Molecular phylogenetics of swimming crabs (Portunoidea Rafinesque, 1815) supports a revised family-level classification and suggests a single derived origin of symbiotic taxa
- Published
- Accepted
- Received
- Academic Editor
- Mohammad Shamsur Rahman
- Subject Areas
- Biodiversity, Evolutionary Studies, Marine Biology, Taxonomy, Zoology
- Keywords
- Evolution, Taxonomy, Phylogeny, Portunidae, Portunoidea, Symbiosis, Decapoda, Brachyura, Systematics, Swimming crab
- Copyright
- © 2018 Evans
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2018. Molecular phylogenetics of swimming crabs (Portunoidea Rafinesque, 1815) supports a revised family-level classification and suggests a single derived origin of symbiotic taxa. PeerJ 6:e4260 https://doi.org/10.7717/peerj.4260
Abstract
Portunoidea is a diverse lineage of ecologically and economically important marine crabs comprising 8 families and 14 subfamilies. Closely related portunid subfamilies Caphyrinae and Thalamitinae constitute some of this group’s greatest morphological and taxonomic diversity, and are the only known lineages to include symbiotic taxa. Emergence of symbiosis in decapods remains poorly studied and portunoid crabs provide an interesting, but often overlooked example. Yet the paucity of molecular phylogenetic data available for Portunoidea makes it challenging to investigate the evolution and systematics of the group. Phylogenetic analyses, though limited, suggest that many putative portunoid taxa are para- or polyphyletic. Here I augment existing molecular data—significantly increasing taxon sampling of Caphyrinae, Thalamitinae, and several disparate portunoid lineages—to investigate the phylogenetic origin of symbiosis within Portunoidea and reevaluate higher- and lower-level portunoid classifications. Phylogenetic analyses were carried out on sequences of H3, 28S rRNA, 16S rRNA, and CO1 for up to 168 portunoid taxa; this included, for the first time, molecular data from the genera Atoportunus, Brusinia, Caphyra, Coelocarcinus, Gonioinfradens, Raymanninus, and Thalamonyx. Results support the placement of all symbiotic taxa (Caphyra, Lissocarcinus, and two Thalamita) in a single clade derived within the thalamitine genus Thalamita. Caphyrina Paulson, 1875, nom. trans. is recognized here as a subtribe within the subfamily Thalamitinae. Results also support the following taxonomic actions: Cronius is reclassified as a thalamitine genus; Thalamonyx is reestablished as a valid genus; Goniosupradens is raised to the generic rank; and three new genera (Zygita gen. nov., Thranita gen. nov., and Trierarchus gen. nov.) are described to accommodate some Thalamita s.l. taxa rendered paraphyletic by Caphyrina. A new diagnosis of Thalamitinae is provided. Results also support a more conservative classification of Portunoidea comprising three instead of eight extant families: Geryonidae (Geryonidae + Ovalipidae; new diagnosis provided), Carcinidae (Carcinidae + Pirimelidae + Polybiidae + Thiidae + Coelocarcinus; new diagnosis provided) and Portunidae. Finally, 16s rRNA data suggests family Brusiniidae might not be a portunoid lineage.
Introduction
The superfamily Portunoidea Rafinesque, 1815 (455 spp.; De Grave et al., 2009) is a diverse clade of marine crabs that includes commercially important species, significant invasives (Brockerhoff & McLay, 2011) and several ecologically divergent lineages that radiated across tropical, temperate and deep-ocean habitats (e.g., Figs. 1 and 2). Collectively referred to as “swimming crabs,” members of this clade are known for being aggressive opportunistic omnivores that are agile and well adapted to swimming (Hartnoll, 1971; Hazlett, 1971; Spiridonov, Neretina & Schepetov, 2014; Williams, 1981). Morphologically, portunoid crabs are characterized by having a broad, compressed, laterally streamlined carapace and paddle-shaped posterior “natatory” legs (Hartnoll, 1971). Yet this clade also includes several atypical lineages that are morphologically and ecologically divergent. Among these, members of the tropical Indo-Pacific subfamily Caphyrinae Paulson, 1875 (28 spp.) have evolved symbiotic relationships with algae, anemones, echinoderms, and soft corals (Caulier et al., 2013; Hay et al., 1989; Spiridonov, 1999; Stephenson & Rees, 1968). Relative to most portunoids, members of this group are smaller, less streamlined and exhibit highly modified “natatory” legs adapted for grasping onto or burying beneath their hosts (Figs. 3A–3D, 3I and 4B–4F). Additional adaptations to symbiosis found in these crabs include cryptic coloration (Ayotte, 2005), attraction to host chemical defense compounds (Caulier et al., 2013; Hay et al., 1989), consumption of host tissue (Caulier et al., 2014; Hay et al., 1989; Steudler, Schmitz & Ciereszko, 1977), and social monogamy (Caulier et al., 2012; for significance see Baeza & Thiel, 2007). Despite its novelty among portunoid crabs, the nature of symbiosis in Caphyrinae remains poorly studied and underreported (Baeza, 2015; Castro, 2015). Unlike Caphyrinae, most well-studied symbiotic crustaceans fall within clades that are species-rich and dominated by or exclusively composed of symbiotic taxa (Baeza, 2015). This has led some to hypothesize that the emergence of symbiosis in crustaceans promotes large evolutionary radiations (Baeza, 2015). However, this hypothesis remains to be tested, requiring phylogenetic analyses of multiple clades with symbiotic and free-living lineages.
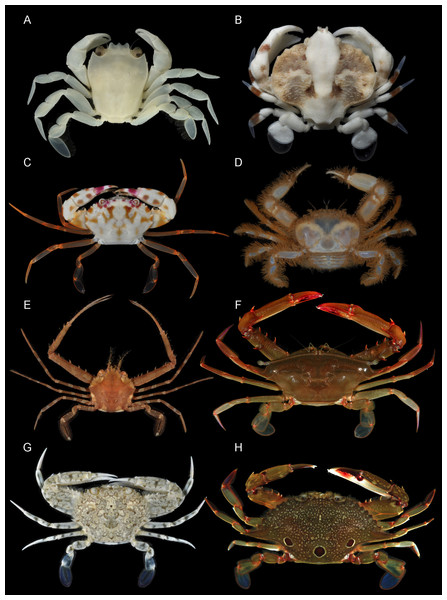
Figure 1: Representatives of various Portunoidea taxa included in this study.
(A) Brusinia profunda (USNM 277519; New Caledonia; preserved color); (B) Coelocarcinus foliatus (UF 40176; Guam); (C) Carupa tenuipes (UF 39918; Palau); (D) Libystes (UF 23926; Moorea Is.); (E) Lupocyclus cf. philippinensis (UF 41639; Luzon Is.); (F) Podophthalmus vigil (UF 24543; Moorea Is.); (G) Portunus (Cycloachelous) granulatus (UF 40021; Guam); (H) Portunus (Portunus) sanguinolentus (UF 24538; Moorea Is.). Photographs (A–C, G) by Nathaniel Evans; photographs (D–F, H) by Gustav Paulay.Figure 2: Representative non-symbiotic Thalamitinae species.
(A) Cronius ruber (UF 35672; Florida); (B) Thalamitoides spinigera (UF 36697; Farasan Banks); (C) Gonioinfradens paucidentatus (UF 37141; Red Sea); (D) Goniosupradens acutifrons (UF 7114; Okinawa); (E) Charybdis orientalis (UF 41638; Luzon Is.); (F) Thalamonyx gracilipes (UF 42972; Mindoro Is.); (G) Thalamita admete (UF 40031; Guam); (H) Thalamita chaptalii (UF 39917; Palau); (I) Thranita coeruleipes, comb. nov. (UF 40078; Guam); (J) Thalamita cf. philippinensis (UF 43302; Mindoro Is.). Photographs (A, G–I) by Nathaniel Evans; photographs (B–F, J) by Gustav Paulay.Figure 3: Representative putative symbiotic Thalamitinae species.
(A) Caphyra loevis (UF 39060); (B) Lissocarcinus cf. laevis (UF 39136; New Caledonia); (C) Lissocarcinus holothuricola (UF 30182; Marquesas); (D) Lissocarcinus orbicularis (UF 23972; Moorea); (E) Zygita murinae, comb. nov. (UF 36721; Farasan Banks); (F) Trierarchus woodmasoni, comb. nov. (UF 40079; Guam); (G) Trierarchus cf. cooperi sp. A, comb. nov. (UF 16023; Moorea Is.); (H) Trierarchus cf. cooperi sp. B, comb. nov. (UF 40100; Guam); (I) Trierarchus rotundifrons, comb. nov. (UF 40067; Guam); (J) Trierarchus squamosus, comb. nov. (USNM 102963; Bikini Atoll; preserved specimen, grayscale, left frontal margin damaged). Photographs (A–C, F, H–J) by Nathaniel Evans; photographs (D, E, G) by Gustav Paulay.Figure 4: Representative portunid natatory leg morphology and divergent, symbiotic caphyrine forms.
Typical portunid P5 morphology and terminology: (A) Thranita cf. rubridens (UF 43834). Typical symbiotic caphyrine P5 morphology: (B) Caphyra cf. fulva (UF 11748; host xeniid soft coral); (C) Caphyra loevis (UF 38881; host xeniid soft coral); (D) Lissocarcinus holothuricola (UF 30302; host holothurians); (E) Lissocarcinus laevis (UF 41571; host cerianthids and actinodendronid anemones); (F) Caphyra rotundifrons (=Trierachus rotundifrons, comb. nov., UF 40067A; host Chlorodesmis algae).Recently, Evans & McKeon (2016) provided compelling evidence that some species of the portunid genus Thalamita also exhibit symbiotic relationships (with soft coral). It has long been suggested that Caphyrinae shares a close, even derived relationship with Thalamita and other taxa in the diverse portunid subfamily Thalamitinae Paulson, 1875 (162 spp.; e.g., see Stephenson & Campbell, 1960). Thalamitinae radiated across the same Indo-Pacific habitats where Caphyrinae and their reef-associated host taxa are found. Consequently, Caphyrinae and Thalamitinae provide an interesting group to investigate the evolution of symbiosis in decapod crustaceans. Unfortunately, like much of Portunoidea, little phylogenetic work has been done on Thalamitinae or Caphyrinae.
The original aim of this study was to investigate the molecular phylogenetic relationships within and between Thalamitinae and Caphyrinae, providing important context for understanding the evolution of symbiosis within portunids. However, preliminary analyses revealed that inclusion of the non-symbiotic Caphyrinae genus Coelocarcinus required analyses be expanded to include the entire superfamily Portunoidea. Consequently, this study compiles and augments the best available molecular data for all of Portunoidea (as of January 2017). Given this broader scope, here I also reevaluate family classifications within the superfamily Portunoidea and subfamily classifications within Portunidae. Finally, for Thalamitinae and Caphyrinae, where taxon sampling is now the densest of any portunoid clade, generic level classifications are also reevaluated and new genera and morphological diagnoses proposed where appropriate.
A brief review of portunoid systematics
Considerable systematic work was carried out on Portunoidea during the 19th and 20th centuries, often in conjunction with work on the morphologically similar Cancroidea (reviewed in Davie, Guinot & Ng, 2015a; Karasawa, Schweitzer & Feldmann, 2008; Schubart & Reuschel, 2009). Toward the end of this period W. Stephenson revised and largely stabilized portunoid classification (Stephenson, 1972). However, morpho-taxonomic work has continued for the group, sometimes revealing surprisingly unique new lineages (e.g., Atoportunus Ng & Takeda, 2003). In recent years genetic data has increasingly been combined with morphology to resolve species complexes (Keenan, Davie & Mann, 1998; Lai, Ng & Davie, 2010; Robles et al., 2007), but neither molecular nor morphological phylogenetic analyses have been widely applied to the group.
To date, only three studies have conducted higher-level molecular phylogenetic analyses of Portunoidea, using 16S rRNA or combinations of CO1, H3, 16S and 28S rRNA for up to 43 portunoid taxa (Mantelatto et al., 2009; Schubart & Reuschel, 2009; Spiridonov, Neretina & Schepetov, 2014). Of these studies, the latter two are the only to include a caphyrine species (Lissocarcinus orbicularis), which was recovered falling sister to, or derived within Thalamitinae (comprised of one and six thalamitine taxa, respectively). Though these studies have significantly improved our understanding of portunoid systematics, synthesis of this work is complicated by a lack of overlap in both taxa and molecular data sampled.
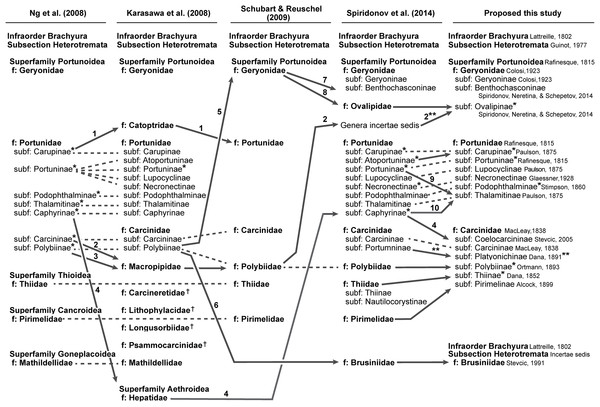
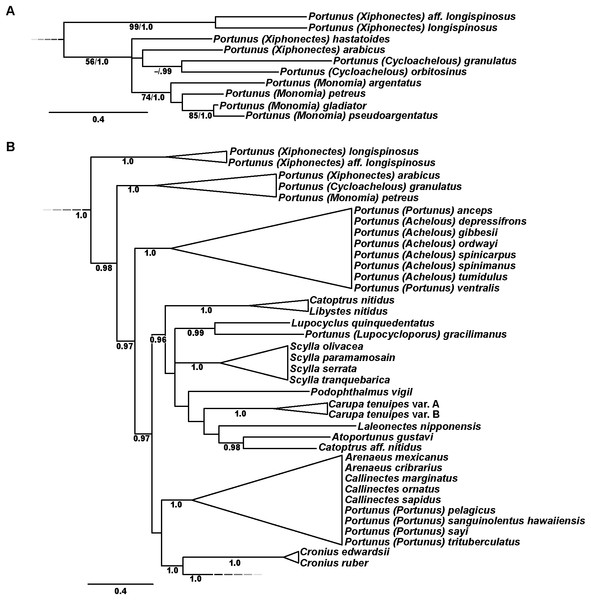
In addition to molecular work, only the generic level morphological cladistic analyses of Portunoidea by Karasawa, Schweitzer & Feldmann (2008) have significantly contributed to our understanding of higher-level phylogenetic relationships within the clade. None of this work analyzed more than approximately 40 of the 455 extant portunoid taxa. Nevertheless, beginning with Ng, Guinot & Davie (2008) four different schemes have been proposed for the familial and subfamilial classification of Portunoidea (Fig. 5). While additional revisions will likely be needed, here I propose a new, more conservative classification scheme for extant portunoids based on more comprehensive molecular phylogenetic analyses of the superfamily.
Figure 5: Summary of major recent changes to Portunoidea familial and subfamilial classification and a new proposed scheme.
Dashed and arrowed lines trace recognized taxa between studies. Solid arrowed lines highlight notable changes, with numbers indicating the movement of specific genera: 1, Catoptrus and Libystes; 2, Echinolatus and Nectocarcinus; 3, Bathynectes, Macropipus, Necora, Parathranites and Raymanninus; 4, Coelocarcinus; 5, Benthochchascon and Ovalipes; 6, Brusinia; 7, Benthochchascon; 8, Ovalipes; 9, Cronius; 10, Caphyra and Lissocarcinus. Single asterisk*, corresponding study considers subfamily composition and status uncertain given morphological or phylogenetic results, or lack there of; double asterisks**, change made following Davie, Guinot & Ng (2015b); †, extinct family. Figure modeled after Fig. 8 in Spiridonov, Neretina & Schepetov (2014).Materials and Methods
Voucher material and taxonomic identifications
Sequence data generated for this study was derived from 137 vouchered specimens listed in Table 1 and Table S1 from the following collections: the National Museum of Marine Biology and Aquarium, Taiwan (NMMBCD); the Florida Museum of Natural History, University of Florida, Gainesville, Florida, USA (UF); the National Museum of Natural History, Smithsonian Institution, Washington, DC, USA (USNM); the Zoological Reference Collection of the Lee Kong Chian Natural History Museum, National University of Singapore, Singapore (ZRC). Additional information regarding UF and USNM vouchers can be obtained by searching digital collection records (http://specifyportal.flmnh.ufl.edu/iz/ and https://collections.nmnh.si.edu/search/iz/) or through the iDigBio portal (https://www.idigbio.org/portal/search). Morphological work was conducted using these and other specimens in UF and USNM holdings. Species identifications were made using taxonomic literature (Edmondson, 1954; Stephenson, 1972; Stephenson & Hudson, 1957; Wee & Ng, 1995) and with reference to material (including types) previously identified by M.J. Rathbun, W. Stephenson, or V. Spiridonov. Identification and taxon sampling was also aided through analyses of a large unpublished data set of CO1 DNA barcode sequences generated from over 1,000 USNM and UF portunoid specimens. Inclusion of all DNA barcode data is beyond the scope of this study but is forthcoming in several investigations led by C.P. Meyer, G. Paulay or N. Evans. The classification scheme of Ng, Guinot & Davie (2008) was primarily followed here including, for the sake of clarity, the Portunus subgeneric classification scheme. However, some modifications were made to be consistent with Spiridonov, Neretina & Schepetov (2014). Specifically, Cycloachelous was treated as a valid subgenus and Lupocycloporus a valid genus. Lineage specific taxa counts were taken from Davie, Guinot & Ng (2015b), De Grave et al. (2009) and Spiridonov, Neretina & Schepetov (2014) and typically do not reflect changes made after these works. Finally, following clarification by V. Spiridonov (2017, personal communication) the authorship of Caphyrinae, Carupinae, Lupocylinae, and Thalamitinae are attributed to Paulson (1875). This avoids the widely used misspelling Paul’son, which resulted from an improper English translation of Paulson (1875) from Cyrillics. Original translations of the author’s name in Latin were Paulson and Paulsohn, but never Paul’son.
| Taxon | 16S rRNA | CO1 | H3 | 28S rRNA | Notes | Voucher ID |
|---|---|---|---|---|---|---|
| Cancroidea: Cancridae: | ||||||
| Cancer pagurus Linnaeus, 1758 | FM207653 | *JQ306000 | **DQ079668 | **DQ079781 | A, B | SMF32764/*MB89000194/**BYU-KC2158 |
| Carpilioidea: Carpiliidae: | ||||||
| Carpilius convexus (Forskal, 1775) | FM208748 | *JX398091 | *JX398111 | *JX398073 | A | SMF32771/*ZMMUMa3438 |
| Corystoidea: Corystidae: | ||||||
| Corystes cassivelaunus (Pennant, 1777) | FM208781 | *JQ306006 | FM208801 | NA | A | SMF32770/*MB89000203 |
| Eriphioidea: Menippidae: | ||||||
| Menippe rumphii (Fabricius, 1798) | HM637976 | HM638051 | HM596626 | NA | ZRC2003.211 | |
| Parthenopoidea: Parthenopidae: | ||||||
| Daldorfia horrida (Linnaeus, 1758) | GQ249177 | *HM638031 | GQ249174 | NA | ZRC2003.0651 | |
| Xanthoidea: Xanthidae: | ||||||
| Etisus utilis Jacquinot, 1853 | HM798456 | HM750981 | *JX398108 | NA | ZRC2002.0586/*NA | |
| Portunoidea: Carcinidae: Carcininae | ||||||
| Carcinus maenas (Linnaeus, 1758) | FM208763 | *FJ581597 | FM208811 | **DQ079798 | A, B | SMF32757/*NA/**BYU-KACmapu |
| Portunoidea: Carcinidae: Coelocarcininae | ||||||
| Coelocarcinus aff. foliatus | KT365545 | NA | NA | NA | A | UF27553 |
| Coelocarcinus foliatus Edmondson, 1930 | KT365601 | KT365724 | KT425058 | NA | UF40056 | |
| Portunoidea: Carcinidae: Pirimelinae | ||||||
| Pirimela denticulata (Montagu, 1808) | FM208783 | NA | FM208808 | NA | A | SMF32767 |
| Sirpus zariquieyi Gordon, 1953 | FM208784 | NA | FM208809 | NA | A | SMF32768 |
| Portunoidea: Carcinidae: Platyonichinae | ||||||
| Portumnus latipes (Pennant, 1777) | FM208764 | NA | FM208812 | NA | A | SMF32758 |
| Portunoidea: Carcinidae: Polybiinae | ||||||
| Liocarcinus corrugatus (Pennant, 1777) | GQ268542 | GQ268536 | *FM208820 | NA | NA/*SMF32760 | |
| Liocarcinus depurator (Linnaeus, 1758) | FM208767 | *FJ174948 | *FJ174852 | *FJ036939 | A | MNHNB31439/*NA |
| Liocarcinus holsatus (Fabricius, 1798) | FM208766 | *GQ268538 | FM208817 | NA | A | SMF32750/*NA |
| Liocarcinus maculatus (Risso, 1827) | FJ174892 | FJ174949 | FJ174853 | FJ036940 | NA | |
| Liocarcinus marmoreus (Leach, 1814) | GQ268547 | GQ268535 | NA | NA | NA | |
| Liocarcinus navigator (Herbst, 1794) | GQ268541 | GQ268537 | *FM208821 | NA | NA/*SMF32775 | |
| Liocarcinus vernalis (Risso, 1816) | FM208768 | *JX123455 | NA | NA | A | SMF32761/*CCDB-1739 |
| Bathynectes longispina (Risso, 1816) | KT365526 | *KT365693 | NA | KT365627 | A, B | UF9383/*UF15140 |
| Bathynectes maravigna (Prestandrea, 1839) | FM208770 | *JQ305966 | FM208814 | NA | A | MNHNB31441/*NA |
| Macropipus tuberculatus (Roux, 1830) | FM208769 | *GQ268530 | FM208815 | NA | A | MNHNB31440/*NA |
| Necora puber (Linnaeus, 1767) | FM208771 | *FJ755619 | FM208813 | **DQ079800 | A, B | SMF32749/*NA/**BYU-KAC2161 |
| Parathranites orientalis (Miers, 1886) | KJ132616 | NA | KJ133173 | NA | NTOUB00090 | |
| “Polybius” henslowii Leach, 1820 | FM208765 | *JQ306041 | FM208816 | NA | A | SMF32759/*MB89000200 |
| Portunoidea: Carcinidae: Thiinae | ||||||
| Thia scutellata (Fabricius, 1793) | FM208782 | NA | FM208810 | NA | A | SMF32769 |
| Portunoidea: Geryonidae: Benthochasconinae | ||||||
| Benthochascon hemingi Alcock & &erson, 1899 | FM208772 | *HM750955 | FM208826 | NA | A | ZRC2000.102 |
| Portunoidea: Geryonidae: Geryoninae | ||||||
| Chaceon granulatus (Sakai, 1978) | FM208775 | *AB769383 | FM208827 | NA | A | SMF32762/*NA |
| Geryon longipes A. Milne-Edwards, 1882 | FM208776 | *JQ305902 | FM208828 | NA | A | SMF32747/*MB89000638 |
| Raymanninus schmitti (Rathbun, 1931) | KT365560 | NA | NA | KT365656 | A, B | UF9676 |
| Portunoidea: Geryonidae: Ovalipinae | ||||||
| Ovalipes iridescens (Miers, 1886) | FM208774 | NA | FM208825 | NA | A | ZRC1995.855 |
| Ovalipes punctatus (De Haan, 1833) | KJ132597 | *KF906404 | KJ133154 | NA | NTOUB00011/*NA | |
| Ovalipes stephensoni Williams, 1976/*O.floridanus Hay & Shore, 1918 | DQ388050 | NA | NA | *KT365648 | B | ULLZ5678/*UF28577 |
| Ovalipes trimaculatus (De Haan, 1833) | FM208773 | *JN315648 | FM208823 | NA | A | MNHNB19785/*NA |
| Portunoidea: Portunidae: Carupinae | ||||||
| Atoportunus gustavi Ng & Takeda, 2003 | KT365590 | KT365692 | NA | NA | UF1266 | |
| Carupa ohashii Takeda, 1993 | FM208759 | NA | FM208790 | NA | A | SMF32756 |
| Carupa tenuipes (var. A) Dana, 1852 | FM208758 | *KT365703 | FM208789 | NA | A | MNHNB31436/*UF16185 |
| Carupa tenuipes (var. B) Dana, 1852 | KT365533 | KT365704 | NA | NA | A | UF15565 |
| Catoptrus aff. nitidus | KT365534 | KT365706 | NA | NA | A | UF18451 |
| Catoptrus nitidus A. Milne-Edwards, 1870/*C. aff. nitidus | FM208755 | *KT365705 | NA | NA | A | MNHNB31435/*UF1024 |
| Laleonectes cf. nipponensis/*L. nipponensis (Sakai, 1938) | KT365548 | KT365727 | *FM208792 | NA | A | UF7342/*MNHNB31434 |
| Libystes edwardsii Alcock, 1899 | FM208761 | NA | NA | NA | A | MNHNB31437 |
| Libystes nitidus A. Milne-Edwards, 1867 | FM208762 | *KT365728 | NA | NA | A | MNHNB31438/*UF12587 |
| Richerellus moosai Crosnier, 2003 | FM208756 | NA | FM208788 | NA | A | MNHNB22838 (paratype) |
| Portunoidea: Portunidae: Lupocyclinae | ||||||
| Lupocycloporus gracilimanus (Stimpson, 1858) | AM410523 | *JX398092 | *JX398124 | *JX398076 | NA/*ZMMUMa3381 | |
| Lupocyclus philippinensis Semper, 1880 | FJ152156 | NA | *JX398119 | *JX398077 | NA/*ZMMUMa3443 | |
| Lupocyclus quinquedentatus Rathbun, 1906 | KT365603 | KT365734 | NA | KT365647 | B | UF10568 |
| Lupocyclus rotundatus Adams & White, 1849 | NA | NA | JX398110 | JX398075 | C | ZMMUMa3441 |
| Portunoidea: Portunidae: Necronectinae | ||||||
| Scylla olivacea (Herbst, 1796) | FJ827760 | FJ827760 | NA | NA | A | NA |
| Scylla paramamosain Estampador, 1949 | FJ827761 | FJ827761 | NA | NA | A | NA |
| Scylla serrata (Forskal, 1775) | FJ827758 | FJ827758 | *FM208793 | NA | A | NA/*MZUF3657 |
| Scylla tranquebarica (Fabricius, 1798) | FJ827759 | FJ827759 | NA | NA | A | NA |
| Portunoidea: Portunidae: Podophthalminae | ||||||
| Euphylax robustus A. Milne-Edwards, 1874 | FJ152153 | NA | NA | NA | CCDB-1122 | |
| Podophthalmus nacreus Alcock, 1899 | NA | JX398093 | NA | JX398078 | C | ZMMUMa3440 |
| Podophthalmus vigil (Fabricius, 1798) | KT365553 | KT365735 | *FM208787 | NA | A | UF18116/*ZRCY4821 |
| Portunoidea: Portunidae: Portuninae | ||||||
| Arenaeus cribrarius (Lamarck, 1818) | FM208749 | *JX123439 | FM208799 | NA | A | SMF32753/*CCDB-3182 |
| Arenaeus mexicanus (Gerstaecker, 1856) | JX123470 | JX123446 | NA | NA | MZUCR2430-4 | |
| Callinectes marginatus (A. Milne-Edwards, 1861) | KT365527 | KT365694 | NA | NA | A | UF11403 |
| Callinectes ornatus Ordway, 1863 | KT365528 | NA | NA | KT365628 | A, B | UF19804 |
| Callinectes sapidus Rathbun, 1896 | AY363392 | AY363392 | *FM208798 | **AY739194 | A, B | NA/*ULLZ3895/**NA |
| Lupella forceps (Fabricius, 1793) | FJ152155 | NA | NA | NA | USNM284565 | |
| Portunus (Achelous) asper (A. Milne-Edwards, 1861) | FJ152158 | NA | NA | NA | CCDB1738 | |
| Portunus (Achelous) depressifrons (Stimpson, 1859) | DQ388064 | *KT365738 | NA | NA | ULLZ4442/*UF26120 | |
| Portunus (Achelous) floridanus Rathbun, 1930 | DQ388058 | NA | NA | NA | ULLZ4695 | |
| Portunus (Achelous) gibbesii (Stimpson, 1859) | DQ388057 | *KT365739 | NA | **KT365650 | B | ULLZ4565/*UF1134/**UF19561 |
| Portunus (Achelous) ordwayi (Stimpson, 1860) | FM208751 | *KT365689 | FM208794 | NA | A | SMF31988/*UF6426 |
| Portunus (Achelous) rufiremus Holthuis, 1959 | DQ388063 | NA | NA | NA | USNM151568 | |
| Portunus (Achelous) sebae (H. Milne Edwards, 1834) | DQ388067 | NA | NA | NA | ULLZ4527 | |
| Portunus (Achelous) spinicarpus (Stimpson, 1871) | DQ388061 | *KT365746 | NA | NA | ULLZ4618/*UF3969 | |
| Portunus (Achelous) spinimanus Latreille, 1819 | KT365558 | *KT365690 | NA | KT365654 | A, B | UF28417/*UF6692 |
| Portunus (Achelous) tumidulus Stimpson, 1871 | KT365589 | KT365691 | NA | NA | UF32157 | |
| Portunus (Cycloachelous) granulatus (H. Milne Edwards,1834) | KT365605 | KT365740 | NA | KT365651 | B | UF4169 |
| Portunus (Cycloachelous) orbitosinus (Rathbun, 1911) | NA | JX398097 | JX398115 | JX398082 | C | ZMMUMa3378 |
| Portunus (Monomia) argentatus (A. Milne-Edwards, 1861) | NA | JX398096 | JX398107 | JX398081 | C | ZMMUMa3365 |
| Portunus (Monomia) gladiator Fabricius, 1798 | NA | JX398095 | JX398113 | JX398080 | C | ZMMUMa3366 |
| Portunus (Monomia) petreus (Alcock, 1899) | KT365606 | KT365743 | NA | NA | UF188 | |
| Portunus (Monomia) pseudoargentatus Stephenson, 1961 | NA | JX398094 | JX398121 | JX398079 | C | ZMMUMa3368 |
| Portunus (Portunus) anceps (Saussure, 1858) | KT365604 | KT365736 | NA | NA | UF32492 | |
| Portunus (Portunus) hastatus (Linnaeus, 1767) | FM208780 | NA | FM208796 | NA | SMF31989 | |
| Portunus (Portunus) inaequalis (Miers, 1881) | FM208752 | NA | FM208795 | NA | A | SMF32754 |
| Portunus (Portunus) pelagicus (Linnaeus, 1758) | FM208750 | *JX398106 | *JX398116 | *JX398074 | A | CSIRO uncatalogued/*NA |
| Portunus (Portunus) sanguinolentus hawaiiensis Stephenson, 1968 | KT365557 | KT365744 | NA | KT365653 | A, B | UF8949 |
| Portunus (Portunus) sayi (Gibbes, 1850) | KT365607 | KT365745 | NA | NA | UF26156 | |
| Portunus (Portunus) trituberculatus (Miers, 1876) | AB093006 | AB093006 | *FM208829 | NA | A | NA/*NA |
| Portunus (Portunus) ventralis (A. Milne-Edwards, 1879) | KT365559 | KT365747 | NA | KT365655 | A, B | UF32351 |
| Portunus (Xiphonectes) arabicus (Nobili, 1905) | KT365554 | KT365737 | NA | KT365649 | A, B | UF7735 |
| Portunus (Xiphonectes) hastatoides Fabricius, 1798 | NA | JX398098 | NA | JX398083 | C | ZMMUMA3392 |
| Portunus (Xiphonectes) aff. longispinosus | KT365555 | KT365741 | NA | KT365652 | A, B | UF10477 |
| Portunus (Xiphonectes) longispinosus (Dana, 1852) | KT365556 | KT365742 | NA | NA | A | UF187 |
| Portunus (Xiphonectes) tenuipes (De Haan, 1835) | NA | JX398099 | NA | JX398087 | C | NA |
| Portunoidea: Portunidae: Thalamitinae | ||||||
| Caphyra bedoti (Zehntner, 1894) | KT365591 | KT365695 | KT425019 | NA | NMMBCD 4091 | |
| Caphyra cf. fulva | KT365529 | KT365696 | KT424990 | KT365629 | A, B | UF11748 |
| Caphyra loevis (A. Milne-Edwards, 1869) | KT365592 | KT365697 | KT425009 | NA | NMMBCD 4090 | |
| Caphyra tridens Richters, 1880 | KT365532 | KT365701 | KT425003 | KT365632 | A, B | UF15907 |
| Caphyra yookadai Sakai, 1933 | KT365593 | KT365702 | KT424993 | NA | NMMBCD 4089 | |
| Caphyra sp. A | KT365531 | KT365699 | NA | NA | A | UF5061-A |
| Caphyra sp. B | NA | KT365700 | KT425046 | KT365631 | B, C | UF14454 |
| Charybdis acuta (A. Milne-Edwards, 1869) | KT365594 | NA | KT425049 | NA | UF13466 | |
| Charybdis anisodon (De Haan, 1850) | KT365536 | NA | NA | NA | A | UF11429 |
| Charybdis annulata (Fabricius, 1798) | KT365595 | KT365708 | KT425027 | KT365634 | B | UF22076 |
| Charybdis bimaculata (Miers, 1886) | KT365596 | KT365709 | KT425036 | *JX398089 | ZRC 2017.0508/ZMMUMa3396 | |
| Charybdis callianassa (Herbst, 1789) | KT365537 | KT365710 | KT425035 | NA | A | ZRC1993.378-384 |
| Charybdis feriata (Linnaeus, 1758) | KT365538 | KT365712 | KT425051 | KT365636 | A, B | UF3739 |
| Charybdis granulata (De Haan, 1833) | NA | JX398102 | JX398118 | JX398090 | C | NA |
| Charybdis hellerii (A. Milne-Edwards, 1867) | KT365540 | KT365715 | KT424999 | KT365638 | A, B | UF11430 |
| Charybdis hongkongensis Shen, 1934 | NA | JX398100 | JX398112 | JX398088 | C | ZMMUMa3363 |
| Charybdis japonica (A. Milne-Edwards, 1861) | FJ460517 | *KT365716 | *KT425042 | NA | A | NA/*ZRC2008.0567 |
| Charybdis longicollis Leene, 1938 | KT365541 | KT365717 | KT425054 | NA | A | UF3179 |
| Charybdis lucifera (Fabricius, 1798) | KT365542 | *KT365718 | *KT425034 | *KT365639 | A, B | UF7667/*UF7684 |
| Charybdis natator (Herbst, 1794) | KT365543 | KT365719 | *KT424998 | NA | A | UF3707/*UF21403 |
| Charybdis orientalis Dana, 1852 | KT588234 | KT588225 | KT781074 | NA | USNM112062 | |
| Charybdis rathbuni Leene, 1938 | KT365599 | KT365722 | KT425056 | NA | UF25655 | |
| Charybdis sagamiensis Parisi, 1916 | KT365598 | KT365721 | NA | KT365641 | B | UF29479 |
| Charybdis variegata (Fabricius, 1798) | KT365600 | KT365723 | KT425043 | NA | ZRC2012.1115 | |
| Cronius edwardsii (Lockington, 1877) | FJ152147 | *KT588227 | NA | NA | A | ULLZ8673/*USNM112311 |
| Cronius ruber (Lamarck, 1818) | KT365546 | *KT365725 | KT425008 | KT365642 | A, B | UF26364/*UF25995 |
| Gonioinfradens paucidentatus (A. Milne-Edwards, 1861) | KT365547 | KT365726 | *KT588216 | NA | A | UF5109/*UF30184 |
| Goniosupradens acutifrons (De Man, 1879) | KT365535 | *KT365707 | *KT425033 | *KT365633 | A, B | UF7114/*UF17047 |
| Goniosupradens erythrodactylus (Lamarck, 1818) | KT365597 | KT365711 | NA | KT365635 | B | UF1398 |
| Goniosupradens hawaiensis (Edmondson, 1954), comb. nov. | KT365539 | KT365714 | KT425023 | KT365637 | A, B | UF25871 |
| Goniosupradens obtusifrons (Leene, 1937) | KT365544 | KT365720 | KT425007 | KT365640 | A, B | UF16599 |
| Lissocarcinus arkati Kemp, 1923 | KT365549 | KT365729 | KT425045 | KT365643 | A, B | UF36296 |
| Lissocarcinus holothuricola (Streets, 1877) | KT365551 | KT365731 | KT425041 | KT365645 | A, B | UF30203 |
| Lissocarcinus laevis Miers, 1886 | KT365550 | KT365730 | *KT425020 | *KT365644 | A, B | UF204/*UF39136 |
| Lissocarcinus orbicularis Dana, 1852 | KT365552 | KT365732 | *KT425032 | NA | A | UF15741/*UF15429 |
| Lissocarcinus polybiodes Adams & White, 1849 | KT365602 | KT365733 | KT424994 | KT365646 | B | UF35245 |
| Thalamita admete (Herbst, 1803) | KT365562 | *KT365749 | *KT425014 | *KT365658 | A, B | UF7688/*UF16971 |
| Thalamita aff. admete | KT365561 | KT365748 | KT424995 | KT365657 | A, B | UF17745 |
| Thalamita auauensis Rathbun, 1906 | KT365563 | KT365750 | KT425022 | NA | A | UF12320 |
| Thalamita bevisi (Stebbing, 1921) | KT365564 | KT365751 | KT425048 | KT365659 | A, B | UF197 |
| Thalamita bouvieri Nobili, 1906 | KT365565 | KT365752 | *KT425016 | KT365660 | A, B | UF24801/*UF17562 |
| Thalamita chaptalii (Audouin, 1826) | KT365568 | KT365758 | *KT425047 | *KT365663 | A, B | UF13103/*UF206 |
| Thalamita cf. gatavakensis sp. A | KT365576 | KT365767 | KT424997 | KT365670 | A, B | UF16649 |
| Thalamita cf. gatavakensis sp. B | KT365575 | *KT365766 | KT424992 | KT365669 | A, B | UF17469/*UF17486 |
| Thalamita gloriensis Crosnier, 1962 | KT365582 | KT365779 | KT425038 | KT365678 | A, B | UF25902 |
| Thalamita granosimana Borradaile, 1902 | KT365577 | KT365769 | KT425005 | KT365671 | A, B | UF24790 |
| Thalamita integra Dana, 1852 | KT365578 | *KT365770 | *KT425028 | *KT365672 | A, B | UF587/*UF22085 |
| Thalamita kagosimensis Sakai, 1939 | KT365612 | KT365771 | KT425011 | KT365673 | B | ZRC 2017.0514 |
| Thalamita aff. kukenthali | KT365608 | KT365753 | KT425052 | NA | UF33634 | |
| Thalamita malaccensis Gordon, 1938 | KT365614 | KT365774 | KT425010 | NA | ZRC 2017.0512 | |
| Thalamita mitsiensis Crosnier, 1962 | KT365580 | KT365775 | *KT425053 | KT365675 | A, B | UF21937/*UF190 |
| Thalamita oculea Alcock, 1899 | KT365616 | KT365777 | KT425044 | NA | ZRC 2017.0513 | |
| Thalamita parvidens (Rathbun, 1907) | KT365567 | KT365757 | KT425037 | KT365662 | A, B | UF17595 |
| Thalamita philippinensis Stephenson & Rees, 1967 | KT365579 | KT365772 | KT425006 | KT365674 | A, B | UF24920 |
| Thalamita picta Stimpson, 1858 | KT365581 | KT365778 | KT425013 | KT365677 | A, B | UF24881 |
| Thalamita pseudoculea Crosnier, 1984 | KT365610 | KT365754 | KT425050 | NA | UF13877 | |
| Thalamita pseudopoissoni Stephenson & Rees, 1967 | KT365609 | KT365755 | KT425055 | NA | UF5051 | |
| Thalamita quadrilobata Miers, 1884 | KT365585 | KT365782 | *KT425015 | *KT365680 | A, B | UF14254/*UF14608 |
| Thalamita savignyi A. Milne-Edwards, 1861 | KT365618 | KT365784 | KT425061 | KT365682 | B | UF7689 |
| Thalamita seurati Nobili, 1906 | KT365587 | KT365785 | KT425004 | KT365683 | A, B | UF12832 |
| Thalamita sima H. Milne Edwards, 1834 | KT365619 | KT365786 | *KT588217 | **JX398086 | UF35869/*UF36191/**ZMMUMa3373 | |
| Thalamita aff. spinifera | KT365621 | KT365788 | KT425001 | NA | UF33379 | |
| Thalamita stephensoni Crosnier, 1962 | KT365623 | KT365790 | KT425059 | NA | UF17070 | |
| Thalamitoides quadridens A. Milne-Edwards, 1869 | KT365588 | *KT365792 | KT425017 | NA | A | UF18495/*UF15637 |
| Thalamitoides spinigera Nobili, 1905 | KT365625 | KT365793 | NA | KT365687 | B | UF32881 |
| Thalamitoides tridens A. Milne-Edwards, 1869 | KT365626 | KT365794 | NA | KT365688 | B | UF18231 |
| Thalamonyx gracilipes A. Milne-Edwards, 1873 | KT365611 | KT365768 | KT425000 | NA | USNM274300 | |
| Thranita coeruleipes (Hombron & Jacquinot, 1846), comb. nov. | KT365569 | KT365759 | KT425057 | KT365664 | A, B | UF3232 |
| Thranita crenata (Rüppell, 1830), comb. nov. | KT365572 | KT365763 | *KT424991 | **JX398085/*KT365667 | A, B | UF8950/*UF17752/**ZMMUMa3343 |
| Thranita danae (Stimpson, 1858), comb. nov. | KT365573 | *KT365764 | *KT425031 | KT365668 | A, B | UF22114/*UF25992 |
| Thranita foresti (Crosnier, 1962), comb. nov. | KT365574 | KT365765 | KT425040 | NA | A | UF2222 |
| Thranita cf. prymna (Herbst, 1803), comb. nov. | KT365583 | KT365780 | KT425025 | *JX398084 | A | UF14613/*ZMMUMa3346 |
| Thranita pseudopelsarti (Crosnier, 2002), comb. nov. | KT365584 | KT365781 | KT425039 | KT365679 | A, B | UF16218 |
| Thranita rubridens (Apel & Spiridonov, 1998), comb. nov. | KT365586 | KT365783 | KT425060 | KT365681 | A, B | UF7700 |
| Thranita aff. rubridens | KT365566 | KT365756 | KT425021 | KT365661 | A, B | UF25803 |
| Thranita spinicarpa (Wee & Ng, 1995), comb. nov. | KT365620 | KT365787 | KT425012 | KT365684 | B | UF36225 |
| Thranita spinimana (Dana, 1852), comb. nov. | KT365622 | KT365789 | NA | KT365685 | B | UF36209 |
| Trierarchus cf. cooperi sp. A, comb. nov. | KT365570 | KT365760 | KT424996 | KT365665 | A, B | UF16152 |
| Trierarchus cf. cooperi sp. B. comb. nov. | KT365571 | KT365761 | KT425029 | KT365666 | A, B | UF16949 |
| Trierarchus rotundifrons (A. Milne-Edwards, 1869), comb. nov. | KT365530 | KT365698 | *KT424989 | *KT365630 | A, B | UF4079/*UF4057 |
| Trierarchus squamosus (Stephenson & Hudson, 1957), comb. nov. | KU737571 | NA | NA | NA | USNM102963 | |
| Trierarchus woodmasoni (Alcock, 1899), comb. nov. | KT365624 | KT365791 | KT425026 | KT365686 | B | UF4114 |
| Zygita longifrons (A. Milne-Edwards, 1869), comb. nov. | KT365613 | KT365773 | KT425002 | NA | UF7343 | |
| Zygita murinae (Zarenkov, 1971), comb. nov. | KT365615 | KT365776 | KT425018 | KT365676 | B | UF36525 |
Notes:
Bolded Genbank numbers represent data generated for this study, for voucher locality data and source references of all other sequences see Table S1.
A, 16S rRNA data include tRNA-Leu and partial NADH1 sequences.
B, 28S rRNA sequences > 500 bps and were included in analyses of 28S only data.
C, included only in single marker and 174 OTU concatenated analyses.
Voucher prefixes refer to the following institutions: BYU, Monte L. Bean Life Science Museum, Brigham Young University, Provo; CCDB, Crustacean Collection of the Department of Biology, University of São Paulo, São Paulo; CSIRO, CSIRO Marine Research collections, Hobart; MB, Museu Nacional de Historia Natural, Universidade de Lisboa, Lisbon; MNHN, Muséum National d’Histoire Naturelle, Paris; MZUCR, Zoology Museum, Universidad de Costa Rica, San José; MZUF, La Specola, Museo Zoologico Universita di Firenze, Florence; NMMBCD, National Museum of Marine Biology and Aquarium, Taiwan; NTOU, National Taiwan Ocean University, Keelung; SMF, Senckenberg Research Institute and Natural History Museum in Frankfurt; UF, Florida Museum of Natural History, University of Florida, Gainesville; ULLZ, Zoological Collection, University of Louisiana at Lafayette, Lafayette; USNM, Smithsonian National Museum of Natural History, Washington; ZMMU, Zoological Museum of the Moscow University, Moscow; ZRC, the Zoological Reference Collection of the Lee Kong Chian Natural History Museum, Singapore.
Morphological terminology
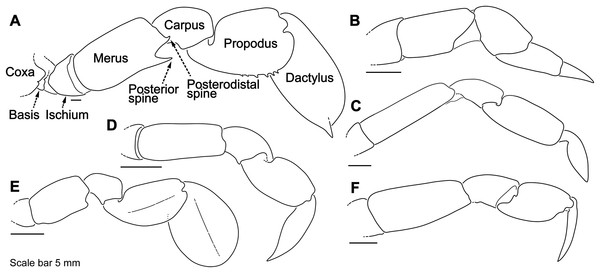
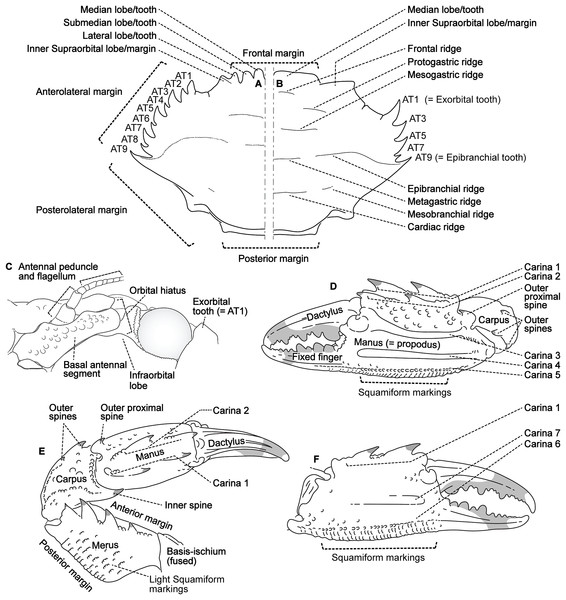
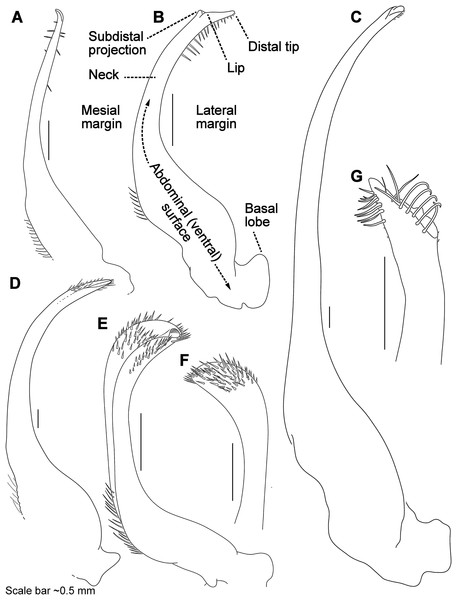
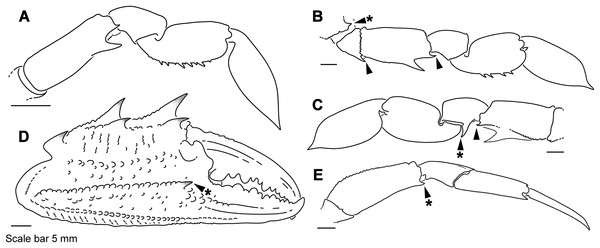
Descriptive work on portunoid crabs has not always used consistent morphological terminology. Morphological terms used here are illustrated in Figs. 4, 6 and 7, and mostly conform to those used by Apel & Spiridonov (1998), Crosnier (1962), Stephenson & Hudson (1957), and Wee & Ng (1995). As in these works, here the demarcation of teeth (or lobes) along the frontal margin of the carapace does not include the inner supraorbital margins, but discussion (or counts) of the teeth along the anterolateral margins does include the exorbital tooth (as tooth number one; Figs. 6A and 6B). Standard pereiopod abbreviations are also followed: P1, cheliped; P2–P4, ambulatory legs; P5, natatory (swimming) leg (Fig. 4). Likewise, G1 and G2 denote male first and second gonopods, respectively (Fig. 7).
Figure 6: Morphological terminology for the carapace, antenna, and cheliped.
Carapace dorsal surface: (A) Cronius edwardsii (USNM 1254607); (B) Thalamita gatavakensis (UF 24660). (C) Stylized ventral surface of antenna and orbit. (D–F) Stylized thalamitine left cheliped: (D), outer surface; (E) dorsal surface; (F) inner surface. AT, positionally homologous portunid anterolateral tooth number.Figure 7: Morphology and terminology for stylized left male first gonopod (G1) from representative taxa.
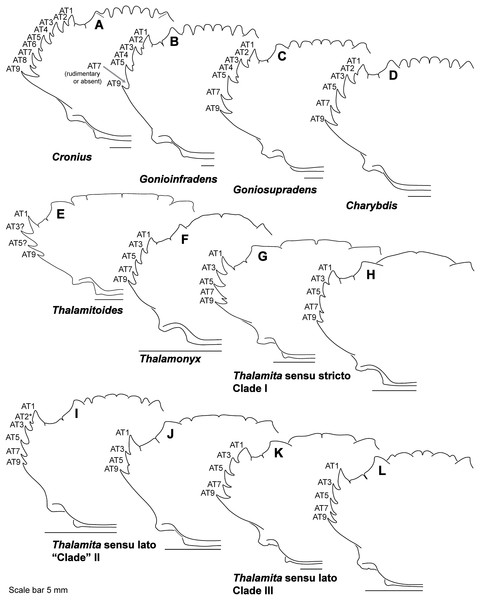
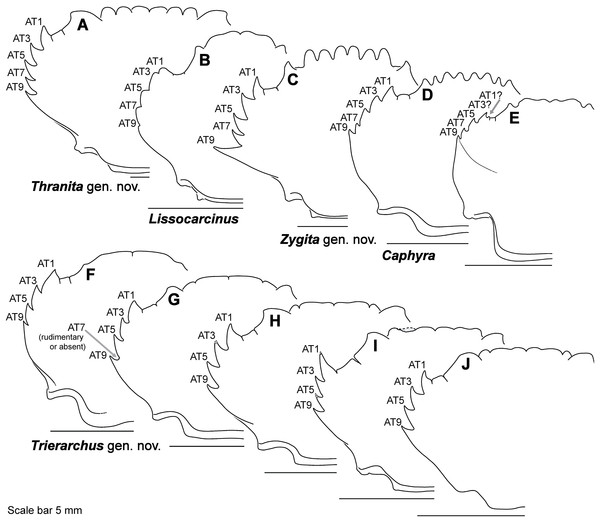
(A) Thalamita granosimana (Thalamita sensu stricto clade 1; composite redrawn from Stephenson & Rees, 1967a, Fig. 27A and 27B); (B) Thalamita spinifera (Thalamita sensu lato “clade” II; redrawn from Crosnier, 1962, Fig. 214); (C) Thranita crenata (“Thalamita” sensu lato clade III; composite redrawn from Crosnier, 1962, Figs. 232 and 233); (D) Zygita murinae (composite redrawn from Spiridonov & Neumann, 2008, Figs. 6 and 7); (E, F) Trierarchus woodmasoni (redrawn from Crosnier, 1975a, Figs. 8J and 8I, respectively); (G) Thalamonyx gracilipes (redrawn from Stephenson & Rees, 1967b, Fig. 2H). (A–E) depict the abdominal (ventral) G1 surface, (F, G) depict distal portion of the sternal (dorsal) G1 surface.Finally, here I propose new terminology in the form of two numbering schemes to respectively characterize carapace anterolateral teeth and cheliped carinae in Portunidae (Figs. 6A, 6B, 6D and 6F). In both cases, indicated structures clearly share positional homology across Portunidae (likely Portunoidea) and adoption of the proposed schemes should bring greater clarity to taxonomic descriptive work on portunids. For example, anterolateral teeth counts are often diagnostic for Thalamita where five teeth are standard, but the fourth is often absent and the first sometimes exhibits a subsidiary tooth. Confusion can arise when diagnoses of Thalamita discuss the form or presence of the “fourth tooth” in disparate species exhibiting a total of four, five or six anterolateral teeth (e.g., compare Figs. 8G–8J). Under the proposed scheme such confusion is avoided; the diagnostic “fourth” anterolateral tooth typically refers to portunid tooth AT7, and is better discussed as such in each of these cases. Likewise a simple count of spines on the upper surface of the cheliped can lead to confusing descriptions when standard spines are absent from different cheliped carinae for different taxa. Although a determination of positional homology for anterolateral teeth may be difficult for select taxa (e.g., Figs. 8E and 9E), “transitional” forms may significantly help. For example, while exhibiting nine anterolateral teeth is clearly plesiomorphic within Portunidae (Spiridonov, Neretina & Schepetov, 2014), in Cronius these teeth alternate in size such that each of its five large teeth are separated by a reduced (or subequal) tooth (Fig. 8A). This suggests that the five anterolateral teeth typical to Thalamita likely correspond (in order) to teeth numbers one, three, five, seven, and nine in portunine taxa (compare Figs. 6A and 6B). This is supported by additional intermediate forms present in other Thalamitinae taxa (Figs. 8B–8D). Last, it is worth noting that some positionally homologous anterolateral teeth are likely homoplastic, reappearing within derived clades through reversal or parallelism (e.g., AT2* in Fig. 8I).
Figure 8: Representative partial carapace outlines of Thalamitinae genera, Part 1.
(A) Cronius edwardsii (USNM 1254607); (B) Gonioinfradens paucidentatus (UF 1411-A); (C) Goniosupradens obtusifrons (UF 16599); (D) Charybdis orientalis (USNM 112062); (E) Thalamitoides quadridens (UF 1962); (F) Thalamonyx gracilipes (USNM 127103-A); (G) Thalamita admete (UF 26950-A); (H) Thalamita parvidens (USNM 32855-A; Holotype); (I) Thalamita spinifera (UF 33379); (J) Thalamita bouvieri (UF 41652); (K) Thalamita sima (USNM 1254584-A); (L) Thalamita malaccensis (USNM 274290-A). AT, positionally homologous portunid anterolateral tooth number (see Figs. 6A and 6B and text). Asterisks indicate a homoplastic anterolateral tooth that arose through parallelism or reversal (see text).Figure 9: Representative partial carapace outlines of Thalamitinae genera, Part 2.
(A) Thranita crenata, comb. nov. (UF 39965); (B) Lissocarcinus laevis (UF 41571); (C) Zygita longifrons, comb. nov. (UF 199); (D) Caphyra loevis (UF 38881); (E) Caphyra cf. fulva (UF 38855; epibranchial ridge depicted); (F) Trierarchus rotundifrons, comb. nov. (UF 40143-A); (G) Trierarchus woodmasoni, comb. nov. (UF 40079); (H) Trierarchus cooperi sp. B, comb. nov. (USNM 41125-A); (I) Trierarchus squamosus, comb. nov. (USNM 102963); (J) Trierarchus acanthophallus, comb. nov. (stylized outline redrawn from Chen & Yang, 2008). AT, positionally homologous portunid anterolateral tooth number (see Figs. 6A and 6B and text).Nomenclatural acts
The electronic version of this article in portable document format will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:90E97894-9BBE-452C-A6A8-AFF7C1B78874. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
DNA extractions, amplification and sequencing
Molecular work was conducted at the Florida Museum of Natural History and the Smithsonian Institution’s Laboratories of Analytical Biology. DNA was extracted using a standard phenol–chloroform protocol by hand or on an Autogen Prep 956 Extractor (AutoGen Inc., Holliston, MA, USA). A total of 345 sequences from four molecular markers (16S rRNA, CO1, 28S rRNA, and H3) were generated from 114 portunoid species, 76 of which have never before been sequenced. Amplifications were carried out following protocols outlined in Evans & Paulay (2012), Lasley, Klaus & Ng (2015), and Leray & Knowlton (2015). Typically this included the use of a “step-down” PCR profile (Evans & Paulay, 2012). This approach involves using a higher annealing temperature for the first five PCR cycles followed by 30 cycles at a lower annealing temperature. Table 2 lists primer pairs, annealing temperatures and resulting fragment sizes for each marker. Amplification of 16S rRNA resulted in at least 500 bps of sequence, but one primer set yielded a 1.2 kb fragment that includes tRNA-Leu and partial NADH1. Both 16S fragments were combined into a single data set that, unless otherwise stated, is referred to here as 16S data (fragment distinctions indicated in Table 1 notes). Clean up, cycle sequencing and purification were carried out on all successful PCR products using Exosap-It (Affymetrix Inc., Santa Clara, CA, USA), ABI BigDye terminator V3.1 reactions and a Sephadex G-50 protocol. Resulting products were bidirectionally sequenced on an ABI 3130xl genetic analyzer (Applied Biosystems, Foster City, CA, USA). Consensus sequences were generated using Geneious v. 7.1.8 (Kearse et al., 2012) and submitted to GenBank. GenBank accession numbers are listed in Table 1.
| Primer pairs—forward/reverse | 5′–3′ Forward primer sequence | 5′–3′ Reverse primer sequence | Ta (°C) | Approximate amplicon size (bps) | Reference |
|---|---|---|---|---|---|
| CO1 | |||||
| dgLCO1490/dgHCO2198 | GGTCAACAAATCATAAAGAYATYGG | TAAACTTCAGGGTGACCAAARAAYCA | 50 and 45 | 650 | Meyer (2003) |
| jgLCO1490/jgHCO2198 | TITCIACIAAYCAYAARGAYATTGG | TAIACYTCIGGRTGICCRAARAAYCA | 50 and 45 | 650 | Geller et al. (2013) |
| 16S rRNA + tRNA-Leu + NADH1 | |||||
| NDH5/16L2 | GCYAAYCTWACTTCATAWGAAAT | TGCCTGTTTATCAAAAACAT | 48 and 44 | 1,200 | Schubart, Cuesta & Felder (2002) |
| 16S rRNA | |||||
| 16H11/16L2 | AGATAGAAACCRACCTGG | See above | 48 and 44 | 580 | Schubart (2009) |
| crust16sF1/crust16sR2 | CCGGTYTGAACTCAAATCATGTAAA | TTGCCTGTTTATCAAAAACATGTYTRTT | 50 and 45 | 515 | Lai et al. (2009) |
| 28S (D1–D2 region) | |||||
| LSUfw1brach/LSUrev1brach | AGCGGAGGAAAAGAAACYA | TACTAGATGGTTCGATTAGTC | 50 and 45 | 1,300 | This study* |
| LSUfw2brach/LSUrev2brach | ACAAGTACCGTGAGGGAAAGTTG | ACAATCGATTTGCACGTCAG | 55 and 50 | 890 | This study* |
| F635/LSUrev2brach | CCGTCTTGAAACACGGACC | See above | 55 and 50 | 600 | Medina et al. (2001) |
| H3 | |||||
| H3af/H3ar | TGGCTCGTACCAAGCAGACVGC | TATCCTTRGGCATRATRGTGAC | 50 and 47 | 327 | Colgan et al. (1998) |
Notes:
Ta, annealing temperatures used here in a “step-down” PCR approach (see text).
Taxon sampling and composition of molecular data sets
A molecular data set comprised of 174 operational taxonomic units (OTUs) was constructed for this study. This data set combined 344 newly generated sequences with 176 previously published fragments of 16S rRNA, CO1, 28S rRNA, and H3 data. Published sequences were mostly drawn from recent phylogenetic studies on Portunoidea, including Mantelatto et al. (2009), Schubart & Reuschel (2009), and Spiridonov, Neretina & Schepetov (2014). With some exceptions, taxon sampling was designed to include portunoid lineages at or above the species-level, avoiding genetically and morphologically highly conserved species complexes, especially those previously investigated (e.g., Callinectes by Robles et al., 2007; Portunus pelagicus by Lai, Ng & Davie, 2010). The complete data set includes 168 ingroup portunoid taxa and six outgroup taxa. The relative position of Portunoidea within Brachyura remains poorly resolved (Tsang et al., 2014) so outgroup taxa were selected with reference to previous studies. Details of each OTU are listed in Table 1 and Table S1, including taxonomy, GenBank accession numbers, voucher information, and source publications. One hundred eight of these OTUs consist of sequences generated from a single vouchered specimen. For most of the remaining multi-specimen OTUs species-level matches were confirmed with additional newly generated or previously published CO1 or 16S rRNA data (including some unpublished DNA barcode data; analyses not shown). This approach permitted the inclusion of longer, more complete sequence data, but OTUs with missing data were unavoidable.
In an effort to mitigate the impact of missing data, two reduced concatenated data sets were also constructed from the original. The first included 163 taxa, representing all OTUs with at least 16S rRNA data. The second included 138 taxa, representing all OTUs with at least 16S rRNA and CO1 data. Additionally, each molecular marker was analyzed separately before concatenation, thus constituting four additional data sets. However, for the 28S rRNA only data set, just 66 of the total 85 sequences were included. This approach avoided all 28S sequences with less than 500 bps of data, most of which span the uninformative D1 region. Finally, preliminary analyses of 16S rRNA recovered the putative portunoid taxon Brusinia profunda falling far outside Portunoidea. Consequently, newly generated 16S rRNA data for this important taxon (voucher USNM 277519, GenBank KX425018, Fig. 1A) was not included in the above data sets. Instead, this 517 bps sequence was added to an additional “Brusinia-16S” data set that combined all 163 sequences from the 16S rRNA only portunoid data set and 145, mostly brachyuran, 16S rRNA sequences analyzed by Tsang et al. (2014). Taxon identity, GenBank numbers, and voucher IDs for all data used from Tsang et al. (2014) appear as taxon labels in the analyzed data set and resulting phylogeny. In summary, eight molecular data sets were constructed for phylogenetic analyses. Each data set is summarized in Table 3 including marker composition, alignment length and the number of parsimony informative sites.
| Dataset name | Taxon sampling | Dataset composition | Alignment length (bps) | Parsimony informative sites (bps) |
|---|---|---|---|---|
| 16S-only | 163 taxa | 16S rRNA | 1,105 | 521 |
| CO1-only | 148 taxa | CO1 | 657 | 260 |
| 28S-only | 66 taxa | 28S rRNA D1–D2 region (>500 bps) | 1,224 | 184 |
| H3-only | 123 taxa | H3 | 327 | 106 |
| 174 taxa concatenated | 174 taxa | 16S rRNA − 163 taxa/CO1 − 148 taxa/28S rRNA − 85 taxa/H3 – 123 taxa | 3,313 | 1,080 |
| 163 taxa concatenated | 163 taxa | 16S rRNA − 163 taxa/CO1 − 138 taxa/28S rRNA − 74 taxa/H3 − 115 taxa | 3,313 | 1,074 |
| 138 taxa concatenated | 138 taxa | 16S rRNA − 138 taxa/CO1 − 138 taxa/28S rRNA − 70 taxa/H3 − 103 taxa | 3,313 | 1,039 |
| Brusinia-16S | 309 taxa | 16S rRNA − 163 taxa (as above) + Brusinia profunda + 145 taxa (Tsang et al., 2014) | 447 | 237 |
Modified identifications of published sequences
Several published portunoid sequences appear to have been misidentified and were addressed as follows. The CO1 sequence data for Charybdis natator analyzed in Spiridonov, Neretina & Schepetov (2014) matched that of Charybdis granulata (GenBank KT365713; Voucher ZRC-2000.0771; Phuket, Thailand; specimen examined, identity confirmed) and not Ch. natator used in this study (Table 1). Consequently, CO1, H3 and 28S rRNA sequence data for Ch. natator from Spiridonov, Neretina & Schepetov (2014) were included in this study but identified as Ch. granulata. Likewise, phylogenetic analyses of H3 sequence data for Thalamita sima from Spiridonov, Neretina & Schepetov (2014; GenBank JX398122) strongly suggests that it represents contamination from a separate Charybdis bimaculata specimen. That is, this sequence matches that of Ch. bimaculata generated for this study and that from Spiridonov, Neretina & Schepetov (2014). This sequence was not included in this study. However, 28S data and CO1 data from this specimen (GenBank JX398086 and JX398105, respectively) are not similarly suspect. A comparison of CO1 data with additional newly generated sequences for Th. sima (GenBank KT588224 and KT365786) confirm that Spiridonov, Neretina & Schepetov (2014) collected and sequenced a correctly identified Th. sima specimen.
Sequence alignment and phylogenetic analyses
Sequence alignments were constructed using MAFFT v 7.123b (Katoh & Standley, 2013) under the E-INS-i setting. Unreliably aligned columns for 16S and 28S rRNA data sets were identified and removed using Guidance2 (Sela et al., 2015), similarly employing MAFFT’s E-INS-i settings (–genafpair –maxiterate 1,000). Each Guidance2 run evaluated 400 alternative alignments generated from 100 alternative guide trees. Columns with a confidence score below 0.9 were trimmed from the final alignment. The Brusinia-16S data set was similarly aligned, but its total length was trimmed to just 447 bps, covering only those sites available in the 16S data of Tsang et al. (2014). Substitution models and partition schemes were evaluated for each data set using the BIC criterion and a greedy search algorithm in Partitionfinder v.1.1.1 (Lanfear et al., 2012). For each data set all models were evaluated as well as just the reduced set available in MrBayes (Ronquist et al., 2012). A single partition and a GTR+I+G model were chosen for the Brusinia-16S data set. The best scoring schemes for the remaining seven data sets are outlined in Table 4 and Table S2 and were used in subsequent partitioned phylogenetic analyses. Maximum likelihood (ML) phylogenetic analyses were carried out on all data sets using GARLI 2.0 (Zwickl, 2006). For each concatenated data set and the Brusinia-16S data set, ML analyses consisted of at least 100 independent searches and included both random and fast ML stepwise starting trees (attachmentspertaxon = 50, 100, or 2N+1). For single marker data sets at least 20 independent ML searches were performed with stepwise starting trees (attachmentspertaxon = 100). Nodal support for each of the best scoring ML topology was evaluated with 500 bootstrap replicates generated using the same tree search parameters. Bayesian analyses (BI) were performed on each concatenated data sets using MrBayes v3.2.5 (Ronquist et al., 2012). A standard MrBayes MCMC analysis (nruns = 2, nchains = 4) was run on each data set and lasted 25 million generations, sampling every 10,000 generations. An arbitrary burn-in value of 2.5 million generations was used for the 138 OTU and 163 OTU concatenated data sets. A higher burn-in value of seven million generations was needed for the 174 OTU concatenated data set. The standard deviation of split frequencies was confirmed to be less than 0.01 for each analysis. Convergence was further evaluated using Tracer v.1.6 (Rambaut et al., 2014) and included confirmation that each run attained ESS values greater than 200. All phylogenetic analyses were carried out on the CIPRES Science Gateway (Miller, Pfeiffer & Schwartz, 2010). FigTree v1.4.0 was used to visualize trees and generate resulting figures. Sequence alignments and phylogenetic results were deposited to TreeBASE (accessible at https://treebase.org/treebase-web/search/study/summary.html?id=21486).
| Marker | Marker subset | Alignment positions | 174 taxa concatenated data | 163 taxa concatenated data | 138 taxa concatenated data | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Model for ML runs | ML partition ID | Model for BI runs | BI partition ID | Model for ML runs | ML partition ID | Model for BI runs | BI partition ID | Model for ML runs | ML partition ID | Model for BI runs | BI partition ID | |||
| 16S rRNA | 16S rRNA | 1–583 | TVM+I+G | 1 | GTR+I+G | 1 | TVM+I+G | 1 | GTR+I+G | 1 | TVM+I+G | 1 | GTR+I+G | 1 |
| tRNA-LEU | 584–653 | TVM+I+G | 1 | GTR+I+G | 1 | TVM+I+G | 1 | GTR+I+G | 1 | TVM+I+G | 1 | GTR+I+G | 1 | |
| ND1 | 654–1,105 | GTR+I+G | 2 | GTR+I+G | 2 | GTR+I+G | 2 | GTR+I+G | 2 | TrN+I+G | 2 | GTR+I+G | 2 | |
| CO1 | Codon Pos. 1 | 1,106–1,762\3 | SYM+I+G | 3 | SYM+I+G | 3 | SYM+I+G | 3 | SYM+I+G | 3 | SYM+I+G | 3 | SYM+I+G | 3 |
| Codon Pos. 2 | 1,107–1,762\3 | F81+I+G | 4 | F81+I+G | 4 | F81+I+G | 4 | F81+I+G | 4 | F81+I+G | 4 | F81+I+G | 4 | |
| Codon Pos. 3 | 1,108–1,762\3 | GTR+I+G | 5 | GTR+I+G | 5 | GTR+I+G | 5 | GTR+I+G | 5 | GTR+I+G | 5 | GTR+I+G | 5 | |
| 28S rRNA,H3 | D1 and D2 region | 1,763–2,986 | GTR+I+G | 6 | GTR+I+G | 6 | GTR+I+G | 6 | GTR+I+G | 6 | GTR+I+G | 6 | GTR+I+G | 6 |
| Codon Pos. 1 | 2,988–3,313\3 | SYM+I+G | 3 | SYM+I+G | 3 | TrNef+I | 8 | SYM+I+G | 3 | TrNef+I | 8 | SYM+I+G | 3 | |
| Codon Pos. 2 | 2,989–3,313\3 | F81+I+G | 4 | F81+I+G | 4 | TrNef+I | 8 | JC+I | 8 | TrNef+I | 8 | JC+I | 8 | |
| Codon Pos. 3 | 2,987–3,313\3 | GTR+I+G | 7 | GTR+I+G | 7 | GTR+G | 7 | GTR+G | 7 | GTR+G | 7 | GTR+G | 7 | |
Results and Discussion
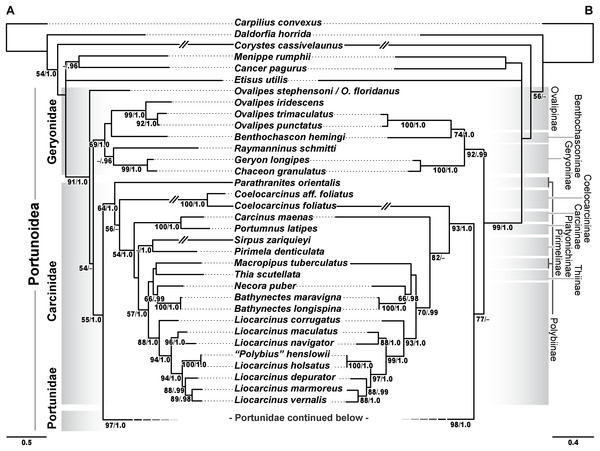
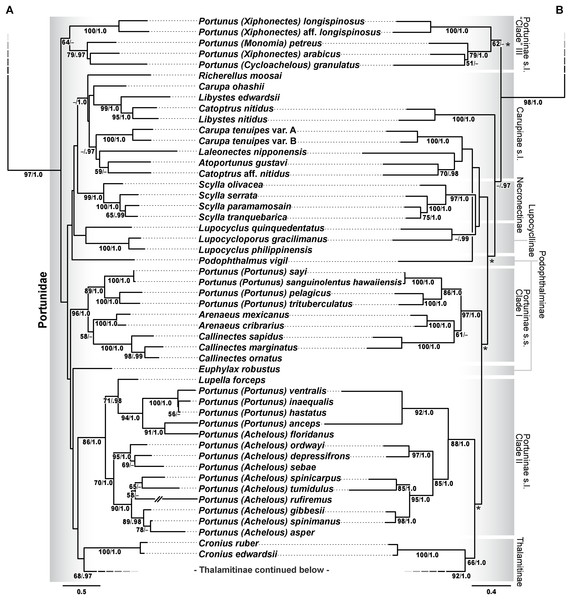
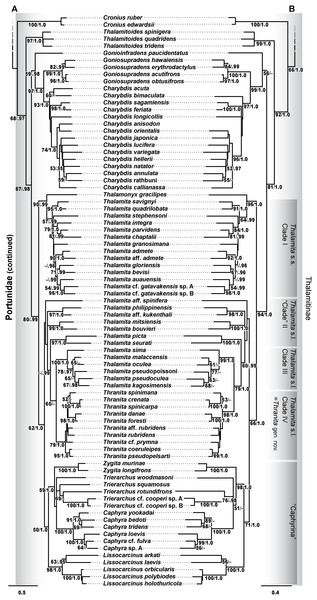
Phylogenetic analyses of up to four molecular markers (16S rRNA, CO1, 28S rRNA, and H3) were carried out on 168 portunoid OTUs, 76 for the first time. Resulting topologies and support values are summarized in Figs. 10–13 and Figs. S1–S6. With few exceptions phylogenetic analyses of the three concatenated data sets recovered consistent topologies that displayed significant support for most of the same clades (Figs. 10–12; Fig. S1). However, analyses of the 174 OTU data set, which had the greatest proportion of missing data, often recovered lower support for each clade (Fig. S1). Clades typically exhibited the greatest support in analyses of the 138 OTU data set, which contained the least amount of missing data (Figs. 10B, 11B and 12B). Nevertheless some topological incongruence was recovered between ML and BI analyses of this 138 OTU concatenated data set (compare nodal asterisks, Figs. 11B and 13B). This conflict was associated with deeper nodes in Portunidae and involved the relative placement of a well-supported “Achelous” sensu lato clade (discussed below). This conflict may be an artifact of the low taxon sampling available for non-thalamitine portunids, a general shortcoming in all analyses. Single marker ML analyses generally recovered poorly resolved topologies, but displayed no significant well-supported conflict with concatenated results (Figs. S2–S5). The following sections present a clade-by-clade discussion of the results for the ML and BI analyses of the 163 and 138 OTU concatenated data sets. The ML topologies for these two data sets are presented together in Figs. 10–12. In text, bootstrap support values (bs) and Bayesian posterior probabilities (pp) are reported together with those for the 163 OTU topology appearing first, followed by those for the 138 OTU topology (e.g., Fig. 10; bs 70%, 100%, pp 0.95, 1.0). Results of the other analyses, including those for the 16S-Brusinia data set, are discussed where relevant.
Figure 10: Maximum likelihood (ML) phylograms of Portunoidea based on analyses of 163 and 138 OTUs and a 3,313 bp alignment of 16S rRNA, CO1, 28S rRNA, and H3 sequence data, Part 1 (of 3).
(A) ML phylogram based on analyses of 163 OTUs, each with at least 16S rRNA data; (B) ML phylogram based on analyses of 138 OTUs, each with at least 16S rRNA and CO1 data. Support values appear below relevant branches with ML bootstrap values ≥50% (based on 500 replicates) appearing first followed by BI posterior probabilities ≥0.95.Figure 11: Maximum likelihood (ML) phylograms of Portunoidea based on analyses of 163 and 138 OTUs and a 3,313 bp alignment of 16S rRNA, CO1, 28S rRNA, and H3 sequence data, Part 2 (of 3).
(A) ML phylogram based on analyses of 163 OTUs, each with at least 16S rRNA data; (B) ML phylogram based on analyses of 138 OTUs, each with at least 16S rRNA and CO1 data. Support values appear below relevant branches with ML bootstrap values ≥50% (based on 500 replicates) appearing first followed by BI posterior probabilities ≥0.95. Asterisk* denotes nodes that topologically conflict with corresponding BI topology (see text and Fig. 13B).Figure 12: Maximum likelihood (ML) phylograms of Portunoidea based on analyses of 163 and 138 OTUs and a 3,313 bp alignment of 16S rRNA, CO1, 28S rRNA, and H3 sequence data, Part 3 (of 3).
(A) ML phylogram based on analyses of 163 OTUs, each with at least 16S rRNA data; (B) ML phylogram based on analyses of 138 OTUs, each with at least 16S rRNA and CO1 data. Support values appear below relevant branches with ML bootstrap values ≥50% (based on 500 replicates) appearing first followed by BI posterior probabilities ≥0.95.Figure 13: Subsections of ML and BI topologies for Portunoidea based on analyses of 174 and 138 OTUs and a 3,313 bp alignment of 16S rRNA, CO1, 28S rRNA, and H3 sequence data.
(A) A subsection of the 174 OTU ML phylogram representing the Portunus subgenera Cycloachelous, Monomia, and Xiphonectes. Support values appear below relevant branches with ML bootstrap values ≥50% (based on 500 replicates) appearing first followed by BI posterior probabilities ≥0.95. (B) Relevant subsection of the 138 OTU BI majority consensus tree exhibiting topological conflict with the ML phylogram generated from the same concatenated data set (see text and Fig. 11). BI posterior probabilities (≥0.95) appear below each relevant node.Superfamily Portunoidea Rafinesque, 1815
Analyses recovered a strongly supported monophyletic Portunoidea (Fig. 10; bs 91%, 99%, pp 1.0, 1.0) comprised of three major, moderately well supported lineages (but see discussion regarding Ovalipes). These three lineages include taxa from seven of the eight currently valid portunoid families, and their relative composition is consistent with, but display greater resolution than that recovered in Schubart & Reuschel (2009) and Spiridonov, Neretina & Schepetov (2014). Summarizing these previous works, Davie, Guinot & Ng (2015a) suggested that the composition and status of each portunoid family may need to be reappraised, but only after all genera have been considered. However, given a shared morphology (discussed in detail by Davie, Guinot & Ng (2015b), Guinot, Tavares & Castro (2013), Karasawa, Schweitzer & Feldmann (2008) and Spiridonov, Neretina & Schepetov (2014)), and in light of the results presented below, the current number of valid portunoid families appears overstated. Here I propose a more conservative classification scheme for Portunoidea comprised of three instead of eight extant families: Geryonidae, Carcinidae, and Portunidae (Figs. 5 and 10). Included in this proposal, results discussed below also suggest that Brusiniidae Števčić (1991), is still a valid brachyuran family, but that it may not be a member of Portunoidea.
Family Geryonidae Colosi, 1923
The portunoid family Geryonidae sensu Schubart & Reuschel (2009) was recovered here as a well-supported clade comprised of Benthochascon, Chaceon, Geryon, Ovalipes, and Raymanninus (Fig. 10; bs 69%, 92%, pp 1.0, 0.99). These results challenge recent actions taken by Spiridonov, Neretina & Schepetov (2014) in which Ovalipes was removed from Geryonidae and the new family Ovalipidae established. Here I propose a more conservative classification in which Ovalipes is retained within Geryonidae in the subfamily Ovalipinae, status nov. (Fig. 5). However, further study is needed as both the 174 and 163 OTU concatenated analyses recovered a poorly supported placement of the hybrid OTU Ovalipes stephensoni + Ovalipes floridanus as sister to all other portunoids, thus rendering Ovalipes polyphyletic and Geryonidae paraphyletic (Fig. 10A; Fig. S1). This placement should be approached with caution and may very well be artifactual. That is, this placement is clearly unstable and was based on limited 16S and 28S rRNA data (461 bps and 618 bps, respectively). Furthermore, this OTU’s relative placement is also poorly resolved in both single gene analyses (Figs. S2 and S4), but was recovered with Raymanninus (with nominal support) as sister to all other Ovalipes in the Brusinia-16S ML analyses (Fig. S6). Nevertheless, the relative placement of this OTU is taxonomically important. Morphologically O. stephensoni and O. floridanus are sister species most closely related with the unsampled generic type O. ocellatus (Herbst, 1799) (see cladistic analyses of Parker, Mckenzie & Ahyong (1998)). If additional work finds further support for the polyphyly of Ovalipes, then Ovalipidae would be a valid family and species derived within Geryonidae would constitute a distinct genus, likely Aeneacancer Ward, 1933. Nevertheless, a new diagnosis of Geryonidae is provided below that incorporates Ovalipidae sensu Davie, Guinot & Ng (2015b).
Family Carcinidae MacLay, 1838
The second major well-supported portunoid clade recovered in this study consists of members from the portunoid families Carcinidae, Pirimelidae, Polybiidae, and Thiidae, as well as the inclusion of the caphyrine genus Coelocarcinus (Fig. 10; bs 64%, 93%, pp 1.0, 1.0). Here I propose that each of these lineages be recognized as a subfamily in the family Carcinidae (Fig. 5). A new diagnosis of Carcinidae is provided below. The composition and diagnoses of carcinid subfamilies will mostly follow that outlined (as families) by Spiridonov, Neretina & Schepetov (2014) and Davie, Guinot & Ng (2015b) but a more detailed treatment of the relationships within the family will be needed. For example, Parathranites’ position as the earliest diverging carcinid lineage renders Polybiinae polyphyletic (Fig. 10A). However, while Parathranites is morphologically distinct, the relatively low ML support in the backbone of the Carcinid topology suggests this placement is not robustly supported. Future efforts would benefit from analyses of more complete sequence data (i.e., less missing data) and greater taxon sampling (e.g., including more than one of the eight Parathranites spp.).
The novel placement of the Caphyrinae genus Coelocarcinus may be expected. These crabs are morphologically peculiar (Fig. 1B) and unlike most caphyrine crabs, they are not symbiotic—instead being found in association with Halimeda-sand, possibly mimicking dead segments of calcified algae (Ng, 2002; N. Evans, 2014, personal observation). Noting its peculiar morphology, Karasawa, Schweitzer & Feldmann (2008) proposed that Coelocarcinus belonged to the family Hepatidae Stimpson, 1871 (now Aethridae Dana, 1851). However, here I recover two Coelocarcinus taxa as a single long-branched clade within a well-supported Carcinidae. While phylogenetically long-branched taxa are more vulnerable to artifactual placement (Evans et al., 2010), additional analyses suggest that this was not the case for Coelocarcinus. ML analyses of the Brusinia-16S data set recovered the same placement for Coelocarcinus even though taxon sampling included hundreds of other brachyuran taxa (Fig. S6). Consequently, here I propose that Coelocarcininae Števčić (2005), is a valid carcinid subfamily.
Finally, concatenated analyses also recover Polybius henslowii as derived within a strongly supported Liocarcinus clade, as sister to Liocarcinus holsatus (Fig. 10). This result is consistent with previous molecular work (Plagge et al., 2016; Schubart & Reuschel, 2009; Spiridonov, Neretina & Schepetov, 2014), and given that L. holsatus and P. henslowii are generic types, the genera should be synonymized. However, while Polybius Leach, 1820, is the senior name, Plagge et al. (2016) proposed that the more widely used Liocarcinus, Stimpson, 1871, should take priority. Nevertheless, it is thought that a more detailed taxonomic revision will be needed and a final ruling by the ICZN may be prudent (Plagge et al., 2016; V. Spiridonov, 2017, personal communication).
Family Portunidae Rafinesque, 1815
The third well-supported major portunoid clade recovered here consists only of taxa belonging to Portunidae sensu Spiridonov, Neretina & Schepetov (2014), excepting Coelocarcinus (Figs. 10 and 11; bs 97%, 98%, pp 1.0, 1.0). These results confirm those of Schubart & Reuschel (2009) by recovering Portunidae as a distinct lineage that does not include carcinid crabs. Results regarding portunid subfamilies and genera are discussed in more detail below. For a diagnosis of the family see Davie, Guinot & Ng (2015b).
Family Brusiniidae Števčić, 1991
Brusinia is a morphologically peculiar genus of small, deep-sea crabs exhibiting many morphological features consistent with membership in Portunoidea (Fig. 1A). Originally assigned to the geryonid genus Benthochascon, this distinct lineage was raised to a generic rank by Števčić (1991) who also erected the tribe Brusiniini Števčić (1991). This clade was subsequently moved from Geryonidae to Carcininae (Crosnier & Moosa, 2002; Števčić, 2005), then to Polybiinae (Ng, Guinot & Davie, 2008; Karasawa, Schweitzer & Feldmann, 2008), and finally raised to family level status by Spiridonov, Neretina & Schepetov (2014). Nevertheless, some have noted that morphologically Brusinia remains an outlier in this family with “all male pleomeres free, somite 3 [lacking] a transverse keel, and the carapace [being] longer than wide” (Karasawa, Schweitzer & Feldmann, 2008). Here I generated the first molecular data for this genus consisting of 16S rRNA from Brusinia profunda. However, preliminary ML analyses failed to recover a placement of this species near or within Portunoidea and thus this sequence was left out of subsequent concatenated analyses. Consideration of lab procedures and extensive analyses of available Brachyura sequence data indicate that this sequence is not likely a contaminant so further analyses were conducted. ML analyses were carried out on Brusinia profunda in a data set comprised of 309 taxa using all 16S rRNA data from this study and all 16S data analyzed in Tsang et al. (2014). Results recovered Brusinia well outside a monophyletic Portunoidea (Fig. S6) albeit, with very low support. With some exceptions (and little to no support) the topology of Brachyura in this analysis was consistent with that recovered by Tsang et al. (2014) from a concatenated data set of eight genes. These results suggest that Brusiniidae may be a distinct lineage within the brachyuran subsection Heterotremata. However, further molecular and morphological work is needed to resolve the specific placement of this clade. For a diagnosis of the family see Davie, Guinot & Ng (2015b).
Portunidae subfamilies
The validity and composition of portunid subfamilies have long been debated (reviewed in Davie, Guinot & Ng (2015a), Karasawa, Schweitzer & Feldmann (2008), Mantelatto et al. (2009), Nguyen (2013), Schubart & Reuschel (2009), Spiridonov, Neretina & Schepetov (2014)). There is consensus that most portunid subfamilies may not represent reciprocally monophyletic clades but are taxonomically useful groupings that should be retained until additional work is conducted (Davie, Guinot & Ng, 2015a). Chief among these, Portuninae and its largest genus Portunus are widely understood to be polyphyletic. However, Karasawa, Schweitzer & Feldmann (2008)—and to some extent Spiridonov, Neretina & Schepetov (2014)—departed from Portuninae sensu Ng, Guinot & Davie (2008) by recognizing the portunid subfamilies Atoportuninae, Lupocyclinae, Necronectinae, and Portuninae, in addition to the more generally accepted Caphyrinae, Carupinae, Podophthalminae, and Thalamitinae (Fig. 5). To the extent possible, the status of each of these portunid subfamilies is reevaluated here in light of the results of this study. However, while Thalamitinae and Caphyrinae are well sampled, it should be understood that most other portunid subfamilies are not. The greater phylogenetic resolution and higher support values recovered for Thalamitinae demonstrate that increased taxon sampling for other subfamilies should significantly improve future analyses of these groups. Yet results of this and other work also suggest that the molecular markers used here will likely never fully resolve deeper nodes in the family (e.g., see Lasley, Klaus & Ng, 2015; Thoma, Guinot & Felder, 2014).
Carupinae Paulson, 1875, sensu lato
Carupinae (Figs. 1C and 1D) is a fascinating group of morphologically peculiar, highly modified portunid crabs. Relative to other portunids members of this group are often smaller, smoother, with reduced eyes and much narrower natatory legs. Most attribute these modifications to their ecology as rubble-dwelling, cavernicolous, or even anchialine crabs (Fujita & Naruse, 2011; Ng, 2011; Ng & Takeda, 2003). This subfamily includes the genera Carupa, Catoptrus, Kume, Libystes, Richerellus and Pele. Atoportunus is also sometimes considered (Ng, 2011; Ng & Takeda, 2003); however, Karasawa, Schweitzer & Feldmann (2008) found morphological cladistic support for the subfamily Atoportuninae Števčić (2005), being comprised of Atoportunus and Laleonectes. Molecular phylogenetic work has subsequently supported an affinity of Laleonectes with Carupinae (Schubart & Reuschel, 2009; Spiridonov, Neretina & Schepetov, 2014). Together these findings led Spiridonov, Neretina & Schepetov (2014) to suggest that Carupinae sensu lato likely includes Atoportuninae. The present study includes the first molecular data generated for Atoportunus. Phylogenetic analyses of the 163 OTU concatenated data set recover a weakly supported monophyletic Carupinae + Atoportuninae clade (Fig. 11A; bs <50%, pp 1.0), but analyses of the 138 OTU data set do not (although they do not provide support against it; Fig. 11B). Consistent with previous molecular work (Schubart & Reuschel, 2009) and morphological discussions (Ng, 2011; Takeda, 2010), these analyses also recover Carupa, Catoptrus and Lybistes as poly- and paraphyletic. These findings include a placement of Catoptrus nitidus derived within or sister to Lybistes (Fig. 11; bs 99%, 100%, pp 1.0, 1.0). However, a second Catoptrus OTU (Catoptrus aff. nitidus) shared no affinity with Lybistes, instead grouping with Atoportunus (Fig. 11; bs 59%, 70%, pp <0.95, 0.98). These results should be approached with caution until more comprehensive molecular and morphological work are conducted on a well sampled Carupinae. Inclusion of Kume Naruse & Ng, 2012, and Pele Ng, 2011 may be particularly important given their close morphological affinity to Lybistes and Catoptrus (Naruse & Ng, 2012; Ng, 2011). Nevertheless, there is now some (though very weak) molecular support for a Carupinae sensu lato that includes Atoportunus and Laleonectes.
Lupocyclinae Paulson, 1875
Lupocyclinae sensu Karasawa, Schweitzer & Feldmann (2008) includes Lupocyclus and Carupella, while Lupocyclinae sensu Spiridonov, Neretina & Schepetov (2014) includes Lupocyclus and Lupocycloporus, but does not explicitly place Carupella anywhere. However, V. Spiridonov (2017, personal communication) has some doubt about the validity of Carupella, questioning whether it may instead represent juvenile specimens of one or more known portunine species (e.g., consider specimens examined by Vijaylaxmi, Padate & Rivonker (2016)). Data from Carupella was not available for analysis and here only weak support was recovered for a poorly sampled monophyletic Lupocyclinae (Fig. 11; bs <50%, <50%, pp <0.95, 0.99). The placement of Lupocyloporus renders Lupocyclus paraphyletic (Fig. 11). This is yet another fascinating, morphologically peculiar lineage of portunids that needs further work.
Necronectinae Glaessner, 1928
Necronectinae is comprised of the Indo-Pacific Scylla and monotypic West African Sanquerus Manning, 1989. The carapace of Sanquerus is similar to that of Scylla, but its chelipeds have a prismatic shape similar (at least superficially) to that of Euphylax (N. Evans, 2014, personal observation; e.g., see Manning, 1989). The present study did not include data for Sanquerus but analyzed all four Scylla species. Results recover strong support for the monophyly of Scylla (Fig. 11; bs 99%, 97%, pp 1.0, 1.0) with species relationships consistent to those recovered by Keenan, Davie & Mann (1998; based on CO1, 16S rRNA and allozyme data). Scylla demonstrates some phylogenetic affinity to Podophthalmus and Carupinae but this relationship exhibits no strong support. Additional analyses must include Sanquerus.
Podophthalminae Stimpson, 1860
This subfamily is comprised of the genera Euphylax and Podophthalmus (including Vojmirophthalmus Števčić, 2011 [=Podophthalmus minabensis Sakai, 1961]). These crabs exhibit unusually long eyestalks that render the orbital regions enormous and the frontal margin greatly reduced (Fig. 1F). The affinity of these genera has never been significantly challenged, but Garth & Stephenson (1966) noted significant differences between the morphology of the eyestalks, anterolateral carapace margin and G1s. Results presented here are the first to analyze the placement of these two genera together. Though data was limited for Euphylax (16S rRNA only), single marker and concatenated analyses failed to recover a monophyletic Podophthalminae (Fig. 11; Figs. S1 and S2). Podophthalmus demonstrated some topological affinity to Necronectinae and Carupinae, but always with little or no support. Euphylax showed no relative affinity to any portunid clade, instead always diverging alone from deeper nodes in Portunidae, but bearing no support. These results neither significantly challenge nor resolve the validity or composition of Podophthalminae.
Portuninae Rafinesque, 1815
As previously discussed, the monophyly of Portuninae and its largest genus, Portunus (98 extant species), has long been challenged. Some of this controversy can be attributed to an expansion of the genus by Stephenson & Campbell (1959) and Stephenson (1972), which included the incorporation of several morphologically similar but previously separate genera. Ng, Guinot & Davie (2008) mostly followed this classification, but retained many of these synonymized genera as subgenera (as did Sakai, 1976). A number of recent studies have provided evidence that these clades are morphologically and phylogenetically distinct, with some clearly worthy of generic status (Karasawa, Schweitzer & Feldmann, 2008; Mantelatto et al., 2009; Nguyen, 2013; Schubart & Reuschel, 2009; Spiridonov, Neretina & Schepetov, 2014). Consistent with these studies, phylogenetic analyses here recover a Portuninae comprised of at least three clades and a Cronius lineage (sensu Mantelatto et al., 2009) falling sister to Thalamitinae (Fig. 11; discussed below). The first of these clades, Portuninae sensu stricto (Clade I), is strongly supported and comprised of Arenaeus, Callinectes and some Portunus species, including the generic type P. pelagicus (Linnaeus, 1758) (Fig. 11; bs 96%, 97%, pp 1.0, 1.0). The second clade, Portuninae sensu lato Clade II, also exhibits significant support (Fig. 11; bs 86%, 88%, pp 1.0, 1.0) and is comprised mostly of Portunus (Achelous), some Portunus (Portunus) and the monotypic Lupella forceps. Following Mantelatto et al. (2009) many have treated Achelous as a distinct but not fully revised genus (Spiridonov, Neretina & Schepetov, 2014; Nguyen, 2013). The third clade, Portuninae sensu lato “Clade” III, was weakly supported and comprised of the Portunus subgenera Cycloachelous, Monomia and a paraphyletic Xiphonectes (Fig. 11; bs 64%, 66%, pp <0.95, <0.95). Only the 174 OTU data set included multiple members of Cycloachelous and Monomia and analyses recovered strong support for the monophyly of Monomia (Fig. 13A; bs 74%, pp 1.0) but less support for the monophyly of Cycloachelous (Fig. 13A; bs <50%, pp 0.99). Finally, the 174 OTU analyses also recovered an unusual but poorly supported placement of Portunus (Xiphonectes) tenuipes within the portunid subfamily Thalamitinae (Fig. S1; bs <50%, pp <0.95). Using the same data for this species (CO1 and 313 bps of 28S rRNA) Spiridonov, Neretina & Schepetov (2014) discussed some concern when the same unusual placement was recovered. However, this result is likely artifactual and finds no other support from morphology or the molecular results presented here. Further work is clearly needed to resolve the systematics of Portunus sensu lato and Portuninae, neither of which were recovered here as monophyletic.
Thalamitinae Paulson, 1875
Following Stephenson (1972) Thalamitinae was placed in Portuninae where it stayed until Apel & Spiridonov (1998) reestablished the subfamily and provided a new morphological diagnosis of the group. Although Thalamitinae now represents the most diverse portunid subfamily (162 spp.; Spiridonov, Neretina & Schepetov, 2014; Spiridonov, 2017), many continue to question the validity of this group (Davie, Guinot & Ng, 2015a). This is partly attributable to the portunine genus Cronius (sensu Mantelatto et al., 2009) which exhibits a morphology intermediate to that of Portunus and the thalamitine genus Charybdis (Garth & Stephenson, 1966; Spiridonov, Neretina & Schepetov, 2014). This has suggested to some researchers that Thalamitinae may be derived within Portuninae. Lending credence to this, the molecular study of Mantelatto et al. (2009) recovered and discussed a derived clade comprised of the portunine genera Cronius + Laleonectes and a monophyletic Thalamitinae. However, results reported in Mantelatto et al. (2009) actually provide no significant support for this “clade,” with NJ and parsimony bootstrap values below 50% and a BI pp of 0.59. Conversely, while some have argued that Cronius may actually share a greater affinity with Charybdis than Portunus (Garth & Stephenson, 1966), only recently has it been suggested, based on morphological grounds, that Cronius might group with Thalamitinae rather than Portuninae (Spiridonov, Neretina & Schepetov, 2014). Results presented here support this view, recovering Cronius sister to Thalamitinae with little to moderate support (Figs. 11 and 12; bs <50%, 66%, pp <0.95, 1.0). Consequently, consideration of morphology (discussed below) and molecular data suggests that Cronius is more appropriately classified as a thalamitine crab. A new diagnosis of Thalamitinae is provided here which accommodates Cronius.
Cronius aside, results presented here also display strong support for a Thalamitinae that includes the Caphyrinae genera Caphyra and Lissocarcinus (Figs. 11 and 12; bs 68%, 92%, pp 0.97, 1.0). Furthermore, these two symbiotic genera also appear highly derived within an otherwise moderately supported Thalamita clade (Fig. 12; bs 62%, 66%, pp 1.0, 1.0). This result is not entirely novel given that the morphological affinity of Caphyrinae to Thalamitinae has long been recognized (Stephenson & Campbell, 1960), and its derived position has received some molecular support (Spiridonov, Neretina & Schepetov, 2014). However, results presented here represent the first comprehensive phylogenetic analyses of both subfamilies, including all described genera, and 70 of 162 Thalamitinae taxa and 12 of 26 Caphyrinae taxa (excluding Coelocarcinus, see above). Furthermore, while Caphyrinae’s placement renders both Thalamita and Caphyra paraphyletic, the derived monophyletic clade Lissocarcinus + Caphyra + Thalamita (=Zygita, gen. nov. and Trierarchus, gen. nov.) includes two Thalamita sensu lato species (=Z. longifrons, comb. nov. and Z. murinae, comb. nov.) recently demonstrated to be symbiotic (Evans & McKeon, 2016). Given the results of this work, Thalamitinae is redefined here to also include Caphyra and Lissocarcinus. For the sake of discussion, the Lissocarcinus + Caphyra + Zygita + Trierarchus clade is ascribed the subtribe name Caphyrina Paulson (1875), nomen translatum. Although the nature, degree, and phylogenetic pattern of symbiosis within Caphyrina clearly needs further study, this clade is dominated by commensal, symbiotic taxa (discussed below), which suggests a single origin of symbiosis for the group. Further highlighting the significance of this clade, symbiotic relationships have not been demonstrated in any other portunid taxa (but see the fascinating epibiotic ecology of Portunus sayi on floating Sargassum algae; Hartnoll, 1971; Russell & Dierssen, 2015; Turner & Rooker, 2006; West, 2012). One notable exception may be the numerous anecdotal observations of juvenile specimens of different portunoid species on gelatinous, nektonic organisms (V. Spiridonov, 2017, personal communication).
Finally, given the results of this study the following taxonomic changes are also discussed below: Thalamonyx A. Milne-Edwards, 1873, is reinstated as a valid genus; the subgenus Goniosupradens Leene, 1938, is raised to a generic rank; three new genera are described to accommodate some of the Thalamita sensu lato lineages rendered paraphyletic by Caphyrinae.
Thalamitinae genera and subclades
Cronius Stimpson, 1860
Using 16S rRNA, Mantelatto et al. (2009) resurrected the species Cronius edwardsii (Fig. 8A), demonstrating that it was a genetically distinct geminate species of the generic type Cronius ruber (Fig. 2A). The same analyses also revealed that the remaining Cronius species, Cronius timidulus, is actually a member of Achelous [=Portunus (Achelous)]. These results are confirmed here with 16S rRNA and CO1 data from new specimens for all three species (Fig. 11).
Thalamitoides A. Milne-Edwards, 1869
Thalamitoides is a morphologically peculiar thalamitine genus with a short, laterally expanded carapace, exceptionally wide set eyes and a wide frontal margin (Fig. 2B). Though sometimes thought to have a greater affinity to Thalamita, phylogenetic results now place the genus sister to the remaining Thalamitinae, with moderate to strong support (Fig. 12; bs 68%, 92%, pp 0.97, 1.0).
Gonioinfradens Leene, 1938
Once classified as a subgenus of Charybdis, the monotypic Gonioinfradens (Fig. 2C) is easily distinguished from Charybdis by having four instead of six well-developed anterolateral teeth, and one to three subsidiary teeth (compare Figs. 8B–8D). The presence of such subsidiary anterolateral teeth occurs in only a few other Charybdis species. Leene (1938) recognized this morphology as distinct and to accommodate these species, described the Charybdis subgenera Gonioinfradens and Goniosupradens. More recently, Gonioinfradens (but not Goniosupradens) was raised to the generic rank (Apel & Spiridonov, 1998). Phylogenetic analyses presented here are the first to include either subgenus. Concatenated analyses recover Gonioinfradens as sister to a well-supported Charybdis sensu lato clade (including Goniosupradens). However, support for this placement is moderate or weak (Fig. 12; bs 59%, 56%, pp 1.0, <0.95).
Goniosupradens Leene, 1938, status nov.
Concatenated analyses recovered strong support for a reciprocally monophyletic clade including all three Goniosupradens species and Charybdis hawaiensis (Fig. 12; bs 99%, 100%, pp 1.0, 1.0). Moreover, this clade was strongly supported falling sister to a monophyletic Charybdis sensu stricto clade (Fig. 12; bs 97%, 99%, pp 1.0, 1.0). Although Ch. hawaiensis (=Goniosupradens hawaiensis, comb. nov.) was thought to be closely related to Ch. orientalis (Edmondson, 1954), a reevaluation of it morphology (discussed below) suggests that these similarities are superficial. Here Goniosupradens (Figs. 2D and 8C) is raised to the generic rank and a new diagnosis is provided that incorporates G. hawaiensis.
Charybdis De Haan, 1833
Concatenated analyses recovered a monophyletic Charybdis lineage (excluding Goniosupradens) with strong support (Fig. 12; bs 93%, 97%, pp 1.0, 1.0). There was no support for other proposed Charybdis subgenera (e.g., Goniohellenus and Gonioneptunus), although analyses included only 18 of 65 Charybdis species.
Thalamonyx A. Milne-Edwards, 1873, status nov.
The status of Thalamonyx has long been questioned as these crabs exhibit a peculiar morphology with similarities to Thalamita, Charybdis and Caphyra (Leene, 1938). This genus was synonymized with Thalamita by Stephenson & Hudson (1957). While this synonymy was widely accepted (Ng, Guinot & Davie, 2008) some continued to treat Thalamonyx as valid (Crosnier, 1962, 1984; Spiridonov, Neretina & Schepetov, 2014). Analyses presented here are the first to include molecular data for the genus and results recover strong support for Thalamonyx gracilipes falling sister to a Thalamita sensu stricto clade (Fig. 12; bs 90%, 96%, pp 0.99, 1.0). Given this taxon’s distinct morphology and that several Thalamita sensu lato clades will constitute additional genera (discussed below), the generic status of Thalamonyx is formally reinstated and a new diagnosis provided.
Thalamita Latreille, 1829
With 91 species, Thalamita is the largest portunid genus (Spiridonov, 2017). Unlike Portunus, the taxonomy of this group has been less controversial. However, Thalamita is morphologically diverse (sometimes confusingly so) and has always been thought to have a close affinity to Charybdis. Some have even suggested that the two genera may “constitute an unbroken series,” one blending into the other (Stephenson & Hudson, 1957). Results presented here do not support this view, instead recovering each genus in phylogenetically distinct clades. Nevertheless, the derived placement of Caphyrinae (=Caphyrina nom. trans.) within Thalamita renders this genus paraphyletic. With the exception of those Thalamita species falling within Caphyrina, three clades and one “grade” of Thalamita taxa were recovered. Each of these four clades are labeled in Fig. 12B and discussed below.
Thalamita admete (Herbst, 1803) is the generic type. With few exceptions members traditionally grouped with this species (e.g., see Stephenson & Hudson, 1957) are recovered here falling within a moderately supported Thalamita sensu stricto clade (Fig. 12; bs 57%, 64%, pp 0.99, 0.99). This clade includes only small to moderate sized Thalamita species that are morphologically similar and often hard to distinguish. They all exhibit two wide frontal lobes; often with equally wide, mostly parallel inner orbital margins (Figs. 8G and 8H). Male first gonopods (G1s) are long, less stout and never significantly flared relative to similarly sized Thalamita sensu lato taxa (compare Figs. 7A and 7B). Fourteen species were recovered in this clade, but the group likely includes many additional species not considered here. Nevertheless, some species traditionally assigned to this group were not recovered in the clade. Specifically, Thalamita oculea and Th. sima exhibit a similar size and carapace morphology to Th. admete but their gonopod morphology is different and phylogenetically they group with members of Thalamita sensu lato Clade III (discussed below). Unfortunately, at this time, developing a new diagnosis for Thalamita sensu stricto would be premature. While the present study does include over half of all Thalamita sensu lato taxa, several morphologically important species have not been included (e.g., Th. annulipes, Th. margaritimana, and Th. platypenis) and poor phylogenetic resolution at several critical nodes complicates the delineation of clades within the group. Additional work on Thalamita sensu lato is underway by V. Spiridonov and N. Evans both separately and in collaboration.
The remaining Thalamita sensu lato taxa and Caphyrina form a moderately well-supported clade (Fig. 12; bs 62%, 66%, pp 1.0, 1.0). In this clade the earliest diverging Thalamita sensu lato taxa form a grade (“Clade” II; Fig. 12) paraphyletic to the remaining Thalamita sensu lato clades (Clades III and IV). While carapace morphologies (e.g., frontal lobes and anterolateral teeth; Figs. 8I and 8J) vary substantially across this grade of small sized Thalamita, their G1s are diagnostically stout often with a laterally flared tip (Fig. 7B). However this G1 morphology is also shared among members of the Thalamita sensu lato Clade III. Clade III forms a distinct, strongly supported lineage (Fig. 12; bs 100%, 99%, pp 1.0, 1.0) of small to medium sized species. However, the diagnosis of this clade is complicated by some species having a two-lobed frontal margin striking similarity to that of Thalamita sensu stricto (Clade I), while others exhibit six frontal lobes similar to some members of Thalamita sensu lato “Clade” II (compare Figs. 8K with 8G and 8L with 8I). Finally, the monophyly of Thalamita sensu lato Clade IV is strongly supported (Fig. 12; bs 79%, 98%, pp 1.0, 1.0) and comprised only of large, morphologically similar Thalamita species. Given their morphology (discussed below) and the monophyly of this group here I establish the new genus Thranita to accommodate these species.
Caphyrina Paulson, 1875, nom. trans.
Here I recognize a moderately well supported clade (Fig. 12; bs 50%, 71%, pp 1.0, 1.0) comprised of Caphyra, Lissocarcinus, and six former Thalamita species as a redefined Caphyrina Paulson, 1875, nomen translatum (Figs. 3 and 9B–9J). Monophyly of Lissocarcinus was strongly supported (Fig. 12; bs 98%, 100%, pp 1.0, 1.0), and fell sister to a well-supported clade comprised of the remaining Caphyrina taxa (Fig. 12; bs 59%, 100%, pp 1.0, 1.0). This latter clade is morphologically diverse and includes a Caphyra sensu stricto clade as well as two lineages comprised of Caphyra rotundifrons and species formerly assigned to Thalamita. The first of these two lineages is comprised of the morphologically distinct geminate species Th. longifrons and Th. murinae. These species are both facultative commensals of nephtheid soft coral (Evans & McKeon, 2016) and have long been considered worthy of a generic status (Spiridonov & Neumann, 2008; Stephenson & Rees, 1967a). The new genus Zygita is described here to accommodate these species. The second lineage was recovered with poor support but includes Thalamita woodmasoni and Thalamita cooperi; species likewise considered part of a morphologically distinct Thalamita clade (the “Woodmasoni” group; Vannini, 1983). Although morphology strongly unites these taxa, phylogenetic results do not yet resolve whether they form a grade or a true clade within a well-supported Caphyrina. Though additional work is needed, here I establish the new genus Trierarchus comprised of these species (and all other “Woodmasoni” species) as well as Thalamita squamosa (=Trierachus squamosus, comb. nov.) and C. rotundifrons (=Trierarchus rotundifrons, comb. nov.). Limited but compelling data suggests that members of Trierarchus are symbiotic, forming facultative or obligate associations with algae (see Ecological remarks below). Furthermore, the placement of the morphologically divergent, obligate algal-commensal C. rotundifrons within Trierarchus, leaves a strongly supported Caphyra sensu stricto clade (Fig. 12; bs 100%, 100%, pp 1.0, 1.0). Caphyra now includes only species known to be commensal on soft corals. Finally, though analyses considered no more than seven of the 16 Caphyra sensu stricto species, results suggest that the genus may consist of two morphologically and ecologically distinct subclades; members of one clade (Caphyra bedoti, Caphyra tridens, and Caphyra yookadai) appear to primarily be obligate commensals of alcyoniid soft corals, where members of the other (including Caphyra loevis and Caphyra cf. fulva) are likely obligate commensals of xeniid soft corals (Crosnier, 1975b; Stephenson & Rees, 1968; collection data from UF holdings).
Taxonomic Account
Portuniod family-level morphological diagnoses presented here amend those of Davie, Guinot & Ng (2015b), but do not address or impact diagnoses of Portunidae Rafinesque, 1815, or Brusiniidae Števčić (1991). New diagnoses are also presented for the portunid subfamily Thalamitinae and three new, and two revalidated thalamitine genera. Post-revision names are used for “Species included” lists, with junior synonyms in brackets “[ ].”
Superfamily Portunoidae Rafinesque, 1815
Family Geryonidae Colosi, 1923
Geryonidae Colosi, 1923: 249. Type genus Geryon Krøyer, 1837
Ovalipidae Spiridonov, Neretina & Schepetov (2014: 420). Type genus Ovalipes Rathbun, 1898
Diagnosis: Carapace ovate, hexagonal or subhexagonal, broader than long (sometimes only slightly so), smooth to moderately granular; regions variously expressed; epibranchial ridge of diffuse granules sometimes present, other carapace ridges not developed; frontal margin shorter than posterior margin, typically divided into even number of lobes or teeth (but sometimes three) with median notch present; supraorbital margin with one or two fissures, often indistinct (if two and distinct, central lobe toothed); infraorbital margin not separated from outer orbital lobe by fissure or notch; anterolateral margin with three to five teeth, shorter than posterolateral margin; posterolateral re-entrant not developed. Basal antennal segment free or fixed, longer than broad. Mesial lobe of first maxilliped endopod not well developed. Chelipeds heterochelous (sometimes weakly so) and heterodontous; merus typically without spines; carpus often with an outer spine; manus with dull, knob-like outer proximal spine. Meri of P2–P5 with antero-distal lobes or spines, sometimes reduced. P5 propodi laterally compressed, sometimes ovate; dactyli ovate, styliform, or lanceolate. Pleon with six somites plus telson typically distinguishable in both sexes with somites three to five in males separated by sutures but immovable. G1 long, opening anterolaterally. G2 thin, more or less approximately as long as G1. Diagnosis modified from Geryonidae and Ovalipidae sensu Spiridonov, Neretina & Schepetov (2014) and Davie, Guinot & Ng (2015b).
Genera included: Benthochascon Alcock & Anderson, 1899; Chaceon Manning & Holthuis, 1981; Echinolatus Davie & Crosnier, 2006; Geryon Krøyer, 1837; Nectocarcinus A. Milne-Edwards, 1861; Ovalipes Rathbun, 1898; Raymanninus Ng, 2000; Zariquieyon Manning & Holthuis, 1989.
Remarks: The placement of Echinolatus and Nectocarcinus within this family is tentative, following Davie, Guinot & Ng (2015b), but may be more appropriately judged “genera incertae sedis” considering remarks by Spiridonov, Neretina & Schepetov (2014). Additional morphological and phylogenetic work will be needed to resolve their placement.
Family Carcinidae MacLeay, 1838
Carcinidae MacLeay, 1838: 59. Type genus Carcinus Leach, 1814
Pirimelidae Alcock, 1899: 95. Type genus Pirimela Leach, 1814
Polybiidae Ortmann, 1893: 66. Type genus Polybius Leach, 1820
Thiidae Dana, 1852: 1425. Type genus Thia Leach, 1816
Diagnosis: Carapace hexagonal, subhexagonal, pyriform, or subcircular, rarely quasi-quadrate, typically broader than long; frontal margin sometimes entire, typically with two to four lobes or teeth, and shorter than posterior margin; inner supraorbital lobe weakly defined, significantly reduced, or absent; one or two supraorbital fissures sometimes reduced, rarely absent; anterolateral margin typically convex with five (sometimes four) teeth or lobes (count excluding exorbital tooth when small or poorly developed, e.g., as in some Pirimelinae); posterolateral re-entrant sometimes not developed, rarely absent. Basal antennal segment fixed, longer than wide. Well defined mesial lobe on endopod of first maxilliped sometimes present. Chelipeds typically heterochelous and heterodontous, sometimes symmetrical; merus typically without spines; carpus often with an outer spine; manus sometimes with dull, knob-like outer proximal spine; proximal inner surface of manus fixed finger concave. Meri of P2–P5 typically without antero-distal lobes or spines. P5 dactyli ovate (paddle-like), styliform, ensiform, or lanceolate. Sutures between sternites and episternites incomplete or partially incomplete. Pleonal somites three to five in males typically fused sometimes with traces of sutures, rarely all six somites and telson free (in Thiinae). G1 straight to slightly or distinctly curved, sometimes with spinules and soft setae. G2 distinctly shorter than G1. Vulva typically rounded or ovate, sometimes broader than long. Diagnosis modified from Carcinidae, Pirimelidae, Polybiidae, and Thiidae sensu Spiridonov, Neretina & Schepetov (2014) and Davie, Guinot & Ng (2015b), and including Coelocarcinus sensu Ng, 2002.
Genera included: Bathynectes Stimpson, 1871; Carcinus Leach, 1814; Coelocarcinus Edmondson, 1930; Coenophthalmus A. Milne-Edwards, 1873; Liocarcinus Stimpson, 1871; Macropipus Prestandrea, 1833; Nautilocorystes H. Milne-Edwards, 1837; Necora Holthuis, 1987; Parathranites Miers, 1886; Pirimela Leach, 1816; Polybius Leach, 1820; Portumnus Leach 1814; Sirpus Gordon, 1951; Thia Leach, 1816; Xaiva MacLeay, 1838.
Family Portunidae Rafinesque, 1815
Subfamily Thalamitinae Paulson, 1875
Thalamitinae Paulson, 1875: 69. Type genus Thalamita Latreille, 1829
Caphyrinae Paulson, 1875: 69. Type genus Caphyra Guérin, 1832
Diagnosis: Carapace subcircular, subhexagonal or subtrapezoidal; slightly to substantially broader than long. Anterolateral margin with two to nine teeth, but typically four to six, and rarely nearly entire (e.g., some Lissocarcinus and Caphyra); if teeth number greater than six, five are typically large, well developed, and correspond to portunid teeth AT1, AT3, AT5, AT7 and AT9; the remaining being small, subsidiary, or rudimentary teeth appearing between the larger teeth (e.g., Figs. 8A–8C); rarely the first anterolateral tooth appears truncate and notched somewhat resembling a poorly developed additional tooth (e.g., Charybdis feriata). Basal antennal segment transversely broadened or lying obliquely, and entering or filling orbital hiatus; antennal peduncle and flagellum completely or nearly completely excluded from orbit (Fig. 6C). Chelipeds (P1) the same length or longer than ambulatory legs (P2–P4), typically bearing spines on the merus, carpus and manus; manus usually bearing one or more spines along both Carina 1 and Carina 2 and a well-developed outer proximal spine (Figs. 6D–6F; notable exceptions include many Caphyra and Lissocarcinus species). P5 typically with paddle-shaped propodi and dactyli, but sometimes otherwise modified (e.g., Figs. 4 and 14A). G1 with subdistal spinules, spines, bristles, or “hairs.” Diagnosis modified from Thalamitinae and Caphyrinae sensu Apel & Spiridonov (1998), Cronius sensu Garth & Stephenson (1966), and Caphyra sensu Apel & Steudel (2001).
Figure 14: Relevant diagnostic morphology of Trierarchus gen. nov. and Zygita gen. nov.
(A) Dorsal surface right P5, Trierarchus cooperi (UF 23802). (B–E) Zygita longifrons (UF 199): (B) dorsal surface right P5; (C) ventral surface right P5; (D) outer surface right cheliped; (E) right P4, ambulatory leg. Solid arrows, morphology discussed. Asterisks, important diagnostic traits.Genera included: Caphyra Guérin, 1832; Charybdis De Haan, 1833; Cronius Stimpson, 1860; Gonioinfradens Leene, 1938; Goniosupradens Leene, 1938, status nov.; Lissocarcinus Adams & White, 1849; Thalamita Latreille, 1829; Thalamitoides A. Milne-Edwards, 1869; Thalamonyx A. Milne-Edwards, 1873, status nov.; Thranita, gen. nov.; Trierarchus, gen. nov.; Zygita, gen. nov.
Remarks: With the addition of Caphyra, Cronius and Lissocarcinus, Thalamitinae now includes 190 species (Spiridonov, Neretina & Schepetov, 2014; Spiridonov, 2017) and is the largest portunoid subfamily. Cronius notably expands the diagnosis of the subfamily to include two species with nine anterolateral teeth. However, Cronius clearly exhibits morphology diagnostic of Thalamitinae including exclusion of the antennal flagellum from the orbit by the basal antennal joint, no more than six large anterolateral teeth, and a G1 with subterminal bristles or “hairs” (Garth & Stephenson, 1966; Spiridonov, Neretina & Schepetov, 2014).
Genus Goniosupradens Leene, 1938, status nov.
Figures 2D, 4A and 8C
Type species: Portunus erythrodactylus Lamark, 1818, subsequent designation by Davie (2002); gender masculine.
Diagnosis: Carapace subhexagonal, slightly broader than long. Frontal margin with six well-developed teeth or lobes of approximately equal width. Anterolateral margin with five large, well-developed, forward-sweeping teeth corresponding to portunid teeth AT1, AT3, AT5, AT7 and AT9 and one or two subsidiary teeth corresponding to teeth AT2 (always present) and AT4 (sometimes present); subsidiary teeth not significantly swept forward but terminating approximately perpendicular to the anterolateral margin; epibranchial tooth (AT9) subequal to and never significantly extending laterally beyond tooth AT7. Posterior margin of carapace forming a curve with the posterolateral margin. Cheliped carinae 3–5 always well developed. Diagnosis modified from Leene (1938) to include G. hawaiensis, comb. nov., after Edmondson (1954).
Species included: Goniosupradens acutifrons (De Man, 1879); Goniosupradens erythrodactylus (Lamarck, 1818) [=Thalamita pulchra Randall, 1840; Thalamita teschoiraei A. Milne-Edwards, 1859]; G. hawaiensis (Edmondson, 1954), comb. nov.; Goniosupradens obtusifrons (Leene, 1937).
Remarks: Historically, G. hawaiensis (=Charybdis hawaiensis) was considered closely related to Ch. orientalis (Edmondson, 1954), but similarities are superficial. Once thought diagnostic of these species, the first “subsidiary” anterolateral tooth (AT2) is more reduced in G. hawaiensis in a manner consistent with other Goniosupradens. Furthermore, G. hawaiensis, like all Goniosupradens, bears an epibranchial, anterolateral tooth (AT9) subequal to, but never significantly extending laterally beyond the preceding tooth (AT7). The opposite condition is present in Ch. orientalis and most Charybdis (compare Figs. 8C and 8D).
Genus Thalamonyx A. Milne-Edwards, 1873, status nov.
Figures 2F, 7G and 8F
Type species: Goniosoma danae A. Milne-Edwards, 1869, subsequent designation by Rathbun, 1922; gender masculine.
Diagnosis: Carapace subhexagonal, approaching subcircular; moderately convex dorsally; mature specimens always slightly broader than long. Frontal margin of carapace not much wider than posterior margin and comprised of two lobes separated by a small, distinct notch and extending forward well beyond the inner supraorbital margin; lobes frequently slightly sinuous or concave near the inner margin such that each appears subtly bilobed. Inner supraorbital margin arched and less than one third as wide as frontal lobes. Five, sharp anterolateral teeth (AT1, AT3, AT5, AT7, and AT9); AT1 largest and directed forward; remaining subequal and swept forward forming an oblique, inclined border similar to that in Charybdis. Chelipeds subequal, not robust, and lightly granular all over; posterior border of merus subtly squamous; manus with one spine present along Carina 1, one spine along Carina 2, and a well-developed outer proximal spine; Carina 3–5 granular but increasingly well developed; Carina 6 granular or smooth and poorly developed; Carina 7 sometimes granular and well developed. Posterior border of P5 propodi without spinules. G1 short, stout, curved, broadening slightly toward a obliquely ending tip; subdistal lateral margin bearing stout, mostly paired bristles numbering approximately nine, preceded by additional thinner bristles; subdistal mesial margin with approximately five long, hook-shaped bristles followed by approximately four mostly straight, variously angled bristles. Female genital opening relatively large, located near anterior margin of sternite.
Species included: Thalamonyx danae (A. Milne-Edwards, 1869) [=Thalamita anomala Stephenson & Hudson, 1957]; Th. gracilipes A. Milne-Edwards, 1869.
Remarks: G1 morphology of this genus is diagnostic (Fig. 7G; see also Stephenson & Rees, 1967b, Fig. 2D; Nguyen, 2013, Fig. 15), as is the female genital opening (pers. comm. V. Spiridonov, 2017). Ng, Guinot & Davie (2008, Note 25, p. 158) created some confusion by misidentifying the type species of this genus which is G. danae A. Milne-Edwards, 1869, not Th. danae Stimpson, 1858. When Stephenson & Hudson (1957) designated Thalamonyx a junior synonym of Thalamita, the species Th. danae (A. Milne-Edwards, 1869) became a secondary homonym of Th. danae Stimpson, 1858. Consequently, Th. danae (A. Milne-Edwards, 1869) was given the new specific epithet Th. anomala (Stephenson & Hudson, 1957). In accordance with Article 59.4 of ICZN (1999), Th. danae (A. Milne-Edwards, 1869) is reinstated here as a valid species and Th. anomala (Stephenson & Hudson, 1957) its junior synonym. Though no Th. danae (A. Milne-Edwards, 1869) specimens were examined for this study, its sister status with Th. gracilipes (sampled here) is without question. The synonymization of these species has been discussed, but has never been fully investigated or formally adopted (discussed in Stephenson & Rees, 1967b). Finally, as others have noted, the Thalamonyx specimen illustrated by Crosnier (1962, Fig. 153) is that of an immature male; both its carapace and G1 are not fully developed and should not be used to diagnose adult specimens.
Genus Thranita, gen. nov.
Figures 2I, 4A, 7C and 9A
urn:lsid:zoobank.org:act:8122DA1E-5C68-45E2-91B0-76583068FF80
Type species: Thalamita crenata Rüppell, 1830, by present designation; gender feminine.
Diagnosis: Carapace subhexagonal, always broader than long, somewhat flattened and never significantly convex; frontal margin with six well-developed, bluntly rounded lobes of approximately even width; anterolateral margin with five (rarely four) well-developed sharp teeth corresponding to portunid teeth AT1, AT3, AT5, AT7, and AT9 (Fig. 9A); exorbital tooth (AT1) always entire; AT7 sometimes reduced or absent (e.g., in Thranita pseudopelsarti). Basal antennal segment always transversely broadened, never laying significantly oblique to orbital hiatus. G1 long, slightly tapering (Fig. 7C), rarely with a laterally recurved tip (e.g., as in Thranita foresti); never stout with a laterally flared tip.
Species included: Thranita cerasma (Wee & Ng, 1995), comb. nov. [=Thalamita cerasma rectifrons Crosnier & Moosa, 2002]; Thranita coeruleipes (Hombron & Jacquinot, 1846), comb. nov.; Thranita crenata (Rüppell, 1830), comb. nov.; Thranita danae (Stimpson, 1858), comb. nov. [=Thalamita stimpsoni A. Milne-Edwards, 1861; Thalamita prymna var. proxima Montgomery, 1931]; Th. foresti (Crosnier, 1962), comb. nov. [=?Thalamita helleri Hoffmann, 1874]; Thranita gurjanovae (Tien, 1969), comb. nov.; Thranita holthuisi (Stephenson, 1975), comb. nov.; Thranita kotoensis (Tien, 1969), comb. nov.; Thranita pelsarti (Montgomery, 1931), comb. nov.; Thranita prymna (Herbst, 1803), comb. nov. [=Thalamita crassimana Dana, 1852; Thalamita pyrmna var. annectans Laurie, 1906]; Thranita pseudopelsarti (Crosnier, 2002), comb. nov.; Thranita rubridens (Apel & Spiridonov, 1998), comb. nov.; Thranita spinicarpa (Wee & Ng, 1995), comb. nov.; Thranita spinimana (Dana, 1852), comb. nov.; Thranita starobogatovi (Tien, 1969), comb. nov.; Thranita tenuipes (Borradaile, 1902), comb. nov.; Thranita williami (Spiridonov, 2017), comb. nov.
Remarks: Sometimes referred to as the “Prymna” group (after Thalamita prymna; Stephenson & Hudson, 1957), members of this clade include nearly all large species of Thalamita sensu lato and have long been recognized as morphologically similar. Though several of these species were not available (or suitable) for molecular analyses (e.g., Th. cerasma, Th. pelsarti, and Th. williami), they have always been considered morphologically most similar to species that were included here (e.g., Wee & Ng, 1995; Spiridonov, 2017). Although there is no striking single synapomorpy for Thranita, all members share a similar shaped carapace with six frontal lobes of approximately uniform shape and width (Fig. 9A) and a relatively long, gradually tapering G1 (Fig. 7C). This combination of characters is not seen in any other Thalamita clade.
Etymology: Thalamita Latreille, 1829 (and its suppressed, objective synonym Thalamites Guérin, 1829, a nomen oblitum; Low, Ng & Evenhuis, 2013) was named after Thalamite, the title given to oarsmen occupying the lowest tier of a trireme (a three-tiered ancient Greek warship). Keeping with this tradition, Thranita originates from Thranite, the title given to oarsmen occupying the upper tier of a trireme. Gender feminine.
Genus Trierarchus, gen. nov.
urn:lsid:zoobank.org:act:5E5BD1B5-524D-46C4-9FFD-EA5FBF1C5239
Type species: Thalamita woodmasoni Alcock, 1899, by present designation; gender masculine.
Diagnosis: Carapace subhexagonal to subcircular, typically broader than long and somewhat convex; frontal margin flat or rounded and comprised of one to six (typically four) weakly distinguished lobes; four lobed specimens typically with median lobes approximately three times the width of lateral lobes; inner supraorbital margin sometimes nearly absent (e.g. Tr. rotundifrons) but typically subtly rounded and oblique with a breadth never greater than one-third the total breadth of the frontal lobes; anterolateral margin not reduced nor exhibiting a significantly concave epibranchial ridge (e.g., as in some Caphyra; Fig. 9E); anterolateral margin with four well-developed teeth swept forward corresponding to portunid teeth AT1, AT3, AT5 and AT9; a rudimentary tooth AT7 sometimes present (Figs. 9F–9J). Carapace and chelipeds subtly to substantially granular and covered with plumose setae. Chelipeds with posterior surface of merus bearing distinct granular squamiform markings; manus with weakly squamiform markings extending ventrally from Carina 5 to a poorly defined Carina 6. P5 dactyli typically lancelet-shaped, especially in juveniles, but approaching paddle-shaped in larger specimens (Figs. 14A and 3F, respectively). G1 curved and swelling slightly toward a club-shaped end with a bluntly rounded tip; subterminal bristles always present on abdominal-mesial surface, typically dense and comprised of several rows extending sparsely or densely to the sternal surface, merging with bristles of the lateral abdominal surface that extend to tip (Figs. 7E and 7F); larger subterminal bristle sockets distinct and often visible when bristles are damaged or missing.
Species included: Trierarchus acanthophallus (Chen & Yang, 2008), comb. nov.; Trierarchus cooperi (Borradaile, 1902), comb. nov.; Trierarchus corrugatus (Stephenson & Rees, 1961), comb. nov.; Trierarchus crosnieri (Vannini, 1983), comb. nov.; Trierarchus demani (Nobili, 1905), comb. nov. [=?Thalamita trilineata Stephenson & Hudson, 1957; ?Thalamita invicta Thallwitz, 1891]; Trierarchus hanseni (Alcock, 1899), comb. nov.; Trierarchus procorrugatus (Dai et al., 1986), comb. nov.; Trierarchus quadridentatus (Dai, Cai & Yang, 1996), comb. nov.; Trierarchus rotundifrons (A. Milne-Edwards, 1869), comb. nov.; Trierarchus sankarankuttyi (Crosnier & Thomassin, 1974), comb. nov.; Trierarchus squamosus (Stephenson & Hudson, 1957), comb. nov.; Trierarchus woodmasoni (Alcock, 1899), comb. nov.; Trierarchus taprobanicus (Alcock, 1899), comb. nov.
Remarks: The most diagnostic morphology of Trierarchus includes the G1, anterolateral margin and presence of squamiform markings and plumose setae. The G1 can be particularly useful (e.g., see also Crosnier, 1975a, Fig. 8; Crosnier & Thomassin, 1974, Fig. 8D; Chen & Yang, 2008, Fig. 7; Dai et al., 1986, Fig. 137A), however, both Tr. squamosus and Tr. rotundifrons possess divergent G1s (Stephenson & Hudson, 1957, Figs. 2K and 3K; Stephenson & Campbell, 1960, Figs. 1H and 2J). Additionally, Tr. rotundifrons is overall morphologically highly divergent from other members of this genus, likely due to its ecology as an obligate commensal. This species is smooth and lacks squamiform markings, most plumose setae, and most carinae on the chelipeds. Furthermore, its P5 dactyli are highly modified for firmly grasping host algae (Fig. 4F). Nevertheless, Tr. rotundifrons clearly displays a morphological affinity with other Trierarchus, most notably Tr. woodmasoni (compare Figs. 3F, 3I, 9F and 9G). Likewise, while Tr. rotundifrons was originally described in Camptonyx Heller, 1861, (an available junior synonym of Caphyra) it shares no close morphological or ecological affinity to C. polita (Heller, 1861), the type species of this invalid genus. Caphyra polita is a soft coral commensal with close morphological affinity to C. fulva (sampled here) and other Caphyra sensu stricto taxa (Crosnier, 1975b). Finally, it is worth noting that species delineations within Trierarchus remain problematic and a revision of this new genus is needed (e.g., see Crosnier, 1975a). Morphologically Tr. sankarankuttyi and Tr. procorrugatus have a strong affinity with Tr. cooperi, but they were described from limited material and interspecific differences were inadequately addressed (see Crosnier & Thomassin, 1974; Dai et al., 1986). Furthermore, while two well supported, genetically distinct Tr. cf. cooperi lineages were recovered here (sp. A and sp. B; Figs. 3G and 3H), examination of multiple DNA barcoded specimens from each lineage failed to reveal clear morphological distinctions between the taxa (from preliminary analyses with unpublished data; but see discussion of color below). Moreover, many individuals from both OTUs fit a diagnosis of Tr. corrugatus (Stephenson & Rees, 1961). This inter- and intraspecific variation likely explains why Crosnier (1962) synonymized this species with Tr. cooperi, though they are currently treated as distinct (Ng, Guinot & Davie, 2008; Nguyen, 2013). Comparison of sequenced specimens of Tr. woodmasoni from across the Indo-Pacific (from preliminary analyses with unpublished data) also suggests that Tr. crosnieri, Tr. taprobanicus, and Tr. woodmasoni may be intraspecific variants. Thus, Trierarchus is likely comprised of fewer valid species than currently recognized, but more detailed studies will be needed.
Ecology: Members of Trierarchus typically inhabit high-energy, shallow marine environments, often in association with algae (Vannini, 1983; Hay et al., 1989; UF collection data; personal observations). In Guam Tr. rotundifrons is always found in association with Chlorodesmis algae in exposed reefs, Tr. cf. cooperi is recovered by sieving living Halimeda (note light green live color in sp. B; Fig. 3H), and Tr. woodmasoni is reliably recovered from sieving Sargassum and other co-distributed algae. Around Moorea Island, French Polynesia, Tr. cf. cooperi is typically recovered by sieving and breaking coral rubble from fore reef environments. However, unlike Tr. cf. cooperi recovered in Guam, the species collected in Moorea (sp. A; Fig. 3G) displays a live color mottled with red, orange and purple hues—shades common among coralline algae, sponges and other encrusting marine life in such substrate. Nevertheless, with the exception of Tr. rotundifrons, which is demonstrably an obligate commensal (Hay et al., 1989), other symbiotic associations suggested for this genus remain speculative and need further study. Finally, in contrast to other species, the rarely collected Tr. squamosus appears to prefer protected lagoonal waters, but no further microhabitat or live color data are available for the species.
Etymology: A trierach (Latin trierachus) is the captain of a trireme, an ancient Greek warship. For context see Etymology of Thranita (above). Gender masculine.
Genus Zygita, gen. nov.
Figures 3E, 7D, 9C, 14B–14E
urn:lsid:zoobank.org:act:5CED217C-3FD0-4090-973D-529226942966
Type species: Goniosoma longifrons A. Milne-Edwards, 1869, by present designation; gender feminine.
Diagnosis: Carapace subhexagonal, approximately 1.5 times broader than long; frontal margin with six well-developed teeth of approximately even width separated by deep notches; inner supraorbital margin oblique and spiniform; anterolateral margin with five large, well-developed sharp teeth forming an oblique, inclined border reminiscent of Thalamonyx and Charybdis (Fig. 9C). Infraorbital lobe well developed and terminating in a spiniform or blunt point. Carapace, chelipeds and ambulatory legs subtly to substantially granular and covered with plumose setae (easily worn away in preserved specimens). Cheliped meri with a ventral anterodistal spine; carpus with additional dorsal spine between the typical three outer spines and a well-developed inner spine; manus Carina 4 distinct, granular and ending distally in a sharp or dull spinule (Fig. 14D), squamiform sculpture extending ventrally from Carina 5 to a poorly defined Carina 6. Meri of P2–P4 bearing a ventral posterodistal spine (Fig. 14E). P5 coxae bearing a stout, well-developed spinule dorsad; ischia with granular to spiniform distal border; meri with both a dorsal and ventral posterodistal spine; carpi with a well-developed spine on ventral posterodistal border; dactyli lancelet-shaped (especially in juveniles), but approaching paddle-shaped in larger individuals (Figs. 14B and 14C). G1 curved and tapering with a row of 1–12 subterminal bristles on lateral margin beginning just behind the tip; bristles continue sparsely across sternal surface extending to the mesial margin; mesial margin with similar, sometimes numerous spines beginning immediately behind tip (Fig. 7D).
Species included: Zygita longifrons (A. Milne-Edwards, 1869), comb. nov. [=Thalamita spinimera Stephenson & Rees, 1967; Thalamita yoronensis Sakai, 1969]; Zygita murinae (Zarenkov, 1971), comb. nov.
Remarks: The distinct morphology of this rarely collected genus is well known and deserving of generic rank (Stephenson & Rees, 1967a; Spiridonov & Neumann, 2008). The most diagnostic traits include the presence of a sharp or dull spinule at the distal end of manus Carina 4; meri of the ambulatory legs bearing a ventral posterodistal spine (Fig. 14E; asterisked); P5 coxa bearing a stout, well-developed dorsal spinule (Fig. 14B; asterisked); P5 carpus with a well-developed spine on the ventral posterodistal border (Fig. 14C; asterisked).
Ecology: In their original description of Thalamita spinimera, Stephenson & Rees (1967a) suggested these crabs were “ectocommensal” on Alcyonaria (=Octocorallia). However, this was based on one specimen collected from soft coral. A subsequent revision of this group by Spiridonov & Neumann (2008) failed to confirm this association, but only considered seven specimens. Evans & McKeon (2016) compiled compelling in situ photographs and collections records for 24 specimens and found that 46% (11 specimens) were found in association with soft corals (seven on nephtheid soft corals) in what is likely a facultative association.
Etymology: Zygita originates from Zygite, the title given to oarsmen occupying the middle tier of a trireme (a three-tiered ancient Greek warship). For context see Etymology of Thranita (above). Gender feminine.
Conclusion
This study constitutes the most comprehensive molecular phylogenetic analyses of Portunoidea to date, but highlights numerous areas where additional work is needed. Results support a more conservative classification of Portunoidea with three instead of eight extant families: Geryonidae (Geryonidae + Ovalipidae; new diagnosis provided), Carcinidae (Carcinidae + Pirimelidae + Polybiidae + Thiidae + Coelocarcinus; new diagnosis provided) and Portunidae. Limited molecular data also suggest that the family Brusiniidae may still be valid, but might not be a portunoid lineage. A major aim of this study was to investigate the molecular phylogenetic origin of symbiosis within Portunoidea by substantially increasing taxon sampling of the subfamilies Caphyrinae and Thalamitinae. Results support a shared ancestry of all symbiotic taxa (Caphyra, Lissocarcinus, and two Thalamita) derived within the thalamitine genus Thalamita. Consequently, Caphyrina Paulson, 1875, nom. trans., should be considered a subtribe within the subfamily Thalamitinae. Although the nature, degree, and phylogenetic pattern of symbiosis within Caphyrina needs further study, this clade is clearly dominated by symbiotic taxa and likely originated from a symbiotic ancestor. Results presented here also support the following taxonomic actions within Thalamitinae: Cronius is reclassified as a thalamitine rather than a portunine genus; Thalamonyx is reinstated as a valid genus; Goniosupradens is raised to the generic rank; and three new genera (Zygita gen. nov., Thranita gen. nov., and Trierarchus gen. nov.) are described to accommodate some Thalamita sensu lato taxa rendered paraphyletic by Caphyrina. A new diagnosis of Thalamitinae has also been provided.
Supplemental Information
ML phylogram of Portunoidea based on analyses of 174 OTUs and a 3313 bp alignment of partial 16S rRNA, CO1, 28S rRNA, and H3 sequence data.
Support values appear below relevant branches with ML bootstrap values ≥50% (based on 500 replicates) appearing first followed by BI posterior probabilities ≥0.95.
ML phylogram of Portunoidea based analyses of 163 taxa and an 1105 bp alignment of partial mitochondrial 16S rRNA (+tRNA-Leu and NADH1) sequence data.
Support values (%) appear above each relevant node and are based on 500 bootstrap replicate ML searches.
ML phylogram of Portunoidea based analyses of 148 taxa and an 657 bp alignment of partial mitochondrial CO1 sequence data.
Support values (%) appear above each relevant node and are based on 500 bootstrap replicate ML searches.
ML phylogram of Portunoidea based analyses of 66 taxa and an 1224 bp alignment of partial 28S rRNA sequence data.
Support values (%) appear above each relevant node and are based on 500 bootstrap replicate ML searches.
ML phylogram of Portunoidea based analyses of 123 taxa and an 327 bp alignment of partial H3 sequence data.
Support values (%) appear above each relevant node and are based on 500 bootstrap replicate ML searches.
ML phylogram of Brusinia profunda and 308 mostly brachyuran taxa based on analyses of a 447 bp alignment of 16S rRNA sequence data.
Support values (%) appear above each relevant node and are based on 500 bootstrap replicate ML searches. Brusiniidae and Portunoidea are highlighted.