Identification of a R2R3-MYB gene regulating anthocyanin biosynthesis and relationships between its variation and flower color difference in lotus (Nelumbo Adans.)
- Published
- Accepted
- Received
- Academic Editor
- Renate Scheibe
- Subject Areas
- Molecular Biology, Plant Science
- Keywords
- Flower color, Anthocyanin, MYB5 transcription factor, Nelumbo
- Copyright
- © 2016 Sun et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2016. Identification of a R2R3-MYB gene regulating anthocyanin biosynthesis and relationships between its variation and flower color difference in lotus (Nelumbo Adans.) PeerJ 4:e2369 https://doi.org/10.7717/peerj.2369
Abstract
The lotus (Nelumbonaceae: Nelumbo Adans.) is a highly desired ornamental plant, comprising only two extant species, the sacred lotus (N. nucifera Gaerten.) with red flowers and the American lotus (N. lutea Willd.) with yellow flowers. Flower color is the most obvious difference of two species. To better understand the mechanism of flower color differentiation, the content of anthocyanins and the expression levels of four key structural genes (e.g., DFR, ANS, UFGT and GST) were analyzed in two species. Our results revealed that anthocyanins were detected in red flowers, not yellow flowers. Expression analysis showed that no transcripts of GST gene and low expression level of three UFGT genes were detected in yellow flowers. In addition, three regulatory genes (NnMYB5, NnbHLH1 and NnTTG1) were isolated from red flowers and showed a high similarity to corresponding regulatory genes of other species. Sequence analysis of MYB5, bHLH1 and TTG1 in two species revealed striking differences in coding region and promoter region of MYB5 gene. Population analysis identified three MYB5 variants in Nelumbo: a functional allele existed in red flowers and two inactive forms existed in yellow flowers. This result revealed that there was an association between allelic variation in MYB5 gene and flower color difference. Yeast two-hybrid experiments showed that NnMYB5 interacts with NnbHLH1, NlbHLH1 and NnTTG1, and NnTTG1 also interacts with NnbHLH1 and NlbHLH1. The over-expression of NnMYB5 led to anthocyanin accumulation in immature seeds and flower stalks and up-regulation of expression of TT19 in Arabidopsis. Therefore, NnMYB5 is a transcription activator of anthocyanin synthesis. This study helps to elucidate the function of NnMYB5 and will contribute to clarify the mechanism of flower coloration and genetic engineering of flower color in lotus.
Introduction
In most species, flower color is attributed to the accumulation of anthocyanins (Quattrocchio et al., 2006). Anthocyanin are the major water-soluble pigments, produced by the flavonoid biosynthesis pathway, widely distributed in flowering plants and responsible for pink, purple, red, and blue of many flowers and fruits (Chandler & Tanaka, 2007; Wessinger & Rausher, 2014). Currently, all structural genes involved in the anthocyanin synthesis have been well characterized in many species (Schwinn & Davies, 2004; Grotewold, 2006; Guo et al., 2014). Anthocyanins are synthesized in the cytosol, and are not stabilized until they are transported into vacuole (Kitamura, Shikazono & Tanaka, 2004; Gomez et al., 2011). Glutathione S-transferase (GST) genes are thought to be involved in anthocyanins transport in many species, such as Arabidopsis TT19, maize Bz2, petunia AN9, grape VvGST1 and VvGST4, cyclamen CkmGST3 and peach Riant (Marrs et al., 1995; Alfenito et al., 1998; Kitamura, Shikazono & Tanaka, 2004; Conn et al., 2008; Kitamura et al., 2012; Cheng et al., 2015).
The structural genes are highly conserved among species (Holton & Cornish, 1995). Regulation of structural genes at the level of transcription appears to be the major mechanism that leads to the diversity of flower color in plants (Quattrocchio et al., 1998; Yamagishi et al., 2010). In all species studied to date, the anthocyanin biosynthesis is regulated by a complex of R2R3-MYB and basic helix-loop-helix (bHLH) transcription factors (TF) as well as WD40 proteins (Broun, 2005; Koes, Verweij & Quattrocchio, 2005; Petroni & Tonelli, 2011). Among them, the R2R3-MYB proteins can directly bind to the promoters of structural genes to activate their expression with the help of bHLH and WD40 proteins and are thought to be the most key TF (Takos et al., 2006; Zhang, Butelli & Martin, 2014; Tian et al., 2015). Anthocyanin-regulating R2R3-MYB genes were isolated from many ornamental plants and economic crops, such as grape VvMybA1, tomato ANT1, apple MdMYB10, gerbera GMYB10, orchid OgMYB1, gentian GtMYB3, pear PyMYB10, lily LhMYB12, crabapple McMYB10 and peach PpMYB10.1 (Kobayashi et al., 2002; Mathews et al., 2003; Espley et al., 2007; Chiou & Yeh, 2008; Laitinen et al., 2008; Nakatsuka et al., 2008; Feng et al., 2010; Yamagishi, Yoshida & Nakayama, 2012; Tian et al., 2015; Tuan et al., 2015). Examples of the interaction between anthocyanin-related MYBs and bHLHs include Zea mays ZmC1 and ZmB, gerbera GMYC1 and GMYB10, Arabidopsis PAP1, PAP2, MYB113 & MYB114 and TT8, petunia AN2 and AN1, apple MdMYB10 and MdbHLH33, and litchi LcMYB1 and LcbHLH1, LcbHLH3 (Goff, Cone & Chandler, 1992; Elomaa et al., 2003; Zimmermann et al., 2004; Quattrocchio et al., 2006; Espley et al., 2007; Lai et al., 2016).
The lotus (Nelumbonaceae: Nelumbo Adans.) is an ancient aquatic plants with large, showy and fragrant flowers, comprising only two extant species, the sacred lotus (N. nucifera Gaerten.) and the American lotus (N. lutea Willd.). N. nucifera is native to Asia and is the national flower of India; N. lutea is native to North America (Tian et al., 2008). Although these two species are allopatric, their hybrids are fertile. Among external morphologies (e.g., plant size, leaf shape, petal shape and color), petal color is the most obvious difference that distinguishes these two species of Nelumbo. The petal color of the wild sacred lotus is mainly red, whereas that of the American lotus is yellow. In Asia, the sacred lotus, N. nucifera, has been cultivated for more than 7,000 years for its beautiful flower as well as medicinal and cooking uses (Zhang, Chen & Wang, 2011). Lotus is one of the top ten traditional garden flowers in China, and over 800 cultivars are now under cultivation (Zhang, Chen & Wang, 2011). The petals of lotus cultivars possess a variety of bright colors including red, pink, white with red borders, plain white, and yellow.
So far, the relationship between flower color and pigments composition and content was elucidated among lotus cultivars using HPLC (Katori et al., 2002; Chen et al., 2013; Deng et al., 2013). The results showed that the red and pink flower cultivars contained all types of anthocyanins except for pelargonidin, while no anthocyanins were detected in white and yellow flower cultivars; flavones and flavonols were detected in all cultivars and small amounts of xanthophylls and β-carotene were detected in some yellow cultivars. Based on the analyses of metabolomic analysis, a putative flavonoid biosynthetic pathway was proposed in lotus (Chen et al., 2013). The whole-genome sequence of lotus has been reported, which facilitate the functional genomics studies in this species (Ming et al., 2013). The flower color difference between red and white lotus cultivars may be attributed to the different methylation intensities on the promoter sequences of the ANS gene (Deng et al., 2015). Although these studies have provided valuable insights into the mechanism of flower coloration in lotus cultivars, the regulation mechanism of anthocyanin biosynthesis and the cause of flower color difference between two species in Nelumbo remain unclear. In this study, regulatory genes were identified from lotus genome and analyzed in two lotus species. Three regulatory genes (MYB5, bHLH1 and TTG1) were isolated from red flowers. The interaction among three regulatory proteins and the function of NnMYB5 were tested.
Materials and Methods
Plant materials
The seeds of the wild lotus N. nucifera and N. lutea were collected from Yilan population of Heilongjiang province of China and Lake Jackson population of Florida of United States of America (USA), respectively. Seeds were germinated and grown at the Wuhan Botanical Garden (WBG), Chinese Academy of Sciences, under the same natural conditions. The flowers of these plants were collected in pre-anthesis while the petals were deeply pigmented. These flowers were immediately frozen with liquid nitrogen and stored at −80 °C for RNA extraction and anthocyanin extraction. The fresh leaves of each species were also collected and stored at −20 °C for DNA extraction.
To test whether there is a relationship between flower color difference of two species and regulatory genes, a total of 97 individuals from 16 wild lotus populations were further collected, including 49 individuals from eight populations of N. nucifera with red flower collected from China and Thailand and 48 individuals from eight populations of N. lutea with yellow flowers collected from USA (Table S1). For this analysis, fresh leaves of each individual were collected and dried immediately with silica gel and stored at room temperature. Voucher specimens from all populations were deposited in the herbarium of the WBG.
Anthocyanin extraction and measurement
Total anthocyanins were extracted from the petals according to the methods described previously (Rahim, Busatto & Trainotti, 2014) and anthocyanin contents were measured using spectrophotometry according to the formula, QAnthocyanins = (A530 − 0.25 × A657) × M−1 (Mehrtens et al., 2005), where QAnthocyanins is the amount of anthocyanins, A530 and A657 is the absorption at 530 and 657 nm wavelength, respectively, and M is the fresh weight (g) of the tissues.
DNA, RNA extraction and qRT-PCR analysis
Total RNA was isolated from petals using Quick RNA Isolation Kit for Polysaccharides and Polyphenolics-rich Plant (Zoman, Beijing, China). A DNA extraction kit (ComWin, Beijing, China) was used to extract DNA from leaves. Each RNA sample was treated with RNase-free DNase I (TaKaRa) to eliminate contaminating genomic DNA before the reverse transcription (RT) reaction. RT-PCR was performed according to the standard instructions of the PrimeScript RT Reagent Kit with gDNA Eraser (TaKaRa).
Considering a lot of flavones and flavonols in red and yellow flowers in lotus (Deng et al., 2013), which indicates that the upstream genes in the anthocyanin biosynthetic pathway are active, we only investigated the expression levels of downstream genes (DFR, ANS, UFGT and GST) by quantitative reverse transcription–PCR (qRT-PCR) and semi-quantitative RT-PCR (sqRT-PCR). qRT-PCR was conducted using StepOne Real-Time PCR System (Applied Biosystems, Foster City, CA, USA). A total reaction volume of 20 μL contained 10 μL of 2 × SYBR premix Ex Taq™ II (DRR082A, TaKaRa), 0.3 μM of each primer, about 100 ng of template cDNA. The amplification condition was as follows: incubation at 95 °C for 30 s, followed by 40 cycles of 95 °C for 3 s, 57 °C for 30 s and 72 °C for 30 s. A lotus actin gene (GenBank ID: EU131153) was used as a constitutive control. Gene relative expression levels of target genes was calculated by 2−ΔΔCt comparative threshold cycle (Ct) method, and three replicates were performed. Primer sequences (Table S2) were designed on the whole genome data of N. nucifera (GenBank accession: AQOG00000000; Ming et al., 2013) and the whole-genome resequencing data of N. lutea (∼10× coverage depth; J. M. Chen, 2015, unpublished data).
Identification of candidate R2R3-MYB, bHLH and WD40 transcription factors in the lotus genome
To identify the candidate R2R3-MYB, bHLH and WD40 proteins in the lotus genome, the R2R3 motif of Arabidopsis AtPAP1 (NP_176057.1) and the whole amino acid sequences of petunia PhAN11 (NP_197840.1) and Arabidopsis AtTTG1 (AAG25927.1) were used to tblastn against our local whole genome database of N. nucifera. The matched sequences with the E-value (Expect value) less than 1e−10 were used. Amino acid sequence alignments were performed using the ClustalW (Thompson, Higgins & Gibson, 1994). A phylogenetic tree was subsequently constructed according to the neighbor-joining (NJ) statistical method (Perrière & Gouy, 1996) using the program MEGA5 (Tamura et al., 2011). Confidence in the nodes was tested by performing 1,000 bootstrap replicates (Felsenstein, 1985).
Based on the result of our phylogenetic analyses, the specific primer pairs, such as MYBU1F/U1R, MYBU2F1/R1, MYBU2F2/R2 and MYBU3F/U3R for MYB genes and bHLH205F/1420R for bHLH gene, were designed in the conserved regions (Table S2) to determine which MYB genes and bHLH genes involved in anthocyanin synthesis were expressed in petals of N. nucifera. The red flowers with high content of anthocyanin were then chosen to isolate the MYB and bHLH genes. The PCR reactions were carried out using the specific primers designed above to amplify cDNAs of red flowers. The amplified fragments were then cloned and sequenced. A number of cDNAs encoding R2R3-MYB domains were obtained using the primer MYBU1F/U1R and all these cDNA clones showed highest identity to NnMYB5. Only one bHLH gene (thereafter named “NnbHLH1”) was detected in petals of N. nucifera. In addition, only one WD40 protein, named “NnTTG1,” was considered to possibly regulate anthocyanin biosynthesis in lotus according to our phylogenetic analyses. In order to study the regulatory gene involved in flower color, so in our subsequent analyses in N. nucifera, we only focused on the three regulatory genes (NnMYB5, NnbHLH1 and NnTTG1) expressed in red flower.
Cloning and sequencing of MYB5, bHLH1 and TTG1 in lotus
For two species in Nelumbo, the full-length cDNAs sequences and genomic sequences of MYB5, bHLH1 and TTG1 genes were amplified by using the primer pairs, MYBU1F/MYB5gR, bHLHgF/gR and TTG1gF/R, respectively. The promoter regions of MYB5 in two species were also cloned using MYBpF/R primer. The details of primer sequences were provided (Table S2).
Yeast two-hybrid analysis
To investigate protein interactions between NnMYB5 and NnbHLH1 or NlbHLH1 (obtained from N. lutea), between NnTTG1 and NnMYB5, and between NnTTG1 and NnbHLH1 or NlbHLH1, the Matchmaker Two-Hybrid System 3 (Clontech, Mountain View, CA, USA) was employed in the yeast two-hybrid (Y2H) assay. The full-length coding sequences of NnMYB5, NnbHLH1, NlbHLH1 and NnTTG1 were cloned into vector pGBK-T7, and then transformed into yeast strain Y2HGOLD according to manufacturer’s instructions (Yeastmaker, Clontech) to check self-activation. The open reading frame (ORF) sequences of NnMYB5 and NnTTG1 were cloned into the pGAD-T7 (with GAL4AD), and then transformed into yeast strain Y187. The primers used in Y2H system are shown (Table S2). After mating, yeast was plated onto SD–Trp–Leu plates. Positive colonies were validated by PCR amplification and then streaked on new SD–Trp–Leu and SD–Trp–Leu–His–Ade plates. Finally, positive colonies were transferred onto SD–Trp–Leu–His–Ade + X-α-Gal plates to further validate positive interaction between the two tested proteins.
Transgenic plasmid construction and plant transformation
To confirm the function of NnMYB5 gene in lotus, the genomic DNA of NnMYB5 was amplified and cloned into the pCAMBIA1301 vector under the control of CaMV 35S promoter with the primers 5′-CGAGCTCATGGATGGTGGTTTGGGTTT-3′ (forward) and 5′-CGGGATCCTCAATAACTCCACCACCTATG-3′ (reverse).
The above recombinant construct was then transformed into Agrobacterium tumefaciens strain GV3101 and introduced into Arabidopsis thaliana WT (Col-0) plants using the floral dip method (Clough & Bent, 1998) to obtain NnMYB5 over-expressing plants. Arabidopsis seeds were sterilized with 70% (v/v) ethyl alcohol, 10% (w/v) NaCl and deionized water. After stratification at 4 °C for 3 d in darkness, transgenic Arabidopsis seeds were selected on half-strength Murashige and Skoog (MS) media containing 30 mg/L Hygromycin. Hygromycin-resistant T1 seedlings were transferred to soil and grown at 23 °C in a growth chamber with a 16-h day length. T2 seedlings were sowed in soil. The nutrient solution was watered from below in pots with soil-grown plants twice every week. The transgenic plants were confirmed by PCR analyses. Additionally, the expression levels of NnMYB5 and TT19 (At5g17220) TT19 gene were determined by semi-quantitative RT-PCR (sqRT-PCR) and the actin gene was used as an internal control. The primers used for sqRT-PCR are shown (Table S2).
The analysis of relationships between flower color differences among two species and MYB5 genes
To further test whether the flower color difference between red flowers and yellow flowers is related to MYB5 genes, the genomic sequences of MYB5 were amplified using the total DNA of 97 individuals from 16 wild populations of lotus (eight populations each species) and the primers 5′-GATAAGTGGAGAAGGCTATGACTGG-3′ (forward) and 5′-TCAATAACTCCACCACCTATGATTCA-3′ (reverse). PCR products were purified using the SanPrep Column PCR Product Purification Kit (Sangon, Shanghai, China) and were sequenced by Sangon Biotech. Co., Ltd. (Shanghai, China).
Accession numbers
Sequence data used in this study can be retrieved from NCBI (http://www.ncbi.nlm.nih.gov/) under the following accession numbers: ZmC1 (AAK09327.1), ZmP1 (AAA19821.1), VvMYBA2 (BAD18978.1), VvMYBA1 (BAD18980.1), VvMYB5b (AAX51291.1), VvMYBA3 (BAD18979.1), VvMYBCs1 (AAS68190.1), SlANT1 (AAQ55181.1), PhODO1 (AAV98200.1), PhMYB3 (CAA78388.1), PhMYB2 (CAA78387.1), PhMYB1 (CAA78386.1), PhAN2 (AAF66727.1), PH4 (AAY51377.1), OsMYB4 (BAA23340.1), IpMYB1 (BAE94388.1), InMYB3 (BAE94710.1), InMYB2 (BAE94709.1), InMYB1 (BAE94389.1), GhBNLGHi233 (AAK19611.1), CaMYB (ABN11121.1), AtPAP1 (AAG42001.1), AtPAP2 (AAG42002.1), AmVENOSA (ABB83828.1), AmROSEA1 (ABB83826.1), AmROSEA2 (ABB83827.1), AmMIXTA (CAA55725.1); NnbHLH1 (KU198709), NnbHLH2 (XP_010278105.1), NnbHLH3 (XP_010255365.1), NnbHLH4 (XP_010241898.1), NnbHLH5 (XP_010273162.1), NnbHLH6 (XP_010275210.1), NnbHLH7 (XP_010242395.1), NnbHLH8 (XP_010256776.1), NnbHLH9 (XP_010267440.1), NnbHLH10 (XP_010252341.1), LhbHLH2 (BAE20058.1), PhAN1 (AAG25927.1), AtTT8 (NP_192720.2), InbHLH2 (BAE94394.1), ZmIN1 (AAB03841.1), OsRc (ABB17166.1), PfMYC-RP (BAA75513.1), PfF3G1 (BAC56998.1), OsRa (AAC49219.1), ZmLc (NP_001105339.1), PhJAF13 (AAC39455.1), InbHLH3 (BAE94395.1), InbHLH1 (BAE94393.1), GMYC1 (CAA07615.1), AtGL3 (NP_680372.1), AtEGL1 (NP_176552.1), AmDEL (AAA32663.1), AtMYC1 (BAA11933.1); NnTTG1 (XP_010251384.1), NnLWD1 (XP_010250423.1), NnLWD1-like (XP_010258659.1) and NnLWD1-like variant X1 (XP_010271596.1), ZmPAC1 (AAM76742), PhAN11 (AAC18914), PFDS (BAB58883), MtWD40-1 (ABW08112), IpWDR1 (BAE94396), InWDR1 (BAE94398), GhTTG1(AF336281.1), GhTTG3 (AAM95645), AtTTG1 (Q9XGN1).
Results
Anthocyanin content and expression analysis of DFR, ANS, UFGT and GST genes between red flowers and yellow flowers
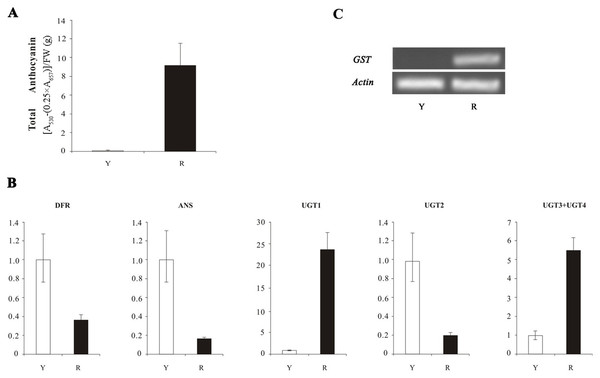
The result indicated a lot of anthocyanin in red flowers and no anthocyanin in yellow flowers (Fig. 1A). The qRT-PCR results showed that the expression of DFR, ANS, and UGT2 in yellow petals was higher than that of in red petals (Fig. 1B), which was contradicted with anthocyanin content. The expression levels of three UFGT genes (UGT1, UGT3 and UGT4) in yellow petals of N. lutea were lower than in red petals of N. nucifera and no transcripts of GSTF11 gene were detected in N. lutea with yellow flowers, which was consistent with anthocyanin content (Fig. 1C).
Figure 1: The anthocyanin content and the expression analyses of four structural genes (DFR, ANS, UFGT and GST) in Nelumbo.
(A) Total anthocyanin content in petals in two species. Error bars are ± SD of the means of triplicates. (B) The expression profile of three structural genes (DFR, ANS and UFGT) in two species as determined by qRT-PCR. Due to high homology of UGT3 and UGT4, the expression level of two genes was detected together by a pair of primers. (C) The transcription levels of GST in N. lutea and N. nucifera as determined by sqRT-PCR. Actin gene was used as internal control. Y, yellow petal in Nelumbo lutea; R, red petals in N. nucifera.Identification of R2R3-MYBs, bHLHs and WD40 transcription factors in the lotus genome
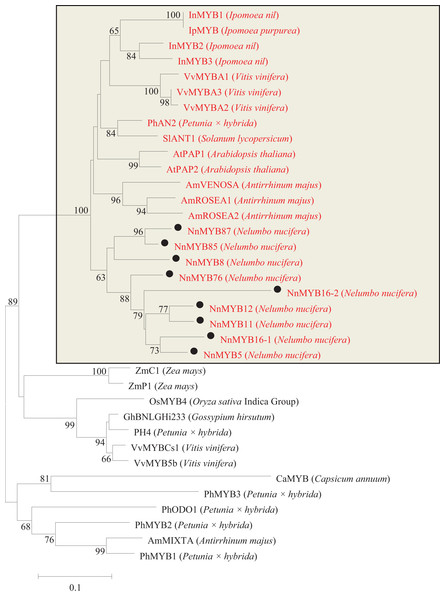
By local blast search, 95 MYBs, 10 bHLHs and 17 WD40s were found in the genome database of N. nucifera (Table S3). Based on the highly conserved R2R3 motifs in plants, a total of 92 R2R3 motifs of R2R3-MYBs were identified in the genome database of N. nucifera. Phylogenetic analysis revealed that nine MYB TFs in N. nucifera were clustered with the known MYB TFs involved in anthocyanin biosynthesis in other species, e.g., Arabidopsis AtPAP1 and AtPAP2, petunia PhAN2, grape VvMYBA1-A3, Ipomoea nil InMYB2, and snapdragon AmROSEA1-2 (Figs. 2 and S1).
Figure 2: Phylogentic relationships of MYB proteins from lotus and other species.
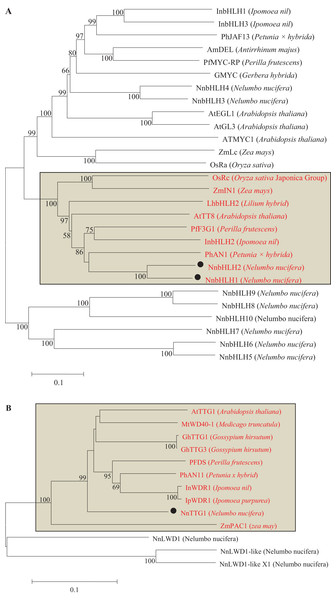
Neighbor-joining tree showing relationships between the nine lotus MYB proteins with MYBs of other species. Numbers close to the nodes indicate bootstrap values, with only values above 50 shown, and scale shows 0.1 amino acid substitutions per site. The deduced amino acid sequences were retrieved from NCBI. Black circles represent lotus MYB proteins that were clustered with MYBs of other species known to be involved in the regulation of anthocyanin biosynthesis. All MYBs involved in anthocyanin synthesis were located in the rectangle and labeled as red letters.Based on the similarity of bHLHs among plant species, the full-length cDNA sequences of 10 bHLHs were predicted from the genome database of N. nucifera. Only two bHLH (NnbHLH1 and NnbHLH2) TFs in N. nucifera were clustered together with Arabidopsis AtTT8 and petunia PhAN2, which are known to be involved in the regulation of anthocyanins synthesis (Fig. 3A).
Figure 3: Phylogentic relationships of bHLH and WD40 proteins from lotus and other species.
(A) Neighbor-joining tree of bHLH TFs in lotus and other species; (B) Neighbor-joining tree of WD40 proteins in lotus and other plants; Numbers close to the nodes indicate bootstrap values, with only values above 50 shown, and scale shows 0.1 amino acid substitutions per site. The deduced amino acid sequences were retrieved from NCBI. Black circles represent lotus bHLH TFs and WD40 proteins that were clustered correspondingly with bHLHs and WD40s of other species known to be involved in the regulation of anthocyanin biosynthesis. All bHLHs and WD40s involved in anthocyanin synthesis were located in the rectangle and labeled as red letters.From the genome database of N. nucifera, the full-length cDNA sequences of four WD40 genes and partial fragments of thirteen WD40 genes were identified by local blast. Among the thirteen partial fragments of WD40 gene, no WD40 genes were clustered with known WD40s involved in regulating anthocyanins. Thus, only WD40s proteins translated from four full-length cDNA sequences in lotus were aligned with other WD40 proteins from other species to create a phylogenetic tree. The result shows that only NnWD40-1 was clustered with Arabidopsis AtTTG1, which is known to be involved in the regulation of anthocyanin synthesis, thereafter NnWD40-1 was further named as NnTTG1 (Fig. 3B).
Isolation of NnMYB5, NnbHLH1 and NnTTG1 genes from red flowers of N. nucifera
Specific primers were designed based on the R2R3 domains of nine MYBs and conserved region of two bHLHs, and cDNA of red flowers were used as templates to isolated MYB and bHLH genes involved in flower coloration. A 289-bp product (GenBank accession: KU198707) was obtained from red flowers. Although many clones were sequenced, all clones only showed the highest identity to NnMYB5 gene. Only a bHLH gene, NnbHLH1, was isolated from cDNA of red flowers of N. nucifera. Therefore, we inferred that NnMYB5 and NnbHLH1 genes may be involved in anthocyanins synthesis in red flowers and further studied.
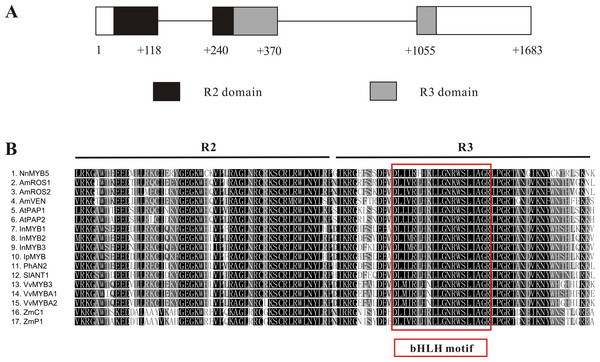
The 876-bp full-length cDNA sequence of NnMYB5 (GenBank accession: KU198708) was obtained and its predicted protein comprises 291 amino acids. A comparison between cDNA sequence and the genomic sequence of NnMYB5 (1,683 bp; GenBank accession: KU198697) revealed that the NnMYB5 gene comprises three exons and two introns (intron 1 is 122 bp in length, whereas intron 2 is 685 bp long) (Fig. 4A). The R2 domain spans exons 1 and 2, and the R3 domain spans exons 2 and 3. Alignment of the predicted protein sequence of NnMYB5 and the other anthocyanin-related MYB TFs at the R2R3 domain revealed a high degree of homology (Fig. 4B). The NnMYB5 has the amino acid residues ([D/E]Lx2[K/R]x3 Lx6Lx3R) that specify interaction with R-like bHLH protein (red box on Fig. 3A). In addition, NnMYB5 also contained three amino acids that are present in the R2 (R instead of G) and R3 domain (V instead of E or D & A instead of E) (Heppel et al., 2013; Kelemen et al., 2015), indicating that NnMYB5 maybe involved in anthocyanin biosynthesis instead of proanthocyanidin. NnMYB5 is closely related to the petunia AN2, with 80.0% amino acid identity over the R2R3 DNA-binding domain and 46.1% identity to the entire protein. For Arabidopsis PAP1 and PAP2, these amino acid percentage identities are 78.1, 42.5 and 79, 43.4%, respectively, whilst for other species the overall identities are as follows: grapevine VvMYBA1 43.9%, maize C1 34.5% and maize P1 34.9%. Phylogenetic analysis revealed that NnMYB5 forms a cluster with the other MYB TFs known to be involved in anthocyanin synthesis.
Figure 4: Characterization of NnMYB5 gene and alignment of NnMYB5 and other anthocyanin-related MYBs from other species.
(A) Characterization of NnMYB5 gene structure. Exons and introns are labeled. Numbers refer to position relative to the first nucleotide of the start codon. Exons are indicated by boxes, and introns are indicated by lines. Location of R2 and R3 domains are highlighted using black and gray backgrounds, respectively. (B) Protein sequence alignment of the R2R3 DNA-binding domains of NnMYB5 and other known anthocyanin MYB regulators in other species. Red rectangle indicates specific residues that interact with bHLH protein. The same residues are evident in NnMYB5, suggesting a protein-protein interaction. Identical residues are shown in black, conserved residues in dark grey and similar residues in light grey.The NnbHLH1 (GenBank accession: KU198709) comprises a 2,124-bp cDNA region encoding a 707-residue protein. Alignment of the predicted protein sequence of NnbHLH1 and the other anthocyanin-related bHLH TFs at the bHLH domain revealed a high degree of homology (Fig. S2). The phylogenetic tree showed that the NnbHLH1 protein clustered together with petunia AN1 and Arabidopsis TT8, with the deduced amino acid sequences exhibiting 56.1 and 42.7% identities, respectively.
The full-length cDNA sequence of NnTTG1 (GenBank accession: KU198711) comprises a 1,020-bp coding region representing a putative protein of 339 amino acids. NnTTG1 exhibited high similarity to its homolog from Arabidopsis AtTTG1 (77.5% identity at protein-level).
A comparison of three regulatory genes (MYB5, bHLH1 and TTG1) among two species
A comparison of the genomic sequences of NnMYB5 and NlMYB5 (GenBank accession: KU198698) revealed forty-three single-nucleotide sequence polymorphisms (SNP), three deletions (3, 2 and 1 bp in size) and three insertions (24, 3 and 46 bp in size) in the coding region, as well as many indels and SNPs in the non-coding region. Among these differences, two nucleotide substitutions (GAA/TAG) in the second exon resulted in a premature stop codon, leading to an incomplete protein in N. lutea. By comparison, the promoter of NlMYB5 (GenBank accession: KU198706) is 107 bp longer than that of NnMYB5 (GenBank accession: KU198705). In addition, the promoter of NlMYB5 contained many insertions and SNPs.
Nine amino acids substitutions and a deletion of up to four amino acids were detected in the alignment of the amino acid sequences of NnbHLH1 and NlbHLH1 (GenBank accession: KU198710). Among these differences, one amino acid change occurred in the bHLH domain and four amino acid changes occurred in the N-terminal of bHLH proteins. However, the protein sequence of NlTTG1 (GenBank accession: KU198718) of N. lutea is identical to that of NnTTG1 of N. nucifera.
Analysis of MYB5 gene sequences among populations
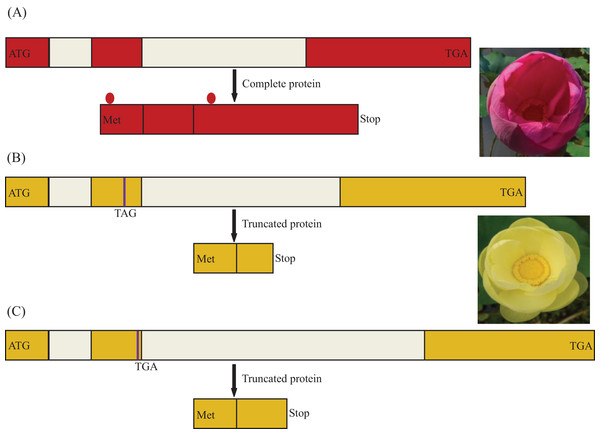
Population analysis allowed the identification of three variants at the MYB5 locus: (i) the functional allele, encoding a 291 amino acid polypeptide present in the homozygous state in red flowers of N. nucifera, (ii) two nucleotide substitutions (GAA/TAG) in the second exon leading to a premature stop codon, (iii) a single-base deletion in the second exon causing a premature stop codon. Remarkably, these two putatively inactive forms of the gene were recognized only in yellow flowers of N. lutea (Fig. 5). Nonsense mutations existed in all eight populations, whereas frameshift mutations existed in six populations of eight populations (NLP1 and NLP8). All frameshift mutations coexisted with nonsense mutations as a heterozygous genotype. So far, we did not find homozygous individuals only with framshift mutations.
Figure 5: Schema of the MYB5 allele structures and related encoded proteins in Nelumbo.
Different colors for the coding regions are reflective of distinct sequence haplotypes. The premature stop codon is indicated in purple line. The region between two red dots is R2R3 motif. (A) A putatively functional form of the NnMYB5 gene (Genbank accession: KU198697), encoding a complete protein in sacred lotus; (B) Haplotype (Genbank accession: KU198698) with two nucleotide substitutions in second exon of NlMYB5 gene causing a premature stop codon, resulting in a truncated protein in American lotus; (C) Haplotype (Genbank accession: KU198699) with 1 bp nucleotide deletion in second exon of NlMYB5 gene causing a frameshift mutation, resulting in a truncated protein in American lotus.Protein–protein interaction of MYB5, bHLH1 and TTG1
NnMYB5 contains a motif necessary for interactions with R-like bHLH proteins, but no such motif is found in NlMYB5. Thus, whether NnMYB5 interacts with NnbHLH1 or NlbHLH1 was investigated using a GAL4-based yeast two-hybrid system in this study. In addition, the protein-protein interactions between NnTTG1 and other two regulatory factors were also investigated. A construct of the NnMYB5 gene as a full-length cDNA was tested for transcriptional activation activity in yeast in this study and the result showed that the NnMYB5 has transcriptional activity in yeast (Fig. 6). Thus, the interaction study was carried out with DNA-binding domain-fused NnbHLH1 and NnTTG1, and activation domain-fused NnMYB5 and NnTTG1. Only the yeast harboring the combination of NnMYB5-NnbHLH1, NnMYB5-NnTTG1, NnTTG1-NnbHLH1, NnMYB5-NlbHLH1 and NnTTG1-NlbHLH1 survived on quadruple dropout medium, and their color turned to blue when grown on SD–Trp–Leu–His–Ade + X–α-Gal media (Fig. 6). This suggests that NnMYB5 interacts with NnbHLH1, NlbHLH1 and NnTTG1, as well as the interaction between NnTTG1 and NnbHLH1 and NlbHLH1.
Figure 6: Cooperative interaction of MYB5, NnbHLH1, and NnTTG1 in yeast two-hybrid experiments.
Functional test of NnMYB5 in transgenic plants
To test the function of NnMYB5, we constructed the expression vector of NnMYB5 with CaMV 35S promoter and transformed it into Arabidopsis plants. Some seeds collected from Hygromycin-resistant T1 plants were brown, which were similar to the seeds of wild-type plants. However, other seeds of T1 plants were purple in color. Although the brown seeds of T1 plants had a higher seed germination rate in soil than purple seeds, almost all the seedlings that germinated from brown seeds were not transgenic plants. Most of transgenic T2 seedlings showed no visible pigmentation anywhere in the entire plant. Only a few transgenic T2 seedlings showed pale purple in flower stalks. Under a stereomicroscope, there was clear ectopic pigmentation in immature seeds (< 10 days after anthesis) and flower stalks of transgenic plants, but not in immature seeds and flower stalks in wild-type control plants (ecotype Columbia) (Figs. 7A and 7B). Our sqRT–PCR results suggested that NnMYB5 was only expressed in the transgenic plants (Fig. 7C). Additionally, sqRT-PCR results also showed that the expression level of TT19 gene was up-regulated in NnMYB5 over-expression plants.
Figure 7: Functional analysis of NnMYB5 in transgenic plants.
Photographs of flower stalks from Arabidopsis ecotype Columbia: (A) untransformed plants and (B) transformed with NnMYB5 DNA. Arrow indicates accumulated anthocyanins in flower stalks. Photographs of immature seeds (less than 10 d after anthesis) from Arabidopsis ecotype Columbia: (C) untransformed plants and (D) transformed with NnMYB5 DNA. (E) The expression levels of NnMYB5 and AtTT19 in wild-type and transgenic plants as determined by semi-quantitative RT-PCR. AtACTIN2 gene was used as the internal control.Discussion
The variation of regulatory genes in the anthocyanin biosynthetic pathway could contribute to the diversity of plant flower color (Nakatsuka et al., 2005). In this study, three transcriptional genes were isolated from cDNAs of red flowers, named NnMYB5, NnbHLH1 and NnTTG1, respectively. Our results demonstrated that the above three TF can mutually interact, and MYB5 is a transcriptional regulator of anthocyanin synthesis. Finally, we found a relationship between MYB5 gene and flower color difference of two species in Nelumbo.
MYB5, bHLH1, and TTG1 can interact simultaneously
The complex composed of three types of regulatory genes (R2R3-MYB, bHLH and WD40) activates transcription of flavonoid biosynthetic genes and thus regulates anthocyanin synthesis (Koes, Verweij & Quattrocchio, 2005; Ramsay & Glover, 2005). In this study, three regulatory genes (NnMYB5, NnbHLH1 and NnTTG1) involved in anthocyanin synthesis were isolated from red flowers. It is well known that R2R3-MYBs interact with bHLHs, such as the interaction between MrbHLH1 and MrMYB1 in bayberry and GtbHLH1 interacting with GtMYB3 in gentian (Nakatsuka et al., 2008; Liu et al., 2013). In this study, NnMYB5 interacts with NnbHLH1 or NlbHLH1. It is uncertain whether WD40 can interact with MYB. For example, in Arabidopsis, TTG1 (WD40) can interact with TT2; in apple, MdTTG1 interacts with bHLH but not MYB proteins (An et al., 2012). In Nelumbo, NnTTG1 not only interacts with two bHLHs (NnbHLH1 and NlbHLH1) but also interacts with NnMYB5. The interaction of NlbHLH1-NnMYB5 was weaker than the interaction of NnbHLH1-NnMYB5, which indicated that amino acid differences between NlbHLH1 and NnbHLH1 affected the interaction with NnMYB5.
NnMYB5 is a transcriptional activator in anthocyanin biosynthesis
Amino acid sequence similarity, protein structures, and phylogenetic analysis suggested that NnMYB5 may exhibit a similar function to other MYB homologues regulating anthocyanin synthesis. However, methods for genetic transformation of lotus have not been established. Therefore, we introduced the full-length gene of NnMYB5 into Arabidopsis plants in order to test the function of NnMYB5. The transgenic plants developed partially red seeds and purple pedicels, indicating that NnMYB5 can function as a transcriptional activator in anthocyanin biosynthesis. This result is consistent with the regulatory gene MdMYB1 in red apple and RsMYB1 in radish (Takos et al., 2006; Lim et al., 2016).
MYB TFs lead to anthocyanin accumulation by up-regulating the transcripts of some structural genes (Tohge et al., 2005). For example, in tomato, over-expression of AN1(MYB) led to up-regulation of GST (Mathews et al., 2003); in Arabidopsis, the expression of TT19 (GST) is up-regulated in the PAP1 over-expressing plants (Tohge et al., 2005); in apple, MdMYB10 activates the transcription of DFR (Espley et al., 2007); in grape, the ectopic expression of VvMYB1 activates the expression of UFGT and GST (Cutanda-Perez et al., 2009); in nectarine, MYB10 positively regulates the promoters of UFGT and DFR (Ravaglia et al., 2013); in ever-red leaf crabapple, McMYB activates McF3′H and later structural genes (Tian et al., 2015). Due to the lack of GST transcripts and inactivation of MYB5 simultaneously occurred in yellow flowers of N. lutea, so we investigated whether NnMYB5 can regulate the expression of GST genes in transgenic Arabidopsis. The results showed that the expression of TT19 (TT19) was up-regulated in NnMYB5 over-expressing plants. Therefore, we inferred that MYB5 transcriptional factor may regulates the expression level of GST in Nelumbo. The direct activation of MYB5 on NnGST needs to be investigated. In addition, the expression of DFR and ANS in yellow flower is higher than that of red flowers, indicating that they maybe controlled by other MYB instead of MYB5.
A relationship between flower color difference of two species in Nelumbo and MYB5 gene
To date, most of previous studies on the genetic basis of flower coloration suggest that variation in regulatory genes is central to variation in pattern and intensity of pigmentation (Schwinn et al., 2006; Yamagishi et al., 2010). Due to the high specificity of MYB in regulating anthocyanin biosynthetic pathway, mutations in MYB genes are expected to incur the least pleiotropic effects and are most likely to be involved in the evolution of flower color (Schwinn et al., 2006; Streisfeld & Rausher, 2011). For example, in Petunia axillaris, the absence of anthocyanins in white flowers is closely related to loss of AN2 function (Quattrocchio et al., 1999; Hoballah et al., 2007); in Mimulus, gains of pigmentation in petal lobes are attributable to the evolution of R2R3-MYB TF (Cooley et al., 2011); in Antirrhinum, variation in three R2R3-MYB regulatory genes might account for the flower color variation among species (Schwinn et al., 2006); in Oncidium, the red/yellow color of floral lip depends on whether OgMYB1 is expressed or not (Chiou & Yeh, 2008); in apple, differential expression of MYB10 led to differences in anthocyanin levels between red and green strips (Telias et al., 2011); in gentian, the loss of functional GtMYB3 expression leads to the absence of anthocyanin in white flower (Nakatsuka et al., 2008). In this study, a striking difference in MYB5 gene was detected in two lotus species. In all populations of N. lutea with yellow flowers, MYB5 is a nonfunctional gene; however, in all populations of N. nucifera with red-flowers, MYB5 is a functional gene. It indicated a relationship between flower color difference and loss of functional MYB5 gene.
Conclusions
In this study, three regulatory genes (NnMYB5, NnbHLH1 and NnTTG1) involved in anthocyanin synthesis were identified from red flower of N. nucifera. Our investigations revealed that MYB5 is a functional transcription activator of anthocyanin synthesis. The flower color difference between red flowers and yellow flowers may be related to the variation of MYB5 gene. The exact of flower color difference needs further investigation.
Supplemental Information
Phylogenetic tree showing relationships between the 92 lotus MYB TFs with MYBs of other species.
Numbers close to the nodes indicate bootstrap values, with only values above 50 shown, and scale shows 0.1 amino acid substitutions per site. Black circles represent lotus MYB TFs that were clustered MYBs with other species known to be involved in the regulation of anthocyanin biosynthesis.
Alignment of bHLH domain of NnbHLH1 and other anthocyanin-related bHLHs from other species.
Identical residues are shown in black, conserved residues in dark grey and similar residues in light grey.