ASB3 ablation has no detectable effects on spermatogenesis and fertility in male mice
- Published
- Accepted
- Received
- Academic Editor
- Mahendra Tomar
- Subject Areas
- Biochemistry, Cell Biology, Developmental Biology, Genomics, Andrology
- Keywords
- Asb3, Testis, Spermatogenesis, Male fertility
- Copyright
- © 2025 Xu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. ASB3 ablation has no detectable effects on spermatogenesis and fertility in male mice. PeerJ 13:e19738 https://doi.org/10.7717/peerj.19738
Abstract
Background
As a member of the Ankyrin repeat and SOCS box (Asb) family, the Asb3 is enriched in the testes and highly conserved in multiple species. The knockout of the Asb12 gene not significantly affect spermatogenesis, but led to a compensatory increase in the mRNA expression level of the Asb3. Although it has been reported that the Asb12 is not required for spermatogenesis and male fertility in mice, the functional role of Asb3 remains not to be clearly elucidated.
Methods and results
Asb3 was predominantly expressed in mouse testis and primarily localized to the elongated spermatids, as determined by real-time fluorescent quantitative PCR and fluorescence in situ hybridization. The Asb3-KO mice were successfully generated using CRISPR Cas9 technology. Sperm quantity and motility from the cauda epididymidis were assessed via the hemocytometer. Histological analysis and immunostaining confirmed that normal fertility, normal spermatozoa and normal spermatogenesis in Asb3-KO mice. Additionally, no significant differences were observed between Asb3-KO mice and heterozygous mice regarding seminiferous tubule apoptosis via the TUNEL analysis.
Conclusions
There is no significant difference in fertility between Asb3-KO mice and heterozygous mice. Despite a significant increase in the relative mRNA expression level of the Asb3 gene due to the absence of the Asb12, the deficiency of ASB3 did not adversely affect fertility or spermatogenesis in males. Hence, we demonstrated that ASB3 ablation has no detectable effects on spermatogenesis and fertility in male mice.
Introduction
Spermatogenesis is a highly intricate, dynamic, and systematic process, mainly including the proliferation and differentiation of spermatogonia, meiosis of spermatocytes, and the formation of mature sperm (De Kretser et al. 1998; Neto et al., 2016). As transcriptional activity of the genome gradually decreases during spermatogenesis, the later stages of sperm development become increasingly reliant on protein post-translational modifications (PTMs) (Dai et al., 2019; Kang et al., 2022). Abnormal PTMs can disrupt spermatogenesis and impair male fertility (Azhar et al., 2023; Wu et al., 2022b). For instance, the knockout of Ttll3 and Ttll8 results in aberrant tubulin glycosylation, which reduces dynein activity in the axoneme of sperm flagella, leading to sperm circling and an inability to move forward in a straight line (Bosch Grau et al., 2013; Rocha et al., 2014).
Proteins involved in spermatogenesis undergo various PTMs, including methylation, phosphorylation, ubiquitination, glycosylation, palmitoylation, SUMOylation and acetylation (Azhar et al., 2023; Gou et al., 2017; Goudarzi et al., 2016; Lei et al., 2021; Rocha et al., 2014; Rodriguez & Pangas, 2016; Zhang et al., 2023). Ubiquitination, distinct from lysosomal autophagy, is a crucial pathway for protein degradation in eukaryotic cells (Xiong, Yu & Zhang, 2022). Ubiquitination plays a critical role in spermatogenesis, stabilizing and maintaining protein activity, reshaping and renewing cells, eliminating misfolded proteins, and degrading proteins that are no longer required (Bose et al., 2014; Gou et al., 2017; Guo et al., 2018; Pohl & Dikic, 2019; Suresh et al., 2015; Zhao et al., 2023). The ubiquitin-proteasome system (UPS) is primarily composed of ubiquitin (Ub), ubiquitin-activating enzyme (E1), ubiquitin conjugating enzyme (E2), ubiquitin ligase enzyme (E3), deubiquitinating enzymes (DUBs) and the 26S proteasome (Hou & Yang, 2013). For instance, the CEP78 stabilizes sperm junction fragments through ubiquitination and mediates the degradation of tektins during sperm flagellar formation. The absence of CEP78 leads to a loss of male fertility (Zhang et al., 2022b); ARRDC binds to E3 ubiquitin ligase to participate in the ubiquitination modification of spermatogenesis. The absence of ARRDC5 prevents the conversion of round spermatids into mature sperm, resulting in the inability of sperm in the cauda epididymis to complete capacitation (Giassetti et al., 2023); PHF7, an E3 ubiquitin ligase for histone H3, mediates histone ubiquitination and regulates the stability of BRDT. Deficiency in PHF7 results in sperm dysfunction and abnormal nuclear condensation in elongated spermatids (Kim et al., 2020).
The Ankyrin repeat and SOCS box (Asb) protein family contains two typical functional domains: the ankyrin repeat (ANK) domain at the N-terminus and the SOCS box at the C-terminus, which serves as a cytokine signaling inhibitor (Kile et al., 2002; Piessevaux et al., 2008). The ANK domain is essential for substrate recognition, while the SOCS box recruits elongin B/C, Cullin-2/5, and RING-box protein Rbx1/2 to form the elongin-Cul-SOCS (ECS) E3 ubiquitin ligase complex, promoting the polyubiquitination of target proteins and subsequent degradation via the proteasome (Kile et al., 2002; Liu, Verhaar & Peppelenbosch, 2019). The ASB protein family contains 18 members in both humans and mice (Piessevaux et al., 2008). Previous studies have shown that ASB proteins are enriched in mouse testes and play a crucial role in spermatogenesis. For instance, the knockout of Asb1 impairs spermatogenic function and significantly reduces germ cell numbers in the seminiferous tubules (Kile et al., 2001); Asb8 plays a significant role in the later stages of sperm differentiation (Chiba et al., 2007); During the pachytene phase of spermatocyte development, there is a significant increase in the expression level of Asb9, which is involved in the second meiotic division and the restructuring of haploid round spermatids into mature sperm (Lee et al., 2008); ASB17 promotes the ubiquitination and degradation of BCLW and MCL1, thereby regulating the apoptosis of differentiated germ cells. The absence of ASB17 leads to the excessive accumulation of ESPN, preventing timely degradation of the ES junction between Sertoli cells and elongated spermatids (Shen et al., 2021).
Based on our team’s previous research, the knockout of the Asb12 gene did not significantly affect spermatogenesis. However, surprisingly, the knockout of the Asb12 led to a compensatory increase in the mRNA expression level of the Asb3 (Zhang et al., 2022a). Therefore, we speculate that there is a potential mutual compensation mechanism between ASB12 and ASB3. Although ASB3 is one of the 18 members of the ASB protein family and is highly conserved and preferentially expressed in mouse testis, its function in mouse spermatogenesis has not been thoroughly analyzed (Kile et al., 2000; Zhang et al., 2022a). In this study, we have generated a Asb3-knockout mouse model to investigate whether Asb3 is involved in spermatogenesis in mice.
Materials and Methods
Animals
The experimental C57BL/6J mice were purchased and raised in the Experimental Animal Center of Nanjing Medical University. The professional keepers at the animal center regularly assessed the physical condition of the experimental mice and provided them with care. The mice are randomly assigned to cages, with no more than 5 mice in each cage. The mice were maintained under standard conditions (50–70% humidity; 20–22 °C; 12-hour light/dark cycle), provided with ample food and drinking water, and kept on clean and dry bedding. All animals used in the study were at least eight weeks old, mature in male reproductive development, and in good physical condition. After each experiment, mice were sacrificed using CO2 and according to the ethical guidelines for animal experiments. Experiments were conducted under a project license (IACUC-2402015) issued by the Animal Ethics and Welfare Committee, in compliance with the guidelines of the Institutional Animal Care and Use Committee of Nanjing Medical University.
Generation of Asb3-KO mice
We utilized CRISPR-Cas9 gene editing technology to generate the Asb3 knockout model of C57BL/6J mice. Four guide RNA (gRNA) target sequences were designed to direct Cas9 nuclease to cleave double-stranded DNA, resulting in amino acid sequence deletions, which ultimately led to frameshift mutations and premature termination of protein translation. The four single-guide (sg)RNA sequences were as follows: 5′-TCTCTGATACGGGTTGACTAAGG-3′, 5′-GTGAGGTTGATAATGCCGGATGG-3′, 5′-ACTAGATAAGTACAGGTCTAGGG-3′and 5′-AGAAGTGAGGTTGATAATGCCGG-3′.
Genotyping
Mouse genomic DNA was extracted from toe or tail tissue, and the absence of the targeted amino acid segments was assessed via PCR amplification and agarose gel electrophoresis. The following primers were used to identify the founders (F0) of the Asb3−/− edited mice: Primer F1:5′-TCTGACTCTTAGAATCTTCCCACCTCT-3′, Primer R1:5′-GAAGCA GTCAGCACCTTACTTGAT-3′ and R2:5′-GCAGCTGAGTGTGCAAGAAGGTATA-3′. The thermal cycling conditions were as follows: initial denaturation at 94 °C for 3 min; 35 cycles of 94 °C for 30 s, 60 °C for 35 s and 72 °C for 35 s; followed by a final extension at 72 °C for 5 min. Sanger DNA sequencing was performed on Asb3−/− mice, and the results were visualized using SnapGene (version 1.1.3). Each mouse was labeled with a unique genotype, gender, date of birth, and parentage information.
Fertility test
The fertility tests for male mice were conducted by mating adult Asb3−/− male mice with wild-type female mice in a 1:2 ratio with the Asb3+/− male mice serving as the control group. Six adult female wild-type mice were randomly allocated into three cages, with two mice per cage, and acclimatized to the strange environment for a predetermined period. During the nocturnal phase, male knockout mice were introduced into the female mouse cages at a 1:2 male-to-female ratio for co-housing. Vaginal plug inspection was initiated approximately 12 h post-cohabitation, typically in the morning. In the absence of detectable plugs, subsequent examinations were conducted at regular intervals, with the entire observation period not exceeding 24 h. Data on the litter were collected. To meet statistical standards, the fertility tests were repeated in triplicate.
RNA extraction and real-time reverse transcription quantitative polymerase chain reaction
Total RNA was extracted from various tissues and the testicular tissues of each week-old using the TRIzol™ Reagent (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. RNA extraction was performed in an RNase-free environment. Subsequently, the total RNA was extracted from various tissues including heart, liver, spleen, lung, kidney, brain, muscle, testis, adipose tissue, ovary, uterus, intestine, and stomach. mRNA quantification was performed using a spectrophotometer, and equal amounts of mRNA (one µg) were reverse-transcribed into cDNA using a reverse transcription system according to the manufacturer’s protocol. Quantitative real-time PCR (qPCR) was performed in a 20 µl reaction volume containing: 10 µl of 2 × SYBR Green Master Mix (Applied Biosystems, Waltham, MA, USA), 0.4 µl of each forward and reverse primer (10 µM), one µl of cDNA template, and nuclease-free water to adjust the final volume to 20 µl. The thermal cycling conditions were as follows: initial denaturation at 95 °C for 30 s, followed by 40 cycles of amplification (95 °C for 10 s and 60 °C for 30 s), and a final melting curve analysis using the instrument’s default program. Gene-specific primers were designed for Asb3 amplification, generating a 192-bp product with the following sequences: the forward primer: 5′-CAGCAGCTCTTGGGTTGATT-3′, and the reverse primer: 5′-CTGGCCCGAATTTCCAAACG-3′. The 18S rRNA was used as an internal control with the following primer sequences: the forward primer: 5′-AAACGGCTACCACATCCAAG-3′ and the reverse primer: 5′-CCTCCAATGGATCCTCGTTA-3′. In the primer design process for forward and reverse primers, we adhered to strict standards regarding primer length, guanine-cytosine (GC) content, and melting temperature (Tm) values. To ensure the primers’ specificity for our fluorescence—based quantitative PCR experiments, we employed the Primer—BLAST bioinformatics tool. This tool enabled us to conduct a comprehensive comparison of primer sequences against relevant genomic databases, thereby confirming that the primers would bind specifically to the target genes pertinent to reproductive research. All reactions were performed in triplicate, and the relative gene expression levels were calculated using the 2(−ΔΔCt) method.
Fluorescence in situ hybridization
Paraffin-embedded sections of testicular tissue were hydrated and washed once with 1× PBS. The hydrated sections were incubated in PBST (containing 0.1% Triton X-100) for 20 min and washed three times with 1× PBS, each for 5 min. Each slide was pre-hybridized with 200 µL of pre-hybridization solution (prepared by mixing Blocking Solution and Pre-hybridization Buffer in a 1:99 ratio) at 37 °C for 30 min. Under dark conditions, 2.5 µL of 20 µM nucleic acid probe stock solution was added to 100 µL of hybridization solution, and the pre-hybridization solution was removed. The slides were then hybridized overnight at 37 °C with the probe-containing hybridization solution. The following day, the slides were washed three times with Hybridization Wash I (4× SSC, 0.1% Tween-20), once with Hybridization Wash II (2× SSC), and once with Hybridization Wash III (2× SSC). Finally, the slides were washed once with 1× PBS for 5 min, stained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) for 5 min, and mounted with glycerol. Fluorescent signals were observed using LSM800 confocal fluorescence microscopy (Carl Zeiss AG, Jena, Germany). The Fluorescence in situ hybridization (FISH) kit and the specific probe designed for Asb3 were purchased from RiboBio (Guangzhou, China).
Epididymal sperm analysis
Epididymal sperm from Asb3+/− and Asb3−/− mice were analyzed using a hemocytometer, focusing on the quantity and quality of sperm in the cauda epididymidis. The epididymal sacs were cut, and sperm were extruded and suspended in human fallopian tube liquid culture medium (In Vitro Care, Frederick, MD, USA) and maintained at 37 °C for 30 min in a water bath. A 10 µl aliquot of the sperm suspension was loaded onto a hemocytometer, covered with a cover slip, and examined under a Primo Star microscope (Zeiss) at 400× magnification to count and assess sperm. The integrity of the sperm membrane was evaluated using the hypo-osmotic swelling test (HOST), which serves as an indirect method for assessing sperm viability. In this procedure, a minimum of 200 sperm cells were randomly counted to ensure statistical reliability. For the assessment of sperm motility, we placed the slide on a 37 °C thermostat of the microscope. The same microscopic field of view was observed for 2–3 s, during which at least 200 sperm cells were randomly counted. These sperm cells were then classified based on their movement trajectories and velocities. Sperm with progressive motility were classified when their heads moved in a straight line or in large circular patterns at a velocity of ≥25 µm/s (approximately half the sperm length/s). Non-progressive motility was assigned to sperm whose heads exhibited oscillatory movements, moved in small circles, or showed only flagellar undulations without forward progression, with velocities <25 µm/s. Sperm were classified as immotile when they remained completely stationary or displayed only minimal tail fluttering. Each experiment requires a pair of Asb3+/− and Asb3−/− mice over 8 weeks old. Experiments were performed on three experimental units with identical genotypes to ensure statistical significance.
Histological analysis
Tissue samples were fixed in modified Davidson’s fluid, dehydrated, and embedded in paraffin. Sections (five µm thick) were cut, mounted on glass slides, deparaffinized with xylene, and stained with hematoxylin and eosin (H&E). Both testis and epididymis were extracted for light microscope assessment. Murine epididymis and testes tissues were fixed in modified Davidson’s fluid overnight, followed by dehydration in a graded ethanol series (70%, 80%, 90%, and 100%). After paraffin embedding, five µm-thick sections were cut, mounted on glass slides, and deparaffinized with xylene. The sections were then stained with H&E. Additionally, smears of cauda epididymal sperm were fixed in PBS containing 4% paraformaldehyde for 30 min, rinsed, and stained with H&E.
Immunofluorescence analysis
Paraffin-embedded sections of testicular tissue from Asb3+/− and Asb3−/− mice were deparaffinized and rehydrated. Antigen retrieval was performed by boiling the sections in sodium citrate buffer for 10 min. The slides were cooled, washed three times with peripheral blood smear (PBS) for 5 min each, and blocked with antibody dilution buffer (1% bovine serum albumin in PBS) at room temperature for 2 h. The sections were then incubated with primary antibodies overnight at 4 °C. After washing with PBST (0.05% Tween-20 in 1× PBS) three times for 5 min each, the sections were incubated with secondary antibodies at room temperature for 2 h. Finally, the slides were stained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) for 5 min, mounted with glycerol, and imaged using LSM800 confocal fluorescence microscopy (Carl Zeiss AG, Jena, Germany).
Terminal deoxynucleotidyl transferase-dUTP nick-end labeling assay
Cell apoptosis levels were detected using the Terminal deoxynucleotidyl transferase-dUTP nick-end labeling (TUNEL) BrightRed Apoptosis Detection Kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions. After labeling with BrightRed labeling buffer, the control and knockout group sections were washed with PBS for 5 min and soaked and washed three times in a dark environment. The slides were then stained with Hoechst 33342 (Invitrogen, Carlsbad, CA, USA) for 5 min at room temperature, mounted with glycerol, and imaged using LSM800 confocal fluorescence microscopy (Carl Zeiss AG, Jena, Germany).
Statistical analysis
All data were statistically analyzed using Prism 8.0 software. The control group and the experimental group each consisted of three experimental units. Experiments were performed on three experimental units with identical genotypes to ensure statistical significance. Data are presented as mean ± standard deviation (SD). The differences between the Asb3+/− and the Asb3−/− group were compared using the unpaired two-tailed t-tests and non-parametric tests. A P value < 0.05 was considered statistically significant.
Results
Compensatory increase in Asb3 mRNA expression following the knockout of Asb12
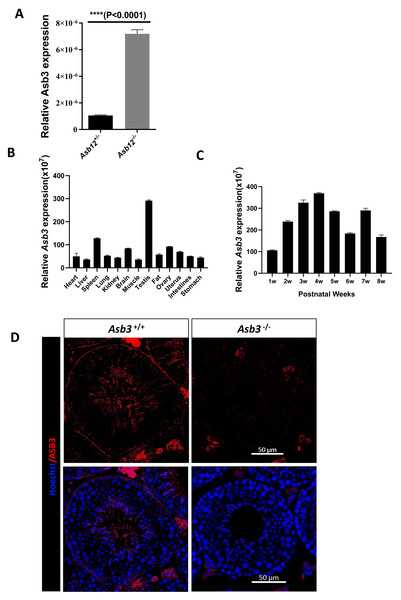
We initially performed real-time RT-qPCR to evaluate the relative transcription level of Asb3 in the testes of Asb12+/− and Asb12−/− mice. The results indicated that the mRNA expression level of Asb3 was significantly increased in the testes of Asb12 knockout mice compared to Asb12+/− mice (Fig. 1A), which was highly consistent with our previous study (Zhang et al., 2022a). The difference was statistically significant with the P value less than 0.05. These above results suggested that the knockout of Asb12 led to a compensatory increase in the relative expression level of Asb3 mRNA.
Figure 1: The mutual compensation mechanism, expression and localization of Asb3.
(A) The mRNA expression level of Asb3 was elevated in Asb12-KO mice. (B) Asb3 mRNA expression was assessed across various organs, with predominant expression observed in the testes. (C) The temporal expression pattern of Asb3 mRNA in the testis revealed that it began to be highly expressed from the fourth week of age. (D) The fluorescence in situ hybridization of Asb3, which was localized to the elongated spermatids in the testis. Nonspecific red fluorescence signals from interstitial cells, present both outside the seminiferous tubules and in testicular sections of wild-type and KO mice, were also observed. Scale bar: 50 µm.Tissue-specific expression and localization of Asb3
We used real-time fluorescent quantitative PCR to detect Asb3 mRNA expression across various organs and at different stages of mouse testes. The RT-PCR analysis revealed that Asb3 was predominantly expressed in the testes (Fig. 1B). In the testes of mice at each week of age, Asb3 gradually increased in the first week, and reached the peak in the fourth week (Fig. 1C). To determine the cellular localization of ASB3/Asb 3 within the mouse testis, we conducted fluorescence in situ hybridization (FISH) and confirmed the immunofluorescence specificity by comparing it with Asb3-KO mice as a negative control. Our findings showed that Asb3 was primarily localized to elongated spermatids in wild-type (WT) mice, while no signal was detected in Asb3-KO mice (Fig. 1D). Based on these observations, we hypothesized that Asb3 may play a functional role in spermatogenesis.
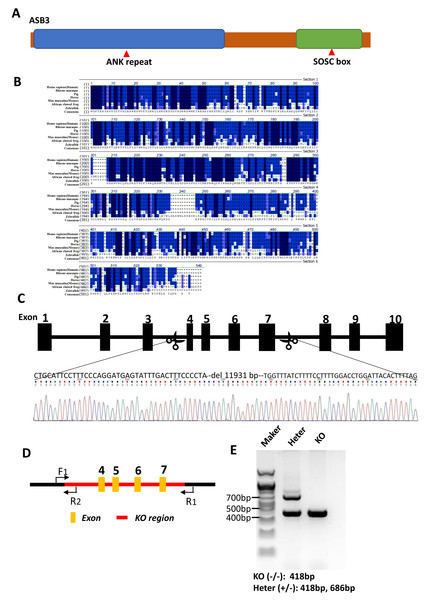
Figure 2: Generation of Asb3-KO mice.
(A) Schematic representation of the ASB3 protein structure, highlighting its typical functional domains, including the ankyrin (ANK) repeat and suppressor of cytokine signaling (SOCS) box. (B) Sequence alignment of ASB3 across multiple species, indicating high conservation. Dark blue shading denotes identical residues, while light blue represents weakly similar residues. (C) CRISPR-Cas9-mediated gene editing strategy for generating Asb3-KO mice, with black boxes representing exons. (D) Primer design for polymerase chain reaction (PCR) verification of the knockout fragments. F1 indicates the first forward primer, R1 and R2 are the first and second reverse primers, respectively. Orange boxes represent exons, and the red box highlights the knockout region. (E) PCR amplification and agarose gel electrophoresis results (above). Asb3−/− mice showed a single fragment of 418 bp, whereas Asb3+/− mice exhibited two fragments of 418 bp and 686 bp, respectively (below).Evolutionary conservation and successful generation of Asb3-KO mice
As the member of the ASB family, ASB3 features N-terminal ankyrin (ANK) repeats and a C-terminal suppressor of cytokine signaling (SOCS) box (Fig. 2A). A protein sequence alignment demonstrated that Asb3 is an evolutionarily conserved gene present in Human, Rhesus macaque, Pig, Horse, Mouse, African clawed frog and Zebrafish (Fig. 2B). We successfully generated Asb3-KO mice by CRISPR-Cas9, which resulted in a 11,931 bp deletion between exon 3 and exon 8, forming a frameshift mutation (Fig. 2C). The successful generation of Asb3-KO mice was confirmed through sequencing of PCR products and agarose gel electrophoresis band analysis (Figs. 2D–2E).
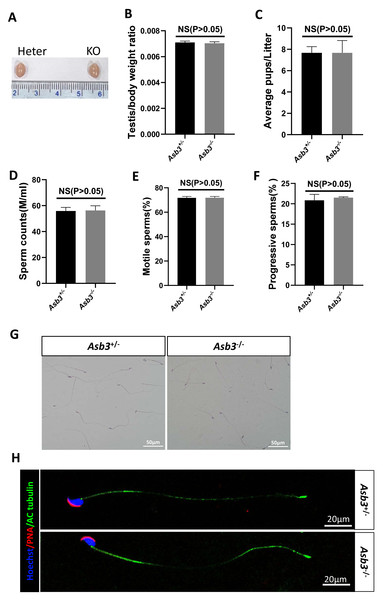
Figure 3: Evaluation of fertility in Asb3-KO mice.
(A) Gross appearance of testes from heterozygous and Asb3-KO mice; (B) Average testis weight/body weight, n = 3, P = 0.4918, 95% confidence interval: −0.0003115 and 0.0001782; (C) Average pups per litter for heterozygous and KO mice, n = 3, P = 0.400, 95% confidence interval: −2.069 and 2.069; (D) Sperm counts from heterozygous and KO mice, n = 3, P = 0.8780, 95% confidence interval:−6.869 and 7.729; (E) Sperm motility in heterozygous and KO mice, n = 3, P = 0.9480, 95% confidence interval: −2.600 and 2.733; (F) Progressive sperm from heterozygous and KO mice, n = 3, P = 0.4972, 95% confidence interval: −1.724 and 2.991; (G) H&E staining of sperm smears from heterozygous and KO mice. Scale bar: 50 µm; (H) Fluorescent detection of AC-tubulin (green), PNA (red) and Hoechst 33342 (blue) from heterozygous and KO mice spermatozoa. Scale bar: 20 µm.Normal fertility and normal spermatozoa in Asb3-KO mice
Male Asb3-KO mice were viable and exhibited normal fertility. Compared with heterozygous mice, the adult Asb3-KO mice appeared normal morphology of the testicles. The p-value for the average testis weight/body weight was 0.4918, indicating no significant difference in testicular weight (n = 3) (Figs. 3A–3B). Then, through fertility tests, we found that the p-value for the average number of pups per litter was 0.400, indicating no significant difference in the average number of pups per litter between the Asb3+/− and the Asb3−/− mice (Fig. 3C). Subsequently, the sperm quality, including sperm count, motility, and progressive motility, was assessed using a hemocytometer, and the p-values for these parameters were 0.8780, 0.9480 and 0.4972, respectively (Figs. 3D–3F). Additionally, the proportion of normal sperm in male Asb3-KO mice was comparable to that of the control group (Figs. 3G–3H).
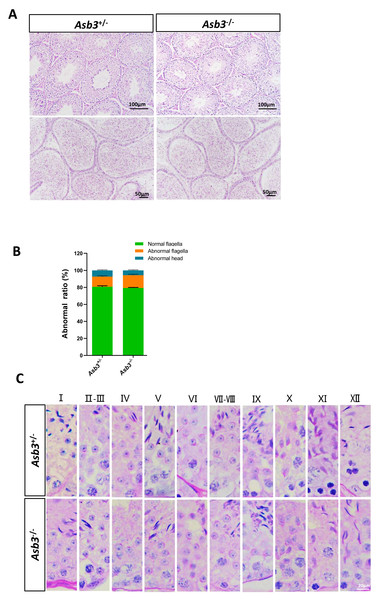
Figure 4: Normal spermatogenesis in Asb3.-KO mice.
(A) Stained sections of the seminiferous tubules and the cauda of the epididymis from heterozygous and Asb3-KO mice via hematoxylin and eosin staining. Scale bar for seminiferous tubules: 100 µm; Scale bar for the cauda of the epididymis for: 50 µm; (B) Abnormal epididymal sperm count from heterozygous and Asb3-KO mice, n = 3, P > 0.05. The green box for normal flagella, the orange box for abnormal flagella and the cyan box for abnormal head; (C) The epithelial cycle was divided into 12 stages recognized by PAS, according to changes in the acrosome and nuclear morphology of spermatids. Scale bar: 20 µm.Normal spermatogenesis in Asb3-KO mice
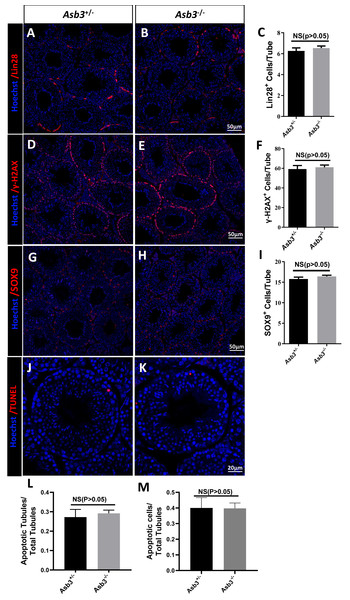
We next examined the seminiferous tubules and the cauda of the epididymis in the Asb3+/− and the Asb3−/− male mice, and observed that the spermatogenic cells in Asb3-KO mice were normal (Fig. 4A). H&E staining of sperm smears did not show any significant differences in sperm morphology between Asb3-KO and control mice (Fig. 4B). Additionally, histological analysis of the seminiferous tubules at all stages of the spermatogenetic cycle revealed the presence of post-meiotic round spermatocytes in Asb3-KO mice compared to heterozygous mice (Fig. 4C). Immunofluorescence staining with Lin28, γ-H2AX, PNA, and Sox9, along with quantitative analysis, was used to visualize spermatogonia, spermatocytes, spermatids, and Sertoli cells. The p-values for Lin28, γ-H2AX and Sox9 were 0.4817, 0.6992 and 0.3157, respectively (Figs. 5A–5I). Fluorescence images confirmed normal spermatogenesis in Asb3-KO mice. To assess the impact of ASB3 absence on germ cell apoptosis, TUNEL analysis was performed on testicular tissue slides. Although occasional red apoptotic signals were observed in the seminiferous tubules, there was no significant difference in the intensity of red fluorescence signals between Asb3-KO and heterozygous mice (Figs. 5J–5K). Further t-test analysis revealed that the p-values for apoptotic cells/total tubules and tubules/total tubules were 0.4750 and 0.9517 (Figs. 5L–5M).
Figure 5: Spermatogenic markers and apoptotic cells in Asb3-KO mice.
Lin28 (spermatogonia marker) immunostaining in testis sections from both (A) heterozygous and (B) Asb3-KO mice. (C) Quantification of Lin28-positive cells in testis sections from heterozygous and Asb3-KO mice, n = 3, P = 0.4817, 95% confidence interval: −0.7061 and 1.253, Scale bar = 50 µm; γ-H2AX (spermatocyte marker) immunostaining in testis sectionsfrom both (D) heterozygous and (E) Asb3-KO mice. (F) Quantification of γ-H2AX-positive cells in testis sections from heterozygous and Asb3-KO mice, n = 3, P = 0.6992, 95% confidence interval: −10.23 and 13.83, Scale bar = 50 µm; Sox9 (Sertoli cell marker) immunostaining in testis sections from both (G) heterozygous and (H) Asb3-KO mice. (I) Quantification of Sox9-positive cells in testis sections from heterozygous and Asb3-KO mice, n = 3, P = 0.3157, 95% confidence interval: −0.8916 and 2.1145, Scale bar = 50 µm; TUNEL analysis of testicular sections from (J) heterozygous and (K) Asb3-KO mice. (L) Average number of apoptotic tubules per seminiferous tubule; (M) Average number of apoptotic cells per seminiferous tubules, n = 3, P = 0.9517, 95% confidence interval: −0.1225 and 0.1170, Scale bar = 20 µm.Discussion
The ubiquitin-proteasome system (UPS) plays a critical role in spermatogenesis through selective protein degradation and the precise regulation of mitosis in spermatogonia, meiosis in spermatocytes, and sperm cell morphogenesis (Forné et al. 2011; Pohl & Dikic, 2019). The UPS accurately regulates key enzymes that recognize substrates, guiding germ cells to differentiate into spermatogonia (Manku, Wing & Culty, 2012). UPS is involved in the processes of mitosis and meiosis of spermatogonia, including the transition from mitosis to meiosis, which is mediated by ubiquitin-activating enzymes, and the differentiation and self-renewal of spermatogonia, which are controlled by ubiquitin ligases (Fok et al., 2017; Hogarth et al., 2011; Liu et al., 2007). Additionally, the UPS participates in the repair of DNA damage during spermatogenesis, ensuring the genetic stability of chromosomes (Gou et al., 2017; Lu et al., 2010; Zhang et al., 2021). Ubiquitination facilitates the rapid degradation of target proteins, enabling their conversion into new structural protein molecules, such as acrosomal vesicles formed by the fusion of Golgi complex vesicles (Shen et al., 2021). Ubiquitin modification also allows for the degradation of cytoplasm, preventing the accumulation of excess cytoplasmic residues and proteins in mature sperm cells. Despite the essential role of ubiquitination in spermatogenesis, the specific localization and mechanisms of ubiquitination proteins in this process remain poorly understood.
Existing researches have confirmed that ASB1, ASB8, ASB9, and ASB17 are all involved in ubiquitination modification during spermatogenesis (Hogarth et al., 2011; Kile et al., 2001; Lee et al., 2008; Shen et al., 2021). The absence of these proteins can impact the spermatogenesis. However, the deletion of Asb12 and Asb15 does not compromise male fertility or lead to the arrest in sperm production (Wu et al., 2022a; Zhang et al., 2022a). Although Asb 12 is not essential for spermatogenesis and male fertility in mice, its knockout significantly increases the relative mRNA expression of Asb3 (Zhang et al., 2022a). These findings suggest a potential compensatory mechanism between ASB3 and ASB12, indicating that Asb3 may be involved in spermatogenesis. In this study, we successfully generated Asb3-KO mice. Through fertility tests and histological analysis, we found that the absence of ASB3 did not impair male fertility or affect the spermatogenesis. Thus, we confirmed that ASB3 ablation has no detectable effects on spermatogenesis and fertility in male mice, indicating that not all members of the ASB protein family serve irreplaceable specialized functions. Although the deletion of Asb12 and Asb3 had no impact on mouse fertility, it cannot be ruled out that there are compensatory mechanisms among some ASB family members. Subsequent “double knockout” and “triple knockout” studies are necessary to elucidate the synergistic mechanisms of ASB protein ubiquitination during spermatogenesis.
Existing studies primarily focus on phenotypic analysis using knockout models but lack detailed elucidation of the specific functions, localization, interaction sites, and signaling pathways of each protein component. Investigating the role of Asb3 in spermatogenesis provides deeper insight into the molecular mechanisms underlying protein ubiquitination during this process. Additionally, research into the mechanisms of E3 ubiquitin ligase inactivation, pathway interruption, functional interference, and other relevant ubiquitin-related inhibitors offers medical evidence and a foundational research framework for the development and translation of male contraceptives. Furthermore, this research serves as a reference point for subsequent clinical diagnosis and genetic screening, aiding researchers in conserving resources for further ubiquitination studies.
Conclusions
We found that the Asb3 was predominantly expressed in mouse testis and primarily localized to the elongated spermatids. We successfully constructed the Asb3-KO mice. Although ASB3 is one of the 18 members of the E3 ubiquitin ligase ASB protein family, its absence does not significantly affect spermatogenesis or male fertility. The study of ASB3 can provide a reference basis for the subsequent comprehensive analysis of ASB protein complexes and offers new insights into protein ubiquitination modifications during spermatogenesis.