Role of pyroptosis in pregnancy-related diseases
- Published
- Accepted
- Received
- Academic Editor
- Tokuko Haraguchi
- Subject Areas
- Cell Biology, Gynecology and Obstetrics, Internal Medicine
- Keywords
- Pyroptosis, Pregnancy, Preeclampsia, Recurrent spontaneous abortion, Neonatal developmental dysplasia, Preterm birth, Gestational diabetes mellitus
- Copyright
- © 2025 Li et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Role of pyroptosis in pregnancy-related diseases. PeerJ 13:e19922 https://doi.org/10.7717/peerj.19922
Abstract
Pyroptosis, a form of programmed cell death characterized by inflammasome-mediated cellular swelling and membrane perforation. This pathway is involved in diverse pathophysiological processes, including inflammatory diseases and tumors. Notably, the role of pyroptosis in pregnancy-related diseases such as preeclampsia, recurrent spontaneous abortion, neonatal developmental dysplasia, preterm birth, and gestational diabetes mellitus has not been elucidated yet. This review aims to systematically dissect the molecular basis of pyroptosis in pregnancy-related diseases and summarize emerging therapeutic strategies targeting pyroptosis and inflammasomes. We first outline the mechanistic link between pyroptosis, inflammasome activation, and maternal-fetal immune regulation. Subsequent sections focus on the putative roles of pyroptotic pathways in the pathogenesis of major pregnancy complications, integrating recent findings from preclinical and clinical studies. Elucidating pyroptosis-mediated mechanisms may pave the way for developing targeted therapies to improve outcomes in pregnancy-related diseases.
Introduction
Pyroptosis is a programmed cell death which relies largely on the inflammatory caspase and gasdermin (GSDM) protein family (Shi, Gao & Shao, 2017). Pyroptosis is involved in regulating the host’s pathogen defense and immune inflammation processes, and it is crucial in maintaining the body’s immune balance. Cellular pyroptosis, as a new proinflammatory programmed cell death, differs significantly from apoptosis in terms of cellular mechanisms of occurrence and morphological changes. Pyroptosis is characterized by continuous cell swelling until lyse, which leads to the release of cellular contents causing a strong inflammatory reaction (Kesavardhana, Malireddi & Kanneganti, 2020). Pyroptosis is involved in a number of physiological and pathological processes.
Pregnancy is a state of immune-inflammatory balance during which the mother and the fetus must tolerate each other while maintaining sufficient immune capacity to resist potential hazard. Placenta and fetus express both maternal and paternal antigens, which function as semi-allogeneic grafts (Ander, Diamond & Coyne, 2019). Thus, maintaining the balance of inflammatory responses is crucial for a successful pregnancy, including the whole gestational period, throughout imbedding, embryo growth and development, pregnancy maintenance, and delivery. However, once this balance is disrupted, inflammasome activation mediated pyroptosis may lead to pathological changes in the mother or fetus, including preeclampsia (PE), abortion, preterm birth, and neonatal development dysplasia.
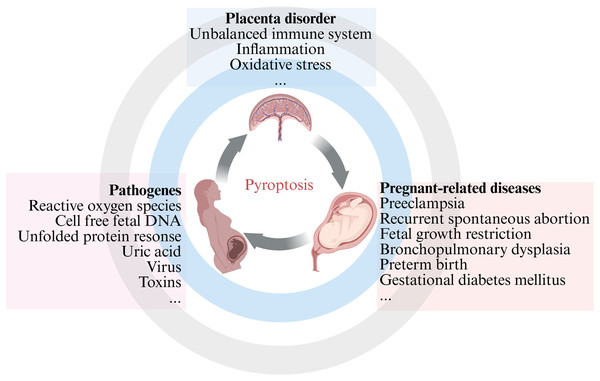
Our comprehensive review emphasized the current knowledge and understanding of the crosstalk between pyroptosis and pregnancy-related diseases, as well as the potential role of inflammasome and pyroptosis in cells involved in the placenta. The aim of the present review is to compile the latest literature on the involvement of pyroptosis in pregnancy-related diseases, with the aim of organizing and supplementing knowledge in this field and provide reference and guidance for basic research and clinical work (Fig. 1). This review is intended for different audiences, including obstetric patients, healthcare professionals, members of the scientific community, and medical researchers, with a focus on PE, recurrent spontaneous abortion (RSA), and other fetal growth-related diseases. Further research on pregnancy-related diseases mediated by apoptosis may provide new insights into reducing pregnancy complications and improving birth outcomes.
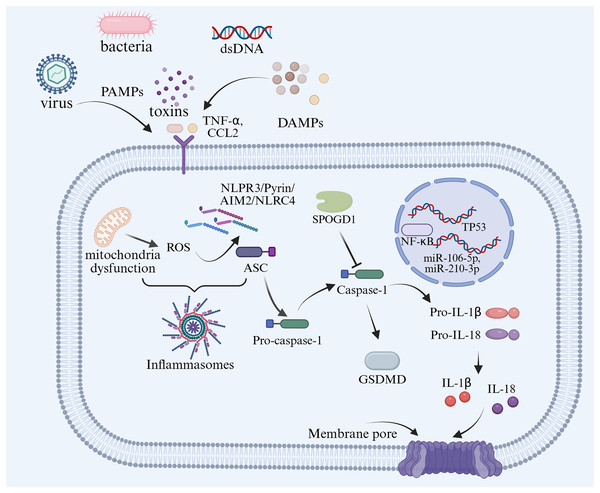
Figure 1: An overview of pyroptosis in pregnancy-related diseases.
Methodology
The literature search for this review was performed through the PubMed digital platforms and Google Scholar. Boolean operators (AND, OR) and predetermined keywords related with pyroptosis (NLRP and inflammasomes), placenta, pregnant, pregnancy related disease (PE, RSA and preterm birth) and fetus were applied. The final selected references included studies on human, rodent models, and cell line studies. Majority publications with significant contribution to this topic between 2019 to 2025. We have selected 161 publications that have made significant contributions to the current topic. We have included original research articles, short communication/letters, narrative reviews, systematic reviews and meta-analysis which encompass all of the subjects areas covered in this review. Editorials, case report, commentary/viewpoint and book review were excluded from the references. To maintain the integrity of our review, full-text literature including open access and subscription resources were included. We only included obstetric related diseases and excluded literature related to gynecology, such as vaginitis, uterine and adnexal malignancies. We excluded articles not emphasizing pyroptosis related molecular biomarkers in pregnant related diseases.
Molecular mechanisms of pyroptosis
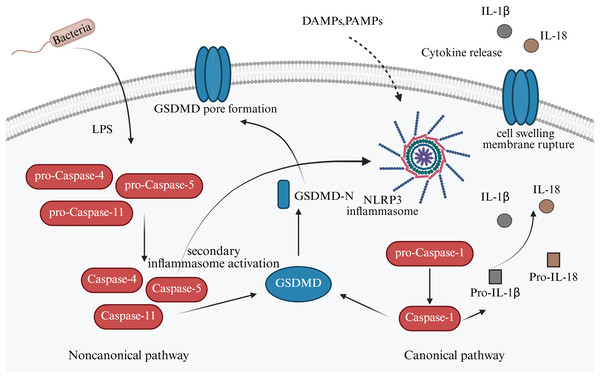
It has so far been elucidated that there are two signaling pathways of pyroptosis: (i) Canonical; and (ii) noncanonical pathways. In the canonical pathway, inflammasomes such as NLR family pyrin domain-containing (NLRP)1, NLRP3, NLRP4, absent in melanoma 2 (AIM2), and pyrin are activated and cleave procaspase-1 to form active caspase-1 (Sandstrom et al., 2019). GSDM family protein-mediated pyroptosis, which is mostly caspase or granzymes dependent. Caspase-1 can cleave the gasdermin D (GSDMD) protein molecule to form active N-terminus and C-terminus, which induce cell membranous pore formation and cell death (Newton et al., 2024). Meanwhile, caspase-1 can also process the activation of interleukin (IL)-1β and IL-18, which are released into the extracellular space via the membrane pores, recruiting inflammatory cell aggregation and inducing the inflammatory response (He et al., 2015).
The non-canonical mechanism of cell pyroptosis is mediated by cysteine protease-4,-5,-11 (caspase-4,-5,-11), contrary to the canonical pathway, depends on caspase-1 (Fig. 2). However, when lipopolysaccharides (LPS) enter the cytoplasm, they bind to caspase-4,-5,11, cleave GSDMD, release the N-terminal domain, and cause cell perforation and contents to leak out. Alternatively, they can activate NLRP3, encourage GSDMD cutting, and release active IL-1β and IL-18 (Newton et al., 2024). An in vitro study reported that LPS stimulation could induce the expression of NLRP1, NLRP3, NLRC4, caspase-1, and IL-1β in trophoblast, decidual stromal, and endometrial cells in early pregnancy (Pontillo et al., 2013).
Figure 2: Canonical and non-canonical pathway of apoptosis in pregnancy-related diseases.
Compared with women in full-term pregnancy, the expression of NLRP3, caspase-1, and inflammatory factors was decreased in the amniotic fluid, the placenta, and the uterine muscle layer in women delivering full-term (Gomez-Lopez et al., 2019). According to another study, the membranes of women delivering full-term delivery had higher concentrations of NLRP7 inflammasomes than the control group. It is noteworthy that after treatment with fibroblast-stimulating lipopeptide-1 (FSL-1) has been found to induce NLRP7 inflammasome formation in human primary amnion epithelial cells (Lavergne et al., 2020). According to the aforementioned studies, the activation of inflammasomes and their mediated cell pyroptosis may not only participate in the immune defense at the maternal fetal interface in the placenta during pregnancy, but also have prominent role in placental formation and delivery initiation.
Pyroptosis in pregnancy physiology
Under normal physiological conditions, pyroptosis serves a crucial role in protecting the host defense against pathogen infections, such as implantation, placentation and immune response regulation (Yu & Li, 2021). Pyroptosis, as a kind of regulated cell death, and its role in pregnancy still controversial. Pyroptosis was found at the maternal-fetal interface in epithelial cells at the implantation site (Li et al., 2024). Pyroptosis could be observed in mouse uterus on day 4 pregnant, and the levels of the proteins NLRP3, apoptosis-associated speck-like protein containing a C-terminal caspase recruitment domain (ASC), cleaved caspase 1, and GSDMD-N increased on day 4 of pregnancy compared with pseudopregnancy group, which suggested caspase1-dependent classical pathway was the main pathway in the beginning of pregnancy (Li et al., 2024).
Pyroptosis in PE
PE is a complex multisystem disorder defined by sudden onset of hypertension after 20 weeks of gestation, accompanied by at least one additional complication, including proteinuria, placental dysfunction or maternal organ dysfunction (Dimitriadis et al., 2023). The incidence rate range of PE is about 3–5%, usually begins after 20 weeks of pregnancy and late onset if more frequent than early onset (Fox et al., 2019). Pyroptosis markers are upregulated in PE with inflammatory state. Accumulating evidence indicates upregulated expression of NLRP3 inflammasome components in peripheral blood cells and placental tissues from women with PE compared to normotensive healthy pregnant controls (Shirasuna, Karasawa & Takahashi, 2020). By immunohistochemistry and quantified by reverse transcription-qPCR (RT-qPCR) analysis, pregnant women with PE have higher levels of NLRP3 inflammasome in placenta tissue than healthy pregnant women (Weel et al., 2017). The results of human tissue and in vivo experiments have also shown that extracellular vesicles activate maternal platelets and NLRP3 inflammasomes in trophoblasts, which inducing placental aseptic inflammation and PE (Dimitriadis et al., 2023). Inhibiting this pathway can alleviate PE symptoms (Kohli et al., 2016). In vitro study shown that human trophoblast cells treated with serum from patients with PE could induce NLRP3 inflammasome activation and cell pyroptosis, releasing the inflammatory factors IL-1β and IL-18 in cell models mimicking pathophysiological conditions (Cheng et al., 2019). Results have shown that the gene expression of NLRP1, NLRP3, caspase-1, IL-1β, and tumor necrosis factor α (TNF-α) in monocytes from the PE group was elevated compared with that in the control group (Matias et al., 2015).
Numerous studies consistently report elevated NLRP3 inflammasome activation in women with PE. For example, monocytes from PE patients exhibit significantly higher basal expression of NLRP3, caspase-1, IL-1β, and TNF-α compared to normotensive pregnant controls (Matias et al., 2015). Similarly, placental tissues from PE patients show increased NLRP3, ASC, and caspase-1 expression in syncytiotrophoblasts and trophoblasts, with co-localization of NLRP3 and complement factors like C5a and terminal complement complex (TCC) (Stødle et al., 2018). While exact prevalence varies by tissue type and study design, NLRP3 upregulation is observed in PE cases across placental, peripheral blood, and monocytes and endothelial cell samples (Socha et al., 2020). PE is pathologically characterized by excessive oxidative stress, systemic inflammation, endothelial dysfunction, and fetal complications (Shirasuna et al., 2015; Nunes, Mattioli & Sandrim, 2021). The pathological process may be triggered by the activation of NLRP3 inflammasome mediated by reactive oxygen species (ROS), which triggers the release of pro-inflammatory cytokines, including IL-1β and IL-18 (Nunes, Mattioli & Sandrim, 2021). These cytokines amplify the inflammatory cascade and exacerbate oxidative stress within the endothelium. As a key regulator of immune function, nitric oxide modulates inflammatory signaling pathways and maintains vascular homeostasis (Guerby et al., 2021). This sustained pro-inflammatory state exacerbates endothelial dysfunction, indicating that endothelial inflammation may be a key driving factor in the occurrence and progression of PE (Phipps et al., 2019).
The implication of pyroptosis-related inflammasomes in the pathophysiology of PE has also been recognized through in vivo experiments. Mechanistically, the inflammatory signal pathways and main inflammasomes have been shown to be associated with pyroptosis in PE (Banerjee et al., 2021). NLRP3 could induce pyroptosis through the toll-like receptor (TLR4)/NF-κB/PFKFB3 signaling pathway in preeclamptic trophoblast models (Zhang et al., 2021). The use of drugs that can inhibit NLRP3 may be a good choice for the future treatment of PE (Nunes, Mattioli & Sandrim, 2021). GSDMD, the important executor of pyroptosis, and caspase-1 were found upregulated expression in the placenta tissue of women with early onset PE (Cheng et al., 2019). It has been reported that IL-11 could activate pyroptosis associated with NLRP3, causing PE in human first trimester placental villi (Menkhorst et al., 2023). Gross pathology in the preeclamptic placenta has revealed vascular sclerosis and multiple placental infarctions (Ezeigwe et al., 2018). The insufficient local oxygen supply nutrients can induce trophoblast cell death, which leads to endothelial activation and release of inflammatory mediators, including damage-associated molecular patterns (DAMPs), such as cell-free fetal DNA, uric acid, IL-1α, and high mobility group box 1 (HMGB1) (Nadeau-Vallée et al., 2016). DAMPs, as endogenous danger molecules, activate the innate immune system and release proinflammatory cytokines, which cause the damage of endothelial cells and dysfunction of trophoblast cells, and then lead to PE (McCarthy & Kenny, 2016). Another study showed that AIM2, as a cytosolic DNA sensor, could be activated by free fetal DNA and lead to the development of PE (Li et al., 2020). Silencing NEAT1 could promote Treg/TH17 immune cell balance and downregulate the microRNA (miR)-458-5p/AIM2 axis in PE (Chen et al., 2021).
Moreover, early-onset and late-onset PE exhibit distinct pathophysiological mechanisms, while syncytiotrophoblaset shown increasing number of pyroptotic markers in late-onset PE (Redman, Staff & Roberts, 2022). The downregulation of histone deacetylase 2 in women with PE was accompanied by an increase of FOXO3 and PERK, along with an increase in the expression of IL-1 β and IL-18, inhibiting cell proliferation, migration, and invasion, ultimately leading to pyroptosis in trophoblasts (Liu & Yang, 2023). Liu et al. (2020) reported that caspase-1 may increase pyroptosis by angiotensin II type 1 receptor exposure in women with PE. Lipoxin A4 could inhibit AT1-AA in PE by regulating caspase-1 expression (Liu et al., 2020). TDRKH-AS1 in small extracellular vesicles, isolated from oxidative stress trophoblasts, may be involved in the pathogenesis of PE by inducing pyroptosis and exacerbating endothelial dysfunction (Chen et al., 2023). Therefore, the activation of inflammasomes may participate in the pathogenesis of PE by raising inflammation (Table 1).
| Model | Compounds/drugs | Pyroptotic component | Function | Related pathways | Refs. |
|---|---|---|---|---|---|
| C57BL/6 mice, human placenta tissue, human trophoblast-like cells (BeWo, JEG-3) | Maternal extracellular vesicles and platelets | NLRP3, caspase-1, IL-1β, ATP | Pregnancy failure, elevated blood pressure, increased plasma soluble fms-like tyrosine kinase 1, and renal dysfunction | Purinergic and inflammasome signaling pathway | Kohli et al. (2016) |
| Atg4BC74A cell line, human placenta tissue, primary human trophoblasts | Hypoxia, autophagy lysosome dysfunction, protein aggregates |
DAMP, alarmins, NLRP3, GSDMD, caspase-1 | Unfolded protein response, ER stress, autophagy-deficient | TXNIP-NLRP3-caspase-1 pathway | Cheng et al. (2019) |
| Isolated monocytes from human blood | Monosodium urate | NLRP1, NLRP3, caspase-1, IL-1β, IL-18 and TNF-α | Increased expression of NLRP1 and NLRP3 receptors | / | Matias et al. (2015) |
| HTR-8/SVneo cell line | Metformin | NLRP3, caspase-1, ASC, GSDMD | Glycometabolism reprogramming and redox disorders | TLR4/NF-κ B/PFKFB3 pathway | Zhang et al. (2021) |
| C57BL6 mice, human placenta tissue | PEGIL11 | ASC, NLRP3, IL-11 | Fibrosis | Asc/NLRP3 pathway | Menkhorst et al. (2023) |
| Human blood, HTR-8/SVneo cell line | Cell-free fetal DNA | AIM2, IFI16, IL-8, IL-6, CCL2, sFlt-1 and CXCL10 | Trigger cytosolic DNA sensor activation, disrupts the immunity balance, antiangiogenic responses | DNA sensor signaling pathways | Li et al. (2020) |
| Human peripheral blood mononuclear cells | lncRNA NEAT1 | NEAT1, miR-485-5p, AIM2 | Treg/Th17 imbalance | miR-485-5p/AIM2 axis | Chen et al. (2021) |
| Human placenta tissue, HTR8/SVneo cell line | HDAC2 | FOXO3, PERK, H3K27ac, IL-1β, IL-18 | H3K27 acetylation | / | Liu & Yang (2023) |
| Human placenta tissue, C57BL/6J mice, HTR8/SVneo cell line | Extracellular vesicles | TDRKH-AS1, DDIT4 | Endothelial dysfunction | PDIA4/DDIT4 axis | Chen et al. (2023) |
Note:
NLRP3, NLR family pyrin domain-containing 3; ATP, adenosinetriphosphate; DAMP, damage-associated molecular patterns; ER, endoplasmic reticulum; AIM2, melanoma 2; IFI16, interferon-inducible protein 16; CCL2, CC chemokine ligand 2; sFlt-1, fms-like tyrosine kinase-1; NEAT1, nuclear enriched abundant transcript 1, HDAC2, Histone deacetylase 2; FOXO3, forkhead box O3; PERK, protein kinase R-like endoplasmic reticulum kinase, DDIT4, DNA damage-inducible transcript 4; PDIA4, protein disulfide isomerase family a member 4.
While no clinical trials explicitly target pyroptosis in PE, several ongoing studies investigate related pathways. A small molecule inhibitor, MCC950, as a selective NLRP3 antagonist, reduces maternal hypertension, oxidative stress, and fetal reabsorption in the reduced uterine perfusion pressure (RUPP) rat model (Wang et al., 2023). Aspirin, anti-inflammatory agents, commonly used for PE prophylaxis, indirectly modulates NLRP3 by reducing thromboxane synthesis and oxidative stress (Lin et al., 2022).
Pyroptosis and RSA
RSA usually refers to the occurrence of three or more consecutive fetal losses before 28 weeks of pregnancy (Deng, Liao & Zhu, 2022). The etiology of RSA is complex and not clear, and the immune inflammatory imbalance between mother and fetus is one of the possible mechanisms of RSA. NLRP3-induced abnormal inflammatory response on the maternal-fetal interface of women with RSA, leads to unsuccessful embryo implantation (Gao et al., 2020). Zhu et al. (2021) reported pyroptosis in the decidual tissue of women with RSA patients and in a mouse model. It was shown that the expression of HMGB1, TLR2, TLR4, receptor for advanced glycation end-products (RAGE), NLRP3, caspase-1, GSDMD, and NF-κB was increased in the maternal-fetal interface tissue (Zhu et al., 2021). After treatment with aspirin, the expression of HMGB1, RAGE, TLR2, TLR4, and NLRP3 inflammasome components was decreased, confirming the possible involvement of the NLRP3 inflammasome in RSA and providing evidence for the application of aspirin in RSA (Zhu et al., 2021). MITA-triggered pyroptosis in the maternal-fetal interface, which interrupted the balance of immune microenvironment and caused abortion during pregnant (Liu et al., 2023).
Emerging evidence implicates NLRP3 inflammasome-mediated pyroptosis as a critical driver of RSA pathogenesis. The canonical NLRP3 inflammasome pathway has been implicated in RSA. Triggered by DAMPs like urate crystals, NLRP3 activation recruits ASC and activates caspase-1, which leads to GSDMD cleavage, pore formation, and release of pro-inflammatory cytokines such as IL-1β and IL-18, disrupting the maternal-fetal interface (Shi et al., 2015). NLRP3 activation contributes to implantation failure by inducing excessive inflammation. Study shown that in pregnancy failure models, NLRP3 activation increases IL-1β release, which reduces embryo survival. In RSA, this may impair trophoblast invasion and increase macrophage activity, hindering successful implantation (Gao et al., 2020). Molecularly, RSA is associated with upregulated NLRP3, ASC, and caspase-1 in placental tissues, along with altered GSDMD cleavage. Maternal circulation also shows elevated IL-1β and IL-18, disrupting placental vascular remodeling and nutrient supply (D’Ippolito et al., 2016). In recent research, pyroptosis was found essential in the microenvironment of the chorionic villus tissue of women with RSA (Wang et al., 2024). A total of 16 genes were found associated with pyroptosis in the GSE22490 and GSE76862 placental datasets (Wang et al., 2024). It was also found that BTK, TLR8, NLRC4, and TNFSF13B were closely related to immune cells in the RSA placenta tissue (Wang et al., 2024). Exposure to BPDE stimulates the Inc-HZ14/ZBP1/NLRP3 axis, which in turn causes miscarriage and trophoblast cell pyroptosis (Wang et al., 2023). One of the pathogenesis in RSA was facilitated by the activated NLRP3 inflammasome by regulated Th17 and T cell imbalance (Lu et al., 2019). Novel treatment therapy for targeting NLRP3 may help reduce miscarriage rates and improve neonatal prognosis (Zhou, Li & Zhang, 2020; Zhao et al., 2024). So far, a number of promising inflammasome complex activation inhibitors have been described such as MCC950 and beta hydroxybutyrate, which can affect NALP3 expression (Di Nicuolo et al., 2018).
Pyroptosis and fetal growth restriction
Fetal growth rate (FGR) is a condition that affects 5–10% of pregnancies and is a leading cause of fetal mortality (Nardozza et al., 2017). There is high expression of pyroptosis-related factors such as NLRP7, IL-1β, ASC, and cleaved caspase-1 in the placenta of pregnant women with FGR (Abi Nahed et al., 2019). The exposure of prenatal IL-1 has shown adverse reactions and effects on the development during the perinatal period in mice (Nadeau-Vallée et al., 2017). The maternal serum GSDMD concentration and its mediated placental pyroptosis were found higher in pregnant women who were complicated by intrauterine growth restriction (Kobal et al., 2023). Diabetic pregnant mice had increased expression of chemerin, which could induce the activation of pyroptosis and a cognitive disorder in the brain of the fetal mice (Liang et al., 2019). By contrast, docosahexaenoic acid could improve cognitive impairment by attenuating hippocampal pyroptosis in rats with intrauterine growth restriction (Wan et al., 2023).
Pyroptosis and bronchopulmonary dysplasia
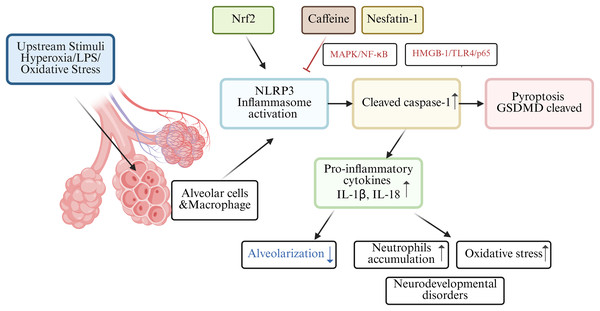
Among those premature infants, bronchopulmonary dysplasia (BPD) is a familiar clinical abnormality in preterm infants. In the BPD mouse model, nuclear factor e2-related factor 2 (Nrf2) may lead to the activation of the NLRP3 inflammasome, which acts as one of the molecular mechanisms of pyroptosis in BPD pathogenesis (Wang et al., 2023). Nrf2 deficiency increased pyroptosis and BPD in Nrf2-/-mice with intrauterine growth restriction by interrupting GSDMD transcription (Chen et al., 2024). Rapamycin reduced LPS-induced pyroptosis in neonatal rats with BPD by inhibiting mTOR phosphorylation and inducing autophagy (Zhang et al., 2023). Infants with BPD often suffer from lung and brain damage, leading to long-term neurodevelopmental disorders. A study involving C57/BL6 mouse pups revealed high oxygen-activated NLRP1 inflammasome and increased the expression of IL-1β and GSDMD, which are key inflammasomes involved in lung and brain tissue pyroptosis (Dapaah-Siakwan et al., 2019).
The NLRP3 inflammasome, triggers caspase-1-mediated pyroptosis, leading to the release of pro-inflammatory cytokines (IL-1β and IL-18) and decreased alveolarization (Liao et al., 2015). Excessive release of proinflammatory cytokines, particularly IL-1β, from alveolar macrophages is believed to play a key role in impairing alveolar development in BPD patients (Ryan, Ahmed & Lakshminrusimha, 2008). Interestingly, research found caffeine could significantly reduce NLRP3 expression in LSP-1induced THP-1 macrophage by suppressing MAPK/NF-κB signaling and A2aR-mediated ROS production (Zhao et al., 2019). In both rats models and alveolar epithelial cell line study, nesfatin-1 reduced neutrophils accumulation and inhibited inflammatory response by modulating HMGB-1/TLR4/p65/NLRP3 signal pathway in BPD (Yang, Luo & Lou, 2025). Hydrogen has been shown to alleviate cellular injury by suppressing the inflammatory response and oxidative stress, which is mediated through the Nrf2-dependent regulation of NLRP3 and NF-κB signaling pathways (Hu, Wang & Han, 2022). A holistic understanding of pyroptosis in BPD as shown in Fig. 3.
Figure 3: Schematic diagram of pyroptosis in BPD.
Pyroptosis with preterm birth
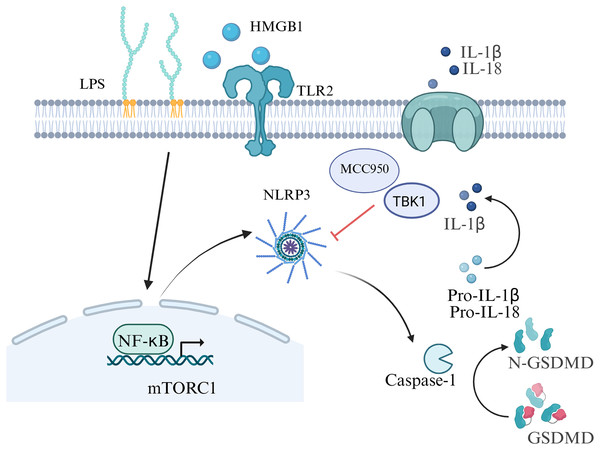
Preterm birth refers to the birth of a baby before 37 weeks of pregnancy, the rate range of preterm birth is 4–16% (Vogel et al., 2018), which is the main cause of neonatal mortality (Perin et al., 2022). There are multiple causes of preterm birth. Most preterm births occur spontaneously, but there are also some caused by pathological factors such as infections or other pregnancy-related complications. Among them, the inflammatory response at the maternal-fetal interface is a key factor in the occurrence of preterm birth (Humberg et al., 2020). Gotsch et al. (2008) first discovered the relationship between cell pyroptosis and the initiation of labor in 2008. There is sufficient evidence that inflammasomes, specifically NLRP3, play a vital role in preterm birth (Motomura et al., 2022; Galaz et al., 2023). In a study, incubation of chorioamniotic membranes with HMGB1 (mimicking preterm birth) increased mature IL-1β, IL-6, and mRNA levels of proinflammatory mediators (NFKB1, IL6, TNF, etc.), HMGB1 receptors (RAGE, TLR2), inflammasome components (NLRP3, AIM2), cleaved caspase-1, and MMP-9 (Plazyo et al., 2016). Inflammation in the amniotic fluid is one of the key factors in preterm birth, including both sterile and non-sterile inflammatory responses (Gomez-Lopez et al., 2019). Women delivering pre-term with intra-amniotic infection had increased levels of GSDMD, caspase-1 and IL-1β than those women delivering full-term (Gomez-Lopez et al., 2019). Gotsch et al. (2008) also showed that the level of caspase-1 in the amniotic fluid of spontaneously preterm pregnant women with combined intrauterine infection was markedly higher than that for spontaneously preterm birth in women without combined infection. In the placental tissues of human spontaneous preterm labor with acute histological chorioamnionitis, upregulation of NLRP3, caspase-4, IL-1β and IL-18 expression were observed, which indicating the activation of pyroptosis pathways in preterm labor (Gomez-Lopez et al., 2017). Zhu et al. (2021) also showed elevated NLRP1, NLRP3, AIM2, and NLRC4 inflammasome activation in the placenta tissue of women with premature rupture of membranes. Besides, women with sterile intra-amniotic inflammation had a lower level of GSDMD in their amniotic fluid than those women with intra-amniotic infection, and its expression in the chorioamniotic membranes was related to IL-1β and caspase-1 (Gomez-Lopez et al., 2019). Consequently, both the canonical and noncanonical pathways of pyroptosis are involved in the occurrence of preterm birth.
Inhibition of inflammasome activation may be a new direction for future research on preterm birth prevention and treatment. In an animal model of LPS-induced intra-amniotic inflammation, the NLRP3 inflammasome in the fetal membranes and decidua of the preterm birth group was notably increased compared with the control group (Faro et al., 2019) . After further treatment with the NLRP3 inhibitor MCC950, the birth rate was decreased in the preterm birth group, which improved the fetal outcome in an in vivo study (Faro et al., 2019). Lee et al. (2021) also provided evidence for the role of LPS-induced NLRP3 inflammasome in placental inflammation, indicating that targeting TBK1 attenuates LPS-induced NLRP3 inflammasome activation by regulating the mTORC1 pathways in trophoblast cells. The authors indicated that the rate of preterm birth induced by trophoblast inflammation could be decreased by omega-3 fatty acids (Chen et al., 2018). The aforementioned results provide new insights for the mechanism of premature birth, which offering possibilities for the development of drugs targeting pyroptosis and inflammasome to prevent premature birth in the future (Fig. 4).
Figure 4: Signaling pathways of pyroptosis in preterm birth.
Pyroptosis and GDM
GDM is one of the most common complications of pregnancy-related metabolic diseases during pregnancy, which not only increases the risk of PE, type 2 diabetes and others, but also has an adverse impact on embryonic intrauterine development and newborn health (Ye et al., 2022). Insulin resistance underlies the pathogenesis of GDM by impairing glucose homeostasis and leading to elevated blood glucose levels. Compared with healthy pregnant women, obese pregnant women or women with GDM tend to have more severe insulin resistance (Pantham, Aye & Powell, 2015). Changes in insulin sensitivity are a normal physiological state during pregnancy, which can lead to the pregnancy-induced insulin resistance (Liu et al., 2020). In maternal adipose tissue, increased protein expression of caspase-1 and IL-1β could be used as evidence of insulin resistance in women with GDM (Lappas, 2014). During pregnancy, inflammasomes are activated to produce proinflammatory cytokines such as IL-1β and IL-18, which lead to pyroptosis and insulin resistance (Gayatri et al., 2024). Han et al. (2024) proposed that the serum concentration of NLRP3 and the expression of its effector molecules, caspase-1, IL-1β, and IL-18, mid pregnancy can serve as biomarkers for predicting adverse pregnancy outcomes. Deficiency of hydrogen sulfide synthase in the placenta of women with GDM may lead to excessive activation of the NLRP3 inflammasome (Wu et al., 2022). Pyroptosis-associated inflammasomes have not only been found in the serum level of women with GDM, but also in the adipose tissue. Based on the evidence to date, TNFα, adipocyte fatty acid-binding protein, leptin, and adiponectin seem to be the closest adipokines related to the pathophysiology of GDM (Fasshauer, Blüher & Stumvoll, 2014). Through miRNA analysis of pregnant women’s plasma, it was found that 13 miRNAs were upregulated when NLRP3 increased, among which miR-106-5p and miR-210-3p were significantly increased in GDM (Monti et al., 2023). As a critical step in pyroptosis, the NLRP3 inflammasome, encompassing the priming signal model and activation signal model, plays a pivotal role in the occurrence, development and complication development of diabetes (Yu et al., 2020).
The correlation between pyroptosis and insulin resistance has been extensively reported (Lin et al., 2020; Wei & Cui, 2022). Inflammatory environment disorders of the placenta and maternal adipose tissue are the main factors of insulin resistance in GDM (Olmos-Ortiz et al., 2021). Han et al. (2024) described the elevated expression of pyroptosis factors in the serum of women with GDM compared with healthy pregnant women. Compared with the control group, the serum levels of caspase-1, IL-1β, IL-18, and NLRP3 in the GDM group were significantly increased (Han et al., 2024). In trophoblast, TP53-induced glycolysis and apoptosis regulator deficiency could induce inflammation and pyroptosis by activating the NLRP3-ASC-caspase1 signaling pathway (Guo et al., 2023). In contrast, targeting the expression of inflammasomes with anti-inflammatory drugs could effectively reduce the inflammation response in GDM (Fig. 5). Treatment with procyanidins could alleviate insulin resistance by reducing the activation of inflammatory via the NF-κB/NLRP3 signal pathway in a GDM mouse model (Liu et al., 2022). An increased expression of SPOCD1 in villi mesenchymal stem cells could attenuate glucose-induced pyroptosis through β-catenin in human umbilical cells, proposing a new therapeutic avenue for GDM treatment in the future (Wang et al., 2023).
Figure 5: Summary of the molecular mechanisms of pyroptosis-regulated cell death in GDM.
Pyroptosis in other pregnancy related diseases
Pyroptosis occurs in the maternal or fetal body caused by some biological factors such as infection, viral or parasitic inflammation. Congenital cytomegalovirus infection could trigger neurodevelopmental disorders, such as brain calcification and neuroinflammation, which pathological analysis shown cell pyroptosis (Zhou et al., 2022). Zika virus could induce adverse fetal outcomes by activating pyroptosis of placenta cells (Zhao et al., 2022). Immunohistochemical analysis showed increased expression of NLRP1, NLRP2, NLRP3, AIM2, IL-1 IL-1β, IL-18, and IL-33, and caspase 1 in Zika virus-positive stillborn brain tissue compared with controls (de Sousa et al., 2018). Inflammasomes are activated by Zika virus infection, leading to the activation of NLRP3-dependent pyroptosis (Wen et al., 2022). In addition, Monti et al. (2023) reported that SARS-CoV-2 infection could also increase the NLRP3-mediated pyroptosis in pregnant women which can induce adverse pregnancy outcomes. Nascimento & Alves-Filho (2023) described the key role of gut microbiota disorder during pregnancy in triggering excessive macrophage pyroptosis, which has raised concerns to the pathogenesis of sepsis. Quan et al. (2023) reported that Toxoplasma gondii infection induced ROS production and CatB activation in placental cells, which could trigger NLRP1/NLRP3/NLRC4/AIM2 inflammasome dependent caspase-1-mediated pyroptosis.
Subclinical hypothyroidism is one of the common diagnoses during pregnancy, as its prevalence in pregnant women is approximately 3.5% (Dong et al., 2020). The Chinese herb Bushen Antai could reduce inflammatory factors expression and improve TSH concentration by inactivating pyroptosis in a subclinical hypothyroidism rats study (He et al., 2022). In animal study, maternal hypothyroidism could activate pyroptosis by increasing GSDMD, NLRP3, and IL-1β at the maternal-fetal interface of rats (Santos et al., 2023). Smoking during pregnancy increases the risk of congenital fetal malformations. As report, in animal experiments, pregnant women exposure to cigarette smoke exacerbated congenital clubfoot in fetal mice (Lou et al., 2021).
Conclusion and future perspectives
Pyroptosis and inflammasomes have a ‘double-edged sword’ role in the process of pregnancy. Pyroptosis not only plays a role in the physiology of pregnancy, but the excessively activated inflammasomes are closely related to the onset of various pregnancy-related diseases. Abnormal expression of apoptosis-related signaling factors in the maternal serum could lead to immune homeostasis disorder of placental tissue, which resulting in adverse pregnancy outcomes. Therefore, numerous studies have focused on the role of pyroptosis in obstetrics and gynecology-related diseases, which providing potential strategies for treatment through research on relevant signaling pathways. Further research is required to identify the specific pathogenesis of pyroptosis and inflammasomes in pregnancy-related diseases, and provide insights into the prevention and management of such diseases by blocking or inhibiting their activation offering new directions for clinical intervention.