Infection of army ant pupae by two new parasitoid mites (Mesostigmata: Uropodina)
- Published
- Accepted
- Received
- Academic Editor
- Marcio Pie
- Subject Areas
- Animal Behavior, Biodiversity, Ecology, Taxonomy, Zoology
- Keywords
- Army ants, Myrmecophile, Parasite, Anactinotrichida, Pupal infection, Macrodinychus extremicus, Macrodinychus vietnamensis, Mites, Malaysia, Acari
- Copyright
- © 2017 Brückner et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Infection of army ant pupae by two new parasitoid mites (Mesostigmata: Uropodina) PeerJ 5:e3870 https://doi.org/10.7717/peerj.3870
Abstract
A great variety of parasites and parasitoids exploit ant societies. Among them are the Mesostigmata mites, a particularly common and diverse group of ant-associated arthropods. While parasitism is ubiquitous in Mesostigmata, parasitoidism has only been described in the genus Macrodinychus. Yet information about the basic biology of most Macrodinychus species is lacking. Out of 24 formally described species, information about basic life-history traits is only available for three species. Here we formally describe two new Macrodinychus species, i.e. Macrodinychus hilpertae and Macrodinychus derbyensis. In both species, immature stages developed as ecto-parasitoids on ant pupae of the South-East Asian army ant Leptogenys distinguenda. By piercing the developing ant with their chelicera, the mites apparently suck ant hemolymph, ultimately killing host individuals. We compare infection rates among all studied Macrodinychus species and discuss possible host countermeasures against parasitoidism. The cryptic lifestyle of living inside ant nests has certainly hampered the scientific discovery of Macrodinychus mites and we expect that many more macrodinychid species await scientific discovery and description.
Background
In 1982 David H. Kistner published an influential book chapter with the title “The Social Insects’ Bestiary” (Kistner, 1982), a metaphor referring to the many thousand arthropod species exploiting social insect societies (Kistner, 1979; Kistner, 1982; Hölldobler & Wilson, 1990). Among them are such diverse groups as beetles, flies, wasps, ants, millipedes, silverfish, and mites (Donisthorpe, 1927; Rettenmeyer, 1961; Kistner, 1979; Kistner, 1982; Hölldobler & Wilson, 1990; Buschinger, 2009; Parker, 2016). The latter are particularly abundant guests of social insect colonies (Kistner, 1982; Eickwort, 1990; Gotwald Jr, 1996). The mite order Mesostigmata is notable in this respect because 20 out of 109 of its families are considered to have some kind of relationship with ants (Walter & Proctor, 1999; Beaulieu et al., 2011). While most of the myrmecophilous mites use ant workers solely as transportation vehicles, some species are ectoparasitic (Kistner, 1982; Eickwort, 1990). For instance, Macrocheles rettenmeyeri (Krantz, 1962) (Mesostigmata: Macrochelidae) is an ectoparasite of Neotropical army ants (Eickwort, 1990). This ‘myrmecophile’ (ant lover) specifically attaches to the pulvilli of Eciton dulcium Forel, 1912 legs (Krantz, 1962; Gotwald Jr, 1996), where it probably sucks hemolymph from the ants’ arolium, an adhesive organ at the tip of legs enabling ants to climb smooth or steep surfaces (Hölldobler & Wilson, 1990). While the negative impact of this ectoparasitic myrmecophile on host fitness is supposedly small, some of the ant-associated mites are parasitoids (Lachaud, Klompen & Pérez-Lachaud, 2016) and therefore, by definition (Godfray, 1994; Godfray, 2004), kill host individuals.
Given the great diversity of mite myrmecophiles, it is surprising that a parasitoid lifestyle is only known in a single mite family, i.e., the Macrodinychidae (Mesostigmata) (González, Gómez & Mesa, 2004; Breton, Takaku & Tsuji, 2006; Krantz, Gómez & González, 2007; Lachaud, Klompen & Pérez-Lachaud, 2016). In the most recent revisions of the group, the family’s only genus, Macrodinychus Berlese, 1917, contained 24 valid species which are distributed throughout tropical regions and some temperate regions (Kontschán, 2011; Kontschán, 2017). Information about the basic biology and life history of most Macrodinychus species is lacking. The life cycle is only well known for three out of 24 species, i.e., M. sellnicki Hirschmann & Zirngiebl-Nicol, 1975 (González, Gómez & Mesa, 2004; Krantz, Gómez & González, 2007), M. multispinosus Sellnick, 1973 (Lachaud, Klompen & Pérez-Lachaud, 2016), and Macrodinychus yonakuniensis Hiramatsu, 1979 (Breton, Takaku & Tsuji, 2006). These species develop on ant pupae where immatures suck the host’s hemolymph to an extent that is lethal to the ants (Lachaud, Klompen & Pérez-Lachaud, 2016). In 1975, Werner Hirschmann, a pioneer in the taxonomy of Uropodina, i.e., an infraorder within the order Mesostigmata (Beaulieu et al., 2011), hypothesized that all Macrodinychus species are parasites of ants (Hirschmann, 1975):
“Bei den Bodenfunden von Macrodinychus -Arten […] handelt es sich wohl um einzelne Zufallsfunde; denn der eigentliche Lebensraum der Macrodinychus-Arten scheint das Ameisennest zu sein, wo die Tiere als Paraphagen oder Parasiten an Ameisen leben dürften.”
(Translation: The Macrodinychus species […] collected from soil samples are probably chance finds, because the actual living environment of the Macrodinychus species seems to be the ant nest, where the animals live as paraphages or parasites on ants.)
When Hirschmann wrote these lines, his hypothesis was speculative and lacked solid evidence. For most Macrodinychus species we still lack information about their basic biology including possible symbiosis with ants. Today it is known that about one third of the Macrodinychus species are indeed associated with ants, with three definite examples of parasitoidism (Lachaud, Klompen & Pérez-Lachaud, 2016). In the present study, we provide further support for Hirschmann’s hypothesis by adding two additional species to the list of Macrodinychus parasitoids. We formally describe and provide life history information for two hitherto undescribed Macrodinychus species, Macrodinychus hilpertae Brückner, Klompen & von Beeren sp. nov. and Macrodinychus derbyensis Brückner, Klompen & von Beeren sp. nov. Both species were collected from colonies of the South-East Asian army ant Leptogenys distinguenda. Like other Macrodinychus parasitoids, the entire juvenile development of the new species took place as ecto-parasitoids on host pupae, ultimately killing the host individuals.
Materials & Methods
Collection and specimen depository
Two Macrodinychus (Mesostigmata: Uropodina) species were discovered during a project aiming to uncover the interactions of the army ant Leptogenys distinguenda and its diverse myrmecophile fauna (Witte et al., 1999; Witte et al., 2002; Kistner, Witte & Maschwitz, 2003; Witte et al., 2008; Maruyama, von Beeren & Hashim, 2010; Maruyama, von Beeren & Witte, 2010; Mendes, von Beeren & Witte, 2011; Ott et al., 2015). The mites were initially hidden, enclosed in ant pupal cocoons, and collection took place incidentally by collecting ant pupae (Fig. 1). The latter were collected during army ant colony emigrations using aspirators and forceps (for more information see von Beeren et al., 2011b). Collection took place in Malaysia, primarily at the Ulu Gombak Field Studies Centre of the University Malaya (latitude: 3.325, longitude: 101.750, elevation: 260) and additionally at the Biodiversity Institute Bukit Rengit (latitude: 3.596, longitude: 102.182, elevation: 72), between April and May 2008, August and September 2008, February and March 2009, August and September 2009, February and March 2010, and March and April 2011 (approx. 11 months in total). The specimens were stored in absolute ethanol and deposited at the Ohio State University Acarology Collection, Columbus, Ohio, USA (OSAL). Macrodinychid mites are vouchered together with their respective ant pupa. Further specimens are deposited at the Adam Mickiewicz University in Poznań (three specimens of M. hilpertae labeled as “Tank mite” and two specimens of M. derbyensis labeled as “Smooth shell”). Borrowing the latter specimens for morphological analysis was not possible in a reasonable time frame, because of an entire re-organisation of the department‘s mite collection. All other specimens have been lost during several institutional moves of one of the authors (CvB).
Figure 1: Host pupa infected with Macrodinychus parasitoid.
Pupae were collected during colony emigrations of Leptogenys distinguenda. Pupal cocoons are opaque but become transparent in ethanol (white arrow). The highlighted pupa is infected with Macrodinychus hilpertae.Note that the host ant is an undescribed species (K Arimoto, pers. comm., 2017). It was designated previously as Leptogenys sp. 1 (Maschwitz et al., 1989) and as L. distinguenda (see Maschwitz & Steghaus-Kovac, 1991; Witte & Maschwitz, 2000; Witte & Maschwitz, 2002; Witte et al., 2008; von Beeren et al., 2011a). To be consistent with the most recent publications we use the name Leptogenys distinguenda for the species, which is in fact a nomen nudum. Specimen images of L. distinguenda have been published previously (denoted there as L. distinguenda (Maruyama, von Beeren & Hashim, 2010)). Voucher ant specimens are deposited at the Southwest Forestry University Ant Collection, Kunming, Yunnan Province, China (collection identifiers: A11-5936–A11-5942).
The electronic version of this article in Portable Document Format (PDF) will represent a published work according to the International Commission on Zoological Nomenclature (ICZN), and hence the new names contained in the electronic version are effectively published under that Code from the electronic edition alone. This published work and the nomenclatural acts it contains have been registered in ZooBank, the online registration system for the ICZN. The ZooBank LSIDs (Life Science Identifiers) can be resolved and the associated information viewed through any standard web browser by appending the LSID to the prefix http://zoobank.org/. The LSID for this publication is: urn:lsid:zoobank.org:pub:84ADDB13-56F3-431D-9244-E19C3A2F7E04. The online version of this work is archived and available from the following digital repositories: PeerJ, PubMed Central and CLOCKSS.
Prevalence of mites
To evaluate mite prevalence and screen for different development stages we gently opened a total of 2,360 L. distinguenda pupal cocoons from a total of six different colonies. Since many adult specimens found during these dissections have been lost, we were not able to reliably identify all Macrodinychus specimens to the species level. As a consequence of this, we could not determine the prevalence for each of the two species separately, but instead evaluated the overall parasitism rate among Macrodinychus spp., i.e., the total number of pupal infections by Macrodinychus mites. In addition, after an initial screening of 1,391 pupal cocoons for adult Macrodinychus mites in 2009 and 2010, all L. distinguenda pupae from different colonies were combined in 2012 for storage at the LMU Munich. Therefore, we only have limited data about the colony of origin of Macrodinychus mites.
For three additional L. distinguenda colonies we estimated the total number of pupae allowing us to estimate the number of pupal infections per colony. For this, Leptogenys bivouac sites were marked with tape and checked every 30 min for ongoing colony emigrations. Upon the start of an emigration, defined as workers carrying larvae or pupae to the new nest site, the number of Leptogenys workers carrying pupae and heading toward the new bivouac site was repeatedly counted for 30 s, followed by a 150 s break till the emigration was finished. We did not collect pupae for dissections from these colonies.
Morphological protocol and imaging
Specimens were dissected and slide mounted in Hoyer’s medium or lactophenol (Walter & Krantz, 2009) and studied with bright-field, differential interference contrast and phase contrast microcopy. Morphological structures were drawn based on images taken during the phase contrast microscopy. In addition, focus-stacked images were taken with a Keyence VHX-5000 digital microscope (Keyence Deutschland GmbH, Neu-Isenburg, Germany) using the VH-Z50L lens. All measurements were taken using internal scale function as implemented in the Keyence system software (version 1.5.1.1; system version 1.0.4). A total of 37 images were uploaded to the Barcode of Life Database. Images can be accessed using the sample ID (provided in results) as search term. Images of all immature stages can be found on BOLD (search using the sample ID). Holotype label information is listed verbatim, with the different labels separated by forward slashes.
Observations in laboratory nests
Interactions between host ants and adult Macrodinychus specimens were studied in laboratory nests containing 110–170 ant workers, 44–55 ant pupae, 22–30 callows (freshly hatched workers) and three to six clusters of ant larvae. Behavioral tests were carried out with workers of the myrmecophile’s colony of origin. Details about the nest set-ups were described previously (von Beeren et al., 2011a). Myrmecophiles were tested individually. Frequently, myrmecophiles behaved excitedly for a short period after transferring them to laboratory nests, which sometimes initiated ant aggression. To avoid biases caused by the specimen transfer we gave myrmecophiles two minutes settling time before recording the ant behaviors. We then observed the interactions of the myrmecophile in the first 50 consecutive encounters with host ant workers (for definition of behavioral categories see Table S1). At the study time, we did not realize that there are two different Macrodinychus species and therefore the data presented here cannot be assigned to the species level. Nonetheless, we consider the behavioral data as valuable because behavioral interactions with host ants have not been studied systematically for any Macrodinychus species. To compare the host-symbiont interactions of Macrodinychus spp. with those of other L. distinguenda myrmecophiles, we additionally tested the following associates: the silverfish Malayatelura ponerophila (Mendes, von Beeren & Witte 2011) the spider Sicariomorpha maschwitzi (Wunderlich, 1994), the snail Allopeas myrmecophilos (Janssen & Witte, 2002), and the rove beetles Maschwitzia ulrichi (Kistner, 1989), Witteia dentrilabrum (Maruyama, von Beeren & Hashim, 2010), and Togpelenys gigantea (Kistner, 1989). Data on rove beetles were published previously (von Beeren et al., 2011a).
Data analysis
Behavioral counts were expressed as compositional data (%) by standardizing for the total number of interactions per specimen (approx. 50 interactions per specimen: mean ± SD = 50.83 ± 3.20 interactions, N = 97). These multivariate data were analyzed with a permutational analysis of variance (PERMANOVA) with 9,999 permutations based on Bray-Curtis similarities. Due to the rareness of certain associates, some specimens were tested multiple times (Table S2). This was considered in the PERMANOVA design (Myrmecophile species = fixed factor; Specimen ID = random factor). In addition to the multivariate analysis of behavioral interactions, we calculated an aggression index (AI in (%)) to measure the total aggression of ants towards a focal myrmecophile. For this, the sum of aggressive behaviors (chased, snapped, stung) was divided through the total number of interactions. We applied PERMANOVA for univariate cases based on Euclidean distances with the same design as described above. PERMANOVAs were run with the software Primer 7 (Primer-E Ltd., Ivybridge, UK, vers. 7.0.12) with the add-on PERMANOVA+1 (Anderson, Gorley & Clarke, 2008).
Results—Taxonomic Section
Species descriptions
| Infraorder UROPODINA Kramer, 1881 |
| Family MACRODINYCHIDAE Kontschán, 2017 |
| Genus MACRODINYCHUS Berlese, 1917 |
Systematic note: For this study, we follow the classification of Kontschán (2011) and Kontschán (2017) in recognizing a single genus, Macrodinychus Berlese, 1916, in the family Macrodinychidae Kontschán, 2017. Within this genus four subgenera are recognized (largely corresponding to the “Stadiengattungen” of Hirschmann, 1979): Macrodinychus, Monomacrodinychus Hirschmann, 1975 (= Baloghmacrodinychus Hirschmann, 1979, see Halliday, 2015), Bregetovamacrodinychus Hirschmann, 1979, and Loksamacrodinychus Hirschmann, 1979. Both of the new species belong in the subgenus Macrodinychus (Monomacrodinychus) based on the shape of the peritremes.
Diagnosis of the genus Macrodinychus (based on Kontschán, 2011, Kontschán, 2017 and Hirschmann, 1975)
Within Uropodina, the genus Macrodinychus is characterized by the following characters: Idiosoma large, oval or sometimes oblong, posterior margin rounded, anterior margin sometimes angular. Color yellow-brown to darkish brown. All legs short, but well developed. Tritosternum trifurcate with narrow basis. Gnathosoma with long hypostomal setae, horn-/peanut-like corniculi, pilose internal malae, pilose gnathotectum and chelicera with sclerotized nodes and without processes on tip fixed digit. Gnathosoma usually largely covered by coxae I. Genital shield of females small relative to the body (when compared to other Uropodina) and comparable in size to that of the males. Females and males do not differ in the shape and structure of the peritremes. Potentially viviparous.
Macrodinychus (Monomacrodinychus) hilpertae Brückner, Klompen & von Beeren sp. nov.
Type-host: Leptogenys distinguenda (Formicidae: Ponerinae)
Type-locality: Ulu Gombak Field Studies Centre of the University Malaya (03°19.479′N, 101°45.163′E, altitude 230 m), Selangor, Malaysia.
Type-specimens: Holotype: female, accession number OSAL 0119286 (Fig. 2), stored in absolute ethanol, field sample code: cvb757macro008, deposited at OSAL (URL: https://acarology.osu.edu/database). Paratypes: on type host and from type locality: female, OSAL 0100050, 95% ethanol, cvb800macro002; male, OSAL 0106708, slide, dissected; on type host but from MALAYSIA: Pahang, Bukit Rengit (3.596 N 102.180 E, 72 m), female, OSAL 0103942, slide; female, OSAL 0103943, slide; female, OSAL 0103944, slide. All paratypes deposited at OSAL. Other specimens: all on type host from type locality: female, OSAL 0119279, 95% ethanol, cvb757macro001; 5 deutonymphs, OSAL 0119280-281, 0119283-284, 0119290, 95% ethanol; 5 deutonymphs, OSAL 0102594, 0102596, 0106709-711, slide; 1 protonymph, OSAL 0119282, 95% ethanol.
Figure 2: Macrodinychus (Monomacrodinychus) hilpertae holotype.
(A)Dorsal, (B) lateral, and (C) ventral view of the Macrodinychus hilpertae holotype. Scale bars are 250 µm.ZooBank registration: Details of the new species have been submitted to ZooBank to comply with the current regulation of the ICZN. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:84ADDB13-56F3-431D-9244-E19C3A2F7E04. The LSID for the new name Macrodinychus (Monomacrodinychus) hilpertae is urn:lsid:zoobank.org:act:88FADEC7-D4A5-4491-A45E-8F8176B65D31.
Etymology: Dedicated to Andrea Hilpert, for her advice, long lasting skillful technical assistance and support of AB and CvB.
Description: General: Length of the idiosoma 2,100 µm, width 1,250 µm (holotype). Shape oblong, posterior margin rounded, color darkish brown.
Dorsal (Fig. 2A): Dorsal shield rough with micro-ornamentation and an alveolar pattern. Completely sclerotized, middle part of the dorsal shield pronounced in a characteristic shape (also in lateral view). Dorsum hypertrichous. Dorsal shield covered by distinct and regularly distributed bulbiform setae. Setae covered with additional hairs on their margins. Dorsal and marginal shield not fused anteriorly. Tips of marginal shield not fused anteriorly. Marginal shield with a crenellation-like pattern of alveolae and ridges. Isolated pygidial shield with alveolar patterns, but without setae.
Ventral (Fig. 2C): Fused sternal and ventral shields bear an alveolar pattern with further micro-ornamentation on the rest of the cuticle. Female operculum between coxae II-III, length 188 µm, male operculum round, between coxae III, length 102 µm. Genital shields in both sexes without ornamentation. Scabellum covered by fish scale-like pattern. All ventral setae bulbiform. Position of sternal setae (St): St 1 and St 2 placed between coxae I and II. St 3 inserted near the posterior margin of coxae II. Setation around the genital shield hypertrichous. An additional row of four pair of setae at the posterior margin of the operculum. Stigmata between coxae II and III, peritreme species-specific with finger-like branches (see Fig. S1C).
Gnathosoma: Gnathotectum triangular, extending in single peak with large barbs (length 190 µm). Salivary stylets (105 µm) long relative to gnathosoma. Subcapitulum: Corniculi peanut-shaped, blunt, length 40 µm, width 18 µm (N = 2). Hypostomal setae long (h1 59–62, h2 35–38, h3 41–55 µm), setiform, barbed; subcapitular (sc) setae rod-shaped, barbed (29–39 µm) (generally similar in arrangement and shape as in M. derbyensis, Fig. S1A, but setae shorter). Deutosternum poorly developed with 3 rows of 2 teeth each. Base of the tritosternum cylindrical, vase-like, with a smooth surface. Tritosternum trifurcate, laciniae with fine bristles (Fig. S1D). Chelicera with a distinct “nodus”, and lacking a membranous extension on the fixed digit; distal end of fixed and movable digit with a small tooth, creating a bifid impression (Fig. S1E); moveable digit 70–76 µm, fixed digit 87 µm, entire fixed segment 280 µm (maximum width 41 µm), basal segment 232 µm (N = 2). Palp length 245 µm, width 42–49 µm (N = 2); tibia and tarsus fused, pretarsus in form of 4-tined apotele. Setation trochanter 2, femur 5, genu 4 setae (tibiotarsus not studied); trochanteral seta v1 long, rod-shaped with increasing density of barbs towards tip (190 µm), v2 setiform, substantially shorter, with much shorter barbs.
Legs: Legs short relative to body: leg I 1,050 µm, leg II 837 µm, leg III 859 µm, leg IV 950 µm. Note that the measurements provided here are better treated as approximations as the legs were folded into the pedofossae during measurements. Femora I–IV with small posterior flange. Leg setae long and barbed. Chaetotaxy (leg setation formula’s following Evans, 1963, Evans, 1972): coxae 2-2-2-1; trochanters 4-5-5-5; femora 1 4/3 1, 1 4/2 1, 1 3/2 0, 1 3/2 0; genua 1 2/1 2/1 1, 1 2/1 2/1 1, 1 2/1 2/0 1, 1 2/1 2/0 1; tibiae 1 1/1 2/1 1, 1 1/1 2/1 1, 1 1/1 2/1 1, 1 1/1 2/1 1, tarsi II–IV 18 setae. Dorsal setae, especially on tibiae and genua, much shorter than lateral or ventral setae (measurements from single individual): on leg I tibia, respectively, 37 µm, 111 µm and 219 µm, leg II 45 µm, 75 µm, 123 µm; on genu leg I, respectively, 37 µm, 81 µm, 134 µm, leg II 50 µm, 74 µm, 93 µm. Setae ad4 and al4 on basitarsus IV long, well-developed, pd4 and pl4 much shorter and less barbed. Similar difference, though less pronounced, on other segments. Pretarsi of legs I small with a well-developed claw. Pretarsi of legs II –IV with long stalk, small pulvillus, and two claws.
Immatures: Larvae unknown. One protonymph, not studied. Deutonymphs weakly sclerotized. Dorsal cuticle distinctly ornamented, mid-dorsal setae distinctly shorter than marginal dorsal setae. Ventral setae generally short (30–40 µm), longer near the body margin. All sternal setae relatively short, St4 also short relative to St1-3 (26 µm vs. 70 µm, N = 2). Gnathosoma unclear in all available specimens. Chelicera with reduced, though distinct, fixed digit (∼1/2 as long as movable digit). Leg chaetotaxy as in adults except for femur I which carried 2 ventral setae (three ventral setae in adults); tibia IV lacking seta pd1. Legs I with small ambulacrum carrying claws.
Differential diagnosis: Within the genus Macrodinychus, this species can be distinguished from most others by its bulbiform setae. This character is only shared with M. extremicus (Kontschán, 2011), for which it was mistakenly identified in a previous publication (Lachaud, Klompen & Pérez-Lachaud, 2016). However, M. hilpertae can be unambiguously discriminated from M. extremicus by the following characters (for images of the M. extremicus holotype see Fig. S2): most prominently, species differ in the shape of their peritremes and the lateral shape of the dorsal shield (also clearly visible from the dorsal view as ring-like cavities). While M. extremicus has an undulating lateral shape of the dorsal shield with three mounds (Fig. S2), M. hilpertae possess just one mound without any subdivision (compare Fig. 2A and Fig. S2A). In addition, M. hilpertae has a highly structured micro-ornamentation on the dorsal shield in contrast to M. extremicus (Figs. 2A and 2B). Alveolae on dorsal shield are bigger in M. hilpertae. Bulbiform setae are slenderer in M. hilpertae and distributed more evenly on the dorsal shield, while the setae are flap-like in M. extremicus, and are condensed at certain areas of the dorsal shield (Fig. S2), and are sometimes overlapping.
The nymphs of M. hilpertae differ substantially from those previously described from M. (Macrodinychus) sellnicki or M. (Bregetovamacrodinychus) multispinosus. In both of those species, nymphs have highly regressed idiosomal and leg setation, lacking nearly all idiosomal and non-tarsal leg setae. They also show highly reduced chelicera with fixed digits completely absent (Krantz, Gómez & González, 2007; H Klompen, pers. obs., 2017).
Macrodinychus (Monomacrodinychus) derbyensis Brückner, Klompen & von Beeren sp. nov.
Type-host: Leptogenys distinguenda (Formicidae: Ponerinae)
Type-locality: Ulu Gombak Field Studies Centre of the University Malaya (03°19.479′N, 101°45.163′E, altitude 230 m), Selangor, Malaysia.
Type-specimens: Holotype: female, accession number OSAL 0119286 (Fig. 3), stored in absolute ethanol, field sample code: cvb757macro009, deposited at OSAL. Paratypes: On type host from type locality: male, OSAL 0106707, slide dissected; female, OSAL 0119292, cvb800macro001. Other specimens. On type host from type locality: three deutonymphs, OSAL 0119285, 0119288-289, 95% ethanol; four deutonymphs, OSAL 0102593, 0103953-955, slide; 1 protonymph, OSAL 0119291, 95% ethanol, cvb757macro013; 1 protonymph, OSAL 0102595, slide. All specimens deposited at OSAL.
Figure 3: Macrodinychus (Monomacrodinychus) derbyensis holotype.
(A) Dorsal, (B) lateral, and (C) ventral view of the Macrodinychus derbyensis holotype. Scale bars are 325 µm.ZooBank registration: Details of the new species have been submitted to ZooBank to comply with the current regulation of the ICZN. The Life Science Identifier (LSID) of the article is urn:lsid:zoobank.org:pub:84ADDB13-56F3-431D-9244-E19C3A2F7E04. The LSID for the new name Macrodinychus (Monomacrodinychus) derbyensis is urn:lsid:zoobank.org:act:83C74026-EF1F-41A8-915F-49B0B2188E2A.
Etymology: The name of the new species refers to the fact that both new species (M. hilpertae and M. derbyensis) co-occur in the same host species. We further picked this name to honor one of the most diversity-loving, integrative and awesome sports—roller derby—with all its players, flamingos, zebras and enthusiastic supporters.
Description: General: Length of the idiosoma 2,370 µm, width 1,480 µm (holotype). Shape oblong, anterior and posterior margins rounded, color ocher-brown/ brown.
Dorsal (Fig. 3A): Dorsal shield completely sclerotized and smooth without ornamentation, but with deep alveolae in the middle part of the dorsal shield. Dorsal shield covered by distinct and regularly distributed smooth, needle-like setae. Setation hypertrichous. Dorsal and marginal shield not fused anteriorly. Tips of marginal shield not clearly distinct anteriorly and sub-marginal shield apically fused with marginal shield. Marginal shield with a crenellation-like pattern posterior, no pattern medial and an alveolar pattern anterior. Isolated pygidial shield with eight setae and deep pits.
Ventral (Fig. 3C): Ventral shield posterior smooth without ornamentation and covered with setae, but ventral shield anteriorly covered with a fine structured micro-alveolar pattern without setae (starting at the peritremes). Sternal and ventral shield fused. Cuticle posterior to genital shield between coxae III and IV with deep pit-like ornamentation. Female operculum between coxae II-III, length 196 µm, male operculum round, between coxae III, length 140 µm. Genital shields in both sexes without ornamentation. Scabellum covered by a fine ornamentation. All ventral setae smooth and needle-like. Only two pairs of sternal setae (St1 and St2) clearly distinct from a row of setae along the endopodal line, and rows of additional pairs of setae at the posterior margin of the operculum. Position of sternal setae (St): St 1 placed between coxae I and II. St 2 inserted near the anterior margin of coxae II. Stigmata between coxae II and III, peritreme species-specific with finger-like branches (Fig. 3B; Fig. S1B).
Gnathosoma: Gnathotectum triangular, extending in single peak with strong barbs (length 254 µm). Salivary stylets thick and long (236 µm). Subcapitulum (Fig. S1A): Corniculi peanut-shaped, blunt, length 61 µm, width 22 µm (N = 2). Hypostomal setae long (h1 101–108 µm, h2 58–61 µm, h3 91 µm), setiform, barbed, subcapitular setae shorter (46–48 µm), rod-shaped, barbed. Deutosternum poorly developed with three rows of two teeth each. Chelicera with “nodus”, lacking membranous extensions on fixed digit; movable digit 82 µm, fixed digit 102 µm, entire fixed segment 390 µm (maximum width 46 µm), basal segment 390 µm. Palp length 310 µm, width 42–47 µm (N = 2); tibia and tarsus fused, pretarsus in form of 4-tined apotele. Setation trochanter 2, femur 5, genu 4 setae (tibiotarsus not studied); trochanteral seta v1 rod-shaped with increasing density of long barbs towards tip (length 124 µm), v2 setiform, substantially shorter.
Legs: Legs short relative to body: leg I 1,184 µm, leg II 1,119 µm, legs III–IV 1,130 µm (N = 1). Femora I–IV with small posterior flange. Setal shape and chaetotaxy as in M. hilpertae. Dorsal setae on tibiae and genua much shorter than lateral or ventral setae (measurements from single individual): on leg I tibia, respectively, 59 µm, 192 µm and 365 µm, leg II 45 µm, 75 µm, 123 µm; on genu leg I, respectively, 34 µm, 85 µm, 169 µm, leg II 77 µm, broken, 154 µm. Differentiation of setae al4 and ad4 vs. pd4 and pl4 on basitarsus IV distinct, but less so than in M. hilpertae. Pretarsi of legs I small with a single claw. Pretarsi of legs II–IV each with a long stalk, well-developed pulvillus including a pair of setiform structures, and two claws. Pulvillus distinctly larger than in M. hilpertae.
Immatures: Larvae unknown. Two protonymphs, not studied. Deutonymphs weakly sclerotized. Dorsal cuticle without distinct ornamentation, mid-dorsal setae as long as marginal dorsal setae. Ventral setae fewer than in M. hilpertae, but longer (80–95 µm). Sternal setae long St4 about 2/3 the length of St1-3 (81 µm vs. 128 µm, N = 3). Gnathosoma well developed compared to other American macrodinychid species. Chaetotaxy as in adults: h1 41 µm, h2 67 µm, h3 91 µm, sc 74 µm (N = 1), corniculi peanut–shaped, 28 × 15 µm. Chelicera shorter than in adult, fixed digit reduced, but distinct (∼1/2 length of movable digit). Palps weakly developed, palp apotele present, 3–4-tined. Leg chaetotaxy as in adults but femur I with only two, rather than three, ventral setae; ventral setae slightly longer than dorsal ones. Legs I with small ambulacrum carrying claws.
Differential diagnosis: Within the genus Macrodinychus, this species can be distinguished from most others by its isolated pygidial shield bearing smooth and needle-like setae. This character is only shared with M. vietnamensis (Hirschmann, 1983), which is the morphologically closest relative. The holotype of M. vietnamensis is lost and not deposited at the Natural History Museum in Budapest, Hungary, as stated in the formal description of the species (Hirschmann, 1983). However, we found a slide-mounted specimen designated by Hirschmann as M. vietnamensis at the Bavarian State Museum of Zoology. We used this specimen for comparisons (see Fig. S3). The following characters can be used to discriminate the species: the cuticle of M. derbyensis posterior to genital shield between coxae III and IV possess a deep pit-like ornamentation (Fig. 3C), a character which is absent in M. vietnamensis. Furthermore, the sub-marginal shield of M. derbyensis is apically fused with the marginal shield, while the sub-marginal shield is apically distinct from the dorsal shield and the marginal shield in M. vietnamensis. In addition, M. derbyensis has rows of setae along the endopodal line and additional rows of paired setae at the posterior margin of the operculum, while M. vietnamensis has only five pairs of sternal setae.
Descriptions of immature morphology of Macrodinychus are hitherto really limited. Comparing the two species described here, the deutonymphs of M. derbyensis differs from those of M. hilpertae in relative length of dorsal and ventral setae (especially relative lengths of St4 vs. St1-3), lack of ornamentation of the dorsum, and presence (vs. absence) of seta pd1 on tibia IV.
Key to species of the subgenus Monomacrodinychus (updated from Kontschán, 2011 and Kontschán, 2017)
To aid in differentiation of the new species from previously described species, we updated the key to species in the subgenus Monomacrodinychus (Kontschán, 2011; Kontschán, 2017):
| 1. Peritreme without branches | other subgenera (see Kontschán, 2011) |
| Peritreme with finger-like branches | 2 |
| 2. Isolated pygidial shield absent | M. multipennus |
| Isolated pygidial shield present | 3 |
| 3. Dorsal and ventral shields with bulbiform setae | 4 |
| Dorsal and ventral shields without bulbiform setae | 5 |
| 4. Dorsal shield with rough alveolae without microstructural reticular ornamentation, bulbiform seate big and flap-like, two half ring-form cavities in the central region of the dorsal shield | M. extremicus |
| Dorsal shield alveolae with microstructural reticular ornamentation, bulbiform setae smaller and distinct from each other, half ring-form cavities less pronounced | M. hilpertae |
| 5. Dorsal setae smooth | 6 |
| Dorsal setae with hairs on their margins | 9 |
| 6 Isolated pygidial shield with setae | 8 |
| Isolated pygidial shield without setae | 7 |
| 7. Pygidal shield narrow, anterior horns absent | M. kaszabi |
| Pygdial shield hemispherical, anterior horn present | M. tanduk |
| 8. No rows of setae along endopodal line, five pairs of sternal setae, cuticle posterior to genital shield between coxae III and IV without ornamentation, sub-marginal shield apically distinct form dorsal shield and marginal shield | M. vietnamensis |
| Rows of setae along the endopodal line, additional rows of paired setae at the posterior margin of the operculum, only St1 and St2 clearly distinguishable, cuticle posterior to genital shield between coxae III and IV with deep pit-like ornamentation, sub-marginal shield apically fused with marginal shield | M. derbyensis |
| 9. Isolated pygidial shield with setae | M. shibai |
| Isolated pygidial shield without setae | 10 |
| 10. Apical part of dorsal setae wide and bear short hairs | M. yoshidai |
| Dorsal setae needle-like with hairs on their margins | 11 |
| 11 Alveolar ornamentation on the lateral part of the dorsal shield, genital shield of female with alveolar pattern | M. baloghi |
| Alveolar ornamentation on the whole dorsal shield, genital shield of female without pattern | M. hirschmanni |
Results—Life History Section
Host infection rate
Out of 2,360 inspected L. distinguenda pupae 40 were infected with one of the two Macrodinychus species, i.e., the pupal infection rate at Ulu Gombak was 1.69%. Each pupa was only infected by a single Macrodinychus specimen. The inspection of host pupae from a single colony in 2009 demonstrated that Macrodinychus species can co-occur in the same colony (M. hilpertae = 2 infected pupae; M. derbyensis = 4 infected pupae).
The pupal number per colony was estimated for three different L. distinguenda colonies: 6,456 pupae, 5,845 pupae, and 9,846 pupae. With an infection rate of 1.69%, the total number of pupal infection per colony was estimated to be 109, 99, and 166, respectively.
Life-history of M. hilpertae and M. derbyensis
The dissection of 2,360 ant pupae recovered 20 immature and 20 adult mite stages. The following two immature development stages were found: three protonymphs (M. derbyensis, N = 2; M. hilpertae, N = 1) and 17 deutonymphs (M. derbyensis, N = 7; M. hilpertae, N = 10). All parasitized ant pupae had small, brownish scars (Fig. 4), which were not present in unparasitized ant pupae. Adult mites and deutonymphs that were detached from ant pupae left behind a conspicuous abnormal cavity in pupal bodies (Fig. 4). We did not find Macrodinychus larvae. However, we detected larval exuviae of three M. hilpertae individuals (Fig. 4). Exuviae of proto- and deutonymph were frequently detected inside pupal cocoons, often still attached to the mite or to the ant specimen (e.g., see BOLD image of sample ‘cvb757macro002’).
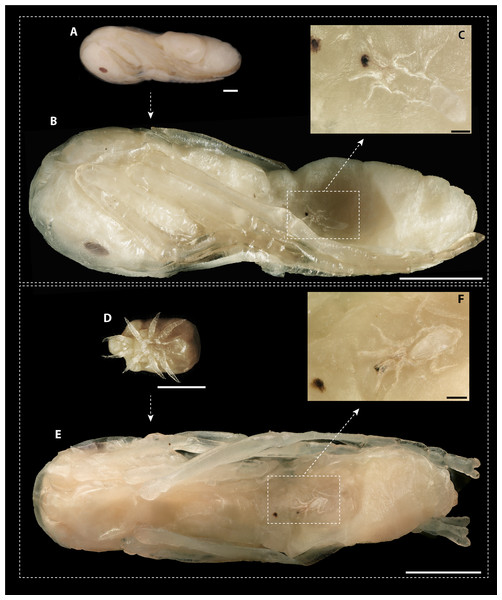
Figure 4: Macrodinychus hilpertae attached to ant pupae.
(A, D) Deutonymphs of M. hilpertae (A) attached to and (D) detached from developing ant pupae (silk cocoon removed). (B, E) Respective ant specimens with mites removed exposing the abnormal intrusion in the ants’ gasters and the brownish scars. Larval exuviae of M. hilpertae are still sticking to the ants (dashed square). (C, F) Enlarged view of the larval exuviae. The cheliceral cuticles are still sticking to the ant‘s wound. Scale bars are 1 mm except for images c and f where it is 0.1 mm.Observations in laboratory nests
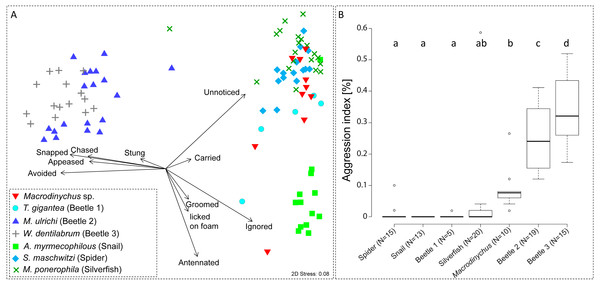
Myrmecophile species differed in their behavioral interactions with host ants (PERMANOVA, pseudo-F = 37.27, P < 0.001; Fig. 5A). Macrodinychus specimens generally walked slowly in the laboratory nests among host workers, which primarily did not notice (mean ± SD: 24 ± 10 events, N = 10) or ignored the mites (mean ± SD: 18 ± 8 events, N = 10; Fig. 5). Out of ten behavioral tests, mites were picked up in nine cases by ant workers (see Video S1). This interaction was initiated by intense antennation by an approaching ant (mean ± SD: 4 ± 4 events, N = 10). Ants then carried around the mites (mean ± SD: 1 ± 0 events, N = 10; Table S2), an interaction uniquely found in Macrodinychus mites and in the snail Allopeas myrmekophilos (Witte et al., 2002). We observed that ants often dumped the mites at the ants’ refuse site (see Video S1).
Figure 5: Ant-symbiont interactions and ant aggression towards symbionts.
(A) Nonmetric-multidimensional scaling (NMDS) plot visualizes the differences in behavioral interactions between host ants and seven symbiont species. Each data point represents approx. 50 encounters of an individual symbiont with host ants. Length and direction of arrows visualize the contribution of behavioral categories to data separation. For clarity, the origin of arrows is not centered in the plot. “Stress” quality measure of the NMDS. (B) Aggressive behaviors of ants towards symbionts. Depicted is the aggression index (AI), which is the proportion of aggressive behaviors (sum of chased, snapped, and stung) towards symbiont specimens relative to their total number of interactions (approx. 50 for all specimens). Different letters depict significant differences (p < 0.05; PERMANOVA pairwise tests).Ant aggression towards separate myrmecophile species differed (PERMANOVA, pseudo-F = 15.00, P < 0.001; Fig. 5B). Compared to other myrmecophiles of L. distinguenda, ants attacked Macrodinychus mites at a moderate level (mean (AI) ± SD: 0.08 ± 0.07, N = 10; Fig. 5B). During the ∼50 encounters with host ants, ants chased (mean ± SD: 0.10 ± 0.32 events, N = 10), snapped (mean ± SD: 2.40 ± 1.78 events, N = 10) and tried to sting the mites (mean ± SD: 2.0 ± 2.05 events, N = 10). All Macrodinychus specimens survived the ∼50 encounters with host ants.
Discussion
Life-history of macrodinychid mites
Adopting Kistner’s metaphor of a social insect bestiary, the two herein described Macrodinychus parasitoids are extraordinary examples of specialized beasts invading ant colonies. Both species fulfill their immature development inside army ant colonies, which constitutes a stable and predator-free space with sufficient supply of food (Kistner, 1979; Hölldobler & Wilson, 1990; Hughes, Pierce & Boomsma, 2008). More specifically, Macrodinychus immatures were attached to and most likely fed on defenseless ant pupae.
While we do not provide direct evidence here that M. hilpertae and M. derbyensis were feeding on the host’s hemolymph or tissue, this seems to be the most parsimonious explanation to us. First, parasitized pupae possessed scars which can be interpreted as feedings marks. We consider it most likely that scars represent areas where mites used their chelicera to pinch the ant’s cuticle in order to feed on host tissue and/or drink from the excreting hemolymph. Second, we found exuviae of different development stages inside individual pupal cocoons, indicating that the mites grew by feeding on the ant pupae. Consumption of host tissue/hemolymph is also indicated by the fact that detached mites left behind physical impressions constituting substantial parts of the ants’ gasters.
In ant-associated Mesostigmata, parasitoidism has only been described in the genus Macrodinychus (Lachaud, Klompen & Pérez-Lachaud, 2016). The five more extensively studied Marodinychus species (including M. hilpertae and M. derbyensis) seem to share the following key life-history traits (González, Gómez & Mesa, 2004; Breton, Takaku & Tsuji, 2006; Krantz, Gómez & González, 2007; Lachaud, Klompen & Pérez-Lachaud, 2016): all species seem to fulfill their entire immature development, including larval, proto- and deutonymphal stage, by feeding on individual ant pupa. For this, they seem to pierce the pupal cuticle with their chelicera to consume host tissue and/or to suck host hemolymph leaving behind small, brownish feeding marks. The larvae have well-developed legs and hence seem to be the mobile instar to find suitable hosts, while proto- and deutonymphs are more likely immobile feeding instars. Macrodinychus adults finally occupy a substantial part of the pupa’s body. Once removed from the ant, they leave behind a conspicuous cavity, providing visual evidence for a lethal feeding strategy.
Parasitism rates of macrodinychid mites—native versus invasive host ants
Besides similarities among species, we also detected a notable difference between the Macrodinychus species studied previously and those studied here. The prevalence of infection, measured as the percentage of infected to non-infected host pupae, was markedly lower in M. hilpertae and M. derbyensis (approx. 2% vs. 15%–90% in other Macrodinychus species; see González, Gómez & Mesa, 2004; Breton, Takaku & Tsuji, 2006; Krantz, Gómez & González, 2007; Lachaud, Klompen & Pérez-Lachaud, 2016). Various explanations could be responsible for the vastly different parasitism rates among studied macrodinychid mites. For example, the particular sampling methods or seasonal and spatial differences in parasitoid prevalence could conceivably cause such variation. Another possible cause is that Macrodinychus hilpertae and M. derbyensis have been studied in a native host-parasitoid system, while other Macrodinychus spp. have exclusively been studied in association with invasive ant species. Parasitoids are often a major source of host mortality and intense selection on the host to evolve counter-defenses against parasitoid attacks can be expected (Godfray, 1994). In species drifts across continents, however, local and alien interaction partners have no coevolutionary history (Thompson, 2005; Simberloff, 2013). In such situations, naïve hosts can suffer from extremely high parasitism rates (Kirk, 2003; Prenter et al., 2004; Lymbery et al., 2014), which can, in extreme cases, lead to the decline of local host populations (Holdich & Reeve, 1991). Invasive ants, in particular, might be predisposed to parasitism by local species due to mass propagation coupled with genetic depletion (Sakai et al., 2001; Holway et al., 2002; Tsutsui & Suarez, 2003; Lester & Gruber, 2016). In fact, a recent host switch of local Macrodinychus parasitoids to invasive ants has been suggested to be responsible for the high parasitism rates found in M. yonakuniensis (15%) (Breton, Takaku & Tsuji, 2006), M. multispinosus (26%) (Lachaud, Klompen & Pérez-Lachaud, 2016) and M. sellnicki (up to 90%) (González, Gómez & Mesa, 2004; Krantz, Gómez & González, 2007).
Possible counter-adaptations against macrodinychid mites
Hidden inside the pupal silk cocoons, the immature mites studied here are practically invisible to adult host workers. In contrast, once eclosed from the pupal cocoon, adult mites are exposed and thus are accessible for host inspection. Similar to socially integrated species such as the spider Sicariomorpha maschwitzi (Witte et al., 2009; von Beeren, Hashim & Witte, 2012) and the silverfish Malayatelura ponerophila (Witte et al., 2009; von Beeren et al., 2011b), adult Macrodinychus spp. were mostly ignored or unnoticed by host ants. Nonetheless, host workers regularly antennated adult parasitoids and ultimately attacked them, although at a relatively low level. Low levels of aggression towards myrmecophiles are still biologically relevant. For instance, soft bodied myrmecophiles such as the silverfish M. ponerophila were occasionally killed in behavioral assays (Witte et al., 2008; von Beeren et al., 2011b). We interpret the occasional attacks towards Macrodinychus mites as a behavior to fight off the adult parasitoid before host brood become infected with parasitoid larvae. However, Macrodinychus specimens survived these attacks unscathed owing to their protective morphology, embodied by a well-sclerotized cuticle and the possibility to retract all extremities into cuticular cavities (pedofossae) (see Figs. 2 and 3). A more efficient host defense might be the ants’ behavior following the initial attacks. Macrodinychus spp. were often picked up by workers in laboratory nests and dumped at the ants’ refuse site, outside the inner nest part where the parasitoid target, i.e., ant brood, is located. The adult mites were mobile and regularly re-entered the brood chambers in laboratory nests, only to be picked up and dumped at the refuse site again. In addition to this, the frequent emigrations of army ants might represent another counter-measurement to reduce a colony’s total fitness loss imposed by parasites and parasitoids (Witte et al., 2008; von Beeren et al., 2011a) because parasites/parasitoids can be left behind at the abandoned nest site (Witte, 2001). Support for this hypothesis comes from an observation during a nest emigration of Leptogenys distinguenda initiated in the laboratory at the field site. We collected three Macrodinychus spp. adults at the abandoned nest site (a 1 m ×1 m ×1 m plastic box filled with leaf litter), in other words, the emigrated colony shed off these parasitoids.
Conclusions
Parasitoidism and also parasitism of ants by mites is likely more common than hitherto known (Campbell, Klompen & Crist, 2013; Lachaud, Klompen & Pérez-Lachaud, 2016) and the cryptic lifestyles of mites inside ant nests has certainly hampered their discovery (Skoracka et al., 2015). In fact, the species studied here were chance finds that were initially overlooked. It is safe to say that many more macrodinychid mites await scientific discovery (see e.g., Lachaud, Klompen & Pérez-Lachaud, 2016) and we thus would like to encourage researchers to specifically screen ant brood for these fascinating and rather unexplored parasitoids.
Supplemental Information
Supplemental Material
Figure S1. (A) Subcapitulum of M. derbyensis (F, OSAL 0106707). Peritrema of (B) M. derbyensis (OSAL 0119286) and (C) M. hilpertae (F, OSAL 0119286). (D) Tritosternum and (E) chelicera of M. hilpertae (F, OSAL0106708). Scale bars are 100 µm.
Figure S2. Macrodinychus extremicus holotype.
Figure S3. Museum voucher of Macrodinychus vietnamensis.
Table S1. Definition of behavioral interactions.