Genome-wide identification of SHMT family genes in C3, C3-C4, and C4 Salsoleae s.l. species
- Published
- Accepted
- Received
- Academic Editor
- Kamil Steczkiewicz
- Subject Areas
- Bioinformatics, Genomics, Plant Science
- Keywords
- SHMT, Expression pattern, Photorespiration, C4 photosynthesis, Chenopodiaceae
- Copyright
- © 2025 Peng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Genome-wide identification of SHMT family genes in C3, C3-C4, and C4 Salsoleae s.l. species. PeerJ 13:e19978 https://doi.org/10.7717/peerj.19978
Abstract
C4 photosynthesis is a carbon-concentrating mechanism that evolved to enhance photosynthetic efficiency under conditions favoring photorespiration, such as high temperature, low atmospheric CO2, and aridity. Photorespiration is considered the primary driving force on the evolution of C4 photosynthesis. Serine hydroxymethyltransferase (SHMT) plays a crucial role in one-carbon metabolism and photorespiration. However, there is a lack of comprehensive bioinformatics investigation on the SHMT gene family across different photosynthetic types, specifically comparing C3, C4, and C3-C4 intermediate species. In this study, we conducted a systematic analysis of the SHMT gene family regarding gene structure, phylogenetic relationships, expression patterns, and cis-acting element in four Salsoleae species, including C3 species Salsola junatovii, C3-C4 intermediate species Oreosalsola laricifolia, and two C4 species Xylosalsola arbuscula and Soda foliosa. The results indicated that 4–5 SHMT members were identified in these four species. No fragment duplication were identified, which may explain the lower number of SHMT members in each Salsoleae species. The range of exon numbers varied from 4 to 15. Phylogenetic analysis showed that the SHMTs from Salsoleae species can be classified into four distinct classes, with most members displaying conserved gene structure and motif numbers, except for OlSHMT3 and XaSHMT3, which had divergent gene structures. The SHMTs in Salsoleae species did not exhibit organ-specific expression patterns; however, variations in expression were observed among the different members. Analysis of newly sequenced Salsoleae transcriptomes data and published data from five other genera (Flaveria, Heliotropium, Mollugo, Alternanthera, and Neurachne) revealed that, compared to C3 and C3-C4 intermediate species, only mitochondrial-localized, leaf preferential SHMT1 showed a low expression among SHMT members, probably evolved in C4 photosynthesis evolution. The MYB transcription factors were predicted to be the most significant regulators of SHMT1 in three Salsoleae species and the second most significant in X. arbuscula. These results may provide valuable information for further analyses, particularly in the evolutionary study of Salsoleae SHMT1.
Introduction
Photorespiration is a consequence of the dual affinity of the enzyme ribulose −1, 5-bisphosphate carboxylase/oxygenase (Rubisco) for both CO2 and O2. The carboxylation reaction catalyzed by Rubisco produces two molecules of 3-phosphoglycerate (3-PGA), which can be reconverted into ribulose bisphosphate (RuBP) via the Calvin cycle. In contrast, the oxygenation reaction yields one molecule of 3-PGA and one molecule of 2-phosphoglycolate (2-PG). Given that 2-PG is toxic to plants, its conversion back to 3-PGA occurs through a reaction sequence known as the photorespiratory carbon cycle (Bowes, Ogren & Hageman, 1971; Voll et al., 2006; Bauwe, Hagemann & Fernie, 2010; Bräutigam & Gowik, 2016). This process consumes ATP and NADPH, ultimately resulting in a net release of CO2 from the plant. Under hot and dry conditions, photorespiration can reduce the efficiency of carbon fixation in plants by as much as 30% (Bauwe, Hagemann & Fernie, 2010; Raines, 2011). Furthermore, aside from its primary role in the photorespiratory carbon cycle, this pathway may also serve secondary functions, such as contributing to the synthesis of glycine and serine (Wingler et al., 2000) or playing a role in pathogen defense (Foyer et al., 2009).
C4 plants evolved independently approximately 61 times from C3 ancestors, involved the modifications in leaf anatomy, physiology and gene expression (Sage, 2017). Compared with C3 plants, the most well-documented C4 plants develop Kranz anatomy, where the outer layer consists of mesophyll cells that fix atmospheric CO2 in the C4 cycle, and the inner layer comprises bundle sheath cells that facilitate the effective donation of CO2 from the decarboxylation of C4 acids to Rubisco, thereby minimizing competition with O2 and reducing photorespiration (Edwards & Voznesenskaya, 2011). C3-C4 intermediates (C2 species) are characterized by a photorespiration pump, which restricts the activity of the glycine decarboxylase complex (GDC) to the bundle sheath cells (Schulze, Westhoff & Gowik, 2016). These intermediates are considered as evolutionary stepping stones toward C4 photosynthesis based on the current model of C4 evolution (Sage, Khoshravesh & Sage, 2014; Bräutigam & Gowik, 2016; Lundgren, 2020; Schlüter & Weber, 2020). Photorespiration is regarded as a major driving force on C4 evolution (Bräutigam & Gowik, 2016). In comparison to C3 plants, there is a notable decrease in the expression of photorespiratory genes in C4 plants, particularly the core enzymes of the photorespiration pathway, while the transcript and protein levels in C2 plants remain constant or even higher compared to C3 plants (Mallmann et al., 2014; Lauterbach et al., 2017; Siadjeu, Lauterbach & Kadereit, 2021; Lauterbach et al., 2024).
Serine hydroxymethyltransferase (SHMT, EC 2.1.2.1), which depends on pyridoxal 5′-phosphate, is one of the eight core enzymes in the canonical photorespiratory pathway (Hagemann et al., 2016). SHMT plays a catalytic role in the transformation between serine and glycine with GDC (Hanson, Gage & Shachar-Hill, 2000), as well as in the synthesis of tetrahydrofolate (H4PteGlun, THF)/5,10-methylenetetrahydrofolate (5,10-CH2-H4PteGlun), the synthesis of methionine, and the maintenance of redox balance during photorespiration (Schirch, 1982; Appaji Rao et al., 2003; Zhang et al., 2010). It is widely distributed across plants, animals, and microorganisms (Prabhu et al., 1996; Hanson, Gage & Shachar-Hill, 2000). Members of the SHMT gene family have been reported in many species, including Arabidopsis, soybean (Lakhssassi et al., 2019), rice (Pan et al., 2024), cucumber (Gao et al., 2022), tomato (Liu et al., 2022), and alfalfa (Gao et al., 2024). The SHMT gene family members range from five (rice, Pan et al., 2024) to eighteen (soybean, Lakhssassi et al., 2019). Depending on their subcellular localization, there are four kinds of SHMTs, distributed in mitochondria, chloroplast, cytoplasm, and the nucleus, respectively (Zhang et al., 2010; Nogués et al., 2022), indicating their diverse roles in metabolic pathways (Voll et al., 2006; Hagemann et al., 2016; Lakhssassi et al., 2019; Liu et al., 2022; Gao et al., 2024). Currently, mitochondrial-localized SHMTs has been extensively studied and are known to participate in the process of photorespiration, one carbon metabolism, plant growth, and stress response (Voll et al., 2006; Liu et al., 2022; Yuan et al., 2022). In Arabidopsis, there are two mitochondrial SHMTs, namely AtSHM1 and AtSHM2. AtSHM1 is predominantly expressed in leaves, whereas AtSHM2 is mainly expressed in shoot and roots (Voll et al., 2006). Only AtSHM1 is involved in the photorespiratory carbon cycle, and the mutation of this gene causes a photorespiratory phenotype in Arabidopsis thaliana (Voll et al., 2006). Additionally, AtSHM1 plays a regulatory role in sucrose accumulation and the homeostasis of reactive oxygen species (ROS), both of which are crucial for primary root growth (Yuan et al., 2022). Mitochondrial OsSHMT1 from rice and GmSHMT08 from soybean are involved in defense mechanisms against abiotic and biotic stress (Wang et al., 2015; Lakhssassi et al., 2020). Furthermore, the mitochondrial SlSHMT from tomato interacts with chaperonin 60α1 (SlCPN60α1) to regulate photosynthesis and photorespiration processes (Ye et al., 2020).
The family Chenopodiaceae s.s. (Amaranthaceae s.l.), as classified by APG IV (2016) comprises approximately 558 species, making it the third largest group of C4 species. The tribe Salsoleae encompasses over half of the known C4 species (310) within Chenopodiaceae s.s. (Sage, 2017), and it also includes C3 and C3-C4 species (Voznesenskaya, 2001; Wen & Zhang, 2015). The diversity in habitats, life forms and photosynthetic characteristics in the assimilation organs of Salsoleae is particularly complex (Edwards & Voznesenskaya, 2011). Unlike most C4 lineages dominated by herbaceous species, these C4 Salsoleae species also include subshrubs and shrubs, and even rarely small trees (Zhu, Mosyankin & Clemants, 2003). Furthermore, many C4 Chenopodiaceae s.s. plants frequently dominate warm temperate and tropical grasslands and savannas, particularly in environments such as sand dunes, salt marshes, semideserts, and deserts (Kadereit et al., 2003).
Due to the limited number of whole genomes sequenced to date, no comparisons of SHMTs across different photosynthetic species have been made to elucidate the genetic evolution and function of SHMTs. This study investigates the genome-wide identification and characterization of SHMT genes in four species from Salsoleae. Salsola junatovii (C3 species), Oreosalsola laricifolia (C2 species), Xylosalsola arbuscula (C4 species), and Soda foliosa (C4 species). A systematic analysis was conducted on gene family number, gene structures, conserved motifs, evolutionary relationships, collinear relationships, cis-acting element distributions, and tissue patterns. Additionally, the published leaf transcriptome data from five genera, including the dicots Flaveria, Heliotropium, Mollugo, Alternanthera, as well as the monocot Neurachne, were utilized. These genera encompass various photosynthetic species to assess SHMTs gene expression along the emergence of C4 species.
Materials and Methods
Genome-wide identification of SHMT gene family members
In this study, we utilized genome sequencing data for Salsola junatovii, Oreosalsola laricifolia, Soda foliosa, and Xylosalsola arbuscula, provided as unpublished genome data by Institute of Genetics and Developmental Biology, Chinese Academy of Sciences. We extracted protein and coding sequences (CDS) for these four Salsoleae species using the ‘GXF Sequence Extract’ and ‘Batch Translate CDS’ modules in TBtools (v2.154) (Chen et al., 2023). Reference protein sequences of Arabidopsis thaliana SHMTs were obtained from the TAIR database (https://www.arabidopsis.org/), and the Hidden Markov Model (HMM) of the SHMT conserved domain (PF00464) was downloaded from the Pfam database (https://www.ebi.ac.uk/interpro/entry/pfam/). Using the SHMT HMM model as a template, we employed HMMER (v3.0) to perform a whole-genome scan of these Salsoleae species to identify potential SHMTs. To validate these candidates, we constructed a local protein database for each Salsoleae species using BLAST (v2.14), with A. thaliana SHMT protein sequences as queries (E-value threshold set at 1e−5) (Ahmad et al., 2024). The candidate genes were further refined by integrating results from HMMER and BLAST, and protein sequences were extracted using the ‘Fasta Extract’ module in TBtools. All candidate genes were confirmed for structural integrity using the NCBI Conserved Domains Database (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi). The SHMT numbers of these Salsoleae species were designated according to homologous genes in A. thaliana. Finally, molecular weight (Mw) and isoelectric point (pI) predictions for all SHMT protein-coding genes were conducted using the ExPASy online tool (https://web.expasy.org/compute_pi/) (Wilkins et al., 1999).
SHMT protein and gene structure analysis
Secondary structure predictions for SHMT proteins were performed using the SOPMA online tool (https://npsa.lyon.inserm.fr/cgi-bin/npsa_automat.pl?page=/NPSA/npsa_sopma.html). The three-dimensional structure of SHMT was predicted utilizing AlphaFold3 (https://alphafoldserver.com/), and the resulting models were simulated and visualized with PyMOL (v3.1.0) (Jumper et al., 2021; Mooers, 2020). The subcellular localization of SHMT proteins was inferred through the CELLO online tool (http://cello.life.nctu.edu.tw/) (Yu et al., 2006), while signal peptide predictions were performed using SignalP (v5.0) (https://services.healthtech.dtu.dk/services/SignalP-5.0/) (Nielsen et al., 2019).
For gene structure and conserved motif analysis, the locations of SHMT genes and their exon-intron structures in these species were extracted from the General Feature Format (GFF) annotation files and visualized using the ‘Gene Location Visualize from GTF/GFF’ function in TBtools. Conserved motifs within SHMT protein sequences were identified using MEME (https://meme-suite.org/meme/tools/meme), with the motif number maintained at 20 and other parameters set to default (Bailey et al., 2015). These results were visualized using the ‘Gene Structure View’ function in TBtools.
Phylogenetic analysis
SHMT protein sequences from A. thaliana, Glycine max, Solanum lycopersicum, Populus trichocarpa, Cucumis sativus, Beta vulgaris and Oryza sativa were retrieved from Phytozome (v13) and CuGenDB (http://cucurbitgenomics.org/). These sequences were aligned using ClustalW, with the Delay Divergent Cutoff (%) set to 30 (Liu et al., 2022), while all other options remained at their default settings. A phylogenetic tree comprising 71 SHMT protein sequences was constructed using the maximum likelihood method in MEGA-X (v10.1.8), applying the Jones-Taylor-Thornton (JTT) amino acid substitution model with uniform rates among sites (no discrete gamma categories or invariant sites), the Nearest-Neighbor-Interchange (NNI) heuristic search (initial tree generated automatically by NJ/BioNJ), and 1,000 bootstrap replicates; all other parameters were left at their defaults (Gao et al., 2024). The resulting phylogenetic tree was subsequently visualized using iTOL (v7.0) (https://itol.embl.de/).
SHMT gene family syntenetic analysis
The gene location information for SHMT family members in four Salsoleae species was analyzed using the ‘Gene Location Visualize from GTF/GFF’ function in TBtools (Chen et al., 2023). Gene density was calculated utilizing the ‘Gene Density Profile’ function. Synteny within each species was assessed using the ‘One Step MCScanX’ module in TBtools, with results visualized through the ‘Advanced Circos’ function. Furthermore, synteny relationships among these species, A. thaliana, and B. vulgaris were examined by downloading the genomic data of Arabidopsis and B. vulgaris from Phytozome (v13) and employing the ‘One Step MCScanX’ Synteny analysis plots were generated to illustrate the syntenic relationships of homologous SHMT genes across these species.
RNA extraction and reverse transcription-qPCR
Seeds from S. junatovii, O. laricifolia, X. arbuscula, and S. foliosa were collected in October 2023 in Xinjiang. The seeds were air-dried at room temperature for 2 weeks and subsequently stored at 4 °C in a refrigerator. Following sterilization (Guo et al., 2024), the seeds were sown on 1/2 MS solid medium (1/2 MS + 15 g L−1 sucrose + 8 g L−1 agar) and incubated in a growth chamber for 3 days. The growth chamber was maintained under a 14 h light/10 h dark cycle at 25 °C during the light period and 15 °C during the dark period. The light intensity was maintained at approximately 300 μmol·m−2·s−1. Subsequently, healthy seedlings were selected and transferred to a Hoagland nutrient solution for hydroponic culture, with the solution being changed every 3 days. After 6 to 8 weeks of growth, leaf tissue samples were collected at 11:00 AM, immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent total RNA extraction. Root, stem, and leaf tissue samples were collected from these Salsoleae species, with each tissue sample weighing approximately 100 mg. The samples were immediately frozen in liquid nitrogen and ground into a fine powder.
Total RNA was extracted using the TaKaRa MiniBEST Plant RNA Extraction Kit. The quality and concentration of the RNA were assessed using a NanoDrop spectrophotometer, and only RNA samples with a 260/280 ratio between 1.8 and 2.1 were selected for further analysis. To eliminate DNA contamination from the samples and synthesize complementary DNA (cDNA), we employed the PrimeScript™ RT Reagent Kit with gDNA Eraser. Reverse transcription- qPCR (RT-qPCR) primers specific to the SHMTs in these species were designed using the NCBI Primer Design Tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/index.cgi). The designed primers were submitted to Sangon Biotech for synthesis via the PAGE purification method. Quantitative RT-PCR was performed utilizing species-specific β-actin genes as internal reference controls for normalization (Zhang et al., 2019b), with the primer sequences for both SHMT and β-actin genes in each species provided (Table S1). RT-qPCR experiments were conducted with the TB Green Premix Ex Taq™ II kit. All experiments were carried out in the molecular laboratory of the research group at the Xinjiang Institute of Ecology and Geography Chinese Academy of Sciences (Zhang et al., 2019a). For each species, the average expression level of all gene members in the root tissue was used as the control, and the relative expression levels of SHMTs in the root, stem, and leaf tissues were calculated using the 2−ΔΔCT method (Livak & Schmittgen, 2001). The expression data were log-transformed (log10), and the results were visualized using the ggplot2 package (v3.5.1).
Gene expression level analysis based on RNA-seq data
To further investigate the expression patterns of SHMT family members associated with different photosynthetic types (C2, C3, and C4) across various plant groups, we selected five plant groups that include species exhibiting C2, C3, and C4 photosynthesis based on prior studies. Subsequently, we downloaded 27 RNA-seq datasets corresponding to each of the 27 species for further analysis. These datasets comprise dicotyledonous plants from Alternanthera (Amaranthaceae), Flaveria (Asteraceae), Heliotropium (Boraginaceae), and Mollugo (Molluginaceae), as well as monocotyledonous plants from Neurachne (Poaceae) (Chinthapalli et al., 2000; Voznesenskaya et al., 2013; Stata et al., 2014; Wen & Zhang, 2015; Tao, Lyu & Zhu, 2016; Thulin et al., 2016; Lundgren, 2020; Lyu et al., 2020; Bernardo et al., 2023; Lauterbach et al., 2024). Additionally, newly sequenced RNA-seq data from four species of Salsoleae (Amaranthaceae) were included (Table S2). All RNA-seq data were generated using a paired-end sequencing strategy and underwent quality control using FastQC. The transcriptomes were assembled de novo using Trinity (v2.11.0) with default parameters (Haas et al., 2013). Subsequently, Cluster Database at High Identity with Tolerance (CD-HIT) (v4.8.1) was employed to cluster the transcript sequences at a 0.95 similarity threshold, effectively removing redundant transcripts (Fu et al., 2012). To assess the quality of the transcript assembly, Bowtie2 (v2.4.4) was utilized to map the reads back to their respective transcriptomes (Langmead & Salzberg, 2012), while Salmon (v1.10.3) was used for the quantitative analysis of the assembled transcripts (Patro et al., 2017). The assembled 31 transcripts were annotated using the Arabidopsis database (https://www.arabidopsis.org/), and the transcipts per million (TPM) values of the SHMT gene family members for each species were extracted to measure transcript abundance. Finally, stacked bar plots were generated using the ggplot2 package (v3.5.1) to display the expression levels of SHMT in Salsoleae plants and the five aforementioned genera. Individual bar plots were created for each SHMT member to illustrate the expression of SHMTs in plants with different photosynthetic types.
Prediction of cis-acting elements and transcription factor binding sites in promoter sequences
Prediction of cis-acting elements and transcription factor binding sites in promoter sequences located 2,000 bp upstream of the SHMT coding sequence (CDS) were extracted using the GTF/GFF3 Sequences Extract function in TBtools. These sequences were then submitted to the PlantCARE website (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) for predicting cis-acting elements (Lescot, 2002), while transcription factor binding sites were predicted utilizing the PlantTFDB database (https://planttfdb.gao-lab.org/) (Tian et al., 2020). The results were systematically organized and visualized through the ‘Gene Structure View (Advanced)’ and ‘Heatmap’ functions available in TBtools.
Results
Genome-wide identification of SHMT gene family member
Based on the whole genome data of four Salsoleae species, we identified four to five members of each SHMT gene family. O. laricifolia and X. arbuscula each contained five SHMTs, including SHMT1, SHMT2, SHMT3, SHMT4, and SHMT7. In contrast, S. junatovii and S. foliosa each possessed four members, namely SHMT1, SHMT2, SHMT4, and SHMT7 (Table 1). All identified SHMTs contained the characteristic SHMT domain (Pfam: PF00464) (Table S3). Further analysis of the physicochemical properties of these SHMT members (Table 1) revealed that the amino acid lengths ranged from 458 (OlSHMT3) to 615 (SjSHMT7, OlSHMT7, SfSHMT7, and XaSHMT7). Notably, SHMT7 across these species exhibited the same amino acid length. Among these members, XaSHMT7 had the largest molecular weight at 68,405.64 Da, while OlSHMT3 had the smallest molecular weight at 50,244.21 Da. Additionally, the isoelectric points (pI) of these SHMTs ranged from 5.98 (OlSHMT7) to 8.86 (XaSHMT4). It was noteworthy that SHMT3 and SHMT7 were classified as acidic proteins (pI < 7).
| Gene name | Gene ID | Gene locus | Amino acids(aa) | Molecular weight (kDa) |
pI | SHMT domain location |
|---|---|---|---|---|---|---|
| SjSHMT1 | ts3g08640_T02 | Chr3 | 539 | 59,960.89 | 8.07 | 54–475 |
| SjSHMT2 | ts2g04227_T01 | Chr2 | 514 | 57,033.97 | 7.18 | 52–449 |
| SjSHMT4 | ts2g06369_T02 | Chr2 | 540 | 59,438.62 | 8.59 | 81–481 |
| SjSHMT7 | ts3g08322_T02 | Chr3 | 615 | 68,383.58 | 6.08 | 157–562 |
| OlSHMT1 | sy2g05957_T04 | Chr2 | 515 | 56,965.33 | 8.38 | 51–451 |
| OlSHMT2 | sy1g02520_T01 | Chr1 | 515 | 57,113.13 | 8.59 | 53–450 |
| OlSHMT3 | sy8g29094_T01 | Chr8 | 458 | 50,244.21 | 6.17 | 105–402 |
| OlSHMT4 | sy1g00078_T01 | Chr1 | 540 | 59,350.55 | 8.47 | 81–481 |
| OlSHMT7 | sy2g06541_T03 | Chr2 | 615 | 68,303.52 | 5.98 | 43–515 |
| SfSHMT1 | jg1g00713_T01 | Chr1 | 515 | 57,035.35 | 7.70 | 54–451 |
| SfSHMT2 | jg2g04576_T01 | Chr2 | 516 | 57,141.09 | 7.18 | 54–451 |
| SfSHMT4 | jg2g02805_T02 | Chr2 | 540 | 59,431.62 | 8.47 | 81–481 |
| SfSHMT7 | jg1g01220_T02 | Chr1 | 615 | 68,302.56 | 6.03 | 157–562 |
| XaSHMT1 | mbsg23959_T02 | Scf7 | 512 | 56,804.11 | 7.67 | 51–448 |
| XaSHMT2 | mb1g00716_T01 | Chr2 | 517 | 57,284.28 | 7.67 | 55–452 |
| XaSHMT3 | mb5g11984_T01 | Chr5 | 532 | 58,316.41 | 6.21 | 86–476 |
| XaSHMT4 | mb4g10902_T02 | Chr4 | 541 | 59,569.08 | 8.86 | 82–482 |
| XaSHMT7 | mb5g13083_T01 | Chr7 | 615 | 68,405.64 | 6.31 | 160–562 |
Note:
Sj, Salsola junatovii; Ol, Oreosalsola laricifolia; Sf, Soda foliosa; Xa, Xylosalsola arbuscula; SHMT, Serine Hydroxymethyltransferase; pI, Isoelectric point.
Prediction of SHMT protein secondary structure and subcellular localization
The secondary structures of the SHMT proteins were analyzed (Table 2). The results indicated that the SHMT family across all four Salsoleae species was predominantly composed of α-helix, extended strands, β-turns, and random coils, with proportions ranging from 37.4% to 48.05%, 9.27% to 11.87%, 2.15% to 3.93%, and 38.13% to 49.27%, respectively. Subcellular localization analysis revealed that SHMT proteins were primarily distributed in the mitochondria, chloroplasts, and nuclei: SHMT1 and SHMT2 in each species were predicted to localize mainly to mitochondria, while SHMT3 and SHMT4 were predicted to localize to chloroplasts, and SHMT7 to nuclei. Signal peptide prediction indicated that none of the SHMT members possessed a typical signal peptide region. Additionally, to further analyze their structural characteristics, we predicted and visualized the three-dimensional structures of SHMT1, SHMT2, SHMT4, SHMT7, and SHMT3 (Fig. S1), with confidence scores are provided (Table S4). The results demonstrated that the overall fold and the distribution of the SHMT domain (pfam00464) were highly conserved among all four species, and each SHMT isoform assembled into a homotetrameric structure. However, distinct local structural variations were evident among different SHMT members; although the global fold was maintained, these local conformational differences may underlie the substrate-binding specificity or regulatory functions of each isoform.
| Gene name | Alpha helix | Extended strand | Beta turn | Random coil | CELLO |
|---|---|---|---|---|---|
| SjSHMT1 | 48.05% | 10.58% | 2.60% | 38.78% | Mitochondrial |
| SjSHMT2 | 47.08% | 11.09% | 3.70% | 38.13% | Mitochondrial |
| SjSHMT4 | 43.52% | 10.19% | 2.78% | 43.52% | Chloroplast |
| SjSHMT7 | 38.70% | 9.76% | 2.44% | 49.11% | Nuclear |
| OlSHMT1 | 46.80% | 11.07% | 3.11% | 39.03% | Mitochondrial |
| OlSHMT2 | 47.96% | 11.07% | 2.52% | 38.45% | Mitochondrial |
| OlSHMT3 | 46.51% | 10.70% | 3.93% | 38.86% | Chloroplast |
| OlSHMT4 | 44.07% | 11.30% | 2.41% | 42.22% | Chloroplast |
| OlSHMT7 | 38.05% | 10.24% | 2.44% | 49.27% | Nuclear |
| SfSHMT1 | 46.80% | 10.87% | 2.72% | 39.61% | Mitochondrial |
| SfSHMT2 | 44.77% | 10.27% | 3.49% | 41.47% | Mitochondrial |
| SfSHMT4 | 44.63% | 11.67% | 2.59% | 41.11% | Chloroplast |
| SfSHMT7 | 44.07% | 9.27% | 2.28% | 44.39% | Nuclear |
| XaSHMT1 | 44.73% | 10.55% | 2.15% | 42.58% | Mitochondrial |
| XaSHMT2 | 46.81% | 10.83% | 2.71% | 39.65% | Mitochondrial |
| XaSHMT3 | 43.61% | 10.71% | 3.20% | 42.48% | Chloroplast |
| XaSHMT4 | 46.40% | 10.35% | 2.22% | 41.04% | Chloroplast |
| XaSHMT7 | 37.40% | 11.87% | 2.60% | 48.13% | Nuclear |
Note:
Sj, Salsola junatovii; Ol, Oreosalsola laricifolia; Sf, Soda foliosa; Xa, Xylosalsola arbuscula; SHMT, Serine Hydroxymethyltransferase.
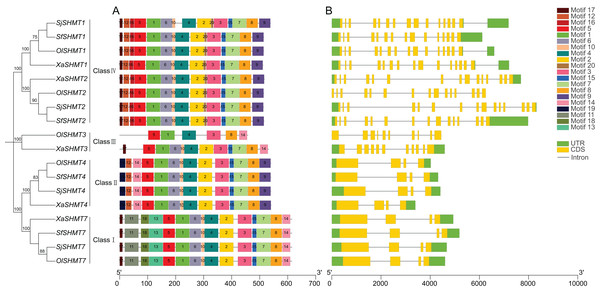
Gene structure and conserved motif analysis of SHMT members
To further elucidate the functional differences among SHMTs, we analyzed their gene structures and conserved motifs. The conserved motif analysis revealed the presence of 20 conserved motifs, with lengths ranging from 8 to 50 amino acids (Table S5). Utilizing TBtools software, we categorized the SHMTs into four distinct classes based on phylogenetic analysis and conserved motifs (Fig. 1A). Specifically, SHMT1 and SHMT2 from each species were classified into Class IV, while SHMT3 from O. laricifolia and X. arbuscula was assigned to Class III. All SHMT4s were placed in Class II, and SHMT7s were categorized into Class I. Notably, SHMT proteins within the same group exhibited similar motif compositions (Fig. 1A), with the exception of SHMT3. Motifs 1, 3, 4, 5, and 8 were present in all SHMT members. Furthermore, the number of exons was consistent within each class (Fig. 1B). SHMTs in Class I, Class II, Class III, and Class IV contained 4, 4, 10, and 15 exons, respectively. These analyses suggested structural differences among SHMT members across different classes, implying potential functional differences.
Figure 1: Conserved motifs (A) and exon-intron structure (B) of the SHMT members in four Salsoleae species.
Different colors represent different motifs. The phylogeny tree was constructed based on the full length of SHMT protein sequences using MEGA 7.0. Sj, Salsola junatovii; Ol, Oreosalsola laricifolia, Sf, Soda foliosa; Xa, Xylosalsola arbuscula.Phylogenetic analysis of the SHMT gene family
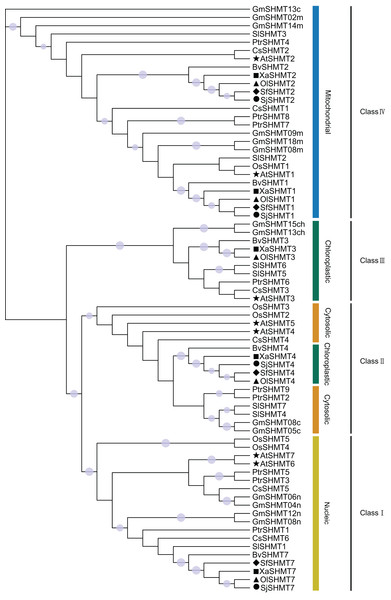
To investigate the phylogenetic relationships among members of the SHMT gene family, this study selected seven representative species, including six dicot species (A. thaliana, G. max, S. lycopersicum, B. vulgaris, C. sativus, and P. trichocarpa) and one monocot species (O. sativa) (Table S6). Based on the topology of the phylogenetic tree constructed using the maximum likelihood method, the 71 SHMTs were classified into four classes (Fig. 2). Members of Class I, Class III, and Class IV were localized in the nucleus, chloroplast, and mitochondrion, respectively. Group II exhibited two predicted subcellular localizations: SHMTs from B. vulgaris and the Salsolee species were localized to the chloroplast, while other SHMTs were localized in the cytosol.
Figure 2: The unrooted phylogenetic tree of SHMT members based on the maximum likelihood method.
SHMT translated protein sequences come from Arabidopsis thaliana (7 protein sequences), Glycine max (14 protein sequences), Solanum lycopersicum (7 protein sequences), Populus trichocarpa (9 protein sequences), Cucumis sativus (7 protein sequences), Beta vulgaris (5 protein sequences), Oryza sativa (5 protein sequences), Salsola junatovii (4 protein sequences), Oreosalsola laricifolia (5 protein sequences), Soda foliosa (4 protein sequences), and Xylosalsola arbuscula (5 protein sequences). The proteins in Oreosalsola laricifolia (Ol), Salsola junatovii (Sj), Soda foliosa (Sf), Xylosalsola arbuscula (Xa), and Arabidopsis thaliana are marked with triangles, circles, rhombuses, squares, and pentagrams, respectively. The four classes are colored differently. Only bootstrap values above 0.7 are shown in the phylogenetic tree. The purple circles on the branches represent support values, with larger circles indicating higher support.Collinearity analysis among Salsoleae species, A. thaliana and B. vulgaris
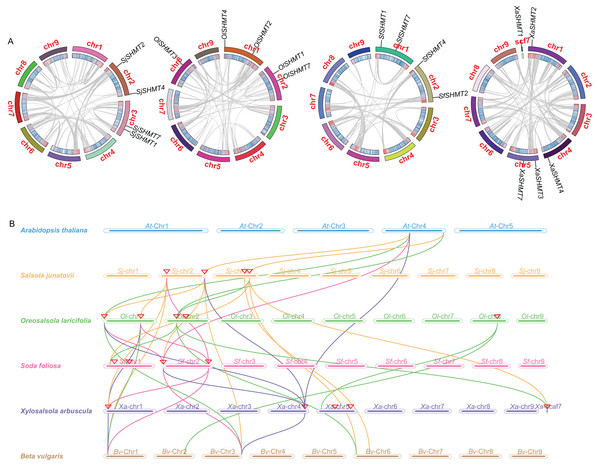
We investigated the gene synteny relationships among the four Salsoleae species, as well as their syntenic relationships with A. thaliana and B. vulgaris. No segmental or tandem duplication events were observed among the Salsoleae species. Chromosomal localization analysis (Table 1, Fig. S2) revealed that the SHMTs of S. junatovii and S. foliosa were distributed across two chromosomes, while the SHMTs of O. laricifolia and X. arbuscula were distributed on three chromosomes. Furthermore, in S. junatovii, O. laricifolia, and S. foliosa, the SHMT1 and SHMT7, as well as SHMT2 and SHMT4, were each located on the same chromosome, respectively. However, in O. laricifolia, the OlSHMT3 gene was located on a separate chromosome. X. arbuscula possessed five SHMTs, and exhibited a distinct distribution pattern. Specifically, XaSHMT3 and XaSHMT7 were located on the same chromosome, while the other genes were distributed across different chromosomes, with one gene (XaSHMT1) found in a scaffold region. This suggests that X. arbuscula may have undergone unique genomic rearrangements during its evolutionary history.
Through the analysis of the genetic relationships of SHMT genes among Salsoleae species, Arabidopsis, and B. vulgaris, we found that SHMT1, SHMT2, and SHMT4 exhibited strong collinearity across the six species. Notably, the collinearity network of SHMT1 encompassed all analyzed species (Fig. 3 and Table S7), suggesting that SHMT1 may exhibit a significant degree of functional conservation across different species. Conversely, SHMT3 and SHMT7 displayed significant species-specific evolutionary traits. Specifically, OlSHMT3 from O. laricifolia showed collinearity with SHMT3 from B. vulgaris, whereas XaSHMT3 from X. arbuscula completely lacked this conserved collinearity. The SHMT7 collinearity network was more limited, with a clear homologous relationship observed solely between S. junatovii and B. vulgaris. Furthermore, SHMT4 gene pairs were identified across all species, suggesting that this gene might have existed prior to the divergence of the ancestral species. Notably, Salsoleae species exhibited 1–2 pairs of homologous genes with Arabidopsis, while there were more homologous genes (2–4 pairs) with B. vulgaris. This may be attributed to the close phylogenetic relationship between Salsoleae and B. vulgaris, as both belong to the Amaranthaceae family, leading to greater homology in their SHMTs.
Figure 3: Gene duplication and collinearity analysis of SHMTs in four Salsoleae species.
(A) Schematic representation of the chromosomal distribution and interchromosomal relationships of SHMTs in a Circos plot. In the Circos plot, gray lines represent the syntenic relationships within each species genome, the innermost ring indicates gene density, and the outermost ring represents the chromosome numbers of each species. (B) Collinearity analysis of the SHMT gene family in Oreosalsola laricifolia, Salsola junatovii, Soda foliosa, Xylosalsola arbuscula, Arabidopsis thaliana, and Beta vulgaris. Different colored lines delineate the syntenic SHMTs pairs: orange, green, pink, blue represents syntenic relationships in Salsola junatovii, Oreosalsola laricifolia, Soda foliosa, and Xylosalsola arbuscula, respectively.Quantitative real-time PCR analysis in different tissue
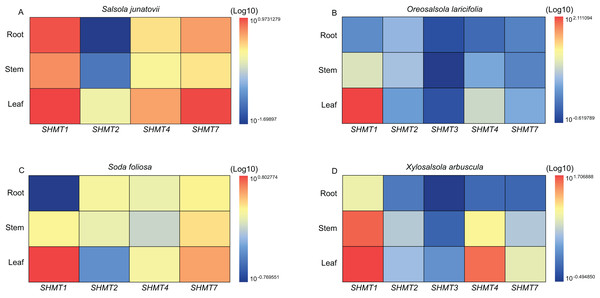
Quantitative real-time PCR (RT-qPCR) was employed to analyze the expression levels of SHMTs in the roots, stems, and leaves of various Salsoleae species (Fig. 4). The results demonstrated that all SHMTs were expressed in all three tissues, indicating a lack of strict tissue specificity. Among these SHMT members, SHMT1 exhibited the highest expression levels, with its transcripts predominantly accumulating in the leaves. Additionally, SHMT4 and SHMT7 demonstrated significantly higher expression in leaves compared to roots and stems. The expression pattern of SHMT2 varied across species, in O. laricifolia and S. foliosa, SHMT2 expression was higher in roots and stems than in leaves, whereas in S. junatovii and X. arbuscula, expression levels were elevated in stems and leaves compared to roots. Notably, SHMT3 was detected only in O. laricifolia and X. arbuscula, with markedly lower expression compared to other members. These findings suggest that distinct SHMT homologs may fulfill specialized functional roles in different organs across Salsoleae species.
Figure 4: Relative expression levels of SHMT members in roots, stems, and leaves of four Salsoleae species.
The relative expression levels were calculated using the 2 −ΔΔCT method, and the data were log-transformed (log10) and standardized for visual comparison. The color scale indicates expression levels, with blue representing lower expression and red representing higher expression.Transcriptional expression analysis of SHMTs in different groups
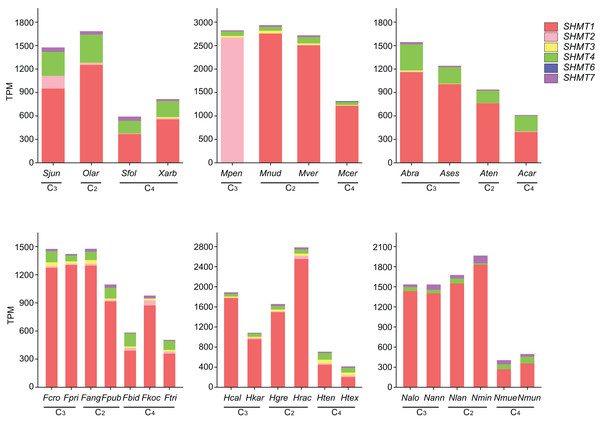
To further investigate the expression of SHMT in Salsoleae and other plant groups, we analyzed SHMTs across four Salsoleae species and five additional genera: Alternanthera (Amaranthaceae), Flaveria (Asteraceae), Heliotropium (Boraginaceae), Mollugo (Molluginaceae), and Neurachne (Poaceae) (Figs. 5 and S3, Table. S2). The results indicated that, except for MpenSHMT1, FkocSHMT4, and McerSHMT7, which were not detected in Mollugo pentaphylla, Flaveria kochiana, and M. cerviana, respectively, all other species exhibited the expression of SHMT1, SHMT4, and SHMT7. Additionally, SHMT2, SHMT3, and SHMT6 were detected in some species. Although SHMT genes were widely distributed across various plant groups, significant differences existed in the number of gene members and their expression levels. Notably, the types and expression patterns of SHMT members varied among different species within these genera. In general, the expression levels of SHMT6 and SHMT7 were relatively low compared to other SHMT members, while SHMT2, SHMT3, and SHMT4 exhibited relatively higher expression levels in specific groups. Furthermore, SHMT1 was the most predominantly expressed member in most species and showed higher expression levels in C2 and C3 plants compared to C4 plants.
Figure 5: The expression levels of SHMTs in leaves across six different plant groups.
With distinct colors representing different SHMT members. Transcript levels are presented as transcripts per million (TPM). All data are derived from published RNA-seq studies. species abbreviations are as follows: Sjun (Salsola junatovii), Sfol (Soda foliosa), Olar (Oreosalsola laricifolia), Xarb (Xylosalsola arbuscula), Fcro (Flaveria cronquistii), Fpri (Flaveria pringlei), Fang (Flaveria angustifolia), Fpub (Flaveria pubescens), Fbid (Flaveria bidentis), Fkoc (Flaveria kochiana), Ftri (Flaveria trinervia), Hcal (Heliotropium calcicola), Hkar (Heliotropium karwinskyi), Hgre (Heliotropium greggii), Hrac (Heliotropium racemosum), Hten (Heliotropium tenuifolium), Htex (Heliotropium texanum), Mpen (Mollugo pentaphylla), Mnud (Mollugo nudicaulis), Mver (Mollugo verticillata), Mcer (Mollugo cerviana), Ases (Alternanthera sessilis), Abra (Alternanthera brasiliana), Aten (Alternanthera tenella), Acar (Alternanthera caracasana), Nalo (Neurachne alopecuroideae), Nann (Neurachne annularis), Nlan (Neurachne lanigera), Nmin (Neurachne minor), Nmue (Neurachne muelleri), Nmun (Neurachne munroi); data sources and other relevant information are provided in Table S5.Cis-acting elements and transcription factor binding site analysis
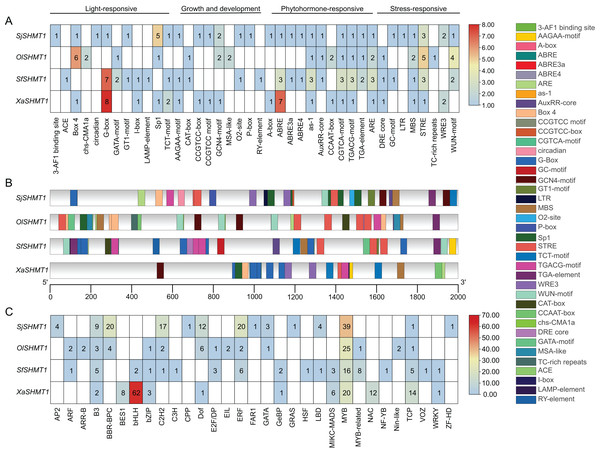
In our study, SHMT1 was identified as the predominantly expressed SHMT. Consequently, we conducted a detailed analysis of the 2,000 bp upstream promoter region of SHMT1 across four Salsoleae species. A total of 40 key cis-acting elements was detected, including 12 light-responsive elements, eight growth and development-related elements, 11 hormone-responsive elements, and nine stress-responsive elements (Fig. 6A). The types of cis-acting elements presented in the SHMT1 promoter region varied among these four species. Specifically, S. junatovii (C3) exhibited the highest number of distinct cis-acting element types, with 29 identified, while O. laricifolia (C2) had the fewest, with only 19. Further analysis revealed that the frequency of occurrence of different cis-acting elements also varied among these species. For instance, the Sp1 element appeared five times in S. junatovii, whereas its occurrence was lower in the other species. The Box-4 element occurred most frequently in O. laricifolia, with six occurrences. We further analyzed the positions of cis-acting elements within the SHMT1 promoter regions across various species. Our findings revealed significant variations in both the quantity of elements and their specific locations within these regions among different species. For example, OlSHMT1 exhibited a higher concentration of cis-acting elements near the transcription start site, whereas XaSHMT1 displayed fewer elements that were more dispersed (Fig. 6B).
Figure 6: Prediction of cis-elements and bounding transcription factors on the SHMT promoters of four Salsoleae species.
(A) The types and numbers of cis-elements on the promoters. (B) The locations of cis-elements on the genes. (C) Putative transcription factors binding to the SHMT promoters.Additionally, we predicted the transcription factor binding sites (Fig. 6C). The results indicated that X. arbuscula possessed only 11 transcription factor binding sites in its SHMT1 promoter, with the BasicHelix-Loop-Helices (BHLH) binding site being particularly prevalent, occurring a total of 62 times. In contrast, the SHMT1 promoter of S. foliosa exhibited the greatest diversity, identifying 18 different types of transcription factor binding sites. Our analysis further revealed that v-myb avian myeloblastosis viral oncogene homolog (MYB) binding sites were abundant in the SHMT1 promoters across all species. Moreover, certain transcription factor binding sites displayed distinct species specificity, for instance, APETALA2 (AP2), Cysteine-rich Polycomb-like (CPP), and Far-red Impaired Response 1 (FAR1) binding sites were detected solely in the SHMT1 promoter of S. junatovii. In summary, these findings reveal significant species-specific differences in the types, numbers, and distribution of cis-acting elements and transcription factor binding sites within the SHMT1 promoter among different Salsoleae species, suggesting that these elements may play unique roles in environmental adaptation and the regulation of photosynthesis.
Discussion
SHMT catalyzes the reversible interconversion of glycine and serine with GDC, and a crucial enzyme in the cell-carbon metabolic pathway (Schirch, 1982; Hanson, Gage & Shachar-Hill, 2000; Appaji Rao et al., 2003; Zhang et al., 2010). Genes encoding the SHMT family have been identified in various higher plants, such as five in rice (Pan et al., 2024); seven in each A. thaliana (Zhang et al., 2010) and tomato (Liu et al., 2022), 9 in P. trichocarpa (Li & Cheng, 2020), 15 in alfalfa (Gao et al., 2024), 18 in soybean (Lakhssassi et al., 2020). In this study, these species contained the close identified SHMT number in rice but less than the most published higher plants. We identified 4 SHMTs in both S. junatovii and S. foliosa, and they are distributed across two chromosomes, respectively. Additionally, 5 SHMTs were identified in both O. laricifolia and X. arbuscula, and they are distributed on three chromosomes, respectively (Table 1, Fig. S2). The number of SHTMs appears to be correlated with gene duplication event and the number of whole-genome duplications. SHMTs in cucumber (Gao et al., 2022), alfafa (Gao et al., 2024), and soybean (Lakhssassi et al., 2020) participate in a fragment duplication event, respectively. The soybean genome contains 18 SHMTs and has likely undergone a larger-scale genome replication event (Gao et al., 2024). However, there was no fragment duplication events, and no gene is involved in tandem duplication in four Salsoleae species (Fig. 3), which maybe cause the less SHMT members in each species.
To investigate the relationship between the SHMT members of four Salsoleae species and other species including A. thaliana, O. sativa, G. max, S. lycopersicum, P. trichocarpa, C. sativus, B. vulgaris, we classified these SHMTs into four clades, which generally corresponded to their subcellular localizations, with the exception of in Class II (Fig. 2). In Class III, consistent with previous studies (Gao et al., 2022, 2024; Pan et al., 2024), we observed the absence of Class III SHMT in rice. Additionally, Class III SHMT was also absent in S. junatovii and S. foliosa. However, the predicted chloroplast-localized SjSHMT4 from S. junatovii and SfSHMT4 from S. foliosa were present in Class II, suggesting that gene duplication may have compensated for the loss of Class III. Furthermore, OsSHMT3 from rice, originally classified within the cytoplasmic-localized group, has been found to be localized in chloroplasts within rice protoplasts (Pan et al., 2024). And there were no cytoplasmic-localized SHMT was predicted in these four Salsoleae species. The overexpression of PtSHMT2 from Populus promotes growth by enhancing biomass production and the release of sugars such as glucose and xylose (Zhang et al., 2019a). Whether a specific SHMT loss event occurred in these Salsoleae species still requires extensive experimental validation. Furthermore, genes belonging to the same class exhibited high similarity in both gene structure and motif numbers, with the exception of OlSHMT3 and XaSHMT3 in terms of gene structure (Fig. 1). Collinearity analysis revealed that OlSHMT3 is collinear with SHMT3 from Beta, but no collinear relationship was found between XaSHMT3 and SHMT3 from Beta (Fig. 3, Table S7), indicating that these two genes have undergone evolutionary and adaptive structural changes. Differences in exon numbers were observed across different classes rather than in between species. The range of exon numbers were from 4 to 15 (Fig. 1). The diverse gene structures of the SHMTs in the four Salsoleae species may. result from an evolutionary process characterized by intron loss or gain (Ding et al., 2014; Lakhssassi et al., 2019).
To investigate the roles of SHMTs during the growth and development of four Salsoleae species, this study analyzed the expression levels of SHMTs in leaves, stem and root, revealing that these SHMTs are expressed across all tissues examined. Each gene exhibited distinct tissue expression patterns. Notably, the mitochondrial-localized SHMT1 from the four Salsoleae species showed higher gene expression levels in leaves compared to stem and root (Fig. 4). This finding aligns with previous reports in Arabidopsis (Moreno, Martín & Castresana, 2005), alfalfa (Gao et al., 2024), soybean (Lakhssassi et al., 2019). Conversely, the mitochondrial-localized SHMT2 demonstrated different expression patterns among in the Salsoleae species. In O. laricifolia (Fig. 4B) and S. foliosa (Fig. 4C), SHMT2 expression was higher in stem and roots than in leaves. In contrast, For S. junatovii (Fig. 4A) and X. arbuscula (Fig. 4D), exhibited higher SHMT2 expression in stem and leaves compared to root. Moreover, no collinear relationship was observed between SHMT1 and XaSHMT1 across Salsoleae species, Beta, and Arabidopsis (Fig. 3, Table S7). The diversification of predominant tissue expression pattern between mitochondrial-localized SHMT1 and SHMT2 in these Salsoleae species may indicate function diversification of these genes (Voll et al., 2006). Furthermore, while mitochondrial-localized SHMT1 and SHMT2 in Arabidopsis exhibit different tissue expression patterns and are not functionally redundant (Voll et al., 2006), the transcript accumulation of AtSHMT4 in Arabidopsis is restricted to the roots of seedlings (Moreno, Martín & Castresana, 2005). In contrast, chloroplast-localized SHMT4 in the four Salsoleae species and SHMT3 in X. arbuscula demonstrated higher gene expression in leaes thanin stem and root (Fig. 4). In O. laricifolia, SHMT3 exhibited higher gene expression in roots (Fig. 4B). Although both SHMT4 and SHMT3 are localized in chloroplasts, the expression level of SHMT4 is greater than that of SHMT3 in X. arbuscula (Fig. 4D) and O. laricifolia (Fig. 4B). Chloroplastic SHMT plays a crucial role in photoreception and the biosynthesis related to one carbon metabolism (Hanson, Gage & Shachar-Hill, 2000; Zhang et al., 2010).
In C4 species, the repression of the Rubisco oxygenation reaction and the absence of toxic byproducts result in the low expression of key genes associated with most enzymes in the photorespiratory cycle (Mallmann et al., 2014). We utilized the newly sequenced leaf transcriptome of four Salsoleae species, along with published leaf transcriptome data from five genera were used to examine the expression of SHMTs along the emergence of C4 species. Compared with other SHMTs, only mitochondrial-localized SHMT1 exhibited significantly high transcript abundance in leaves, with the exception of M. pentaphylla, which did not express SHMT1. Furthermore, the expression of SHMT1 in C4 species was lower compared to that in C3 and C2 species. Notably, the transcript levels of SHMT1 in C2 plants remained constant or were even higher than those observed in C3 plants (Fig. 5). AtSHMT1 and AtSHMT4 from Arabidopsis are regulated by the circadian clock, aligns with their role in photorespiration (McClung et al., 2000). However, the expression of SHMT4 in C4 species was inconsistent compared to that in C3 and C2 species within these genera (Fig. 5). AtSHMT1, the SHMT coding gene from Arabidopsis, plays a crucial role in the photorespiratory cycle (Voll et al., 2006). It is concluded that the low expression of the leaf preferential mitochondrial-localized SHMT1 has evolved during the evolution of C4 photosynthesis. The cis-acting element and the transcription factors bound to the SHMT1 promoters in four Salsoleae species were predicted (Fig. 6). Our analysis revealed that the SHMT1 promoter regions in these species contained various cis-acting elements associated with light response, growth and development, phytohormone response, and stress response (Fig. 6A). This indicates that the function of SHMT1 in these Salsoleae species may encompass these four aspects. The MYB factors were predicted to be the most significant transcription factors binding to SHMT1 in three Salsoleae species, and the second most significant in X. arbuscula (Fig. 6C). Further verification through additional experiments is needed to confirm the binding of MYB factors to SHMT1 in Salsoleae. MYB factors are implicated in various aspects of C4 photosynthesis, including cell/division/size (Rao et al., 2016), bundle sheath wall formation (Rao et al., 2016), sulfur metabolism, glucosinolate biosynthesis (Aubry et al., 2014) and achieving cell specific expression (Dickinson et al., 2020; 2023).
Conclusions
In this study, we systematically analyzed the SHMT gene family across four Salsoleae species: C3 species S. junatovii, C3-C4 intermediate O. laricifolia, and C4 species X. arbuscula and S. foliosa. We identified four or five SHMTs in each species, with no instances of fragment duplication detected, which may account for the relatively low number of family members. The number of exons in SHMTs varied from four to fifteen, and these genes were classified into four distinct phylogenetic groups. SHMTs within each phylogenetic group shared similar exon-intron structures and conserved motif compositions. Expression analysis revealed that, although SHMTs are not strictly organ-specific, the mitochondrial-localized and leaf-preferential SHMT1 exhibited significantly lower expression levels in C4 species compared to those in C3 and C3-C4 intermediate species. This observation suggests that the regulation of this gene may have evolved in the C4 photosynthesis. Furthermore, cis-acting element analysis predicted that MYB transcription factors may serve as key regulators of SHMT1 in several Salsoleae species. Overall, these findings establish a foundation for further exploration of the function and evolution of the SHMT gene family in Salsoleae, contributing to a deeper understanding of the molecular mechanisms underlying the evolution of photosynthetic pathways.