MicroRNA-597-3p targets MACC1 to suppress proliferation and invasion of human ovarian cancer cells
- Published
- Accepted
- Received
- Academic Editor
- Hilal Ozdag
- Subject Areas
- Cell Biology, Molecular Biology, Oncology
- Keywords
- Ovarian cancer, MiR-597-3p, MACC1, Tumor suppressor, Metastasis, Apoptosis
- Copyright
- © 2025 Al Awadh et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. MicroRNA-597-3p targets MACC1 to suppress proliferation and invasion of human ovarian cancer cells. PeerJ 13:e20316 https://doi.org/10.7717/peerj.20316
Abstract
Background
Ovarian cancer is a lethal gynecological malignancy, largely due to late-stage diagnosis and poor prognosis. MicroRNA-597-3p (miR-597-3p) has been identified as a tumor suppressor in several cancers, while metastasis-associated colon cancer 1 (MACC1) functions as an oncogene that promotes metastasis. This study investigated the role of miR-597-3p and its regulation of MACC1 in ovarian cancer progression.
Methods
Ovarian cancer cell lines and the normal ovarian epithelial cell line IOSE-398 were used. Quantitative real-time PCR (qRT-PCR) measured the expression of miR-597-3p and MACC1. Functional assays (MTT, colony formation, AO/EB staining, and Transwell invasion) evaluated cell proliferation, cell death, and invasion. Dual-luciferase reporter assays confirmed the interaction between miR-597-3p and MACC1, while bioinformatics analysis identified potential targets. Western blotting was used to validate MACC1 and downstream proteins, MMP-2, and MMP-9 at the protein level.
Results
MiR-597-3p was significantly downregulated (P < 0.05) in ovarian cancer cells, whereas MACC1 was upregulated. Overexpression of miR-597-3p suppressed cell viability, colony formation, and invasion, and increased cell death with a pro-apoptotic shift in Bax/Bcl-2 expression. Mechanistically, miR-597-3p directly targeted MACC1, leading to reduced expression of MMP-2 and MMP-9 at both transcript and protein levels. Notably, MACC1 overexpression reversed the tumor-suppressive effects of miR-597-3p.
Conclusions
MiR-597-3p functions as a tumor suppressor in ovarian cancer by directly targeting MACC1, thereby inhibiting proliferation, invasion, and survival. These findings highlight the miR-597-3p/MACC1 axis as a potential therapeutic target and suggest miR-597-3p as a promising biomarker for ovarian cancer.
Introduction
Ovarian cancer stands as the eighth most common type of cancer in women worldwide and remains the second major cause of cancer-related deaths (Webb & Jordan, 2024). Ovarian cancer is very lethal, with approximately 0.31 million new cases and 0.2 million new deaths annually, largely due to its diagnosis at advanced stages, with a median diagnosis age of 63 years in developed countries (Bray et al., 2024; Ali, Al-Ani & Al-Ani, 2023). This late-stage presentation is primarily caused by the non-specific symptoms that overlap with those of menopause and the absence of reliable screening methods (Ali, Al-Ani & Al-Ani, 2023). Prognosis in ovarian cancer continues to be bleak, thereby necessitating the elucidation of its more precise molecular mechanisms to identify better therapeutic targets (Konstantinopoulos & Matulonis, 2023). One promising area of investigation is the role of MicroRNAs (miRs), which are small non-coding RNA molecules that modulate gene expression at the post-transcriptional stage (Shi et al., 2021). Known to carry out critical functions in a diversity of biological processes such as cell proliferation and metastasis, miRs are considered agents for regulating various diseases in living organisms (Kargutkar, Hariharan & Nadkarni, 2023). In the context of cancer, depending on the genes they regulate, miRNAs can serve as either promoters of tumor development or inhibitors, acting as oncogenes or tumor suppressors (Zhang et al., 2007). Having diverse roles in cancer progression, miRNAs have attracted interest as therapeutic targets and as biomarkers of early-stage diagnosis (Medina & Slack, 2008). MiR-597-3p has been suggested as a tumor suppressor in several malignancies (Yu, Li & Zhong, 2020; Kang, Kim & Choi, 2022; Li et al., 2023; Zhang et al., 2023). In non-small cell lung cancer (NSCLC), it inhibits tumor progression by inducing cell cycle arrest and reducing tumor growth (Yu, Li & Zhong, 2020). In myelodysplastic syndromes, miR-597-3p promotes apoptosis by repressing FOSL2, an anti-apoptotic transcription factor (Kang, Kim & Choi, 2022). Similarly, in colorectal cancer, it functions as an in vivo inhibitor of tumor growth by targeting CXCL5 (Li et al., 2023), while in gastric cancer, its overexpression reduces cell viability and enhances apoptosis (Zhang et al., 2023). Collectively, these findings highlight the broad tumor-suppressive potential of miR-597-3p across diverse cancer types. Despite this evidence, its role in ovarian cancer remains poorly defined. Given its consistent tumor-inhibitory effects in other malignancies, we hypothesized that miR-597-3p may exert similar suppressive functions in ovarian cancer, particularly through regulation of oncogenic drivers.
Metastasis-associated in colon cancer 1 (MACC1), an oncogene that stimulates tumor growth, invasion, and metastasis, is a crucial factor in ovarian cancer progression. MACC1 expression is upregulated in many cancers, such as ovarian cancer, and has been linked to poor prognosis, higher stage of tumors, and chemotherapy resistance (Guo et al., 2016; Zhang et al., 2019; Huang et al., 2013). MACC1 primarily exerts its effects through the activation of diverse signaling pathways that play pivotal roles in regulating cell migration, survival, and tumor metastasis (Xiong et al., 2022). Despite its well-established role in ovarian cancer, the regulation of MACC1 by miRNAs has not been well understood, and the potential involvement of miR-597-3p in modulating MACC1 expression remains an area of active research. Based on increasing evidence that miR-597-3p plays a role as a tumor suppressor and its possible roles in regulating major oncogenes, we set out to study its effects in terms of ovarian cancer cell proliferation and invasion. Furthermore, in this work, the regulatory interaction of miR-597-3p and MACC1 is examined. Through more detailed understanding of the molecular interactions between miR-597-3p and MACC1, this work is expected to contribute to developing novel therapeutic strategies against ovarian cancer progression and metastasis.
Materials and Methods
Cell culture
In this study, ovarian cancer cell lines (CAOV-3, SKOV3, OVCAR-3, and OV90) along with the normal ovarian epithelial cell line IOSE-398 were utilized. These cell lines were obtained from the American Type Culture Collection (ATCC). The cells were grown in RPMI-1640 medium, which was supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The cultures were maintained in a humidified incubator at 37 °C, with an atmosphere containing 5% CO2.
Expression analysis
Quantitative real-time PCR (qRT-PCR) was utilized to assess the transcript levels of miR-597-3p, MACC1 and related genes. Total RNA was isolated using the RNeasy Mini Kit following the manufacturer’s guide. MiRNA expression was analysed by synthesizing complementary DNA (cDNA) of miScript II RT Kit and miR-597-3p expression was measured by miR-specific primers. For gene expression, cDNA was synthesized using the iScript cDNA Synthesis Kit, and MACC1 expression was quantified using MACC1-specific primers. Target gene expression levels were normalized to internal control, U6 for miRNAs and GAPDH for mRNA, then analysed using the 2−ΔΔCt method. Primers are shown in Table S1.
Transfection
Ovarian cancer cells were seeded at a density of 2 ×105 cells per well in 6-well plates and transfected the next day when they reached ∼70% confluency. Transfections were performed using Lipofectamine 3000 reagent (Invitrogen, Waltham, MA, USA) at a ratio of two µL Lipofectamine per 50 nM oligonucleotide or two µg plasmid DNA, according to the manufacturer’s protocol. The following constructs were used: miR-597-3p mimics (50 nM), siRNA targeting MACC1 (50 nM), pcDNA-MACC1 plasmid (two µg), and the corresponding negative controls (miR-NC, si-NC, and empty pcDNA vector). Transfection complexes were prepared in Opti-MEM medium and incubated with cells for 6 h, after which the medium was replaced with complete RPMI-1640 medium containing 10% FBS. Cells were then maintained under standard culture conditions for 48 h before downstream assays.
MTT assay
Cell viability was assessed using the MTT assay. Transfected cells were seeded into 96-well plates at a density of 1 × 104 cells per well in 100 µL of medium and incubated for 24, 48, 72 and 96 hours under standard culture conditions. At each time point, 10 µL of MTT solution (five mg/mL in PBS) was added to each well, and the plates were incubated at 37 °C for 4 hours. The resulting formazan crystals were dissolved by adding 100 µL of dimethyl sulfoxide (DMSO) to each well, and absorbance was measured at 570 nm using a microplate reader (BioTek Synergy HT, Winooski, VT, USA). Cell viability of the control group was taken as 100%, and the viability of other groups was calculated relative to this value.
Colony formation assay
For the colony formation assay, 500 cells per well were seeded into 6-well plates and cultured under standard conditions for 10–14 days, with medium replaced every 3 days. At the end of the incubation period, colonies were gently washed with PBS, fixed with 4% paraformaldehyde for 20 minutes, and stained with 0.1% crystal violet for 30 minutes at room temperature. The plates were rinsed with distilled water and air-dried. Colonies containing more than 50 cells were counted under an inverted microscope, and results were expressed relative to the control group.
Bioinformatics analysis
To identify potential targets of miR-597-3p, bioinformatics prediction tools including TargetScan, miRDB, and TarBase were employed. Venn diagram analysis was performed to determine the overlapping candidate genes predicted by these databases. Among the identified targets, MACC1 was prioritized based on its well-established role in tumor progression and metastasis. In addition, gene expression profiling was carried out using the GEPIA database (http://gepia.cancer-pku.cn/) to compare MACC1 expression levels between ovarian cancer tissues and normal ovarian tissues.
Dual luciferase assay
Luciferase activity was quantified using the Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) according to the manufacturer’s instructions. Luminescence was measured with a GloMax® 96 Microplate Luminometer (Promega, Madison, WI, USA), which allows sequential detection of firefly and Renilla luciferase signals using dual injectors. Firefly luciferase activity was normalized against Renilla luciferase activity to control for transfection efficiency. Raw luminescence data were acquired with GloMax software and further processed, statistically analyzed, and visualized using GraphPad Prism 8 (GraphPad Software, San Diego, CA, USA).
AO/EB staining
Apoptosis was detected by acridine orange/ethidium bromide (AO/EB) staining. SKOV-3 cells were harvested 48 h after transfection with miR-597-3p mimics or controls. Cells were washed twice with PBS and resuspended in 100 µL PBS. A staining solution containing AO (10 µg/mL) and EB (10 µg/mL) was added, and cells were incubated for 10 min at 37 °C in the dark. Stained cells were immediately examined under a fluorescence microscope. Viable cells emitted green fluorescence, whereas dead cells emitted orange/red fluorescence.
Transwell assay
The invasive potential of ovarian cancer cells was assessed using Transwell chambers (eight µm pore size; Corning, Corning, NY, USA) coated with Matrigel (diluted 1:8 in serum-free medium, 50 µL per insert, incubated at 37 °C for 1 h to solidify). SKOV-3 cells (1 ×105) were resuspended in 200 µL of serum-free medium and added to the upper chamber, while 600 µL of complete medium containing 10% FBS was placed in the lower chamber as a chemoattractant. After 48 h of incubation at 37 °C in 5% CO2, non-invading cells on the upper surface were removed with a cotton swab. Invading cells on the underside of the membrane were fixed with 4% paraformaldehyde for 20 min and stained with 0.1% crystal violet for 30 min. Invaded cells were counted under an inverted microscope (five random fields per insert). The invasion rate was expressed relative to the control group, which was set as 100%, and experimental groups were normalized accordingly.
Statistical analysis
All data are presented as mean ± standard deviation (SD) from at least three independent experiments. Statistical significance between two groups was assessed using the paired Student’s t-test, while comparisons among multiple groups were performed using one-way ANOVA followed by Dunnett’s post hoc test against the control group. A value of P < 0.05 was considered statistically significant. All analyses were conducted using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA).
Results
miR-597-3p suppresses ovarian cancer cell proliferation
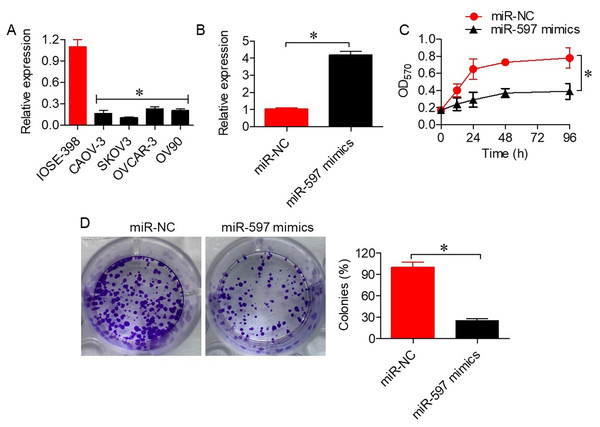
To investigate the role of miR-597-3p in ovarian cancer, we first examined its expression in normal ovarian epithelial cells and ovarian cancer cell lines. qRT-PCR analysis revealed that miR-597-3p expression was significantly downregulated (P < 0.05) in ovarian cancer cells (CAOV-3, SKOV3, OVCAR-3, and OV90) compared with normal IOSE-398 cells (Fig. 1A). Transfection with miR-597-3p mimics markedly increased miR-597-3p expression in SKOV-3 cells (Fig. 1B). Functional assays demonstrated that miR-597-3p overexpression significantly (P < 0.05) inhibited cell viability (Fig. 1C) and colony formation (Fig. 1D), supporting its tumor-suppressive role in ovarian cancer.
Figure 1: miR-597-3p suppresses the proliferation of ovarian cancer cells.
(A) Relative expression levels of miR-597-3p in normal ovarian cells and ovarian cancer cells. (B) Relative miR-597-3p expression in SKOV-3 cells transfected with miR-NC or miR-597-3p mimics. (C) Cell viability of SKOV-3 cells transfected with miR-NC or miR-597-3p mimics. (D) Colony formation ability of SKOV-3 cells transfected with miR-NC or miR-597-3p mimics. All experiments were performed in triplicate, and data are presented as mean ± SD (*P < 0.05).miR-597-3p directly targets MACC1
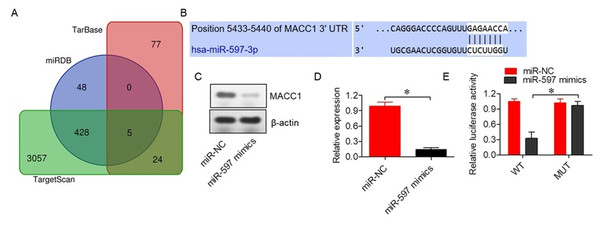
To identify the molecular targets of miR-597-3p, bioinformatic analysis was performed using three independent databases, which revealed five potential target genes (Fig. 2A, Table S2). Among the identified targets, MACC1 was selected as the miR-597-3p target given the role in cancer proliferation and metastasis (Guo et al., 2016). Furthermore, MACC1 showed interactions with miR-597-3p via the 3′ untranslated region (UTR), as confirmed by Target Scan (Fig. 2B). Transfection with miR-597-3p mimics into SKOV-3 cells significantly (P < 0.05) reduced expression of MACC1 (Figs. 2C and 2D). A dual luciferase reporter assay also confirmed the direct interaction of miR-597-3p with the 3′ UTR of MACC1 (Fig. 2E).
Figure 2: miR-597-3p directly targets MACC1.
(A) Venn diagram illustrating the five common target genes of miR-597-3p identified across three indicated databases. (B) TargetScan prediction showing the interaction between miR-597-3p and the 3′ UTR of MACC1. (C) MACC1 protein expression in SKOV-3 cells transfected with miR-NC or miR-597-3p mimics. (D) Relative MACC1 mRNA expression in SKOV-3 cells transfected with miR-NC or miR-597-3p mimics (E) Dual-luciferase assay confirming the interaction between miR-597-3p and the MACC1 3′ UTR in SKOV-3 cells. All experiments were performed in triplicate, and data are presented as mean ± SD (*P < 0.05).Silencing MACC1 inhibits ovarian cancer cell proliferation
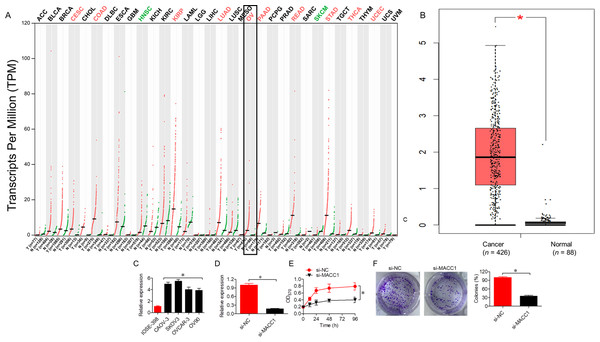
Gene expression profiling analysis (GEPIA database) showed MACC1 upregulated across different cancer types such as ovarian cancer (Fig. 3A). Data from the GEPIA database showed significantly (P < 0.05) elevated levels of MACC1 in ovarian cancer tissues compared to normal tissues (Fig. 3B). Expression analysis showed upregulation of MACC1 in ovarian cancer cell lines relative to normal cell line (Fig. 3C). Knockdown of MACC1 by siRNA (si-MACC1) in SKOV-3 cells significantly (P < 0.05) decreased MACC1 expression at both mRNA and Protein leve (Fig. 3D). Functional assays showed that knockdown of MACC1 reduced cell viability (Fig. 3E) and colony formation (Fig. 3F), corroborating its oncogenic role in ovarian cancer.
Figure 3: Silencing MACC1 inhibits the proliferation of ovarian cancer cells.
(A) Expression profile of MACC1 across cancer types from the GEPIA database. (B) Comparison of MACC1 expression in normal and cancer tissues, as retrieved from the GEPIA database. (C) MACC1 expression in SKOV-3 cells transfected with si-NC or si-MACC1. (D) Cell viability of SKOV-3 cells transfected with si-NC or si-MACC1. (E) Colony formation ability of SKOV-3 cells transfected with si-NC or si-MACC1. All experiments were performed in triplicate, and data are presented as mean ± SD (*P < 0.05).Overexpression of MACC1 reverses the tumor-suppressive effects of miR-597-3p
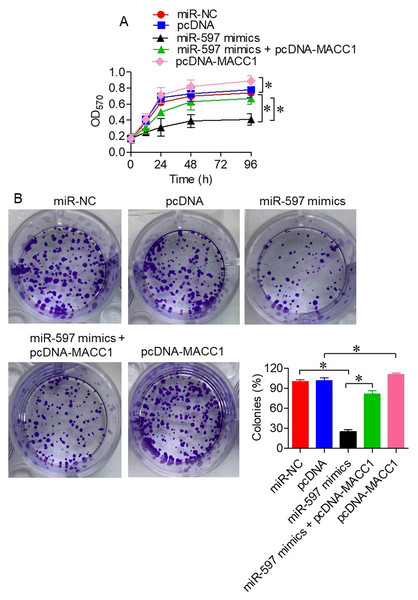
To better understand the functional relationship between miR-597-3p and MACC1, we co-transfected SKOV-3 cells with miR-597-3p mimics and pcDNA-MACC1, which carries a wild-type gene encoding MACC1. Overexpression of MACC1 significantly (P < 0.05) rescued the reduction in cell viability (Fig. 4A) and colony formation (Fig. 4B) induced by miR-597-3p mimics, indicating that miR-597-3p exerts its tumor-suppressive effects by targeting MACC1.
Figure 4: Overexpression of MACC1 reverses the tumor-suppressive effects of miR-597-3p.
(A) Cell viability and (B) colony formation ability of SKOV-3 cells transfected with miR-NC, pcDNA, miR-597-3p mimics, or a combination of miR-597-3p mimics and pcDNA-MACC1. All experiments were performed in triplicate, and data are presented as mean ± SD (*P < 0.05).miR-597-3p promotes apoptosis in ovarian cancer cells
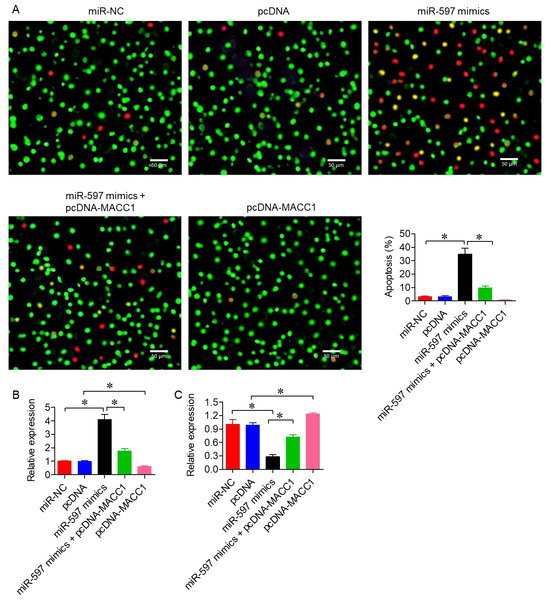
Next, AO/EB staining revealed a significantly higher proportion of non-viable cells in SKOV-3 cells transfected with miR-597-3p mimics compared with controls (P < 0.05; Fig. 5A). To confirm apoptotic induction, we further assessed apoptosis-related proteins. MiR-597-3p overexpression significantly increased Bax expression (pro-apoptotic) and decreased Bcl-2 expression (anti-apoptotic) (Figs. 5B–5C). Together, these findings indicate that miR-597-3p promotes apoptosis in ovarian cancer cells, with AO/EB staining supporting general cell death and Bax/Bcl-2 expression confirming apoptotic involvement.
Figure 5: miR-597-3p promotes apoptosis in ovarian cancer cells.
(A) AO/EB staining of SKOV-3 cells transfected with miR-NC, pcDNA, miR-597-3p mimics, or co-transfected with miR-597-3p mimics and pcDNA-MACC1. (B) Relative mRNA expression of Bax and (C) Relative mRNA expression of Bcl-2 under the same conditions, as measured by qRT-PCR. All experiments were performed in triplicate. Data are presented as mean ± SD (*P < 0.05).miR-597-3p inhibits ovarian cancer cell invasion
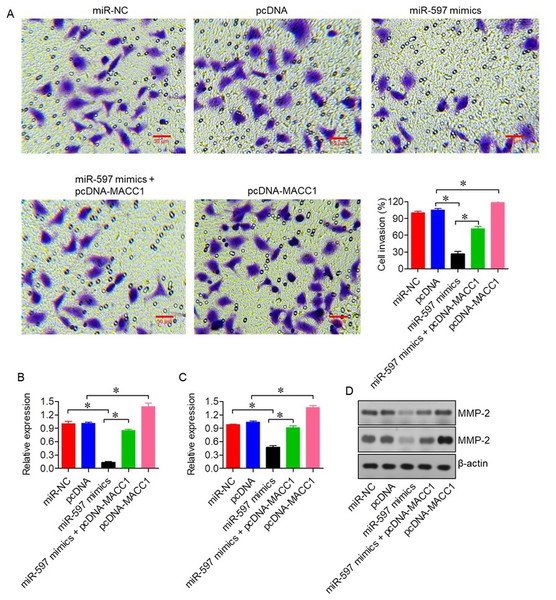
We next evaluated the effect of miR-597-3p on the invasive capacity of ovarian cancer cells. Transwell invasion assays demonstrated that miR-597-3p overexpression significantly reduced the invasive potential of SKOV-3 cells (P < 0.05; Fig. 6A). This inhibitory effect was reversed by MACC1 overexpression, highlighting the central role of the miR-597-3p/MACC1 axis in regulating ovarian cancer cell invasion. In line with these observations, qRT-PCR and western blot analysis showed that miR-597-3p overexpression decreased the expression of the matrix metalloproteinases MMP-2 and MMP-9 (Figs. 6B–6D), which are key mediators of extracellular matrix degradation and tumor invasion.
Figure 6: miR-597-3p inhibits the invasion of ovarian cancer cells.
(A) Transwell invasion assay of SKOV-3 cells transfected with miR-NC, pcDNA, miR-597-3p mimics, or co-transfected with miR-597-3p mimics and pcDNA-MACC1. (B) Relative mRNA expression of MMP-2 and (C) MMP-9 measured by qRT-PCR (D) Western blot analysis of MMP-2 and MMP-9 protein levels under the same transfection conditions. All experiments were performed in triplicate. Data are presented as mean ± SD (*P < 0.05).Discussion
Ovarian cancer is one of the leading causes of mortality among gynecological malignancies, largely due to late-stage diagnosis and poor prognosis. Despite advances in understanding its biology and the introduction of novel therapies, survival rates remain unsatisfactory (Tossetta & Inversetti, 2023). This stagnation may be attributed to the complex molecular mechanisms driving ovarian cancer progression and its intrinsic resistance to chemotherapy (Aliyuda et al., 2023). MicroRNAs (miRs), which post-transcriptionally regulate gene expression, have gained attention for their involvement in diverse cancer-related processes such as proliferation, invasion, and metastasis (Wu et al., 2017). In this study, we investigated the role of miR-597-3p and its regulatory interaction with the oncogene MACC1, providing novel insights into ovarian cancer progression. MiR-597-3p has been reported to act as a tumor suppressor in several malignancies, including NSCLC, myelodysplastic syndromes, colorectal cancer, and gastric cancer, through mechanisms such as cell cycle arrest and induction of apoptosis (Yu, Li & Zhong, 2020; Kang, Kim & Choi, 2022; Li et al., 2023; Zhang et al., 2023). These findings provided the rationale to evaluate its potential role in ovarian cancer. Our results are consistent with previous studies, demonstrating that miR-597-3p overexpression inhibits ovarian cancer cell proliferation and invasion. Importantly, we identified MACC1 as a direct target of miR-597-3p, thereby linking miR-597-3p activity to a well-established oncogenic driver in ovarian cancer.
MACC1 is a recognized oncogene associated with tumor growth, invasion, and metastasis in multiple cancers, including ovarian cancer (Wu et al., 2017; Yu et al., 2019). Clinical studies have shown that high MACC1 expression correlates with poor prognosis, advanced tumor stage, and chemotherapy resistance in epithelial ovarian cancer (Li et al., 2015b; Jeong et al., 2018). In agreement with these findings, our study demonstrated that silencing MACC1 reduced ovarian cancer cell proliferation and invasion, whereas MACC1 overexpression reversed the tumor-suppressive effects of miR-597-3p. These results highlight MACC1 as a key downstream effector of miR-597-3p, consistent with previous work reporting MACC1-mediated metastasis through activation of c-MET signaling (Sheng et al., 2014).
Beyond ovarian cancer, MACC1 promotes invasion and metastasis in other malignancies. In hepatocellular carcinoma, it enhances invasiveness by upregulating MMP-2 and MMP-9 (Gao et al., 2013), and similar effects have been observed in squamous cell carcinoma (Li et al., 2015a). Our results align with these observations, showing that miR-597-3p reduces the expression of MMP-2 and MMP-9 in ovarian cancer cells at both the transcript and protein levels, as confirmed by qRT-PCR and Western blotting. This provides direct mechanistic evidence that the miR-597-3p/MACC1 axis regulates extracellular matrix degradation and invasion in ovarian cancer.
In addition to regulating invasion, miR-597-3p promoted apoptosis in ovarian cancer cells. AO/EB staining demonstrated increased cell death in miR-597-3p mimic-transfected cells, and this was corroborated by changes in apoptosis-related proteins. Specifically, miR-597-3p upregulated Bax, a pro-apoptotic protein, and downregulated Bcl-2, an anti-apoptotic protein (Chota, George & Abrahamse, 2021), thereby shifting the Bax/Bcl-2 balance toward apoptosis. The reversal of these effects by MACC1 overexpression further supports the role of the miR-597-3p/MACC1 axis in apoptotic regulation. It should be noted, however, that AO/EB staining alone cannot reliably distinguish between apoptosis and necrosis. Therefore, we interpreted these findings in conjunction with Bax and Bcl-2 expression to provide stronger evidence of apoptosis. Additional assays, such as caspase activation or flow cytometry, would further validate this conclusion.
Despite the promising implications of our findings, several limitations should be acknowledged. First, the experiments were conducted primarily in vitro using a limited number of ovarian cancer cell lines, and validation in additional models, including patient-derived samples, is necessary to account for the molecular heterogeneity of ovarian cancer. Second, while we identified MACC1 as a direct target of miR-597-3p, other downstream pathways potentially regulated by miR-597-3p remain unexplored. A broader transcriptomic or proteomic approach could provide deeper insights into its regulatory network. Finally, in vivo studies are required to confirm the therapeutic potential of miR-597-3p and assess its clinical applicability as a biomarker or therapeutic target.
Conclusion
In conclusion, this study demonstrates the tumor-suppressive role of miR-597-3p in ovarian cancer, as evidenced by its ability to inhibit cell proliferation, promote apoptosis, and reduce invasion. By directly targeting the oncogene MACC1, miR-597-3p downregulates key downstream effectors, including MMP-2 and MMP-9, thereby impeding critical processes in tumor progression and metastasis. These findings suggest that miR-597-3p holds promise as both a biomarker and a therapeutic target in ovarian cancer. Future in vivo studies and clinical investigations will be essential to validate the translational potential of miR-597-3p-based therapeutic strategies.