The CD8+/Foxp3+ ratio, but not the number of OX40+ TILs, is an independent predictor of tumor recurrence in non-muscle-invasive bladder cancer: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Xin Zhang
- Subject Areas
- Oncology, Pathology, Urology
- Keywords
- OX40, CD8, Foxp3, Tumor infiltrating lymphocytes, Non-muscle-invasive bladder cancer
- Copyright
- © 2025 Han et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. The CD8+/Foxp3+ ratio, but not the number of OX40+ TILs, is an independent predictor of tumor recurrence in non-muscle-invasive bladder cancer: a retrospective study. PeerJ 13:e20322 https://doi.org/10.7717/peerj.20322
Abstract
Non-muscle-invasive bladder cancer (NMIBC) frequently recurs and progresses into an aggressive and lethal entity within five years. The clinical management of recurrent tumors remains limited. Therefore, identifying individual patients who are at a high risk of recurrence is crucial for early clinical monitoring and appropriate medical intervention, which may lead to improved outcomes. OX40 is a dual modulator that stimulates effector T cells and suppresses Tregs. It appears to be an ideal molecule for predicting survival outcomes, surpassing the predictive power of single or combined T cell signatures. It has been shown to act as an independent tumor prognostic predictor in various cancers, including non-small cell lung cancer, melanoma, and colorectal cancer. However, its potential as a prognostic tool for tumor recurrence in NMIBC has yet to be investigated. The present study aimed to investigate the potential value of OX40 as a predictor of recurrence risk in patients with NMIBC. Additionally, its downstream effectors, Foxp3 and CD8, were also evaluated. Tissue samples were collected from a cohort of 110 patients diagnosed with NMIBC. Immunohistochemistry was performed to assess the density of stromal OX40+, Foxp3+, and CD8+ tumor infiltrating lymphocytes (TILs). Following survival analysis using the Kaplan-Meier method and log-rank test, we found that tumor recurrence was associated with a decreased density of OX40+ and CD8+ TILs, an elevated density of Foxp3+ TILs, and lower ratios of OX40+/Foxp3+ and CD8+/Foxp3+ TILs. However, after adjustment, multivariate COX regression analysis indicated that only the ratio of CD8+/Foxp3+ was an independent predictor of recurrence risk. The prediction power was assessed by a receiver operating characteristic (ROC) curve analysis. The results demonstrated that the AUC value for the CD8+/Foxp3+ ratio was better than the other predictive markers. Although the expression of OX40 in TILs was associated with tumor recurrence, our results suggest that the predictive efficacy of a combination of CD8 and Foxp3 was more robust after adjustment. Future research utilizing advanced immunotyping techniques is necessary to validate these findings in larger cohorts.
Introduction
Urothelial bladder carcinoma (UBC) is the fourth most prevalent cancer in men and the second leading cause of cancer-related mortality in genitourinary tumors (Lenis et al., 2020). Non-muscle-invasive bladder cancer (NMIBC) accounts for about 70% of UBC cases (Schneider, Chevalier & Derré, 2019). About 50% of patients with NMIBC experience recurrence or progression in tumor grade within five years (Lenis et al., 2020). Therefore, identifying high-risk groups for recurrence is crucial for personalizing treatment and optimizing therapeutic strategies. Previous studies have linked various clinicopathologic factors, such as tumor size, multifocality, and histological variants, to the risk of recurrence (Babjuk et al., 2019). Based on this information, the European Organization for Research and Treatment of Cancer (EORTC) and the Spanish Urological Club for Oncological Treatment (CUETO) developed risk scores for predicting recurrence and progression (Fernandez-Gomez et al., 2009; Sylvester et al., 2006). However, when these scoring systems were validated on a large European cohort, they demonstrated limited effectiveness (Vedder et al., 2014). Consequently, there is a pressing need to identify new biomarkers that can more effectively predict the risk of tumor recurrence in NMIBC.
Tumor-infiltrating lymphocytes (TILs) are a major component of the tumor microenvironment (TME). Those cells can differentiate into various subpopulations with distinct immunologic profiles upon receiving exogenous stimulation. They play a vital role in modulating immune responses to cancer, monitoring tumor escape mechanisms, and facilitating metastasis through their interactions with tumor cells and immune networks (Nair et al., 2020; Qayoom, Sofi & Mir, 2023). The accumulation of distinct TIL subgroups within the TME can also serve as a significant predictor for therapeutic responses and clinical outcomes (Vargas et al., 2024). OX40, a T-cell co-stimulatory receptors belonging to the tumor necrosis factor receptor superfamily (TNFRSF), controls bidirectional T cell activation and function (Thapa et al., 2024). It binds to its ligand OX40L to form the OX40-OX40L complex, which triggers signal transduction pathways that result in two primary functions: promoting the survival of CD4+ and CD8+ T cells on one hand, and inhibiting the induction of Foxp3+ Tregs by suppressing Foxp3 gene expression on the other hand (Watts et al., 2025). Consequently, OX40 may serve as an ideal biomarker for predicting tumor behavior and prognosis (Croft, 2010; Webb, Hirschfield & Lane, 2016). For instance, a high density of OX40-positive immune cells in primary high-grade serous ovary carcinoma was associated with chemosensitivity (Ramser et al., 2018). Additionally, patients with advanced lung adenocarcinoma exhibiting elevated serum OX40 levels have shown reduced overall survival rates (Yokouchi et al., 2021). However, the predictive role of OX40 in NMIBC remains undetermined.
Furthermore, CD8 and Foxp3, the downstream molecules controlled by OX40 (Vu et al., 2007), have been found to play crucial roles in predicting tumor prognosis in bladder cancer (Krpina, Babarović & Jonjić, 2015). Liu et al. (2018) found that high infiltration of CD8+ TILs predicted better overall survival (OS) in NMIBC. In addition, infiltration of Foxp3+ lymphocytes has been linked to improved OS and longer progression-free survival (PFS) in urinary bladder cancer (Winerdal et al., 2011). Olkhov-Mitsel et al. (2020) reported that rapid tumor relapse was associated with low levels of CD3, CD4, and CD8, but not with Foxp3 expression in patients with MIBC.
This retrospective study aims to determine if the distribution of OX40+ TILs can serve as an effective prognostic factor for tumor recurrence in NMIBC. Additionally, the potential predictive value of its downstream molecules, CD8+ and Foxp3+ TILs, were also explored.
Materials & Methods
Patients
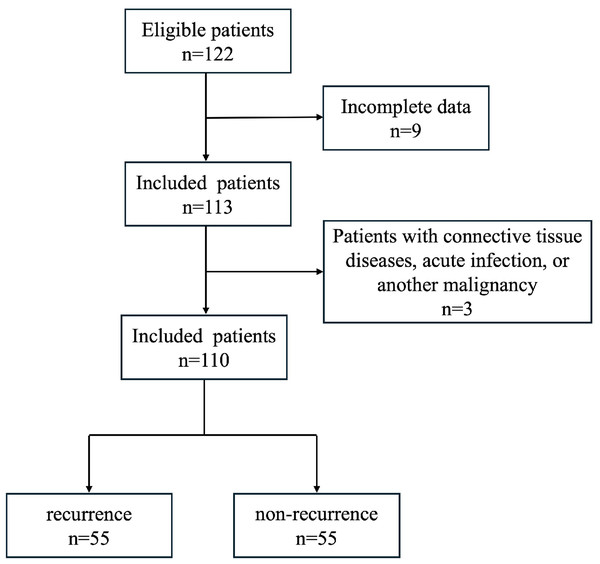
A total of 122 patients with NMIBC who underwent transurethral resection from February 2016 to October 2019 at the Qingdao Municipal hospital were enrolled in this study. Nine patients were excluded for unavailable follow-up data or insufficient FFPE samples and three patients were excluded for having connective tissue diseases, acute infection, or another malignancy. Finally, the study cohort was composed of 110 cases (Fig. 1). All patients did not receive chemotherapy, radiotherapy or ICIs therapy before surgery. Clinical parameters including gender, age, treatment, and recurrence were obtained from clinical records. All sections were submitted to be reevaluated by two senior genitourinary pathologists. The discrepancies in diagnosis were resolved by discussion to reach a consensus. Tumor grade and stage was assigned according to the World Health Organization (WHO) 2016 classification and the 8th edition of the AJCC TNM Staging Manual (Humphrey et al., 2016; Wang & McKenney, 2019). Written consent was obtained from each patient involved. The study was approved by the Ethics Review Committee of Qingdao Municipal Hospital (Qingdao, Shandong, China). Approval No: 2024-LW-065. All tissue specimens were obtained from the initial TURBT (transurethral resection of bladder tumor) prior to any intravesical therapy or recurrence.
Figure 1: Schematic diagram of study subject screening process.
The flowchart illustrates the process of patient selection and outcomes.Histologic evaluation
Non-invasive low grade papillary urothelial carcinoma (NILGPUC) had delicate fibroblast cores covered by neoplastic urothelium with uniform polarity, slight nuclear irregularity, and small nucleoli. Non-invasive high grade papillary urothelial carcinoma (NIHGPUC) displayed cellular disorder with irregular and pleomorphic nuclear and prominent nucleoli. Mitotic activity was usually low in the low-grade lesion and distracted away from the basement membrane if present, while numerous mitoses were randomly distributed among any epithelial layer of the high-grade lesion. We used 5% as the minimum proportion to render a high-grade diagnosis. Invasive urothelial carcinoma (IUC) was characterized by nests or clusters of single tumor cells infiltrating muscularis propria. Desmoplastic reaction was seen surrounding the infiltrating foci.
Immunohistochemistry (IHC)
Formalin-fixed paraffin-embedded tissue blocks were cut into 3–5-µm sections and mounted onto adhesive slides. All sections were stained with mouse monoclonal anti-CD8 antibody (C8/144B 1:30 MXB Biotechnology), mouse monoclonal anti-Foxp3 antibody (236A/E7, 1:50, Invitrogen), and mouse monoclonal anti-OX40 antibody (ACT35, 1:100; Invitrogen), utilizing a BenchMark ULTRA automated immunohistochemical slide staining system (Ventana Medical Systems, Inc.). The appropriate positive and negative controls were concurrently performed.
Scoring
Five tumor areas with a high density of positive cells (hotspots) for each marker were photographed under a Nikon Eclipse microscope equipped with a 40 × objective (per 0.25 mm2) and a C630 digital camera (Nikon, Japan). The number of positive cells was manually calculated on each image using Image ProPlus 6.0 analysis software (Media Cybernetics Inc., Rockville, MD, USA). The density of OX40+, CD8+, and Foxp3+ TILs (positive cell number per 0.25 mm2) was determined by the average of cell counts. In addition, the cells were counted independently by two pathologists, and the final score was determined by the average of each count. Consensus meetings were held for obvious discrepancy in counting.
Statistical analysis
Continuous variables were compared using Student’s t-test between two groups. Chi-square test was utilized to analyze the differences between categorical variables. The correlations between specific immune cell counts and clinicopathological features were evaluated by Spearman’s rank correlation coefficients. Recurrence-free probability (RFP) was defined as the time from the date of surgery to the date of disease recurrence, estimated by the Kaplan–Meier method, and compared using the log-rank test. Multivariate analyses were performed using the Cox proportional hazards regression model. Receiver operating characteristic (ROC) curves were used to determine the predictive accuracy. The corresponding area under the curves (AUC) was calculated. All statistical analyses were conducted using the IBM SPSS statistical software package (version 25.0) and GraphPad Prism v7.0. The data are presented as the mean ± standard error of the mean (SEM), and statistical significance was considered when a two-sided P < 0.05 was established.
Results
Clinicopathological characteristics
The clinicopathological features of the patients are illustrated in Table 1. The cohort comprised 91 males and 19 females. The median follow-up time was 27.42 months (range: 2–66 months). A total of 66/110 (60%) patients had non-invasive carcinoma with the tumors at Ta stage or Tis stage and 44 patients had T1 stage tumors (invasion into the lamina propria). Seventy-four cases had single tumor lesion and 36 had more than two tumor lesions, including with 28 cases with two lesions, six cases with three lesions, and two cases with four lesions.
| Variables | Recurrence | Non-recurrence | P value |
|---|---|---|---|
| Age (median) | 70 | 66 | 0.348 |
| Sex | 0.449 | ||
| Male | 47 (85.5) | 44 (80.0) | |
| Female | 8 (14.5) | 11 (20.0) | |
| Tumor stage | 0.602 | ||
| Tis | 1 (1.8) | 0 | |
| Ta | 32 (58.2) | 33 (60.0) | |
| T1 | 22 (40.0) | 22 (40.0) | |
| Tumor size | 0.543 | ||
| ≥3 cm | 20 (36.4) | 16 (29.1) | |
| <3 cm | 35 (63.6) | 39 (70.9) | |
| Histology | 0.511 | ||
| CIS | 1 (1.8) | 0 | |
| NILGPUC | 19 (34.6) | 24 (43.6) | |
| NIHGPUC | 13 (23.6) | 9 (16.4) | |
| IUC | 22 (40.0) | 22 (40.0) | |
| Multifocality | 0.311 | ||
| 1 | 35 (63.6) | 39(70.9) | |
| 2 ∼ 3 | 18 (32.7) | 16(29.1) | |
| >3 | 2 (3.7) | 0 |
Notes:
CIS, carcinoma in situ; NILGPUC, non-invasive low grade papillary urothelial carcinoma; NIHGPUC, non-invasive high grade papillary urothelial carcinoma; IUC, invasive urothelial carcinoma.
A total of 55/110 (50%, 47 males and eight females) experienced tumor recurrence during the follow-up period, including 16 cases of non-invasive low-grade UC, 17 cases of non-invasive high-grade UC, and 22 cases of invasive UC. The mean number of OX40+, CD8+, and Foxp3+ TILs was 8.7 (1–30 cells, mean ± SD: 8.67 ± 8.61), 18.2 (2–40 cells, mean ± SD: 18.06 ± 11.48), and 16.1 (3–50 cells, mean ± SD: 15.8 ± 12.74) per 0.25 mm2 in the recurrence group and 14.4 (2–40 cells, mean ± SD: 14.31 ± 10.29), 37.9 (12–80 cells, mean ± SD: 37.67 ± 17.96), and 8.1 (1–30 cells, mean ± SD: 8.49 ± 7.55) per 0.25 mm2 in the non-recurrence group, respectively (Fig. 2). No significant differences were observed in sex, age, tumor size, multifocality, tumor stage, histology, and chemotherapy treatment between recurrence and non-recurrence group.
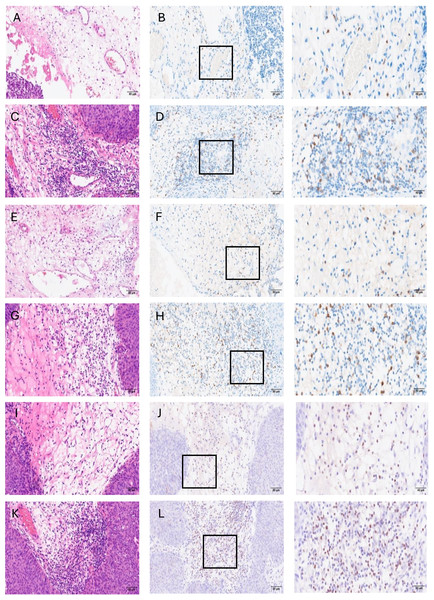
Figure 2: Representative images for H&E and immunohistochemical stains showing low or high infiltration of OX40+, CD8+, and Foxp3+ immune cells in stroma area of tumor.
(A) low OX40+cell density-H&E (20×), (B) low OX40+ cell density-IHC (20×), (C) high OX40+ cell density-H&E (20×), (D) high OX40+ cell density-IHC (20×), (E) low CD8+ cell density-H&E (20×), (F) low CD8+ cell density-IHC (20×), (G) high CD8+ cell density-H&E (20×), (H) high CD8+ cell density-IHC (20×), (I) low Foxp3+ cell density-H&E (20×), (J) low Foxp3+ cell density-IHC (20×), (K) high Foxp3+ cell density-H&E (20×), (L) high Foxp3+ cell density-IHC (20×). Scale bars represent 80 µm. The third column shows enlarged views of the boxed area in B, D, F, H, J, and L, respectively (40×). Scale bars in the third column represent 40 µm.Discriminant distribution of OX40+, CD8+, and Foxp3+ TILs
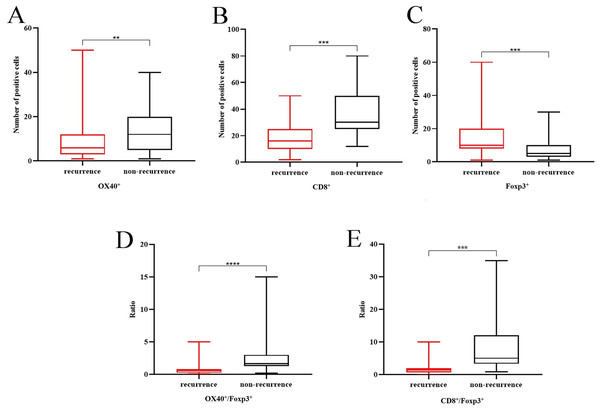
The distribution of OX40+, CD8+, and Foxp3+ cells was compared between the two groups. A lower density of OX40+ and CD8+ (P < 0.001, P = 0.001) (Figs. 3A, 3B), but higher density of Foxp3+ cells (P < 0.001) (Fig. 3C) was found in patients with recurrence than those without. We also observed a significantly lower ratio of OX40+/Foxp3+ (P < 0.001) (Fig. 3D) and CD8+/Foxp3+ (P < 0.001) (Fig. 3E) in recurrence group compared to non-recurrence group.
Figure 3: The distribution of TILs in recurrence and non-recurrence group.
Decreased number of OX40+ and CD8+ TILs (P = 0.001, P < 0.001) and increased number of Foxp3+ (A–C) TILs in the recurrent group (P < 0.001,); lower ratio of OX40+/Foxp3+ (D) and CD8+/Foxp3+ (E) in recurrence group (P < 0.001).Associations of OX40+, CD8+, and Foxp3+ TILs with clinicopathological features
The density of OX40+ cells was positively correlated with tumor stage, resulting in an increased number of OX40+ cells in tumors with a higher stage (P = 0.001) (Table 2). The density of Foxp3+ cells was associated with sex, and male patients had more abundant Foxp3+ cells infiltrates than females (P = 0.032) (Table 2). We also found moderate correlations between OX40+/Foxp3+ and CD8+/Foxp3+ cell ratio and sex (P = 0.012 and P = 0.008, respectively) (Table 2). Finally, no associations were observed between the density of CD8+ cells and clinicopathological parameters.
| Characteristics | OX40 | CD8 | Foxp3 | OX40/Foxp3 | CD8/Foxp3 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | r | P | r | P | |
| Age | 0.015 | 0.877 | 0.023 | 0.812 | 0.036 | 0.709 | 0.046 | 0.636 | 0.020 | 0.836 |
| Sex | −0.101 | 0.292 | 0.017 | 0.863 | 0.205 | 0.032 | 0.238 | 0.012 | 0.250 | 0.008 |
| Tumor stage | 0.319 | 0.001 | 0.047 | 0.626 | 0.094 | 0.330 | 0.165 | 0.085 | −0.048 | 0.617 |
| Tumor size | 0.174 | 0.068 | 0.013 | 0.895 | 0.192 | 0.045 | 0.119 | 0.215 | −0.034 | 0.726 |
| Multifocality | −0.072 | 0.452 | −0.034 | 0.727 | −0.113 | 0.239 | −0.023 | 0.809 | 0.033 | 0.736 |
| Histology | −0.013 | 0.893 | 0.101 | 0.292 | 0.118 | 0.218 | 0.071 | 0.460 | 0.029 | 0.762 |
Predictive significance of OX40+, CD8+, and Foxp3+ TILs
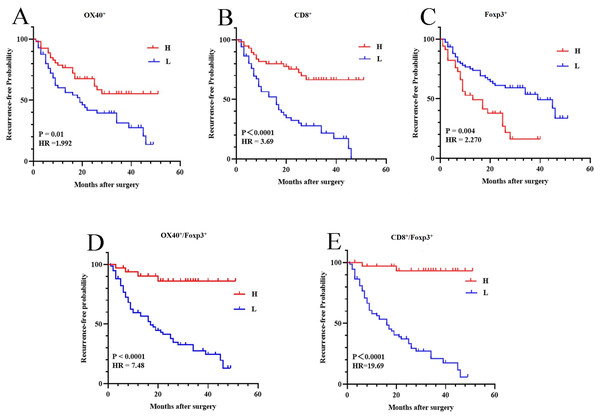
The associations of RFP with the density of OX40+, CD8+, and Foxp3+ cells were analyzed by Kaplan–Meier method and compared using log-rank test. The dichotomy of the density level was based on the median value: positive cell counts above the median value were considered high-density, and while positive cell counts below the median value were considered low-density. The cutoff values of the number of OX40+, CD8+, and Foxp3+ cells were nine cells/0.25 mm2, 24 cells/0.25 mm2, and 10 cells/0.25 mm2, respectively.
The patients with a high density of OX40+ or CD8+ cells had a significantly prolonged RFP (95% CI [1.179–3.366]; HR 1.992; P = 0.01; 95% CI [2.154–6.345]; HR 3.690; P < 0.0001) (Figs. 4A, 4B). The RFP of patients with a high density of Foxp3+ cells was significantly lower than those with a low density of Foxp3+ cells (95% confidence interval (CI) [1.392–4.167]; hazard ratio (HR): 2.270; P = 0.004) (Fig. 4C). Patients with a low OX40+/Foxp3+ and CD8+/Foxp3+ ratio had significantly shorter time intervals of RFP compared to those with a high ratio (95% CI [4.328–12.94]; HR 7.483; P < 0.0001; 95% CI [11.54–33.60]; HR 19.69; P < 0.0001) (Figs. 4D, 4E).
Figure 4: Illustration of Kaplan–Meier survival curves.
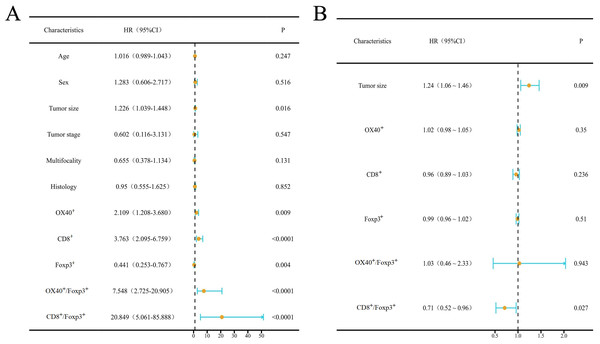
A, B, C, D, and E are the corresponding Kaplan–Meier survival curves for high and low density of OX40+ (A), CD8+ (B) and Foxp3+ (C) TILs, OX40+/Foxp3+ cell ratio (D), and CD8+/Foxp3+ cell ratio (E), respectively.The univariate COX regression regarding associations of RFP with clinicopathological parameters and the density of OX40+, CD8+, and Foxp3+ cells were analyzed. RFP was found to be significantly associated with tumor size (P = 0.016), OX40+ (P = 0.009), CD8+ (P < 0.0001), and Foxp3+ (P = 0.004) cells counts, and OX40+/Foxp3+ and CD8+/Foxp3+ ratio (P < 0.0001) (Fig. 5A). After adjustment, multivariate COX analysis showed that the ratio of CD8+/Foxp3+ was an independent predictor (95% CI [0.52–0.96]; HR 0.71; P = 0.027) (Fig. 5B).
Figure 5: Results of univariable and multivariable Cox regression analysis.
Forest plots illustrate the results of univariable (A) and multivariable (B) Cox regression analysis. Blue dots represent point estimates, orange horizontal lines denote 95% confidence intervals (CIs), and dashed vertical lines indicate the null effect (HR = 1.0). Statistical significance thresholds: Analyses were weighted by inverse variance and adjusted for covariates.Receiver operating characteristic curve analysis
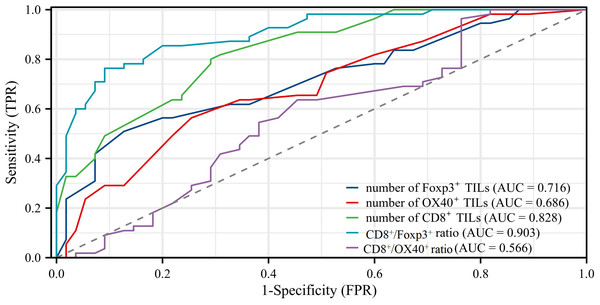
We generated ROC curves and calculated the corresponding areas under the ROC curves (AUC). The AUC values obtained were as follows: 0.903 (95% CI [0.848–0.958]) for the CD8+/Foxp3+ ratio, 0.828 (95% CI [0.755–0.902]) for the number of CD8+ TILs, 0.716 (95% CI [0.620–0.812]) for the number of Foxp3+ TILs, 0.686 (95% CI [0.588–0.785]) for the number of OX40+ TILs, and 0.566 (95% CI [0.457–0.675]) for the CD8+/OX40+ ratio. The AUC value for the CD8+/Foxp3+ ratio in predicting recurrence showed a significant difference compared to the values for the number of Foxp3+ TILs (P < 0.001), the number of OX40+ TILs (P = 0.0008), and the CD8+/OX40+ ratio (P < 0.001). The prediction power of the CD8+/Foxp3+ ratio was higher than that of the number of CD8+ TILs, while no statistical significance was found between their AUC values (AUC: 0.903 vs 0.828, P = 0.0828) (Fig. 6).
Figure 6: Results of receiver operating characteristic (ROC) curve analysis.
ROC analyses showed that the result of CD8+/Foxp3+ ratio (AUC = 0.903) was better than other markers (AUC: Foxp3+ TILs = 0.716, OX40+ TILs = 0.686, CD8+ TILs = 0.828, CD8+/OX40+ ratio = 0.566).Discussion
Non-muscle invasive bladder cancer (NMIBC) often recurs and can progress to a higher-grade neoplasm, which may lead to treatment failures. Identifying molecular biomarkers that can stratify the risk of tumor recurrence is crucial for tailoring therapeutic approaches and improving patient prognosis. In this context, the emerging role of OX40, an upstream modulator that bidirectionally influences T cell functions, presents a promising candidate for improving prognosis prediction. In our study, we investigated the predictive value of OX40+, CD8+, and Foxp3+ tumor-infiltrating lymphocytes (TILs) for tumor recurrence in patients with NMIBC. Our results demonstrate that tumor recurrence rates were associated with the density of OX40+, CD8+, and Foxp3+ TILs, as well as the ratios of OX40+/Foxp3+, and CD8+/Foxp3+. After adjustment in multivariate analysis, the CD8+/Foxp3+ immune cell ratio emerged as an independent predictive factor for recurrence rates. In addition, the results of ROC analysis showed that the predictive accuracy for CD8+/Foxp3+ was better than that of the other biomarkers.
Over the past decade, the tumor immune microenvironment (TME)—which reflects autoimmune responses—has been shown to play various biological roles in promoting tumor development and predicting clinical outcomes (Nair et al., 2020). Notably, the accumulation of different T cell subtypes within the TME has been identified as a predictive factor for tumor prognosis, including CD8+ T cells and Foxp3+ Treg cells. CD8+ T cells dominate anti-tumor immune responses by eliminating tumor cells. Conversely Foxp3+ Treg cells drive immunosuppressive responses during tumor development (Reiser & Banerjee, 2016; Shitara & Nishikawa, 2018). However, the reliability of a single marker, whether CD8+ TILs or Foxp3+ Treg cells, has often been inconsistent due to the complexity and plasticity of T cell differentiation, which can lead to conflicting results (Maimela, Liu & Zhang, 2019; Vargas et al., 2024). For instance, the number of CD8+ lymphocytes was lower in the non-recurrent group of NMIBC patients compared to the recurrent group (Krpina, Babarović & Jonjić, 2015). Conversely, neither CD8 expression in peritumoral lymphocytes nor in peritumoral lymphocytes impacted tumor recurrence and grade progression in NMIBC, according to Sharma’s study (Eich et al., 2019; Sharma et al., 2007). Similarly, Foxp3+ TILs exhibited variable relevance: NMIBC group with a higher density of Foxp3+ TILs had a better RFS that Foxp3+ low group (Balçık & Yılmaz, 2025; Wing, Tanaka & Sakaguchi, 2019), while those with a high percentage of Foxp3+ T cells showed worse recurrence-free survival rates for intravesical recurrence (Murai et al., 2018; Shang et al., 2015). The controversial results may stem from the unsustainable anti-tumor functions of these cells, which can be influenced by chemokine receptors, costimulatory molecules, and other immune-related signals. Our study showed the increased density of CD8+ TILs and decreased density of Foxp3+ TILs were correlated with prolonged RFP, consistent with their immune-activating and immune-suppressing roles, respectively.
OX40 serves as an upstream modulator with dual immunomodulatory properties. The OX40-OX40L interaction promotes the proliferation and survival of tumor-specific CD4+ and CD8+ T cells, while also enhancing the cooperation between CD8+ T cells and non-CD8+ T cells (Fu et al., 2020). Conversely, OX40 signaling inhibits Foxp3 expression and the induction of regulatory T cells (Tregs) in primitively activated CD4+ T cells by stimulating the BATF3/BATF and AKT-mTOR signaling pathways (Zhang et al., 2018). This suggests that OX40 could serve as an ideal alternative to the combinations of tumor-infiltrating lymphocytes (TILs) subpopulations as a prognostic biomarker. It has been shown that a high infiltration of OX40+ lymphocytes is associated with better overall survival (OS) and holds independent predictive significance in multivariate analyses for non-small cell lung cancer, small cell lung cancer, and colorectal cancer (Massarelli et al., 2019; Weixler et al., 2015; Yokouchi et al., 2021). Our data showed that patients with a high density of OX40+ TILs had a longer RFP in NMIBC. However, its independent prognostic significance was not determined in the multivariate analysis.
Notably, the prognostic prediction associated with TILs can be enhanced by combining different markers (Zhang et al., 2017). For example, in gastric cancer patients, a high Foxp3+ Tregs/CD8+ ratio and sole Foxp3+ Tregs were both associated with poor prognosis; however, the former proved to be an independent predictor in multivariate analysis (Shen et al., 2010). A high Foxp3+/CD8+ ratio was also found to independently predict poor overall survival in bladder cancer, based on both protein levels and mRNA expression levels (Horn et al., 2016). Additionally, Kinoshita et al. (2020) classified Foxp3+ and CD8+ TIL infiltration into four categories: CD8-Low/Foxp3-Low, CD8-High/Foxp3-Low, CD8-Low/Foxp3-High, and CD8-High/Foxp3-High in stage IA lung adenocarcinoma, revealing that the CD8-Low/Foxp3-High group is an independent prognostic factor for short disease-free survival. Furthermore, the combined analysis of CD8 and OX40 demonstrated a superior ability to stratify tumor prognosis. Patients with a high density of both CD8+ and OX40+ cells in the tumor stroma showed significantly prolonged recurrence-free periods in non-small cell lung cancer (Yokouchi et al., 2021). In advanced stages (IIB, IIC, and III) of colorectal cancer, patients with high densities of OX40 and CD8 infiltration had overall survival rates comparable to those of stage I colorectal cancer (Weixler et al., 2015). In accordance with the previous findings, the CD8+/Foxp3+ immune cell ratio was found to be a significantly independent prognostic indicator for RFP in the multivariate COX regression analysis in our study. ROC analysis was performed to determine the predictive accuracy. The results for the CD8+/Foxp3+ ratio yielded an AUC value of 0.903, better than the other biomarkers. Previous studies found that the AUC value for EORTC model in predicting NMIBC disease recurrence and progression were 0.597 and 0.662, and for CUETO were 0.523 and 0.616, respectively (Xylinas et al., 2013). Notably, the AUC value for CD8+/Foxp3+ ratio was 0.903 in our study, indicating an enhanced efficacy in tumor recurrence prediction.
Despite these findings, the current study has some limitations: the study cohort was relatively small, and Foxp3 immunohistochemistry cannot differentiate between the various Foxp3+ Treg subpopulations. Additionally, evaluating the predictive roles of TILs based solely on cell counts presents certain limitations.
In conclusion, our results highlight that the validation of CD8+/Foxp3+ ratio showed improved efficacy beyond the upstream modulator and single marker for recurrence prediction in NMIBC. The combined analysis may represent the functional dynamics of individual markers, better reflect the status of tumor immune responses, thus provide more accurate information for predicting tumor recurrence. Further large-scale clinical studies are necessary to assess clinical significance and investigate the underlying molecular mechanisms accurately.
Supplemental Information
Raw data
Primary observational and measurement results for our research. A series of quantitative and qualitative records relevant to the key variables under study. It serves as the foundation for all the statistical analyses and findings presented in our manuscript. Specifically, it includes detailed numerical values, categorical data, and other relevant information that has been carefully collected over the course of our investigation.