The role of a water bug, Sigara striata, in freshwater food webs
- Published
- Accepted
- Received
- Academic Editor
- Dezene Huber
- Subject Areas
- Aquaculture, Fisheries and Fish Science, Ecology, Entomology, Environmental Sciences, Zoology
- Keywords
- Predation, Predator–prey interactions, Food webs, Foraging, Heteroptera, Corixidae
- Copyright
- © 2014 Klecka
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2014. The role of a water bug, Sigara striata, in freshwater food webs. PeerJ 2:e389 https://doi.org/10.7717/peerj.389
Abstract
Freshwater food webs are dominated by aquatic invertebrates whose trophic relationships are often poorly known. Here, I used laboratory experiments to study the role of a water bug, Sigara striata, as a potential predator and prey in food webs of stagnant waters. Multiple-choice predation experiment revealed that Sigara, which had been considered mostly herbivorous, also consumed larvae of Chironomus midges. Because they often occur in high densities and are among the most ubiquitous aquatic insects, Sigara water bugs may be important predators in fresh waters. A second experiment tested the role of Sigara as a potential prey for 13 common invertebrate predators. Mortality of Sigara inflicted by different predators varied widely, especially depending on body mass, foraging mode (ambush/searching) and feeding mode (chewing/suctorial) of the predators. Sigara was highly vulnerable to ambush predators, while searching predators caused on average 8.1 times lower mortality of Sigara. Additionally, suctorial predators consumed on average 6.6 times more Sigara individuals than chewing predators, which supports previous results hinting on potentially different predation pressures of these two types of predators on prey populations. The importance of these two foraging-related traits demonstrates the need to move from body mass based to multiple trait based descriptions of food web structure. Overall, the results suggests that detailed experimental studies of common but insufficiently known species can significantly enhance our understanding of food web structure.
Introduction
The view of ecological communities as networks of interacting species has revolutionized research of community structure, stability and responses to environmental changes (Ings et al., 2009). Studies that combine modelling and field data are attempting to provide general explanations of mechanisms structuring natural communities (Bascompte et al., 2003; Beckerman, Petchey & Warren, 2006; Bascompte & Jordano, 2007; Petchey et al., 2008) and to predict their responses to various threats, such as climate change (Petchey, Brose & Rall, 2010; O’Gorman et al., 2012) and habitat destruction (Melián & Bascompte, 2002; Fortuna & Bascompte, 2006). Having accurate and detailed information about trophic interactions of individual species forming food webs is a necessary condition for these attempts to succeed. Although the resolution of published food webs has increased considerably in recent years (Thompson, Dunne & Woodward, 2012), trophic position of many common species remains uncertain. This is troubling because a vast body of theoretical research has shown that food web structure is a key to understanding food web dynamics (de Ruiter, Neutel & Moore, 1995; McCann, Hastings & Huxel, 1998). Limited knowledge of food web structure in individual habitats thus calls into question the reliability of predictions of consequences of climate change or habitat destruction on food web diversity and stability.
Freshwater food webs are dominated by invertebrates, such as adults and larvae of insects, many of them carnivorous. Predatory aquatic insects have been traditionally considered generalists (Peckarsky, 1982) and food web studies often include them in relatively low resolution. This approach runs the risk of missing many crucial details given the tremendous diversity of aquatic insects. For example, there is over 150 species of diving beetles (all of them predatory) in the Czech Republic (Boukal et al., 2007), where this study has been conducted. Tens of species can coexist locally (Klecka & Boukal, 2011), possibly thanks to pronounced prey selectivity (Klecka & Boukal, 2012). Selective feeding in predatory aquatic insects is characterized not only by interspecific differences in prey selectivity and diet breadth (Cooper, Smith & Bence, 1985; Allan, Flecker & McClintock, 1987; Culler & Lamp, 2009; Klecka & Boukal, 2012), but also by marked ontogenetic diet shifts (Woodward & Hildrew, 2002; Klecka & Boukal, 2012). Prey mortality results from the interaction of several key predator and prey traits, particularly body size, predator foraging behaviour and prey vulnerability traits, such as its ability of rapid escape (Klecka & Boukal, 2013). The insect component of freshwater food webs is thus complex, but we are beginning to understand mechanisms structuring its interactions. The importance of predatory insects is underscored by findings of trophic cascades elicited by individual species, such as Dytiscus alascanus (Cobbaert, Bayley & Greter, 2010) and Notonecta sp. (Arnér et al., 1998).
Although detailed laboratory studies have been performed with a range of species, descriptions of food web structure have relied mostly on gut contents analyses of field-collected specimens (Warren, 1989; Woodward & Hildrew, 2002; Layer et al., 2010). The most serious downside of this approach is that it cannot be reliably used to study the diet of suctorial predators, such as water bugs (Heteroptera). Even recent studies rely on expert knowledge or previous literature data to infer trophic relationships of suctorial predators (e.g., Layer et al., 2010). This way of circumventing the problem of identifying prey of suctorial predators may be dangerous. Feeding relationships of most species are virtually unknown and existing literature provides conflicting information in many other cases, including a decades-long controversy about herbivorous or predatory nature of corixid bugs (Heteroptera: Corixidae) (Hutchinson, 1993; Popham, Bryant & Savage, 1984) studied in this paper. Despite several early attempts at identification of prey remains in the gut of suctorial water bugs by techniques of molecular biology (Giller, 1982; Giller, 1984; Giller, 1986), little progress has been achieved (Tate & Hershey, 2003; Morales et al., 2003). Modern molecular assays detecting prey DNA in the gut using species-specific markers have not yet been widely employed in freshwater predators, although they are increasingly used in terrestrial invertebrates (Günther et al., in press; Foltan et al., 2005; Symondson, 2002) and hold a significant promise for the future (Pompanon et al., 2012). In this situation, traditional laboratory experiments still offer a unique opportunity to obtain reliable high-resolution data on predator–prey interactions of individual species in freshwater food webs.
The aim of this study was to evaluate the role of a common freshwater insect, Sigara striata, in freshwater food webs. Sigara and other corixid water bugs are among the most ubiquitous and abundant aquatic insects (Schilling, Loftin & Huryn, 2009) and often reach densities of tens (Tolonen et al., 2003) or even hundreds of individuals m−2 (Bendell & McNicol, 1995), but their trophic position is not well understood (Hutchinson, 1993). I performed two complementary experiments addressing this knowledge gap. First, I tested whether Sigara can consume seven locally abundant species of aquatic invertebrates. Second, I tested the mortality of Sigara water bugs caused by 13 common predatory insects (nine species, multiple life stages in three cases). The same set of species, both predators and prey, as in Klecka & Boukal (2012) and Klecka & Boukal (2013) was used. The first experiment addressed a decades-old controversy concerning whether Sigara is strictly herbivorous (scraping algae from submerged vegetation, stones etc.) or whether it also feeds on other invertebrates. If carnivorous, Sigara could be an important freshwater predator because of its frequently very high population density.
Methods
Experiments
The water bug Sigara striata (Heteroptera: Corixidae) was collected in small pools in a reclaimed sandpit near Suchdol nad Lužnicí in South Bohemia, Czech Republic. Some of its potential predators and preys were collected at the same site and others in various small fishless water bodies close to the city of České Budějovice. Species were selected to represent a wide variety of regionally dominant species and to form a taxonomically and functionally diverse assemblage. Experiments were carried out in May and June 2007 in a climate room with a regular temperature cycle (day: max. 22 °C, night: min. 18 °C; mean 20 °C) and 18L:6D photoperiod. The experimental procedures followed Klecka & Boukal (2012) and Klecka & Boukal (2013) and the same species of predators and prey, except for Sigara, were used. All animals were kept in the lab for 2–5 days prior to the experiments to allow for acclimation to the laboratory conditions. Predators were fed daily ad libitum with prey different from Sigara (mainly larvae of Trichoptera) and starved for 24 h prior to the experiment. Experiments were performed in plastic boxes filled with 2.5 l of aged tap water (bottom dimensions 24 × 16 cm, water depth ca. 8 cm). The vessels had no substrate on the bottom but contained simple perching sites formed by four stripes of white plastic mesh suspended vertically in the water column. The vessels were shielded by sheets of brown carton from all sides to prevent disturbance of the experiments.
The first experiment tested whether Sigara striata can feed on six species of invertebrates which co-occur with it frequently in natural habitats (Table 1). In each replicate, six juvenile Lymnaea snails, 10 Chironomus midge larvae, 10 Cloeon mayfly larvae, 10 Culex mosquito larvae, 10 adult Asellus isopods and 30 adult Daphnia cladocerans were introduced into a vessel with water (see above) and 10 Sigara individuals were added after ca. 10 min. Ten Sigara individuals were used to increase the chance of detecting predation even if it occurs only rarely. Six replicates with Sigara as a potential predator and six without Sigara were performed to compare prey mortality in the presence/absence of Sigara. Qualitative observations of Sigara and prey behaviour were conducted in the beginning of the experiment and than occasionally during the experiment. All Sigara individuals used in the experiment and a sample of all prey species were preserved in 80% ethanol, their body length excluding appendages was measured to nearest 0.1 mm and their body mass was weighed after 48 h of drying at 50 °C.
| Body length (mm) | Body mass (mg) | |||||
|---|---|---|---|---|---|---|
| Species | N | Mean | SD | Mean | SD | Microhabitat |
| Asellus aquaticus adult | 10 | 7.21 | 0.99 | 1.69 | 0.38 | benthic |
| Chironomus sp. larva | 10 | 9.12 | 0.71 | 0.31 | 0.069 | benthic |
| Cloeon dipterum larva | 10 | 6.87 | 0.89 | 1.02 | 0.21 | benthic |
| Culex sp. larva | 10 | 8.92 | 0.41 | 0.62 | 0.17 | pelagic |
| Daphnia sp. adult | 20 | 2.21 | 0.19 | 0.041 | 0.029 | pelagic |
| Lymnaea stagnalis juvenile | 10 | 9.65a | 0.77 | 7.84b | 2.01 | pelagicc |
The second experiment aimed to estimate mortality rate of Sigara inflicted by different predators (Table 2). In each replicate, ten adults of Sigara were released first and one predator was added after ca. 10 min. The number of surviving Sigara individuals was counted after 24 h. All individuals of Sigara and the predators were used only once. Four control trials were run to evaluate natural mortality of Sigara; no individual died in any of these control trials, suggesting that mortality observed in the presence of predators was caused entirely by predation. All predators were preserved in 80% ethanol, their body length measured and their body mass weighed as in the first experiment. I also classified their microhabitat use and made qualitative observations of their behaviour during the experiments.
| Body length (mm) | Body mass (mg) | |||||||
|---|---|---|---|---|---|---|---|---|
| Species | N | Mean | SD | Mean | SD | Foraging mode | Feeding mode | Microhabitat |
| Coleoptera | ||||||||
| Acilius canaliculatus adult | 4 | 15.6 | 0.57 | 61.74 | 9.34 | searching | chewing | benthic |
| Acilius canaliculatus L2 | 4 | 11.8 | 0.63 | 2.74 | 0.66 | ambush | suctorial | pelagic |
| Acilius canaliculatus L3 | 4 | 22.2 | 1.75 | 14.66 | 4.70 | ambush | suctorial | pelagic |
| Dytiscus marginalis adult | 4 | 31.7 | 0.89 | 528.43 | 50.88 | searching | chewing | benthic |
| Dytiscus marginalis L3 | 4 | 49.1 | 3.01 | 176.43 | 76.12 | ambush | suctorial | pelagic |
| Hydaticus seminiger adult | 4 | 14.5 | 0.32 | 64.94 | 8.42 | searching | chewing | benthic |
| Hemiptera | ||||||||
| Ilyocoris cimicoides adult | 4 | 13.9 | 0.60 | 34.43 | 6.85 | searching | suctorial | benthic |
| Notonecta glauca adult | 5 | 15.3 | 0.44 | 39.43 | 8.08 | ambush | suctorial | pelagic |
| Odonata | ||||||||
| Anax imperator F-0 | 4 | 47.6 | 2.61 | 267.0 | 54.42 | ambush | chewing | pelagic |
| Coenagrion puella F-0 | 4 | 12.8 | 0.87 | 4.80 | 0.99 | ambush | chewing | pelagic |
| Libellula depressa F-0 | 5 | 22.3 | 1.11 | 58.41 | 19.51 | ambush | chewing | benthic |
| Libellula depressa F-2 | 4 | 15.7 | 0.72 | 20.94 | 5.57 | ambush | chewing | benthic |
| Sympetrum sanguineum F-0 | 4 | 16.1 | 0.97 | 20.82 | 4.49 | ambush | chewing | pelagic |
Notes:
- L2
larvae of the second instar
- L3
larvae of the third instar
- F-0
larvae of the last instar
- F-2
larvae of the second before the last instar
- N
number of replicates
Data analysis
I used Bayesian methods to estimate predation rates of Sigara consuming other invertebrates in the first experiment. The same approach was used to estimate mortality rate of Sigara caused by different predators and to test the role of predator traits for Sigara mortality in the second experiment. Overdispersed binomial distribution and logistic link function was used in all cases.
To estimate predation rates of Sigara on different prey species, I used a model contrasting mortality rate of prey species i in the presence of Sigara and in control trials without Sigara. This approach was needed because some prey species had non-zero mortality in the control trials. I assumed that mortality mi of prey i depends on the presence of Sigara in a species-specific way. To account for overdispersion, I used a two-stage model: (1) where Yi is the number of individuals of a prey species i dying in an experiment, ni is the initial number of prey individuals, mi is the mortality of prey i, ai is the natural mortality of prey i in the absence of Sigara, bi is the effect of the presence of Sigara on the mortality of prey i, Si denotes the presence of Sigara (Si = 1 when Sigara was present, Si = 0 otherwise), and zi is the overdispersion term.
To estimate the mortality rate of Sigara caused by individual predators, I used a model where the mortality of Sigara is a function of predator species. Predator species was used as a random factor. Two-stage model accounting for overdispersion was used similarly as above: (2) where Yi is the number of Sigara individuals dying in an experiment, ni is the initial number of individuals, mi is the mortality of Sigara, a0 is the intercept, bi is the effect of predator i on the mortality of Sigara and zi is the overdispersion term.
To estimate the dependence of Sigara mortality on predator traits, I used a hierarchical model where the mortality of Sigara mi is a function of predator species (a random factor) as in Eq. (2). The mortality of Sigara further depends on a combination of several predator traits with additive effects at the scale of the linear predictor. Two-stage model accounting for overdispersion was used as above; linear predictor in Eq. (3) contains all explanatory variables: (3) where Yi is the number of Sigara individuals dying in an experiment, ni is the initial number of individuals, mi is the mortality of Sigara, a0 is the intercept, a1, …, a5 are parameters describing the effect of individual predator traits, wi is body mass of predator i, is mean body mass of all predators (i.e., predator body mass is centered in Eq. (3)), Ai is the foraging mode of predator i (Ai = 1 for ambush predators and Ai = 0 for searching predators), Bi is the feeding mode (Bi = 1 for ambush predators and Bi = 0 for searching predators), Ci is the microhabitat preference of predator i (Ci = 1 for ambush predators and Ci = 0 for searching predators) and zi is the overdispersion term. The analysis of posterior parameter distributions revealed that parameters a2 and a5 were approximately zero; the corresponding terms in Eq. (3) were dropped to obtain the reduced model reported in the results (Table 3).
| Full model | Reduced model | |||
|---|---|---|---|---|
| Parameter | Mean estimate (SE) | Median (95% CI) | Mean estimate (SE) | Median (95% CI) |
| Intercept (a0) | −3.06 (0.023) | −3.02 (−6.29, −0.08) | −3.35 (0.012) | −3.32 (−5.79, −1.08) |
| Predator mass (linear; a1) | 1.05 (0.006) | 1.02 (0.02, 2.30) | 1.00 (0.004) | 0.98 (0.13, 1.96) |
| Predator mass (quadratic; a2) | −0.19 (0.003) | −0.19 (−0.81, 0.39) | – | – |
| Foraging—ambush (a3) | 2.25 (0.041) | 2.21 (−2.26, 7.03) | 2.38 (0.014) | 2.38 (−0.33, 5.09) |
| Feeding—suctorial (a4) | 2.20 (0.018) | 2.14 (−1.02, 5.81) | 2.15 (0.009) | 2.11 (−0.29, 4.77) |
| Microhabitat—pelagic (a5) | 0.27 (0.036) | 0.30 (−4.45, 4.79) | – | – |
| SD interspecific | 2.24 (0.012) | 2.04 (1.13, 4.46) | 1.89 (0.004) | 1.77 (1.04, 3.42) |
| SD intraspecific | 0.29 (0.001) | 0.25 (0.08, 0.68) | 0.28 (0.001) | 0.25 (0.08, 0.68) |
| Explained variance (%) | 64.1% | 64.3% | 63.2% | 63.3% |
Uninformative priors were used for all parameters in all models. Specifically, normal distribution with μ = 0 and σ2 = 103 was used for all parameters (see code in Supplemental Information for implementation). Model parameters were estimated using Markov Chain Monte Carlo (MCMC) simulations with three chains, each with 106 steps with thinning of 100; i.e., 104 values per chain. A burn-in of 2⋅103 steps was used in all cases. All data analyses were conducted in R 3.0.1 (R Core Team, 2013); Bayesian analysis was performed using JAGS (Plummer, 2003) through rjags package for R (Plummer, 2013); coda package for R (Plummer et al., 2006) was used to analyse the MCMC output and to perform convergence diagnostics. A complete set of the raw data and the code is available as Supplemental Information. Posterior distributions of all parameter estimates obtained using Gibbs sampling in JAGS are provided in Supplemental Information together with the results of convergence diagnostics and autocorrelation analysis of MCMC chains.
Results
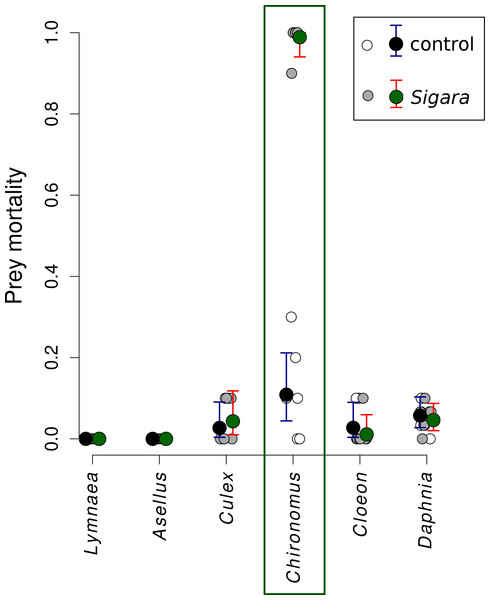
Sigara consumed on average 95% of Chironomus midge larvae in the first experiment, while it did not feed on the remaining five species (Fig. 1). Direct observations confirmed that increased mortality of Chironomus midge larvae was indeed caused by consumption by Sigara and that they were captured alive and subsequently consumed. Sigara used its forelegs to grab Chironomus larvae on the bottom of the experimental vessel, then ascended to the water surface, where it pierced the cuticle of its prey with a proboscis and sucked out the majority of soft tissues in the Chironomus body. Only small part of the prey body was discarded after consumption.
Figure 1: Mortality of six freshwater invertebrates in the presence/absence of Sigara striata.
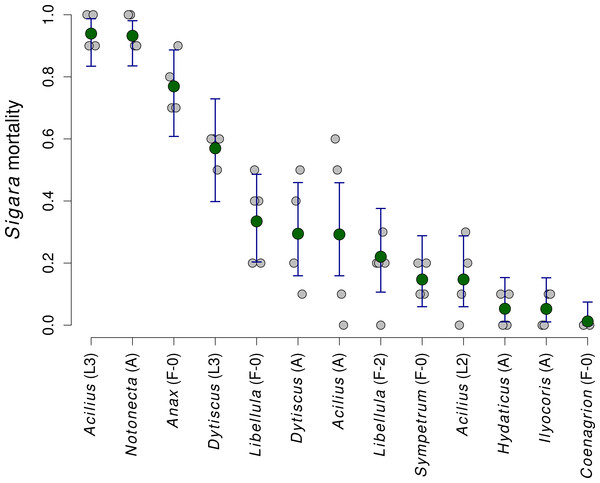
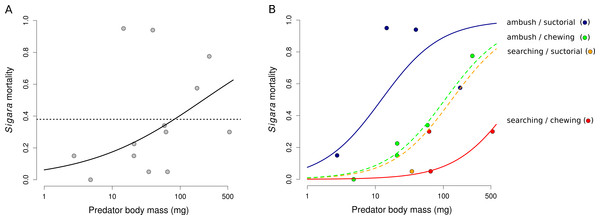
Estimated proportion of prey individuals dying during a 24 h long experiment. The initial number of prey individuals was six Lymnaea, 10 Chironomus, 10 Cloeon, 10 Culex, 10 Asellus and 30 Daphnia. In the predation treatment, 10 Sigara bugs were added. Large circles denote mean values and vertical bars are 95% credible intervals. Small circles show values observed in individual replicates.Mortality of Sigara caused by individual predators varied widely (Fig. 2). The analysis of the role of predator traits for Sigara mortality showed that the mortality of Sigara depended on body mass, foraging mode and feeding mode of the predators. On average, larger predators caused higher mortality of Sigara (Fig. 3, Table 3). Parameter estimate for the quadratic term of predator body mass was around zero, which indicates that Sigara mortality increases in a logistic way (i.e., linearly at the scale of the linear predictor) within the range of predator body masses used in the experiment (Fig. 3, Table 3). Interestingly, body mass played a significant role only when other predator traits were included; alone it explained only ca. 0.2% of variation in mortality rate. In addition to the effect of body mass, the data indicate that predator’s foraging mode and feeding mode affect the mortality rate of Sigara. Model which included these traits in addition to body mass explained over 60% of variation in mortality rate (Table 3). As expected, ambush predators caused higher mortality of Sigara than searching predators (Fig. 3); 8.1 times higher when ambush and searching predators of average body mass are compared. Suctorial predators killed more individuals than chewing predators (Fig. 3); 6.6 times more when suctorial and chewing predators of average body mass are compared. On the other hand, microhabitat preference of predators had no effect on the mortality of Sigara (Table 3).
Figure 2: The mortality of Sigara striata caused by 13 different predators.
Estimated proportion of Sigara individuals killed by individual predators. Large green circles denote mean values, vertical bars are 95% credible intervals and small grey circles are individual observed values.Figure 3: The dependence of the mortality of Sigara striata on predator traits.
Mortality of Sigara predicted by a model with predator body mass only (A) and with a combination of body mass, foraging mode and feeding mode (B). Black line in (A) shows model prediction and dotted line shows mean mortality. Lines in (B) show the predicted proportion of Sigara killed by a predator as a function of predator body mass, foraging mode and feeding mode. Small circles are mean values of Sigara mortality caused by individual predator species. Note that the x-axis has a logarithmic scale.Discussion
This study demonstrates that our knowledge of trophic links in freshwater food webs in still insufficient and can be enhanced by detailed laboratory experiments. Food web theory has been attempting to shed light on mechanisms underlying the maintenance of biodiversity (de Ruiter, Neutel & Moore, 1995; McCann, Hastings & Huxel, 1998) and more recently also to predict the consequences of climate change (Petchey, Brose & Rall, 2010; O’Gorman et al., 2012), habitat fragmentation (Melián & Bascompte, 2002) and other threats to natural communities, as well to study ecosystem recovery (Layer et al., 2010). However, empiricists have been frequently critical of food web approaches especially because they have relied on very crude data (e.g., Polis, 1991). Early food web studies lumped species to broad functional groups (Cohen, Briand & Newman, 1990) and although recent datasets have improved the resolution of food web descriptions considerably (Thompson, Dunne & Woodward, 2012), there is still much room for improvements and refinements. Although food web research has been theory-driven for several decades; changes in the availability of empirical data are responsible for many recent advances. When May (1972) reported that complexity decreases stability, he used a simple model where feeding links were assigned at random. It later turned out that non-randomness of feeding links and uneven distribution of interaction strengths allow complex communities to persist (de Ruiter, Neutel & Moore, 1995; McCann, Hastings & Huxel, 1998). More detailed data collected over the past two decades led to the rediscovery of the key role of body size for food web structure (Elton, 1927; Brose et al., 2006), which has important implications for food web stability (Brose, Williams & Martinez, 2006; Heckmann et al., 2012). New, even more detailed data can reveal additional hidden levels of complexity (Melián et al., 2011; Gilljam et al., 2011). Detailed observational studies of individual species are thus needed to drive future progress.
Attempts to explain food web structure have recently used body size of predators and prey as a major factor deciding on who eats whom (Petchey et al., 2008; Williams, Anandanadesan & Purves, 2010; Williams & Purves, 2011). However, it is becoming clear that this approach is oversimplified and can be substantially improved by the inclusion of multiple species traits, mostly related to predator foraging behaviour, prey vulnerability, and microhabitat use of both predators and prey (Rohr et al., 2010; Rossberg, Brännström & Dieckmann, 2010; Wirtz, 2012; Klecka & Boukal, 2013). Mortality of Sigara in my experiment depended not only on predator’s body mass, but also on its foraging mode (ambush/searching) and feeding mode (chewing/suctorial). Ambush predators were more efficient in capturing Sigara probably because Sigara is capable of rapid escape behaviour which may be more effective against searching predators. Suctorial predators also consumed more Sigara individuals than chewing predators. Both these results support conclusions reached in multiple-choice experiments with the same set of predators but a larger set of seven different prey species (Klecka & Boukal, 2013).
Surprisingly, Sigara can also be an important predator in freshwater food webs because the first experiment revealed that it feeds on Chironomus midge larvae. The possibility that Sigara water bugs could be carnivorous has been debated for several decades (Popham, Bryant & Savage, 1984; Hutchinson, 1993). Gut content analyses by Popham, Bryant & Savage (1984) suggested considerable variation in feeding habits of bugs of the family Corixidae. Several genera, mostly of larger species, such as Corixa and Cymatia, seem to be mostly carnivorous. However, smaller species of a diverse genus Sigara seemed to feed on algae or detritus or on a mixed diet. This reportedly included some unspecified animal components (Popham, Bryant & Savage, 1984). Other authors reported remains of microscopic invertebrates, namely rotifers, in the gut of Sigara (Hutchinson, 1993). Because these bugs are suctorial, only remains of very small organisms which are consumed completely can be found in the gut and majority of the gut contents is unidentifiable (Popham, Bryant & Savage, 1984). Sigara in my experiment fed on Chironomus midge larvae larger than itself by sucking out their body fluids, while holding the prey using the forelegs. It is very unlikely that any identifiable remains could be detected in its gut. The possibility that Sigara could feed on larger invertebrates, including insect larvae, has been rarely considered. For example the review of Shaalan & Canyon (2009) on predatory insects feeding on mosquitoes lists only one study reporting predation by Sigara hoggarica on mosquitoes (Alahmed, Alamr & Kheir, 2009); all other hemipterans in their review were members of the family Notonectidae. Nevertheless, the ability to feed on large prey, even larger than the predator, seems to be common among suctorial predators (Klecka & Boukal, 2013; Nakazawa, Ohba & Ushio, 2013). They also kill on average larger amounts of prey than equally sized chewing predators, such as adult beetles or dragonfly larvae, which makes them potentially more likely to have stronger interactions with prey (Klecka & Boukal, 2013). Unfortunately, because their diet cannot be reliably studied using gut content analyses, they are underrepresented in studies of food web structure. Given their high species diversity, numerical abundance and voraciousness, this limitation of data availability can significantly distort our understanding of the dynamics of freshwater food webs, especially in small fishless water bodies, where invertebrate predators dominate. It is plausible that Sigara is an important freshwater predator, because the abundance of Sigara can reach hundreds on ind. m−2 (Bendell & McNicol, 1995). This makes Sigara one of the most abundant insects in suitable habitats (Tolonen et al., 2003; Schilling, Loftin & Huryn, 2009). Molecular gut content assays have been employed to study the diet of Notonecta water bugs (Heteroptera: Notonectidae) in several early studies with mixed results (Giller, 1982; Giller, 1984; Giller, 1986) and in narowly targeted studies to identify predators of mosquitoes (Morales et al., 2003; Ohba et al., 2010). Although these methods are not widely used, methodical advancements, such as next generation sequencing, hold promise for the future (Pompanon et al., 2012). At present, careful laboratory experiments thus remain the only viable option to evaluate the role of the whole group of suctorial predators in freshwater food webs. Supplementing gut content analyses with laboratory predation experiments is a feasible way to boost the reliability and resolution of next generation food web data.
Simple laboratory experiments, such as the ones presented here, are not without limitations. Specifically, they are conducted over short periods of time in small vessels with very simple structure. Short-term measurements of consumption rates may not correlate well with long-term measures of per-capita interaction strength (Wootton, 1997). Less clear is how the lack of habitat complexity in laboratory conditions affects prey selectivity of predators. Habitat structure in the natural environment may provide refuges for some prey species against some predators but not against others, as demonstrated in numerous experiments testing the effects of the presence or density of vegetation for the foraging success and selectivity of predatory fish (Eklöv & Diehl, 1994; Horinouchi et al., 2009). The availability of perching sites also affects foraging behaviour and predation rates in damslefly larvae (Convey, 1988). Many other predators adjust their foraging strategies in response to habitat structure; e.g., Dytiscus larvae decrease their activity in structured environment and employ more strongly ambushing tactics than in simple environment (Michel & Adams, 2009). It is not clear how the relative importance of different predators for the mortality of Sigara might differ under natural conditions from the results of my experiment. Importantly, Sigara water bugs often live in newly created water bodies lacking dense vegetation, which the simple experimental conditions may represent reasonably well. However, the experimental vessels contained no substrate on the bottom. Many chironomid midge larvae, including the ones used in the experiment, normally bury themselves in soft sediments on the bottom. This is likely to reduce the encounter rates of Sigara and Chironomus larvae under natural conditions. Estimating the potential of Sigara to exert predation pressure sufficient to control the population density of Chironomus in the field will thus require observational or experimental data collected under natural conditions.
The findings of this study, particularly predation by Sigara on Chironomus larvae and high vulnerability of Sigara to ambushing and suctorial predatory insects, have important implications for understanding the structure of freshwater food webs. In suitable habitats, Sigara water bugs could be a key resource for certain groups of predators and they may also inflict large predation pressure on chironomid midge larvae, and possibly other invertebrates. It has been recently proposed that Sigara hoggarica can regulate mosquito populations by consuming their larvae and pupae (Alahmed, Alamr & Kheir, 2009). Although their trophic role is still not well understood, Sigara water bugs may thus represent a key group in many freshwater food webs. Another, perhaps trivial, conclusion is that understanding food web structure requires paying attention to details of biology of individual species. In this particular case, information about the possibility that Sigara water bugs may play an important role as predators of insect larvae could be gleaned from specialized natural history papers, which are usually not published in high-profile journals and are thus missed by most ecologists. Food web research can progress only if feeding links are assigned correctly and comprehensively with at least species-level resolution. Detailed experimental studies of uncharismatic non-model species may identify a number of unexpected but potentially important trophic links.