The potential biofertilization effect of H2 is accompanied by a modest impact on the composition of microbial communities in the rhizosphere of common vetch
- Published
- Accepted
- Received
- Academic Editor
- Michael LaMontagne
- Subject Areas
- Microbiology, Soil Science
- Keywords
- Biostimulant, Molecular hydrogen, Rhizosphere, Soil, Microbiome, Hydrogen-oxidizing bacteria
- Copyright
- © 2025 Dip and Constant
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. The potential biofertilization effect of H2 is accompanied by a modest impact on the composition of microbial communities in the rhizosphere of common vetch. PeerJ 13:e20019 https://doi.org/10.7717/peerj.20019
Abstract
Background
N2-fixing nodules release molecular hydrogen (H2) in the rhizosphere of legumes. The process activates H2-oxidizing bacteria (HOB) in soil, leading to multiple effects on biogeochemical processes and a potential biofertilization effect. The legacy effect of the energy potential of H2 on the soil microbial community structure and the population density of HOB has received little attention. The aim of the current study is to evaluate how the legacy effect of HOB, previously activated in soil microcosms exposed to elevated H2 concentrations (eH2), affects biomass production yield of common vetch (Vicia sativa), the abundance of HOB, and the composition of the rhizosphere microbiome.
Methods
Contrasting soil samples displaying more than 60% difference in H2 oxidation activity were used as growth substrate for vetch. Soil microbial community composition and diversity were examined by bacterial 16S rRNA polymerase chain reaction (PCR) amplicon sequencing, and dry weight (DW) of the above- and below-ground biomass of vetch was analyzed to assess the impact of HOB enrichment on plant growth. The population density of high-affinity HOB was estimated by using the droplet digital polymerase chain reaction (ddPCR) technique to target the hhyL gene, encoding for the large subunit of group 1H/5 [NiFe]-hydrogenase.
Results
The abundance of HOB possessing group 1H/5 [NiFe]-hydrogenase was indistinguishable between the treatments, indicating that soil nutrient content (inorganic and organic carbon) and the energy potential of H2 were insufficient to support their growth. Aeromicrobium spp. and Ramlibacter spp. were favored by eH2 exposure at the activation stage, but their response was lost after vetch growth. The root biomass and the root/shoot ratio were reduced in soil conditioned with eH2 compared to control soil exposed to ambient H2, suggesting that the plant growth-promotion activity of HOB reduces root proliferation for nutrient prospection. These results provide new experimental evidence suggesting the biofertilization effect of H2 is not universal and requires specific conditions that are yet to be identified.
Introduction
N2-fixing nodules of legume plants release H2 as a by-product of the N2 fixation reaction. In the case of symbiosis involving rhizobia without the [NiFe]-hydrogenase (hup- genotype), the release of H2 creates a concentration gradient from approximately 10,000 ppmv at the soil-nodule interface to sub-atmospheric concentration (<0.5 ppmv) five cm away from the source (Dong & Layzell, 2001; Piché-Choquette, Khdhiri & Constant, 2018). At least 40 phyla of H2-oxidizing bacteria (HOB) exploit the energy potential of H2 (Greening et al., 2016). In carbon limitation conditions, HOB can survive by relying on H2 as the sole energy source or by a mixotrophic strategy combining H2 with organic carbon (Bay et al., 2021; Greening et al., 2015; Islam et al., 2020; Liot & Constant, 2016). According to metagenomic surveys, approximatively 12% of soil bacteria use H2-derived electrons to fix CO2 (Xu et al., 2021).
An elevated H2 concentration in the rhizosphere of legume plants promotes the enrichment of HOB (La Favre & Focht, 1983). Incubating soil samples in modified atmospheres comprising different H2 concentrations isolates the impact of H2 on the composition and activity of soil microbial communities. Soil exposure to 250 nmol H2 g(soil)−1 h−1 mimics H2 fluxes from N2-fixing nodules and enriches CO2 fixation activity from HOB (Stein et al., 2005). The number of cells affiliated to Cytophaga-Flavobacterium-Bacteroides was also impacted by H2, as shown by phylotype enumeration by fluorescence in situ hybridization analyses (Stein et al., 2005). Soil exposure to elevated H2 concentrations promotes the activity of HOB degrading persistent organic pollutants in soil (Xu et al., 2023). The activation of HOB in soil exposed to H2 for a few weeks led to variable impacts on the composition of soil microbial communities (Khdhiri et al., 2017; Khdhiri et al., 2018), calling into question the impact of H2 on soil microbiota.
Soil fumigation by H2 enhances plant growth. For instance, wheat biomass increased by 48% in soil samples exposed to a H2-enriched atmosphere compared to control soil samples (Dong et al., 2003). This phenomenon is known as the H2 fertilization effect (Golding & Dong, 2010). The exact mechanism is currently unknown, but some evidence suggests that it involves HOB activated by H2, including Variovorax spp., displaying plant growth-promotion activity (Maimaiti et al., 2007). The current study was conducted to determine whether a high concentration of H2 in soil activates and increases the population of HOB and if HOB activation modifies the composition of the soil microbial community and enhances the growth of V. sativa. These hypotheses were tested by setting up a two-stage experimental design. The first stage consisted of conditioning two contrasting HOB populations by exposing a sandy loam soil to either (i) sub-atmospheric (<0.5 ppmv) or (ii) supra-atmospheric (5,000 ppmv) H2 concentrations. Soil exposure to H2 lasted two months to favour the activation and enrichment of HOB in soils exposed to high H2 concentration. The second stage consisted of growing V. sativa using the enriched soils as a substrate, without further exposure to the modified atmosphere. The plant biomass and microbial communities of the plant rhizosphere were analyzed to examine the legacy effect of H2 soil exposure. The legacy effect is defined here as a modification of the composition or activity of the microbial community during the activation stage that persists in the second stage of the experiment, without an external supply of H2. The legacy effect includes stimulation of soil biological processes through plant-soil feedback caused by the plant growth-promotion effect of activated HOB. The activation of HOB did not support apparent growth of the HOB population and caused little impact on the composition of the soil microbial community. Elevated H2 exposure reduced the below-ground biomass of V. sativa and no difference was noticed for the above-ground biomass. This manuscript was previously published as a preprint (bioRxiv 2025.02.12.637852; doi: https://doi.org/10.1101/2025.02.12.637852).
Material and Methods
Activation of HOB in soil microcosms
Sandy loam soil (80 g organic matter kg−1, C/N = 10 and pH = 6) was collected in an experimental field trial as described by Agoussar et al. (2021). Soil was homogenized and sieved (two mm mesh size). The water content was adjusted at 30% water holding capacity, and a defined amount (300 g) was transferred into 500 mL (nominal) Wheaton borosilicate bottles, with water content composing approximately half of the total volume. Bottles were sealed with a gas tight rubber septum cap. Two different H2 treatments were applied: elevated H2 concentration (eH2) and atmospheric H2 concentration (control). Soil exposure to eH2 was achieved by injecting a defined volume of certified 99.999% compressed H2 (Praxair Distribution Inc., PA, USA) in bottles to reach 5,000 ppmv in the static headspace. Injections were conducted every 2–3 days after 30 min of equilibration of the headspace with the ambient atmosphere. For the control treatments, bottles were left open for 30 min to equilibrate the headspace with atmospheric H2 (approximatively 0.5 ppmv). Static headspace was replaced every 2–3 days. Microcosms were incubated for two months in dark conditions at room temperature to activate HOB (De la Porte et al., 2024; Piché-Choquette et al., 2016). Each experimental unit was represented by 20 repetitions (2 treatments × 20 repetitions = 40 microcosms). Soil subsamples (0.25 g) were collected after the incubation for DNA extraction, and the balance was transferred into pots for vetch cultivation.
Hydrogen oxidation rate
After seven incubation days, H2 oxidation rate was monitored sporadically. Measurements were performed on a random selection of three soil microcosms representative of each H2 exposure treatment. A gas chromatographic assay was utilized to measure the first-order oxidation rate under 5,000 ppmv H2 concentration (De la Porte et al., 2024). A defined volume of a certified gas mixture containing 100% H2 (Distribution Praxair Canada, Saint-Laurent, Quebec, Canada) was injected into the static headspace of soil microcosms. First-order oxidation rates were computed by integrating H2 time series recorded over 1 h (eH2 treatment) or 2 h (control treatment). H2 was measured with a gas chromatograph (Agilent Technologies, Santa Clara, California, USA) equipped with a thermal conductivity detector. Calibrations were conducted using a certified H2 gas mixture (10,000 ppmv H2, balance air). Linear regression was integrated from three dilutions of the certified gas mixture: 0.1% (1,000 ppmv), 0.5% (5,000 ppmv), and 1% (10,000 ppmv) H2.
Vicia sativa pot experiment
Soil microcosms representative of each H2 exposure were selected after the H2 activation stage (10 repetitions × 2 treatments = 20 pots in total). Soil samples (approximately 300 g) were mixed with vermiculite (5:1, soil:vermiculite) and transferred to 1 L pots. V. sativa L. seeds (EBENA BRAND-FF 2023-0261, Meuneries Mondou) were surface disinfected and allowed to germinate in petri dishes containing 1% water-agar solution at 25 °C, 50% with a photoperiod of 16h/8 h light/darkness, respectively. Seedlings were transferred into the pots and randomly placed in a growth room under full spectrum LED lights for two months. Pots were regularly watered with tap water. The biomass of V. sativa was harvested to determine the fresh and dry weight of aerial and roots biomass. The rhizosphere soil was collected for subsequent DNA extraction, namely “legacy-stage”.
PCR amplicon sequencing and quality control of the bacterial 16S rRNA gene
Total DNA was extracted from soil microcosms after the activation stage and from rhizosphere soil after plant harvest with the PowerLyzer PowerSoil kit (Qiagen), following the instructions from the manufacturer. PCR amplicon sequencing of the bacterial 16S rRNA gene was performed with the primers 515F and 806R, targeting the V4 hypervariable region of the 16S rRNA gene (Caporaso et al., 2011). The procedure for library preparation was followed as described by Saavedra-Lavoie et al. (2020). A few DNA samples were excluded during the library preparation process due to low DNA quality and quantity, leaving a total of 33 samples for the activation stage and 20 samples for the legacy stage. The sequencing and quality control were performed at the Centre d’expertise et de service Génome Québec (Montreal, Québec, Canada) with the Illumina MiSeq PE-250 platform. Downstream analyses of raw sequences were performed on RStudio software using Cutadapt for primer removal and the DADA2 pipeline for quality control, an amplicon sequence variant (ASV) table, and taxonomic assignation (Callahan et al., 2016; Martin, 2011). The taxonomic assignation was based on the SILVA v128 database (Quast et al., 2012). ASVs representing less than 0.005% relative abundance were not considered for downstream analysis. Species richness, Shannon diversity, and Simpson diversity were computed with iNEXT v3.0.1 (Chao et al., 2014; Hsieh, Ma & Chao, 2016). Raw sequence reads were deposited in the Sequence Read Archive of the National Center for Biotechnology Information under BioProject PRJNA1200744.
Quantification of hhyL gene abundance
The abundance of the hhyL gene, encoding for the large subunit of [NiFe]-hydrogenases group 5/1 h, was determined in soil samples collected during the activation stage. The droplet digital PCR (ddPCR) assay described by Baril et al. (2022) was utilized. Randomly selected DNA from each treatment (control and eH2) were used for the ddPCR assays. Negative controls were tested using DNA-free sterile water. The threshold was set manually to include rain in the positive fraction (considering only those with up to 12% error), and samples with more than 10,000 droplets were selected for further analyses. Copy number concentrations were converted to copy per gram of dry soil.
Statistical analyses
Statistical analyses were performed using the software Rstudio v4.2.3 (Team, 2013). Graphics were generated with ggplot2 v3.5.2 (Wickham, 2016). ASVs aggregated at the genus level were subjected to ANCOM-BC analyses to assess the response of bacteria to the activation phase using microbiome v1.20 and ANCOM-BC v2.0.3 (Lahti & Shetty, 2018; Lin & Peddada, 2020). Comparison of biomass dry weight (DW) between treatments and alpha diversity of microbial communities was examined with one-way ANOVA using de stats package v4.2. A t-test was computed to compare the absolute abundance of hhyL genes between the two H2 exposure treatments. Raw datasets utilized for statistical analyses are provided as supplementary material (Tables S1–S8).
Results
HOB activation stage
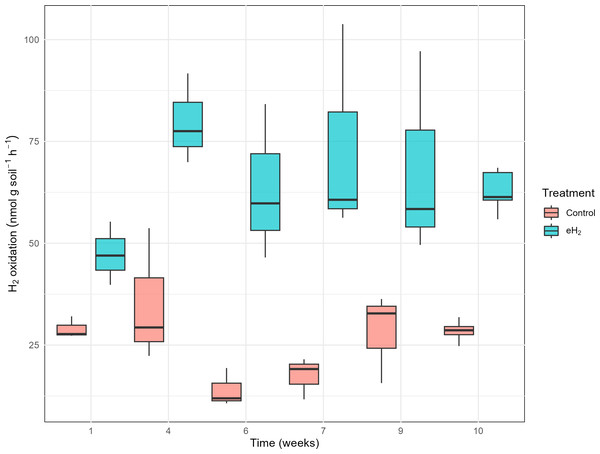
The H2 oxidation rate measured in the control treatment was relatively stable, at 29 ± 3 nmol g(soil−dw)−1 h−1 on average, without significant temporal pattern (Fig. 1). H2 oxidation rate of the control soil decreased in weeks 6 and 7 prior to increasing again at weeks 9 and 10. Repetition of the experiment would be required to verify if the transient loss of activity was caused by technical issues or biological processes. No variations in soil water content or incubation conditions were noticed. Activation of HOB in microcosms exposed to elevated H2 concentration was proved to be efficient with the H2 oxidation rate varying from 47 ± 8 to 80 ± 11 nmol g(soil−dw)−1 h−1 after 30 days of exposure to 63 ± 5 nmol g(soil−dw)−1 h−1 (Fig. 1). The plateau of the H2 oxidation rate indicates the energy potential of H2 and nutrient content in soil were not sufficient to support proliferation of HOB. This conclusion was supported by indistinguishable hhyL gene copy numbers among control, with 2.2 ± 1.7 ×106 copies per g, and eH2 treatments, with 2.9 ± 2.2 ×106 copies per g (t-test, p-value = 0.56; Table S5).
Figure 1: H2 oxidation rates (nmol gsoil−1 h−1) in soil microcosms exposed to elevated (eH2) or atmospheric (Control) H2 concentration.
Each measurement routine was conducted on three randomly selected replicates during the activation stage of the experiments.Soil microbial communities
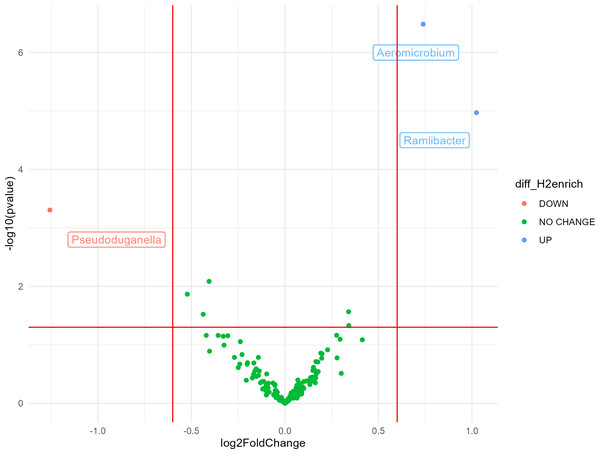
Most bacterial 16S rRNA gene sequences were affiliated with the phyla Actinobacteria (28%), Proteobacteria (27%), Thaumarchaeota (16%), and Acidobacteria (10%). The impact of H2 exposure during the activation stage on soil bacterial communities was negligible, as variations of alpha diversity were not significantly influenced by the eH2 treatment (Tables S2 and S3). Aggregation of the ASV at the genus level led to the identification of three genera displaying contrasting differential abundances between the two treatments. A higher log2 fold-change (LFC) was observed in the eH2 treatment compared to the control, and two groups were identified as being favored by H2 exposure, namely Aeromicrobium spp. (LFC = 1.02, p-value = 0.002) from the Actinobacteria phylum and Ramlibacter spp. (LFC = 0.74, p-value = 0.00005) from the Proteobacteria phylum (Fig. 2). A single group of ASVs aggregated to the genus Pseudoduganella spp. appeared more favored by the control treatment than by the eH2 treatment, but the difference was not significant (LFC = −1.25, p-value = 0.08). No legacy effect on the eH2treatment was observed on soil microbial communities in the rhizosphere of vetch as H2 exposure neither influenced the alpha diversity nor the distribution of ASVs aggregated at the genus level (Tables S1 and S3).
Figure 2: Volcano plot representing the ANCOM-BC analysis (n = 10) for the activation stage of microbial communities.
Log2 fold change (eH2/Control) of ASV aggregated at the genus level in response to H2 enrichment at the activation stage of the experiments. Blue dots represent genera favored by eH2 soil exposure (top-right quadrant), and the red dot represents the single genus favored in the Control treatment, meaning not favoured in the eH2 treatment (top-left quadrant). Green dots show non-responsive bacterial genera to H2 exposure (low-mid quadrant).The legacy of HOB activation of vetch
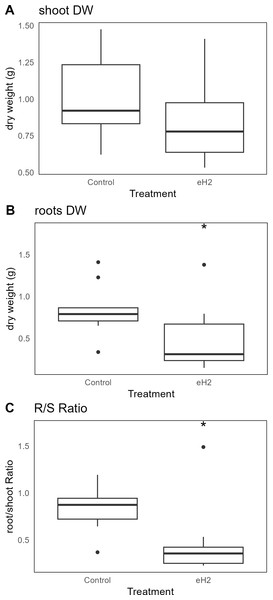
The eH2 treatment did not influence the aerial biomass of V. sativa (Fig. 3A), whereas it did reduce root biomass (p-value= 0.034; Fig. 3B). The root/shoot ratio, usually used as an observation of the plant response to the environment, was reduced in eH2 exposure treatment (p-value = 0.028; Fig. 3C).
Figure 3: Differences in dry weight (g) of V. sativa after harvest in soil conditioned under elevated (eH2) or atmospheric (Control) H2 concentrations.
The dry weight (g) of (A) aerial, (B) root, and (C) root/shoot ratio is represented (n = 10).Discussion
The production of H2 in N2-fixing nodules comprising rhizobia not possessing [NiFe]-hydrogenase creates steep H2 concentration gradients in the rhizosphere of legume plants. These concentration gradients expand at long distances, up to several centimeters away from nodules. In addition to redefining the spatial boundaries of the rhizosphere effect, these gradients are expected to play a role in modifying the microbial community structure and function (De La Porte et al., 2020). This notion was first supported by the enrichment of HOB activity in soil surrounding N2-fixing nodules in pioneering investigations performed in pot experiments (La Favre & Focht, 1983). Subsequent efforts were invested in artificial soil microcosms exposed to elevated H2 concentrations. H2 exposure enhanced net CO2 fixation in soil, which is due to the facultative chemolithoautotrophic life strategy of numerous HOB in soil (Stein et al., 2005). The application of terminal restriction fragment length polymorphism (T-RFLP) led to the conclusion that only a few members of the microbial community were favored by H2 exposure (Osborne, Peoples & Janssen, 2010). This observation was contrasted in subsequent investigations by applying high-throughput PCR amplicon and metagenomic sequencing techniques coupled with loose differential abundance analyses, suggesting H2 exposure induced significant alteration of microbial communities, mainly in the rare biosphere (Khdhiri et al., 2017; Piché-Choquette et al., 2016). Revisiting these experiments with more stringent statistical tools tailored to the sparse microbiome data in the current study and in a recent work (De la Porte et al., 2024) confirmed conclusions supported by earlier T-RFLP techniques that H2 exerts negligible impact on the soil microbial community. The exact mechanisms responsible for variations in biogeochemical processes upon soil exposure to H2 are therefore decoupled from HOB proliferation and compositional change of microbial communities.
Only two ASV groups, Aeromicrobium and Ramlibacter, were positively influenced by the eH2 treatment during the activation stage (Fig. 2). Pumphrey, Ranchou-Peyruse & Spain (2011) reported the enrichment of labelled DNA from a phylotype affiliated with Aeromicrobium ginsengisoli from hairy vetch rhizospheric soil incubated with 100 ppm of H2. There is, therefore, evidence for chemolithoautotrophic metabolism in Aeromicrobium spp., but demonstration of the activity in isolates has not been reported. In addition to the negligible alteration of microbial communities by H2, there is also no evidence supporting the proliferation of HOB encoding for group 1H/5 [NiFe]-hydrogenase in soil exposed to eH2 as quantification of hhyL was indistinguishable between treatments (Table S5). The enrichment of HOB encoding [NiFe]-hydrogenase from other groups cannot be ruled out as they were not detected in the quantitative PCR assay. For instance, HOB encoding for [NiFe]-hydrogenase encompassing group 2a (Bay et al., 2021; Cordero et al., 2019; Islam et al., 2020), group 1f (Hyo type; Myers & King, 2016), group 1l (Hyl type; Ortiz et al., 2021), and group 1d (6C type; Pandelia, Lubitz & Nitschke, 2012) were not detected by hhyL PCR assays and are potential contributors of the H2 oxidation activity measured in the current study. The stabilization of the H2 oxidation activity in soil exposed to eH2 was suggested in a previous microcosms experiment where the response of high-affinity H2 oxidation activity plateaued after a one-week H2 exposure period (Piché-Choquette et al., 2016). Extended incubation under a headspace enriched with H2 was insufficient to induce a secondary enrichment after the enrichment plateau (Fig. 1). The level of CO2 in the static headspace of soil microcosms during the activation stage as well as organic carbon and other nutrients were likely insufficient to support the proliferation of HOB.
Another aspect of H2 exposure in agroecosystems is the fertilization effect of this gas. This was first reported in pot experiments where soil substrate was fumigated with H2 prior to planting (Dong et al., 2003). The plant biomass increased by 15–48% in fumigated soil compared to controls. The fertilization effect was attributed to HOB displaying the plant growth-promotion effect (Maimaiti et al., 2007), such as 1-aminocyclopropane-1-carboxylate (ACC) deaminase activity reducing the accumulation of ethylene, which acts as a stress phytohormone (Yang & Hoffman, 1984). This is indirect evidence suggesting that recruitment of symbiotic N2-fixing rhizobia releasing H2 (Hup−) is an evolutionary advantage for legume plants. Rhizobia possessing [NiFe]-hydrogenase (Hup+) to recycle H2 produced by nitrogenase exist, but they appeared less represented by their Hup− counterparts in nature (Annan et al., 2012). Consideration of the fertilization effect of H2 and the selection of Hup- symbioses in nature led to the suggestion that H2 could be a missing link in clarifying why there are multiple benefits of crop rotation that have not been explained by nitrogen fertilization alone (Golding & Dong, 2010).
The benefits of H2 on plants are likely multifactorial. The fertilization effect was not observed for wheat grown in soil subjected to fumigation by H2, CO, or CH4 (De la Porte et al., 2024). The aerial biomass of vetch in the present study was not enhanced in soil conditioned with eH2 exposure treatment (Fig. 3). The reduction in root biomass, as well as in root/shoot ratio, is thought to be related to the plant growth-promotion activity of HOB, including ACC deaminase reducing root proliferation for nutrient prospection. If this mechanism holds true, it was realized in the absence of evident HOB proliferation. These results must be considered in trials integrating irrigation treatments with H2-saturated water, enhancing stress resistance in plants. The antioxidant potential of H2, microbial activation of immune system in plants, and HOB are potential contributors to plant benefits (Wang et al., 2024). A more holistic analysis, examining both plant and microorganism interactions in soil exposed to different H2 levels or in contrasting Hup+/Hup- symbioses, is recommended to disentangle the contributions of microorganisms and plant metabolism in explaining the benefit of H2.
Conclusions
In conclusion, soil exposure to eH2 led to an activation of HOB without supporting their growth. H2 was therefore sufficient to support the persistence energy requirement of HOB, probably by rewiring their energy metabolism toward an inorganic energy source and a repression of carbon metabolism. Examination of the potential synergetic effect of H2 with nutrients will decipher the limiting factors for HOB proliferation and activation in soil.