Microecological recombination of Angelica sinensis driven by the transplanting of “alpine seedling–cellar planting–dam cultivation”
- Published
- Accepted
- Received
- Academic Editor
- Ahmet Tansel Serim
- Subject Areas
- Agricultural Science, Biodiversity, Ecology, Microbiology, Plant Science
- Keywords
- Angelica sinensis, Transplanting, Microecosystem, Microecological recombination, High-throughput sequencing
- Copyright
- © 2025 He et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Microecological recombination of Angelica sinensis driven by the transplanting of “alpine seedling–cellar planting–dam cultivation”. PeerJ 13:e19208 https://doi.org/10.7717/peerj.19208
Abstract
Transplanting is important for obtaining and maintaining excellent germplasm of cultivated plants. During plant transplantation, the endophytic microbial community regularly reorganizes, which may be crucial for plant germplasm rejuvenation. Angelica sinensis, a widely used medicinal and edible plant, relies on transplanting for its exceptional quality. To explore the microecological recombination of A. sinensis during the transplanting process of “alpine seedling–cellar planting–dam cultivation”, this study analyzed shifts in endophytic and soil microbial communities across the three transplanting stages in Min County, Gansu Province, China. High-throughput sequencing revealed significant changes, with 82.27% to 84.65% of bacteria and 93.19% to 93.49% of fungi species altering in transplanted Angelica. Main findings indicate that Mortierellomycota, Actinobacteriota, and Myxococcota were dominant in cellar planting root and cellar rhizosphere soil, contrasting with Firmicutes predominance in alpine and dam areas. Notably, potentially pathogenic endophytes like Fusarium and Xanthomonas decreased post-alpine seedling and cellar planting, favoring a healthier plant environment. Cellar planting root exhibited a rich accumulation of psychrophilic flora, including Tetracladium, Pseudomonas, and Flavobacterium, alongside a unique dominance of Mortierella fungi. Microbial co-occurrence network analysis highlighted cellar planting root as pivotal, suggesting its importance in microbial interactions. In conclusion, transplanting significantly reshaped A. sinensis’s endophytic flora, with fungi showing more pronounced recombination than bacteria. Soil microbial communities emerged as crucial drivers of this recombination, facilitating the overwintering of A. sinensis, reducing diseases, and rejuvenating the germplasm. Transplanting-driven microecological reorganization is an important scientific mechanism for the high-quality production of cultivated medicinal plants.
Introduction
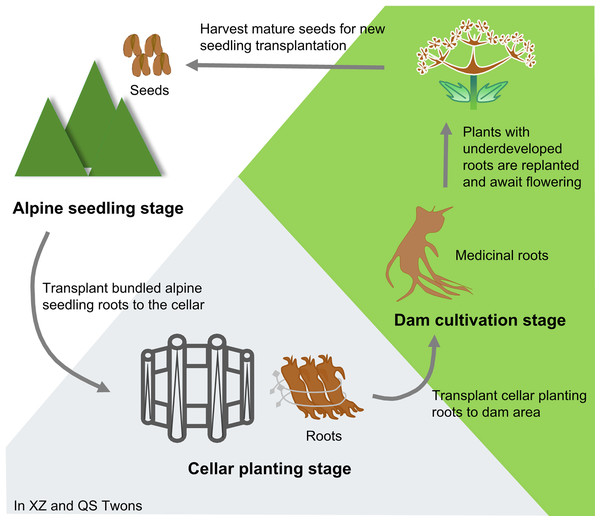
Angelica sinensis (Oliv.) Diels (Umbelliferae) is a medicinal and edible plant. It is widely used in China and has a long history of use. Because its roots have the effects of tonifying blood and activating blood, regulating menstruation and relieving pain, moistening intestine and relaxing bowels, it is commonly used in clinical treatment of anemia, rheumatism, menstrual disorders and other problems (Chinese Pharmacopoeia Commission, 2020). It has also been incorporated into health care products and cosmetics (Zhang et al., 2012). A. sinensis is suitable to grow at alpine and cold climates. Min County in Gansu Province, China is its primary production area. The rhizosphere microecosystem of A.sinensis in this area is healthy and stable, which is conducive to the growth and reproduction of A. sinensis (Jiang et al., 2009; Wang, 2014). Historical accounts from the “Min Rural and Local History” provide insights into the cultivation of Min A. sinensis (Wang, 2014). The transplanting technology of A. sinensis “alpine seedling–cellar planting–dam cultivation” has been formed in the Ming and Qing Dynasties. (Fig. 1). In detail, in the spring of the first year, seedlings were raised at an altitude of 3,000 m; in October of the same year, the roots of the seedlings were dug out, tied up and transplanted into the cellar for storage. In the spring of the second year, the roots excavated from the cellar were transplanted to the dam area at an altitude of 1,000∼2,000 m for cultivation, and the medicinal plants could be harvested by October. Any plants with underdeveloped roots continued to be cultivated, and entered the reproductive stage in the spring of the third year. When the seeds were summed up, they could be used for a new round of A. sinensis seedling transplanting (Gong et al., 2016; Gong et al., 2022; Liu et al., 2021).
Figure 1: Traditional cultivation pattern of A. sinensis.
Xizhai Twon (XZ) and Qingshui Twon (QS), Min County, Dingxi City, Gansu Province, China.In addition to the traditional seedling transplanting, the current cultivation technology of A. sinensis also has the way of seed direct seeding (Gong et al., 2018). Although direct seeding of seeds shortens the time cost of cultivation and reduces the economic cost, A. sinensis is smaller (Liu et al., 2021; Zhang et al., 2016). For the way of seedling transplanting, it mainly includes wasteland seedling and mature-land seedling (Gong et al., 2022). The growth effect of wasteland seedling is better, but the seedling raising in mature-land belongs to the continuous cultivation of medicinal plants in the same place. This method can easily reduce the microbial diversity of plant rhizosphere, reduce the adaptability of plants to the environment, and increase the incidence of plant diseases (Berg & Cernava, 2022; Huo et al., 2018; Zhang & Zhang, 2008; Zhao et al., 2016) An important reason for the continuous cropping obstacle of medicinal plants is the imbalance of rhizosphere microbial community structure (Wu & Lin, 2020). Therefore, optimizing the microecosystem is crucial for the growth and disease resistance of medicinal plants (Peng et al., 2020).
Transplantation is a widely employed method in cultivating medicinal plants, offering disease mitigation and enhancing germplasm quality. For instance, Ligusticum chuanxiong Hort., a plant in the same Umbelliferae as A. sinensis, is traditionally cultivated using the “mountain breeding–dam cultivation” technique, effectively preserving its germplasm quality against degradation from prolonged asexual propagation. Previous research has demonstrated that transplantation fosters a periodic recombination of endophytic microbial communities, crucial for rejuvenating L. chuanxiong germplasm (Kang et al., 2021; He, 2016). However, the scientific significance of this recombination phenomenon remains unexplored in other medicinal plants.
The “alpine seedling–cellar planting–dam cultivation” technique developed in Min County is an important way to ensure that ‘Min A. sinensis’ has excellent germplasm and authentic (Dao Di) quality. Multiple transplanting is of great significance to regulate the rhizosphere microecological balance of A. sinensis, and its microecological mechanism needs to be elucidated. Therefore, we hypothesize that transplanting-driven microecological reorganization of A.sinensis is a key factor in ensuring the production and quality of A.sinensis. This study utilizes high-throughput sequencing technology to analyze the microbial community structure and microecological recombination patterns in ‘Min A. sinensis’ across its three transplanting stages. The aim is to explain the scientific connotation of transplanting-driven plant germplasm rejuvenation, and to provide new solutions for reducing the incidence of medicinal plant diseases, restoring germplasm vitality and improving the quality of medicinal materials.
Materials & Methods
Transplantation experiment design
Two concurrent field experiments were conducted in Xizhai Town (XZ: 103°48′E, 34°29′N, average altitude 2,397 m, annual average temperature 5.0 °C, annual average precipitation 587 mm) and Qingshui Town (QS: 103°54′E, 34°27′N, average altitude 2,362 m, annual average temperature 5.3 °C, annual average precipitation 592 mm), Min County, Dingxi City, Gansu Province, China. In the first year, A. sinensis seeds were sown in the alpine region for seedling production in mid-to-late May; the seedlings were then excavated and transplanted to the cellar for cellar planting in early October of the same year. In March of the next year, the cellar planting roots were transplanted to the dam area for medicinal plant cultivation, and A. sinensis could be harvested by October (Fig. 1). Three sample plots were established at each transplanting stage in both XZ and QS towns.
Test materials and pretreatment
The roots and soil samples of A. sinensis from XZ and QS towns were collected at three specific time points: the end of seedling production (in October of the first year), the end of cellar planting (in March of the second year) and prior to the harvest of medicinal materials (in October of the second year). These three time points precisely represent the specific sampling times of the three transplanting stages of A. sinensis ‘alpine seedling–cellar planting–dam cultivation’. The detailed sampling methods and processing protocols are as follows.
(1) Plant samples
The plant sampling method of the three sample collection time points was equidistant sampling method, with 20 healthy A. sinensis plants randomly selected from each sample plot. The aerial portions of the plant were excised and the roots were temporarily preserved in an ice box before being promptly transported to the laboratory for surface sterilization. The roots underwent a series of sterilization steps, including washing with running water, immersion in 75% ethanol for 30 s, treatment with 5% hypochlorite for 5 min, and rinsing with sterile water thrice. Subsequently, longitudinal sections were made using a sterile blade within an laminar flow hood to ensure each segment retained both cortex and xylem tissues. The processed samples were then transferred to sterile tubes and stored at −20 °C for subsequent DNA extraction.Triplicate repetitions per treatment.
(2) Soil samples
At the same time of plant sampling, the rhizosphere soil samples at the three time points were collected. In each sample plot, a total of 20 rhizosphere soil samples of A. sinensis were obtained by shaking the roots, followed by de-adulteration and homogenization in sterile tubes (Zhang et al., 2021). These samples were promptly preserved in an icebox and transported back to the laboratory at −20 °C to preserve their DNA integrity. Before the harvest of medicinal materials, the non-rhizosphere soil was collected from a distance of five cm to 20 cm away from the A. sinensis plants and were processed in a manner consistent with the handling of rhizosphere soil samples. Triplicate repetitions per treatment.
DNA extraction
Utilizing a mixed sampling method, A. sinensis root or soil samples from individual sample plots were pulverized with liquid nitrogen, yielding approximately 0.1 g of sample powder for DNA extraction using the Zymo Research BIOMICS DNA Microprep Kit (Cat # D4301). The integrity, purity, and concentration of the DNA samples were assessed through 0.8% agarose gel electrophoresis (model DYY-6D; Beijing Liuyi Biotechnology Co., Ltd.) and nucleic acid microprocessor (model DS-11+; Dannoer) analysis. Upon successful evaluation, the samples were forwarded to Chengdu Rhonin Biosciences Co., Ltd. for high-throughput DNA sequencing. Sample information is shown in Table 1.
| Transpanting stage | Sample type | Origin and sample number | |||||
|---|---|---|---|---|---|---|---|
| Xizhai twon (XZ) | Qingshui twon (QS) | ||||||
| Alpine seedling stage | M | XZM1 | XZM2 | XZM3 | QSM1 | QSM2 | QSM3 |
| MS | XZMS1 | XZMS2 | XZMS3 | QSMS1 | QSMS2 | QSMS3 | |
| Cellar planting stage | J | XZJ1 | XZJ2 | XZJ3 | QSJ1 | QSJ2 | QSJ3 |
| JS | XZJS1 | XZJS2 | XZJS3 | QSJS1 | QSJS2 | QSJS3 | |
| Dam cultivation stage | Y | XZY1 | XZY2 | XZY3 | QSY1 | QSY2 | QSY3 |
| YS | XZYS1 | XZYS2 | XZYS3 | QSYS1 | QSYS2 | QSYS3 | |
| GS | XZGS1 | XZGS2 | XZGS3 | QSGS1 | QSGS2 | QSGS3 | |
PCR amplification and high-throughput sequencing
Fungi
The fungal ITS region was amplified with primers ITS1F/ITS2R (ITS1F: 5′-CTTGGTCATTTAGAGGAAGTAA-3′; ITS2R: 5′-GCTGCGTTCTTCATCGATGC-3′). The reaction system was 10 × Buffer two µL, 2.5 mM dNTPs two µL, 0.8 µL each of forward and reverse primers, rTaq polymerase 0.2 µL, BSA 0.2 µL, Template DNA 10 ng, supplemented ddH2O to 20 µL. The reaction parameters were 95 °C for 3 min; 95 °C for 30 s, 55 °C for 30 s, 72 °C for 45 s, 35 cycles; and 72 °C for 10 min. PCR products were detected by 2% agarose gel electrophoresis, and samples that passed the test were recovered and quantified using Qubit@ 2.0 Fluorometer (Thermo Fisher Scientific). Libraries were constructed using the NEBNext Ultra II DNA Library Prep Kit (NEB # E7645L) and PE250 sequencing was performed using the HiSeq Rapid SBS Kit v2 (FC-402-4023 500 Cycle).
Bacteria
The bacterial 16S rDNA V4 region was amplified with primers 515F/806R (515F: 5′-GTGYCAGCMGCCGCGGTAA-3′; 806R: 5′-GGACTACHVGGGTWTCTAAT-3′). In order to prevent contamination of host plant DNA, PCR was conducted utilizing the Green Shield Sequence (GSS) technique, which involved the incorporation of two additional pairs of primers, MTR-F/MTR-R (MTR-F: 5′-GTCGAACGTTGTTTTCGG-3′; MTR-R: 5′- CTTCACCCCAGTCGAAGA-3′) and CHP-F/CHP-R (CHP-F: 5′-GTCGAACGGGAAGTGGT-3′; CHP-R: 5′-CTTCACTCCAGTCGCAAGC-3′). These primers were designed to flank homologous sequences in the host mitochondria and plasmid, thereby minimizing the amplification of host genes. The reaction system was 10 × Buffer five µL, GSS Depletion Mix two µL, 2.5 mM dNTPs five µL, 25 mM MgSO4 three µL, 1.5 µL each of forward and reverse primers, KOD-Plus-Neo (one U/µL) one µL, Template DNA two µL, and supplemented with H2O to 50 µL. The reaction parameters were 94 °C for 1 min; 94 °C for 20 s, 54 °C for 30 s, 72 °C for 30 s, 30 cycles; 72 °C for 5 min. Quantitative detection of PCR products, library construction and sequencing were the same as for fungi.
Statistical analysis
DNA fragments from microbial communities were bipartite sequenced on the Illumina MiSeq platform. QIIME (v1.9.1; http://qiime.org/) was used to analyze sequences. After removing low-quality or ambiguous sequences, the bipartite sequences were spliced using Flash (https://ccb.jhu.edu/software/FLASH/index.shtml). The OTU clustering was performed based on Uparse (v11; https://drive5.com/uparse/) at a 97% consistency level. Species annotations were obtained using the RDP classifier Bayesian algorithm (v2.13; https://john-quensen.com/classifying/rdp-classifier-updated/) combined with the SILVA database (https://www.arb-silva.de/). Utilize Mothur (v1.30.2; https://mothur.org/) for calculating the alpha diversity index and employ the Student’s t-test to determine significant differences in index values between each pair of groups. The Kruskal–Wallis H test was applied to assess significant differences among species within multiple sample groups. All additional bioinformatics analyses were conducted using the Shanghai Majorbio Cloud Platform (http://www.majorbio.com/).
Results
Analysis of the Illumina sequencing data
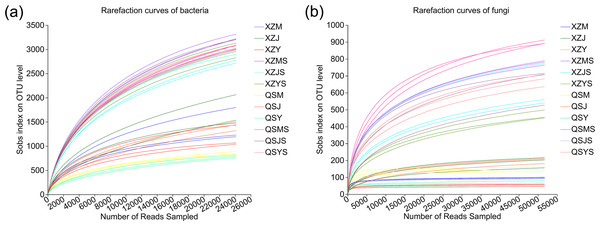
A total of 1,673,293 optimized bacterial sequences (average length: 296 bp) and 2,751,905 optimized fungal sequences (average length: 234 bp) were obtained. The OTU clustering obtained 12,107 bacterial OTUs and 937 fungal OTUs, of which endophytic bacteria were annotated to 28 phyla, 78 classes, 207 orders, 349 families, 730 genera, 1,505 species and 5,947 OTUs, and soil bacteria were annotated to 38 phyla, 116 classes, 281 orders, 460 families, 920 genera, 2,123 species and 10,457 OTUs; endophytic fungi were annotated to four phyla, 12 classes, 32 orders, 61 families, 95 genera and 121 species and 159 OTUs, and soil fungi were annotated to 12 phyla, 32 classes, 71 orders, 148 families, 295 genera, 443 species, 853 OTUs. The rarefaction curves tend to flatten as the number of sample sequencing reads increases (Fig. 2), indicating the sufficient sample sequencing depth.
Figure 2: Rarefaction curves of endophytic and soil microorganisms in A.sinensis.
(A) Rarefraction curves of bacteria in endophyte and soil. (B) Rarefraction curves of fungi in endophyte and soil.Alpha diversity analysis
The α-diversity index serves as a measure of the richness, diversity, and evenness of a microbial community. The results of α-diversity index of A. sinensis at three transplanting stages in both XZ and QS twons revealed fungal Coverage exceeding 0.99 and bacterial Coverage exceeding 0.95 (Table S1 online), suggesting that the sequencing depth effectively captured the true characteristics of the samples under study.
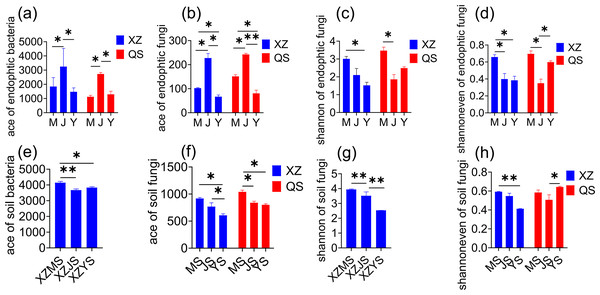
In plant samples, the Ace of endophytic bacteria (Fig. 3A) and endophytic fungi (Fig. 3B) exhibited a significant trend of cellar planting root (J) > alpine seedling root (M) > dam medicinal root (Y) at both origins (p < 0.05). The Shannon (Fig. 3C) and Shannon even (Fig. 3D) of endophytic fungi were the highest in alpine regine at both origins (p < 0.05). In rhizosphere soil samples, the Ace of bacteria (Fig. 3E) was significantly higher in alpine rhizosphere soil (MS) at XZ town (p < 0.05). Similarly, the Ace of fungi (Fig. 3F) followed the trend of alpine rhizosphere soil (MS) > cellar rhizosphere soil (JS) > dam rhizosphere soil (YS) at both origins (p < 0.05). Furthermore, the Ace index in the dam cultivation stage showed a pattern of dam non-rhizosphere soil (GS) > dam rhizosphere soil (YS) > dam medicional root (Y) (Table S1). In conclusion, the diversity of transplanted A. sinensis endophytes was most pronounced in the cellar, while the diversity of rhizosphere soil microbes was the highest in alpine regine. The microbial richness decreased progressively from the soil to the root.
Figure 3: Alpha diversity index of A. sinensis with significant differences at three transplanting stages.
(A) Ace index of endophytic bacteria in both XZ and QS towns. (B) Ace index of endophytic fungi in both XZ and QS towns. (C, D) Shannon and Shannon even index of endophytic fungi in XZ and QS towns respectively. (E) Ace index of soil bacteria in XZ town. (F, H) Ace and Shannon even index of soil fungi in both XZ and QS towns. (G) Shannon index of soil fungi in XZ town (**p < 0.01, *p < 0.05).Microbial community structure analysis
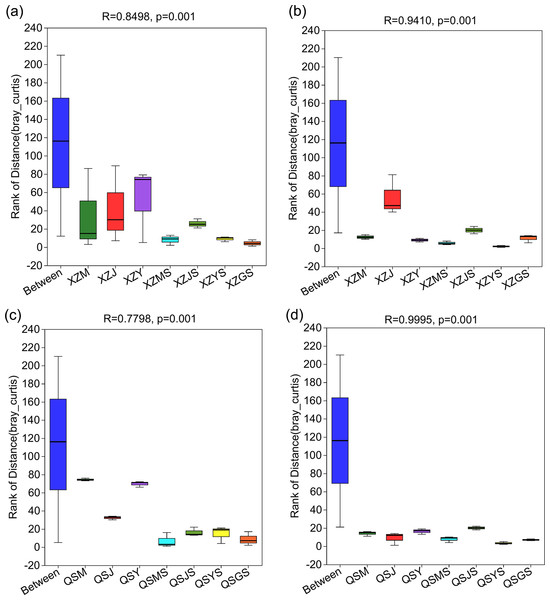
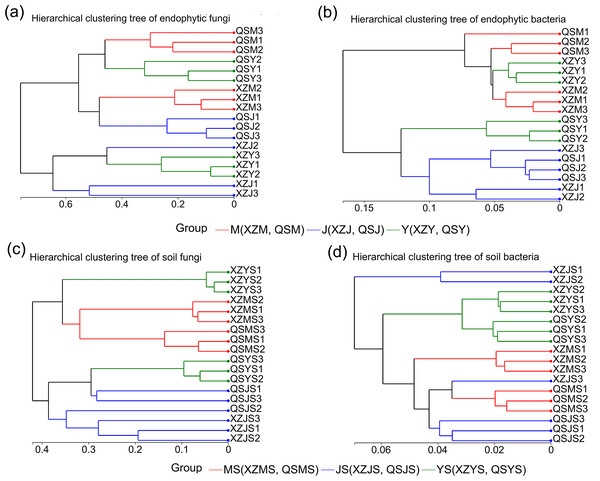
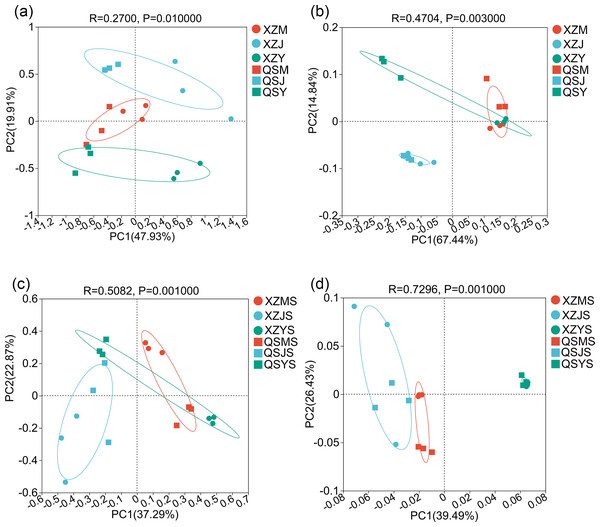
The structure of intergroup microbial communities was assessed through the utilization of ANOSIM, hierarchical clustering, and principal coordinates analysis (PCoA) at the operational taxonomic unit (OTU) classification level. ANOSIM analysis employed the Bray-Curtis distance algorithm to evaluate the statistical significance of group dissimilarities. The results show (Fig. 4) that the differences between the microbiological groups are more significant than those within the group. Additionally, the results of the hierarchical clustering analyses showed (Fig. 5) that plant or soil samples from the same transplanting stage and origin generally clustered together.
Figure 4: ANOSIM analysis of A. sinensis microbe in XZ and QS.
(A, B) The between and within group differences of bacteria and fungi in A. sinensi from XZ town. (C, D) The between and within group sample differences of bacteria and fungi in A. sinensis from QS town.Figure 5: Hierarchical cluster analysis of endophytic and soil microbes of A. sinensis in XZ and QS.
(A) Endophytic fungi. (B) Endophytic bacteria. (C) Soil fungi. (D) Soil bacteria.The results of PCoA analysis for endophytic fungi (Fig. 6A) revealed that each sample could be clearly distinguished from one another during transplantation. Samples from cellar planting roots (J) and dam medicinal roots (Y) were notably separated into distinct groups based on their respective origins. Similarly, the PCoA analysis results of endophytic bacteria showed (Fig. 6B) that most samples could be well separated from each other during transplantation. Samples from dam medicinal root samples (Y) exhibited a clear division into two groups based on their origin, and alpine seedling root samples (M) also displayed a tendency towards grouping by origin. These findings suggest significant alterations in endophytic fungi and bacterial communities during the transplantation process of A. sinensis. Furthermore, PCoA analysis results of soil fungi and bacteria showed (Figs. 6C, 6D) that soil samples from varying transplanting environments exhibit distinct separation. Soil fungi and soil bacterial samples from the dam (YS) and alpine (MS) environments demonstrate noticeable differences in origin, aligning with the observed pattern of A. sinensis endophytes varying with transplanting and displaying origin-specific distinctions.
Figure 6: PCoA analysis of endophytic and soil microorganisms in A. sinensis from two places of origin.
(A, B) Endophytic fungi and endophytic bacteria in XZ and QS. (C, D) Soil fungi and soil bacteria in XZ and QS.Microbial species composition and difference analysis during transplantation
Species composition and difference analysis of endophytic and soil bacteria in A. sinensis
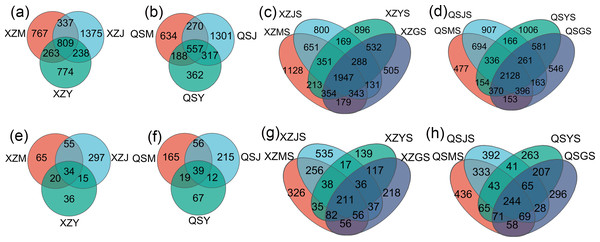
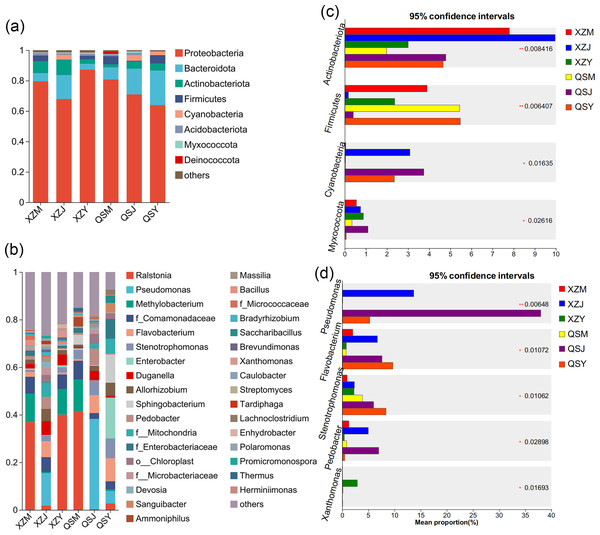
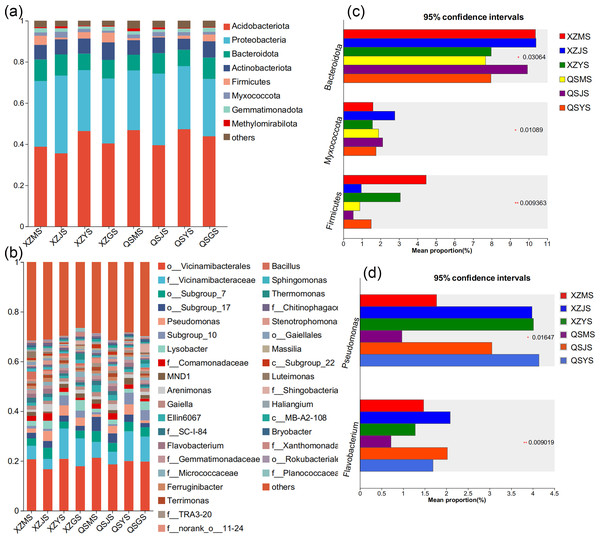
Analysis based on the Venn diagram at the OTU level of endophytic bacteria (Figs. 7A, 7B) reveals that shared endophytic bacteria across the three transplanting stages constituted 15.35% to 17.73% of the entire transplanting process. This suggests that only a fraction of endophytic bacteria persists during A. sinensis transplanting, while most species are either lost or introduced. Further examination of endophytic bacteria at the phylum and genus levels reveals significant insights. Bacterial groups with a relative abundance exceeding 1% were considered dominant in this study. At the phylum level (Figs. 8A, 8C), eight dominant bacterial phyla were identified in A. sinensis across the three transplanting stages in the two places of origin. Notably, cellar planting roots (J) exhibited a higher abundance of Actinobacteriota (p < 0.01), Myxococcota (p < 0.05) and Cyanobacteria (p < 0.05) compared to alpine seedling roots (M) and dam medicinal roots (Y), while Firmicutes (p < 0.01) were less prevalent. At the genus level (Figs. 8B, 8D), 28 dominant bacterial genera were observed across the transplanting stages in the two places of origin. The relative abundance of beneficial bacteria such as Pseudomonas (p < 0.01), Flavobacterium (p < 0.05) and Pedobacter (p < 0.05) was significantly higher in cellar planting roots (J) than in the other materials. Conversely, after transplanting to the dam area, the relative abundance of Stenotrophomonas (p < 0.05) and the potentially pathogenic bacteria Xanthomonas (p < 0.05) increased significantly.
Figure 7: Venn diagram of A. sinensis endophytes and soil microbes under OTU unit.
(A, B) The endophytic bacteria in XZ and QS towns respectively. (C, D) The soil bacteria in XZ and QS towns respectively. (E, F) The endophytic fungi in XZ and QS towns respectively. (G, H) The soil fungi in XZ and QS towns respectively.Figure 8: Dominant species composition and difference analysis of endophytic bacteria in A. sinensis.
(A, B) The endophytic bacterial species composition at the phylum and genus levels respectively. (C, D) The endophytic bacterial differential species at the phylum and genus level respectively.The Venn diagram based on the OTU level of soil bacteria (Figs. 7C, 7D) showed that 74.48% to 77.13% of the soil bacterial species differed among the three transplanting environments. Similarly, at the phylum and genus classification levels, bacterial groups with a relative abundance exceeding 1% were defined as dominant bacterial groups. At the phylum level, eight dominant bacterial phyla of soil bacteria were observed across the three transplanting environments (Figs. 9A, 9C), with six of these phyla overlapping with the dominant bacterial phyla of A. sinensis endophytes. The cellar rhizosphere soil (JS) exhibited a higher abundance of Actinobacteriota and Myxococcota (p < 0.05) and a lower abundance of Firmicutes (less than 1%, p < 0.01) compared to alpine (MS) and dam (YS) soil. It can be seen that the dominant phyla of soil bacteria have a similar abundance distribution to that of endophytic bacteria. Furthermore, analysis at the genus level revealed sixteen dominant bacterial genera across the three transplanting stages of A. sinensis from the two production areas (Figs. 9B, 9D). The relative abundance of Flavobacterium (p < 0.01) in the cellar rhizosphere soil (JS) was higher than that in the alpine (MS) and dam (YS) soil, while the relative abundance of Pseudomonas (p < 0.05) in the soil increased significantly after transplanting to the dam area. In summary, the soil bacterial species associated with A. sinensis varied significantly under different transplanting environments. These differences in soil bacteria likely contribute to the alterations in species composition and abundance of endophytic bacteria in A. sinensis.
Figure 9: Dominant species composition and difference analysis of soil bacteria in A. sinensis.
(A, B) The soil bacterial species composition at the phylum and genus levels respectively. (C, D) The soil bacterial differential species at the phylum and genus level respectively.Species composition and difference analysis of endophytic and soil fungi in A. sinensis
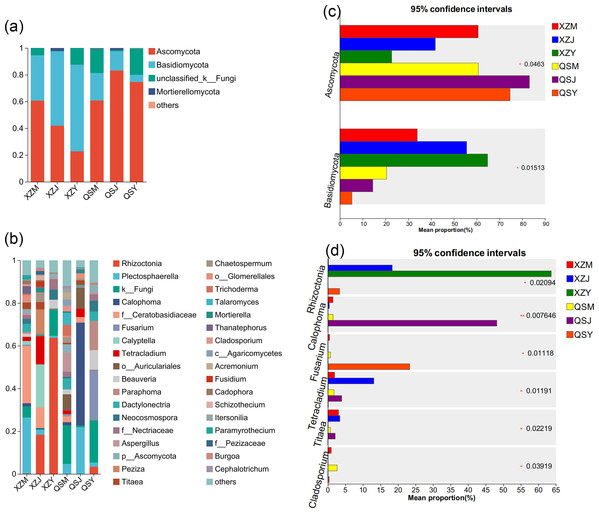
Analysis based on the Venn diagram at the OTU level of endophytic fungi (Figs. 7E, 7F) reveals that only 6.51% to 6.81% of endophytic fungi in A. sinensis were shared across all three transplanting stages, indicating a substantial turnover of endophytic fungi during the transplantation process. Analysis at the phylum level (Figs. 10A, 10C) revealed four dominant fungal phyla in A. sinensis from the two origins, with Ascomycota and Basidiomycota ranking highest and second-highest in relative abundance, respectively. Notably, Mortierellomycota were the unique dominant fungal phyla of cellar planting roots (J). In addition, analysis of endophytic fungi at the genus level showed (Figs. 10B, 10D) that a total of 20 dominant genera existed in the three transplanting stages of A. sinensis from the two origins. The relative abundance of beneficial fungi such as Cadophora, Tetracladium (p < 0.05) and Titaea (p < 0.05) was significantly higher in alpine seedling roots (M) and cellar planting roots (J) compared to dam medicinal roots (Y). Conversely, the relative abundance of Rhizoctonia (p < 0.05), Cladosporium (p < 0.01) and potential pathogenic fungus Fusarium (p < 0.05) peaked after transplanting to the dam area, with Mortierella uniquely present in cellar planting roots (J).
Figure 10: Dominant species composition and difference analysis of endophytic fungi in A. sinensis.
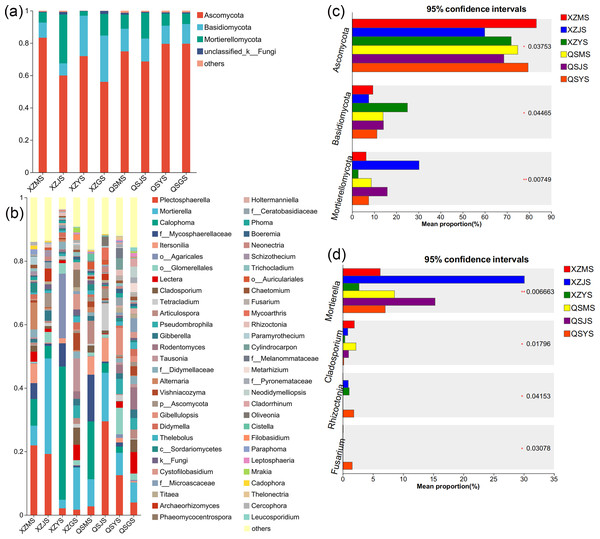
(A, B) The endophytic fungal species composition at the phylum and genus levels respectively. (C, D) The endophytic fungal differential species at the phylum and genus level respectively.The Venn diagram of soil fungal OTU levels (Figs. 7G, 7H) indicates that 90.23% to 90.65% of soil fungal species differ across the three transplanting environments. Similar to the dominant phyla of endophytic fungi, Ascomycota and Basidiomycota were the top two dominant fungal phyla in relative abundance within A. sinensis soil fungi (Fig. 11A). Mortierellomycota (p < 0.01) had the highest relative abundance in the cellar rhizosphere soil (JS) (Fig. 11C). Analysis at the genus level (Figs. 11B, 11D) identified 28 dominant genera in the three transplanting stages from the two origins, among which Cladosporium (p < 0.05) and Mortierella (p < 0.01) had the highest relative abundance in alpine (MS) and cellar (JS) soils respectively. The relative abundance of soil fungi Rhizoctonia (p < 0.05) and Fusarium (p < 0.05) peaked after transplantation to the dam area. In summary, the soil fungi of A. sinensis in different transplanting environments vary greatly. Soil fungi and endophytic fungi have similar changing patterns throughout all transplanting stages.
Figure 11: Dominant species composition and difference analysis of soil fungi in A. sinensis.
(A, B) The soil fungal species composition at the phylum and genus levels respectively. (C, D) The soil fungal differential species at the phylum and genus level respectively.Microbial co-occurrence network analysis of transplanted A. sinensis
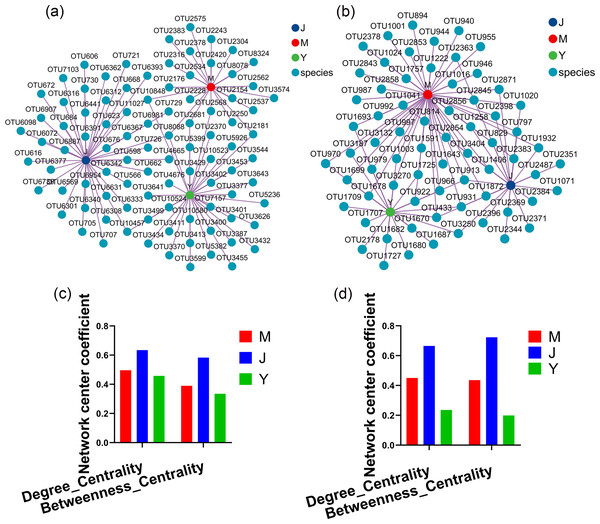
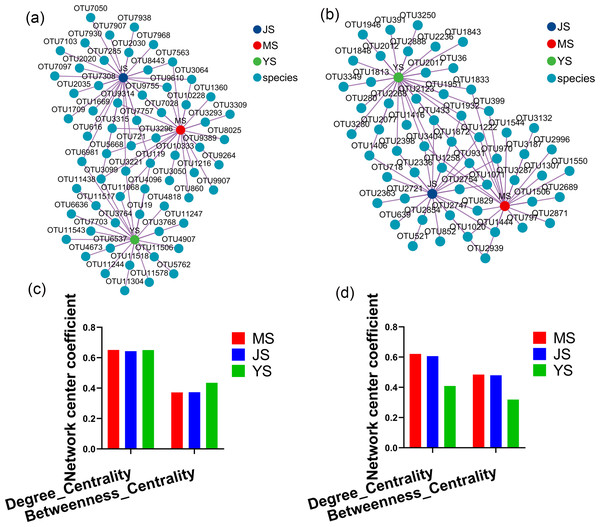
Evaluating the relative impact of the three transplanting stages on the formation of A. sinensis microbial communities through microbial co-occurrence network. Degree centrality and betweenness centrality serve as crucial indicators of the contribution of different transplanted sample nodes to network formation. A higher degree of centrality signifies stronger connectivity between a node and other nodes within the network, while a larger betweenness centrality indicates the node’s pivotal role in maintaining tight network connectivity. Analysis of degree centrality and betweenness centrality of A. sinensis endophytic bacteria (Figs. 12A, 12C) and endophytic fungi (Figs. 12B, 12D) at the nodes of cellar planting root (J) revealed higher values compared to nodes from the other two transplanting samples (J, Y), while nodes from dam medicine root (Y) exhibited the lowest degree centrality and betweenness centrality, indicating that A. sinensis endophytes in the cellar planting stage play a crucial role in maintaining the overall co-occurrence network’s tight connectivity. There is little difference in the degree centrality and betweenness centrality of A. sinensis soil bacteria (Figs. 13A, 13C) and soil fungi (Figs. 13B, 13D) in the alpine rhizosphere soil (MS) nodes and the cellar rhizosphere soil (JS) nodes, but they are both higher than those in the dam rhizosphere soil (YS) nodes, indicating that alpine and cellar soil make a relatively greater contribution to the soil microbial network structure of A. sinensis throughout the transplanting process.
Figure 12: Co-occurrence network analysis of endophytic microbes in transplanted A. sinensis.
(A, C) Network diagram of endophytic bacteria and their degree centrality and betweenness centrality values in three transplanted nodes: alpine seedling roots (M), cellar planting roots (J) and dam medicinal roots (Y). (B, D) Network diagram of endophytic fungi and their degree centrality and betweenness centrality values in three transplanted nodes: M, J, Y.Figure 13: Co-occurrence network analysis of soil microbes in transplanted A. sinensis.
(A, C) Network diagram of soil bacteria and their degree centrality and betweenness centrality values in three transplanted nodes: alpine rhizosphere soil (MS), cellar rhizosphere soil (JS) and dam rhizosphere soil (YS). (B, D) Network diagram of soil fungi and their degree centrality and betweenness centrality values in three transplanted nodes: MS, JS, YS.Function prediction analysis of microbial communities
Function prediction analysis of A. sinensis bacterial community
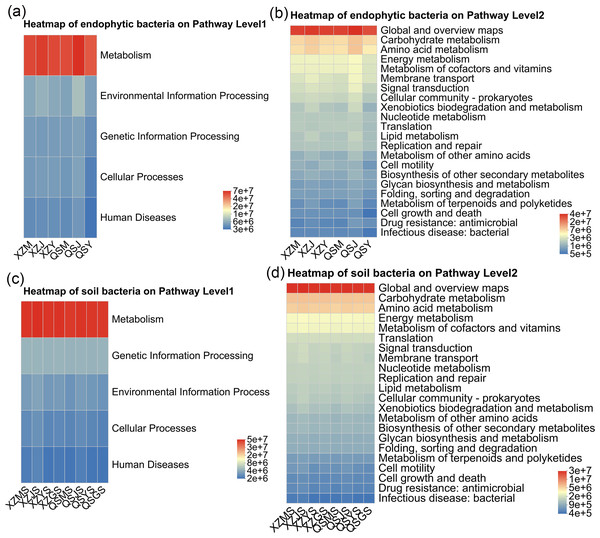
PICRUSt2 was employed to forecast the functional profiles of endophytic and soil bacteria within A. sinensis across various transplanting stages. Utilizing the KEGG pathway database, we selected the top five and top 20 abundant functions for first and second-level analyses, respectively. The predictions for endophytic bacterial functions revealed that, at pathway level 1 (Fig. 14A), “metabolism” represented the predominant category. At pathway level 2 (Fig. 14B), endophytic bacteria exhibited heightened activity in “carbohydrate metabolism” and “amino acid metabolism” during the cellar planting stage (J), whereas the expression of “bacterial infectious disease” was comparatively lower during the alpine seedling stage (M). Conversely, the soil bacterial function predictions indicated that “metabolism” was also the primary category at pathway level 1 (Fig. 14C). At pathway level 2 (Fig. 14D), the expression of “infectious disease” was lower in alpine rhizosphere soil (MS) and higher in cellar rhizosphere soil (JS).
Figure 14: Heatmap diagram of PICRUSt 2 functional prediction of endophytic and soil bacteria in A. sinensis under different transplanting stages.
(A, B) The function and abundance information of endophytic bacteria on Pathway Level 1 and Pathway Level 2 respectively. (C, D) The function and abundance information of soil bacteria on Pathway Level 1 and Pathway Level 2 respectively.Function prediction analysis of A. sinensis fungal community
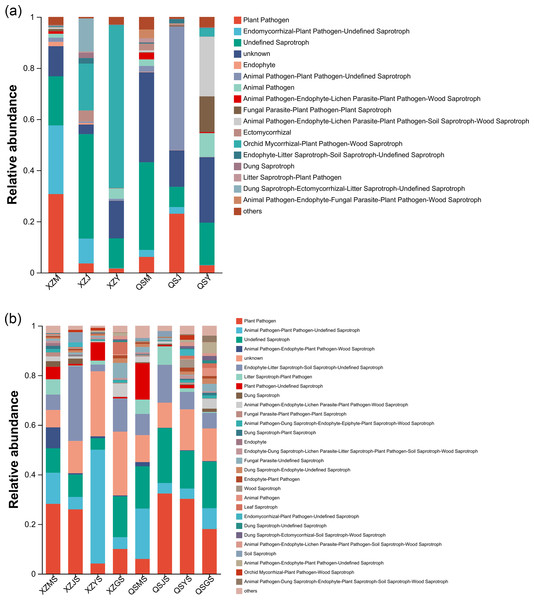
FUNGuild was employed to forecast the functional profiles of endophytic and soil fungi within A. sinensis across various transplanting stages. Employing the three nutritional modes of pathotroph, saprotroph and symbiotroph, endophytic fungi were further divided into 16 main guilds (Fig. 15A) based on their strategies for resource absorption and utilization. Symbiotrophic endophytic fungi were notably prevalent in the alpine seedling root (M) and cellar planting root (J), such as Ectomycorrhizal and Epiphyte. Endophytic fungi with symbiotrophic-saprotrophic type appeared more frequently in the cellar planting root (J). Conversely, endophytic fungi displaying a pathological-saprophytic-symbiotrophic type were predominantly observed in dam medicinal roots (Y). In addition, a total of twenty-seven major guilds were predicted from soil fungi (Fig. 15B). Soil fungi with symbiotrophic-saprotrophic type were prominent in cellar rhizosphere soil (JS), while those with pathological-saprotrophic type were mostly found in alpine and dam environments.
Figure 15: FundGuild function histogram of endophytic and soil fungi of A. sinensis at different transplanting stages.
(A) Functional classification and abundance information of endophytic fungi. (B) Functional classification and abundance information of soil fungi.Discussion
The plant endophytic microbial community is closely related to the soil microbial community. The soil flora provides a “seed bank” for the plant endophytic flora (Liu et al., 2019). Plants selectively recruit certain bacterial flora from the soil through root exudates (Zhong et al., 2022). Therefore, besides the plant’s positive impact on the microbial community composition, the soil environment is also an important factor in determining the structure of the plant endophytic flora (Liu et al., 2014). Zhang et al. (2021) analyzed the differences in the community structure of non-rhizosphere soil, rhizosphere soil and roots of Ligusticum chuanxiong, and found that plants obtain some species from the soil flora and occur regularly accumulation or depletion, eventually forming a specific endophytic flora (Zhang et al., 2021). Xiao et al. (2023) studied the differences in the bacterial community structure of L. chuanxiong in mountainous areas and dam areas based on the “Mountain Breeding and Dam Cultivation” planting model, and found that changes in the soil flora of off-site transplantation shaped the recombination of endophytic bacteria in L. chuanxiong (Xiao et al., 2023).
In this study, A. sinensis developed distinct endophytic microbial communities at different transplanting stages, and the patterns of change in these communities were consistent with those observed in the soil. Significant differences in endophytic microbial communities were observed among different transplanting regions, which aligned with the variations in soil microbial communities under different environmental conditions. At the phylum level, the dominant taxa of endophytic microbes overlapped with those of soil microbes, and their abundance distributions were similar. At the genus level, the abundance dynamics of certain dominant endophytic genera, such as Flavobacterium and Pseudomonas, as well as potential pathogenic genera like Rhizoctonia and Fusarium, mirrored their patterns in the soil across different transplanting stages. Additionally, transplanting induced more pronounced reorganization of endophytic fungi, which was associated with greater differences in soil fungal communities compared to soil bacteria across different transplanting environments. These findings suggest that transplanting essentially drives the reorganization of host endophytic microbiota through changes in the soil microbial community. The soil environment and plant-microbe interactions are crucial for understanding the mechanisms underlying microbial recombination. For instance, soil physicochemical properties can lead to distinct compositions of rhizosphere microbial communities (Shi et al., 2023). Alpine forest soils exhibit higher soil organic carbon content and more acidic pH values, where the “fungal-dominated” soil type plays a significant role (Dong et al., 2023). Additionally, topographic factors (alpine vs. dam area) drive microbial community recombination, with a higher prevalence of gibberellin-producing and auxin-producing strains isolated from alpine environments, indicating the existence of plant growth-promoting microorganisms specifically adapted to montane habitats (Kang et al., 2021). Moreover, the development of rhizosphere effects by plant roots modifies soil properties in the root proximity zone, thereby governing microbial recruitment processes. Changes in rhizosphere microbial communities directly impact the recombination of the endophytic microbiota (Wu et al., 2024). Simultaneously, microorganisms regulate endogenous phytohormones by secreting exogenous phytohormones, demonstrating a symbiotic dialogue between plants and their microbes. This interaction not only influences endophytic microbiota recombination but also modulates plant growth and stress resistance. For example, in L. chuanxiong, endophytic microbiota recombination enhances the development of buds, stem nodes, and internodes, as well as increases the activities of peroxidase, catalase, and phenylalanine ammonia-lyase (Kang et al., 2021). However, the direct physiological impacts of A. sinensis endophyte dynamics on host growth physiology require further experimental investigation. In conclusion, transplanting fundamentally represents a process of host endophytic flora recombination, driven by alterations in the soil microbial community. This process involves multiple mechanisms, including soil properties, topographic factors, rhizosphere effects, and plant-microbe interactions.
Microbial community reorganization holds positive significance for the overwintering of A. sinensis in the cellar planting stage and for reducing the disease threat in dam cultivation satge. During the cellar planting stage, A. sinensis accumulates some psychrophilic flora, which helps A. sinensis adapt to the low-temperature environment and reduces the physiological damage during overwintering. Notably, several microbial taxa show significantly higher relative abundance during this stage, including endophytic fungi Tetracladium and Titaea, endophytic bacteria Pseudomonas, Flavobacterium, Pedobacter, and Duganella, as well as soil fungus Cadophora and soil bacterium Gaiella (Gupta et al., 2020; Goh, Jeon & Mun, 2022; Zhao et al., 2022). The enrichment of these psychrophilic microorganisms contributes to the plant’s survival and physiological maintenance under low-temperature stress conditions. In addition, endophytic fungi Rhizoctonia, Fusarium, Cladosporium and endophytic bacteria Xanthomonas are common plant pathogenic microorganisms (Weller et al., 2002). Fusarium is a typical A. sinensis pathogenic fungi (Zhang, 2021), and Xanthomonas can cause bacterial black stem rot of A. sinensis (Han et al., 2002). The relative abundance of these pathogens in the alpine seedling and cellar planting stages of A. sinensis is significantly lower compared to the dam cultivation stage. This suggests that the transplantation process may reduce the ecological niche occupation by pathogens, thereby mitigating the disease threat in the final dam cultivation stage. Transplanting also encourages the accumulation of beneficial microbes. The dim environment of the cellar promotes the growth of saprophytic functional fungi, such as Mortierella, which reaches its highest relative abundance during the cellar planting stage. These fungi contribute to the decomposition of soil organic matter, replenishing soil nutrients and supporting A. sinensis growth in a cold and dark conditions (Li et al., 2020; Wang et al., 2022). Moreover, Mortierella has a competitive advantage in soils that inhibit Fusarium pathogens (Xiong et al., 2017). In the dam cultivation soil, the relative abundance of Pseudomonas is the highest. As primary decomposers of simple carbohydrates in soil organic matter, Pseudomonas species actively compete for rhizosphere ecological niches and participate in carbohydrate metabolism processes (Lugtenberg & Kamilova, 2009; Waldrop & Firestone, 2004; Vélez et al., 2020). This microbial activity contributes significantly to the observed high metabolic activity of carbohydrate metabolism in the dam cultivation soil. Furthermore, Pseudomonas may play a protective role through competitive exclusion of pathogens, potentially inhibiting their growth and colonization in the rhizosphere (Haas & Défago, 2005; Kamilova, Lamers & Lugtenberg, 2008).
The transplanting mode of “alpine seedling–cellar planting–dam cultivation” is of great value in alleviating the continuous cropping obstacle of A. sinensis. A. sinensis is not suitable for continuous cultivation, and continuous cultivation can easily lead to the deterioration of cultivated soil conditions, which in turn leads to plant diseases such as ditylenchus destructor and root rot (Zhang, 2009). Even when crop rotation (Bai et al., 2019; Ma et al., 2009) or intercropping (Wang et al., 2013) isemployed, the cultivation efficiency of A. sinensis is still unsatisfactory, and soil microecological factors are an important reason (Bai, 2021). In this study, it is found that during the alpine seedling stage, the bacteria with infectious disease functions are decreased. At the cellar planting stage, more saprotrophic-symbiotrophic fungi are accumulated. And in the dam cultivation stage, the carbohydrate metabolism function of bacteria is enhanced. These factors jointly ensure the successful production of A. sinensis in the final dam cultivation stage.
In summary, transplanting triggers a restructuring of Angelica’s microecology. The development of endophytic microbial communities within A. sinensis plants across various transplantation stages signifies the plant’s adaptive response to environmental shifts. Notably, soil flora dominate this reorganization of endophytic flora. The recombinant flora not only helps A. sinensis to overwinter in the cellar but also mitigates challenges associated with continuous cropping during the alpine seedling stage and minimizes disease risks during dam cultivation. Moreover, this phenomenon endows the practice of transplanting with profound scientific significance, offering a pathway for revitalizing A. sinensis germplasm. Thus, it presents a promising cultivation model worthy of further exploration, applicable not only to crop production (Cheshmi et al., 2023; Chang et al., 2023) but also holding significant implications for the cultivation and production of medicinal plants (Chakraborty et al., 2017).
Conclusion
Transplanting significantly influences the microbial recombination within A. sinensis, particularly impacting the composition of endophytic flora. The variation in soil flora across transplanting emerges as a crucial factor driving this reorganization. Notably, the recombination of fungi appears more pronounced compared to bacteria.
The transplanting process not only diminishes disease threats during A. sinensis cultivation but also fosters the accumulation of beneficial bacteria, aiding the plant’s adaptation to varying transplanting environments. For instance, pathogenic bacteria such as Fusarium and Xanthomonas decrease post-transplanting, while psychrophilic bacterial groups like Tetracladium, Titaea, Pseudomonas, and Flavobacterium thrive during the cellar planting stage.
Furthermore, the altered soil microbiota caused by transplanting creates favorable conditions for regulating the health and stability of the A. sinensis micro-ecosystem, serving as a reliable method to restore and enhance the quality of medicinal plant germplasm.
Supplemental Information
Alpha diversity index of endophyte and soil microorganisms in A. sinensis
Within the same sample type, different lowercase letters indicate significant differences among the three transplanting samples (ANOVA, p < 0.05).