Genome-wide identification and characterization of the NF-Y proteins in Zanthoxylum armatum
- Published
- Accepted
- Received
- Academic Editor
- Rohit Upadhyay
- Subject Areas
- Bioinformatics, Genomics, Plant Science
- Keywords
- Zanthoxylum armatum, NF-Y transcription factor, Nucellar embryo formation, Tissue-specific expression
- Copyright
- © 2025 Zheng et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Genome-wide identification and characterization of the NF-Y proteins in Zanthoxylum armatum. PeerJ 13:e19142 https://doi.org/10.7717/peerj.19142
Abstract
Somatic embryogenesis from nucellar tissues is common in many Zanthoxylum plants. The nuclear factor Y (NF-Y) transcription factor is pivotal in this process. Despite its significance, the identification and functional analysis of the NF-Y transcription factor family in Zanthoxylum armatum (Z. armatum) remains unexplored. This study identified 67 ZaNF-Y transcription factors in the diploid Z. armatum genome, comprising 20 ZaNF-YA, 30 ZaNF-YB, and 17 ZaNF-YC genes. Gene duplication, conserved domain, and motif analyses revealed the similarity and specificity of ZaNF-Y members in functional evolution. Cis-element analysis suggested that plant hormones and various transcription factor families may regulate ZaNF-Y gene expression, impacting nucellar embryo formation. Expression analysis across tissues indicated that the expression of most ZaNF-Y genes, such as ZaNF-YB5, was low in female flowers. In contrast, ZaNF-YC1 was highly expressed in female flowers and young fruit, indicating their potential role in nucellar embryo formation. Additionally, protein association network analysis provided insights into the composition of ZaNF-Y complexes. Our study enhances understanding of ZaNF-Y transcription factors and provides a basis for harnessing apomixis in hybrid crop production.
Introduction
Apomixis is a form of asexual reproduction that occurs without fertilization stage, which is divided into sporophytic apomixis and gametophytic apomixis (Wang & Underwood, 2023). Dandelion is a typical plant that undergoes gametophytic apomixis, where the diploid egg cell forms an embryo through parthenogenesis (Underwood et al., 2022). Existing studies have shown that PARTHENOGENESIS (PAR) is a key regulatory gene for the apomixis in dandelion (Underwood et al., 2022). The ectopic expression of BABY BOOM1 (BBM1) gene in rice egg cell can also induce the formation of somatic embryos without fertilization (Khanday et al., 2019). In sporophytic apomixis, the embryo originates from somatic cells within the ovule, such as nucellus cells. Citrus is a typical plant exhibiting sporophytic apomixis. Previous studies have shown that the RWP-RK transcription factor CitRWP plays a crucial regulatory role in the process of apomixis in citrus (Wang et al., 2017). Zanthoxylum armatum DC., an aromatic plant of the Rutaceae family, is economically important for its applications in traditional Chinese medicine and cuisine (Hui et al., 2020). Several species in the Zanthoxylum genus, including Z. armatum, exhibit sporophytic apomixis, where somatic embryos develop from nucellar cells, a process similar to that in Citrus plants (Hojsgaard & Pullaiah, 2022). Studies on apomixis in Zanthoxylum have identified genes such as Zardc07021, which is homologous to the CitRWP gene in Citrus, known for its role in apomixis (Wang et al., 2017). Heterologous expression in Arabidopsis affects floral organ development (Wang et al., 2021). Overexpression of the D-class MADS-box transcription factor, ZbAGL11, from Zanthoxylum bungeanum, enables seed formation in normally emasculated Arabidopsis lines (Fei et al., 2021). Recently, preliminary analyses explored the function of the RWP-RK transcription factor family in apomixis of Z. armatum (Zheng et al., 2024). However, understanding the transcriptional regulatory mechanisms of somatic embryo formation from the nucellus in Z. armatum remains limited.
Nuclear factor Y (NF-Y), also called the CCAAT-box binding factor (CBF) or heme-associated protein, is a family of transcription factors conserved across eukaryotes (Mantovani, 1999; Myers & Holt 3rd, 2018). The family consists of three subunits: NF-YA, NF-YB, and NF-YC (Nardini et al., 2013). Typically, NF-YB and NF-YC form a heterodimer in the cytoplasm, which then binds to NF-YA in the nucleus to assemble a functional NF-Y heterotrimer complex (Gnesutta et al., 2017). This complex regulates downstream gene expression either through the NF-YA subunit’s N-terminal or by interacting with other regulatory proteins (Nardone, Chaves-Sanjuan & Nardini, 2017; Huang et al., 2015). NF-Y transcription factors play vital roles in plant growth, development, stress responses, and hormone signaling (Kavi Kishor et al., 2023; Zhang et al., 2023). For instance, overexpression of AtNF-YA5 enhanced the drought tolerance by reducing leaf water loss in Arabidopsis (Li et al., 2008). Similarly, overexpression of the homologous gene GmNF-YA5 in soybeans resulted in the same phenotype as AtNF-YA5 (Ma et al., 2020). Under long-day conditions, the overexpression of OsHAP5H (OsNF-YC9) delays heading in rice (Li et al., 2016). In soybean, GmNF-YC9 is a homologous gene to OsNF-YC9 and is involved in the regulation of drought and salt stress resistance, but it does not affect flowering time (Yu et al., 2024). This indicates that the functions of the NF-Y genes are both conserved and unique across different plants.
In recent decades, NF-Y family members have been extensively studied for their roles in embryo development. LEAFY COTYLEDON 1 (LEC1, AtNF-YB9), a key transcription factor, is essential for embryo maturation and exhibits a distinct expression pattern in embryonic tissues (Lotan et al., 1998; Lee et al., 2003). Overexpression of LEC1 in transgenic plants induces somatic embryo-like structures (Harada, 2001). Similarly, in rice, the LEC1 homolog OsNF-YB7 is predominantly expressed in embryos, and its disruption results in abnormal embryo formation (Niu et al., 2021). Other related proteins, like LEC1-like (LIL, NF-YB6), also play critical roles in embryo development (Kwong et al., 2003). In Arabidopsis, NF-YA3 and NF-YA8 are functionally redundant but collectively necessary for embryogenesis (Fornari et al., 2013). GhL1L1 regulates somatic embryogenesis in cotton by influencing auxin distribution (Xu et al., 2019). However, in Z. armatum, the identification and functional analysis of NF-Y transcription factors in nucellar embryo formation are still poorly understood. A clear understanding of expression patterns or CRISPR-based knockouts is vital for improving our knowledge of NF-Y function.
In this study, we identified 67 ZaNF-Y genes in Z. armatum, comprising 20 ZaNF-YA, 30 ZaNF-YB, and 17 ZaNF-YC genes. An in-depth analysis was conducted on these genes, including chromosomal localization, physicochemical properties, subfamily classification, conserved domains, and cis-elements. We also examined the potential functions of ZaNF-Y genes in nucellar embryo formation through tissue expression variations and protein interaction predictions. Our study provides a comprehensive understanding of ZaNF-Y transcription factors in Z. armatum and suggests promising directions for future research on the regulatory mechanisms of nucellar embryo formation.
Materials & Methods
Identification and basic characteristics of ZaNF-Y family members
Using the reported diploid Z. armatum genome data (https://doi.org/10.6084/m9.figshare.14400884.v1) and model profiles of NF-YA (PF02045) and NF-YB/C (PF00808) from the Pfam database (http://pfam-legacy.xfam.org/), we predicted the ZaNF-Y proteins with HMMER software (version 3.0), applying a cut-off value of 0.01 based on previous studies (Liu et al., 2021; Wang et al., 2021; Prakash et al., 2017). All ZaNF-Y proteins were screened using the National Center for Biotechnology Information Conserved Domain Database (https://www.ncbi.nlm.nih.gov/cdd/). We determined the location, strand, and coding sequences (CDS) of ZaNF-Y genes based on the Z. armatum genome data. The CDS of ZaNF-Y genes are shown in File S1. ProtParam is a tool that enables the calculation of physical and chemical properties for a protein sequence provided by the user. The physicochemical properties of ZaNF-Y proteins, including the number of amino acids, molecular weights, and pI, were obtained using ProtParam tools (http://web.expasy.org/protparam/). Subcellular localization of ZaNF-Y proteins was predicted using the WoLF PSORT tool (https://wolfpsort.hgc.jp/), which was trained on protein sequences of more than 14,000 organisms and predicted based on amino acid sequences, with an overall prediction accuracy of over 80%.
Phylogenetic analysis of ZaNF-Y family members
AtNF-Y protein sequences from Arabidopsis were downloaded from the Arabidopsis Information Resource website (http://www.arabidopsis.org/) as previously reported (Siefers et al., 2009). OsNF-Y protein sequences from rice were obtained from the MSU TIGR database (http://rice.uga.edu/) as previously reported (Yang et al., 2017). CgNF-Y protein sequences from Citrus grandis were sourced from the Citrus Pan-genome2breeding database (http://citrus.hzau.edu.cn/index.php/) as previously reported (Mai et al., 2019). The amino acid sequences of NF-Y proteins from Z. armatum, Arabidopsis, rice and Citrus grandis are shown in File S2. The Multiple Alignment using the Fast Fourier Transform program was used to align NF-Y proteins from Z. armatum, Arabidopsis, Oryza sativa, and C. grandis (Katoh, Rozewicki & Yamada, 2019). RaxmlGUI serves as a graphical user interface for RAxML, a highly popular software for phylogenetic inference utilizing maximum likelihood (Edler et al., 2021). Phylogenetic trees were constructed using the maximum likelihood method with 1,000 bootstrap replications in the raxmlGUI program (version 2.0) and only nodes with bootstrap values above 70% were considered reliable. These trees were annotated using EvolView (https://www.evolgenius.info/evolview-v2/).
Chromosomal location and gene duplication
The chromosomal locations of ZaNF-Y genes were determined and visualized using TBtools-II (Chen et al., 2023). MCScanX is one of the commonly used tools for detecting gene colinearity and evolutionary analysis (Wang et al., 2012). Segmental duplications of ZaNF-Y members were identified through the multiple collinear scanning toolkits (MCScanX) using parameters such as a match score of 50, a gap penalty of −1, a match size of 5, an e value of 1e−05, and a maximum gap count of 25 (Wang et al., 2012). The resulting collinearity file was visualized using TBtools-II (Chen et al., 2023). Homologous genes between Z. armatum and other species (Arabidopsis, O. sativa, and C. grandis) were identified, and synteny analysis was conducted using the MCscanX tool and visualized using TBtools-II (Chen et al., 2023; Wang et al., 2012).
Analysis of conserved structural domains and conserved motifs of ZaNF-Y proteins
Conserved structural domains of ZaNF-Y proteins were identified using CD-search (https://www.ncbi.nlm.nih.gov/Structure/bwrpsb/bwrpsb.cgi) (Wang et al., 2023). Conserved motifs in ZaNF-Y proteins were analyzed with MEME (https://meme-suite.org/meme/tools/meme) with the following parameters: A maximum of 10 motifs and motif widths ranging from six to 50. The results were visualized using TBtools-II (Chen et al., 2023).
Cis-element and binding transcription factors prediction of ZaNF-Y promoters
The promoters of ZaNF-Y genes (regions upstream of the start codon within two kb) were extracted from the Z. armatum genome data using TBtools-II software. PlantCare (Plant Cis-acting Regulatory Element) is a database focusing on plant cis-regulatory elements (such as promoters, enhancers, repressors, etc.), but its updates are lagging behind (Lescot et al., 2002). Plant Promoter Analysis Navigator (PlantPAN) is mainly used to predict transcription factor binding sites (TFBS) and binding transcription factors, with a wide range of species coverage and timely data updates (Chow et al., 2024). The promoter sequences of ZaNF-Y genes were submitted to PlantCARE (https://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and Plant Promoter Analysis Navigator (PlantPAN) (version 4.0) (http://plantpan.itps.ncku.edu.tw/plantpan4/promoter_analysis.php) for cis-element and binding transcription factors prediction. The results were visualized using TBtools-II (Chen et al., 2023). The promoter sequences of ZaNF-Y genes are shown in File S3. The cis-acting elements of ZaNF-Y genes are shown in File S4. The transcription factors that may be bound to the ZaNF-Ys’ promoter are shown in File S5.
RNA extraction and reverse transcription
To verify the expression of ZaNF-Y genes in different tissues, we collected female flowers, young fruits, stems, and leaves of Z. armatum, following a previous study (Zheng et al., 2024). Total RNA was extracted using the FastPure® Plant Total RNA Isolation Kit (Polysaccharides and Polyphenolics-rich) (Vazyme, Nanjing, China), and cDNA was prepared using the ABScript III RT Master Mix for qPCR with gDNA Remover (ABclonal, RK20428).
qRT-PCR analysis
qRT-PCR was performed using a 2X Universal SYBR Green Fast qPCR Mix (ABclonal, RK21203). The qRT-PCR reaction protocol consisted of one cycle at 95 °C for 30 s, followed by 40 cycles at 95 °C for 10 s and 60 °C for 30 s, with a melting curve generated automatically. The ZaGAPDH gene was used as an internal control according to our previous study (unpublished results), and the description are detailed in File S6. The relative expression levels of ZaNF-Y genes were calculated using the 2−ΔΔCt method. Statistical analyses were conducted using the International Business Machines Statistical Package for the Social Sciences software (version 27.0). The primers used in qRT-PCR are detailed in File S7. The raw data for Ct values are shown in File S8. The MIQE checklist is shown in File S6.
Protein association network analysis of ZaNF-Y proteins
STRING serves as an online database frequently employed to develop protein-protein interaction networks, scoring each interaction between target proteins (Szklarczyk et al., 2023). The protein association network of ZaNF-Y proteins was analyzed using STRING (version 12.0; https://cn.string-db.org/).
Statistical analysis
The qRT-PCR experiment included three biological and three technical replicates to ensure the reproducibility. Data were analyzed using one-way ANOVA with Tukey’s tests. Significant differences were defined as those with a P value less than 0.05.
Results
Genome-wide identification and physicochemical properties of ZaNF-Y Genes
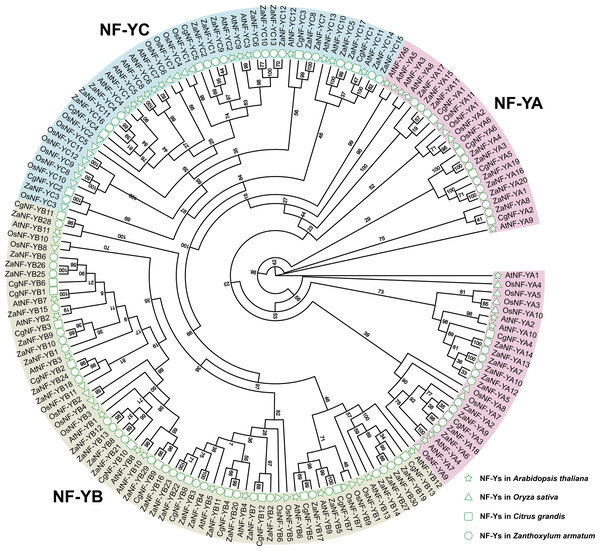
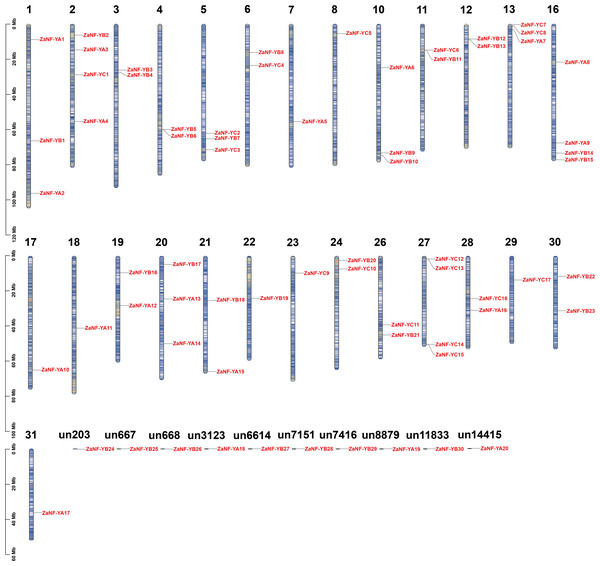
We identified 67 ZaNF-Y transcription factors in the Z. armatum genome using NF-YAs (PF02045) and NFYBs/Cs (PF00808) from the Pfam database. To classify these factors, we constructed a phylogenetic tree using amino acid sequences of 36 Arabidopsis AtNF-Y proteins (Siefers et al., 2009), 34 rice OsNF-Y proteins (Yang et al., 2017), and 24 pummelo CgNF-Y proteins (Mai et al., 2019) based on sequence similarity and conservation of NF-Y proteins among different species (Fig. 1). The ZaNF-Y proteins clustered into three subfamilies: NF-YA, NF-YB, and NF-YC. The NF-YA subfamily contains 21 members (ZaNF-YA1–ZaNF-YA20), the NF-YB subfamily contains 30 members (ZaNF-YB1–ZaNF-YB30), and the NF-YC subfamily contains 17 members (ZaNF-YC1–ZaNF-YC17) (Fig. 1, File S9). Based on chromosomal locations, 10 ZaNF-Y genes (ZaNF-YA18-20 and ZaNF-YB24-30) were not annotated to specific chromosomes (Fig. 2). Analysis of their physical and chemical properties revealed coding sequences ranging from 285 bp (ZaNF-YC3) to 1,020 bp (ZaNF-YA1), corresponding to amino acid sequences from 94 aa (ZaNF-YC3) to 339 aa (ZaNF-YA1), with molecular weights between 10.35 kDa (ZaNF-YC3) and 37.13 kDa (ZaNF-YA15) (File S9). The isoelectric points (pI) values ranged from 4.53 (ZaNF-YB14) to 9.89 (ZaNF-YA14) (File S9). Most ZaNF-Y members were predicted to localize in the nucleus (59), with a few in the cytoplasm (8) (File S9).
Figure 1: Phylogenetic relationships between ZaNF-Y proteins and other plant NF-Y proteins.
RaxmlGUI 2.0 was used to construct the maximum likelihood (ML) tree with 1,000 bootstrap replicates and displayed using EvolView.Figure 2: Chromosomal locations of the ZaNF-Y genes.
The vertical bars represent the chromosomes with numbers at the top of each bar representing chromosome number. “un” represents the unassembled scaffold. ZaNF-Y genes are numbered in the order of chromosomes.Gene duplication of ZaNF-Y genes
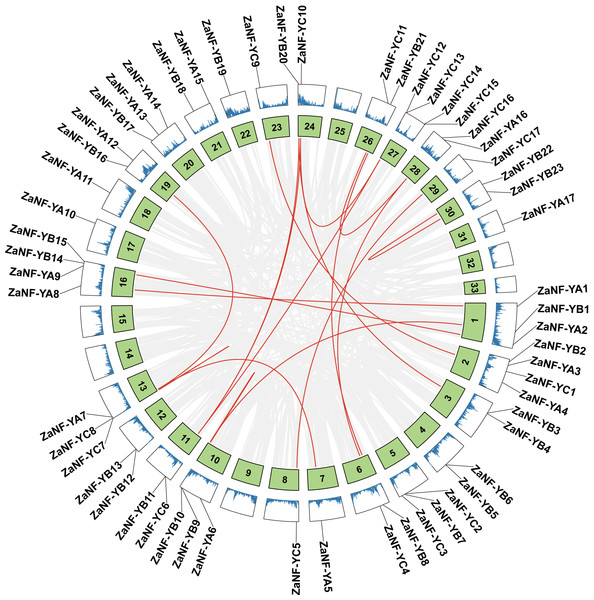
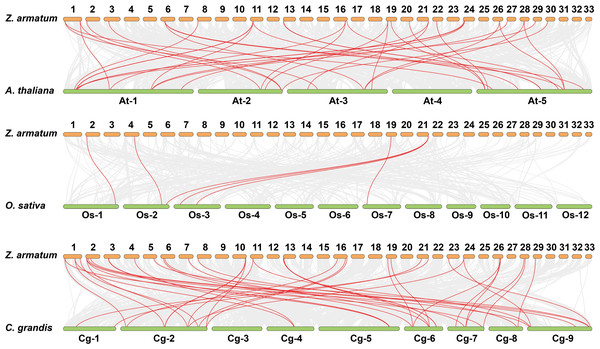
Gene duplications are crucial for gene expansion and evolution. Analyzing gene family expansions and duplications benefits significantly from collinearity information. For ZaNF-Y genes, 16 pairs of segmental duplications and two tandemly duplicated genes (ZaNF-YC12 and ZaNF-YC13) were identified (Fig. 3). Collinearity often indicates homologous sequences with potentially similar functions. We constructed three collinearity maps to examine collinearity between ZaNF-Y and NF-Y genes in Arabidopsis, O. sativa, and C. grandis (Fig. 4). The results identified 33, six, and 39 pairs of orthologous genes between Z. armatum and Arabidopsis, O. sativa, and C. grandis, respectively. Given the extensive research on NF-Y genes in other plants, identifying these homologous pairs can enhance our understanding of ZaNF-Y gene functions in Z. armatum.
Figure 3: Duplication analysis of the ZaNF-Y genes on different chromosomes.
The red lines represent collinearity relationship of ZaNF-Y genes. The gray lines connect all genes with a collinearity relationship in Z. armatum. Chromosome numbers are indicated by the texts in the green boxes. The blue lines in the outermost box indicate gene density. ZaNF-Y genes are indicated on the chromosomes with a black line.Figure 4: Synteny analysis of ZaNF-Y genes between Z. armatum, Arabidopsis thaliana, Oryza sativa, and Citrus grandis.
Za, Z. armatum; At, Arabidopsis thaliana; Os, Oryza sativa; Cg, Citrus grandis. The red lines indicate the collinearity relationship between NF-Y genes. Colinear gene pairs between Z. armatum and three other species are indicated by the gray lines in the backdrop.Conserved structural domains and motifs analysis of ZaNF-Y proteins
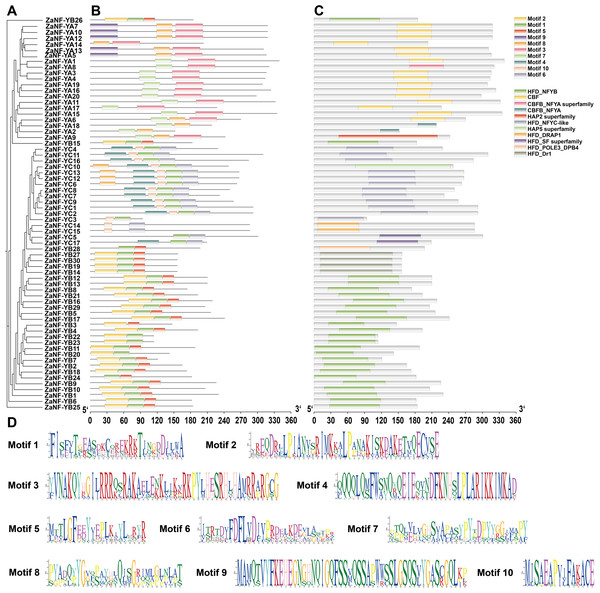
Phylogeny, conserved domains, motif information, and domain conservation were used to assess relationships among ZaNF-Y family members (Fig. 5). Using the MEME software, we identified 10 conserved motifs in ZaNF-Y proteins, ranging from 15 to 50 amino acids (Figs. 5B and 5D). The ZaNF-YB subfamily mainly contains Motifs 1, 2, and 5. Motifs 7 and 3 are predominant in the ZaNF-YA subfamily, while Motifs 4, 6, and 10 are common in the ZaNF-YC subfamily. Additionally, Motif 9 is unique to certain ZaNF-YA members (ZaNF-YA5, ZaNF-YA7, ZaNF-YA10, ZaNF-YA12, and ZaNF-YA13), suggesting it may play a crucial role in these genes. Conserved structural domains of ZaNF-Y proteins were also identified (Fig. 5C). The CBF domain characterizes the main NF-YA subfamily, while the HFD-NFYB domain is typical for most ZaNF-YB members. Most NF-YC subfamily members contain the HFD_NFYC-like domain. Some proteins possess unique domains; for example, ZaNF-YB28 has the HFD_POLE3_DPB4 domain, and the HFD_DRAP1 domain is exclusive to ZaNF-YC14 and ZaNF-YC15. These findings suggest that these proteins may have significant regulatory roles through their unique HFD domains.
Figure 5: Phylogenetic relationships, conserved motifs, and conserved structural domains of ZaNF-Y proteins.
(A) Phylogenetic tree of 67 ZaNF-Y proteins. (B) Distribution of conserved motifs in ZaNF-Y proteins. Colors distinguish each of the ten motifs. (C) Distribution of conserved domains in ZaNF-Y proteins. Each of the eleven domains is distinguished by different colors. (D) Sequences logos of the 10 conserved motifs in ZaNF-Y proteins.Cis-element analysis of ZaNF-Y promoters
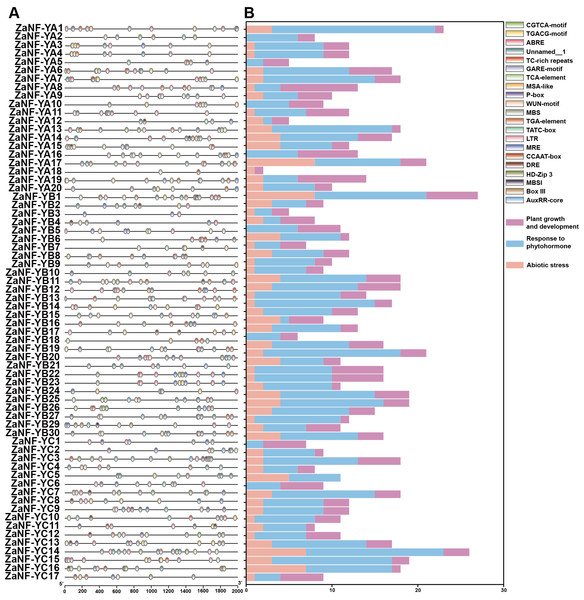
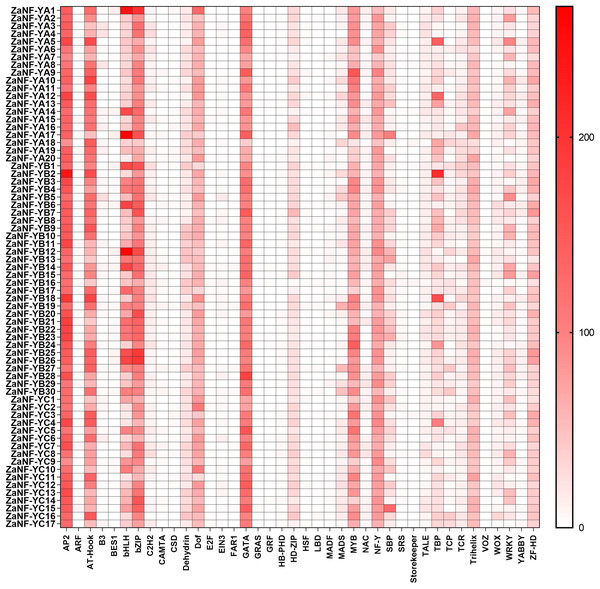
The PlantCare database is commonly used for analyzing cis-elements associated with factors like hormones and abiotic stresses in promoter. In order to determine whether ZaNF-Y genes are regulated by these factors during the formation of nucellar embryo, cis-elements in the promoter regions 2,000 bp upstream of the start codon were identified using PlantCare (Fig. 6, File S4). A total of 21 types of regulatory elements were identified in the promoter regions of 67 ZaNF-Y genes, classified into three categories as previous research: plant growth and development, phytohormone response, and abiotic stress (Hu et al., 2022). Most elements were phytohormone-responsive, including 170 ABA response elements, 34 auxin-responsive elements (23 TGA-element and 11 AuxRR-core), and 65 gibberellin-responsive elements (29 P-box, 20 GARE-motif, and 16 TATC-box). Plant growth and development-related elements formed the second largest category. Certain cis-elements were specific to particular ZaNF-Y genes, such as HD-Zip 3 (ZaNF-YA13) and Box III (ZaNF-YB1 and ZaNF-YB29). In addition, the PlantPan database is a useful tool for identifying transcription factors in promoter region. The results showed that the promoter regions of ZaNF-Y genes contained multiple transcription factor family members, such as AP2, AT-hook, bZIP, bHLH, and GATA (Fig. 7, File S5). These transcription factors may regulate ZaNF-Y gene expression, affecting physiological processes in Z. armatum, including nucellar embryo formation.
Figure 6: Cis-element analysis of the promoter of the ZaNF-Y genes.
(A) Location of cis-elements in the ZaNF-Y promoter regions. Each of the cis-elements is distinguished by different colors. (B) Statistical analysis of cis-elements for each ZaNF-Y gene in three categories.Figure 7: Statistical analysis of transcription factor binding sites in the ZaNF-Y promoter regions.
Binding sites are more abundant in colors with darker tints.Tissue-specific expression of ZaNF-Y genes
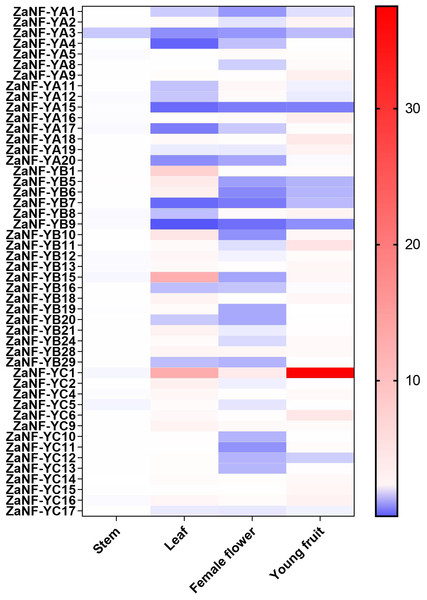
In the apomixis process of Zanthoxylum plants, female flowers can develop into complete fruits without pollination (Fei et al., 2021). During this process, nucellar cells develop into somatic embryos, which ultimately form seeds. To investigate the potential function of ZaNF-Y genes in nucellar embryo formation in Z. armatum, we measured their expression levels in female flowers and young fruits using quantitative RT-PCR (qRT-PCR) (Fig. 8). Expression levels in stems and leaves served as controls. Most ZaNF-Y genes exhibited low expression in female flowers, suggesting their involvement in regulating somatic embryogenesis. Specifically, ZaNF-YA1, ZaNF-YA3, ZaNF-YA15, ZaNF-YB5, ZaNF-YB6, ZaNF-YB7, ZaNF-YB9, and ZaNF-YC12 were significantly downregulated in female flowers and young fruits. The expressions of ZaNF-YB5, ZaNF-YB6, and ZaNF-YC12 were notably lower in female flowers and young fruits compared to stems and leaves. In contrast, ZaNF-YA9, ZaNF-YA16, ZaNF-YA18, and ZaNF-YC6 were highly expressed in young fruits, while ZaNF-YC1 was highly expressed in both female flowers and young fruits, indicating their potential roles in apomixis.
Figure 8: Expression patterns analysis of Za NF-Y genes in different tissues (stem, leaf, female flowers, and young fruit) based on the qRT-PCR data.
Different colours represent different expression levels. The expression levels of stem were used as control.Correlation analysis of apomixis-related ZaNF-Y genes
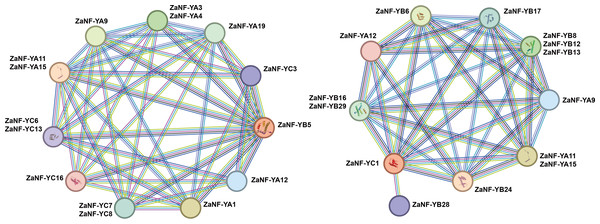
Previous research has demonstrated that ectopic expression of the LEC1 gene can induce somatic embryo-like structures (Harada, 2001). Phylogenetic tree analysis depicts that ZaNF-YB5 in Z. armatum is closely related to AtLEC1 (Fig. 1). Typically, NF-YB and NF-YC proteins form heterodimers, which then associate with NF-YA proteins to form heterotrimers. Using the STRING database, we analyzed the potential interacting proteins of ZaNF-YB5 (Fig. 9). The results indicated that ZaNF-YC3, ZaNF-YC6, ZaNF-YC7, ZaNF-YC8, ZaNF-YC13, and ZaNF-YC16 might interact with ZaNF-YB5. Additionally, ZaNF-YB5 could interact with ZaNF-YA1, ZaNF-YA3, ZaNF-YA4, ZaNF-YA9, ZaNF-YA11, ZaNF-YA12, ZaNF-YA15, and ZaNF-YA19, suggesting that these ZaNF-Y proteins might form heterotrimers with regulatory roles. Considering the high expression of ZaNF-YC1 in female flowers and young fruits, we also predicted its interacting proteins. The results indicated that members of the ZaNF-YA subfamily (ZaNF-YA9, ZaNF-YA11, ZaNF-YA12, and ZaNF-YA15) and members of the ZaNF-YB subfamily (ZaNF-YB6, ZaNF-YB8, ZaNF-YB12, ZaNF-YB13, ZaNF-YB16, ZaNF-YB17, ZaNF-YB24, ZaNF-YB28, and ZaNF-YB29) might interact with ZaNF-YC1. Some ZaNF-YA subfamily members (ZaNF-YA9, ZaNF-YA11, ZaNF-YA12, and ZaNF-YA15) were predicted to interact with both ZaNF-YB5 and ZaNF-YC1, suggesting these proteins might form heterotrimers with key regulatory roles in nucellar embryo formation.
Figure 9: Protein–protein association network analysis of ZaNF-Y proteins visualized by STRING.
Discussion
NF-Y transcription factors play vital roles in biological processes such as flowering, stress response, growth, and development, as shown in species like cabbage, melon, alfalfa, and poplar (Li et al., 2019; Jiang et al., 2023; Li et al., 2023; An et al., 2022; Liu et al., 2021). Despite their importance, NF-Y family members in Z. armatum have remained unexplored. Using the diploid genome of Z. armatum, we identified 67 ZaNF-Y genes: 20 ZaNF-YA, 30 ZaNF-YB, and 17 ZaNF-YC members (File S9, Fig. 1). This represents a significant expansion compared to 36 NF-Y genes in Arabidopsis 34 in rice, and 24 in pummelo. Additionally, ZaNF-Y transcription factors exhibit conserved domain and motif compositions consistent with their corresponding subunits, demonstrating their evolutionary conservatism (Fig. 5).
Gene duplication drives evolutionary diversification and is a key mechanism for expanding gene families (Lawton-Rauh, 2003). In this study, we identified 16 pairs of segmentally duplicated genes and two tandem duplicates in the ZaNF-Y family (Fig. 3). All segmentally duplicated gene pairs were found to belong to the same phylogenetic groups as expected, which indicated that the expansion of the ZaNF-Y family was driven by segmental duplications. These duplications have shaped the functional diversity of ZaNF-Y genes, with some members acquiring new functions or losing existing ones (Xu et al., 2020). For example, in the segmental duplication genes, ZaNF-YC11 shows significantly low expression in female flowers, whereas ZaNF-YC16 is upregulated in both female flowers, leaves, and young fruits (Fig. 8). In another pair of segmental duplications, the expression levels of ZaNF-YB8 and ZaNF-YB21 in female flowers show an opposite trend (Fig. 8). Tandem duplicates ZaNF-YC12 and ZaNF-YC13 show distinct expression patterns: ZaNF-YC12 is minimally expressed in female flowers, while ZaNF-YC13 is highly expressed in young fruits, suggesting functional adaptation to reproductive roles (Fig. 8). Some duplicated gene pairs indicated consistent expression patterns, while others did not. For instance, ZaNF-YC1 is highly expressed in young fruits and female flowers but not in stems, whereas ZaNF-YC9 depicts no significant expression difference across tissues. ZaNF-YA1 and ZaNF-YA8 are significantly downregulated in female flowers, but ZaNF-YA1 is also downregulated in leaves and young fruits. In the female flowers of Z. armatum, the nucellus cells initiate embryo differentiation, whereas in the young fruit, the nucellar embryo has already begun to develop. The initiation of embryo differentiation and development is typically regulated by different genes. Therefore, these gene duplications may have allowed the ZaNF-Y genes to play different regulatory roles at various stages of nucellar embryo development.
NF-Y transcription factors are crucial in the formation of somatic embryos. Ectopic expression of the LEC1 gene in transgenic plants induces somatic embryo-like structures (Harada, 2001). GhL1L1, a LEC1-like gene, regulates somatic embryogenesis in cotton by affecting auxin distribution (Xu et al., 2019). The heterologous expression of OsNF-YB7 in the Arabidopsis lec1 mutant can complement the lec1 deficiency (Niu et al., 2021). Phylogenetic analysis depicts that ZaNF-YB5 is homologous to AtLEC1 and OsNF-YB7 (Fig. 1). However, ZaNF-YB5’s low expression in female flowers suggests a divergent regulatory role compared to its homologs in Arabidopsis, such as AtLEC1, which is essential for somatic embryogenesis (Fig. 8). This may result from functional divergence in different species. Interestingly, ZaNF-YC1 is highly expressed in female flowers and young fruits, indicating it may also play a positive regulatory role in nucellar embryo formation (Fig. 8).
In the Arabidopsis lec1 mutant, the expression level of the BBM gene is significantly down-regulated, while BBM expression in rice ovules induces the formation of apomixis (Pelletier et al., 2017; Khanday et al., 2019). Therefore, we speculate that ZaNF-YB5 may regulate the process of asexual reproduction in Z. armatum by affecting the expression of BBM. In the Z. bungeanum, the MADS transcription factor AGL11 has been reported to be associated with apomixis (Fei et al., 2021). According to the cis-element analysis results, we also found that the promoter regions of many ZaNF-Y genes contain binding sites for MADS transcription factors, including ZaNF-YB5 and ZaNF-YC1. Therefore, we also speculate that AGL11 may affect the apomixis in Z. armatum by regulating the expression of ZaNF-YB5 and ZaNF-YC1. We will further validate the function of these candidate genes in nucellar embryo formation by overexpressing them in other model plants (e.g., Arabidopsis) or mutating these genes in Z. armatum using CRISPR/Cas9 technology.
Studies have found that hormones are capable of inducing the formation of embryos from plant somatic cells (Long et al., 2018). ABA has been proven to induce cell differentiation during the somatic embryogenesis process in Cunninghamia lanceolate (Zhou et al., 2017). In Arabidopsis, an increase in JA content inhibits MYC2 and activates the expression of JAZ1, thereby initiating the formation of somatic embryos (Mira et al., 2016). Similarly, the promoter regions of the ZaNF-Y genes also contain abundant ABA and JA responsive elements, suggesting that they may play a significant role in nucellar embryo formation (Fig. 6). Polar auxin transport and auxin responses are crucial for specifying embryogenic cell fate (Jia et al., 2021). In this study, we also found that some ZaNF-Y genes’ promoter contain auxin response elements. These ZaNF-Y genes may participate in the formation of ovule embryos by responding to auxin signals. Additionally, the promoter regions of the ZaNF-Y genes also contain a large number of abiotic stress response elements. This may be related to the important regulatory role of ZaNF-Y genes in abiotic stress.
The function of NF-Y requires the formation of a heterotrimeric complex involving NF-YA/B/C subunits, which exhibit complex and specific interactions (You et al., 2021). In Arabidopsis, 10 AtNF-YB proteins (AtNF-YB1 to AtNF-YB10) and seven AtNF-YC proteins (AtNF-YC1 to C4, C6, C9, and C12) are strongly interconnected, with 74% of theoretical protein-protein interactions detected by yeast two-hybrid experiments (Hackenberg et al., 2012). In this system, AtNF-YBs and AtNF-YCs rarely interacted with AtNF-YAs, indicating that the AtNF-YB/C heterodimer must form to bind AtNF-YA subunits (Hackenberg et al., 2012). Similar interactions have been observed in rice between OsHAP3A and two OsHAP2s, as well as six OsHAP5s (Thirumurugan et al., 2008). In Arabidopsis, AtNF-YA can only form heterotrimeric complexes with dimers containing the HFD subunit and cannot bind to individual AtNF-YA or AtNF-YB subunits (Hackenberg et al., 2012). However, studies indicate that OsNF-YA8 can interact with OsNF-YB9 through yeast-two-hybrid assays. In this study, we found that ZaNF-YA11, ZaNF-YA12, and ZaNF-YA15 have potential interactions with many ZaNF-YB subfamily proteins (Fig. 9). NF-YA, NF-YB, and NF-YC subfamily proteins form heterotrimeric complexes, which then interact with other NF-YA proteins or regulatory factors to regulate downstream target gene expression (Gnesutta et al., 2017; Nardone, Chaves-Sanjuan & Nardini, 2017; Huang et al., 2015). Specifically, ZaNF-YB5 may interact with ZaNF-YA9 and ZaNF-YA12, while ZaNF-YA9 and ZaNF-YA12 may also interact with ZaNF-YC1, suggesting the formation of heterotrimeric complexes involving ZaNF-YA9, ZaNF-YA12, ZaNF-YB5, and ZaNF-YC1. In Arabidopsis, LEC1 can interact with AtNF-YC2 (Hackenberg et al., 2012). Therefore, ZaNF-YB5 (the homolog of LEC1) and ZaNF-YC1 (the homolog of AtNF-YC2) may also be able to form a complex. Moreover, AtNF-YC2, AtNF-YA2, and AtNF-YA4 have the same expression pattern, suggesting that they may function together. Similarly, ZaNF-YA9, ZaNF-YA12, ZaNF-YB5, and ZaNF-YC1 may also participate in nucellar embryo formation by forming complexes in Z. armatum, We will further validate these interactions through experiments (such as yeast two- and three-hybrid analysis, etc.).
Conclusions
In this study, we identified 67 ZaNF-Y transcription factors from the diploid Z. armatum genome, including 20 ZaNF-YA, 30 ZaNF-YB, and 17 ZaNF-YC genes. We analyzed their basic characteristics, chromosomal localization, and gene duplication events. These ZaNF-Y genes demonstrated functional similarity and specificity based on conserved structural domains and motif analysis. Transcription factor analysis suggested potential regulatory mechanisms of ZaNF-Y genes at the transcriptional level. Differential expression analysis across various tissues indicated the regulatory roles of specific ZaNF-Y members (ZaNF-YB5 and ZaNF-YC1) during nucellar embryo formation. Furthermore, protein association network analysis provided insights into the composition of ZaNF-Y complexes. Overall, the identification of ZaNF-Y genes associated with nucellar embryo formation provides a basis for harnessing apomixis in hybrid crop production.