Morphometric variation of extant platyrrhine molars: taxonomic implications for fossil platyrrhines
- Published
- Accepted
- Received
- Academic Editor
- Philip Reno
- Subject Areas
- Anthropology, Biodiversity, Evolutionary Studies, Zoology
- Keywords
- Molar shape, Platyrrhines, Geometric morphometric, Phylogeny
- Copyright
- © 2016 Nova Delgado et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2016. Morphometric variation of extant platyrrhine molars: taxonomic implications for fossil platyrrhines. PeerJ 4:e1967 https://doi.org/10.7717/peerj.1967
Abstract
The phylogenetic position of many fossil platyrrhines with respect to extant ones is not yet clear. Two main hypotheses have been proposed: the layered or successive radiations hypothesis suggests that Patagonian fossils are Middle Miocene stem platyrrhines lacking modern descendants, whereas the long lineage hypothesis argues for an evolutionary continuity of all fossil platyrrhines with the extant ones. Our geometric morphometric analysis of a 15 landmark-based configuration of platyrrhines’ first and second lower molars suggest that morphological stasis may explain the reduced molar shape variation observed. Platyrrhine lower molar shape might be a primitive retention of the ancestral state affected by strong ecological constraints throughout the radiation of the main platyrrhine families. The Patagonian fossil specimens showed two distinct morphological patterns of lower molars, Callicebus—like and Saguinus—like, which might be the precursors of the extant forms, whereas the Middle Miocene specimens, though showing morphological resemblances with the Patagonian fossils, also displayed new, derived molar patterns, Alouatta—like and Pitheciinae—like, thereby suggesting that despite the overall morphological stasis of molars, phenotypic diversification of molar shape was already settled during the Middle Miocene.
Introduction
Platyrrhine evolution is controversial. However, most researchers agree that they most likely constitute a monophyletic clade derived from African ancestors (Fleagle & Kay, 1997; Takai et al., 2000; Kay et al., 2004; Oliveira, Molina & Marroig, 2009; Bond et al., 2015), although the phylogenetic position of some living taxa and the affinities of some fossil specimens are still uncertain. Currently, two different viewpoints have been proposed regarding the evolutionary history of the earliest platyrrhines and their overall relationships with extant forms. The “long lineages” hypothesis argues that the oldest known Patagonian fossils (16–20 Ma) are to be included within the extant Platyrrhines (Rosenberger, 1979; Rosenberger, 1980; Rosenberger, 1981; Rosenberger, 1984; Rosenberger et al., 2009; Tejedor, 2013), whereas the “layered or successive radiations” hypothesis suggests that these fossils constitute a geographically isolated stem group, phylogenetically unrelated to the crown platyrrhines, that went extinct (along with some Antillean species) (Kay, 2010; Kay, 2014; Kay & Fleagle, 2010; Kay et al., 2008). According to Kay (2014), the divergence of modern lineages occurred in the tropics. The Late Oligocene and Early Miocene platyrrhines would have branched off from the ancestral lineage when climatic conditions in Patagonia became unfavorable and the Andean uplift was a potential barrier to their dispersal. However, Tejedor (2013) has suggested that Chilecebus (20 Ma), a fossil specimen (Tejedor, 2003) from the western Andean cordillera, south of Santiago de Chile, indicates that the Andean mountains did not constitute a biogeographic barrier. Tejedor (2013) argued that a paleobiogeographic corridor throughout western South America would have allowed for a continental connectivity between the north and the southernmost fossil platyrrhines. Unfortunately, dating of the fossil specimens and fossil-based approaches for calibrating the molecular phylogeny support both models. Perez et al. (2013) have estimated a crown platyrrhine origin at around 29 Ma (27–31), which allows for the inclusion of the fossil Patagonian primates into a crown Platyrrhini lineage showing evolutionary continuity with the Middle Miocene lineages. In contrast, Hodgson et al. (2009) have dated their origin between 16.8 and 23.4 Ma, suggesting an unlikely relationship of the early Miocene fossils with the crown platyrrhine clade (but see different temporal models in Goodman et al., 1998; Opazo et al., 2006; Chatterjee et al., 2009; Perelman et al., 2011; Wilkinson et al., 2011; Jameson Kiesling et al., 2014).
Molar morphology has been widely used to determine the phylogenetic positions of extinct specimens with respect to living forms (e.g., Kay, 1990; Rosenberger et al., 1991; Rosenberger, Setoguchi & Hartwig, 1991; Benefit, 1993; Meldrum & Kay, 1997; Miller & Simons, 1997; Horovitz & MacPhee, 1999; Kay & Cozzuol, 2006; Kay et al., 2008), since tooth development is under strong genetic control (Jernvall & Jung, 2000). Recent studies have reported that molar shapes carries strong phylogenetic signals, and can be a useful tool for establishing taxonomic affinities between extanct and extinct catarrhine primates (Nova Delgado et al., 2015a; Gamarra et al., 2016), and also in some Platyrrine taxa (Nova Delgado et al., 2015b) with closely related species exhibiting common phenotypic traits.
Affinities of the fossil platyrrhine primates based on dental morphology
Until now, a total of 31 Early Miocene Platyrrhini fossil genera have been reported in the South American continent and the Caribbean: 13 in La Venta (Colombia), eight in the Argentinian Patagonia, five in the Greater Antilles, five in Brazil, and one each in Chile, Bolivia and Peru (Tejedor, 2013; Bond et al., 2015). Neosaimiri, Laventiana (La Venta, Colombia) and Dolichocebus (Chubut Province, Argentina) have been included in Cebinae (Rosenberger, 2011). Neosaimiri is considered a direct ancestor of the extant Saimiri due to its similar molar shape (Rosenberger, Setoguchi & Shigehara, 1990; Rosenberger et al., 1991). Its molars exhibit sharp cusps, well-developed distal cusps, buccal cingulum, a strong buccal flare, and a distinct post-entoconid notch on molars only found in Saimiri and Laventiana (Rosenberger et al., 1991; Rosenberger, Setoguchi & Hartwig, 1991; Takai, 1994; Tejedor, 2008). Laventiana is sometimes considered a synonym of Neosaimiri (Takai, 1994; Meldrum & Kay, 1997), although it has been suggested to be more primitive than Neosaimiri (Rosenberger, Setoguchi & Hartwig, 1991). Laventiana’s teeth closely resemble those of Saimiri and Cebus-Sapajus; it shows thick-enamel bunodont molars exhibiting a small buccal cingulum and an angular cristid obliqua, lacking buccal flare (Rosenberger, Setoguchi & Hartwig, 1991). Dolichocebus has been suggested to be a member of the Saimiri lineage, mainly for its interorbital fenestra considered a derived feature in squirrel monkeys (Tejedor, 2008; Rosenberger et al., 2009; Rosenberger, 2010). However, Kay and colleagues (Kay et al., 2008; Kay & Fleagle, 2010) argued that Dolichocebus is a stem platyrrhine and that the description of the orbital region was probably affected by postmortem damage.
On the other hand, Aotus dindensis was first described as a sister taxon of extant Aotus (Setoguchi & Rosenberger, 1987), although Kay (1990) has suggested that it is probably conspecific with Mohanamico hershkovitzi, which may be closely related to the callitrichines, especially Callimico, due to their morphological similarities in the canine and the second premolar. Aotus dindensis is included into the Pitheciidae (Rosenberger, Setoguchi & Shigehara, 1990) within the Homunculinae subfamily, along with Aotus, Callicebus and some Argentinian and Caribbean fossil primates (Rosenberger, 1981; Rosenberger, 2002; Rosenberger, 2011). However, molecular phylogenetic analyses have repeatedly rejected a link between Aotus and Pitheciids (e.g., Hodgson et al., 2009; Osterholz, Walter & Roos, 2009; Wildman et al., 2009), placing it as a basal cebid. Tejedor & Rosenberger (2008) proposed that Homunculus is likely an ancestral pitheciid because although it shows a primitive dental morphology, it notably resembles that of Callicebus. The two taxa show rectangular-shaped molars, small incisors and non-projecting canines, a trait shared with Carlocebus (Fleagle, 1990). Nonetheless, unlike Callicebus, the molars of Homunculus exhibit well-marked crests and prominent cusps (Tejedor, 2013), and an unusual paraconid on the lower first molar (also found in Dolichocebus; Kay et al., 2008). Another fossil from the early Miocene known as Soriacebus was initially included as an early Pitheciinae (Rosenberger, Setoguchi & Shigehara, 1990), due to its resemblance on the anterior dentition (Fleagle et al., 1987; Fleagle, 1990; Fleagle & Tejedor, 2002; Tejedor, 2005). However, some dental traits of Soriacebus (premolars-molars size, lower molar trigonid, and reduced hypocone) bear resemblance also with the callitrichines. Indeed, Kay (1990) argues that such similarities found between Soriacebus and pitheciines or with callitrichines are due to homoplasy, rather than phylogenetic relationships among such lineages (Kay, 1990). According to Kay (1990), Soriacebus, Carlocebus, Homunculus and all Patagonian fossils should be considered stem platyrrhines.
Xenothrix is a Late Pleistocene Caribbean fossil from Jamaica that shows a callitrichine-like dental formula (2132; MacPhee & Horovitz, 2004), low relief molars and a narrowing of intercuspal distance and augmentation of the mesial and distal crown breadths (Cooke, Rosenberger & Turvey, 2011), a feature also seen in Insulacebus toussaintiana, another Caribbean primate. Rosenberger (2002) argued that Xenothrix is closely related to Aotus and Tremacebus by the enlargement of the orbits and the central incisors, while MacPhee & Horovitz (2004) suggested a possible Pitheciidae affinity, due to its low relief molar pattern. Nonetheless, the puffed cusps and the lack of crenulation on the molar crown discriminate the Jamaican fossil from the Pitheciidae, suggesting that it is likely that Xenothrix does not belong to crown platyrrhine group (Kay, 1990; Kinzey, 1992).
Cebupithecia and Nuciruptor, two Colombian Middle Miocene genera, also share some traits with the extant Pitheciidae family, mostly in the anterior dentition but also in their low molar cusps and poorly developed crests (Kay, 1990; Meldrum & Kay, 1997). Nuciruptor does not exhibit several of the shared traits among pitheciines (projecting canine and small or absent diastema). Cebupithecia, although considered to be more derived than Nuciruptor (Meldrum & Kay, 1997), was interpreted by Meldrum & Kay (1997) as an example of convergent evolution, and thus, not a direct ancestor of extant pitheciines. Finally, Stirtonia (originally from Colombia but also recovered from Acre State, Brazil) exhibits similar dental size and morphology to extant Alouatta; showing molar teeth with sharp and well-formed crests, a long cristid oblique, small trigonid, and spacious talonid basin (Hershkovitz, 1970; Kay et al., 1987; Kay & Frailey, 1993; Kay & Cozzuol, 2006; Kay, 2014).
Numerous studies have examined landmark-based geometric morphometrics (GM) of molar shape for studying patterns of inter-specific variation and their implication in phylogeny and ecological adaptations (e.g., Bailey, 2004; Cooke, 2011; Gómez-Robles et al., 2007; Gómez-Robles et al., 2008; Gómez-Robles et al., 2011; Martinón-Torres et al., 2006; Singleton et al., 2011; Nova Delgado et al., 2015a; Nova Delgado et al., 2015b; Gamarra et al., 2016). However, in Platyrrhine primates, GM of molar shape has mainly focused on dietary adaptations (Cooke, 2011), rather than to predict the phylogenetic attribution of unclassified specimens (Nova Delgado et al., 2015a).
The aim of the present study is to use two-dimensional (2D) GM to quantify and analyze occulsal shape variation of lower molars (M1 and M2) of extant Platyrrhini primates to asesses the affinities of the Patagonian, Colombian, and Antillanean fossil taxa with the extant forms and to estimating the efficiency of molar shape for discriminating fossil specimens.
Material and Methods
Images of the dental crowns, in occlusal view and including a scale line, of 12 holotype fossil platyrrhine specimens and one fossil from Fayum (Proteopithecus sylviae), were obtained from the literature (Table 2). The platyrrhine fossil specimens included 12 genera (Soriacebus, Dolichocebus, Homunculus, Carlocebus, Neosaimiri, Laventiana, Mohanamico, Aotus, Stirtonia, Nuciruptor, Cebupithecia, and Xenothrix), discovered in Argentina, Colombia, and Jamaica, and dated to between Holocene and early Miocene (Table 1).
| Fossils | Location | Age (Ma) | Phylogenetic position | Specimen number and reference |
|---|---|---|---|---|
| F1 Proteopithecus sylviae | Fayum, Egypt | 33.9–28.4a | Stem anthropoidb | CGM 42209; Miller & Simons (1997) |
| F2 Soriacebus spp. | Pinturas Formation, Santa Cruz Province, Argentina | 17c | Stem platyrrhined/Pitheciidaee | MACN-SC 21, MACN-SC 52 MPM-PV 363; Tejedor (2005) |
| F3 Dolichocebus gaimanesis | Gaiman, Chubut Province, Argentina | 20f | Stem platyrhine/sister to Saimirig | MPEF 5146; Kay et al. (2008) |
| F4 Homunculus spp. | Santa Cruz Formation, Santa Cruz Province, Argentina | 16.5h | Stem platyrrhine/Pitheciidae | MACN-A5969; Tejedor & Rosenberger (2008) |
| F5 Carlocebus spp. | Pinturas Formation, Santa Cruz Province, Argentina | 18–19i | Stem platyrrhine/Pitheciidae | MACN-SC 266; Fleagle (1990) |
| F6 Neosaimiri fieldsi | La Venta, Huila, Colombia | 13.5–11.8j | Sister to Saimirik | IGM-KU 890294, IGM-KU 890195, UCMP 392056, IGM-KU 890027, IGM-KU 390348, IGM-KU 890539, IGM-KU 8913010; Takai (1994) |
| F7 Laventiana annectens | La Venta, Huila, Colombia | 13.5–11.8 | Sister to Saimiri/synonymy with Neosaimiril | IGM-KU 880; Rosenberger, Setoguchi & Hartwig (1991) |
| F8 Mohanamico hershkouitzi | La Venta, Huila, Colombia | 13.5–11.8 | Sister to Callimicom | IGM 181500; Kay (1990) |
| F9 Aotus dindensis | La Venta, Huila, Colombia | 13.5–11.8 | Sister to Aotusn/coespecific with Mohanamicoo | IGM-KU 8601; Kay (1990) |
| F10 Stirtonia spp. | La Venta, Huila, Colombia | 13.5–11.8 | sister to Alouattap | UCPM 38989; Kay et al. (1987) |
| F11 Nuciruptor rubricae | La Venta, Huila, Colombia | 13.5–11.8 | Pitheciidaeq/stem Pitheciinaer | IGM 251074; Meldrum & Kay (1997) |
| F12 Cebupithecia sarmientoni | La Venta, Huila, Colombia | 13.5–11.8 | Pitheciidae/stem Pitheciinae | UCMP 38762; Meldrum & Kay (1997) |
| F13 Xenothrix macgregori | Jamaica | Holocenes stem platyrhine/retaded to Callicebust | AMNHM 148198; MacPhee & Horovitz (2004) |
Notes:
References used in the table: (Miller & Simons, 1997)a; (Kay, 1990)b; (Fleagle et al., 1987)c; (Kay, 2010; Kay, 2014r; Kay & Fleagle, 2010; Kay et al., 2008f); (Rosenberger, 1979g; Tejedor & Rosenberger, 2008h); (Rosenberger, 1979)g; (Fleagle, 1990)i; (Flynn, Guerrero & Swisher, 1997)j; (Rosenberger, Setoguchi & Hartwig, 1991)k; (Takai, 1994; Meldrum & Kay, 1997)l; (Rosenberger, Setoguchi & Shigehara, 1990)m; (Setoguchi & Rosenberger, 1987; Takai et al., 2009)n; Meldrum & Kay, 1997o,q; (e.g., Hershkovitz, 1970; Kay et al., 1987)p; (Cooke, Rosenberger & Turvey, 2011)s; (MacPhee & Horovitz, 2004)t.
- CGM
-
Cairo Geological Museum
- MPM-PV
-
Museo Regional Provincial Padre Manuel Jesús Molina, Río Gallegos, Argentina
- MPEF
-
Museo Paleontológico E. Feruglio, Trelew, Chubut Province, Argentina
- MACN, MACN-SC/A
-
Museo Argentino de Ciencias Naturales “Bernardino Rivadavia,” Buenos Aires, Argentina
- SC/A
-
denotes locality
- IGM, IGM-KU
-
Museo Geologico del Instituto Nacional de Investigaciones Geológico-Mineras, Bogota, Colombia
- KU
-
denotes Kyoto University
- UCPM
-
University of California Museum of Paleontology, Berkeley, California
- AMNHM
-
Division of Vertebrate Zoology Mammalogy, American Museum of Natural History
| Genus/species | M1−2 | Collectiona |
|---|---|---|
| Subfamily: Cebinae | ||
| Cebus (gracile capuchins) | ||
| 1 C. albifrons | 9 | MZUSP, MNRJ |
| 2 C. olivaceus | 6 | MNRJ |
| Sapajus (robust capuchins) | ||
| 3 S. apella | 14 | MZUSP |
| 4 S. libidinosus | 15 | MNRJ |
| 5 S. nigritus | 15 | MNRJ |
| 6 S. robustus | 15 | MNRJ |
| 7 S. xanthosternos | 7 | MNRJ |
| Subfamily: Samiriinae | ||
| Saimiri (squirrel monkeys) | ||
| 8 S. boliviensis | 17 | MZUSP, MNRJ |
| 9 S. sciureus | 25 | MZUSP, MNRJ |
| 10 S. ustus | 18 | MZUSP, MNRJ |
| 11 S. vanzolinii | 8 | MNRJ |
| Subfamily: Callitrichinae | ||
| Callithrix (marmosets from Atlantic Forest) | ||
| 12 C. aurita | 11 | MNRJ |
| 13 C. geoffroyi | 15 | MNRJ |
| 14 C. jacchus | 21 | MZUSP |
| 15 C. kuhlii | 20 | MNRJ |
| 16 C. penicillata | 14 | MNRJ |
| Mico (marmosets from Amazon) | ||
| 17 M. argentata | 21 | MZUSP, MNRJ |
| 18 M. chrysoleuca | 16 | MZUSP, MNRJ |
| 19 M. emiliae | 6 | MZUSP |
| 20 M. humeralifer | 16 | MZUSP |
| 21 M. melanurus | 8 | MZUSP, MNRJ |
| Cebuella (pygmy marmoset) | ||
| 22 C. pygmaea | 7 | MZUSP |
| Callimico (goeldi’s marmoset) | ||
| 23 C. goeldii | 4 | MZUSP |
| Leontopithecus (lion tamarins) | ||
| 24 L. chrysomelas | 5 | MZUSP, MNRJ |
| 25 L. rosalia | 17 | MZUSP, MNRJ |
| Saguinus (tamarins) | ||
| 26 S. fuscicollis | 13 | MZUSP |
| 27 S. imperator | 10 | MZUSP |
| 28 S. labiatus | 9 | MZUSP, MNRJ |
| 29 S. midas | 22 | MZUSP, MNRJ |
| 30 S. mystax | 13 | MZUSP, MNRJ |
| 31 S. niger | 14 | MNRJ |
| Subfamily: Aotinae | ||
| Aotus (owl or night monkeys) | ||
| 31 A. azarae | 4 | MZUSP, MNRJ |
| 32 A. nigriceps | 9 | MZUSP, MNRJ |
| 33 A. trivirgatus | 21 | MZUSP |
| Subfamily: Callicebinae | ||
| Callicebus (titi monkeys) | ||
| 34 C. bernhardi | 5 | MNRJ |
| 35 C. cupreus | 14 | MZUSP, MNRJ |
| 36 C. hoffmannsi | 12 | MNRJ |
| 37 C. moloch | 16 | MZUSP, MNRJ |
| 38 C. nigrifrons | 8 | MNRJ |
| 39 C. personatus | 16 | MZUSP, MNRJ |
| Subfamily: Pitheciinae | ||
| Cacajao (uakaris) | ||
| 40 C. calvus | 14 | MZUSP, MNRJ |
| 41 C. melanocephalus | 9 | MZUSP, MNRJ |
| Chiropotes (bearded sakis) | ||
| 42 C. albinasus | 18 | MZUSP, MNRJ |
| 43 C. satanas | 15 | MZUSP, MNRJ |
| Pithecia (sakis) | ||
| 44 P. irrorata | 17 | MZUSP, MNRJ |
| 45 P. monachus | 7 | MZUSP, MNRJ |
| 46 P. pithecia | 16 | MZUSP, MNRJ |
| Subfamily: Atelinae | ||
| Lagothrix (woolly monkeys) | ||
| 47 L. cana | 7 | MNRJ |
| 48 L. lagotricha | 8 | MZUSP |
| Brachyteles (muriquis) | ||
| 49 B. arachoides | 16 | MZUSP, MNRJ |
| 50 B. hypoxanthus | 5 | MNRJ |
| Ateles spider monkeys) | ||
| 51 A. belzebuth | 2 | RBINS |
| 52 A. chamek | 15 | MNRJ |
| 53 A. marginatus | 20 | MZUSP |
| Subfamily: Alouatinae | ||
| Alouatta (howler monkeys) | ||
| 54 A. belzebul | 15 | MZUSP |
| 55 A. caraya | 15 | MZUSP, MNRJ |
| 56 A. discolor | 10 | MNRJ |
| 57 A. guariba | 5 | MZUSP, MNRJ |
| 58 A. g. clamitas† | 15 | MNRJ |
| 59 A. nigerrima | 10 | MNRJ |
| 60 A. palliata | 15 | HLP |
| 61 A. seniculus | 15 | MZUSP |
| 62 A. ululata | 7 | MNRJ |
The extant comparative samples consisted in 802 adult individuals representing all recognized platyrrhine groups (three families, 18 genera, 61 species; Table 2), whose 2D and 3D morphometric variability of lower molars has alredy been analysed in some platyrrine species (Nova Delgado et al., 2015b). Dental casts were obtained from original specimens housed at Museu de Zoologia Universidade de São Paulo (MZPS), Museu Nacional do Rio de Janeiro (MNRJ) in Brazil, and from Hacienda La Pacífica (HLP) in Costa Rica. The casts were made following published protocols (see Galbany, Martínez & Pérez-Pérez, 2004; Galbany et al., 2006). 2D images of molar occlusal surfaces of the extant specimens were taken with a Nikon D70 digital camera fitted with a 60-mm optical lens held horizontally on the stand base, at a minimum distance of 50 cm. The dental crown was imaged with a 0°of tilt with the cervical line perpendicular to the camera focus (Nova Delgado et al., 2015a). Images of fossil dental crowns were obtained from the literature and imported to Adobe Photoshop, where they were scaled to the same resolution (400 dpi). The images both for the extant and the fossil specimens were scaled to 5 mm and standardized to right side, with the mesial border facing to the right, the distal border to the left, and the lingual and buccal sides facing upward and downward, respectively. All images were saved at high resolution (1600 × 1200 pixel) in JPEG format.
Geometric morphometric analysis
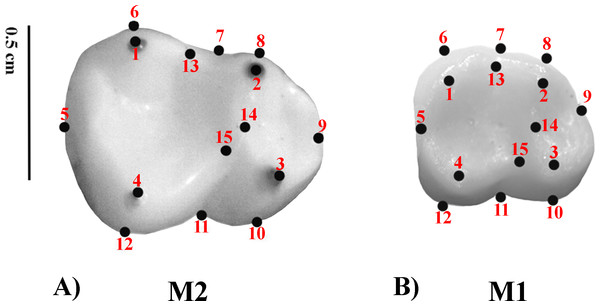
Geometric Morphometrics (GM) quantifies shape differences between biological structures using a set of digitized homologous points (landmarks) in two-dimensional or three-dimensional spaces (Bookstein, 1991; Adams, Rohlf & Slice, 2004; Slice, 2005). Landmarks are numerical values (coordinates) that reflect the location and orientation of each specimen in the morphospace (Slice, 2007). A previously defined two-dimensional (2D) landmark protocol (Nova Delgado et al., 2015a; Nova Delgado et al., 2015b; Gamarra et al., 2016) was adopted. The configuration consisted of 15 landmarks. Molar occlusal polygon was defined by the tips of the four main cusps (protoconid, metaconid, hypoconid, and entoconid). The crown outline was represented by eight landmarks, which included two landmarks on fissure intersections; four corresponding to maximum crown curvatures; and two in the mid mesio-distal line on the crown perimeter. Furthermore, three landmarks were used to represent the positions of crests (Table 3 and Fig. 1) (Cooke, 2011). Landmark recording was performed with TPSDig v 1.40 (Rohlf, 2004) and landmark coordinates were then imported into MorphoJ (Klingenberg, 2011). The most commonly employed method to remove the information unrelated to shape variation is the generalized procrustes analysis (GPA) (Rohlf, 1999; Rohlf, 2005). GPA is based on a least squares superimposition approach that involves scaling, translation and rotation effect so that the distances between the corresponding landmarks are minimized (Rohlf & Slice, 1990; Goodall, 1991; Rohlf & Marcus, 1993; Rohlf, 1999; Adams, Rohlf & Slice, 2004).
Figure 1: Set of landmarks used in the geometric morphometrics analyses.
(A) M2; Alouatta guariba 23177 MNRJ; (B) M1: Sapajus libidinosus 23246 MNRJ.| Landmark | Type | Definition |
|---|---|---|
| 1 | 2 | Tip of the distolingual cusp (entoconid) |
| 2 | 2 | Tip of the mesiolingual cusp (metaconid) |
| 3 | 2 | Tip of the mesiobuccal cusp (protoconid) |
| 4 | 2 | Tip of the distobuccal cusp (hypoconid) |
| 5 | 3 | Most distal point of the mid mesiodistal line on the crown outline |
| 6 | 2 | Point of maximum curvature directly below the entoconida |
| 7 | 3 | Point on the dental crown outline at the lingual groove |
| 8 | 2 | Point of maximum curvature directly below the metaconida |
| 9 | 3 | Most mesial point of the mid mesiodistal line on the crown outline |
| 10 | 2 | Point of maximum curvature directly below the protoconida |
| 11 | 3 | Point on the dental crown outline at the mesial groove |
| 12 | 2 | Point of maximum curvature directly below the hypoconida |
| 13 | 2 | Midpoint between the preentocristid and postmetacristida |
| 14 | 2 | Lowest point on the protocristida |
| 15 | 2 | Lowest point on the crista obliquea |
Notes:
Intra-observer landmark digitizing error was measured in a subsample of five specimens representative of five different species including one fossil taxon (Alouatta belzebul, Aotus dindensis, Callicebus personatus, Callithrix geoffroyi, Pithecia irrorata). The landmarks were digitized nine times during three non-consecutive days. Mean Procrustes distance between paired repetitions was 0.13328, with a standard deviation of 0.04644, and the average Pearson correlation between Procrustes distance matrices (Mantel test) of the repeated measurements was 0.9887. No significant differences in shape configurations among repetitions were obtained with a non-parametric MANOVA test (Anderson, 2001); F = 0.07729; P = 0.9997). The inter-observed error rates were not computed since a single researcher (MND) made all the analyses.
After the procrustes superimposition, the covariance matrix of all the compared shapes was used to derive a Principal Components Analysis (PCA) (Zelditch et al., 2004). The PCA of M1 and M2 morphometric variability of the extant species were used to explore phenetic dental similarities of fossil specimens within the extant comparative platyrrhine sample. The resulting PCA scores were used to conduct a Linear Discriminant Function analysis (LDA) to classify fossil specimens, since PCA removes the irrelevant and redundant dimensions (Zelditch et al., 2004). LDA maximizes differences between groups but allows classifying isolated cases based on their distances to the group centroids of the extant taxa. The probability that a case belongs to a particular group is proportional to the distance to the group centroid (Kovarovic et al., 2011). The reliability of the classification was estimated from the post-hoc correct classification probability after cross-validation (pcc), and the a posteriori probability score was used as the probability that a fossil belongs to a particular group. Several LDAs were made considering different discriminant factors: (1) family (Cebidae, Atelidae, Pitheciidae), (2) the subfamily-level classification proposed by Groves (2005) (Subfamily G) (Cebinae, Saimiriinae, Callitrichinae, Pitheciinae, Callicebinae, Aotinae, Atelinae, Alouattinae), (3) the subfamily classification by Rosenberger (2011) (Subfamily R) (Cebinae, Callitrichinae, Pitheciinae, Homunculinae, Atelinae) (Table 4), and (4) a genus level (Cebus, Sapajus, Saimiri, Callithrix, Mico, Cebuella, Callimico, Leontopithecus, Saguinus, Aotus, Callicebus, Cacajao, Chiropotes, Pithecis, Lagothrix, Brachyteles, Atelles, Allouatta). The LDA analyses were carried out with SPSS v.15 (SPSS, Chicago, IL, USA).
| Genus | Subfamily by Groves (2005) | Subfamily by Rosenberger (2011) |
|---|---|---|
| Cebus | Cebinae | Cebinae |
| Sapajus | ||
| Saimiri | Saimiriinae | |
| Callithrix | Callitrichinae | Callitrichinae |
| Mico | ||
| Cebuella | ||
| Callimico | ||
| Leontopithecus | ||
| Saguinus | ||
| Aotus | Aotinae | Homunculinae |
| Callicebus | Callicebinae | |
| Cacajao | Pitheciinae | Pitheciinae |
| Chiropotes | ||
| Pithecia | ||
| Lagothrix | Atelinae | Atelinae |
| Brachyteles | ||
| Ateles | ||
| Alouatta | Alouattinae |
Results
Principal components analyses
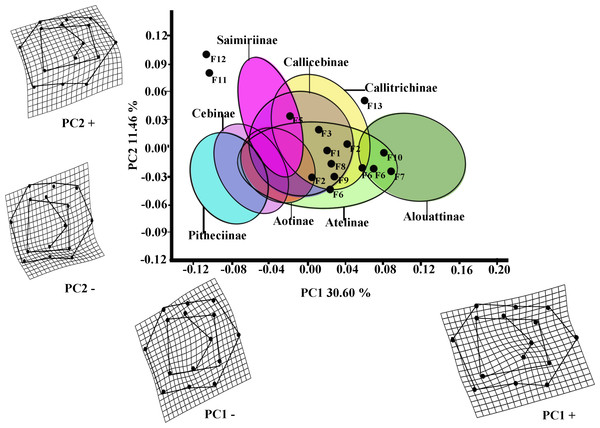
The first two PCs of the PCA analysis of M1 for all platyrrhines (Fig. 2) explain 42.06 % of total shape variance (PC1 30.60%; PC2 11.46%). Positive scores on PC1 correspond to molars with a broad occlusal polygons and a mesiodistally rectangular outline; whereas negative PC1 scores characterize a relatively quadrangular outline and slight buccolingually rectangular occlusal polygon resulted by displacement of distal cusps (entoconid and hypoconid) to mesio-lingually and mesial cusps (metaconid and protoconid) to distal-lingually side respectively. Positive scores on PC2 molar indicate a rectangular occlusal polygon and a mesiodistally rectangular outline, whereas negative score on PC2 reflect molars with relatively quadrangular outline and slight rectangular occlusal polygon more widely displaced to buccally side.
Figure 2: Scatterplot of the first two principal components (PCs) derived from the PCA of M1 shape variability of Platyrrhini.
Grids indicate the deformations associated with the extreme values of each principal component. Ellipses represent the subfamily-level classification proposed by Groves (2005). The letters F and numbers in figure represent the fossils listed in Table 1.Even though the PCA does not distinguish subfamilies, the plot of PC1 versus PC2 (Fig. 2, including 95% confidence ellipses of the subfamily groups) shows clear trends between subfamilies. Alouattinae clearly cluster on the positive scores of PC1, while Pithecinae and Cebinae greatly overlap on the most negative score of PC1. The rest of the groups (Saimirinae, Callicebinae, Callitrichidae, Atellidae, and Aotinae) show intermediate values for PC1 and greatly overlap. For the second PC function (PC2), all groups greatly overlap, though Saimirinae, Callitrichinae and Callicebinae show somewhat higher PC2 scores than the rest. Most of the fossil specimens showed positive PC1 scores, except Carlocebus (F5) and especially Nuciruptor (F11) and Cebupithecia (F12) that had negative PC1 and positive PC2 scores. Most extinct forms overlapped with the extant platyrrhines, within Callicebinae, Callitrichinae, and Atellinae, except Xenothrix (F13), Nuciruptor and Cebupithecia, which do not.
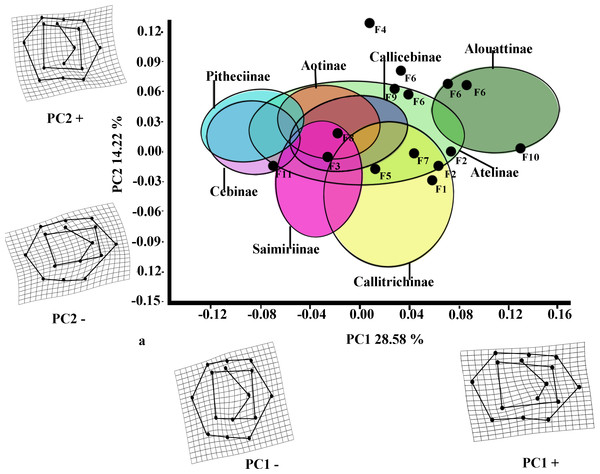
The first two PCs for M2 (Fig. 3) accounted for 42.80% of the total variance (PC1: 28.58%; PC2: 14.22%). The molar shape changes for positive and negative PC1 scores for M2 were relatively similar to those observed for M1, whereas positive PC2 scores for M2 corresponded to the negative ones on PC2 for M1, and negative ones on PC2 for M2 were equivalent to the positive score of PC2 for M1. The PC1 versus PC2 plot (Fig. 3) showed similar distributions of the subfamilies to those for M1, although greater separations between groups were observed. Alouattinae showed the largest, positive scores for PC1, and Pitheciinae and Cebinae the most negative scores, with the other groups showing again intermediate values. Callitrichinae and Saimiriiane were placed mainly on the negative score of the PC2 axis, although they overlapped somewhat with the other groups. Most fossil specimens again clustered on positive scores for PC1 and PC2, mainly within the dispersion of Callitrichinae, although Stirtonia (F10), and some specimens of Neosaimiri clearly fell within the Alouattinae clade, Dolichocebus (F3) within Saimiriinae, and Nuciruptor (F11) was closer to Cebinae and Pitheciinae on the negative scores of PC1. Homunculus (F4) did not fell at all within any extant taxa, showing highly positive PC2 scores.
Figure 3: Scatterplot of the first two principal components (PCs) derived from the PCA of M2 shape variability of Platyrrhini.
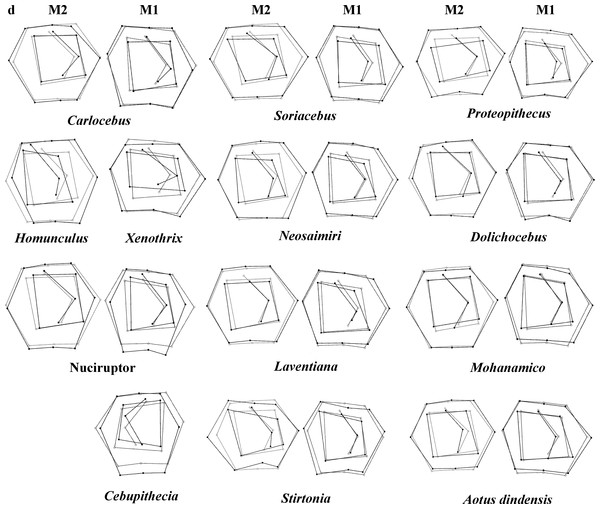
Grids indicate the deformations associated with the extreme values of each principal component. Ellipses represent the subfamily-level classification proposed by Groves (2005). The letters F and numbers in figure represent the fossils listed in Table 1.Figure 4: First and second molar shapes of the extinct fossil platyrhines used in this study.
| (A) M1 | ||||
|---|---|---|---|---|
| Family % | Subfamily by G % | Subfamily by R % | Genus % | |
| DF1 | 56.0 | 50.5 | 42.4 | 49.0 |
| DF2 | 44.0 | 19.1 | 29.1 | 14.2 |
| Classification | 88.7 | 91.3 | 88.2 | 91.0 |
| Cross-validation | 87.4 | 88.0 | 85.7 | 86.3 |
| (M1) | Family | % | Subfamily by G | % | Subfamily by R | % | Genus | % |
|---|---|---|---|---|---|---|---|---|
| Proteopithecus | Cebidae | 99.6 | Saimiriinae | 99.2 | Cebinae | 99.9 | Saimiri | 99.3 |
| Soriacebus1 | Cebidae | 99.9 | Callitrichinae | 99.9 | Callitrichinae | 99.8 | Saguinus | 89.6 |
| Soriacebus2 | Cebidae | 99.1 | Callitrichinae | 76.6 | Callitrichinae | 94.0 | Callithrix | 69.1 |
| Dolichocebus | Cebidae | 86.5 | Callicebinae | 77.9 | Homunculinae | 67.4 | Callicebus | 86.4 |
| Carlocebus | Cebidae | 97.0 | Callitrichinae | 94.2 | Callitrichinae | 83.7 | Callithrix | 87.1 |
| Neosaimiri4 | Pitheciidae | 48.5 | Atelinae | 48.8 | Callitrichinae | 52.2 | Saguinus | 78.7 |
| Neosaimiri5 | Cebidae | 98.4 | Callitrichinae | 97.5 | Callitrichinae | 97.3 | Saguinus | 99.6 |
| Neosaimiri6 | Cebidae | 97.0 | Callitrichinae | 76.5 | Callitrichinae | 94.6 | Saguinus | 72.2 |
| Laventiana | Atelidae | 94.6 | Atelinae | 44.5 | Atelinae | 94.9 | Callicebus | 53.0 |
| Mohanamico | Cebidae | 96.2 | Callitrichinae | 87.3 | Callitrichinae | 70.3 | Leontopithecus | 65.4 |
| Aotus dindensis | Pitheciidae | 59.0 | Aotinae | 99.7 | Homunculinae | 97.4 | Aotus | 98.7 |
| Stirtonia | Atelidae | 98.9 | Alouattinae | 99.9 | Atelinae | 98.2 | Alouatta | 99.9 |
| Nuciruptor | Pitheciidae | 99.7 | Callicebinae | 99.5 | Homunculinae | 83.6 | Callicebus | 63.3 |
| Cebupithecia | Pitheciidae | 96.5 | Pitheciinae | 92.1 | Pitheciinae | 65.3 | Chiropotes | 59.2 |
| Xenothrix | Pitheciidae | 75.8 | Callicebinae | 30.5 | Homunculinae | 61.9 | Callithrix | 90.7 |
| (B) M2 | ||||
|---|---|---|---|---|
| Family% | Subfamily by G % | Subfamily by R % | Genus % | |
| DF1 | 68.3 | 45.6 | 47.6 | 43.5 |
| DF2 | 31.7 | 29.0 | 32.8 | 22.6 |
| Classification | 89.5 | 93.3 | 90.3 | 88.7 |
| Cross-validation | 88.2 | 90.6 | 89.0 | 84.7 |
| (M2) | Family | % | Subfamily by G | % | Subfamily by R | % | Genus | % |
|---|---|---|---|---|---|---|---|---|
| Proteopithecus | Cebidae | 99.4 | Callitrichinae | 82.3 | Callitrichinae | 80.3 | Callimico | 86.7 |
| Soriacebus1 | Cebidae | 65.6 | Callicebinae | 81.6 | Homunculinae | 58.4 | Saguinus | 74.6 |
| Soriacebus3 | Atelidae | 77.1 | Callitrichinae | 96.7 | Callitrichinae | 98.0 | Saguinus | 65.6 |
| Dolichocebus | Cebidae | 50.7 | Callicebinae | 92.6 | Homunculinae | 90.1 | Callicebus | 92.6 |
| Homunculus | Pitheciidae | 91.4 | Callicebinae | 93.7 | Homunculinae | 97.3 | Callicebus | 99.9 |
| Carlocebus | Cebidae | 55.6 | Callitrichinae | 58.8 | Callitrichinae | 50.4 | Mico | 72.5 |
| Neosaimiri7 | Cebidae | 98.3 | Callicebinae | 92.9 | Cebinae | 35.8 | Callicebus | 67.2 |
| Neosaimiri8 | Cebidae | 64.9 | Callicebinae | 61.2 | Homunculinae | 93.7 | Saguinus | 65.1 |
| Neosaimiri9 | Cebidae | 99.5 | Callitrichinae | 61.3 | Callitrichinae | 51.7 | Saguinus | 92.3 |
| Neosaimiri10 | Cebidae | 98.9 | Callicebinae | 84.6 | Callitrichinae | 71.9 | Saguinus | 98.3 |
| Laventiana | Cebidae | 99.9 | Callitrichinae | 99.8 | Callitrichinae | 99.7 | Saguinus | 40.8 |
| Mohanamico | Cebidae | 97.7 | Callitrichinae | 94.9 | Callitrichinae | 94.6 | Saguinus | 99.9 |
| Aotus dindensis | Cebidae | 84.4 | Callicebinae | 88.9 | Homunculinae | 76.1 | Callicebus | 96.5 |
| Nuciruptor | Pithecidae | 89.7 | Pitheciinae | 89.7 | Pitheciinae | 73.0 | Pithecia | 49.4 |
| Stirtonia | Atelidae | 81.8 | Alouattinae | 86.0 | Callitrichinae | 92.1 | Alouatta | 94.0 |
Discriminant analyses of the fossil specimens
The post-hoc percentages of correct classification after cross-validation (pcc) were high both for M1 (Table 4A, range = [85.7–88.0%]) and M2 (Table 4B, range = [84.7–90.6%]). In both cases the highest pcc value was obtained when Groves’ subfamily factor was discriminated. The range of differences between pcc values before and after cross-validation was [1.3–4.7] and in both teeth the genus discriminant factor showed the highest decrease in pcc. The differences in pcc values between Groves’ (Cebinae, Saimiriinae, Callitrichinae, Pitheciinae, Callicebinae, Aotinae, Atelinae, Alouattinae) and Rosenberger’s (Cebinae, Callitrichinae, Pitheciinae, Homunculinae, Atelinae) pcc values were 2.3% for M1 and 1.6% for M2 (Table 5). The percentage of total variance explained by the first two discriminant functions (DF1, DF2; Table 4) for all discriminat factors ranged from 63.3% (genus) to 100% (family) for M1, and from 66.1% (genus) to 100% (family) for M2. The highest percentage of total variance explained by DF1 was 56.0% (family) for M1 and 68.3% (family) for M2, and the highest one for DF2 was 44.0% (family) for M1 and 32.8% (subfamily R) for M2.
Regarding the classification of the fossil specimens, the ranges of the a priori classification probabilities varied depending on the discriminant factors used (Table 5; Fig. 4 shows the landmark configurations of the fossil specimes analysed). Mohanamico showed a high probability of belonging to the callitrichines clade, as well as Carlocebus, although the probability was smaller for M2. Both Neosaimiri and Soriacebus showed high probabilities of belonging to the callitrichines for M1, though to Callicebinae/Homunculinae for M2. Cebupithecia (M2 not available) and Nuciruptor neotypes showed a high probability of belonging to the pitheciid clade in LDAs. In contrast, Xenothrix (M2 not available) likely belonged to Callithrix, despite in the PCA this fossil specimen did not fall within Callitrichinae range. Stirtonia was assigned to the Atelidae clade, and to Alouatta at the genus level, except for Rosenberger’ subfamily factor for M2. Laventiana was also classified into the atelids for M1, but was more closely related to callitrichines for M2. Aotus dindensis showed a high probability of belonging to Aotustaxa for M1, but Callicebus was the group with the greatest affinity for M2. Finally, Proteopithecus showed a high resemblance with Saimiri for M1, but with Callimico for M2.
Discussion
The positions of the anthropoid form Proteopithecus sylviae (F1) in the morphospace and its molar shapes showed pattern resemblance to that of platyrrhines. However, because, many dental and postcranial features of P. sylviae are considered to be symplesiomorphic characters of all anthropoids, it is placed as the stem anthropoid (Kay, 1990; Kay, 2014). The recent discovery of Perupithecus ucayaliensis, probably from the Late Eocene, suggests that this fossil exhibits similarities with Proteopithecus, also with Talahpithecus and Oligopithecidae (Bond et al., 2015). The upper molars of Perupithecus slightly resemble those of the callitrichines, but its morphology is more similar to Proteopithecus and Talahpithecus (Bond et al., 2015). Proteopithecus sylviae differed from the extant and extinct platyrrhines in having a molar distomesially expanded, marked by a rectangular shape of the occlusal polygon (especially on M2) (also seen in Xenothrix). Thus, if the Fayum form likely was a sister taxon to platyrrhines, the interspecific variation of shape would have shown relatively little change. This could mean that the main traits of molars shapes in platyrrhines represent retention of a primitive ancestral form. Moreover, the LDA showed a high probability of P. sylviae belonging to the Cebidae clade, suggesting that the molar of the earliest ancestors of platyrrhines must have exhibited close similarity to Saimiri-Callimico. This resemblance matches with the description of an Oligocene primate fossil found in South America, Branisella (Rosenberger, 2002; Rosenberger et al., 2009), whose morphology indicates that the structural characteristics of M2 may have been Saimiri-like, and the upper P2 Callimico-like (Rosenberger, 1980). However, both molar shapes of P. sylviae more closely resembled Callimico than Saimiri. Furthermore, the subtriangular upper molars of Perupithecus, show relative similarity with Callimico (Bond et al., 2015). Thus, if P. sylviae was a sister taxon of platyrrhines, it is likely that the hypothetical ancestral molar shape of pre-platyrrhine would have been similar to a molar of Callimico. By contrast, if P. sylviae were a stem species, Callimico would show retention of primitive pre-anthropoid platyrrhine molar shape.
Early Miocene platyrrhines from Patagonia
Most of the traits used to identify phylogenetic affinities among Early Miocene platyrine fossils show high levels of homoplasy Kay (1990), Kay (2010) and Kay (2014). The present work alone cannot reject the successive radiations or the long lineages hypotheses, nor can confirm which is correct. However, the PCA showed clear trends at the subfamily level (Figs. 2 and 3). Although the fossils were not very spread out in the morphospace, many of them were located mainly within the Callicebinae and Callitrichinae range (except Homunculus for M2), showing phenetic similarities with these two extant subfamilies
The Early Miocene fossils were mainly assigned to two taxa by the LDA; a Callicebus-shaped and a Sagunus-shaped. For example, Dolichocebus (F3) was classified as a pitheciid, mainly by having a square occlusal polygon (Table 4). However, although the PCA for M1 placed this specimen in the Callicebinae range, a morphological similarity with Saimiriinae was seen for M2 (Fig. 3A). In contrast, Soriacebus (F2) was related mainly to the callitrichine clade, but for M2 the probability of belonging to this group was small (Table 4). Soriacebus showed a rectangular occlusal polygon on M2 and the ectoconid was inclined distolingually. Regarding callitrichines, although Soriacebus also showed differences in cusp configuration, the callitrichines and Soriacebus share a C-shaped distal side and a somewhat straight lingual-side contour (mostly seen in Saguinus). Kay (1990) reported that many dental features of marmosets and Soriacebus were convergent. In contrast, Rosenberger, Setoguchi & Shigehara (1990) suggested that there are some similarities with callitrichines (development of hypoconids and entoconids in the talonid), although, based on the anterior teeth, they concluded that Soriacebus represents the first branch of pitheciines. Although marmosets are considered derived lineages (e.g., Chatterjee et al., 2009; Jameson Kiesling et al., 2014), it is likely that the relation with Soriacebus may be due to the fact that callitrichines exhibit primitive traits on their molars, which means that both taxa share a retention of rectangular contour and occlusal polygon shape. In the case of Carlocebus (F5), it was classified as a Callitrichinae in the DFA. However, it has been shown to be more similar with Callicebus than marmosets, such as the shape contour and quadrate alignment of cusps in both molars. Homunculus (F4), was placed outside the range of Patagonian forms in the PCA (Fig. 2A), whereas the LDA indicated a high probability of belonging to Pitheciidae (ca. 91–99%; Table 4), and especially to Calliecebus. Nonetheless, Homunculus molar showed an asymmetrical shape compared to pitheciid forms. Furthermore, unlike pitheciids, Homunculus cusps were predominantly inclined toward the distal side and the trigonid was almost as broad as the basin-like talonid, which means that although sharing some traits with pitheciids, its position is highly uncertain.
Middle Miocene platyrrhines from Colombia and the Caribbean Xenothrix
Many of these fossils were mostly catalogued as callitrichines, specifically into the Saguinus clade, except Nuciruptor, Cebupithecia, Aotus dindensis, and Stirtonia. One of the major differences between these primates and the extant forms (except Alouatta and Brachyteles) was the rectangular-shaped molar (see Xenothrix below). This phenetic similarity among phyletically distinct groups of extinct primates indicates that a rectangular-shaped molar almost certainly represents a plesiomorphy in the Patagonian fossils. Thus, the trend toward ovoid molar shape might be a derived feature in many living forms. Laventania (F7) exhibited distally oriented cusps on M1, showing considerable resemblance with some atelid groups, which provided a confusing classification between atelids and Callicebus in the LDA (Table 5). Thus, the trend to rectangular shape for M1 in Laventania differs notably from the phylogenetic relationship with Cebinae and Saimiriinae. Nonetheless, when M2 was analyzed, the fossil was classified as member of the Callitrichinae clade. As with Laventania, some neotypes of Neosaimiri (F6) were classified in completely distant taxonomic groups (Table 4). However, despite these results, Neosaimiri was principally associated to the Cebidae family, although the molar shape was found to have more affinities with callitrichines than Saimiri. On the other hand, Mohanamico (F8) and Aotus dindensis (F9) have been considered by Kay and collaborators (Meldrum & Kay, 1997; Kay, 2014) to belong to the same genus,despite Takai et al. (2009) have suggested that A. dindensis should be assigned to distinct genus. According to their molar shape, Mohanamico and A. dindensis may be classified into different species. Both fossils showed a relative rectangular shape of the outline, as well as in the occlusal polygon, although M2 in both species was slightly square shaped. In fact, PCA for M1 (Fig. 2A) showed that the two forms were placed closer to each other. Thus, similar molar shape might be due to the fact that the two forms must have shared relatively similar ecological niches, likely because Mohanamico and A. dindensis were found in the same locality and at the same stratigraphic level (Kay, 1990). However, the LDA indicated that the probability of classification was different for both groups. Aotus dindensis was mainly related to Aotus/Callicebus, whereas Mohanamico was assigned to Callitrichinae (Table 4). In the case of Nuciruptor (F11) and Cebupithecia (F12), the occlusal views in both species were relatively rounded, with a slightly rectangular alignment of cusps, and buccally oriented, which resembles the condition in most extant Pitheciinae. Moreover, the LDA indicated that Cebupithecia and Nuciruptor had a close affinity with the Pitheciidae clade (Table 4). However, despite the two neotypes clustered close to the pitheciids, they were not placed into the extant species range (except Nuciruptor on M2) (Fig. 2A). Several studies have suggested that, although there are important characteristics that have been associated with the living taxa, both fossils should be considered stem pitheciines (Meldrum & Kay, 1997; Kay, Meldrum & Takai, 2013; Kay, 2014).
The sister relationship between Stirtonia and Alouatta was classified in the LDA with a 99.9% probability for M1 and 94.0% for M2. Likewise, the PCA showed that Stirtonia was placed close to howler monkeys (Figs. 2A and 3A). However, differences between Stirtonia and Alouatta were mainly seen in the occlusal polygon of M2. The metaconid of Stirtonia was located near the protoconid and the ectoconid was distolingually inclined, somewhat similar to the Cebuella configuration. This relationship was reflected in the high percentage of probability at the subfamily level, Callitrichinae (Table 5).
Finally, Xenothrix (F13), the Caribbean platyrrhine form, has been allied with pitheciids (Rosenberger, 2002; Horovitz & MacPhee, 1999). In the LDA, Xenothrix was mainly attributed to pitheciids, but at the genus level, it was assigned to Callithrix (Table 4). Thus, some resemblance with marmosets could be interpreted as convergent evolution. However, the relationship between Xenothrix and pitheciids was highly uncertain, given that its molar morphology (especially the occlusal configuration) differs from that of the pitheciids. It is likely that Xenothrix could be a single branch that evolved independently of crown platyrrhines, as was suggested by some investigations that proposed an early Antillen arrival (Iturralde-Vinent & MacPhee, 1999; MacPhee & Iturralde-Vinent, 1995; MacPhee & Horovitz, 2004; Kay et al., 2011; Kay, 2014).
The slow rate of phenotypic changes on molar shapes suggests that morphological stasis (different concept from long lineages hypothesis) explains the low interspecific variation between extinct and extant linages and between Early Miocene platyrrhines (including P. sylviae) and forms from La Venta. This small phenotypic variation could be due to developmental and functional constraints, given the role in occlusion and mastication (Gómez-Robles & Polly, 2012) and the reduced dietary diversification in platyrrhines. This ecological constraint may be related to the fact that the phenotypic adaptation of main platyrrhine families could have happened in Amazon rainforest (Jameson Kiesling et al., 2014). Following an African origin scenario, and taking into account the phenotypic similarity of the most recent discovered and oldest fossil found in Peru, Perupithecus (Bond et al., 2015), it is likely that the ancestor of extant platyrrhines could have exhibited a Callimico-like molar shape. We also observed that Saguinus and Callicebus were the main assigned groups for Patagonian fossils by the LDA, which suggests that there were both Callicebus-like and Saguinus-like morphologies in early stem platyrrhines, dispite extant Saguinus might not represent an early branch according to molecular evidence. Currently, Callicebus and Saguinus present relatively high diversity of species and geographic range (Rylands & Mittermeier, 2009). The Callicebus and Saguinus species richness probably are related to expansion and diversification of both clades in the Amazon basin, during the period of platyrrhine evolution (Ayres & Clutton-Brock, 1992; Boubli et al., 2015). Thus, it is feasible that Callicebus, as well as Saguinus, molar shape would be an ancestral precursor for the existing forms. Moreover, the Middle Miocene platyrrhines indicate continuity in molar shape pattern with the early fossils, incorporating also new molar shapes not observed in the Patagonian forms: the Alouatta-like and the Pitheciinae-like forms.
Conclusions
This study develops a dental model based on molar shapes of M1 and M2 to explore phenotypic variation in extinct and extanct platyrrhines. Our results showed that morphological stasis explains the low phenotypic changes in extinct and exctant platyrrhines, probably due to ecological constraints, caused by phenotypic adaptation of platyrrhines to a relatively narrow ecological niche. Early and Middle Miocene platyrrhines shared some relatively similar molar shape patterns, whereas the Colombian fossils more closely resemble Alouatta and the Pitheciinae. The relation between both fossil samples could be due to: 1. All platyrrhine molar shapes share a primitive retention of the ancestral state. 2. An early divergence between two parallel shapes; a Callicebus-like and a Saguinus-like, which would be the ancestral precursors to all other forms. 3. Callicebus-like and Saguinus-like morphologies independently occurred in the early stem platyrrhines.