Foraging behaviour of an egg parasitoid exploiting plant volatiles induced by pentatomids: the role of adaxial and abaxial leaf surfaces
- Published
- Accepted
- Received
- Academic Editor
- Darren Ward
- Subject Areas
- Entomology, Plant Science
- Keywords
- Chemical ecology, Leaf surface, Oviposition, Walking activity, Trissolcus basalis, Nezara viridula
- Copyright
- © 2017 Frati et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. Foraging behaviour of an egg parasitoid exploiting plant volatiles induced by pentatomids: the role of adaxial and abaxial leaf surfaces. PeerJ 5:e3326 https://doi.org/10.7717/peerj.3326
Abstract
Several phases of herbivorous insect attack including feeding and oviposition are known to induce plant defenses. Plants emit volatiles induced by herbivores to recruit insect parasitoids as an indirect defense strategy. So far, volatiles induced by herbivore walking and their putative role in the foraging behavior of egg parasitoids have not been investigated. In this paper we studied the response of the egg parasitoid Trissolcus basalis toward volatiles emitted by Vicia faba plants as consequence of the walking activity of the host Nezara viridula. Olfactometer bioassays were carried out to evaluate wasp responses to plants in which the abaxial or the adaxial surfaces were subjected to walking or/and oviposition. Results showed that host female walking on the abaxial but not on the adaxial surface caused a repellence effect in T. basalis 24 h after plant treatment. The emission of active volatiles also occurred when the leaf was turned upside-down, indicating a specificity of stress localization. This specificity was supported by the results, which showed that oviposition combined with feeding elicit the induction of plant volatiles, attracting the parasitoid, when the attack occurred on the abaxial surface. Analyses of plant volatile blends showed significant differences between the treatments.
Introduction
Plants belong to complex communities interacting with different organisms (Dicke, Van Loon & Soler, 2009). In particular, plants are continuously under attack from herbivorous insects since they are used as food source, oviposition site and place to meet potential mates. After the attack, plants can activate specific responses that result in defenses against the threats (Karban & Baldwin, 1997; Schoonhoven, Van Loon & Dicke, 2005; Howe & Jander, 2008; Schaller, 2008). These defenses can be direct, affecting negatively the physiology or behaviour of the herbivorous insect, and indirect, attracting herbivore enemies through the synthesis of volatile compounds, named herbivore-induced plant volatiles (HIPVs) (Hare, 2011; Heil, 2014).
Plant attack by phytophagous insects can be divided into different phases acting in sequence or in concert (Hilker & Meiners, 2010). During an attack, herbivorous insects come in contact with plants by touch or walk, and then they can feed and/or oviposit on or into the plant. Plants apparently seem passive but they are capable to sense touch by wind, vibrations (Appel & Cocroft, 2014) and also insects (Hilker & Meiners, 2010). In particular, they have the perception of being touched or scratched by a walking herbivore or the capacity to respond to chemical substances released from the herbivore’s tarsi during the walking activity on the plant substrate (Hilker & Meiners, 2010). During their foraging and/or oviposition activity, herbivorous insects leave their footprints on the plant substrate, which are an ensemble of tarsal pressure with surface and tarsal chemical secretions. Insect footprints have been investigated from different points of view. In the case of bumblebees, chemical footprints left on flowers are considered as an intraspecific signal used by conspecifics to avoid recently visited flowers (Eltz, 2006). Moreover, footprints have been studied considering the role played by the pad secretions in the mechanism of insect adhesion on the surface, and the quantification of the fluid secretion rate in adhesive pads (Gorb, 2001; Dirks & Federle, 2011). Finally, insect footprints can act as interspecific signal between herbivores and parasitoids. The larval parasitoid Cotesia marginiventris Cresson (Hymenoptera: Braconidae) can detect footprints left by the caterpillars of its host Spodoptera frugiperda Smith (Lepidoptera: Noctuidae) during walking activity, since the footprints are adsorbed on the plant wax surface (Rostás et al., 2008). In addition, it is known that footprints left on the substrate by true bugs have kairomonal effect for some Trissolcus and Telenomus species (Hymenoptera: Platygastridae) (Colazza, Salerno & Wajnberg, 1999a; Borges et al., 2003; Conti et al., 2004; Salerno et al., 2006). In particular, footprints left on the substrate by Nezara viridula (L.) (Heteroptera: Pentatomidae) females are perceived by the egg parasitoid Trissolcus basalis (Wollaston) females since they are retained onto the epicuticular waxes (Colazza et al., 2009; Lo Giudice et al., 2010; Lo Giudice et al., 2011). Moreover, the footprints left by Murgantia histrionica Hahn (Heteroptera: Pentatomidae) females on the cabbage leaves are able to induce the emission of contact synomones (Conti et al., 2010; Frati, Salerno & Conti, 2013); in fact, M. histrionica footprints left on leaf surface elicit a behavioural response in the egg parasitoid Trissolcus brochymenae (Ashmead) (Conti et al., 2010). In addition, these footprint-induced synomones are adsorbed by the epicuticular waxes of cabbage leaves and subsequently exploited by the parasitoid female (Frati, Salerno & Conti, 2013). All these examples refer to the parasitoid responses after its landing on plant. However, to date no studies have investigated whether the N. viridula walking activity—i.e., the ensemble of tarsal pressure with surface and the tarsal chemical secretions—is involved in eliciting the induction of specific volatile organic compounds (VOCs), which can affect the foraging behaviour of insect parasitoids. In our paper, using the tritrophic system Vicia faba (L.)—Nezara viridula—Trissolcus basalis, we tested whether herbivore walking induces the emission of plant volatiles eliciting a response of the egg parasitoid in olfactometer with particular attention on the role of leaf surface on which the biotic stress occurred. Behavioural experiments are conducted to test our hypothesis and they are followed by chemical analysis to confirm the recorded behavioural responses.
The walking activity on the substrate during the host plant exploitation represents the early phase of the herbivore attack. This phase can be followed by feeding and egg deposition. Plants are able to perceive herbivore feeding that is characterized by the physical damage on the plant tissue (Hilker & Meiners, 2010). However, the plant artificial wounding is not able to mimic the damage inflicted by herbivore feeding that is associated to the release of herbivore regurgitants into the plant wounds triggering the defensive responses (Hilker & Meiners, 2010). Plants can also sense insect oviposition (Hilker & Meiners, 2006; Hilker & Meiners, 2010; Hilker & Fatouros, 2015; Hilker & Fatouros, 2016). For several systems, it is well known that insect oviposition induces the emission of plant synomones that attract specific egg parasitoids (Meiners & Hilker, 1997; Meiners & Hilker, 2000; Hilker & Meiners, 2002; Hilker, Rohfritsch & Meiners, 2002; Colazza et al., 2004; Colazza, McElfresh & Millar, 2004; Fatouros et al., 2005; Fatouros et al., 2007; Fatouros et al., 2008; Fatouros et al., 2009). Generally, the oviposition-induced synomones are perceived by the parasitoids as olfactory stimuli. However, the egg parasitoids might also respond to contact synomones perceived after they have alighted on the plant (Fatouros et al., 2005; Fatouros et al., 2007; Fatouros et al., 2009; Conti et al., 2010). In our study model it is clearly demonstrated in olfactometer that T. basalis is attracted to OIPVs induced by oviposition combined with feeding of N. viridula (Colazza et al., 2004; Colazza, McElfresh & Millar, 2004). However, the feeding damage alone does not elicit parasitoid response (Colazza et al., 2004). Phytophagous stink bug species most frequently oviposit on the underside of soybean leaves (Tood & Herzog, 1980), and this behaviour guarantees to the herbivore egg masses a great protection from predators and to unfavourable microclimatic conditions (Müller & Hilker, 2001) due for example to sunlight. This was also confirmed for N. viridula on soybean plants where about the 80% of egg masses were laid on the abaxial leaf surface (Colazza & Bin, 1995).
On this account, do plants specifically respond to feeding and oviposition according to stress localization? In order to answer to this question, we tested in the described system (a) whether there is an indirect plant response when N. viridula feeding damage is inflicted on adaxial leaf surface and (b) whether indirect plant response is stronger when the oviposition (combined with feeding) occurs on abaxial part of the leaf (this situation resembles the most common case occurring in nature).
This study could give significant information regarding the ecological consequences of herbivore walking activity on plants and could contribute to understand the role of the footprints as a component of the oviposition-induced plant volatile (OIPV) induction in the studied system. In particular, this paper could give an important contribute in the knowledge of the interaction between host and egg parasitoid mediated by OIPVs.
Material and Methods
Insects
The N. viridula colony was reared in a controlled condition chamber (25 ± 1 °C; 70 ± 10% RH, 14 h:10 h L:D), inside clear plastic food containers (300 mm ×195 mm ×125 mm-high) with 5 cm diameter mesh-covered holes for ventilation. Separate containers were used for nymphs and adults. All stages were fed with a diet of sunflower seeds and seasonal fresh vegetables and food was changed every 2–3 d. Egg masses were collected daily and used to maintain cultures of both N. viridula and the parasitoid T. basalis. The N. viridula colony was supplemented regularly with field collected bugs.
The parasitoid T. basalis was reared on N. viridula egg masses glued on paper strips. Wasps were maintained in 85 ml glass tubes, fed with a honey-water solution and kept in controlled environment room under the same rearing conditions of N. viridula. After emergence, male and female wasps were kept together to allow mating. For all bioassays, naïve 2–4- d old females were used. Females were individually isolated in small vials 1 h before bioassays and then transferred to the bioassay room for acclimation.
Plant growing
Seeds of broad bean plants (V. faba cv. Superaguadulce) were immersed for 24 h in a slurry of water and soil (1:4) to favor root nodulation. Then, seeds were individually planted in plastic pots (9 × 9 × 13 cm) filled with a mixture of agriperlite (Superlite; Gyproc Saint-Gobain PPC Italia, Milan, Italy), vermiculite (Silver; Gyproc Saint-Gobain PPC Italia, Milan, Italy) and sand (1:1:1) and grown in a climate controlled chamber (24 ± 2 °C, 45 ± 10% RH, 12 h:12 h L:D). Plants were watered daily and, from one week post-germination, fertilized with an aqueous solution (1.4 g/l) of fertilizer (5-15-45, N-P-K, Plantfol, Valagro, Italy). Fifteen days old plants with approximately four fully expanded leaves were used for the experiments. Adaxial and abaxial leaf surface of Vicia faba plants were considered for behavioural experiments (Fig. S1).
Plant treatments
Role of the herbivore walking activity in eliciting induction of plant volatiles
To verify the possible volatile induction in V. faba plants by N. viridula walking activity on the abaxial leaf surface, behavioral assays were carried out at different time intervals elapsing after the end of the treatments (0 h, 24 h, and 48 h). To obtain plants treated with only footprints (tarsal pressure and tarsal chemical secretions), preventing bug feeding, gravid females with excised stylets were used. For stylet excision, females were previously anaesthetized at −4 °C for 3 min inside a glass tube, in order to immobilize their labium. Afterwards, the stylets were drawn from the labium with an entomological pin (no. 000), to amputate more than half their length using precision micro-scissors under a stereomicroscope (Zeiss Stemi SV8) with optical fiber illumination (Intralux 5000). The treated females were then placed inside a plastic dish (12 cm diameter) allowing them to recover, and used after 1 h to infest plants. A N. viridula female with excised stylets was placed for 24 h on the abaxial surface of a leaf located at the second foliar stage level. The insect was set inside a small cage, made from two Petri dishes (35 mm diameter, 10 mm height) with the bottoms substituted with a fine nylon mesh and each rim of the opposite side covered with a small foam rubber ring, which was kept tightened to the leaf with the help of a clip. These treated plants were maintained at controlled conditions (24 ± 2 °C, 45 ± 10% RH, 12 h:12 h L:D) for 24 h and then used for olfactometer bioassays.
To understand whether the adaxial V. faba leaf surface plays a different role in the volatile induction associated to herbivore walking activity, N. viridula females with excised stylets were individually placed for 24 h on the adaxial leaf surface as described above. In addition, to exclude the possible role due to the walking position of the bug females and thus the different amount of chemical tarsal secretions due to the insect pressure on the leaf surface, and to exclude the effect of the different light and temperature conditions on the two leaf surfaces, plants were treated with footprints left on the abaxial leaf surface but turning the leaf upside-down. For both treatments, plants were used for olfactometer bioassays 24 h after the end of the treatment.
To evaluate the role of bug gender in the induction of volatiles triggered by walking activity on the abaxial leaf surface and the specificity of these volatiles, V. faba plants were exposed respectively to the footprints left by mated N. viridula males and by Murgantia histrionica females, both with excised stylets. Murgantia histrionica was used because it is not a host of T. basalis (Peri et al., 2013). Considering the difference in weight between N. viridula (0.21 ± 0.01 gr) and M. histrionica (0.087 ± 0.001 gr) females, two bugs were used inside a clip cage during each treatment. The treatments were implemented following the protocol already described and the plants used for the bioassays 24 h after the end of the insect exposure. Healthy plants were used always as control. As for treated plants, an empty clip cage was applied on the leaf of the second foliar stage for 24 h and the plant was used 24 h after the clip removal. In the case of the treatment represented by footprints left on abaxial upside-down turned leaf, the control was obtained as described above but turning the leaf upside-down.
Role of feeding and oviposition localization in plant volatile induction
To elucidate whether the abaxial and adaxial V. faba leaf surfaces, damaged by feeding or by oviposition (oviposition always combined with feeding), play a different role in volatile induction according to stress localization, N. viridula females were individually placed for 24 h on the abaxial or on the adaxial leaf surface as described above. Plants with feeding punctures plus footprints and plants with a combination of feeding punctures, a deposited egg mass and footprints were used for olfactometer bioassays 24 h after the end of the treatment. Healthy plants with an empty clip cage applied on the leaf of the second foliar stage were used as control.
Behavioural assays
Wasp responses to volatile chemicals from V. faba plants subjected to different treatments were investigated with a dual choice Y-tube olfactometer as described by Moujahed et al. (2014). The different treatments were randomly assigned to each olfactometer arm at the beginning of the bioassays and were reversed after testing about 10 parasitoid females. At every switch, the polycarbonate olfactometer was cleaned with water and detergent and the glass parts were changed with cleaned ones. At the end of the bioassays, the glass parts were then cleaned with acetone and baked overnight at 180 °C.
Wasp females were singly introduced into the Y-tube olfactometer at the entrance of the stem and allowed to move freely for 10 min. Their behavior was recorded using a monochrome CCD video camera (Sony SSC M370 CE) fitted with a 12.5–75 mm/F 1.8 zoom lens. The camera lens was covered with an infrared pass filter (Kodak Wratten filter 87 Å) to remove visible wavelengths. Analog video signals from the camera were digitized by a video frame grabber (Canopus®ADVC 110; Grass Valley, CA, USA). Digitized data were processed by XBug, a video tracking and motion analysis software (Colazza et al., 1999). Wasp response was measured in terms of residence time, i.e., the time spent by the wasps in each arm during the entire bioassay.
The Y-tube olfactometer bioassays were carried out as paired choices in which the parasitoids were offered: (1) healthy plant versus plant with N. viridula female walking activity on abaxial leaf surface at 0 h (4 couples of plants assayed with 39 parasitoids), 24 h (4 couples of plants assayed with 41 parasitoids) and 48 h (4 couples of plants assayed with 41 parasitoids) from the end of the treatment; (2) healthy plant versus plant with N. viridula female walking activity on adaxial leaf surface (4 couples of plants assayed with 40 parasitoids); (3) healthy plant with upside-down abaxial leaf versus plant with N. viridula female walking activity on upside-down abaxial leaf (5 couples of plants assayed with 40 parasitoids); (4) healthy plant versus plant with N. viridula walking activity of male on abaxial leaf surface (4 couples of plants assayed with 34 parasitoids); (5) healthy plant versus plant with M. histrionica females walking activity on abaxial leaf surface (4 couples of plants assayed with 39 parasitoids); (6) healthy plant versus plant with N. viridula female walking activity associated with feeding punctures on abaxial (5 couples of plants assayed with 44 parasitoids) or on adaxial leaf surface (4 couples of plants assayed with 34 parasitoids); (7) healthy plant versus plant with N. viridula female walking activity associated with feeding punctures and oviposition on abaxial (6 couples of plants assayed with 44 parasitoids) or on adaxial leaf surface (6 couples of plants assayed with 58 parasitoids).
All bioassays were conducted from ∼09:00 h to 13:00 h under controlled conditions (26 ± 1 °C, 50 ± 5% RH).
Collection and analysis of VOCs
Cylindrical glass chambers (inner Ø= 10 cm, h = 30 cm), with a two semi-circular-part joint base made by Teflon with a 2-cm hole in the center to permit the insertion of the plant at the level of collar were used to collect headspace volatiles only from the epigeous part of the plant. Teflon taping was placed around the plant collar to make it tight on the Teflon base. Before each collection, the glass chamber and Teflon base were washed with water and detergent, rinsed with acetone, and baked overnight at 120 °C. A whole plant was inserted in the chamber and flushed with active charcoal filtered air at flux rate of 300 ml min−1 for 3 h using a pump NMP 830 KNDC 12V (KNF, Milano, Italy).
VOC emissions by plants were collected using adsorbent traps, placed at the chamber outlet, made by glass tubes filled with PorapakQ (SigmaAldrich; 60 mg, 80–100 mesh), which were pre-cleaned with hexane and then heat conditioned for at least 2 h in a stream of nitrogen (100 ml/min) at 130 °C. After 3 h traps were eluted with 700 µl of hexane, and the resulting extracts were stored at −20 °C in glass vials with Teflon cap liners until used for gas chromatography (GC) analysis. On the basis of behavioral results only the treatments significantly affecting the parasitoid response were used for volatile collection. In particular, VOCs were collected from healthy plants as control, plants with N. viridula female walking activity on abaxial leaf surface at 24 h, plants with N. viridula female walking activity on adaxial leaf surface; plants with N. viridula female walking activity + feeding punctures + oviposition on abaxial leaf surface and plants with N. viridula female walking activity + feeding punctures + oviposition on adaxial leaf surface. For each treatment, 6 plants were sampled. Blank measurements were carried out before every set of measurements, by sampling air from the chamber, in this case the Teflon base was closed using Teflon.
Gas chromatography-mass spectrometry (GC-MS) analysis was performed on a Hewlett-Packard 5890 GC system interfaced with an HP5973 quadruple mass spectrometer. For each sample, 1 µl of extract was injected into a HP5-MS column (5% diphenyl–95% dimethyl polysiloxane 30 m × 0.2 mm, 0.25-µm film; J & W Scientific, Folsom CA, USA) in splitless mode. Injector and detector temperatures were 260 °C and 280 °C respectively. Helium was used as the carrier gas. The GC oven temperature program was 40 °C for 5 min, then, increased by 10 °C/min to 250 °C. Electron impact ionization spectra were obtained at 70 eV, recording mass spectra from 40 to 550 amu.
Peak area of each detected compound was calculated. Compounds were tentatively identified, based on comparison of RI and mass spectra with those in Adams (2007), http://www.pherobase.com and the NIST 1998 libraries. For (E)-2-Hexenal, (Z)-3-Hexenyl acetate, Benzaldehyde, β-Ocimene, α-Pinene, α-Myrcene, Linalool, β-Caryophyllene, Octanal, Nonanal, Decanal, Octan-1-ol, Tetradecane and Isomenthone, tentative identifications by GC-MS were confirmed by injection of authentic standards. Standars used were obtained from Sigma-Aldrich (Munich, Germany).
Statistical analysis
Data were analyzed by linear mixed model (LMM) with the plant treatment as fixed effect and parasitoid nested within each plant pairs as random effects to account for pseudoreplication. Significance of the fixed term in the model was determined using likelihood ratio tests (LRTs) comparing the model with and without the factor in question (Crawley, 2007). Because this approach compares models with different fixed effect structures, Maximum Likelihood (ML) was specified in the models instead of Restricted Maximum Likelihood (REML) (Crawley, 2007). Model fit was assessed with residual plots. All statistical analyses were carried out with R software, version 3.1.3 (R Core Team, 2015).
Data from analysis of volatiles were analyzed by multivariate analysis using projection to latent structures discriminant analysis (PLS-DA) with SIMCA-P+12.0 software program (Umetrics AB, Umeå, Sweden). The projection method determines if samples belonging to the different treatment groups can be separated on the basis of quantitative and qualitative differences in their volatile blends.
Results
Role of the herbivore walking activity in eliciting induction of plant volatiles
Trissolcus basalis females were able to discriminate between V. faba healthy plants and plants with N. viridula walking activity on abaxial leaf surface but not with N. viridula walking activity on adaxial leaf surface.
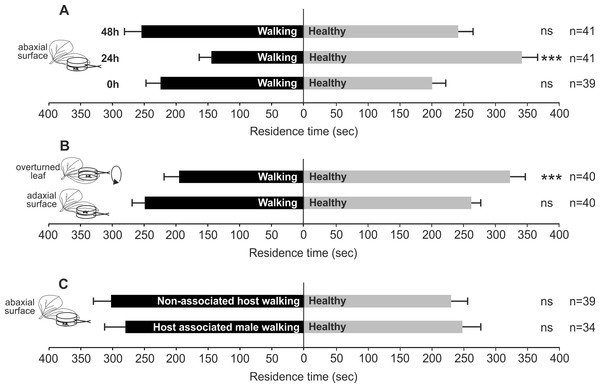
In particular, T. basalis females significantly preferred volatiles emitted by healthy plants as control compared to plants with N. viridula walking activity on abaxial leaf surface, assayed 24 h after the end of the treatment (χ2 = 19.016; df = 1; P < 0.0001). Whereas differences were not significant when 0 h (χ2 = 0.645; df = 1; N = 0.422) and 48 h (χ2 = 0.117; df = 1; N = 0.733) were considered (Fig. 1A).
Figure 1: Possible role of bug walking activity in plant volatile induction.
Response of T. basalis females in a Y-tube olfactometer to volatiles from V. faba plants treated: (A) with N. viridula female walking activity on the abaxial leaf surface, and assayed 0 h, 24 h, and 48 h after the treatment versus healthy plants; (B) with N. viridula female walking activity on overturned leaf versus healthy plants with overturned leaf and plants with N. viridula female walking activity on adaxial leaf surface versus healthy plants; (C) with M. histrionica (non-associated host) walking activity of 2 females on abaxial leaf surface versus healthy plants and treated with N. viridula walking activity of male on abaxial leaf surface versus healthy plants. Bars represent mean (±SEM) of the time spent by wasp females in each arm over an observation period of 600 s. Asterisks (***) indicate p < 0.001 by linear mixed model LMM. ns, not significant; n, number of replicates.Wasps showed a significant preference for volatiles emitted by the control compared to those released by plants with N. viridula walking activity on abaxial leaf turned upside-down (χ2 = 11.162; df = 1; N = 0.0008) (Fig. 1B). When comparing plants with N. viridula walking activity on adaxial leaf surface and healthy plants, no significant differences were displayed between test and control (χ2 = 0.260; df = 1; N = 0.611) (Fig. 1B).
The walking activity of N. viridula male (χ2 = 0.56; df = 1; N = 0.454) or that of M. histrionica females on abaxial leaf surface (χ2 = 2.108; df = 1; N = 0.147) did not stimulate a significant response from wasps compared to healthy plants (Fig. 1C).
Role of feeding and oviposition localization in plant volatile induction
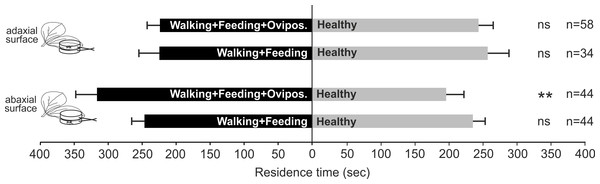
Nezara viridula oviposition, combined with feeding and footprints, on plant surface induces OIPVs triggering the parasitoid response only when egg deposition occurs on abaxial leaf surface. In particular, parasitoid females showed a significant preference for volatiles released by plants exposed to N. viridula walking, feeding and oviposition activities on abaxial leaf surface (χ2 = 7.331; df = 1; N = 0.007) compared to the control (Fig. 2), whilst, when the treatment was located on adaxial leaf surface no significant choice was displayed (χ2 = 0.247; df = 1; N = 0.620) (Fig. 2). In the case of V. faba plants with N. viridula walking and feeding activities on abaxial (χ2 = 0.164; df = 1; N = 0.685) or adaxial leaf surface (χ2 = 0.392; df = 1; N = 0.531), no preference for test and control was shown (Fig. 2).
Figure 2: Role of stress localization in the induction of plant volatiles.
Response of T. basalis females in a Y-tube olfactometer to volatiles emitted by (1) V. faba plants with N. viridula female walking activity associated with feeding punctures and oviposition on the adaxial leaf surface versus healthy plants; (2) plants with N. viridula female walking activity associated with feeding punctures on the adaxial leaf surface versus healthy plants; (3) plants with N. viridula female walking activity associated with feeding punctures and oviposition on the abaxial leaf surface versus healthy plants and (4) plants with N. viridula female walking activity associated with feeding punctures left on the abaxial leaf surface versus healthy plants. Bars represent mean (±SEM) of the time spent by wasp females in each arm over an observation period of 600 s. Asterisks (**) indicate p < 0.01 by linear mixed model LMM. ns, not significant; n, number of replicates.Plant VOC analysis
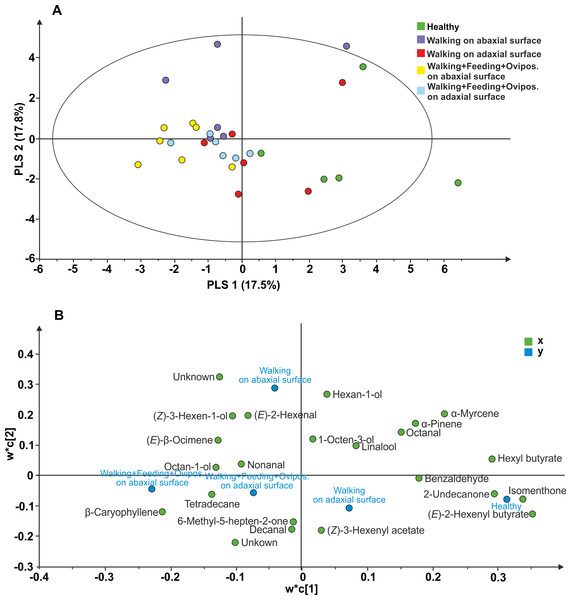
The following 23 compounds were detected in V. faba plants differently treated: (E)-2-Hexenyl butyrate, Isomenthone, Hexyl butyrate, Undecan-2-one, α-Myrcene, unknown 1, Ethylbenzene, β-Caryophyllene, α-Pinene, Hexan-1-ol, unknown 2, Octanal, Benzaldehyde, (Z)-3-hexen-1-ol, (E)-2-Hexenal, Tetradecane, (E)- β Ocimene, (Z)-3-hexenyl acetate, Octan-1-ol, Decanal, Linalool, 6-Methyl-5-hepten-2-one, nonanal, 1-Octen-3-ol. The PLS-DA comparison including samples of all treatments, resulted in a model with one significant principal component (PC1; R2X = 0.175; R2Y =0.148; Q2 =0.055; Fig. 3). The model separated healthy plants and plants with N. viridula walking on adaxial leaf surface from plants with N. viridula walking on abaxial leaf surface and plants with N. viridula walking associated with feeding punctures and oviposition on abaxial or adaxial leaf surface. In particular, the volatile blend emitted by plants with footprints left on abaxial leaf surface is different from that emitted by plants with footprints left on adaxial leaf surface and from healthy plants. Examination of the loading plot showed that a group of 8 compounds contributed the most to explaining the variation in the model (Fig. 3B). These compounds have the following corresponding VIP values (variable importance for the projection): (E)-2-Hexenyl butyrate =1.54; Isomenthone =1.48; Hexyl butyrate =1.31; Undecan-2-one =1.28; α Myrcene =1.21; Unknown =1.10; Ethylbenzene =1.10; β-Caryophyllene = 1.05.
Figure 3: Projection to latent structures discriminant analysis (PLS-DA) comparison of the volatile compounds emitted by individual V. faba plants.
(A) Score plot of the samples, with the percentage of explained variation in parentheses. The PLS-DA resulted in a model with one significant principal components (PCs). The ellipse defines the Hotelling’sT2 confidence region (95%). (B) Loading plot of the first two components of the PLS-DA, showing the contribution of each of the compounds toward the model.Discussion
Our study suggests that the herbivore walking activity on the substrate elicits plant volatile induction. The egg parasitoid T. basalis was able to discriminate between volatiles from healthy plants and volatiles from plants with footprints of N. viridula females. However, the parasitoid response was time interval dependent, as it was recorded after 24 h from the treatment, while no response was shown at the time intervals of 0 and 48 h after the end of bug walking activity. The leaf surface is actively involved in this induction. In fact, only when the herbivore walking activity occurred on the abaxial leaf surface the parasitoid response was shown. This is demonstrated by the fact that turning the leaf upside-down and treating the abaxial leaf surface the parasitoid response did not change. In addition, there were no behavioral responses by the parasitoid when leaf treatment occurred with walking activity of N. viridula males and of the non-associated host M. histrionica females. The combination of walking and feeding activities on adaxial and abaxial leaf surface did not induce wasp behavioral responses. Furthermore, our research reveals that N. viridula oviposition (combined with walking and feeding) elicited the induction of OIPVs when it has occurred on abaxial leaf surface. This result confirms the induction of OIPVs in V. faba by N. viridula, already reported in previous papers where the oviposition was generally recorded on the abaxial leaf surface, as happens in natural conditions, because the plant exposition was carried out in a wood-framed, nylon mesh cage (Colazza et al., 2004) or in a net bag (Moujahed et al., 2014) with N. viridula females free to explore the whole plant.
It is possible to hypothesize that, in our system, the walking activity of N. viridula female on abaxial V. faba leaf surface elicited the emission of putative induced plant volatiles. The behavioural data seem to be supported by volatile analyses since the PLS-DA showed that the whole blend of plant volatiles changes according to stress localization, i.e., when footprints are left on the abaxial or adaxial leaf surface. An effect on wasp behaviour of volatilization of precursors deposited by the walking insects on leaf surface could be excluded considering that the wasp response was recorded only when the abaxial leaf surface, but not the adaxial leaf surface, is contaminated. Although the induced volatiles associated with the herbivore walking activity did not attract the parasitoid but favoured a repellence effect, they actually might act similarly to a synomone. In fact, our hypothesis is that these induced volatiles could give the parasitoid information that the plant has been visited by the herbivore but it is not there anymore. This repellence for ‘old’ (24 h) footprint is not showed for fresh footprints (0 h) as they could indicate the possibility of herbivores around. Considering that the parasitoids are under selection pressure to maximize their foraging efficiency in order to improve their ecological fitness (Tamiru, Zeyaur & Bruce, 2015), it is fundamental that they should not waste time exploiting these chemical cues, which may be unreliable indicators of the egg presence. The emission of putative induced volatiles associated with herbivore walking activity is not due to the simple contact with the herbivorous insect but probably to the secretions released at tarsal level and probably produced by tibial glands (R Romani, pers. comm, 2016). These secretions, which concur to the herbivore adhesion on the leaf surface, interact with the plant in a specific manner depending on whether the interaction occurs with the abaxial or the adaxial leaf surface. In addition, the adhesion of the N. viridula tarsi on the substrate, and in particular of the claws during the stationary contact on the surface, does not provoke evident mechanical damage on both leaf surfaces as morphological investigations by scanning electron microscope reveal no differences between healthy and footprint-treated V. faba leaf surfaces (R Romani, pers. comm., 2016). Our results clearly show that the two leaf surfaces interact differently with N. viridula walking activity. This phenomenon could be explained considering the following morphological, anatomical and structural differences between abaxial and adaxial leaf surfaces. The wax composition of the abaxial leaf surface may differ from that of the adaxial surface (Müller & Hilker, 2001); leaf palisade mesophyll cells are beneath the adaxial leaf surface and spongy mesophyll in the lower half; stomatal density is higher on the abaxial surface respect to adaxial surface of leaves (Willmer & Fricker, 1996a); abaxial guard cells are typically larger and stomatal pores are wider under conditions favouring opening (Willmer & Fricker, 1996b); finally, gas exchange between a leaf or leaflet and the atmosphere occurs mainly via abaxial stomata (Lu, 1988). In the case of V. faba, leaves have stomata on both abaxial and adaxial epidermis, but the number of stomata per mm2, the stomata width and length on the abaxial epidermis of the leaflet is higher than on the adaxial epidermis (Pekşen, Pekşen & Artik, 2006). The pad secretions of N. viridula are released during walking on the substrate (G Salerno, pers. comm., 2016) but how they interact with leaf surface or if /how they move into the leaf remains unknown. Moreover, the exposure to light and/or temperature of the tarsal secretions could vary from abaxial and adaxial leaf surface, but in our bioassays we can exclude the effect of the different light and temperature conditions on the two leaf surfaces since no differences were recorded in the T. basalis responses towards plants with the abaxial leaf surface with N. viridula walking and plants with the upside-down turned leaf.
The two leaf surfaces interact differently not only with N. viridula walking but also with the eggs laid by the herbivore. It is known that plants react to herbivore oviposition activating defensive responses. In particular egg deposition, also associated with wounding, induces the emission of volatiles attracting antagonists of the herbivores. The majority of herbivore insects oviposit on plant leaves (Hilker & Meiners, 2011) preferring the abaxial leaf surface rather than the adaxial (Müller & Hilker, 2001). Oviposition on adaxial leaf surface exposes eggs to sunlight, to unfavourable microclimatic conditions (Willmer, 1986) and to egg predators and parasitoids (Müller & Hilker, 2001). As recorded for other stink bug species (Tood & Herzog, 1980), N. viridula tends to oviposit on the abaxial leaf surface (Colazza & Bin, 1995). Our data show that N. viridula oviposition (combined with feeding and footprints) on plant surface induces OIPVs triggering the parasitoid response only when egg deposition occurs on abaxial leaf surface. However, these results are not explained by the PLS-DA, as the model does not separate between volatiles emitted in response to oviposition occurring on the abaxial or adaxial leaf surfaces. However, in our analysis (E)–β-caryophyllene appears to be a VIP compound confirming its potential role as synomone for T. basalis as previously hypothesized by Colazza, McElfresh & Millar (2004).
PLS-DA analyses take into account all volatiles but the wasps likely use a specific subset of compounds of the total blend (Clavijo McCormick, Unsicker & Gershenzon, 2012). Thus, a mismatch between behavioral responses and chemical analyses could be due to the fact that the parasitoids focused on some key volatiles associated with N. viridula-egg deposition on the abaxial leaf surface which constitute the active blend, whereas PLS-DA takes the whole blend into account.
In conclusion, our results confirm that the signals mediating the interaction between plants, herbivores and parasitoids are effective and finely tuned since they guarantee maximization of the chance to find the suitable host. In particular, data reported in this paper elucidate, first, the role of the herbivore walking activity in a simplified experimental set up (walking alone, without feeding and oviposition, only occasionally occur in nature), and second, the key role of the abaxial leaf surface in mediating the volatile communication between T. basalis and its host.
Supplemental Information
Image of adaxial and abaxial leaf surface of Vicia faba plant
Vicia faba leaf surface. Scanning electron microscopy (SEM) of abaxial (A) and adaxial (B) leaf surface of Vicia faba plant. Bars 200 µm