A systematic review of animal predation creating pierced shells: implications for the archaeological record of the Old World
- Published
- Accepted
- Received
- Academic Editor
- Brian Kraatz
- Subject Areas
- Ecology, Marine Biology, Paleontology, Zoology
- Keywords
- Scaphopoda, Gastropoda, Bivalvia, Shell beads, Interspecies interactions, Jewellery, Predators
- Copyright
- © 2017 Kubicka et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2017. A systematic review of animal predation creating pierced shells: implications for the archaeological record of the Old World. PeerJ 5:e2903 https://doi.org/10.7717/peerj.2903
Abstract
Background
The shells of molluscs survive well in many sedimentary contexts and yield information about the diet of prehistoric humans. They also yield evidence of symbolic behaviours through their use as beads for body adornments. Researchers often analyse the location of perforations in shells to make judgements about their use as symbolic objects (e.g., beads), the assumption being that holes attributable to deliberate human behaviour are more likely to exhibit low variability in their anatomical locations, while holes attributable to natural processes yield more random perforations. However, there are non-anthropogenic factors that can cause perforations in shells and these may not be random. The aim of the study is compare the variation in holes in shells from archaeological sites from the Old World with the variation of holes in shells pierced by mollusc predators.
Methods
Three hundred and sixteen scientific papers were retrieved from online databases by using keywords, (e.g., ‘shell beads’; ‘pierced shells’; ‘drilling predators’); 79 of these publications enabled us to conduct a systematic review to qualitatively assess the location of the holes in the shells described in the published articles. In turn, 54 publications were used to assess the location of the holes in the shells made by non-human predators.
Results
Almost all archaeological sites described shells with holes in a variety of anatomical locations. High variation of hole-placement was found within the same species from the same site, as well as among sites. These results contrast with research on predatory molluscs, which tend to be more specific in where they attacked their prey. Gastropod and bivalve predators choose similar hole locations to humans.
Discussion
Based on figures in the analysed articles, variation in hole-location on pierced shells from archaeological sites was similar to variation in the placement of holes created by non-human animals. Importantly, we found that some predators choose similar hole locations to humans. We discuss these findings and identify factors researchers might want to consider when interpreting shells recovered from archaeological contexts.
Introduction
The adornments of prehistoric people play an important role in our understanding of the evolution of human behaviour (Bednarik, 2001; Szabo, Brumm & Bellwood, 2007; Gutiérrez-Zugasti & Cuenca-Solana, 2013) because they can indicate evolutionary changes in the ethno-linguistic diversity of early humans (Vanhaeren & D’Errico, 2006; Schick & Toth, 2013; Stiner, 2014). These findings help anthropologists to construct a picture of the life of prehistoric human groups, and can give insights into their social status (Bednarik, 1998; Stiner, 1999; Vanhaeren & D’Errico, 2005), group membership, age or marital status (Kuhn et al., 2001). Molluscs are among the most robust material remains. Shells fashioned into personal adornments survive well in most sedimentary contexts (Bar-Yosef-Mayer & Beyin, 2009; Lombardo et al., 2013) and can be interpreted in various ways, depending on the context of the find. Usually the deposits are associated with graves (Vanhaeren et al., 2004; Vanhaeren & D’Errico, 2005), human shelters (Kuhn et al., 2001) and hearths (Douka et al., 2014). Some of the earliest forms of body adornment are shell beads that date back to ∼75 Kya (Henshilwood et al., 2004) and ∼82 Kya (Bouzouggar et al., 2007), possibly even 100–130 Kya (Vanhaeren et al., 2006) or earlier (Bednarik, 2015). However, some researchers argue that this “modern behaviour” was probably established earlier than is reflected in the archaeological record, and is simply not visible due to taphonomic processes (Bowdler & Mellars, 1990; Noble & Davidson, 1996; Botha, 2008; Botha, 2010). Teasing apart pre-depositional effects in mollusc remains, however, can be made more difficult because predators can produce modifications which are similar to those produced by humans through their ability to make holes in shells.
Researchers use detailed analyses of adornments, radiometric dates and stratigraphic information to explain innovations in shell beads and the spread of cultural traditions (Kuhn et al., 2001). The location of piercings in shells can provide information on the placement of the shell bead within the finished adornment (e.g., a necklace; Baysal, 2013; Stiner, Kuhn & Güleç, 2013). Indications of human manipulation can also be detected, such as striations indicating rotary drilling by a tool (Zilhão et al., 2010), notches close to the perforation that might indicate the presence of a suspension system (e.g., cord) and the direction the traction was exerted (Cristiani, 2012). Researchers also use experiments to understand shell anatomy (e.g., mineralogy and structure) and the processes involved in the production of piercings (e.g., Beyin, 2010; Nigra & Arnold, 2013; Tátá et al., 2014; Joordens et al., 2015). Microscopy can provide evidence of the shape of the tools used for piercing shells, as well as other tell-tale signs of human activity (D’Errico et al., 2005; Nigra & Arnold, 2013). For example, piercings are often examined for the presence of residues, such as ochre or polishing by the cord (D’Errico et al., 2005; D’Errico et al., 2009; Stiner, Kuhn & Güleç, 2013). Similarly, microscopic analyses of naturally made holes in molluscs provide insight (e.g., Li, Young & Zhan, 2011; Gorzelak et al., 2013).
Based upon this kind of painstaking evidence-gathering, experts make judgements as to whether perforations in shells from archaeological sites are anthropogenic in origin or formed by natural processes (D’Errico et al., 2005), such as those made by hole-boring predators or parasites (Kowalewski, 2004; Li, Young & Zhan, 2011) or taphonomic processes (e.g., water erosion, crushing, diagenesis; Peacock et al., 2012; Gorzelak et al., 2013). While the location and type of the perforation is only part of a raft of evidence that indicates an operational chain (starting with the collection of the raw material, followed by the manufacture and use, and ending with its discard), some researchers have proposed that the anatomical locations of holes pierced in shells by humans exhibit low variability whereas holes made by non-human animals yield more random perforations (Stiner, 1999; Bouzouggar et al., 2007).
In the Palaeolithic, beads made from molluscs were desirable for body adornments (e.g., necklaces, headdresses), which likely varied due to decorative traditions of prehistoric human groups (Stiner, 2014). Although there is only rare evidence of shell bead arrangements from the Palaeolithic, we might expect beads strung in different arrangements to require differently placed piercings for the shells to hang according to a predetermined design. The evidence indicates that prehistoric people were adept at piercing holes in shells, but also made use of natural perforations when possible (Bar-Yosef Mayer, Vandermeersch & Bar-Yosef, 2009), suggesting that perforation location may also have varied based on opportunistic natural hole placements.
People also appear to have preferred mollusc species with vivid markings and that vary in size and shape (Stiner, 2014). Inter-specific morphological differences in shell size and shape also encompass variations in shell thickness that impacts the ease with which a piercing can be made. All these factors have influenced the attraction of humans to particular species of mollusc and likely contributed to the variability of hole placement in shells, hence, the assumption that humans pierce shells in consistent places, may not be borne out (D’Errico et al., 2005; Kuhn et al., 2009; Stiner, Kuhn & Güleç, 2013).
Furthermore, animal predation on mollusc populations is a widespread phenomenon (Quensen & Woodruff, 1997; Rosin et al., 2011). Such behaviours have been observed for many hole-boring predators, such as naticids, muricids, octopuses, crabs and birds (Grey, Lelievre & Boulding, 2005; Grey, 2005; Rosin et al., 2011; Li, Young & Zhan, 2011). Moreover, predators can be specific in where they attack molluscs because shell strength and location of internal organs can be important in prey selection (Hagadorn & Boyajian, 1997; Dodge & Scheel, 1999; Rosin et al., 2013). For instance, birds usually choose to perforate the part of the gastropod shell near the apex, which is less resistant to crushing than, for example, the labium (Rosin et al., 2013). In contrast, octopuses and predatory snails choose areas close to the bivalve umbo, which tends to be thicker than other areas of the shell, but is near to the heart. This strategy appears to be a compromise between the time taken to pierce the shell and the effectiveness of the injected toxin (Dodge & Scheel, 1999).
Recent evidence also indicates that holes in shells can be made without the action of predators or humans. In a set of shell-rolling experiments that imitate the action of the waves and tides, Gorzelak and co-workers (2013) showed that abrasive action of rolling shells together can create holes in predictable locations that coincide with the holes of predators. It is therefore possible that abrasion may also imitate human actions.
Considering that pierced shells can not only be produced by humans for making Palaeolithic jewellery, but can also be produced by natural processes, we examined if the range of variability of hole location in shells made by humans is less than the variability in hole location in shells made by non-human animals (Stiner, 1999; Bouzouggar et al., 2007). We discuss these findings with a focus on the actions of mollusc predators, and consider factors researchers might want to consider when interpreting shells recovered from archaeological contexts.
Materials & Methods
The first part of this research assessed the variability of hole placement in shell beads made by humans. For this purpose we reviewed information within 316 publications including articles, PhD theses and chapters in books about malacological findings in archaeological contexts (Supplemental Information 3). Most of the gathered literature was written in English, with only a few papers published in other language (e.g., French or Spanish). We searched for these using Google Scholar and SCOPUS, and using keywords such as: shell beads, pierced shells, beads, shells, mollusc, Gastropoda, bivalves, pendant, shell midden, ornaments, shell ornaments, predators. Once the publications were selected, their references (backward search) and citation records (forward search) were analysed to find other articles that could provide relevant data (Table 1). Gathered literature was published between the 1966 and the first quarter of 2015. From these articles we selected papers which contained figures and information about the perforated shells. Related articles, which included the same figures of shells, were rejected. This approach ensured that site data was only assessed once (i.e., data were not replicated). We were able to select 79 papers from 316 gathered scientific articles.
| Mollusc species (Class) | Country | Site (N) | Date | References | HT (n) |
|---|---|---|---|---|---|
| Acanthocardia tuberculata (Bivalvia) | Cyprus | Shillourokambus (1) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 9 (1) |
| France | Balauzerie (1) | 40.000–28.000 BP | Barge (1983) | 9 (1) | |
| Régismont (1) | 40.000–28.000 BP | Fiocchi (1998) | 9 (1) | ||
| Tournal (1) | 40.000–28.000 BP | Fiocchi (1998) | 9 (1) | ||
| Italy | Fanciulli (1) | 40.000–28.000 BP | Barge (1983) | 9 (1) | |
| Riparo Mochi (1) | 34.870–32.280 BP | Barge (1983) | 9 (1) | ||
| Spain | Cueva de los Aviones (1) | 50.000 BP | Zilhão et al. (2010), Vanhaeren & D’Errico (2011) | 9 (1) | |
| Cova de l’Or (4) | 6.720–6.265 BP | Zilhão et al. (2010) | 9 (4) | ||
| Cova del Parpallo (2) | 50.000–10.000 BP | Zilhão et al. (2010) | 9 (1), 10 (1) | ||
| Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 10 (1) | |
| Antalis sp. (Scaphopoda) | Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 5 (1) |
| Portugal | Vale Boi (4) | 20.570–18.859 BP | Tátá et al. (2014) | 5 (4) | |
| Spain | El Cuco (30) | 29.000–22.000 ka | Gutiérrez-Zugasti & Cuenca-Solana (2013) | 5 (30) | |
| Guilanya (5) | 14.160–9.500 BP | Martinez-Moreno, Mora & Casanova (2010) | 5 (5) | ||
| Tito Bustillo (1) | 18.000–10.000 ka | Avezuela (2014) | 5 (1) | ||
| Bolinus brandaris (Gastropoda) | Cyprus | Shillourokambus (1) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 4 (1) |
| Buccinum undatum (Gastropoda) | Italy | Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 4 (1) |
| Cerastoderma sp. (Bivalvia) | Spain | Cova del Parpallo (8) | 50.000–10.000 BP | Zilhão et al. (2010) | 9 (4), 10 (4) |
| Cerithium sp. (Gastropoda) | Italy | Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 7 (1) |
| Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 6 (1) | ||
| Jordan | Wadi Mataha (1) | 15.579–11.042 BP | Janetski & Bar-Yosef (2005) | 2 (1) | |
| Chlamys sp. (Bivalvia) | Italy | Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 10 (1) |
| Clanculus corallines (Gastropoda) | Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 6 (1) |
| Italy | Cala (1) | 40.000–28.000 BP | Fiocchi (1998) | 4 (1) | |
| Riparo Mochi (3) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 4 (3) | ||
| Columbella sp. (Gastropoda) | Austria | Krems-Hundsteig (10) | 40,000–28,000 BP | Fiocchi (1998), Wild et al. (2008) | 2 (5), 3 (5) |
| Croatia | Zala cave (N10) | 11.070–10.500 BP | Komšo & Vukosavljević (2011) | 3 (4), 4 (5), 7 (1) | |
| Cyprus | Shillourokambus (3) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 4 (3) | |
| Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 4 (1) | |
| Italy | Cala (1) | 40.000–28.000 BP | Fiocchi (1998) | 2 (1) | |
| Grotta di Pozzo (2) | 85.000–60.000 BP | Mussi et al. (2000) | 4 (2) | ||
| Riparo Biarzo (16) | 12.000–5.600 BP | Cristiani (2012) | 3 (1), 4 (7), 6 (8) | ||
| Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 4 (1) | ||
| Near East | Ksar Akil (6) | 41.000–39.000 BC | Inizan (1978), Douka (2013) | 3 (2), 7 (3), 4 (1), 11 (1) | |
| Sefunim (1) | 41.000–15.000 BP | Bar-Yosef (1996a) | 4 (1) | ||
| Russia | Kostienki 1 (1) | 36.500–32.600 BP | Sinitsyn (1993) | 2 (1) | |
| Spain | Botiquería de Los Moros (6) | 6.000–4.000 BP | Álvarez-Fernández (2010) | 4 (2), 8 (3), 11 (1) | |
| Turkey | Pınarbaşı (3) | 8.5000–8.000 BC | Baysal (2013) | 8 (1) | |
| Conus sp. (Gastropoda) | Australia | Mandu Mandu Creek rock-shelter (1) | 35.200–30.900 BP | Morse (1993), Balme & Morse (2006) | 8 (1) |
| Cyprus | Shillourokambus (2) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 8 (2) | |
| Italy | Cala (1) | 40.000–28.000 BP | Fiocchi (1998) | 4 (1) | |
| Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998), Stiner (1999) | 2 (1) | ||
| Oman | Sumhuram (1) | 4.000–1.000 BP | Wilkens (2005) | 8 (1) | |
| Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 8 (1) | |
| Cyclope sp. (Gastropoda) | France | Abri Peyrony (13) | 40.000–28.000 BP | Vanhaeren & D’Errico (2011) | 1 (1), 3 (6), 4 (1), 11 (3) |
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2007) | 1 (1) | ||
| Germany | Andernach-Martinsberg (4) | 13.200–12.820 BP | Langley & Street (2013) | 1 (2), 3 (2) | |
| Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 6 (1) | |
| Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (1) | |
| Riparo Biarzo (1) | 9.000–7.000 BP | Cristiani (2012) | 1 (1) | ||
| Riparo Mochi (25) | 34.870–32.280 BP | Kuhn & Stiner (1998), Stiner (1999) | 1 (25) | ||
| Riparo Tagliente (2) | 14.600–11.5000 BC | Fontana et al. (2009) | 1 (2) | ||
| Spain | Cingle Vermell (1) | 9.760 BP | Oliva & Yll (2010) | 1 (1) | |
| La Pena de Estebanvela (4) | 12.000–9.000 BP | Avezuela (2014) | 1 (2), 4 (2) | ||
| Nerja Cave (2) | 25.000–21.000 BP | Jordá Pardo et al. (2010) | 1 (2) | ||
| Tito Bustillo (1) | 18.000–10.000 ka | Avezuela (2014) | 1 (1) | ||
| Cymatium parthenopeum (Gastropoda) | Cyprus | Shillourokambus (1) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 6 (1) |
| Cypraea sp. (Gastropoda) | India | Deccan region (3) | 2.300–900 BC | Deshpande-Mukherjee (2005) | 5 (3) |
| Dentalium sp. (Scaphopoda) | Austria | Krems-Hundsteig (1) | 40.000–28.000 BP | Fiocchi (1998), Neugebauer-Maresch (1999) | 5 (1) |
| Langmannersdorf (5) | 40.000–28.000 BP | Hahn (1972) | 5 (5) | ||
| Senftenberg (1) | 40.000–28.000 BP | Hahn (1972) | 5 (1) | ||
| Willendorf (1) | 28.000–22.000 BP | Kozłowski (1996) | 5 (1) | ||
| Cyprus | Shillourokambus (2) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 5 (2) | |
| France | Abri Peyrony (600) | 40.000–28.000 BP | Vanhaeren & D’Errico (2011) | 5 (600) | |
| Blanchard (2) | 34.000–32.000 BP | Taborin (1993) | 5 (2) | ||
| Caminade Est (1) | 37.200–32.140 BP | Taborin (1993) | 5 (1) | ||
| Castanet (1) | 34.000–32.000 BP | Taborin (1993) | 5 (1) | ||
| Cellier (1) | 40.000–28.000 BP | Taborin (1993) | 5 (1) | ||
| Laouza (1) | 40.000–28.000 BP | Fiocchi (1998) | 5 (1) | ||
| Pecheurs (1) | 28.000–22.000 BP | Barge (1983) | 5 (1) | ||
| Rochette (1) | 40.000–28.000 BP | Movius (1995) | 5 (1) | ||
| Rothschild (2) | 40.000–28.000 BP | Zilhão (2011) | 5 (2) | ||
| Saint-Germain-la-Rivière (1) | 15.570 BP | Vanhaeren & D’Errico (2005) | 5 (1) | ||
| Salpetriere (1) | 22.000–18.000 BP | Barge (1983) | 5 (1) | ||
| Tournal (1) | 40.000–28.000 BP | Fiocchi (1998) | 5 (1) | ||
| Tuto de Camalhot (1) | 40.000–28.000 BP | Taborin (1993) | 5 (1) | ||
| Vachons (1) | 40.000–28.000 BP | Taborin (1993) | 5 (1) | ||
| Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 5 (1) | |
| Italy | Cala (1) | 40.000–28.000 BP | Fiocchi (1998) | 5 (1) | |
| Grotta del Cavallo (1) | 31.000–21.000 BP | Cesnola & Mallegni (1996) | 5 (1) | ||
| Grotta di Pozzo (4) | 85.000–60.000 BP | Mussi et al. (2000) | 5 (4) | ||
| Fanciulli (1) | 40.000–28.000 BP | Barge (1983) | 5 (1) | ||
| Fumane (1) | 41.000–38.000 BP | Fiocchi (1998) | 5 (1) | ||
| Riparo Mochi (3) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 5 (3) | ||
| Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 5 (1) | ||
| Jordan | Wadi Mataha (6) | 15.579–11.042 BP | Janetski & Bar-Yosef (2005) | 5 (6) | |
| Near East | Ksar Akil (1) | 41.000–39.000 BP | Douka (2013) | 5 (1) | |
| Spain | Beneito (1) | 40.000–28.000 BP | Soler-Major (2001) | 5 (1) | |
| Cingle Vermell (1) | 9.760 BP | Oliva & Yll (2010) | 5 (1) | ||
| Cova del Parco (2) | 13.175–12.460 BP | Estrada et al. (2010), Mangado et al. (2010) | 5 (2) | ||
| Cova del Reclau Viver (53) | 39.000–29.000 BP | Avezuela Aristu & Álvarez-Fernández (2012) | 5 (53) | ||
| Nerja Cave (2) | 25.000–21.000 BP | Jordá Pardo et al. (2010) | 5 (2) | ||
| Roc del Migdia (4) | 8.800–8.190 BP | Oliva & Yll (2010) | 5 (4) | ||
| Turkey | Çatalhöyük (5) | 7.200–6.000 BP | Bar-Yosef Mayer, Gümüs & Islamoglu (2010) | 5 (5) | |
| Pınarbaşı (2) | 8.5000–8.000 BC | Baysal (2013) | 5 (2) | ||
| Boncuklu Höyük (3) | 9.000–8.000 BC | Baysal (2013) | 5 (3) | ||
| Engina mendicaria (Gastropoda) | Eritrea | Red Sea Coast (3) | 7.330–5.385 BP | Bar-Yosef-Mayer & Beyin (2009) | 4 (3) |
| Euthria cornea (Gastropoda) | Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 4 (1) |
| Gibbula sp. (Gastropoda) | Italy | Fumane (2) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (2) |
| Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 4 (1) | |
| Glycymeris sp. (Bivalvia) | Cyprus | Shillourokambus (1) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 9 (1) |
| France | Figuier (1) | 40.000–28.000 BP | Taborin (1993) | 9 (1) | |
| Israel | Qafzeh cave (1) | 90.000 y BP | Taborin (1993) | 9 (1) | |
| Italy | Fumane (6) | 41.000–38.000 BP | Vanhaeren & D’Errico (2011), Bertola et al. (2013) | 9 (6) | |
| Portugal | Gruta do Caldeirao (1) | 6.500–5.800 BP | Zilhão et al. (2010) | 4 (1) | |
| Spain | Cova de l’Or (16) | 6.720–6.265 BP | Zilhão et al. (2010) | 9 (16) | |
| Cova del Parpallo (7) | 50.000–10.000 BP | Zilhão et al. (2010) | 9 (7) | ||
| Cueva de los Aviones (2) | 50.000 BP | Zilhão et al. (2010) | 9 (2) | ||
| Hexaplex trunculus (Gastropoda) | Cyprus | Shillourokambus (2) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 4 (2) |
| Homalopoma sanguineum (Gastropoda) | Germany | Andernach-Martinsberg (54) | 13.200–12.820 BP | Álvarez-Fernández (2009), Langley & Street (2013) | 1 (1), 3 (1), 4 (21), 8 (8) |
| Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 6 (1) | |
| Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (1) | |
| Spain | Cova del Parco (1) | 13.175–12.460 BP | Mangado et al. (2010) | 11 (1) | |
| Tito Bustillo (1) | 18.000–10.000 ka | Álvarez Fernández (2006) | 4 (1), 8 (1) | ||
| Lithoglyphus sp. (Gastropoda) | Croatia | Pupićina Cave (1) | 11.070–10.500 BP | Komšo & Vukosavljević (2011) | 6 (1) |
| Zala cave (20) | 11.070–10.500 BP | Komšo & Vukosavljević (2011) | 1 (9), 4 (11) | ||
| Italy | Riparo Biarzo (7) | 12.000–7.000 BP | Cristiani (2012) | 3 (3), 7 (4) | |
| Littorina littorea (Gastropoda) | France | Gargas Cave (1) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 1 (1) |
| Hautes-Pyrénées (3) | 21.000–10.000 BP | Cattelain (2012) | 1 (1), 2 (1), 4 (1) | ||
| Spain | El Cuco (1) | 29.000–22.000 ka | Gutiérrez-Zugasti & Cuenca-Solana (2013) | 2 (1) | |
| Tito Bustillo (1) | 18.000–10.000 ka | Álvarez Fernández (2006) | 4 (1) | ||
| Littorina obtusata (Gastropoda) | France | Gargas Cave (2) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 1 (1), 4 (1) |
| Hautes-Pyrénées (97) | 10.000–6.000 BP | Cattelain (2012) | 1 (1), 2 (10), 4 (11) | ||
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2011) | 1 (1) | ||
| Spain | Cueto de La Mina (1) | 50.000–10.000 BP | Cáceres, Marcos & Diez (2008) | 4 (1) | |
| El Cuco (2) | 29.000–22.000 ka | Gutiérrez-Zugasti & Cuenca-Solana (2013) | 2 (1), 3 (1) | ||
| El Horno (2) | 12.862–12.481 BP | Fano & Álvarez-Fernández (2010) | 1 (2) | ||
| La Garma A (10) | 29.000–22.000 ka | Álvarez-Fernández (2007) | 1 (1), 4 (4), 8 (2) | ||
| Maltravieso cave (1) | 40.000–10.000 BP | Rodríguez Hidalgo et al. (2010) | 1 (1) | ||
| Nerja Cave (2) | 25.000–21.000 BP | Jordá Pardo et al. (2010) | 1 (1), 4 (1) | ||
| Littorina sp. (Gastropoda) | Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (1) |
| Riparo Mochi (1) | 34.870–32.280 BP | Stiner (2010) | 8 (1) | ||
| France | Saint-Jean-De-Verges (6) | 40.000–28.000 BP | Vezian & Vezian (1966) | 1 (2), 4 (2) | |
| Portugal | Vale Boi (9) | 20.570–18.859 BP | Tátá et al. (2014) | 1 (2), 4 (7), 8 (3) | |
| South Africa | Sibudu Cave Middle Stone (3) | 70.000–60.000 BP | D’Errico, Vanhaeren & Wadley (2008) | 1 (2), 4 (1) | |
| Melanopsis sp. (Gastropoda) | Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 3 (1) |
| Mitra corniculata (Gastropoda) | Italy | Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 4 (1) |
| Monodonta sp. (Gastropoda) | Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 4 (1) |
| Nassarius circumcintus (Gastropoda) | Spain | Moroccan cave (1) | 83.000–60.000 BP | D’Errico et al. (2009) | 4 (1) |
| Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (1) | |
| Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 4 (1) | ||
| Nassarius gibbosulus (Gastropoda) | Algeria | Oued Djebbanna (1) | 35.000 BP | Vanhaeren & D’Errico (2006), Douka (2013) | 2 (1) |
| Cyprus | Shillourokambus (6) | 9.000–8.000 BP | Serrand, Vigne & Guilaine (2005) | 6 (6) | |
| France | Blanchard (1) | 34.000–32.000 BP | Taborin (1993) | 4 (1) | |
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2011) | 4 (1), 11 (1) | ||
| Israel | Skhul (2) | 110.000 BP | Vanhaeren & D’Errico (2006) | 1 (1), 2 (1) | |
| Italy | Fumane (1) | 41.000–38.000 BP | Vanhaeren & D’Errico (2011) | 3 (1) | |
| Riparo Mochi (1) | 40.000–28.000 BP | Kuhn & Stiner (1998), Stiner (1999) | 4 (1) | ||
| Morocco | Grotte des Contrebandiers (1) | 40.000–12.500 BP | Vanhaeren & D’Errico (2011) | 4 (1) | |
| Grotte des Pigeons, Taforalt (13) | 83.000–81.000 BP | Vanhaeren & D’Errico (2011), Elias (2012), Douka (2013) | 1 (1), 2 (5), 3 (1), 4 (4), 6 (1), 11 (1) | ||
| Near East | Ksar Akil (2) | 41.000–39.000 BP | Douka et al. (2013) | 1 (1), 4 (1) | |
| Sefunim (1) | 41.000–15.000 BP | Bar-Yosef (1996b) | 4 (1) | ||
| Spain | Moroccan cave (17) | 83.000–60.000 BP | D’Errico et al. (2009) | 2 (4), 3 (3), 4 (8), 11 (2) | |
| Turkey | Üçağızlı (11) | 41.000–39.000 BC | Kuhn et al. (2001), Stiner, Kuhn & Güleç (2013) | 1 (1), 2 (1), 3 (2), 4 (6), 7 (2), 8 (1) | |
| Nassarius incrassatus (Gastropoda) | Italy | Fumane (2) | 41.000–38.000 BP | Vanhaeren & D’Errico (2011), Bertola et al. (2013) | 2 (2) |
| Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 4 (1) | ||
| Riparo Tagliente (1) | 14.600–11.5000 BC | Fontana et al. (2009) | 4 (1) | ||
| Spain | Cingle Vermell (1) | 9.760 BP | Oliva & Yll (2010) | 1 (1) | |
| El Horno (1) | 12.862–12.481 BP | Fano & Álvarez-Fernández (2010) | 3 (1) | ||
| Roc del Migdia (7) | 8.800–8.190 BP | Oliva & Yll (2010) | 4 (7) | ||
| Nassarius kraussianus (Gastropoda) | South Africa | Blombos Cave (2) | 78.000–75.600 BP | D’Errico et al. (2005), Douka (2013), Vanhaeren et al. (2013) | 1 (1), 3 (1) |
| Border Cave (2) | 44.000–22.000 BP | D’Errico et al. (2012) | 3 (1), 4 (1), 11 (2) | ||
| Nassarius mutabilis (Gastropoda) | France | Rothschild (1) | 40.000–28.000 BP | Zilhão (2011) | 4 (1) |
| Italy | Fumane (2) | 41.000–38.000 BP | Vanhaeren & D’Errico (2011), Bertola et al. (2013) | 2 (1), 4 (1) | |
| Spain | Tito Bustillo (1) | 18.000–10.000 ka | Álvarez Fernández (2006) | 1 (1), | |
| Turkey | Üçağızlı (11) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 3 (9), 11 (5) | |
| Nassarius reticulates (Gastropoda) | Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 3 (1) |
| France | Rothschild (1) | 40.000–28.000 BP | Zilhão (2011) | 3 (1) | |
| Russia | Mezmaiskaya Cave (1) | 36.000–28.510 BP | Golovanova, Doronichev & Cleghorn (2010) | 2 (1), 11 (1) | |
| Spain | El Horno (1) | 12.862–12.481 BP | Fano & Álvarez-Fernández (2010) | 3 (1) | |
| Tito Bustillo (1) | 18.000–10.000 ka | Avezuela (2014) | 1 (1) | ||
| Nassarius sp. (Gastropoda) | Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 4 (1) |
| Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998) | 4 (1) | ||
| Jordan | Wadi Mataha (2) | 15.579–11.042 BP | Janetski & Bar-Yosef (2005) | 3 (2) | |
| Morocco | Grotte des Contrebandiers (1) | 40.000–12.500 BP | D’Errico et al. (2009) | 4 (1), 11 (1) | |
| Spain | Cova del Parco (1) | 13.175–12.460 BP | Mangado et al. (2010) | 4 (1) | |
| Turkey | Boncuklu Höyük (1) | 9.000–8.000 BC | Baysal (2013) | 11 (1) | |
| Pınarbaşı (2) | 8.5000–8.000 BC | Baysal (2013) | 4 (2) | ||
| Natica sp. (Gastropoda) | Italy | Fumane (1) | 41.000–38.000 BP | Vanhaeren & D’Errico (2011) | 3 (1) |
| Spain | Cova del Parco (1) | 13.175–12.460 BP | Mangado et al. (2010) | 3 (1) | |
| Naticarius sp. (Gastropoda) | Turkey | Üçağızlı (2) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 1 (2), 8 (1) |
| Neritina picta (Gastropoda) | France | Hautes-Pyrénées (1) | 21.000–10.000 BP | Cattelain (2012) | 4 (1) |
| Abri Peyrony (99) | 40.000–28.000 BP | Vanhaeren & D’Errico (2011) | 1 (1), 4 (54) | ||
| Gargas Cave (1) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 1 (1) | ||
| Nucella lapillus (Gastropoda) | France | Hautes-Pyrénées (2) | 21.000–10.000 BP | Cattelain (2012) | 1 (1), 11 (1), 4 (1) |
| Gargas Cave (2) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 1 (2), 11 (1) | ||
| Rothschild (1) | 40.000–28.000 BP | Zilhao (2010) | 4 (1) | ||
| Saint-Germain-la-Rivière (1) | 15.570 BP | Vanhaeren & D’Errico (2005) | 4 (1) | ||
| Greece | Klisoura (1) | 41.000–38.000 BP | Stiner (2010) | 4 (1) | |
| Spain | La Garma A (1) | 29.000–22.000 ka | Avezuela Aristu & Álvarez-Fernández (2012) | 4 (1), 8 (1), 11 (1) | |
| Ocinebrina edwardsii (Gastropoda) | Spain | Cueto de la Mina (1) | 18.000–10.000 ka | Sella (1916) | 2 (1) |
| Italy | Riparo Mochi (7) | 34.870–32.280 BP | Kuhn & Stiner (1998), Stiner (1999) | 4 (7) | |
| Oliva bulbosa (Gastropoda) | Oman | Sumhuram (9) | 4.000–1.000 BP | Wilkens (2005) | 8 (9) |
| Patella vulgata (Gastropoda) | France | Hautes-Pyrénées (3) | 21.000–10.000 BP | Cattelain (2012) | 10 (3) |
| Spain | La Garma A (1) | 29.000–22.000 ka | Avezuela Aristu & Álvarez-Fernández (2012) | 10 (1) | |
| Maltravieso cave (1) | 40.000–10.000 BP | Rodríguez Hidalgo et al. (2010) | 10 (1) | ||
| Pecten sp. (Bivalvia) | France | Gargas Cave (1) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 10 (1) |
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2007) | 1 (1) | ||
| Spain | Cueva Anton (1) | 38.440–36.810 BP | Zilhão et al. (2010) | 4 (1) | |
| Riparo Mochi (1) | 40.000–28.000 BP | Stiner (1999) | 4 (1) | ||
| Persicula terveriana (Gastropoda) | Eritrea | Red Sea Coast (1) | 7.330–5.385 BP | Bar-Yosef-Mayer & Beyin (2009) | 4 (1) |
| Pirenella plicata (Gastropoda) | France | Hautes-Pyrénées (1) | 21.000–10.000 BP | Cattelain (2012) | 2 (1) |
| Theodoxus fluviatilis (Gastropoda) | France | Gargas Cave (1) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 1 (1) |
| Hautes-Pyrénées (1) | 21.000–10.000 BP | Cattelain (2012) | 1 (1) | ||
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2007) | 1 (1) | ||
| Portugal | Vale Boi (5) | 20.570–18.859 BP | Tátá et al. (2014) | 4 (5) | |
| Russia | Kostienki 14 (4) | 36.500–32.600 BP | Sinitsyn (2003) | 2 (2), 3 (1) | |
| Spain | Cova del Parco (2) | 13.175–12.460 BP | Estrada et al. (2010), Mangado et al. (2010) | 1 (1) 4 (1) | |
| Nerja Cave (4) | 25.000–21.000 BP | Jordá Pardo et al. (2010) | 4 (4) | ||
| Theodoxus sp. (Gastropoda) | Greece | Klisoura (3) | 41.000–38.000 BP | Stiner (2010) | 6 (3) |
| Italy | Riparo Biarzo (2) | 12.000–7.000 BP | Cristiani (2012) | 1 (1), 8 (2) | |
| Turkey | Üçağızlı (1) | 41.000–39.000 BC | Stiner, Kuhn & Güleç (2013) | 4 (1) | |
| Trivia sp. (Gastropoda) | France | Gargas Cave (3) | 26.910–23.590 BP | Juan-Foucher & Foucher (2008) | 3 (3), 11 (2) |
| Hautes-Pyrénées (2) | 21.000–10.000 BP | Cattelain (2012) | 1 (2) | ||
| Rothschild (1) | 40.000–28.000 BP | Zilhão (2011) | 3 (1) | ||
| Saint-Germain-la-Rivière (3) | 15.570 BP | Vanhaeren & D’Errico (2005) | 5 (3) | ||
| Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013) | 11 (1) | |
| Riparo Mochi (1) | 34.870–32.280 BP | Kuhn & Stiner (1998), Stiner (1999) | 3 (1) | ||
| Portugal | Vale Boi (4) | 20.570–18.859 BP | Tátá et al. (2014) | 5 (1), 8 (3) | |
| Spain | Cingle Vermell (2) | 9.760 BP | Oliva & Yll (2010) | 5 (2) | |
| El Horno (1) | 12.862–12.481 BP | Fano & Álvarez-Fernández (2010) | 5 (1) | ||
| La Fragua (1) | 12.960 BP | Zugasti (2010.) | 5 (1) | ||
| La Pena de Estebanvela (4) | 12.000–9.000 BP | Avezuela (2014) | 5 (2) | ||
| Los Canes (6) | 7.930–7.580 BP | Álvarez-Fernández (2010) | 3 (6) | ||
| Nerja Cave (1) | 25.000–21.000 BP | Jordá Pardo et al. (2010) | 3 (1) | ||
| Tito Bustillo (1) | 18.000–10.000 ka | Avezuela (2014) | 5 (1) | ||
| Trophon muricatus (Gastropoda) | Russia | Mezmaiskaya Cave (1) | 36.000–28.510 BP | Golovanova, Doronichev & Cleghorn (2010) | 11 (1) |
| Turritella sp. (Gastropoda) | Italy | Fumane (1) | 41.000–38.000 BP | Bertola et al. (2013), Taborin (1993) | 2 (1) |
| France | Abri Peyrony (25) | 40.000–28.000 BP | Vanhaeren & D’Errico (2011) | 2 (6), 3 (19) | |
| Spain | Cova del Reclau Viver (1) | 39.000–29.000 BP | Avezuela Aristu & Álvarez-Fernández (2012) | 3 (1), 11 (1) | |
| El Horno (4) | 18.000–10.000 ka | Álvarez Fernández (2006) | 2 (4) |
Notes:
- N
-
the number of mollusc species
- n
-
the number of shells with appropriate hole type
- HT
-
hole type
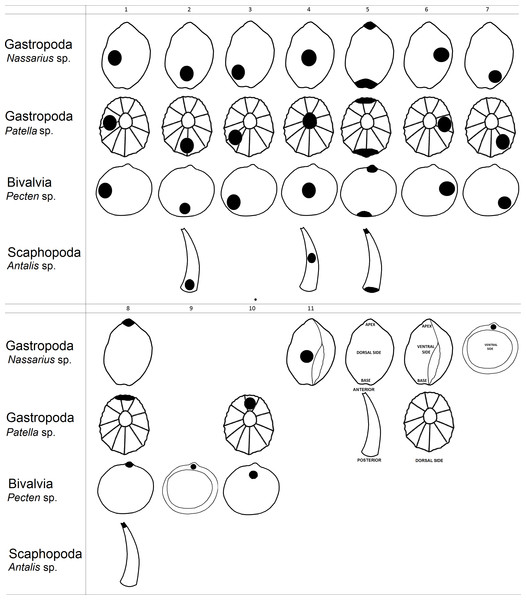
Figure 1: Graphical representation of hole location types in shells.
Information from all 79 papers was assessed for the following information: (1) mollusc species from which the shell beads were made; (2) name and country of the archaeological site where the perforated shells were found; (3) period from which the shell beads came; (4) hole location in the shell beads (Table 1). We made the assumption that analyses performed by experts correctly interpreted holes in shells as human made (i.e., the pierced shells were not predator-made intrusions).
Next, we created a classification of hole location in shell beads which helped us analyse gathered data from the literature (Fig. 1). As an example of shell shape we used species from genus Nassarius, Patella, Pecten and Antalis which are relatively common in the archaeological findings. Then, we assessed hole location in shells found in the archaeological literature (i.e., holes made by humans), based on the figures in each article. Our estimation was based on the figures within the publications, thus the analysis is not quantitative, but qualitative.
In the second part of the research we analysed variation of hole location in shells made by non-human animals. Thus, we searched for information on hole-making predators for each mollusc species recovered from each archaeological site with putative shell beads (Table 2). For this purpose, we gathered information on the location of holes in the shells made by mollusc predators from 54 scientific articles. To be clear, these articles were not associated with archaeological finds. Next, we assessed hole locations in shells made by hole-boring predators using the same classification that was used for the human-made holes in shell beads (Fig. 1). This part of the research was also based on the figures within the publications. We made the assumption that analyses by experts correctly interpreted holes in shells as naturally made and not made by humans (i.e., the shells were pierced by predators).
| Mollusc species recovered from archaeological sites (Class) | Predators class | Predator | References | HT (n) |
|---|---|---|---|---|
| Acanthocardia tuberculata (Bivalvia) | Gastropoda | Naticarius hebraeus | Calvet (1992) | 4 (1), 9 (1) |
| Antalis sp. (Scaphopoda) | No data | |||
| Bolinus brandaris (Gastropoda) | Cephalopoda | Octopus vulgaris | Nixon & Maconnachie (1988), Passini & Garassino (2012) | No data |
| Gastropoda | Naticidae | |||
| Buccinum undatum (Gastropoda) | Gastropoda | Euspira macilenta | Sawyer (2010) | No data |
| Cerastoderma sp. (Bivalvia) | Gastropoda |
Polinices pulchellus Hexaplex trunculus |
Kingsley-Smith, Richardson & Seed (2003), Morton, Peharda & Harper (2007) | 1 (1), 4 (1), 10 (1) |
| Cerithium sp. (Gastropoda) | Gastropoda | Euspira macilenta | Turra, Denadai & Leite (2005), Sawyer & Zuschin (2010), Coleman (2010), Gorman, Sikinger & Turra (2015) | 3 (1) |
| Malacostraca |
Callinectes danae Eriphia gonagra Menippe node frons Panopeus occidentalis |
|||
| Chlamys sp. (Bivalvia) | Asteroidea | Pycnopodia helianthoides | Guerrero & Reyment (1988), Farren & Donovan (2007), Chattopadhyay & Dutta (2013) | 2 (1), 10 (1) |
| Gastropoda | Murex sp. Naticidae | |||
| Clanculus corralinus (Gastropoda) | No data | |||
| Columbella sp. (Gastropoda) | Cephalopoda | Octopus vulgaris | Mather & O’Dor (1991) | No data |
| Conus sp. (Gastropoda) | Gastropoda | Euspira macilenta | Kohn & Arua (1999), Sawyer & Zuschin (2010) | 4 (2), 8 (1), 11 (3) |
| Cyclope sp. (Gastropoda) | Asteroidea | Astropecten sp. | Baeta & Ramón (2013) | No data |
| Cymatium parthenopeum (Gastropoda) | No data | |||
| Cypraea sp. (Gastropoda) | Cephalopoda | Octopus vulgaris | Nixon & Maconnachie (1988) | No data |
| Dentalium sp. (Scaphopoda) | Gastropoda |
Euspira macilenta Euspira obliquata Natica canrena Neverita duplicata Oichnus sp. |
Yochelson, Dockery & Wolf (1983), Sawyer & Zuschin (2010), Li, Young & Zhan (2011) | 4 (25), 8 (1) |
| Engina mendicaria (Gastropoda) | No data | |||
| Euthria cornea (Gastropoda) | Cephalopoda | Octopus vulgaris | Nixon & Maconnachie (1988), Nixon & Young (2003) | 4 (2) |
| Gibbula sp. (Gastropoda) | Cephalopoda | Octopus vulgaris | Guerra & Nixon (1987), Mowles, Rundle & Cotton (2011) | 4 (1) |
| Malacostraca | Carcinus maenas | |||
| Glycymeris sp. (Bivalvia) | Gastropoda |
Cryptonatica sp. Euspira sp. Glossaulax sp. Naticarius hebraeus |
Calvet (1992), Ramsay, Richardson & Kaiser (2001), Amano (2006), Sawyer & Zuschin (2010) | 4 (1), 9 (1) |
| Malacostraca | Cancer pagurus | |||
| Hexaplex trunculus (Gastropoda) | Cephalopoda | Octopus vugaris | McQuaid (1994), Sawyer & Zuschin (2010), Passini & Garassino (2012) | 4 (1), 11 (1) |
| Gastropoda | Naticidae | |||
| Homalopoma sanguineum (Gastropoda) | No data | |||
| Lithoglyphus sp. (Gastropoda) | No data | |||
| Littorina littorea (Gastropoda) | Asteroidea |
Pisastero straceaus Pycnopodia helianthoides |
Pechenik & Lewis (2000), Harley et al. (2013) | 11 (1) |
| Gastropoda | Naticidae | |||
| Littorina obtusata (Gastropoda) | Aves | Calidris canutus | Alerstam, Gudmundsson & Johannesson (1992), Edgell et al. (2008), Edgell & Rochette (2009) | No data |
| Malacostraca | Carcinus maenas | |||
| Littorina sp. (Gastropoda) | Malacostraca | Carcinus maenas | Reimchen (1982) | 1 (1), 4 (1) |
| Melanopsis sp. (Gastropoda) | Gastropoda | Gastropoda | Kowalewski, Rosa & Mancheno (2009) | No data |
| Mitra corniculata (Gastropoda) | Gastropoda | Euspira macilenta | Sawyer & Zuschin (2010), Cardoso & Dias Coelho (2012) | 4 (1) |
| Monodonta sp. (Gastropoda) | Aves | Haematopodidae Laridae | Tongiorgi et al. (1981), Harris (1984) | 4 (1) |
| Gastropoda | Ocinebrina edwardsi | |||
|
Nassarius circumcintus Nassarius gibbosulus Nassarius incrassatus Nassarius kraussianus Nassarius mutabilis Nassarius reticulates Nassarius sp. (Gastropoda) |
Malacostraca | Carcinus maenas | Stenzler & Atema (1977), Kohn & Arua (1999), Sawyer & Zuschin (2010) | 8 (1), 11 (1) |
| Gastropoda |
Euspira macilenta Lunatiaheros, Natica tecta |
|||
| Natica sp. (Gastropoda) | Gastropoda | Euspira macilenta Naticidae, Muricidae | Arua (1989), Zlotnik (2001), Sawyer (2010), Das, Mondal & Bardhan (2013) | 4 (1), 11 (37) |
| Naticarius sp. (Gastropoda) | Asteroidea | Asterina sarasini | Sawyer (2010) | No data |
| Gastropoda | Euspira macilenta | |||
| Neritina picta (Gastropoda) | Gastropoda | Acteocina Muricidae | Zagyvai & Demeter (2008) | 4 (2), 8 (4), 11 (1) |
| Polychaeta | Polychaeta | |||
| Nucella lapillus (Gastropoda) | No data | |||
| Ocinebrina edwardsii (Gastropoda) | No data | |||
| Oliva bulbosa (Gastropoda) | Gastropoda | Naticidae | Kohn & Arua (1999), Passini & Garassino (2012) | 11 (1) |
| Patella vulgata (Gastropoda) | Aves | Haematopus ostralegus | Coleman et al. (1999), Kohn & Arua (1999), Smith (2003), Silva et al. (2008), Silva et al. (2010), Sawyer (2010) | 10 (1) |
| Cephalopoda | Octopus vulgaris | |||
| Gastropoda | Euspira macilenta | |||
| Malacostraca |
Cancer pagurus, Carcinus maenas, Necora puber, Pachygrapsus marmoratus, |
|||
| Pecten sp. (Bivalvia) | Asteroidea |
Asteria srubens Marthasterias glacialis |
Jonkers (2000), Sawyer (2010), Magnesen & Redmond (2011) | 4 (2), 10 (1) |
| Gastropoda | Euspira macilenta | |||
| Persicula terveriana (Gastropoda) | Cephalopoda | Octopus insularis | Leite, Haimovici & Mather (2009) | No data |
| Pirenella plicata (Gastropoda) | Gastropoda | Naticidae | Taraschewski & Paperna (1982) | No data |
| Theodoxus fluviatilis (Gastropoda) | Aves | Gallinula chloropus | Blanco-Libreros & Arroyave-Rincón (2009) | 1 (1), 4 (2) |
| Malacostraca | Macrobrachium sp. | |||
| Theodoxus sp. (Gastropoda) | Gastropoda | Muricidae | Arpad (1993), Zagyvai & Demeter (2008) | 4 (2), 8 (4), 11 (1) |
| Trivia sp. (Gastropoda) | Asteroidea | Asterina sarasini | Sadhukhan & Raghunathan (2013) | No data |
| Trophon muricatus (Gastropoda) | Gastropoda | Naticidae | Gordillo & Archuby (2014) | 11 (1) |
| Turritella sp. (Gastropoda) | Gastropoda |
Euspira macilenta Naticidae Muricidae, Odostomia sp. |
Allmon, Nieh & Norris (1990), Hagadorn & Boyajian (1997), Filipescu & Popa (2001), Sawyer (2010) | 4 (3) |
Notes:
- n
-
the number of shells with appropriate hole type
- HT
-
hole type
Next, we analysed separately the types of hole location in shell beads made by human and non-human animals for normality by using the Shapiro Wilk test, and for homogeneity of variances by using Levene’s test; all were non-significant (P < 0.05). Analysed material did not fulfil the criterion of normality, thus data were log or square root transformed and tested again for normality (Sokal & Rohlf, 1981). After these transformations the data were still not normally distributed; we therefore used nonparametric sign test to analyse variation and differences in types of hole location between shells perforated by humans and non-human animals within mollusc species.
In order to analyse the strength of the differences between holes locations made by human and non-human animals we calculated size of the effect using the following equation: d = (M1 − M2)/SDpooled, where d is the Cohen-d index, M1 is the mean of the first group, M2 is the mean of the second group and SDpooled is the pooled standard deviation (Cohen, 1988). To interpret d values we used the following criteria for effect sizes: d⩾0.1, small; d⩾0.3, medium; d⩾0.5, large (Cohen, 1988).
Results
Human made holes in shells
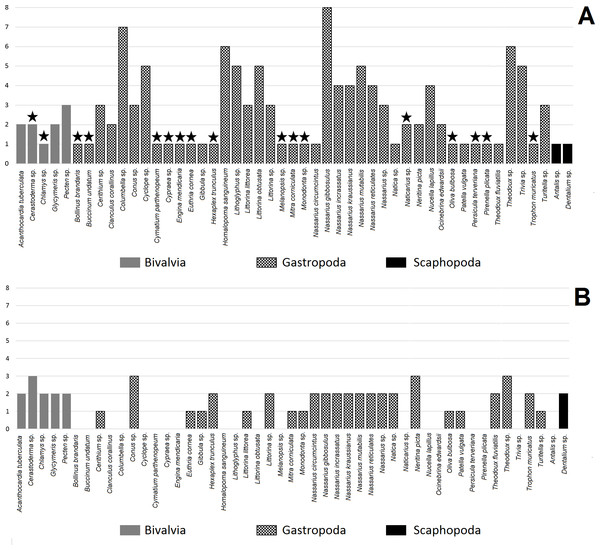
Table 1 shows hole assessment in 49 taxa of Mollusca perforated by humans from archaeological sites. Anthropogenically modified shells come from 21 countries of the Old World, with most archaeological sites located in Spain and France (Table 3). Twenty-seven taxa exhibited more than one type of hole location in their shells, while shells from 22 taxa were classified as having only one type of hole (Fig. 2A). However, 15 taxa from the latter group were recovered at one archaeological site alone.
| Country | Site | Bivalvia | Gastropoda | Scaphopoda | Summary | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | HT | N | HT | N | HT | N | n | ||
| Algeria | Oued Djebbanna | 1 | 2 | 1 | 1 | ||||
| Australia | Mandu Mandu Creek rock-shelter | 1 | 8 | 1 | 1 | ||||
| Austria | Krems-Hundsteig | 1 | 2, 3 | 1 | 5 | 2 | 3 | ||
| Langmannersdorf | 1 | 5 | 1 | 1 | |||||
| Senftenberg | 1 | 5 | 1 | 1 | |||||
| Willendorf | 1 | 5 | 1 | 1 | |||||
| Croatia | Pupićina Cave | 1 | 6 | 1 | 1 | ||||
| Zala cave | 2 | 1, 3, 4, 7 | 2 | 4 | |||||
| Cyprus | Shillourokambus | 2 | 9 | 6 | 4, 6, 8 | 1 | 5 | 9 | 5 |
| Eritrea | Red Sea Coast | 2 | 4 | 2 | 1 | ||||
| France | Abri Peyrony | 3 | 1, 2, 3, 4, 11 | 1 | 5 | 4 | 6 | ||
| Balauzerie | 1 | 9 | 1 | 1 | |||||
| Blanchard | 1 | 4 | 1 | 5 | 2 | 2 | |||
| Caminade Est | 1 | 5 | 1 | 1 | |||||
| Castanet | 1 | 5 | 1 | 1 | |||||
| Cellier | 1 | 5 | 1 | 1 | |||||
| Figuier | 1 | 9 | 1 | 1 | |||||
| Gargas Cave | 1 | 10 | 7 | 1, 3, 4, 11 | 8 | 5 | |||
| Hautes-Pyrénées | 8 | 1, 2, 4, 10, 11 | 8 | 5 | |||||
| Laouza | 1 | 5 | 1 | 1 | |||||
| Pecheurs | 1 | 5 | 1 | 1 | |||||
| Régismont | 1 | 9 | 1 | 1 | |||||
| Rochette | 1 | 5 | 1 | 1 | |||||
| Rothschild | 1 | 1 | 8 | 1, 3, 4, 11 | 1 | 5 | 10 | 5 | |
| Saint-Germain-la-Rivière | 2 | 4, 5 | 1 | 5 | 3 | 2 | |||
| Saint-Jean-De-Verges | 1 | 1, 4 | 1 | 2 | |||||
| Salpetriere | 1 | 5 | 1 | 1 | |||||
| Tournal | 1 | 9 | 1 | 5 | 2 | 2 | |||
| Tuto de Camalhot | 1 | 5 | 1 | 1 | |||||
| Vachons | 1 | 5 | 1 | 1 | |||||
| Germany | Andernach-Martinsberg | 2 | 1, 3, 4, 8 | 2 | 4 | ||||
| Greece | Klisoura | 7 | 4, 6 | 1 | 5 | 8 | 3 | ||
| India | Deccan region | 1 | 5 | 1 | 1 | ||||
| Israel | Qafzeh cave | 1 | 9 | 1 | 1 | ||||
| Skhul | 1 | 1, 2 | 1 | 2 | |||||
| Italy | Cala | 3 | 2, 4 | 1 | 5 | 4 | 3 | ||
| Fanciulli | 1 | 9 | 1 | 5 | 2 | 2 | |||
| Fumane | 1 | 9 | 13 | 2, 3, 4, 11 | 2 | 5 | 16 | 6 | |
| Grotta del Cavallo | 1 | 5 | 1 | 1 | |||||
| Grotta di Pozzo | 1 | 4 | 1 | 5 | 2 | 2 | |||
| Riparo Biarzo | 4 | 1, 3, 4, 6, 7, 8 | 4 | 6 | |||||
| Riparo Mochi | 3 | 4, 9, 10 | 12 | 1, 2, 3, 4, 7, 8 | 1 | 5 | 16 | 9 | |
| Riparo Tagliente | 6 | 1, 4, 6 | 1 | 5 | 7 | 4 | |||
| Jordan | Wadi Mataha | 2 | 2, 3 | 1 | 5 | 3 | 3 | ||
| Morocco | Grotte des Contrebandiers | 2 | 4, 11 | 2 | 2 | ||||
| Grotte des Pigeons, Taforalt | 1 | 1, 2, 3, 4, 6, 11 | 1 | 6 | |||||
| Near East | Ksar Akil | 2 | 1, 3, 4, 7, 11 | 1 | 5 | 3 | 6 | ||
| Sefunim | 2 | 4 | 2 | 1 | |||||
| Oman | Sumhuram | 2 | 8 | 2 | 1 | ||||
| Portugal | Gruta do Caldeirao | 1 | 4 | 1 | 1 | ||||
| Vale Boi | 3 | 1, 4, 5, 8 | 1 | 5 | 4 | 4 | |||
| Russia | Kostienki 14 | 2 | 2, 3 | 2 | 2 | ||||
| Mezmaiskaya Cave | 2 | 2, 11 | 2 | 2 | |||||
| South Africa | Blombos Cave | 1 | 1, 3 | 1 | 2 | ||||
| Border Cave | 1 | 3, 4, 11 | 1 | 3 | |||||
| Sibudu Cave Middle Stone | 1 | 1, 4 | 1 | 2 | |||||
| Spain | Beneito | 1 | 5 | 1 | 1 | ||||
| Botiquería de Los Moros | 1 | 4, 8, 11 | 1 | 3 | |||||
| Cingle Vermell | 3 | 1, 5 | 1 | 5 | 4 | 2 | |||
| Cova de l’Or | 2 | 9 | 2 | 1 | |||||
| Cova del Parco | 4 | 1, 3, 4, 11 | 1 | 5 | 5 | 5 | |||
| Cova del Parpallo | 3 | 9, 10 | 3 | 2 | |||||
| Cova del Reclau Viver | 1 | 3, 11 | 1 | 5 | 2 | 3 | |||
| Cueto de La Mina | 2 | 2, 4 | 2 | 2 | |||||
| Cueva Anton | 1 | 4 | 1 | 1 | |||||
| Cueva de los Aviones | 2 | 9 | 2 | 1 | |||||
| El Cuco | 2 | 2, 3 | 1 | 5 | 3 | 3 | |||
| El Horno | 5 | 1, 2, 3, 5 | 5 | 4 | |||||
| Guilanya | 1 | 5 | 1 | 1 | |||||
| La Fragua | 1 | 5 | 1 | 1 | |||||
| La Garma A | 3 | 1, 4, 8, 10, 11 | 3 | 5 | |||||
| La Pena de Estebanvela | 2 | 1, 4, 5 | 2 | 3 | |||||
| Los Canes | 1 | 3 | 1 | 1 | |||||
| Maltravieso cave | 2 | 1, 10 | 2 | 2 | |||||
| Moroccan cave | 2 | 2, 3, 4, 11 | 2 | 4 | |||||
| Nerja Cave | 4 | 1, 3, 4 | 1 | 5 | 5 | 4 | |||
| Roc del Migdia | 1 | 4 | 1 | 5 | 2 | 2 | |||
| Tito Bustillo | 6 | 1, 4, 5, 8 | 1 | 5 | 7 | 4 | |||
| Turkey | Boncuklu Höyük | 1 | 11 | 1 | 5 | 2 | 2 | ||
| Çatalhöyük | 1 | 5 | 1 | 1 | |||||
| Pınarbaşı | 2 | 4, 8 | 1 | 5 | 3 | 3 | |||
| Üçağızlı | 1 | 10 | 8 | 1, 2, 3, 4, 7, 8, 11 | 9 | 8 | |||
Notes:
- N
-
the number of mollusc species
- n
-
the number of hole types
- HT
-
hole type
Figure 2: Number of hole location types in shell for mollusc species. A, made by humans; B, made by non-human animals.
Star indicates taxon found at one archaeological site.The number of hole location types was diverse amongst mollusc species (Fig. 2A). Bivalves were more diverse in terms of hole location than Scaphopoda with most species exhibiting more than one type of hole location (Table 3). Gastropoda was the most numerous and diverse class in terms of hole location. Nassarius gibbosulus was the most variable species in terms of hole location among all archaeological sites (1, 2, 3, 4, 6, 7, 8 and 11) (Table 1; Fig. 2A). Moreover, shells of Nassarius gibbosulus from Üçağızlı (Turkey) and Grotte des Pigeons (Morocco) had the most variable hole location from single archaeological site (Table 3). Compared to other mollusc species, which are more frequently found at archaeological sites, but are less variable in terms of types of hole location, high variation in hole location in Nassarius gibbosulus does not appear to be a consequence of the relative abundance of this species.
Bivalves were the least common taxa found in archaeological assemblages and are characterised by hole locations 4, 9 and 10 (Table 3). Scaphopoda were more common than Bivalvia, but much less diverse in terms of types of hole location. Shell beads belonging to Scaphopoda had two holes, one in the anterior and the second one in the posterior of the shell (type number 5, Fig. 2A). In turn, gastropod shell beads were recovered at almost all archaeological sites and mostly showed more than one type of hole location.
Fumane (Italy) was the most diverse in context of number of gastropod species (13 taxa, Table 3), while Üçağızlı (Turkey) was characterised by the greatest number of types of hole location in gastropod shells at single archaeological site. Across all species, the most variable placed holes in shells were found at the site of Riparo Mochi (Italy) dated to the earliest Aurignacian (Kuhn & Stiner, 1998; Stiner, 1999).
Predator made holes in shells
Table 2 shows that almost all mollusc taxa recovered from the analysed archaeological sites are preyed upon by hole-making predators (41 taxa). Among these taxa we were able to assess the hole location in the shells made by non-human animals in 30 mollusc species. Most of the assessed species exhibited two types of hole location in their shells, while only four species were classified as having three types of hole (Fig. 2B).
All species from the Bivalvia have predators that make holes in shells (Table 2). Species from this mollusc class are usually associated with more than one predator. Gastropoda is the class with the most numerous predators (nine species), then the Asteroidea (three species) and then Malacostraca with only one predator (Table 2). Almost all taxa from the Gastropoda have non-human predators that attack the prey by piercing holes in the mollusc’s shell.
Assessment of hole location types in shells made by predators was possible only for one Scaphopoda species (Dentalium sp., Table 2). Dentalium sp. can be attacked by five species of the Gastropoda and is pierced by gastropod predators in two places: in the middle or at the apex of the shell. Bivalvia is more diverse than Scaphopoda in terms of the placement of holes made by non-human predators. Gastropoda, Asteroidea and Malacostraca make holes in bivalves in the following locations: 1, 2, 3, 9 and 10 (Fig. 1). In turn, gastropods have predators that usually pierce holes in only one or two locations in particular species (Table 2, Fig. 2B).
Comparison of hole locations made by humans and predators
The number of hole location types per scaphopod and bivalve species were higher for hole-boring predators than for anthropogenically modified shells. In turn, holes in gastropod shells made by humans were characterised by slightly greater variation than holes in shells pierced by non-human predators (Table 4). However, the sign test revealed no significant difference in types of hole location between shells perforated by humans and non-human animals within mollusc species (Table 4, P = 0.398). The result of the sign test was supported by value of the Cohen-d index. For differences between human made and non-human made holes in shell, the Cohen’s d value was small (d = 0.14, Table 4).
| Class | N | Mean | SD | Min | Max | N | P | Z | d |
|---|---|---|---|---|---|---|---|---|---|
| Human made holes in shells | 60 | 0.398 | −0.845 | 0.14 | |||||
| Scaphopoda | 2 | 1.00 | 0.00 | 1 | 1 | ||||
| Bivalvia | 5 | 2.00 | 0.63 | 1 | 3 | ||||
| Gastropoda | 42 | 2.72 | 1.93 | 1 | 8 | ||||
| Non-human made holes in shells | |||||||||
| Scaphopoda | 1 | 2.00 | 0.00 | 2 | 2 | ||||
| Bivalvia | 5 | 2.20 | 0.40 | 2 | 3 | ||||
| Gastropoda | 30 | 1.71 | 0.68 | 1 | 3 | ||||
Notes:
- N
-
the number of mollusc taxa
- SD
-
standard deviation
- Min
-
minimal number of hole location types at archaeological site
- Max
-
maximal number of hole location types at archaeological site
- n
-
the total number of compared taxa
- Z
-
result of sign test (P < 0.05)
Discussion
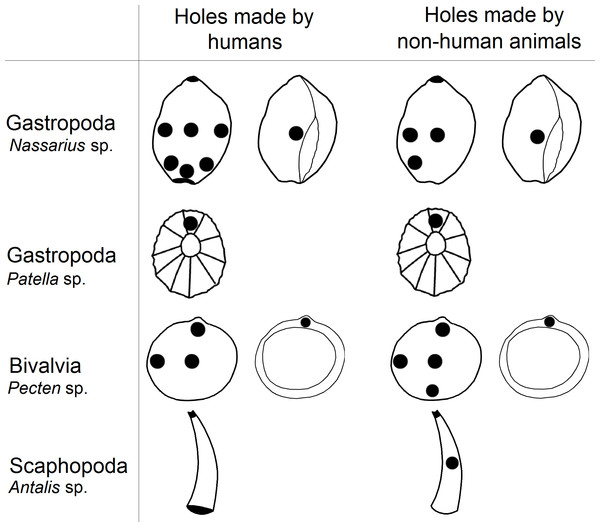
In this study we examined the assertion that the anatomical locations of holes in mollusc shells pierced by prehistoric human groups have lower variability compared to predator made holes, which have been said to be more randomly placed (Stiner, 1999; Bouzouggar et al., 2007). We found that holes in shells reported to have been pierced by humans were as variable as those made by predators. Furthermore, predators and humans pierced shells in similar locations (Fig. 3).
Figure 3: The locations of perforations in shells made by human and non-human animals.
Holes in the shells of Gastropoda
Among molluscs, species within the Gastropoda were found to be the most varied in terms of hole locations in shell beads made by humans (Fig. 2A, Table 4). This may be associated with natural morphological variation (shape, size and shell thickness) among species in this mollusc class (Stiner, 2014). Different styles of Palaeolithic bead adornments using ornate shells (Stiner, 2014) may also increase the variability of hole placements in the analysed shells.
Almost all gastropod taxa reported from archaeological sites are also vulnerable to hole-boring predators (e.g., Fig. 2B). In most cases, the predators belong to the class Gastropoda or Malacostraca (Table 2). The rest belong to Aves, Asteroidea, Cephalopoda and Polychaeta (Table 2). Despite the numerous predators, gastropod taxa had lower variation in the types of hole location per species made by non-human species than by humans (Table 4). These findings are supported by other research which shows that anatomical locations selected by predators of gastropods are not randomly selected, but are strategically located (Arpad, 1993; Zagyvai & Demeter, 2008). For example, in Neritina picta, access to the apex of the shell is preferred as a strategic location by predators belonging to Asteroidea, Muricidae, or Polychaeta (Zagyvai & Demeter, 2008). In turn, Theodoxus sp. usually exhibit a muricid (predatory sea snail) borehole that is often located close to the umbilicus (Arpad, 1993).
Holes in the shells of Scaphopoda
Scaphopoda was the least diverse mollusc class in terms of variation in hole location in shell beads made by humans (Table 4), which is probably a consequence of their characteristic anatomy (tusk shaped). All shell beads belonging to this class have two anatomical holes, one in the anterior (allowing the burrowing foot and captacula to protrude) and a second one in the posterior part of the shell (responsible for respiration; Reynolds, 2002). As such, these shells can be threaded onto a cord without being pierced.
Mean variation of hole location in shells pierced by predators of scaphopods was greater than in shells modified by humans (Table 4). Klompmaker (2011) found that predators pierced holes in the shells of Miocene scaphopods in the middle section of the shell, which is the thickest part. Whereas we found that Dentalium sp. was pierced in two places: in the middle or at the posterior part of the shell. However, we were only able to assess predator made holes in one species of Scaphopoda (Dentalium sp.), therefore results for this class should be interpreted with caution.
Holes in the shells of Bivalvia
Holes pierced by humans in bivalves were slightly more diverse than in scaphopods, but were less variable than in gastropods (Table 4). According to Carter (2008) bivalves in South America were rarely used as beads or pendants due to their size and weight and it is possible that most perforations could be attributed to predation or taphonomic processes. Other evidence, based upon context and use-wear analysis of shells from Palaeolithic sites, suggests the presence of bivalves can often be attributed to utilitarian purposes (e.g., food, receptacles for pigments) rather than use as body adornments (Harper, 2005; Bar-Yosef-Mayer, 2007; Rogalla & Amler, 2007; Douka et al., 2014). Zilhão et al. (2010) suggest that for most species of bivalves recovered from archaeological sites, anthropogenic modifications can be confirmed when (i) the weathering stage and perforation patterns do not agree with those seen in natural death assemblages; (ii) a tool was involved in the perforation, or (iii) the hole is associated with artificial modification of the shell’s geometry.
Predators of bivalves are also diverse and belong to Asteroidea, Gastropoda or Malacostraca. This variation in predators might be associated with higher variations in types of hole placement per species, which is slightly greater than reported in anthropogenically modified shells (Table 4). For example, variation in hole location is very low in Chlamys sp., with naticid and muricid predators usually choosing the region near the adductor muscle (corresponding to number 2 in Fig. 1), which may facilitate access to the viscera (Chattopadhyay & Dutta, 2013). Similar behaviour to non-human predators has been noted in the fossil bivalve Pseudodon in which 33% of holes were made near the anterior adductor muscle by Homo erectus at Trinil (Joordens et al., 2015). In this mollusc species, the adductor muscles are placed near the 1 and 6 of the hole location types (Fig. 1). Location of the holes made by H. erectus may vary from the data presented in our study because Pseudodon shells at Trinil, although engraved, appear to have been perforated in order to open the shells to access the meat rather than to be used as body adornments.
Evidence from the study of bivalve species from the early and middle Pleistocene, indicate that shells were most often pierced by non-human predators close to the umbo or near the centre (Amano, 2006). Only few examples of piercings near the adductor muscle have been described, and these could have been caused by the incomplete drill holes in prey which continue to grow after the attack changing the relative position of the incomplete hole (Chattopadhyay & Dutta, 2013).
Pierced shells; factors to consider
Our results show that within the analysed sample, there were no significant differences between the placement of holes made by non-human animals and those made by humans; both pierce shells in the same locations in most classes of molluscs (Table 4; Fig. 3). For example in Euthria cornea, Gibbula sp. and Mitra corniculata we observed that humans and non-human predators made holes in the same location (type number 4). Similarly, in Glycymeris sp. humans and predators pierced the same part of the shell (types number 4 and 9; Tables 1 and 2). However, for some mollusc species selection of hole location was only partial. For example in Littorina sp. we observed that humans and non-human animals made holes in the centre of shell (type number 4) and near apex (type number 8), but this species is also perforated by humans near the outer lip (type number 1; Tables 1 and 2).
The non-random piercing of holes by the mollusc predators is widely reported (e.g., Johannesson & Ekendahl, 2002; Kingsley-Smith, Richardson c A & Seed, 2003; Dietl & Kelley, 2006; Gorzelak et al., 2013) and it can be associated with shell thickness of prey which varies across the body, probably due to differential age of the shell whorls and predatory pressure on snails (Rosin et al., 2013). In molluscs with ornamental shells, up to five times more force can be required to make a hole (Dalziel & Boulding, 2005). Predatory gastropods can spend between three to twenty minutes locating a piercing site on their prey’s shell surface and, once the location is fixed, it may take from several hours to several days to pierce the shell, depending on the thickness (Hagadorn & Boyajian, 1997). The variation in shell thickness within and between mollusc species may be linked to why holes in shells most often occur near the lip and in the centre (e.g., types number 1, 2, 3 and 4; Fig. 1) and why humans and non-human predators choose similar locations to pierce shells of Bivalvia and Gastropoda (Fig. 3) However, results for scaphopods deviated from this pattern, with humans and non-human predators piercing holes in different locations (type 8 and 4 versus type 5, respectively; Fig. 1). Similarity in the choice of hole location made by humans and non-human predators in most species of mollusc could cause researchers to wrongly assign shell perforations as anthropogenically manipulated because they believe predator made holes are less likely to be pierced in suitable locations for threading (D’Errico et al., 2005; Bouzouggar et al., 2007).
Bicho & Haws (2008) have suggested that the larger biomass of molluscs in the Palaeolithic likely meant that mollusc gathering formed part of hunter-gatherers’ regular foraging behaviour in Portugal. Furthermore, predator drill frequency in gastropods and bivalves has been estimated to range between 2.8%–50.0% and 8.6%–34.1%, respectively. In contrast, scaphopods were pierced at a much lower rates (0.9% in Dentalium sp.; Taraschewski & Paperna, 1982; Yochelson, Dockery & Wolf, 1983; Hagadorn & Boyajian, 1997; Zagyvai & Demeter, 2008; Sawyer, 2010). It is possible that prehistoric people that regularly foraged for molluscs, were more likely exposed to a greater numbers of naturally perforated shells than we might expect. As such, the likelihood of finding shells with predator-made holes in locations suitable for threading could be higher than researchers believe.
Beads made from shells with holes made by natural processes have been identified at archaeological sites, for example, the perforated bead of Antalis sp. from the Early Upper Paleolithic site in El Cuco (Spain; Gutiérrez-Zugasti & Cuenca-Solana, 2013). Some researchers claim that shell beads from the Middle and Lower Palaeolithic could have been perforated by natural processes. For example, Bednarik (2015) proposed that predators and parasitic organisms commonly perforate mollusc shells and that it should be expected that naturally perforated shells were used as beads and pendants. Hahn (1972) emphasized that the signs of human manipulation in shells from Aurignacian sites such as Krems-Hundssteig, Willendorf, Kostienki 1 and Sjuren, are not always present and that some holes could have been made by predators. It is possible that before tools were used to bore holes, finding shells with holes in favourable positions for threading into ornate jewellery may have increased their importance or value. Similarly the natural apertures in fish vertebrae or crinoid discs may have made them attractive as items for threading (e.g., see Mussi, 2002).
Finally we would like to draw attention to the common assumption that predator-made holes are mostly made by chemical processes and tend to be round in outline, while shells pierced by humans have elliptical or irregular outlines (Stiner, 1999; Komšo & Vukosavljević, 2011). We would argue, however, that this statement is probably an overgeneralisation because many predators form holes in their prey which range in shape from nearly perfect circles to ellipsoids (Zagyvai & Demeter, 2008). For example, muricids, naticids and cephalopods use their radula to bore into mollusc shells and they can adapt the size and shape of the pierced hole to the morphology of their prey, as a result, bore holes can differ in shape (Walker & Brett, 2002). Bird beaks can also cause cracks and chips to the shells that imitate stone tool use (Ingolfsson & Estrella, 1978; Harper, 2005; Shumaker, Walkup & Beck, 2011). Thus, detailed analytical methods remain critical to identifying the tell-tale signs of anthropogenic manipulation of shells recovered from archaeological sites.
Conclusions
Some researchers have argued that holes in shells made by predators vary more than holes made by humans. However, our findings show that the variation in hole location on shell beads recovered from archaeological sites did not significantly differ from the locations of predator-made holes. Our assessment of hole location was based on figures from the published literature only, so it is possible that the true level of variation from beads recovered from archaeological sites might be higher (i.e., in collections not described in the literature).
This study highlights how the placement of holes on the shells made by predators can potentially be similar to human activity. Moreover, the likelihood of finding shells with holes made by predators in locations suitable for threading is probably higher than researchers believe. These findings emphasise the importance of the battery of tests currently used to identify whether piercings in shells are made naturally or are anthropogenic modifications. Dispelling assumptions about human and non-human predator hole placements in shells and providing information on the patterns of predation on molluscs can augment these tests in order to contribute to more realistic scenarios of the social and cultural expressions of prehistoric people.