The role of transcriptional regulation in maintaining the availability of mycobacterial adenylate cyclases
- Published
- Accepted
- Received
- Academic Editor
- Matt Hutchings
- Subject Areas
- Microbiology, Infectious Diseases
- Keywords
- Cyclic adenosine monophosphate, Adenylate cyclase, Mycobacterium tuberculosis , Gene regulation, Mycobacterium smegmatis
- Copyright
- © 2014 Casey et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
- Cite this article
- 2014. The role of transcriptional regulation in maintaining the availability of mycobacterial adenylate cyclases. PeerJ 2:e298 https://doi.org/10.7717/peerj.298
Abstract
Mycobacterium species have a complex cAMP regulatory network indicated by the high number of adenylate cyclases annotated in their genomes. However the need for a high level of redundancy in adenylate cyclase genes remains unknown. We have used semiquantitiative RT-PCR to examine the expression of eight Mycobacterium smegmatis cyclases with orthologs in the human pathogen Mycobacterium tuberculosis, where cAMP has recently been shown to be important for virulence. All eight cyclases were transcribed in all environments tested, and only four demonstrated environmental-mediated changes in transcription. M. smegmatis genes MSMEG_0545 and MSMEG_4279 were upregulated during starvation conditions while MSMEG_0545 and MSMEG_4924 were downregulated in H2O2 and MSMEG_3780 was downregulated in low pH and starvation. Promoter fusion constructs containing M. tuberculosis H37Rv promoters showed consistent regulation compared to their M. smegmatis orthologs. Overall our findings indicate that while low levels of transcriptional regulation occur, regulation at the mRNA level does not play a major role in controlling cellular cyclase availability in a given environment.
Introduction
Cyclic adenosine monophosphate (cAMP) is an important second messenger produced by adenylate cyclase enzymes which controls a wide range of cellular responses in both prokaryotic and eukaryotic cells (Botsford & Harman, 1992; Peterkofsky et al., 1993; Tang & Hurley, 1998; Baumann, Lange & Soppa, 2007). cAMP signaling is critical for the regulation of virulence genes in several bacterial pathogens such as Yersinia pestis and Pseudomonus aeruginosa, and recent evidence suggests that cAMP also plays a role in the virulence of Mycobacterium tuberculosis, the causative agent of tuberculosis (Rickman et al., 2005; Petersen & Young, 2002; Smith, Wolfgang & Lory, 2004; Agarwal et al., 2009). A bacterially-derived cAMP burst is responsible for increased levels of cAMP in M. tuberculosis infected macrophages. The increased cAMP leads to decreased phagosome-lysosome fusion (Kalamidas et al., 2006) and increased production of CREB-mediated TNFα (Agarwal et al., 2009). Both physiological responses aid in M. tuberculosis survival after macrophage phagocytosis. Deletion of adenylate cyclase Rv0386 causes a loss of the bacterial-derived intramacrophage cAMP, leading to decreased bacterial survival during mouse infection (Agarwal et al., 2009). Additionally, deletion of the cAMP-controlled transcription factor, cAMP Receptor Protein (CRPMt), from the M. tuberculosis genome causes attenuation of M. tuberculosis in a murine model and reduced bacterial growth rates in vitro and within macrophages (Rickman et al., 2005; Akhter et al., 2008).
The classical model for cAMP regulation in bacteria is based on the well characterized cAMP response in Escherichia coli (Botsford & Harman, 1992). E. coli contains a single class I adenylate cyclase which catalyzes the conversion of ATP to cAMP (Peterkofsky et al., 1993). By comparison, the M. tuberculosis H37Rv genome contains 15 class III adenylate cyclases (McCue, McDonough & Lawrence, 2000), 10 of which have confirmed, biochemically distinct, activity (Castro et al., 2005; Linder, Hammer & Schultz, 2004; Linder, Schultz & Schultz, 2002; Reddy et al., 2001; Abdel Motaal et al., 2006; Cann et al., 2003; Shenoy & Visweswariah, 2006; Guo et al., 2001; Sinha et al., 2005; Tews et al., 2005). Classes of adenylate cyclases contain separate, unrelated gene families with no structural similarities. Class I cyclases are composed of cytoplasmic cyclases found in enterobacteria, class II cyclases are secreted toxins, while class III is the largest and most diverse class. Class III cyclases are typically multidomain proteins which include all of the known eukaryotic cyclases as well as a variety of prokaryotic cyclases (Danchin, 1993). M. tuberculosis class III cyclases include receptor, membrane bound, and soluble type family members, and two (Rv1625c and Rv2435c) belong to the mammalian type adenylyl cyclase grouping (McCue, McDonough & Lawrence, 2000; Shenoy & Visweswariah, 2006; Reddy et al., 2001). This abundance and diversity of adenylate cyclases suggests a complex role for cAMP signaling in M. tuberculosis. Besides M. tuberculosis, other mycobacterial species, including the nonpathogenic M. smegmatis, actinobacteria, alphaproteobacteria, and cyanobacteria also contain a wide diversity of annotated class III adenylate cyclases signifying that the E. coli paradigm is not transferrable to all bacterial species (McCue, McDonough & Lawrence, 2000; Shenoy et al., 2004).
With a high number of adenylate cyclases there is likely to be significant redundancy in mycobacterial cAMP production. Enzymatic regulation of adenylate cyclases in response to changing environments has been identified as a regulatory mechanism in M. tuberculosis (Abdel Motaal et al., 2006; Linder, Hammer & Schultz, 2004; Cann et al., 2003; Linder, Schultz & Schultz, 2002). However we hypothesize that it is unlikely for all cyclases to be present in the cell at the same time. Instead, it is more probable that cyclase regulation is first controlled at the level of transcription, with expression dependent on specific environmental signals or growth stimuli. For instance, Dass et al. (2008) demonstrated that expression of MSMEG_3780 is downregulated under low pH conditions and that downregulation is tied to decreased production of cAMP in that environment (Dass et al., 2008). While Dass et al. (2008) focused on characterizing the detailed regulation of one cyclase we have examined expression of all eight M. tuberculosis cyclase orthologs found in nonpathogenic M. smegmatis to determine the role transcriptional regulation may have on the availability of cyclases in the cell.
Materials and Methods
Bacterial culture
M. smegmatis mc2155 was grown in Tryptic Soy Broth (TSB) supplemented with 0.05% Tween-80. Cultures were grown in ambient air or 5% CO2 at 37°C in 25 cm2 tissue culture flasks rocking with gentle agitation. For gene regulation assays, late log phase cultures were exposed to low pH (TSB adjusted to pH 5.5 with 0.1 M HCl), starvation (incubation in Phosphate Buffered Saline), hydrogen peroxide (5 mM), or nitric oxide (10 mM diethylenetriamine/nitric oxide adduct) for 4 h and gene expression was compared to non-exposed cultures.
RNA preparation
Late log phase culture of M. smegmatis mc2155 was pelleted and resuspended in RNase-free water. Cells were mechanically disrupted using a bead beater (BioSpec Products) for four rounds of beating on high for 1 min each, in a mixture of 0.1 mm zirconia-silica beads (BioSpec Products), 45% TRIzol (Invitrogen), 45% acid phenol, and 10% chloroform-isoamyl alcohol (24:1). RNA was precipitated with isopropanol/3 M sodium acetate (pH 5.2) and resuspended in RNase-free water. RNeasy Mini Kit and RNase-free DNAse (Qiagen) were used to remove contaminating DNA following manufacturer’s specifications.
Semi-quantitative RT-PCR
cDNA was prepared from 0.5 µg of RNA using the iScript cDNA synthesis kit according to the manufacture’s specifications (BioRad). PCR was run using a series of cDNA dilutions (0–1:1000) as templates to ensure reactions chosen for quantitation were in the linear range of the PCR (Table 1 for primers). Reactions were performed at 94°C for 1 min, 57°C for 1 min and 72°C for 1 min followed by a 10 min extension at 72°C. Control reactions were performed against 16S rDNA using cDNA diluted 10−5. PCR products were separated on agarose gels and band densities quantified using ImageJ software (Abramoff, Megalhaes & Ram, 2004). The 16S PCR products from all growth conditions were normalized to one another before quantitation of individual genes, to ensure equal levels of starting RNA in each reaction. 16S rDNA PCR was also performed using total RNA without reverse transcription to ensure the absence of DNA contamination.
| RT-PCR | Promoter:reporter fusions | ||
|---|---|---|---|
| Gene | Sequencea | Gene | Sequence |
| MSMEG_0545 | F-GATCGAGCCGAAGAACTGTG | Rv1264 | F-NNNNGGATCCGACGATGTCGACGTAGTTGT |
| R-ATTGAGGGCGATCAAGTGAG | R-NNNNGGTACCGCGCACGTGGTCTGTCAC | ||
| MSMEG_3578 | F-CGATCGTCAACAAACTGGTG | Rv1318c | F-NNNNGGATCCAGATGCCCGAGGTCCAAG |
| R-CAGGTATCCGTTGTGCAGTG | R-NNNNGGTACCGTGCTCTTGGCCGACAT | ||
| MSMEG_3780 | F-CATACTCTTGCGCCTGTGAA | Rv1319c | F-NNNNGGTACCCGATCGCGGTCATGTACTC |
| R-CCCTGAGGTCTTTCGTGCT | R-NNNNGGTACCTGTGTCGGTCACGCTCTAAG | ||
| MSMEG_4279 | F-CGACCTGTCGGATTTCACC | Rv1359 | F-NNNNGGATCCGGAGGTTCGCCACAAGATT |
| R-CATCTGATGCCGCAGAACT | R-NNNNGGTACCCGATACCTTCCGGCTAAGCA | ||
| MSMEG_4477 | F-AGCCTGGCGTATCAGCTCT | Rv1647 | F-NNNNGGATCCAGCGGGAACCGCTAGGG |
| R- ACGGTCCAGAACAATTCGAC | R-NNNNGGTACCGTAGGTGGTGCGGGCTGAG | ||
| MSMEG_4924 | F-GTGACGCTGGAGAACCTGAC | Rv1900c | F-NNNNGGATCCACCGGATCGATCACTTGC |
| R-AAGATGAAGCCGAACACCAG | R-NNNNGGTACCATGGTCGAGGCGGATCAC | ||
| MSMEG_5018 | F-ATCCAGCCACTCCTGGAAG | Rv2212 | F-NNNNGGATCCGCAGATTGGTGATGCTCAGA |
| R-TGAGCAGCCAGTTGATCAGT | R-NNNNGGTACCGACCATAGCAGGACGTCACC | ||
| MSMEG_6154 | F-CCTGCTCAACGAGTTCTTCC | Rv2435c | F-NNNNGGATCCGTCTGAGTGCGTCGTCGTT |
| R-GCGTCACCCTGGAACTTGT | R-NNNNGGTACCTACCGAGTCCAGTGCCTCAC | ||
| 16S rDNA | F-GCGATACGGGCAGACTAGAG | Rv3645 | F-NNNNGGATCCAATCACCACGATCTGCCAGT |
| R-CCTCCTCCTGATATCTGCGCATT | R-NNNNGGTACCGCATGCTCAGCGAGAACAG | ||
| sigA | F-TCGAGGACGAGGAAGAAGAA | tuf | F-NNNNGGATCCGTGCGGAAGTAGAACTGCGG |
| R-CCTCCAGCAGATGGTTTTTG | R-NNNNGGTACCAGGAAGTTGAGATCGTCGGC | ||
Notes:
NNNN, added nucleotides to aid in restriction digestion, sequence irrelevant.
Gene reporter construction and assay
Promoter:gfp reporter strains were generated for gene expression analysis of M. tuberculosis promoter regions in a M. smegmatis background. The intergeneic DNA sequences of the adenylate cyclase genes were amplified by PCR (Table 1 for primer sequences) and amplified DNA was cloned into pGFPoriM, which carries a promoterless gfpmut2 gene as previously described (Purkayastha, McCue & McDonough, 2002; Florczyk et al., 2003). Constructed plasmids were electroporated into M. smegmatis mc2155 at 2500 mV (Eppendorf 2510 Electroporator). GFP fluorescence from cultured cells was detected using GloMax Multi + Detection System (Promega) and normalized to 106 bacteria based on OD600.
Results and Discussion
Regulation of M. smegmatis adenylate cyclases
The genome of M. smegmatis contains ten annotated adenylate cyclases, eight of which have orthologs in pathogenic M. tuberculosis (Kapopoulou, Lew & Cole, 2011). In order to determine the role of gene expression in adenylate cyclase availability we systematically examined transcription of all eight orthologs using semi-quantitative RT-PCR. Gene expression was examined with a focus on M. tuberculosis orthologs, using a variety of environments known to be relevant for M. tuberculosis infection. Conditions examined include starvation, low pH, oxidative and nitrosative stress and 5% CO2. Evidence suggests that non-growing persistent M. tuberculosis is exposed to nutrient starvation in lung granulomas (Betts et al., 2002; Nyka, 1974) while low pH, oxidative and nitrosative environments are known to occur following macrophage phagocytosis of M. tuberculosis (Smith, 2003; Liao, Fan & Bao, 2013; Chan et al., 1992).
Expression under control conditions (growth in TSB + Tween-80) indicated that all eight adenylate cyclase genes are transcribed at the same time in the cell, albeit with varied levels of transcription (Fig. 1). Out of all conditions tested, starvation and oxidative stress (H2O2 exposure) affect gene expression the most, with three genes showing statistically significant changes in expression under starvation conditions and two demonstrating statistical changes in expression under oxidative stress. This enhances the evidence of cAMP-mediated gene regulation in nutrient-limiting conditions as 30% of the M. tuberculosis CRPMt regulon has previously been linked to nutrient starvation (Bai, McCue & McDonough, 2005). Two genes (MSMEG_0545 and MSMEG_4279) were upregulated 2–3 fold after 4 h of starvation while one gene (MSMEG_3780) was observed to be downregulated approximately 2 fold after starvation and exposure to low pH. Additionally, two genes (MSMEG_0545 and MSMEG_4924) were downregulated approximately 2 fold under oxidative stress (Figs. 1 and 2). No expression differences were seen during nitrosative stress or the presence of CO2 (data not shown).
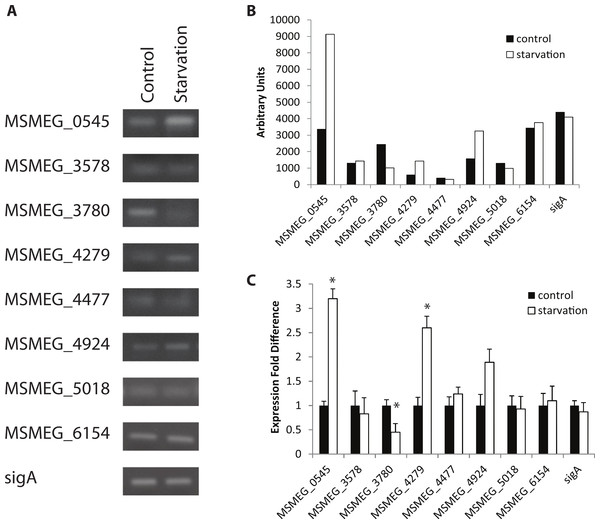
Figure 1: Regulation of M. smegmatis adenylate cyclase genes under starvation conditions.
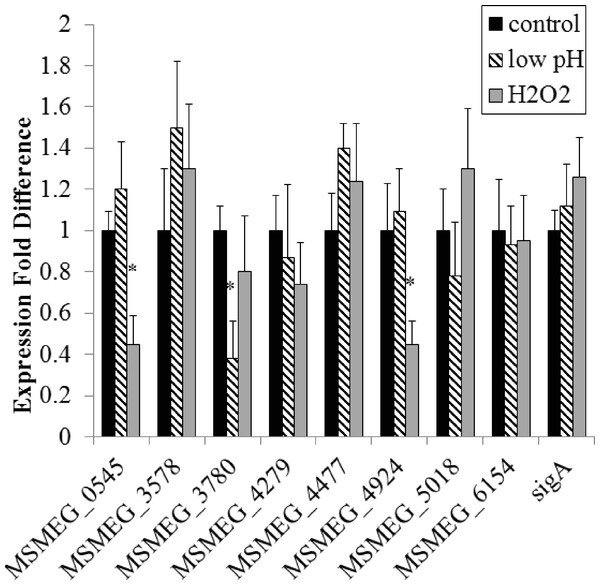
Semi-quantitative RT-PCR was used to compare adenylate cyclase mRNA levels between late-log phase cultures incubated for 4 h in mycomedia (control) or PBS (starvation). (A) A representative depiction of PCR-amplified cDNA separated using agarose gel electrophoresis. (B) Quantification of control (black bars) and starvation (white bars) PCR products depicted in A. (C) Average of three different experiments represented as fold differences of starvation compared to control expression. ∗ indicates statistically significant difference between expression in control and starvation for an individual gene (P < 0.05.)Figure 2: Regulation of M. smegmatis adenylate cyclase genes under low pH and oxidative stress.
Semi-quantitative RT-PCR was used to compare adenylate cyclase mRNA levels between late-log phase cultures incubated for 4 h in mycomedia (control, black bars), mycomedia adjusted to pH 5.5 (low pH, hatched bars), and mycomedia containing H2O2 (H2O2, grey bars). Results are the average of three independent experiments and are expressed in fold difference comparing experimental condition to control. ∗ indicates conditions with statistically significant differences in expression compared to control for an individual gene (P < 0.05).Interestingly, the expression of only four of the eight adenylate cyclases was influenced by the environments examined, and those observed expression changes, though significant, were not dramatic. Combined with the result that all eight genes were transcribed at various levels in all environments tested we conclude that our results counter our hypothesis and indicate that transcriptional regulation plays only a minor role in controlling the availability of various adenylate cyclases in the cell. Three of the four genes with environmentally-altered expression encode for soluble adenylate cyclase proteins suggesting that transcriptional regulation may play a larger role in the availability of soluble cyclases as opposed to the membrane associated and multi-domain structures. It is likely that biochemical regulation of various protein domains has the dominant role in regulation of cAMP production by the redundant cyclases.
Regulation of M. tuberculosis adenylate cyclases
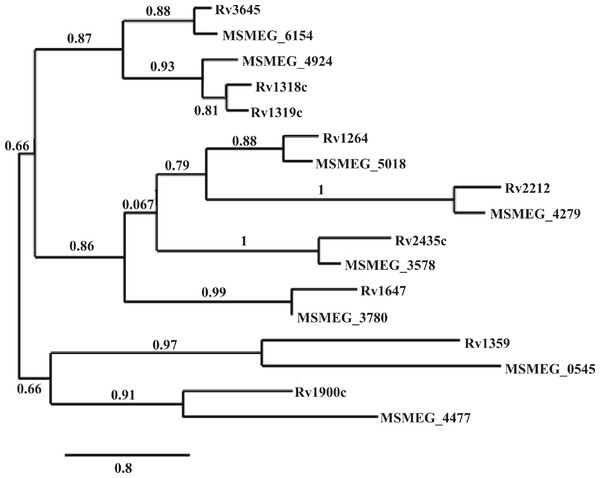
Of the ten annotated M. smegmatis cyclases, eight have reported orthologs in M. tuberculosis and M. bovis, seven in M. marinum, and five in M. avium (Kapopoulou, Lew & Cole, 2011). Phylogenic analysis based on protein sequence alignment was completed using Phylogeny.fr, online software that combines MUSCLE for multiple alignment, PhyML for tree building, and TreeDyn for tree rendering (Dereeper et al., 2008). This analysis confirmed the similarities of the M. tuberculosis and M. smegmatis ortholgos (Fig. 3). Additionally, promoter regions of each ortholog pair, represented by the 500 nucleotides upstream of the putative ATG start site of each gene, were compared for sequence similarities using the sequence alignment program T-Coffee (Notredame, Higgins & Heringa, 2000; McWilliam et al., 2013). Percent identities ranged from 51.94% to 82.08% (Table 2), indicating enough similarity to predict regulation would be similar between orthologs.
Figure 3: Phylogenic analysis of M. tuberculosis and M. smegmatis adenylate cyclase orthologs.
Amino acid sequences obtain from MycoBrowser databases were aligned and used to generate a phylogenic tree using Phylogeny.fr, combining alignment with MUSCLE, tree building with PhyML, and TreeDyn for tree rendering. The percentage of replicate trees in which the associated orthologs clustered together in the bootstrap test is shown above the horizontal branches. The branch lengths are drawn to represent evolutionary distances.| MSMEG gene | H37Rv gene | Percent identityb |
|---|---|---|
| 0545 | Rv1359 | 57.02 |
| 3578 | Rv2435 | 46.3 |
| 3780 | Rv1647 | 51.94 |
| 4279 | Rv2212 | 78.97 |
| 4477 | Rv1900 | 54.42 |
| 4924 | Rv1318c | 54.6 |
| 4924 | Rv1319c/ Rv1320c | 82.08 |
| 5018 | Rv1264 | 54.01 |
| 6154 | Rv2645 | 69.28 |
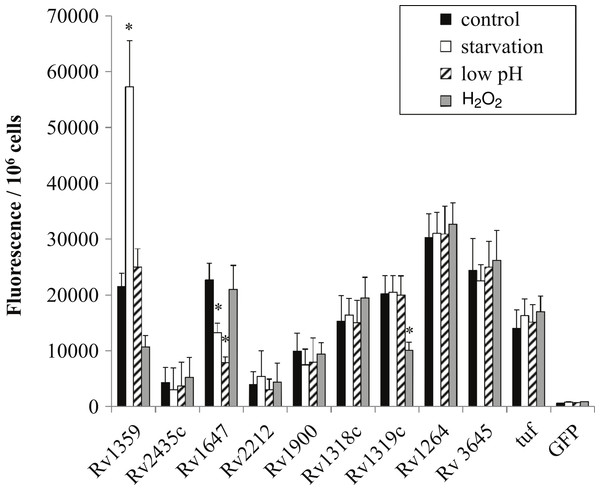
In order to determine if regulation in M. smegmatis was similar to that of the pathogenic orthologs we generated gfp:promoter fusions using the intergenic regions amplified from H37Rv chromosomal DNA. Overall, regulation between species orthologs was very similar. Rv1647 showed downregulation in both starvation and low pH similarly to MSMEG_3780, supporting the observation by Dass et al. (2008) who reported comparable regulation (Fig. 4). Additionally, Rv1359 showed similar regulatory patterns of upregulation in starvation and downregulation in oxidative stress as did MSMEG_0545 (Fig. 4). Oxidative stress and starvation are key environments for M. tuberculosis infection and latency respectively. Interestingly, Rv1359 is one of five H37Rv annotated adenylate cyclases that has not been shown to be biochemically active. The catalytic mechanism of class III adenylate cyclases is predicted to be a 2-metal ion reaction similar to DNA polymerase, where one metal ion, typically magnesium, associates with ATP and the second is involved in the catalysis reaction (Zimmermann, 1998). The Rv1359 protein sequence contains a glycine residue in place of the first of two required metal binding aspartates, along with missing a critical arginine residue, making it likely that this protein cannot function as an enzymatically active homodimer (Shenoy et al., 2004). Blastp alignment of the orthologs Rv1359 and MSMEG_0545 indicates that MSMEG_0545 is also missing the first aspartate residue, containing an arginine in this location instead, suggesting that MSMEG_0545 may also be catalytically inactive.
Figure 4: Regulation of M. tuberculosis adenylate cyclase genes.
GFP: promoter fusions containing M. tuberculosis promoters were electroporated into M. smegmatis and used to examine adenylate cyclase expression in late log phase cultures after 4 h incubation in mycomedia (control, black bar), PBS (starvation, white bar), mycomedia adjusted to pH 5.5 (low pH, hatched bars), and mycomedia containing H2O2 (H2O2, grey bars). Fluorescence was normalized to 106 cells and represented as the average of three independent experiments. ∗ indicates conditions with statistically significant differences in expression compared to control for an individual gene (P < 0.05).Transcriptional regulation of Rv1359 and MSMEG_0545 suggests that these genes do encode functional proteins. Guo et al. (2009) identified interactions between the Rv1359 protein and the promoter regions of rubredoxin encoding genes rubA and rubB and fatty acid metabolic genes fadD26, fadE26, and fadE27 (Guo et al., 2009). These genes encode proteins that may function in oxidative stress or starvation conditions. Rubredoxins are iron containing proteins involved with electron transfer, which have been shown to be upregulated in phagocytized M. tuberculosis (Buchko et al., 2011), an environment known to expose bacterial cells to oxidative stress. FadD26 is a fatty acid synthetase that is downregulated during starvation of M. tuberculosis (Betts et al., 2002) while FadE26 and FadE27 are involved in lipid degradation, a process likely to be important for phagocytized mycobacteria which rely on fatty acids for energy in the phagosome (Schnappinger et al., 2003). We hypothesize that Rv1359, and likely MSMEG_0545, are transcriptional regulators which could play roles in mediating a cAMP signal under oxidative stress and starvation. These proteins may function individually or in conjunction with other proteins as fadD26 has already been identified as part of the CRPMt regulon (Rickman et al., 2005).
The last regulatory similarity observed was the oxidative stress regulation of both MSMEG_4924 and the Rv1320c promoter region. MSMEG_4924 has three predicted orthologs in M. tuberculosis, Rv1318c, Rv1319c and Rv1320c. Rv1319c and Rv1320c comprise an operon and are both represented by the Rv1320c promoter region. The regulatory similarity of MSMEG_4924 to Rv1319c/Rv1320c and not Rv1318c correlates with the higher percent similarity to the promoter (82.08%) compared to Rv1318c’s promoter (54.6%) (Table 2).
Conclusions
Mycobacterial species contain a high number of functional adenylate cyclases when compared to E. coli, the typical bacterial model system. The high level of cyclase redundancy led us to hypothesize that only specific cyclases would be expressed in the cell under any given condition. However, the results in this study counter that hypothesis, indicating that all 8 M. smegmatis cyclases are transcribed in the cell at one time. While cAMP has been shown to be important for M. tuberculosis pathogenesis, adenylate cyclase transcriptional regulation does not appear to have a major role in regulating the availability of cyclases in the cell. Observed changes in cyclase expression in response to varying environments was minor but statistical, and changes occurred under conditions physiologically relevant for M. tuberculosis infection. Additionally, regulation of expression was conserved between mycobacterial orthologs, validating the use of M. smegmatis as a model system for studying the complex mycobacterial adenylate cyclase/cAMP network.