (−/+1), and 14 and 21 (−/+3) are weakly conserved, which correlates well with previous data showing that mutations at these positions in the S. coelicolor ΦBT1 attB site do not affect integration (Zhang et al., 2010). However, a comparison of position −/+3 in attP and the most commonly observed pseudo-attB sites (Fig. 4B), shows that these positions correspond well to one another in each half site, suggesting that matching nucleotides at these positions may be part of the reason that these sites were preferred over others. As with the canonical attB sequence, the pseudo-attB motif identified here also exhibits a weakly conserved inverted repeat centered on the core GT dinucleotide. As mentioned above, the most conserved bases appear between positions −/+11 to −/+16 from the core, representing approximately 1–1.5 helical turns of the DNA; which fits well with the attP-attB-Int binding model that has been previously proposed for phage A118 integrase (Rutherford & Van Duyne, 2014; Rutherford et al., 2013).
The identification of four sequenced pseudo-attB sites out of 52 predicted sites, using our motif as a search query, suggests that there are still additional refinements to be made to the motif. Indeed, as an equal number of mutants were not identified in each species, the contribution of sites from each to the motif is unequal, meaning that DNA compositional effects may have biased our motif sequence. It is possible that production of multiple motifs with each based on the pseudo-attB sequences obtained from an individual species may lead to more accurate prediction of putative pseudo-attB sites within each genome. Further work is required here to understand in detail the impact of changes to individual nucleotides within the ΦBT1 attB sequence.
Overall, we have shown that ΦBT1 integrase can be used as a tool to investigate the transformability of rare actinomycetes and that ΦBT1 Int-catalyzed DNA integration can occur at a number of pseudo sites. This study furthers our knowledge on the integration of serine recombinases and shows that the absence of a specific attB-site does not preclude integration of the phage. Given the potential number of diverse insertion sites in a non-Streptomyces genome, we suggest that caution should be used in interpreting the sites of phage integrase insertions in previously undescribed species.
Conclusion
Plasmid vectors based on the ΦBT1 integrase have been used in multiple Streptomyces species and are important tools in these organisms. Here, we have shown that ΦBT1 Int-based vectors can be transformed into and can integrate stably in a range of Nocardia species. However, integration of pRT801 occurs at pseudo-attB sites in these organisms, and there are multiple pseudo-attB sites in each of these species. An in-depth analysis of these sites showed that only limited homology to the canonical attB sequence from S. coelicolor is required for insertion, although the core GT dinucleotide is essential. Furthermore, the delineation of a pseudo-attB motif has revealed those sites that are important for insertion and provides further information on the nucleotides that are important for recognition and integration by large serine recombinases.
Supplemental Information
Table S2. Concordance of SRA read number with species and attB site, as referred to in the manuscript.
Table S3. Insertion sites in N. brasiliensis, N. arthritidis and N. uniformis genomes and comparison with the S. coelicolor attB site.
Table S4. Streptomyces genomes analysed for ΦBT1 attB sites.
Fig. S1. Pseudo-attB sites in Nocardia species.
Alignment of all unique pseudo-attB insertion sites in the four tested Nocardia species compared with the S. coelicolor attB sequence. The 36 bp minimal attB is underlined and the 9 bp ΦBT1 recombination site is marked with a black box. The ΦBT1 attP site is shown in black at the top of the figure. The core GT dinucleotide is marked with asterisks. Red nucleotides represent those that are conserved with attB.
Fig. S2. ΦBT1 Int-based insertions at pseudo-attB sites result in complete attL and attR sites.
(A) Simplified diagram showing the resulting attL and attR sites that are formed following serine integrase-mediated recombination. (B) Both attL and attR sites of each unique pRT801 insertion event identified in this study are shown. Right hand and left hand arms of attB are shaded in blue, while right hand and left hand arms of attP are shaded in orange. The complete attL and attR sites are marked. The canonical attB and attP sequences for ΦBT1 from S. coelicolor are shown at the bottom, with the core GT dinucleotide indicated by asterisks and the 9bp cross-over region boxed.
Fig. S3. ΦBT1 attB sites in Streptomyces species.
Comparison of ΦBT1 attB sites within 22 Streptomyces genomes to the canonical attB from S. coelicolor. The 36 bp minimal attB is underlined and the 9 bp ΦBT1 recombination site is marked with a black box. The core GT dinucleotide is marked with asterisks. Red nucleotides represent those that differ from the canonical attB sequence.
Fig. S4. The pseduo-attB site motif compared with insertion sites of Nocardia and Streptomyces strains.
Alignment of attB sites from all analysed Nocardia and Streptomyces strains with the pseudo-attB motif. Red nucleotides are those that are identical to the most common nucleotide at each position within the predicted motif.