Analysis of risk factors for poor prognosis in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Erika Braga
- Subject Areas
- Microbiology, Epidemiology, Hematology, Infectious Diseases, Nephrology
- Keywords
- Renal insufficiency, Bloodstream infection, Nomogram
- Copyright
- © 2025 Wang and Zhao
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Analysis of risk factors for poor prognosis in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection: a retrospective study. PeerJ 13:e19993 https://doi.org/10.7717/peerj.19993
Abstract
Background
This study analyzed the risk factors for poor prognosis in patients with Enterobacteriaceae bloodstream infections and co-occurring renal insufficiency. A nomogram prediction model was constructed to aid in clinical diagnosis and treatment.
Methods
Data were retrospectively collected from patients admitted to the First People’s Hospital of Jiande with positive blood culture results of Enterobacteriaceae combined with renal insufficiency. Patients were divided into survival and death groups according to clinical outcome, and independent risk factors for poor prognosis were screened using a multifactorial logistic regression analysis. A nomogram was subsequently established and validated.
Results
The following risk factors and conditions were significantly associated with a higher patient mortality rate: male sex; admission to the ICU; comorbidity with shock, respiratory failure, coma, pneumonia, or leukaemia; the presence of carbapenem-resistant enterobacteriaceae (CRE) organisms; anaemia; thrombocytopenia; elevated D-dimer; hypo-proteinaemia; and hypocalcaemia (P < 0.05). Multifactorial logistic regression analysis suggested that shock, respiratory failure, and CRE bacterial bloodstream infection were independent risk factors for mortality in these patients.
Conclusions
This study established a nomogram prediction model of risk factors for poor prognosis in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection. This tool can assist clinicians in assessing patient prognosis at an early stage and, therefore, allow for more efficient intervention measures to reduce patient morbidity and mortality.
Introduction
Enterobacteriaceae is a large group of Gram-negative bacilli with similar biological traits. These bacteria often reside in the intestinal tracts of humans and animals and are also found in soil, water, and decaying matter (Li et al., 2022). There is a wide variety of Enterobacteriaceae bacteria, and the most commonly observed in clinical practice are Escherichia coli and Klebsiella pneumoniae. Bloodstream infections caused by these two bacteria tend to progress rapidly and, if left untreated, can rapidly progress to sepsis, infectious shock, and multiorgan failure, leading to poor patient outcomes (Skogberg et al., 2012; Leibovici-Weissman, Tau & Yahav, 2021). Studies have shown that the incidence of E. coli and K. pneumoniae bloodstream infections varies significantly among different populations and is particularly common in elderly, immunocompromised, and chronically ill patients with underlying diseases (Yahav et al., 2016; Kreitmann et al., 2024; Cho et al., 2015).
Pan et al. (2022) demonstrated that patients with concomitant renal insufficiency exhibited a significantly higher risk of bloodstream infections, poorer prognosis, and prolonged treatment duration compared with those without renal impairment. Another population-based retrospective cohort study revealed that, compared with patients with an estimated glomerular filtration rate (EGFR) ≥60 ml/min/1.73 m2, patients with an EGFR of 30–59 ml/min/1.73 m2 had a significantly increased risk of community-acquired bloodstream infections, and patients with an EGFR <30 ml/min/1.73 m2 faced an even higher risk (Dagasso et al., 2020). Renal insufficiency not only affects the metabolism and excretion of drugs; it may also lead to electrolyte disorders, acid–base imbalance, and other serious internal environmental disturbances, further aggravating the condition (Laupl et al., 2020).
Nomograms are used as a reliable statistical tool to create simple and intuitive predictive models that can quantify the risk of clinical events (Stark et al., 2019). Nomogram risk prediction models are now widely used in clinical practice (Park, 2018); however, there is a lack of data regarding the ability of nomogram risk models to predict poor prognosis in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infections.
In summary, patients with E. coli and K. pneumoniae bloodstream infections combined with renal insufficiency have severe conditions, high mortality rates, and poor prognoses. The in-depth study of risk factors and the construction of nomogram prediction models can provide new ideas and methods to improve patient prognosis and provide a basis for clinicians to develop more quantitatively-based treatment strategies.
Methods
Study subjects
Data was retrospectively collected from a total of 569 patients with positive blood culture results of Enterobacteriaceae and comorbid renal insufficiency in the First People’s Hospital of Jiande from January 2018 to November 2024. Patient data included: (1) demographic characteristics (age, gender, underlying conditions); (2) clinical manifestations (coma, hypotension); (3) laboratory findings (complete blood count, liver and kidney function, electrolyte levels); and (4) outcome (mortality). All data were independently reviewed by two experienced clinicians to ensure accuracy and completeness. As this investigation was retrospective in nature, informed consent from individual participants was not obtained. This study was approved by the Medical Ethics Committee from the First People’s Hospital of Jiande. All data were anonymized to ensure that patient privacy was fully protected.
Patient information and collection
Patient information was collected from the hospital’s electronic medical record system and laboratory system; this included patients’ basic information (age, gender, underlying conditions), primary infection site, laboratory tests, and outcome. Blood cultures were collected from all patients when their body temperature exceeded 38.5 degrees Celsius. Sample collection was performed by the hospital’s microbiology laboratory, and the testing instruments and operating procedures were in accordance with guidelines or recommended standards.
Grouping
The 569 patients were divided into a survival group (510 cases) and a death group (59 cases). Inclusion criteria were as follows: (1) two or more blood culture results were positive for Enterobacteriaceae, along with concurrent renal insufficiency; (2) patients’ basic information, medical records, and laboratory test data were complete, and the laboratory data included blood routine and biochemistry tests within 48 h before and after the blood culture. Exclusion criteria were as follows: (1) inaccurate or missing records of the patient’s medical course and lack of laboratory test data; (2) the patient was automatically discharged from the hospital; (3) the presence of other serious infectious diseases.
Statistical analysis
Clinical data analysis was completed using SPSS 28.0 (IBM Corp., Armonk, NY, USA) and R 4.4.2 software. The chi-square test was used for categorical variables, and the t-test was used for continuous variables. Independent risk factors for poor prognosis were screened by multifactorial logistic regression analysis for single factors with a threshold of P < 0.05, and the independent risk factors were imported into R software. A nomogram of the risk factors for poor patient prognosis was created and validated by using the ‘rms’ package in R software. The receiver operating characteristic curve (ROC), calibration curve, and decision curve analysis (DCA) of the model were plotted. The ability of the model to predict poor prognosis in patients with bloodstream infections was assessed by calculating the area under curve (AUC). P-value < 0.05 indicated a statistically significant difference.
Results
Demographics of patients with bloodstream infection
Of the 569 patients included in the statistical analysis, 287 (50.4%) were male and 282 (49.6%) were female.
Risk factors for death in patients with Enterobacteriaceae bloodstream infection
Statistical analysis of the 569 patients showed that the following risk factors were associated with a higher mortality rate: male sex; ICU admission; co-occurring shock, respiratory failure, coma, pneumonia, or leukaemia; the presence of carbapenem-resistant Enterobacteriaceae (CRE)- producing organisms; anaemia; thrombocytopenia; elevated D-dimer; hypoproteinaemia; and hypocalcaemia (P < 0.05; Table 1).
| Survivor (n = 510) | Death (n = 59) | X2 | p | |
|---|---|---|---|---|
| Female | 261 (51.2%) | 21 (35.6%) | 5.137 | 0.027 |
| Admitted to ICU | 96 (18.8%) | 37 (62.7%) | 56.871 | <0.001 |
| Coma | 24 (4.7%) | 22 (37.3%) | 75.550 | <0.001 |
| Shock | 56 (11.0%) | 42 (71.2%) | 134.452 | <0.001 |
| Respiratory failure | 36 (7.1%) | 31 (52.5%) | 105.309 | <0.001 |
| Pneumonia | 245 (48.0%) | 44 (74.6%) | 14.900 | <0.001 |
| Leukaemia | 244 (47.8%) | 44 (74.6%) | 7.768 | 0.011 |
| CRE-producing bacteria | 20 (3.9%) | 19 (32.2%) | 66.253 | <0.001 |
| Elevated D-dimer | 368 (72.2%) | 50 (84.7%) | 4.581 | 0.017 |
| Hypocalcaemia | 145 (28.4%) | 26 (44.1%) | 11.647 | <0.001 |
| Elevated or lowered white blood cells | 360 (70.1%) | 48 (81.4%) | 3.022 | 0.093 |
| Decreased red blood cells | 196 (38.4%) | 42 (71.2%) | 23.318 | <0.001 |
| Anaemia | 235 (46.1%) | 45 (76.3%) | 19.288 | <0.001 |
| Thrombocytopenia | 202 (39.6%) | 43 (72.9%) | 23.879 | <0.001 |
| Hypoproteinaemia | 270 (52.9%) | 45 (76.3%) | 11.647 | <0.001 |
Notes:
Statistical analysis showed that the mortality rate of patients with bloodstream infections was higher for the following risk factors: male sex; ICU admission; co-occurring shock, respiratory failure, coma, pneumonia, or leukaemia; carbapenem-resistant Enterobacteriaceae (CRE)-producing organisms; anaemia; thrombocytopenia; elevated D-dimer; hypoproteinaemia; and hypocalcaemia (P < 0.05).
Analysis of independent risk factors for death and construction of a nomogram prediction model
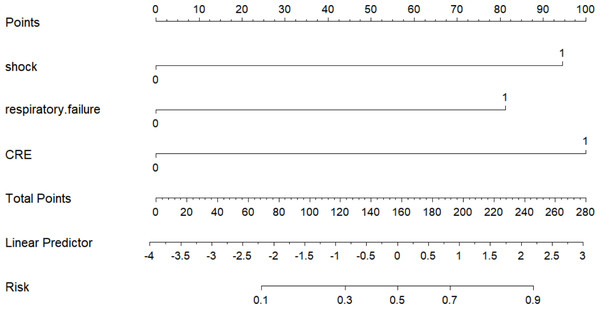
Multifactorial logistic regression analysis showed that shock, respiratory failure, and CRE-producing bacteria were independent risk factors for death in patients with renal insufficiency and Enterobacteriaceae bloodstream infection. These independent risk factors were imported into the R software, and a nomogram was created (Fig. 1). A score value corresponding to each risk factor was calculated, and the sum of these score values was recorded as the total score, which corresponded to the probability of patient death.
Figure 1: Nomogram prediction model for the risk of death in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection.
Multifactorial logistic regression analysis showed that shock, respiratory failure, and CRE-producing bacteria were independent risk factors for death in patients with renal insufficiency and Enterobacteriaceae bloodstream infection. The independent risk factors were imported into the R software and a nomogram was created.Tests of the predictive model-discrimination, calibration, and clinical utility
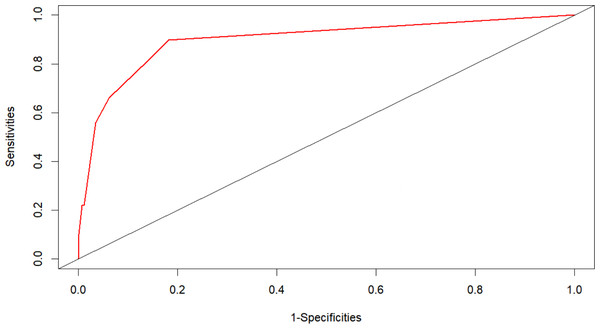
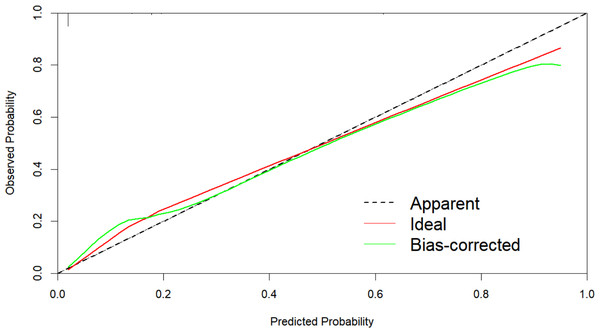
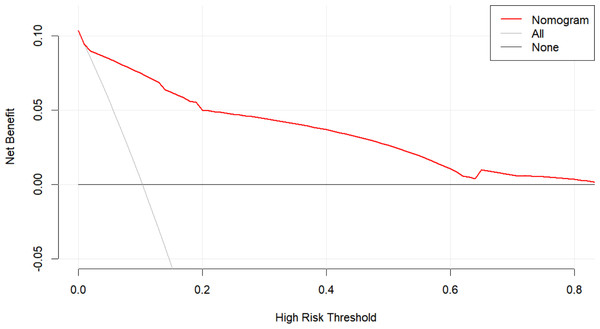
The AUC of the nomogram model was 0.897 (95% CI [0.851–0.943]), indicating that the model had a strong prognostic ability for the risk of death in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection (Fig. 2). The calibration plot (Fig. 3) showed that this model was well calibrated and that its predicted probability was similar to the actual probability. The DCA (Fig. 4) showed that this nomogram model had clinical utility. Specifically, the net benefit curve of the model lies above those of both the “treat-all” and “treat-none” strategies from threshold probabilities of approximately 5% to 75%. Within this interval, clinicians who rely on the nomogram to guide treatment decisions would achieve a higher net benefit—i.e., more true-positive cases identified without an excessive rise in false-positives. This study used the Bootstrap resampling method for model validation with a resampling size of 1,000. Bootstrap resampling showed that this model has good predictive performance.
Discussion
E. coli and K. pneumoniae are two common Gram-negative bacilli that are widely found in nature and in the human gut. These two bacteria not only play an important role in gastrointestinal diseases but also frequently cause severe bloodstream infections. Compared with patients who maintain normal renal function, those who develop acute kidney injury (AKI) during bloodstream infections exhibit a two- to six-fold higher mortality risk and a markedly increased probability of subsequent progression to chronic kidney disease (CKD) (Khwaja, 2012; Hoste et al., 2015). Renal insufficiency itself is a risk factor for bloodstream infections, as it weakens the body’s detoxification and metabolic capacity, complicating the control and treatment of infections (Pan et al., 2022). This study analyzed the clinical characteristics and risk factors of patients with renal insufficiency combined with Enterobacteriaceae bloodstream infections and found that the following were risk factors for patient mortality: male sex; ICU admission; co-occurring shock, respiratory failure, coma, pneumonia, or leukaemia; the presence of carbapenem-resistant Enterobacteriaceae (CRE)- producing organisms; anaemia; thrombocytopenia; elevated D-dimer; hypoproteinaemia; and hypocalcaemia. Multifactorial logistic regression analysis showed that shock, respiratory failure, and CRE-producing bacteria were independent risk factors for death in these patients.
Figure 2: ROC prediction curve for risk of death in patients with Enterobacteriaceae blood stream infections.
The AUC of the nomogram model was 0.897 (95% CI [0.851–0.943]), indicating that the model had a strong prognostic ability for the risk of death in patients with renal insufficiency combined with Enterobacteriaceae bloodstream infection.Figure 3: Calibration curves of the nomogram.
The calibration plot showed that this model was well calibrated and that its predicted probability was similar to the actual probability.Figure 4: Decision curve analysis (DCA) of the prediction model.
The DCA showed that this nomogram model had clinical utility. Specifically, the net benefit curve of the model lies above those of both the “treat-all” and “treat-none” strategies from threshold probabilities of approximately 5% to 75%. Within this interval, clinicians who rely on the nomogram to guide treatment decisions would achieve a higher net benefit—i.e., more true-positive cases identified without an excessive rise in false-positives.Patients with renal insufficiency experience a significant increase in mortality risk when complicated by Enterobacteriaceae bloodstream infections. Studies have shown that the 30-day mortality rate of Enterobacteriaceae bloodstream infections can be as high as 46.2%, with CRE (particularly carbapenem-resistant K. pneumoniae) being the predominant type of pathogen. These pathogens are closely associated with a high incidence of sepsis and septic shock (Zhou et al., 2021). Additionally, patients with renal insufficiency often have poor underlying health conditions, which further increase the mortality risk following infection. Research indicates that severe sepsis or shock, a high Charlson Comorbidity Index, and inappropriate empirical treatment are independent risk factors for mortality (Gutiérrez-Gutiérrez et al., 2016). These factors may be more pronounced in patients with renal insufficiency, as their bodies are already under additional physiological stress.
Shock is an independent risk factor for bloodstream infections. The body’s immune functions significantly decrease in the state of shock, making it easier for pathogenic bacteria to invade the blood circulation system. Additionally, microcirculation disorders and tissue hypoxia triggered by shock further weaken the body’s defense mechanisms, providing favorable conditions for bacterial proliferation and spread. Shock patients are often impacted by multiple organ dysfunction, which not only increases the complexity of treatment but also significantly increases the risk of pathogenic bacterial infection. Therefore, monitoring and preventive measures should be emphasized for patients with shock, especially those with potential risk of infection, to identify and manage possible bloodstream infections in a timely manner.
Respiratory failure is an independent risk factor for bloodstream infection. As the respiratory system represents an important immune barrier in the human body, the impaired function of the lungs directly leads to the weakening of the systemic immune response, and this state of immunosuppression provides an opportunity for bacterial invasion and propagation. Secondly, mechanical ventilation therapy, which often accompanies respiratory failure, improves the oxygenation status of patients to a certain extent, but also increases the risk of hospital-acquired infections (Al-Shukri et al., 2022). Mechanical ventilation tubing, ventilator interfaces, and other equipment may become breeding grounds for bacteria that in turn may lead to bloodstream infections. In addition, patients with respiratory failure are often required to undergo a variety of invasive operations such as central venous cannulation and arterial cannulation, which not only increase the chances of direct bacterial entry into blood circulation but may also lead to infection as a result of contamination during the operation (Ray-Barruel et al., 2019). Therefore, patients with respiratory failure should be highly vigilant and take active preventive measures to reduce the incidence of bloodstream infections and improve their prognosis.
The application of carbapenem antibiotics is a powerful tool for the treatment of drug-resistant Gram-negative bacilli, but in recent years the emergence of CRE organisms, which are resistant to nearly 90% of the antibiotics available in the clinic, has led to failure of initial treatment, prolongation of the course of therapy, and lack of adequate medication in community hospitals that do not have access to advanced antibiotics. CRE has an enormous impact on global public health, as shown in one survey study (Kelly, Mathema & Larson, 2017), with the percentage of community-associated or community-onset CRE percentages ranging from 0.04%–29.5% and prevalence rates ranging from 5.6%–10.8% in the United States alone. CRE is growing and spreading at an alarming rate globally, and carbapenem-resistant Pseudomonas aeruginosa, carbapenem-resistant Acinetobacter baumannii, and carbapenem-resistant Enterobacteriaceae continue to be important pathogens related to hospital-acquired pneumonia and are classified as key pathogens requiring urgent research and drug development by the World Health Organization (WHO) (Brink, 2019). E. coli and K. pneumoniae are common carbapenemase-producing bacteria in clinical settings, and the bloodstream infections they cause often lead to high morbidity and mortality, presenting a serious threat to human life and health. A 2015 multicenter study (Zhang et al., 2018) of 25 Grade A tertiary hospital in 14 provinces in China found that the susceptibility rate of CRE to polymyxin B was >90%, the susceptibility rate of CRE E. coli to tigecycline was 90.2%, and the susceptibility rate of CRE K. pneumoniae to tigecycline was 40.2%. Antibiotics for the treatment of CRE are very limited, highlighting the need for new, effective anti-CRE drugs and treatment regimens.
The limitations of this study include a relatively small sample size, and a retrospective cohort study design. Nonetheless, the results of this study have certain clinical significance and provide a scientific basis for clinicians to develop more effective treatment strategies and preventive measures. Future research should further expand the sample size and adopt a prospective cohort study design to verify the reliability and generalizability of the results of this study.
Conclusion
Patients with renal insufficiency complicated by Enterobacteriaceae bloodstream infections present with severe illness, high mortality, and poor prognosis. The escalating resistance of these pathogens—especially CRE—renders such infections increasingly lethal for critically ill individuals. To address this challenge, we developed and validated a nomogram that quantifies the risk of in-hospital death in this population. Consequently, this decision-support tool may substantially improve the early recognition of high-risk patients and facilitate evidence-based management strategies for controlling bloodstream infections in the setting of renal insufficiency.