The role of pre-pregnancy overweight in gestational diabetes, hypertension, and macrosomia: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Lesley Anson
- Subject Areas
- Diabetes and Endocrinology, Gynecology and Obstetrics, Women’s Health
- Keywords
- Pre-pregnancy overweight, Gestational diabetes mellitus, Gestational hypertension, Macrosomia, Adverse pregnancy outcomes
- Copyright
- © 2025 Zhu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. The role of pre-pregnancy overweight in gestational diabetes, hypertension, and macrosomia: a retrospective study. PeerJ 13:e20277 https://doi.org/10.7717/peerj.20277
Abstract
Purpose
Adverse pregnancy outcomes, including gestational diabetes mellitus (GDM), gestational hypertension (GHp), macrosomia, preterm birth, and low birth weight, pose significant risks to maternal and neonatal health. Pre-pregnancy overweight is a modifiable risk factor for these outcomes. However, comprehensive analyses of multiple adverse outcomes and their dose-response relationships with pre-pregnancy body mass index (BMI) remain limited.
Methods
This retrospective cohort study included 748 women with singleton pregnancies who delivered at Yuyao Maternal and Child Health Hospital from January 1, 2022, to December 31, 2022. Participants were categorized into normal-weight and overweight groups based on pre-pregnancy BMI. Logistic regression models were used to evaluate associations between overweight and adverse pregnancy outcomes, adjusting for confounding variables. Restricted cubic spline (RCS) regression was employed to investigate dose-response relationships between BMI and pregnancy outcomes.
Results
Pre-pregnancy overweight was significantly associated with higher risks of GDM (adjusted OR = 3.122, 95% CI [1.754–5.557], p < 0.001), GHp (adjusted OR = 2.864, 95% CI [1.566–5.239], p = 0.001), and macrosomia (adjusted OR = 2.119, 95% CI [1.076–4.173], p = 0.030). No significant associations were observed with preterm birth or low birth weight. RCS analysis showed no evidence of nonlinear relationships, indicating that the risk of adverse outcomes increased linearly with BMI.
Conclusion
Pre-pregnancy overweight is a significant modifiable risk factor for adverse maternal and neonatal outcomes, particularly GDM, GHp, and macrosomia. These findings underscore the importance of integrating BMI monitoring and personalized weight management strategies into pre-pregnancy care programs to mitigate risks and improve maternal and neonatal health outcomes.
Introduction
Maternal and child health serves as a cornerstone of public health, reflecting not only individual well-being but also the capacity for societal development and sustainability (Kerber et al., 2007). Adverse pregnancy outcomes, including preterm birth, low birth weight, and macrosomia, have profound implications for maternal and neonatal health. These outcomes significantly increase the risks of neonatal mortality, developmental impairments, and long-term chronic illnesses (Jornayvaz et al., 2016; Chawanpaiboon et al., 2019). Additionally, they place substantial burdens on healthcare systems and pose considerable socioeconomic challenges, particularly in low- and middle-income countries (Petrou, Yiu & Kwon, 2019). Addressing these challenges is essential for reducing global health disparities and improving outcomes for mothers and neonates.
In recent decades, the prevalence of overweight and obesity among women of reproductive age has risen dramatically, driven by widespread changes in dietary habits, physical activity, and lifestyle behaviors (Popkin, Adair & Ng, 2012). Currently, approximately 24.8% of Chinese women of childbearing age are classified as overweight, with an additional 4.8% categorized as obese (He et al., 2016). This alarming trend represents a significant threat to maternal and neonatal health, as pre-pregnancy overweight has been identified as a critical risk factor for adverse maternal outcomes such as gestational diabetes mellitus (GDM), gestational hypertension (GHp), and increased rates of cesarean delivery (Mutsaerts et al., 2014; Catalano & Shankar, 2017). Similarly, neonatal outcomes such as macrosomia and low birth weight are strongly associated with pre-pregnancy overweight, underscoring the vital role of maternal metabolic health in fetal development (Liu et al., 2016; Voerman et al., 2019).
Despite substantial evidence from Western populations, the relationship between pre-pregnancy overweight and adverse pregnancy outcomes remains underexplored in Chinese populations. Globally, a meta-analysis of 63 studies from 29 countries estimated the pre-pregnancy overweight and obesity prevalences at 23.0% and 16.3% (Martínez-Hortelano et al., 2020). Another systematic review and meta-analysis reported that 43.8% of pregnancies in 2010–2019 were affected by overweight/obesity and 16.3% by obesity, with modelled current obesity prevalence around 20.9% (Kent, Mcgirr & Eastwood, 2024). Consistently, a global modelling study estimated that in 2014, approximately 38.9 million pregnant women had overweight or obesity and 14.6 million had obesity (Chen, Xu & Yan, 2018). In China, large-scale preconception surveillance from the National Free Preconception Health Examination Project reported that, using Chinese BMI criteria, the combined prevalence of pre-pregnancy overweight/obesity was 24.8% among more than 16 million rural women between 2010 and 2014 (He et al., 2016). This gap is particularly notable given the unique dietary patterns, healthcare access, and sociocultural factors within this population (He et al., 2016). Moreover, existing studies frequently focus on single outcomes—for example GDM (Li et al., 2020), GHp (Zhou et al., 2015), or macrosomia (Li et al., 2021)—rather than jointly assessing multiple maternal and neonatal endpoints within the same cohort. Addressing this evidence gap in the Chinese context, by simultaneously evaluating multiple adverse outcomes and exploring dose–response patterns, can generate more actionable, population-specific evidence to inform preconception and antenatal weight-management strategies.
This study aims to comprehensively evaluate the association between maternal pre-pregnancy overweight and adverse maternal and neonatal outcomes—including GDM, GHp, macrosomia, preterm birth, and low birth weight—within a Chinese population. By providing population-specific evidence, this research seeks to inform the development of tailored public health strategies for weight management and the prevention of adverse pregnancy outcomes. Ultimately, the findings aim to contribute to improved maternal and child health outcomes in China.
Methods
Study design
This retrospective cohort study was conducted at the Yuyao Maternal and Child Health Hospital, utilizing clinical data collected over a one-year period from January 1, 2022, to December 31, 2022. The primary objective of the study was to evaluate the associations between maternal pre-pregnancy overweight status and adverse maternal and neonatal outcomes. By analyzing detailed clinical and demographic data, this study aimed to provide a comprehensive understanding of the impact of pre-pregnancy overweight on pregnancy-related complications and neonatal health.
Participants and sample
Participants were recruited based on clearly defined inclusion and exclusion criteria. The inclusion criteria were as follows: (1) women who were primiparous, primigravid, and in their first marriage; (2) aged between 20 and 35 years; (3) delivered live singleton births at the hospital; and (4) attended regular prenatal care visits. We restricted the analysis to primiparous, primigravid, and first-married women in order to reduce heterogeneity in reproductive history, obstetric complications, and socio-familial characteristics that could confound the associations under study. This also ensured complete capture of clinical records from the first pregnancy. The exclusion criteria included: (1) women where one or both partners were in a remarriage; (2) women classified as part of the migrant population. After applying these criteria, a total of 748 women with singleton pregnancies were included in the final analysis.
Ethical approval for this study was obtained from the local ethics committee of Yuyao Maternal and Child Health Hospital (2023KT007). All participants provided written informed consent prior to participation in accordance with institutional and ethical guidelines.
Data collection methods
Data for this study were extracted from routinely collected clinical records at Yuyao Maternal and Child Health Hospital. The collected data included the following categories: Sociodemographic information: Maternal age, education level, occupation, smoking status, and alcohol consumption habits. Anthropometric measurements: Height and weight recorded during the first prenatal visit, which were used to calculate pre-pregnancy body mass index (BMI). Pregnancy and neonatal outcomes: Gestational age at delivery, mode of delivery, and maternal complications, including GDM and GHp. Neonatal outcomes included preterm birth, low birth weight, normal birth weight, and macrosomia. All data were recorded systematically during routine clinical care, ensuring accuracy and reliability for analysis. Standardized protocols and trained healthcare providers were employed to minimize variability and maintain data integrity.
Measurement tools
Pre-pregnancy BMI was calculated using weight recorded within two months before pregnancy and height measured during the first prenatal visit, the most recent prenatal BMI during pregnancy was not considered, as it does not reflect the baseline pre-pregnancy weight status. BMI was determined as weight (kg) divided by the square of height (m2). Participants were classified based on the criteria established by the Working Group on Obesity in China as follows: Normal weight: BMI of 18.5–23.9 kg/m2; Overweight: BMI of 24–27.9 kg/m2; Obesity: BMI ≥ 28 kg/m2(Huang et al., 2024). Due to the small number of participants categorized as obese, overweight and obese groups were combined into a single “overweight” group. Similarly, participants with underweight BMI (<18.5 kg/m2) were combined with the normal weight group for analysis.
GHp was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg during late pregnancy, or the use of antihypertensive medication(Brown et al., 2001). GDM was diagnosed based on meeting one or more of the following criteria during pregnancy: fasting glucose ≥5.1 mmol/L, 1-hour glucose ≥10.0 mmol/L, or 2-hour glucose ≥8.5 mmol/L following an oral glucose tolerance test (Metzger et al., 2010). Preterm birth was defined as delivery before 37 completed weeks of gestation(Goldenberg et al., 2008). Low birth weight was defined as a neonatal birth weight <2500 g. Macrosomia was defined as a neonatal birth weight ≥ 4,000 g (Huang et al., 2024).
Statistical analysis
Statistical analyses were performed using SPSS (version 25.0; IBM Corp., Armonk, NY, USA) and R (version 4.4.1). Continuous variables were presented as mean ± standard deviation and compared using independent sample t-tests. Categorical variables were expressed as frequencies and percentages, with group comparisons conducted using chi-square tests or Fisher exact tests when appropriate. Univariate and multivariate logistic regression analyses were employed to examine the associations between pre-pregnancy overweight status and adverse maternal and neonatal outcomes. The multivariate models adjusted for potential confounders, including age, education level, occupation, smoking status, and alcohol consumption. Results were summarized as odds ratios (OR) with 95% confidence intervals (CIs) and visualized using a forest plot for clarity. As a sensitivity analysis, we further adjusted the multivariate model for pre-existing diabetes mellitus and chronic hypertension to assess the robustness of the associations. To explore potential dose–response relationships, restricted cubic spline (RCS) regression was applied, allowing for the assessment of non-linear associations between pre-pregnancy BMI and adverse outcomes. The model used three default knots placed at the 10th, 50th, and 90th percentiles of the BMI distribution, following standard statistical conventions. Statistical significance was defined as a two-sided p-value < 0.05 for all analyses.
Results
Baseline characteristics by overweight status
Table 1 summarizes the baseline characteristics of the study population, comprising 620 normal-weight participants and 128 overweight participants. Overweight participants were slightly younger, with a mean age of 26.12 ± 2.61 years compared to 26.77 ± 2.86 years in the normal-weight group, a difference that reached statistical significance (p = 0.018).
| Variables | Total (n = 748) | Normal weight (n = 620) | Overweight (n = 128) | t/χ2 | p |
|---|---|---|---|---|---|
| Age | 26.66 ± 2.83 | 26.77 ± 2.86 | 26.12 ± 2.61 | 2.37 | 0.018 |
| Education level | 14.09 | <0.001 | |||
| Secondary school and below | 104 (13.90) | 81 (13.06) | 23 (17.97) | ||
| Junior college | 217 (29.01) | 166 (26.77) | 51 (39.84) | ||
| Undergraduate and above | 427 (57.09) | 373 (60.16) | 54 (42.19) | ||
| Occupation | 3.54 | 0.170 | |||
| Sedentary work | 621 (83.02) | 522 (84.19) | 99 (77.34) | ||
| Moderate physical activity work | 110 (14.71) | 85 (13.71) | 25 (19.53) | ||
| Physically demanding work | 17 (2.27) | 13 (2.10) | 4 (3.12) | ||
| Smoking | 16.04 | <0.001 | |||
| No | 680 (90.91) | 575 (92.74) | 105 (82.03) | ||
| Passive smoking | 55 (7.35) | 35 (5.65) | 20 (15.62) | ||
| Yes | 13 (1.74) | 10 (1.61) | 3 (2.34) | ||
| Alcohol consumption | 0.84 | 0.358 | |||
| No | 686 (91.71) | 566 (91.29) | 120 (93.75) | ||
| Yes | 62 (8.29) | 54 (8.71) | 8 (6.25) |
Education levels varied significantly between the groups. Among overweight participants, 39.84% had completed junior college, compared to 26.77% in the normal-weight group, whereas only 42.19% of overweight participants had attained undergraduate or higher degrees compared to 60.16% of normal-weight participants (p < 0.001).
Smoking habits also demonstrated significant differences. Passive smoking was more prevalent among overweight participants (15.62%) compared to normal-weight participants (5.65%, p <0.001). Conversely, no significant differences were identified for occupation (p = 0.170) or alcohol consumption (p = 0.358).
Maternal and neonatal outcomes by overweight status
Table 2 presents the comparison of maternal and neonatal outcomes between normal-weight and overweight participants. Overweight participants exhibited a significantly higher incidence of GHp (15.62% vs. 5.81%, p < 0.001) and GDM (17.97% vs. 6.29%, p < 0.001) compared to their normal-weight counterparts.
| Variables | Total (n = 748) | Normal weight (n = 620) | Overweight (n = 128) | t/χ2 | p |
|---|---|---|---|---|---|
| GHp | 14.77 | <0.001 | |||
| No | 692 (92.51) | 584 (94.19) | 108 (84.38) | ||
| Yes | 56 (7.49) | 36 (5.81) | 20 (15.62) | ||
| GDM | 19.04 | <0.001 | |||
| No | 686 (91.71) | 581 (93.71) | 105 (82.03) | ||
| Yes | 62 (8.29) | 39 (6.29) | 23 (17.97) | ||
| Preterm birth | 0.34 | 0.562 | |||
| No | 709 (94.79) | 589 (95.00) | 120 (93.75) | ||
| Yes | 39 (5.21) | 31 (5.00) | 8 (6.25) | ||
| Delivery mode | Fisher | 0.020 | |||
| Spontaneous vaginal delivery | 467 (62.43) | 399 (64.35) | 68 (53.12) | ||
| Cesarean section | 276 (36.90) | 218 (35.16) | 58 (45.31) | ||
| Operative vaginal delivery | 5 (0.67) | 3 (0.48) | 2 (1.56) | ||
| Low birth weight infant | 0.53 | 0.467 | |||
| No | 726 (97.06) | 600 (96.77) | 126 (98.44) | ||
| Yes | 22 (2.94) | 20 (3.23) | 2 (1.56) | ||
| Macrosomia | 5.68 | 0.017 | |||
| No | 701 (93.72) | 587 (94.68) | 114 (89.06) | ||
| Yes | 47 (6.28) | 33 (5.32) | 14 (10.94) |
In terms of delivery mode, cesarean section was more frequent in the overweight group (45.31%) compared to the normal-weight group (35.16%, p = 0.020). Similarly, the prevalence of macrosomia was significantly higher among overweight participants (10.94%) than in the normal-weight group (5.32%, p = 0.017).
Conversely, no significant differences were observed between the two groups for preterm birth (6.25% vs. 5.00%, p = 0.562) or low birth weight infants (1.56% vs. 3.23%, p = 0.467).
Logistic regression of overweight status and adverse outcomes
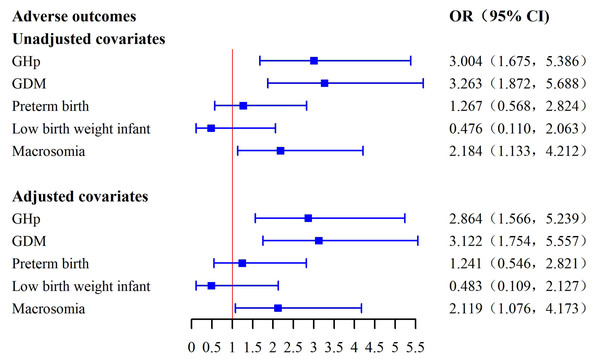
Table 3 and Fig. 1 collectively display the results of univariate and multivariate logistic regression analyses assessing the associations between pre-pregnancy overweight status and adverse maternal and neonatal outcomes. In the unadjusted analysis, pre-pregnancy overweight was significantly associated with increased risks of GHp (OR = 3.004, 95% CI [1.675–5.386], p < 0.001), GDM (OR = 3.263, 95% CI [1.872–5.688], p < 0.001), and macrosomia (OR = 2.184, 95% CI [1.133–4.212], p = 0.020).
| Variables | β | S.E. | Wald | p | OR (95% CI) |
|---|---|---|---|---|---|
| Model 1 Unadjusted covariates |
|||||
| GHp | 1.100 | 0.298 | 13.633 | <0.001 | 3.004 (1.675, 5.386) |
| GDM | 1.183 | 0.283 | 17.406 | <0.001 | 3.263 (1.872, 5.688) |
| Preterm birth | 0.236 | 0.409 | 0.334 | 0.563 | 1.267 (0.568, 2.824) |
| Low birth weight infant | −0.742 | 0.748 | 0.984 | 0.321 | 0.476 (0.110, 2.063) |
| Macrosomia | 0.781 | 0.335 | 5.441 | 0.020 | 2.184 (1.133, 4.212) |
| Model 2 Adjusted covariates |
|||||
| GHp | 1.052 | 0.308 | 11.661 | 0.001 | 2.864 (1.566, 5.239) |
| GDM | 1.138 | 0.294 | 14.973 | <0.001 | 3.122 (1.754, 5.557) |
| Preterm birth | 0.216 | 0.419 | 0.265 | 0.607 | 1.241 (0.546, 2.821) |
| Low birth weight infant | −0.729 | 0.757 | 0.927 | 0.336 | 0.483 (0.109, 2.127) |
| Macrosomia | 0.751 | 0.346 | 4.719 | 0.030 | 2.119 (1.076, 4.173) |
| Model 3 Adjusted covariates + comorbidities |
|||||
| GHp | 1.054 | 0.311 | 11.464 | 0.001 | 2.869 (1.559, 5.282) |
| GDM | 1.183 | 0.296 | 15.980 | <0.001 | 3.264 (1.827, 5.829) |
| Preterm birth | 0.250 | 0.420 | 0.355 | 0.551 | 1.285 (0.564, 2.928) |
| Low birth weight infant | −0.697 | 0.758 | 0.847 | 0.357 | 0.498 (0.113, 2.198) |
| Macrosomia | 0.783 | 0.347 | 5.090 | 0.024 | 2.188 (1.108, 4.321) |
Notes:
Model 1 (Unadjusted covariates): univariate analysis without covariate adjustment.
Model 2 (Adjusted covariates): adjusted for age, education level, occupation, smoking status, and alcohol consumption.
Model 3 (Adjusted covariates + comorbidities): Model 2 further adjusted for pre-existing diabetes mellitus and chronic hypertension.
Figure 1: Forest plot of associations between overweight status and adverse outcomes.
After adjusting for potential confounders, including age, education level, occupation, smoking, and alcohol consumption, these associations remained significant. Overweight participants had higher odds of GHp (adjusted OR = 2.864, 95% CI [1.566–5.239], p = 0.001), GDM (adjusted OR = 3.122, 95% CI [1.754–5.557], p < 0.001), and macrosomia (adjusted OR = 2.119, 95% CI [1.076–4.173], p = 0.030). No significant associations were observed between overweight status and preterm birth or low birth weight in either the unadjusted or adjusted analyses. In a further sensitivity analysis additionally adjusting for pre-existing diabetes mellitus and chronic hypertension, the direction and magnitude of the associations remained essentially unchanged (Table 3), supporting the robustness of our findings.
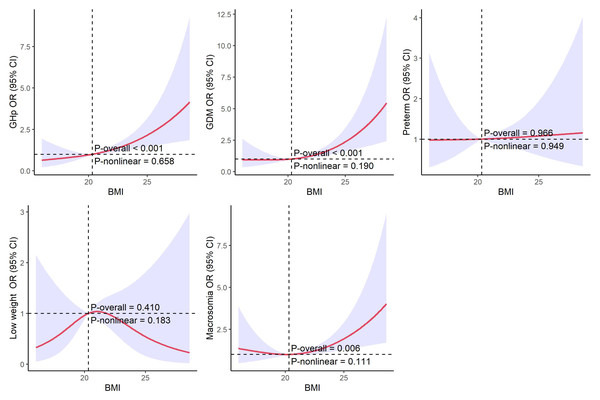
Restricted cubic spline analysis of BMI and adverse outcomes
Figure 2 presents the restricted cubic spline (RCS) regression curves evaluating the relationship between pre-pregnancy BMI and various adverse maternal and neonatal outcomes. The results indicate a positive linear association between BMI and the risks of GHp, GDM, and macrosomia. As BMI increases, the odds of these outcomes rise consistently, with no evidence of non-linear associations, as demonstrated by non-significant non-linearity p-values. In contrast, no significant associations were observed between BMI and preterm birth or low birth weight in the RCS analysis.
Figure 2: RCS of pre-pregnancy BMI and adverse maternal and neonatal outcomes.
Discussion
This study demonstrates that pre-pregnancy overweight is significantly associated with an increased risk of GDM, GHp, and macrosomia. Multivariate logistic regression analysis confirmed that these associations remained statistically significant after adjusting for confounding factors, including age, educational attainment, occupation, smoking, and alcohol consumption. Additionally, no significant association was identified between pre-pregnancy overweight and adverse outcomes such as preterm birth or low birth weight. Using restricted cubic spline analysis, the study revealed a positive dose–response relationship between pre-pregnancy BMI and adverse pregnancy outcomes, indicating a progressive increase in risk with rising BMI. These findings underscore the significant impact of pre-pregnancy overweight on maternal metabolic stress and fetal development, offering a solid scientific basis for optimizing perinatal health management strategies.
The significant association between pre-pregnancy overweight and the risk of GDM is consistent with findings from multiple studies (Song et al., 2023). Obesity and overweight are major risk factors for GDM, with obese women having a 3.26-fold higher risk compared to women with normal weight (Assaf-Balut et al., 2016). A systematic review indicated that for every 1 kg/m2 increase in pre-pregnancy BMI, the prevalence of GDM increases by 0.92% (Torloni et al., 2009). It has been estimated that maintaining a normal BMI in all women could potentially prevent approximately half of GDM cases (Kim et al., 2010). This phenomenon likely reflects the systemic impact of maternal overweight on glucose metabolism. Mechanistically, overweight and obesity are associated with the clustering of metabolic risk factors during early pregnancy, including elevated fasting glucose levels and insulin resistance, which are strongly linked to a higher risk of GDM (Yen et al., 2019). Pre-pregnancy overweight is linked to insulin resistance, primarily due to the release of pro-inflammatory cytokines from adipose tissue, which impairs insulin signaling and glucose metabolism (Tinius et al., 2020).
This study also found that pre-pregnancy overweight significantly increases the risk of GHp, consistent with existing research (Barton et al., 2015; Shin & Song, 2015). Women with pre-pregnancy overweight and a history of prehypertension have a 3.5-fold increased risk of developing hypertensive disorders of pregnancy (Hedderson et al., 2012). Furthermore, the combination of pre-pregnancy overweight and GDM significantly elevates the subsequent risk of developing both diabetes and hypertension (Pirkola et al., 2010). Potential mechanisms include the accumulation of adipose tissue leading to elevated estrogen levels, which, through the renin-angiotensin-aldosterone system, regulate aldosterone secretion, thereby triggering sodium retention and increased blood pressure (Sun et al., 2020; Bernardi et al., 2024). Additionally, dysregulated lipid metabolism may further contribute to GHp development by increasing vascular resistance and inflammation levels (Jarvie & Ramsay, 2010).
This study found that pre-pregnancy overweight significantly increases the risk of macrosomia. A cohort study indicated that both pre-pregnancy overweight and obesity are associated with an elevated risk of macrosomia (Feng et al., 2019). Furthermore, the combination of pre-pregnancy overweight and GDM exhibits a synergistic effect, significantly amplifying the risk of macrosomia and large for gestational age compared to either factor alone (Yang et al., 2019). Mediation analysis further demonstrated that GDM mediates approximately 40% of the effect of pre-pregnancy obesity on macrosomia (Song et al., 2022).
This study found that women with pre-pregnancy overweight were more likely to undergo cesarean delivery, whereas women with normal weight were more likely to deliver vaginally. Other studies have similarly reported a higher prevalence of cesarean delivery among obese women (Vinturache et al., 2014). Pre-pregnancy overweight may influence delivery mode through multiple mechanisms. First, overweight women are more likely to develop pregnancy-related complications, such as GDM and GHp, which increase the clinical need for cesarean delivery (Barber et al., 2011; Scott-Pillai et al., 2013). Second, pre-pregnancy overweight is associated with a higher incidence of macrosomia, where larger fetal size increases the difficulty of vaginal delivery and further raises the likelihood of cesarean section.
Beyond immediate delivery outcomes, cesarean delivery to overweight mothers also contributes to intergenerational obesity transmission, as infants born via cesarean delivery are at greater risk of childhood obesity, particularly in the context of maternal overweight (Mueller et al., 2017; Tun et al., 2018). These findings highlight the importance of perinatal weight management in reducing cesarean delivery rates and mitigating the long-term health risks associated with obesity. This is particularly critical in Asian countries, where cesarean delivery rates remain persistently high (Betran et al., 2021).
This study did not find a significant association between pre-pregnancy overweight and preterm birth or low birth weight. However, some studies have reported an increased risk of preterm birth associated with pre-pregnancy obesity. For example, overweight and obese Chinese women were shown to have a higher risk of preterm birth compared to women with normal weight (Su et al., 2020). Another study reported that the risk of preterm birth increases with higher BMI, particularly among women with class III obesity (Khatibi et al., 2012). The relationship between BMI, weight gain, and preterm birth is complex. In overweight and obese women, gestational weight reduction has been associated with a decreased risk of preterm birth (Masho, Bishop & Munn, 2013). Conversely, studies have also observed a higher risk of preterm birth and low birth weight among women with pre-pregnancy underweight (Yu et al., 2013; Huang et al., 2024). This study did not find significant associations, possibly due to differences in population characteristics, healthcare access, or sample size limitations. This study had limited statistical power to detect small differences in these relatively rare outcomes. Specifically, with the observed incidence of preterm birth (5.21%) and low birth weight (2.94%) in our population, a larger sample size would be needed to definitively rule out associations with pre-pregnancy overweight. Future research should further investigate these complex relationships to better understand the mechanisms linking pre-pregnancy overweight with preterm birth and low birth weight.
Pre-pregnancy overweight is a modifiable risk factor with significant implications for maternal and neonatal health. Effective weight management can reduce pregnancy complications and improve perinatal outcomes. In the context of rising obesity rates and urbanization in China, integrating BMI monitoring and personalized weight management into the national maternal health care system is essential. Promoting healthy lifestyles through pre-pregnancy weight monitoring, health education, and interventions targeting nutrition, physical activity, and mental well-being can lay a strong foundation for maternal and child health.
This study provides critical evidence linking pre-pregnancy overweight to adverse outcomes such as GDM, GHp, and macrosomia, while identifying linear dose–response relationships through restricted cubic spline regression. However, as a single-center study, its generalizability is limited, and the absence of long-term follow-up precludes the assessment of extended impacts. The exclusion of women from remarriages and migrant populations may have further limited the representativeness of our sample and could introduce selection bias. The relatively small number of cases for preterm birth and low birth weight in our cohort may have limited the statistical power to detect significant associations for these outcomes; therefore, we cannot rule out the possibility that true associations exist but were not detected due to insufficient power. Due to limitations in the available clinical records, we were unable to adjust for lifestyle variables such as diet, exercise, or stress levels. Future multicenter cohort studies with larger sample sizes, long-term follow-up, and inclusion of dietary, physical activity, and socioeconomic factors are needed to validate these findings and support the development of precise public health strategies.
Conclusion
This study demonstrates that pre-pregnancy overweight significantly increases the risks of gestational diabetes mellitus, gestational hypertension, and macrosomia, while showing no significant associations with preterm birth or low birth weight. These findings highlight the importance of pre-pregnancy weight management in improving maternal and neonatal health, particularly in the context of rising obesity rates in China. Strengthening pre-pregnancy health education, early BMI monitoring, and personalized weight management plans can reduce adverse outcomes and promote long-term public health benefits.
Supplemental Information
Raw data on maternal BMI and pregnancy outcomes
Individual-level data used in the statistical analyses, including maternal pre-pregnancy BMI, demographic characteristics, and pregnancy outcomes such as gestational diabetes, gestational hypertension, and macrosomia.