DOC concentrations across a depth-dependent light gradient on a Caribbean coral reef
- Published
- Accepted
- Received
- Academic Editor
- Joseph Pawlik
- Subject Areas
- Biochemistry, Biological Oceanography, Ecology, Environmental Sciences, Marine Biology
- Keywords
- Dissolved organic carbon, Coral reef, Depth gradient, Light, Coral, Algae, Photoinhibition
- Copyright
- © 2017 Mueller et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. DOC concentrations across a depth-dependent light gradient on a Caribbean coral reef. PeerJ 5:e3456 https://doi.org/10.7717/peerj.3456
Abstract
Photosynthates released by benthic primary producers (BPP), such as reef algae and scleractinian corals, fuel the dissolved organic carbon (DOC) production on tropical coral reefs. DOC concentrations near BPP have repeatedly been observed to be elevated compared to those in the surrounding water column. As the DOC release of BPP increases with increasing light availability, elevated DOC concentrations near them will, in part, also depend on light availability. Consequently, DOC concentrations are likely to be higher on the shallow, well-lit reef terrace than in deeper sections on the fore reef slope. We measured in situ DOC concentrations and light intensity in close proximity to the reef alga Dictyota sp. and the scleractinian coral Orbicella faveolata along a depth-dependent light gradient from 5 to 20 m depth and compared these to background concentrations in the water column. At 10 m (intermediate light), DOC concentrations near Dictyota sp. were elevated by 15 µmol C L−1 compared to background concentrations in the water column, but not at 5 and 20 m (high and low light, respectively), or near O. faveolata at any of the tested depths. DOC concentrations did not differ between depths and thereby light environments for any of the tested water types. However, water type and depth appear to jointly affect in situ DOC concentrations across the tested depth-dependent light gradient. Corroborative ex situ measurements of excitation pressure on photosystem II suggest that photoinhibition in Dictyota sp. is likely to occur at light intensities that are commonly present on Curaçaoan coral reefs under high light levels at 5 m depth during midday. Photoinhibition may have thereby reduced the DOC release of Dictyota sp. and DOC concentrations in its close proximity. Our results indicate that the occurrence of elevated DOC concentrations did not follow a natural light gradient across depth. Instead, a combination of multiple factors, such as water type, light availability (including the restriction by photoinhibition), and water movement are proposed to interactively determine the DOC concentrations in the close vicinity of BPP.
Introduction
Dissolved organic carbon (DOC) is the largest pool of reduced carbon on tropical coral reefs (Atkinson & Falter, 2003). A lack of a relationship between particulate organic carbon (POC, as proxy for planktonic primary producers) and DOC concentrations (Tanaka et al., 2011), and increased DOC concentrations near the bottom compared to the surface water (Van Duyl & Gast, 2001) indicate that benthic primary producers (BPP) are an important source of this DOC. Reef algae and scleractinian corals release a substantial portion of their photosynthetically fixed carbon as DOC into the surrounding water; reef algae generally release more DOC than corals (e.g., Haas et al., 2011; Haas et al., 2013b). This algal-derived DOC can promote the growth of opportunistic heterotrophic microbes in the water column as well as in the contact zone between corals and algae (Haas et al., 2013a; Haas et al., 2013b; Nelson et al., 2013). Increased microbial respiration in the coral-algal interface causes anoxia (Gregg et al., 2013; Haas et al., 2013a) in combination with the release of secondary metabolites, and can lead to tissue loss or even coral death (Barott & Rohwer, 2012; Morrow et al., 2013). Moreover, while most heterotrophic macroorganisms cannot utilize DOC for their nutrition an increasing number of reef sponges are found to predominantly rely on DOC as carbon source (Yahel et al., 2003; De Goeij et al., 2008; Mueller et al., 2014a). As with microbes, sponges also appear to prefer algal- over coral-derived DOC (Rix et al., 2016). In the so-called sponge loop, these sponges utilize the energy stored in DOC and make it available to higher trophic levels via subsequent detritus production (Alexander et al., 2014; De Goeij et al., 2013). Both heterotrophic microbes and DOC-feeding sponges are therefore likely to benefit from elevated DOC concentrations with potential consequences for carbon cycling and overall coral reef functioning (e.g., Rohwer & Youle, 2010; Barott & Rohwer, 2012; De Goeij et al., 2013; Haas et al., 2016).
Elevated DOC concentrations in close proximity to BPP have been repeatedly observed on tropical coral reefs (Van Duyl & Gast, 2001; Hauri et al., 2010; Mueller et al., 2014b). However, most studies were conducted in shallow reef areas between 5 and 10 m and little attention was given to deeper reef sections or how DOC concentrations change across depth. Light availability decreases exponentially with depth and is an important environmental parameter that structures benthic communities across the reef slope (e.g., Bak, 1974; Veron, 2000; Vermeij & Bak, 2002). Light availability positively affects the DOC release rates of BPP (Crossland, 1987; Haas et al., 2010b; Naumann et al., 2010; Barrón, Apostolaki & Duarte, 2014 and references therein). The occurrence of elevated DOC concentrations near BPP were found to be positively correlated with the availability of light (Mueller et al., 2014b). We therefore hypothesize that DOC concentrations change with depth and that elevated DOC concentrations near BPP are more likely to occur on the shallow, well-lit reef terrace (5 m) than at the drop off (10 m) or in deeper sections of the fore reef slope (20 m). To test this we measured in situ DOC concentrations and light intensity in close proximity to the reef alga Dictyota sp. and the scleractinian coral Orbicella faveolata (former Montastraea annularis) along a depth-dependent light gradient from 5 to 20 m depth and compared these to background concentrations in the water column.
Materials and Methods
Fieldwork was performed under the research permit (#2012/48584) issued by the Curaçaoan Ministry of Health, Environment and Nature (GMN) to the CARMABI foundation.
Study site and general environmental parameters
Sampling was conducted at Snake Bay (12°8′N, 68°59′W) on the leeward coast of the Island of Curaçao in the Southern Caribbean. The site consists of an approximately 100 m wide sandy reef terrace with patchy coral communities. The reef terrace gradually slopes towards a drop-off that starts at around 10 m depth. The reef then slopes down under a steep angle (20–30°; Van Duyl (1985)) and is characterized by a structurally complex reef topography. The hydrodynamics at the study site are mainly dominated by oceanic currents, which generally flows westwards along the island with approx. 50 cm s−1 (Gast et al., 1999). Due to small tidal differences (10–30 cm), tidal currents are usually neglectable on the narrow Curaçaoan fringing reefs and water currents over the reef terraces are typically around 10–15 cm s−1.
Benthic composition was determined from 20 photo quadrats (1 × 1 m) placed at randomized distances and alternatingly on both sides along a 30 m transect line. On March 24, 2017 the benthic cover following the 5, 10, and 20 m isobaths was recorded in the area where the DOC concentrations were quantified before (see DOC concentrations across a depth-dependent light gradient). Percentage cover of most dominant benthic components was quantified from 40 randomly-generated overlaid points on each photograph using Coral Point Count with Excel Extensions (CPCe) (Kohler & Gill, 2006). Two photographs of the 5 m transect and five of the 20 m transect had to be excluded from be analysis due to insufficient quality.
In June, 2015 water samples to access bacterial concentrations in the water column 2 m off the reef slope (towards the open ocean) were taken at 15 m depth, following the protocol described in Dinsdale et al. (2008) to describe background bacterial abundances. Water samples (n = 5) were transported to the nearby CARMABI research station where they were processed and analyzed (for details see Supplemental Information 1).
DOC concentrations across a depth-dependent light gradient
To quantify DOC concentrations across a depth-dependent light gradient, water samples were taken in situ in close proximity (<5 mm) to the reef alga Dictyota sp., the scleractinian coral O. faveolata, and the water column. Both, Dictyota sp. and O. faveolata are considered holobionts, including epi- and endophytes and associated microbial communities (sensu Barott et al., 2011), jointly affecting the water properties (e.g., DOC concentration) in their close vicinities. At midday on July 24, 2012 between 12:00 hrs and 13:00 hrs (when light intensities are the highest) patches of Dictyota sp. and colonies of O. faveolata were sampled at 5 (reef flat; high light), 10 (drop-off; intermediate light) and 20 m depth (fore reef slope; low light) (each n = 5). In addition, the water column 2 m off the reef slope (towards the open ocean) was sampled (n = 5) at the same depths and used to indicate background DOC concentrations (i.e., those not directly affected by DOC release of BPP). Sampling started at 20 m depth and 10 and 5 m were sampled consecutively. At each depth approx. 10 min were spent to collect all samples. The sampling procedure described by Van Duyl & Gast (2001) and modified by Mueller et al. (2014b) was followed. In short, water samples were collected using 100 ml acid-washed, polypropylene syringes equipped with a flexible silicon tube attached to their tips. The tube was moved slowly above the surfaces of Dictyota sp. and O. faveolota, respectively, while collecting water (each n = 5). The water column was sampled using a similar syringes (n = 5). All water samples were collected facing the water current to avoid potential contamination related to the diver’s presence. Ambient light intensity (PAR) was recorded simultaneously while sampling (approx. 10 min; sampling intervals 1 min) using a light meter in a custom-made underwater housing (cosine LI-192SSA underwater quantum sensor connected to LI-1000 data logger; range: PAR 400-700). Water samples were transported (<30 min) to the lab and stored at 4 °C until they were processed later that same day.
Processing of DOC samples
Water samples collected were filtered (<20 kPa Hg suction pressure) over a 0.2 µm polycarbonate filter (25 mm; Whatman, Maidstone, UK). Prior to filtration, filters, glassware and pipette tips were rinsed three times with acid (10 mL 0.4 M HCl) and twice with sample water (10 mL). Afterwards, 20 mL of sample water was filtered and the filtrate containing DOC was transferred to pre-combusted (4 h at 450 °C) Epa vials (40 mL). Samples were acidified with 6–7 drops of concentrated HCl (38%) to remove inorganic C and stored at 4 °C until analysis. DOC concentrations were measured using the high-temperature catalytic oxidation (HTCO) technique in a total organic C analyzer (TOC-VCPN; Shimadzu, Kyoto, Japan). The instrument was calibrated with a standard addition curve of Potassium Hydrogen Phthalate (0; 25; 50; 100; 200 µmol C L−1). Consensus Reference Materials (CRM) provided by DA Hansell and W Chen of the University of Miami (Batch 12; 2012; 41–44 µmol C L−1) were used as positive controls for our measurements. Concentrations measured for the batch gave average values (±SD) of 45 ± 3 µmol C L−1. Average analytical variation of the instrument was <3% (5–7 injections per sample).
Maximum excitation pressure over photosystem II in Dictyota sp.
To explore the occurrence of photoinhibition as a potential explanation for the lack of elevated DOC concentrations observed near Dictyota sp. at 5 m depth , an ex situ experiment to determine maximum light-dependent reduction of the effective quantum yield of photosystem II (ΔF∕Fm′) relative to its maximum at dawn (Fv∕Fm) (Iglesias-Prieto et al., 2004; Enríquez & Borowitzka, 2010) was conducted. On March 16, 2017 30 thalli of Dictyota sp. were collected on the reef terrace (5 m depth) at Buoy 0 (12°12′35″N, 68°97′10″) and transported in a dark insulated box to the nearby CARMABI Research Station. The algae were allowed to recover and acclimatize in a flow-through seawater aquarium (flow rate: 7 L min−1 to ensure stable temperatures throughout the day) until the commencement of the experiment the following day. On March 17, 2017 at 6:00 hrs (sun rise time: 6:41 hrs; https://www.timeanddate.com/sun/curacao/willemstad?month=3year=2017) 10 thalli of Dictyota sp. were randomly selected from the aquarium and placed in a plastic dish with sea water. In the lab Fv∕Fm was measured in triplicates per thalli with a waterproof PAM fluorometer (Diving PAM, Waltz). After the measurements the thalli were discarded. Light intensity (PAR) and water temperature (°C) were recorded with a light logger (ODYSSEY PAR logger, Dataflow systems; sampling interval 1 min) and a temperature logger (Onset HOBO® Pendant, UA-002-08; sampling intervals 1 min), respectively. After sun set, light intensity gradually increased to approximately 200 µmol photons m−2 s−1 at 9:00 hrs (see Supplemental Information 1 for light and temperature data). Due to the positioning of the aquarium the light intensity remained stable until 12:00 hrs, when it steeply increased to an average (±SD) of 1,237 ± 486 µmol photons m−2 s−1 for the following three hours. At 15:00 hrs 10 new thalli of Dictyota sp. were randomly selected, placed in a plastic dish with sea water, and transferred to the lab. After a dark-adaptation period of 30 min to completely relax photochemical quenching, ΔF∕Fm′ was measured in triplicates per thalli.
Data analysis
Differences in DOC concentrations at the substrate-water-interface of Dictyota sp., O. faveolata and the water column from 5, 10 and 20 m were tested using one-way ANOVAs, followed by a Tukey HSD post-hoc test in case of significant differences. DOC concentrations were square root transformed to meet assumptions of the analysis. To further explore the combined effects of water types (Dictyota sp. and O. faveolata contact water, and water column water) and depth, a two-way ANOVA was performed. The maximum excitation pressure over photosystem II (Qm) was calculated as: Qm = 1 − [(ΔF∕Fm′ at noon)∕(Fv∕Fm at dawn)] (Iglesias-Prieto et al., 2004). Values that are close to zero indicate that most of the reaction centers of photosystem II remain open, whereas values close to 1.0 indicate that they are closed, suggesting photoinhibition.
Results
General environmental parameters
Benthic composition differed between the three sampled depths (Table 1; see Supplemental Information 1 for benthic composition to higher taxonomic levels). While the reef terrace (5 m; high light) was mainly dominated by the non-biological components sand, coral rubble, and bare coral rock, the percentage cover of scleractinian corals, macroalgae, as well as other living taxa increased at the drop off (10 m; intermediate light) and the fore reef slope (20 m; low light). Similarly, with increasing depth the cover of the study organisms Dictyota sp. and O. faveolata increased from <1% on the reef terrace to 8.8% and 2.6% at the drop off, and 5.5 % and 3.0% on the fore reef slope, respectively. Mean bacterial concentration (±SD) in the water column at 15 m depth was 9.6 ± 1.2 × 105 cells mL−1.
DOC concentrations across a depth-dependent light gradient
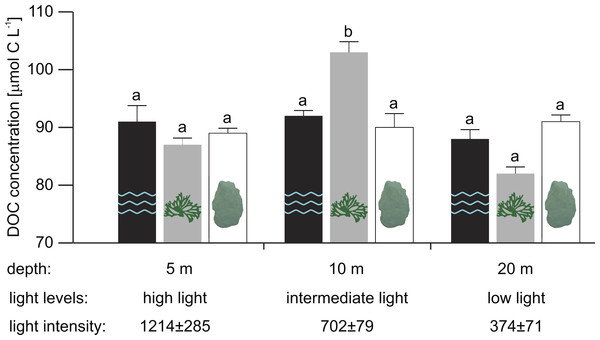
At 10 m depth (intermediate light), mean in situ DOC concentration in close proximity to the reef alga Dictyota sp. was 13 and 11 µmol L−1 higher than for the scleractinian coral O. faveolata (Tukey HSD, p = 0.001) and the water column (Tukey HSD, p = 0.012), respectively (Fig. 1 and Supplemental Information 1 for raw data).
Figure 1: Mean in situ DOC concentrations (n = 5, except for water column at 10 m and Dictyota sp. at 5 m depth with n = 4) measured in the water column (2 m off the reef slope; black) and at the substrate-water interfaces of the reef algae Dictyota sp. (dark grey) and the scleractinain coral Orbicella faveolata (white) at 5, 10, and 20 m depth.
Error bars indicate SE. Different letters indicate differences between water types for each depth (Tukey HSD, p < 0.05). Light intensities (mean ± SD) during the sampling are given in µmol photons m−2 s−1.| Percentage cover (%) | |||
|---|---|---|---|
| Benthic component | 5 m | 10 m | 20 m |
| Macroalgae | 0.3 | 14.0 | 31.7 |
| Turf algae | 0.0 | 9.3 | 3.0 |
| Crustose coralline algae | 0.0 | 3.5 | 6.8 |
| Cyanobacteria | 0.0 | 6.6 | 5.3 |
| Scleractinian corals | 1.9 | 24.4 | 32.7 |
| Fire corals | 0.0 | 0.6 | 0.3 |
| Soft corals/gorgonians | 0.0 | 0.3 | 0.3 |
| Sponges | 0.3 | 1.5 | 6.0 |
| Tunicates | 0.0 | 0.1 | 0.0 |
| Sand | 73.5 | 23.1 | 6.3 |
| Bare coral rock | 11.0 | 4.3 | 6.3 |
| Coral rubble | 13.1 | 11.1 | 1.2 |
| Studied taxa: | |||
| Dictyota sp. | 0.1 | 8.8 | 5.5 |
| Orbicella faveolata | 0.4 | 2.6 | 3.0 |
In contrast, mean in situ DOC concentration at 5 m (high light) and 20 m (low light) did not differ significantly between water types (Table 2A). While mean DOC concentrations in close proximity to Dictyota sp. at 20 m appear to be depleted relative to concentrations in the water column and near O. faveolata, these differences were not found to be significant (Fig. 1). No depth-related differences in mean DOC concentrations were established in any of the tested water types (Table 2B).
| df | F | p | ||
|---|---|---|---|---|
| A. Differences between water types | ||||
| Per depth: | ||||
| High light (5 m) | 2 | 0.357 | 0.707 | |
| Intermediate light (10 m) | 2 | 13.378 | 0.001 | * |
| Low light (20 m) | 2 | 0.129 | 0.880 | |
| B. Differences between depth: | ||||
| Per water type: | ||||
| Water column | 2 | 0.148 | 0.864 | |
| Dictyota sp. | 2 | 3.682 | 0.06 | |
| Orbicella faveolata | 2 | 2.798 | 0.101 | |
However, both water type and depth appear to jointly affect mean in situ DOC concentrations along the tested depth-dependent light gradient (significant interaction: water type × depth, p = 0.026; Table 3). The sampling depths 5, 10, and 20 m corresponded to a light intensity of 1,214 ± 285, 702 ± 79 and 374 ± 71 µmol photons m−2 s−1 (mean ± SD), respectively, during the sampling (between 12:00 and 13:00 hrs).
| Factor | df | F | p | |
|---|---|---|---|---|
| Water type | 2 | 4.731 | 0.015 | * |
| Depth | 2 | 0.054 | 0.947 | |
| Water type × Depth | 4 | 3.169 | 0.026 | * |
Maximum excitation pressure over photosystem II in Dictyota sp.
At dawn, maximum potential quantum yield (Fv∕Fm) in Dictyota sp. was 0.65 ± 0.04 (mean ± SD), which was reduced by 67% to 0.44 ± 0.09 at 15:00 (effective quantum yield; (ΔF∕Fm′) (Supplemental Information 1 for raw data). The corresponding Qm value of 0.33 indicates foremost closed reaction centers of photosystem II and thus suggests the occurrence of photoinhibition at light levels of 1,237 ±486 µmol photons m−2 s−1, which are comparable to those observed in situ on the reef terrace at 5 m depth (high light) during midday (see DOC concentrations across a depth-dependent light gradient).
Discussion
In this study we investigated DOC concentrations in close proximity to the reef alga Dictyota sp., the scleractinian coral O. faveolata, and in the water column across a depth-dependent light gradient between 5 and 20 m. Due to the positive relationship between light availability and DOC release we hypothesized that DOC concentrations near BPP are highest on the shallow, well-lit reef terrace and decrease along the fore reef slope following a depth-dependent pattern. Elevated DOC concentrations compared to the background concentrations in the water column were only observed near Dictyota sp. at an intermediate depths at 10 m, but not at 5 m or 20 m depth, or near O. faveolata at any of the tested depth. No depth- and therefore light-related differences in mean DOC concentrations were established in any of the tested water types, however, water type and depth appear to jointly affect in situ DOC concentrations across the tested depth-dependent light gradient.
Elevated DOC concentrations in close proximity to BPP occur when DOC release exceeds removal processes. Consequently, environmental parameters, such as light availability (Haas et al., 2010b; Barrón, Apostolaki & Duarte, 2012), temperature (Gillooly et al., 2001; Haas et al., 2010b), grazing pressure (Berman & Holm-Hansen, 1974), senescence (Khailov & Burlakova, 1969), nutrient availability (Lopéz-Sandoval, Fernandéz & Marañón, 2011; Mueller et al., 2016), and hydrodynamic conditions (Wild et al., 2012) affect the DOC release of BPP. In combination with factors which affect the accumulation/removal of DOC near them (e.g., morphology of the BPP, hydrodynamic conditions (Losee & Wetzel, 1993; Escartín & Aubrey, 1995) and DOC consumption by heterotrophic microbes and sponges (Gast et al., 1999; Yahel et al., 2003; Scheffers, Bak & Duyl, 2005; De Goeij et al., 2008; Haas et al., 2011; Nelson et al., 2013), these parameters interactively determine the DOC concentrations in close vicinity to BPP. The lack of elevated DOC concentrations near Dictyota sp. under high light levels at 5 m depth could thus be explained by (1) reduced DOC release, (2) high DOC removal, or (3) a combination of both.
Light availability is generally considered to have a strong positive effect on DOC release of reef algae. However, Haas et al. (2010b) reported that this positive correlation in the reef alga Caulerpa sp. only held until a maximum light intensity was reached. At these light intensities DOC release rates steeply decreased to levels comparable to those in the dark. They explained this decrease with the onset of photoinhibition at a species-specific light intensity, which is a common phenomenon in coral reef BPP (Franklin, 1994; Hanelt, Li & Nultsch, 1994; Brown et al., 1999; Hoegh-Guldberg & Jones, 1999; Iglesias-Prieto et al., 2004). While the occurrence of photoinhibition wasn’t specifically tested for during the DOC sampling, the corroborative ex situ experiment to assess excitation pressure over photosystem II in Dictyota sp. suggests that photoinhibition can occur at light levels comparable to those observed in situ at 5 m depth on the aforementioned sampling day. Accordingly, photoinhibition may have reduced the DOC release of Dictyota sp. on the reef terrace at 5 m depth and therefore contributed to the fact that no elevated DOC concentrations in its close proximity were found.
Similar to light availability, hydrodynamic conditions can affect in situ DOC concentrations near BPP in two ways. Either positively, when water movement increases the metabolism and DOC release rates of BPP by alleviating the limitation of the diffusive boundary layer around them (Carpenter, Hackney & Adey, 1991; Lesser et al., 1994; Wild et al., 2012), or negatively, when water movement and water exchange hamper the accumulation of DOC by dilution (Hauri et al., 2010). Water movement generally decreases exponentially as a function of depth (Shashar et al., 1996) and significantly higher water movement rates are reported at 5 m compared to 10 or 20 m depth on the reef slope of Curaçao (Vermeij & Bak, 2003). Thus, a reduced DOC release rate of Dictyota sp. due to photoinhibition in combination with high water movement and water exchange that hamper the accumulation of DOC, could explain the lack of elevated DOC concentrations near Dictyota sp. under high light levels at 5 m depth. It can be further assumed that the negative effect of water movement and water exchange on the accumulation of DOC at 10 m was higher than at 20 m, i.e., a higher DOC release rate was necessary to result in elevated DOC concentrations at 10 m. Yet despite higher water movement, elevated DOC concentrations near Dictyota sp. were only found at 10, but not at 20 m. One explanation for this difference is that DOC release rates were higher at 10 m (intermediate light) than at 20 m (low light), which is in line with the aforementioned positive relation between light availability and DOC release. Interestingly, whilst not significant, DOC concentrations in close proximity to Dictyota sp. at 20 m depth tend to be depleted compared to concentrations near O. faveolata or in the water column. Reduced water movement and thus a prolonged water residence time combined with a low, but steady release of bio-available algal DOC (Nelson et al., 2013) by Dictyota sp., could have stimulated the growth of heterotrophic microbial communities. The bio-available DOC could have further allowed those communities to metabolize otherwise refractory components of the DOC pool and thereby deplete the local DOC stock, as described for the water columns overlying algal-dominated reefs (Dinsdale et al., 2008; Haas et al., 2016).
No elevated DOC concentrations were observed near the scleractinian coral O. faveolata at any of the sampling depths. In general, the DOC release of scleractinian corals is more variable than that of reef algae and an increasing number of studies suggest that scleractinian corals only contribute marginally to the local DOC pool on tropical coral reefs (e.g., Haas et al., 2010a; Naumann et al., 2010; Haas et al., 2011). Furthermore, the massive morphology of O. faveolata is less likely to restrict water exchange than the bushy thalli of Dictyota sp. and is thereby less favorable for the accumulation of DOC in its vicinity (Stocking, Rippe & Reidenbach, 2016). Given the positive effect of light availability on the DOC release by BPP, we expected to find significantly higher DOC concentrations on the shallow and well-lit reef terrace compared to deeper reef sections, following the natural light gradient across depth. Yet no significant differences in the mean DOC concentrations between the sampled depths were observed, neither in close vicinity to the BPP Dictyota sp. or O. faveolata, nor in the water column. Nevertheless, both water type and depth (and thereby light availability) seem to have interactively determined in situ DOC concentrations. This is in accordance with previous findings that suggest that DOC release by, and in situ DOC concentrations near BPP are at least partly determined by a substrate-specific relationship with light availability (Mueller et al., 2014b). The absence of significant differences in DOC concentrations across the water column was also observed in other studies (Torréton et al., 1997; Nelson et al., 2011). To date only Slattery & Lesser (2015) reported a significant decline in DOC concentration with depth from coral reefs on the Bahamas, albeit this decrease occurred at mesophotic depths below 30 m. This may indicate that at least above mesophotic depths, DOC released by BPP is either quickly mixed and diluted throughout the reef overlying water column and/or taken up by DOC feeding organisms (i.e., heterotrophic bacteria and reef sponges). The abundances of open reef sponges and microbes at our study site, and on Curaçaoan reefs in general, are fairly low (Gast et al., 1999; De Goeij & Van Duyl, 2007; Mueller et al., 2014a; De Bakker et al., 2017) compared to abundances at more degraded locations throughout the Caribbean (e.g., Pawlik et al., 2015 and references therein, Haas et al., 2016). However, DOC removal by cryptic sponges living underneath overhangs and in coral cavities, which were not recorded in this study, is estimated to be in the same order of magnitude as gross primary production on these reefs (De Goeij et al., 2013).
Conclusion
While light availability has a strong positive effect on the DOC release of BPP, the occurrence of elevated DOC concentrations near them did not follow a natural light gradient across the reef slope in our study system. Instead, a combination of multiple factors, including water type, light availability, which affects the release of DOC (including the restriction by photoinhibition), and water movement, which affects the accumulation/removal of DOC, are proposed to interactively determine the DOC concentrations in the close vicinity of BPP along the reef slope.