RAD51 expression and prognostic impact in patients with stomach adenocarcinoma
- Published
- Accepted
- Received
- Academic Editor
- Vladimir Uversky
- Subject Areas
- Biochemistry, Bioinformatics, Cell Biology, Oncology
- Keywords
- Stomach neoplasms, RAD51 protein, Biomarkers, Prognosis
- Copyright
- © 2025 Jian et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. RAD51 expression and prognostic impact in patients with stomach adenocarcinoma. PeerJ 13:e19179 https://doi.org/10.7717/peerj.19179
Abstract
Background
Stomach adenocarcinoma (STAD) is the most common gastrointestinal cancer. A clear diagnosis and molecular targeted therapy have important implications for prolonging survival of patients. RAD51 is the central catalyst of homologous recombination that plays important role in maintaining genomic integrity. However, the clinical significance of RAD51 expression in STAD patients remains unclear. This study aimed to assess the association of RAD51 expression with clinicopathological characteristics and patient outcomes.
Methods
In this study, RAD51 mRNA expression in STAD patients was assessed using the UALCAN and GEPIA databases. The diagnostic value of RAD51 was evaluated by analyzing the ROC curve (data from the The Cancer Genome Atlas (TCGA) database). The protein expression level of RAD51 in STAD patients and its relationship with clinicopathological characteristics and prognosis were evaluated by immunohistochemistry. Co-expression analysis of RAD51 in STAD was performed by Coexpedia and Gene Expression Profiling Interactive Analysis (GEPIA) databases. The associations of RAD51 and its co-expression genes with immune infiltrates were analyzed in TIMER database.
Results
Our bioinformatic analysis revealed that RAD51 demonstrates elevated expression in STAD. The ROC curve analysis yielded an AUC value of 0.9366 (95% CI [0.9075–0.9658]), confirming its potential as a biomarker for STAD. Immunohistochemical assessments validated the up-regulation of RAD51 in STAD, highlighting its significant correlation with TNM stage and T stage, but not with age, sex, grade, N stage, M stage, or P53 expression. Patients exhibiting high RAD51 expression exhibited significantly reduced overall survival. Multivariate analysis identified RAD51 expression may serve as an independent prognostic biomarker of poor prognosis in patients with STAD. Additionally, our bioinformatic analysis identified eight RAD51 co-expression genes (AURKA, CKS1B, NUSAP1, PFDN4, CCNE1, CDCA4, KIF4A, and MCM10) in STAD. Moreover, we discovered that RAD51 and its main co-expressed genes were significantly negatively associated with most or all immune cell infiltration.
Conclusions
RAD51 overexpression was related to disease progression and poor prognosis, as well as infiltration of immune cells in gastric cancer.
Background
Stomach adenocarcinoma (STAD) is the most common digestive tract tumor with a high incidence and mortality rate (Zhao, Zhang & Sun, 2022). Despite improvements in the clinical treatment of STAD in recent years, the survival rate of patients with STAD remains very low, this is largely due to the paucity of effective methods to evaluate the prognosis of STAD patients (Guo et al., 2020b).
DNA double-strand breaks (DSBs) are considered the most toxic form of DNA damage (Li et al., 2012). Homologous recombination repair (HRR) is a crucial pathway responsible for repairing DSBs (Liu et al., 2016). It is an error-free repair pathway and only occurs in the late S/G2 phase of the cell cycle when the sister chromatid is in close proximity (IJ et al., 2021). Defects in HR repair not only promote cancer development due to increased genomic instability but also lead to an increased sensitivity to radiotherapy and chemotherapy in cancer treatment (Fernandes et al., 2018).
The RAD51 protein is characterized by a ∼230 amino acid domain containing two ATPase motifs called Walker A and Walker B (Orhan et al., 2021). It is an essential factor for HR as it forms a nucleoprotein filament on single-stranded DNA (ssDNA) that catalyzes strand invasion into intact homologous double-stranded DNA (dsDNA) (Muraszko et al., 2021; Peng et al., 2015). Researchers have demonstrated that alteration in RAD51 levels leads to chromosomal aberrations, mutagenesis, and cell death (Bhatla et al., 2008). Even minor changes in RAD51 may cause genetic instability and cancer (Shkundina et al., 2021). Additionally, RAD51 plays a role in the repair and restart of stalled DNA replication forks (He et al., 2015). Cancer cells treated with genotoxic agents can appear replication fork stalling and even collapse. Therefore, increasing RAD51 levels may prevent massive fork collapse and protect cancer cells from DNA breakage.
RAD51 expression and activity are typically subject to stringent regulation to minimize chromosomal recombination abnormalities. However, numerous studies have reported a significant upregulation of RAD51 in various human cancers. Han et al. analyzed differentially expressed genes in pancreatic cancer cells using cDNA microarrays, identifying 30 upregulated genes, among which RAD51 was notably elevated. RT-PCR analysis of frozen tissue samples from patients further confirmed the overexpression of RAD51. Immunohistochemical analysis using a pancreatic tumor tissue microarray revealed that RAD51 staining significantly increased in 74% of the samples, with strong signals detected in 13% of the samples (Han et al., 2002). Li et al. (2020) investigate the expression of RAD51 in oral squamous cell carcinoma (OSCC) and elucidate its relationship with pathological grade, clinical stage, and lymphatic metastasis potential. Their findings reveal that RAD51 expression is elevated in tumor cells compared to normal mucosal tissues. Moreover, RAD51 expression correlates with increased tumor differentiation. Notably, elevated RAD51 levels were observed in patients exhibiting lymphatic metastases, and these patients also exhibited higher relapse rates (Li et al., 2020). Liu & Weng (2022) conducted a bioinformatics analysis that revealed RAD51 overexpression in 28 distinct cancer types, with a notable association between RAD51 levels and decreased overall survival in 11 of these cancer types. Furthermore, RAD51 has been linked to cancer stemness, tumor mutational burden, and various immunomodulatory factors across diverse cancer types. Collectively, these findings suggest a potential role of RAD51 in the progression of human tumors. Several studies have investigated the association between RAD51 and gastric cancer. Data analysis by Redati et al. (2022) demonstrated that RAD51 is upregulated in adenocarcinoma of the gastroesophageal junction, and high RAD51 levels are associated with a poor prognosis. Additionally, Padua et al. (2022) found that negative expression of nuclear RAD51 is significantly associated with key pathological features in gastric cancer patients, including vascular invasion, lymph node metastasis, increased tumor size, and reduced overall and disease-free survival rates. However, the clinical significance of RAD51 expression in stomach adenocarcinoma (STAD) patients remains unclear. Given this, we carried out the current study to address this question.
Material and Methods
The workflow of the present study is shown in Fig. 1.
Figure 1: The workflow of this study.
Bioinformatics analyses
We analyzed the mRNA expression of RAD51 in carcinoma tissues and paracarcinoma tissues using the TIMER, GEPIA, and UALCAN databases. TIMER (https://cistrome.shinyapps.io/timer/) is a database used for the systematic analysis of immune infiltrates and gene expression across different types of cancer. GEPIA (http://gepia.cancer-pku.cn/index.html) is a web server database that provided differential gene expression analysis of 33 kinds of cancers based on integrated analysis of The Cancer Genome Atlas (TCGA) and Gene Tissue Expression (GTEX) databases, which was developed by Peking University. UALCAN (http://ualcan.path.uab.edu/analysis.html) is a comprehensive database providing diverse in-depth analysis of cancer data from The Cancer Genome Atlas (TCGA) and MET500 cohort data. ROC curves were drawn with GraphPad prism 8 (data from the TCGA database) to evaluate the diagnostic value of RAD51. We constructed the co-expression network of RAD51 in stomach neoplasms using Coexpedia (http://www.coexpedia.org/) which is a database that contains context-associated coexpression networks derived from microarray data inferred from individual series of human and mouse samples. Then we filtered out the coexpression genes of RAD51 in STAD by GEPIA. Correlations between RAD51 expression and various infiltrating immune cell types were investigated using the TIMER database.
Clinical materials
The gastric cancer tissue microarray (HStmA180Su09), which contained 90 paired carcinoma tissues and adjacent tissues, was purchased from Shanghai Outdo Biotech (Shanghai, China). All tissue samples were pathologically diagnosed. All patients with STAD received radical surgery between December 2009 and June 2010, with follow-ups up to June 2016. Detailed clinical characteristics, including patient age and sex, tumor grade, and TNM stage, P53 expression are shown in Table 1.
| Variables | RAD51 expression | Total | χ2 | P value | ||
|---|---|---|---|---|---|---|
| Low | High | |||||
| Age (year) | 0.951 | 0.330 | ||||
| ≤70 | 23 | 19 | 42 | |||
| >70 | 19 | 24 | 43 | |||
| T stage | 5.780 | 0.016 | ||||
| T1/T2 | 13 | 3 | 16 | |||
| T3/T4 | 26 | 29 | 55 | |||
| TNM stage | 3.970 | 0.046 | ||||
| I/II | 22 | 12 | 34 | |||
| III/IV | 18 | 25 | 43 | |||
| N stage | 1.730 | 0.188 | ||||
| N0 /N1 | 22 | 16 | 38 | |||
| N2/N3 | 20 | 26 | 46 | |||
| M stage | 0.000 | 0.987 | ||||
| M0 | 41 | 42 | 83 | |||
| M1 | 1 | 1 | 2 | |||
| P53 | 0.941 | 0.332 | ||||
| Negative | 21 | 17 | 38 | |||
| Positive | 21 | 26 | 47 | |||
| Sex | 2.363 | 0.124 | ||||
| Female | 13 | 7 | 20 | |||
| Male | 29 | 35 | 64 | |||
| Grade | 0.315 | 0.575 | ||||
| I/II | 25 | 23 | 48 | |||
| III/IV | 17 | 20 | 37 | |||
Immunohistochemistry
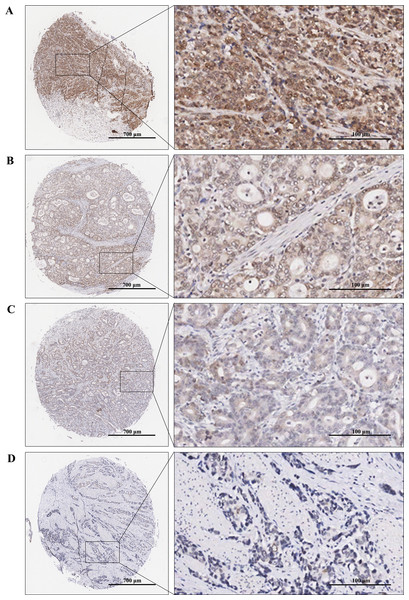
Immunohistochemical staining was performed to examine the expression levels of RAD51 in STAD and matched normal tissue. The tissue chip was placed in the oven at 60 °C for 1 h, then dewaxed and rehydrated by xylene and graded alcohol. Next, antigens were repaired by using the Dako automatic immunohistochemistry pretreatment system. Subsequently, tissue microarrays were stained with primary antibodies against RAD51 (ab133534; Abcam) followed by secondary antibodies and visualized with diaminobenzidine (DAB). Finally, the chip was counterstained by hematoxylin, dried, and sealed. Immunoreactive score (IRS) = SI (staining intensity) × PP (percentage of positive cells). SI was assigned as: 0 = negative; 1 = weak; 2= moderate; 3 = strong (Fig. 2). PP was defined as 0 = 0%; 1 = 0–25%; 2 = 26–50%; 3 = 51–75%; 4 = 76–100%. Finally, patients were divided into two groups: low RAD51 expression group (≤3) and high RAD51 expression group (>3) according to IRS.
Figure 2: TMA-IHC images of different staining intensities of RAD51 in STAD tissues.
(A) Strong intensity of RAD51 in STAD tissues. (B) Moderate intensity of RAD51in STAD tissues. (C) Weak intensity of RAD51 in STAD tissues. (D) Negative intensity of RAD51 in STAD tissues.Statistical analysis
The chi-squared test was used to evaluate the difference in RAD51 expression between STAD tissues and the adjacent non-tumor tissues. Chi-squared test was also employed to analyze the correlations between RAD51 expression and patient clinical parameters. Patient survival was analyzed via Kaplan–Meier analysis and log-rank tests. Independent prognostic factors of survival were identified with a multivariate Cox regression analysis. The factors identified as significant in univariate analysis were included in the multivariate Cox analysis. P values < 0.05 represented statistically significant.
Ethical statements
The ethical statement was obtained from Ethics Committee of Shanghai Outdo Biotech Company (number YBM-05-02).
Results
Patterns of RAD51 mRNA expressions in cancers
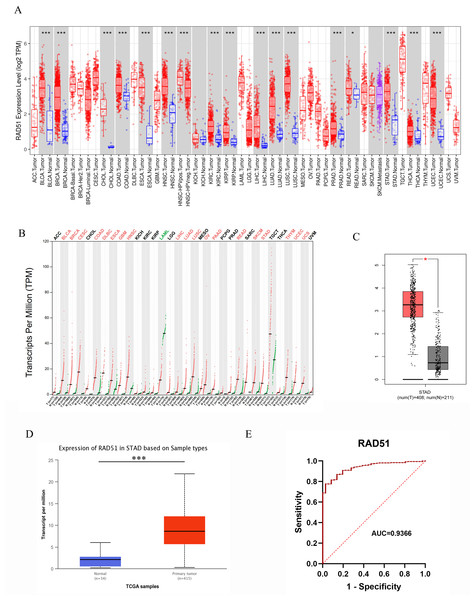
With the help of the TIMER database, we conducted a pan-cancer analyze of RAD51. The data analysis revealed that, among 19 types of solid tumors with normal tissue samples as controls, rad51 mRNA expression was significantly upregulated in tumor tissues of 17 cancer types. These cancer types included bladder urothelial carcinoma (BLCA), breast invasive carcinoma (BRCA), cholangiocarcinoma (CHOL), colon adenocarcinoma (COAD), esophageal carcinoma (ESCA), head and neck squamous cell carcinoma (HNSC), human papillomavirus-positive head and neck squamous cell carcinoma (HNSC-HPVpos), kidney renal clear cell carcinoma (KIRC), kidney renal papillary cell carcinoma (KIRP), liver hepatocellular carcinoma (LIHC), lung adenocarcinoma (LUAD), lung squamous cell carcinoma (LUSC), prostate adenocarcinoma (PRAD), rectal adenocarcinoma (READ), stomach adenocarcinoma (STAD), thyroid carcinoma (THCA), and uterine corpus endometrial carcinoma (UCEC). Notably, only two cancer types, namely kidney chromophobe (KICH) and skin cutaneous melanoma (SKCM), exhibited no statistically significant difference (p > 0.05) in rad51 mRNA expression levels between tumor tissues and normal tissues. Detailed data are shown in Fig. 3A.
Figure 3: RAD51 mRNA expression in cancers based on GEPIA and UALCAN databases.
(A) The RAD51 pan-cancer expression profile was analyzed using the TIMER database. (B) The RAD51 pan-cancer expression profile was analyzed using the GEPIA database. (C) RAD51 mRNA expression in STAD and normal tissues based on GEPIA database. (D) RAD51 mRNA expression in STAD and normal tissues based on UALCAN database. (E) ROC curve of RAD51.Pan-cancer analysis based on the GEPIA database revealed that, among 31 types of solid tumors with normal samples as controls, rad51 mRNA expression was significantly upregulated in tumor tissues of 19 cancer types. These cancer types included BLCA, BRCA, Cervical squamous cell carcinoma and endocervical adenocarcinoma (CESC), COAD, diffuse large B cell lymphoma (DLBC), ESCA, glioblastoma multiforme (GBM), HNSC, LIHC, LUAD, LUSC, ovarian serous cystadenocarcinoma (OV), pancreatic adenocarcinoma (PAAD), READ, skin cutaneous melanoma (SKCM), STAD, thymoma (THYM), UCEC, uterine carcinosarcoma (UCS). Furthermore, no significant difference in rad51 mRNA expression was observed in tumor tissues compared to normal tissues in 11 cancer types, including adrenocortical carcinoma (ACC), CHOL, KICH, KIRC, KIRP, lower-grade glioma (LGG), pheochromocytoma and paraganglioma (PCPG), PRAD, sarcoma (SARC), testicular germ cell tumors (TGCT), and THCA. Notably, rad51 mRNA expression was significantly downregulated in acute myeloid leukemia (LAML). These data are illustrated in Fig. 3B.
RAD51 mRNA expression in STAD
We analyzed the mRNA expression of RAD51 in 408 STAD tissues and 211 normal tissues using GEPIA database. The result indicated RAD51 mRNA levels were higher in STAD tissues than in normal tissues (Fig. 3C). We then used the UALCAN database to analyze the transcriptional levels of RAD51 in 415 STAD tissues and 34 non-tumor tissues (Fig. 3D). The result still showed that RAD51 levels were up-regulated in STAD tissues, consistent with the results of TIMER and GEPIA databases.
The value of RAD51 in diagnosis of STAD
To ascertain the diagnostic potential of RAD51 in STAD, an ROC curve analysis was performed. The resultant AUC value of 0.9366 (95% CI [0.9075–0.9658]) substantiates its promising role as a biomarker for STAD.
RAD51 protein expression and its association with clinicopathological features in STAD
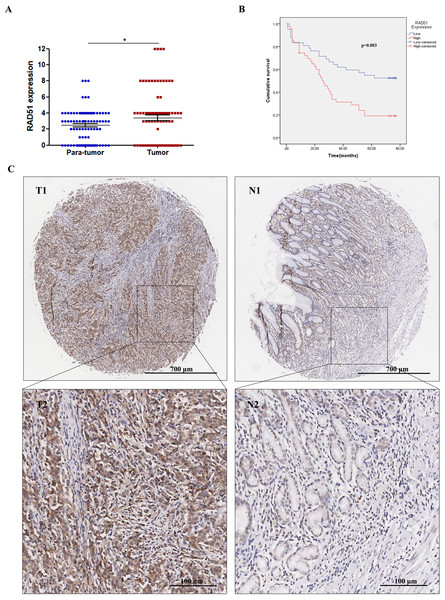
Through IHC staining of 90 paired STAD tumor tissues and adjacent normal tissues, we found that RAD51 was upregulated in STAD tissues compared with the normal adjacent non-cancerous tissues (Fig. 4A). Relationships between RAD51 protein expression and clinicopathological parameters of patients with STAD were investigated. The experimental results showed that RAD51 expression was associated significantly with TNM stage and T stage, but not with age, sex, grade, N stage, M stage, and P53 (Table 1).
Figure 4: RAD51 protein expression and its correlation with survival in STAD patients.
(A) The level of RAD51 expression in STAD tissues was significantly increased compared with the paracancerous tissues (P < 0.05). (B) The patient group with higher RAD51 expression had shorter overall survival than the patient cohort with lower RAD51 expression (P < 0.01). (C) RAD51 protein expression in STAD tissue samples and corresponding non-cancer tissue samples.Relationship of RAD51 expression with STAD patient survival
We investigated the association between RAD51 expression and the overall survival of STAD patients. The results suggested that high RAD51 expression was significantly correlated with poor survival (Fig. 4B). In addition, a Cox univariate survival analysis revealed that RAD51 expression, grade, age, T stage, N stage, M stage, and TNM stage were significant parameters affecting the survival time of patients with STAD (Table 2). These seven significant factors were subsequently substituted into the multivariate Cox survival analysis, which indicated that high RAD51 expression level, grade, and M stage were independent predictors of survival (Table 2).
RAD51 co-expression network analysis in STAD
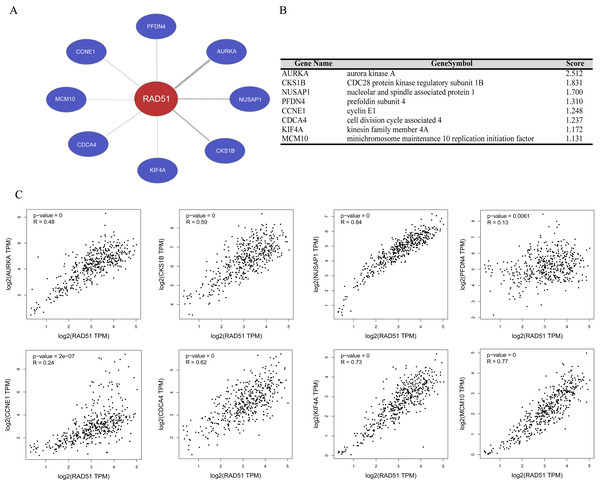
We performed gene co-expression analysis using Coexpedia database, and eight co-expressed genes of RAD51 were identified in stomach neoplasms. As seen in Figs. 5A and 5B, these eight genes are Aurora Kinase A (AURKA), CDC28 protein kinase regulatory subunit 1B (CKS1B), Nucleolar and spindle-associated protein 1 (NUSAP1), prefoldin subunit 4 (PFDN4), cyclin E1 (CCNE1), cell division cycle associated 4 (CDCA4), (kinesin family member 4A) KIF4A and minichromosome maintenance 10 replication initiation factor (MCM10). The GEPIA database mining revealed that there were significant positive correlations between these genes and RAD51 in STAD tissues (Fig. 5C).
Associations of RAD51 and its co-expression genes with immunity
Based on the TIMER database, we analyzed the associations of RAD51 and its co-expression genes with immune infiltration (purity, B cell, CD8+T cell, CD4+T cell, macrophage, neutrophil, dendritic cell) in STAD tissues. As shown in Fig. S1, we showed that the expression of RAD51 was significantly negatively correlated with the infiltration of B cells (partial. cor = −0.27, P = 1.46e−07), CD4+ T cells (partial. cor = −0.251, P = 1.21e−06), and Macrophage (partial. cor = −0.295, P = 6.97e−09). Similarly, co-expressed genes with RAD51 were significantly negatively associated with most or all immune cells (Fig. S1).
| Variables | Univariate analysis | Multivariate analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | |||
| RAD51 expression | 2.286 | 1.306–3.999 | 0.004 | 2.071 | 1.068–4.017 | 0.031 | ||
| Sex | 1.359 | 0.682–2.709 | 0.383 | |||||
| Grade | 1.645 | 1.048–2.584 | 0.031 | 2.019 | 1.053–3.870 | 0.034 | ||
| Age | 1.953 | 1.127–3.383 | 0.017 | 1.297 | 0.701–2.400 | 0.407 | ||
| T stage | 1.860 | 1.222–2.830 | 0.004 | 1.566 | 0.790–3.106 | 0.199 | ||
| N stage | 1.648 | 1.276–2.129 | 0.000 | 1.488 | 0.880–2.516 | 0.138 | ||
| M stage | 9.562 | 2.099–43.548 | 0.004 | 11.044 | 1.209–100.882 | 0.033 | ||
| TNM stage | 2.969 | 1.792–4.918 | 0.000 | 0.835 | 0.286–2.439 | 0.742 | ||
| P53 | 0.665 | 0.389–1.136 | 0.135 | |||||
Figure 5: RAD51 co-expression network analysis in STAD.
(A) Co-expression networks of RAD51 in stomach neoplasms based on Coexpedia database. (B) Co-expression genes with RAD51 were ranked according to their scores. (C) Correlation analysis of RAD51 expression with AURKA, CKS1B, NUSAP1, PFDN4, CCNE1, CDCA4, KIF4A and MCM10 in STAD tissues based on the GEPIA database.Discussion
In the present study, we found that both the mRNA and protein levels of RAD51 were significantly upregulated in STAD patients. The ROC curve analysis yielded an AUC value of 0.9366 (95% CI [0.9075–0.9658]), confirming its potential as a biomarker for STAD. The overexpression of RAD51 results in excessive and improper recombination, which leads to genomic instability, which eventually may lead to malignant transformation, and tumor progression. Also, increasing RAD51 levels can prevent massive fork collapse and protect cancer cells from DNA breakage, which may contribute to tumor drug resistance (Delabaere et al., 2017; Garcin et al., 2019).
We further investigated the relationship between RAD51 expression and the clinicopathological characteristics of patients with STAD. The results showed that RAD51 expression was associated significantly with TNM stage. It has been reported that RAD51 overexpression in two models of triple-negative breast cancer promoted brain metastases (Woditschka et al., 2014). In contrast, loss of RAD51 also inhibited breast cancer metastasis not only in syngeneic mice but human xenografts and changed the metastatic gene expression profile of cancer cells (Wiegmans et al., 2014). There’s also a study finding that RAD51 induced tumor growth and metastasis in esophageal squamous cell carcinoma (Chiu et al., 2020). These studies indicated that high RAD51 expression is correlated with increased metastatic potential.
We also analyzed the association between RAD51 expression levels and the survival of patients with STAD. The results showed that STAD cancer patients with high expression of RAD51 had shorter survival. Several reports have revealed that patients with tumors (such as breast cancer, ovarian cancer, and neuroblastoma) characterized by high RAD51 expression indicated poor survival and decreased drug sensitivity (Feng et al., 2021; Wu et al., 2022; Xu et al., 2019). Based on the above evidence, we conjectured that RAD51 enhances DNA repair capacity, causing chemotherapy resistance in cancer cells and ultimately leading to poor prognosis and low survival rates.
Previous literature reported that P53 can modulate homologous recombination by regulation of RAD51 expression. We determined the expression of RAD51 and P53 in STAD tissues by IHC. The results revealed that there was no obvious correlation between RAD51 expression and P53 expression. Previous studies have indicated that P53 protein detected by immunohistochemistry seems to represent most mutant P53 (Feng et al., 2002). Sabine et al. found that P53 interaction with RAD51 influenced DNA recombination and repair and additional modifications of p53 by mutation affected this interaction (Buchhop et al., 1997). Based on the above results, we conjectured that P53 by mutation may influence its regulatory effect on RAD51.
In addition, we explored the co-expression genes with RAD51 in stomach neoplasms by the Coexpedia website. Our analysis showed that these genes were AURKA, CKS1B, NUSAP1, PFDN4, CCNE1, CDCA4, KIF4A, and MCM10. According to the literature, AURKA is reported to be highly expressed in multiple types of tumors and is significantly negatively correlated with survival (Chuang et al., 2016; Guo et al., 2018; Jian et al., 2021; Long et al., 2020; Wu et al., 2016). CKS1B can accelerate chemotherapeutic resistance in many types of cancers, while a decrease in CKS1B makes these tumor cells sensitive to chemotherapeutic drugs, suggesting that CKS1B is a promising treatment target in cancers (Shi et al., 2020). NUSAP1 promotes the tumorigenesis and progression of various types of cancer, including gastric cancer, prostate cancer, breast cancer, and Osteosarcoma. PFDN4 regulates extracellular vesicle secretion and promotes tumorigenesis in vivo (Gordon et al., 2017; Guo et al., 2020a; Wang et al., 2021; Wu et al., 2021). CCNE1 is found to be an oncogene in multiple cancer types, such as gastric cancer, colorectal cancer, prostate cancer, and non-small cell lung cancer (Zhang et al., 2020). Studies have demonstrated that KIF4A serves as an oncogene and is involved in multiple types of tumors, such as breast, colorectal, and oral cancer (Xu et al., 2018). MCM10 overexpression has also been reported to promote cell proliferation and predict poor prognosis in prostate cancer (Cui et al., 2018). Overall, overexpression of these RAD51 co-expressing genes is associated with cancer development and an unfavorable prognosis. By analyzing the GEPIA database, we found that there were significant positive correlations between these genes and RAD51 in STAD. This suggested that high RAD51 expression may be associated with tumor progression and poor prognosis in patients with STAD.
Immune cell infiltration is an important immune response to cancer cells and is positively correlated with good prognosis in various cancers (Shi et al., 2017). Interestingly, we found that RAD51 and its co-expression were significantly negatively associated with most or all immune cell infiltration. This result suggested that the expression level of RAD51 may indicate the level of immune infiltration level of tumor cells, providing a reference for the immunotherapy of STAD. Taken together with our prior studies, we conjectured that increased RAD51 levels may promote cancer progression and metastasis by inhibiting immune cell infiltration.
Conclusions
The main limitation of our study is that our sample size was not large enough, and the results need to be further verified by expanding the sample size. Clinical correlation studies involve a variety of complex variables, such as genetic polymorphisms in patients, differences in environment and lifestyle, diversity in drug responses, and changes in immune status, among others. These factors interact with each other, collectively influencing the onset, progression, and treatment response of diseases, thereby adding diversity and complexity to the research outcomes. Nevertheless, our data showed that RAD51 was highly expressed in STAD tissues and closely correlated with poor survival of STAD patients.