Antibacterial activity of the endophytic fungal extracts and synergistic effects of combinations of ethylenediaminetetraacetic acid (EDTA) against Pseudomonas aeruginosa and Escherichia coli
- Published
- Accepted
- Received
- Academic Editor
- Amjad Abu Hasna
- Subject Areas
- Microbiology, Molecular Biology
- Keywords
- Antibacterial, Checkerboard assay, Combination, Ethylenediaminetetraacetic acid, Endophytic fungi, Medicinal plants, Synergism, Escherichia coli, Pseudomonas aeruginosa
- Copyright
- © 2025 Rosdee et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Antibacterial activity of the endophytic fungal extracts and synergistic effects of combinations of ethylenediaminetetraacetic acid (EDTA) against Pseudomonas aeruginosa and Escherichia coli. PeerJ 13:e19074 https://doi.org/10.7717/peerj.19074
Abstract
The growing threat of antibiotic resistance in bacteria is a critical public health concern. Combining natural compounds with antimicrobial agents is an alternative approach to improve the antibacterial efficacy and safety of these agents. The strategy is to restore the effectiveness of existing antibiotics while minimizing the required concentrations of antibiotics or antimicrobial agents. This study aimed to isolate the endophytic fungi from medicinal plants, including Lantana camara, Orthosiphon aristatus, Mansonia gagei, Terminalia bellirica, Oroxylum indicum, Elaeagnus latifolia, Talinum paniculatum, and Capsicum annuum, and evaluate the combined antibacterial efficacy with selected antibiotics or ethylenediaminetetraacetic acid (EDTA) against Pseudomonas aeruginosa. The antimicrobial activity of the extracts was assessed using agar well diffusion and broth microdilution methods. The minimum inhibitory concentration (MIC) values of the extracts were 32–64 µg/mL against Escherichia coli, and 512–2,048 µg/mL against P. aeruginosa, respectively. Time-kill assays demonstrated the bacteriostatic effect of the extracts. The checkerboard microbroth dilution method was performed to determine the synergistic effect between endophytic fungal extracts and antibiotics or EDTA. The synergistic effect was observed in the extractions of endophytic fungi isolated from M. gagei, T. bellirica, O. indicum, E. latifolia, T. paniculatum, and C. annuum combined with EDTA against P. aeruginosa. Combinations of endophytic fungi with EDTA, which exhibited a synergistic effect, demonstrated bactericidal action against Gram-negative bacteria. The present study suggests that combining endophytic fungal extracts and EDTA could be an essential strategy for combating pathogenic Gram-negative bacteria.
Introduction
The rise of superbugs, bacteria resistant to common antibiotics, drives a renewed interest in natural alternatives. Overusing antibiotics has led bacteria to develop defenses, rendering the antibiotics ineffective (Algammal et al., 2023). Hospital-acquired infections (HAIs) are a significant concern in healthcare settings. These infections contribute to increased morbidity, mortality, length of stay, and overall healthcare costs (Mehta et al., 2014). Gram-negative hospital-acquired infections (HAIs) are increasingly developing high levels of antibiotic resistance (Morris & Cerceo, 2020). Escherichia coli can produce extended-spectrum beta-lactamase and carbapenemase, leading to becoming Extended- Spectrum beta-lactamase (ESBL) producing Enterobacterales and Carbapenem-resistant Enterobacterales (CRE), respectively (United States Centers for Disease Control and Prevention, 2019). The COVID-19 pandemic was associated with a significant increase in hospital-onset ESBL-producing Enterobacterales and Carbapenem-resistant Enterobacteriaceae (CRE) cases. Estimates suggest a 32% to 35% rise from 2019 to 2020 (United States Centers for Disease Control and Prevention, 2022). Pseudomonas aeruginosa exhibits high resistance to multiple antibiotic classes, leading to the emergence of multidrug-resistant (MDR) P. aeruginosa, which is a significant cause of healthcare-associated infections (HAIs) and is associated with high mortality rates (Li et al., 2024). As the prevalence of antibiotic resistance rises and the cases of resistant bacteria increase, there is an emphasis on the urgency and importance of exploring natural products of animal, bacterial, fungal, and plant origin as promising sources of new antimicrobial agents that could combat these resistant bacteria (Álvarez-Martínez, Barrajón-Catalán & Micol, 2020). In addition to discovering new compounds to combat antibiotic resistance, there is also a need for effective treatment methods. Endophytic fungi, microorganisms that live inside plant cells and secrete secondary metabolites, are considered a source of bioactive compounds with antibacterial activity (Manganyi & Ateba, 2020). Although bioactive substances in endophytic fungal extracts have exhibited potent antibacterial properties, most endophytic fungal extracts are inherently inactive against common Gram-negative bacteria due to their inability to penetrate the outer membrane barrier (Ababutain et al., 2021; Chatterjee, Ghosh & Mandal, 2022; Makuwa & Serepa-Dlamini, 2021; Maliehe et al., 2022). Numerous studies have shown that endophytic fungi possess greater effectiveness against Gram-positive bacteria than against Gram-negative bacteria (Al-Qaralleh, Al-Zereini & Al-Mustafa, 2021; Rani et al., 2017; Sadrati et al., 2023). Culture broth of Aspergillus parasiticus, isolated from Michelia champaca L., containing bioactive compounds such as flavonoids, alkaloids, tannins, and saponins. This culture broth exhibited antibacterial activity, specifically against Gram-positive bacteria (Hastuti et al., 2023). Endophytic fungi from Artemisia sieberi demonstrated greater effectiveness in combating Gram-positive bacteria than Gram-negative bacteria; Staphylococcus aureus ATCC29213 was the most sensitive bacteria (Ababutain et al., 2021). Alternaria sp. RL4 isolated from Rauvolfia serpentina L. Benth. exhibited antibacterial activities against Gram-positive bacteria but was ineffective against Gram-negative bacteria (Ghosh et al., 2018).
Gram-negative bacteria, a considerable concern in multiple-drug resistance (Gauba & Rahman, 2023), have a unique outer layer that acts as a permeability barrier. This outer membrane, composed of lipopolysaccharides, phospholipids, and unique proteins, is designed to slow the entry of harmful substances, including antibiotics. Lipopolysaccharides, in particular, play a vital role in this defense system by being more resistant to breakdown and less permeable to hydrophobic molecules (Coleman & Smith, 2014; Nikaido, 1998). Therefore, to increase the antibacterial efficacy of endophytic fungal extract against Gram-negative bacteria, a combination of endophytic fungal extracts and outer membrane permeabilizers could be employed.
Ethylenediaminetetraacetic acid (EDTA) is a colorless, water-soluble solid that acts as a chelating agent, binding to metals via four-carboxylate and two amine groups. EDTA has several applications, including treating heavy metal poisoning and as a permeating and sensitizing agent in dentistry, medical devices, and veterinary medicine to treat biofilm-associated conditions (Finnegan & Percival, 2015; Mohammadi, Shalavi & Jafarzadeh, 2013). EDTA is also included as a food additive (Xu et al., 2021). EDTA can enhance the efficacy of other antimicrobial agents by removing Mg2+ and Ca2+ ions from the outer membrane of Gram-negative bacteria, leading to the release of up to 50% of the lipopolysaccharides (LPS) molecules and exposure of the inner membrane phospholipids (Finnegan & Percival, 2015). Consequently, combining endophytic fungal extracts with an EDTA could enhance the possibility of entering cells and reaching the site of action.
Previous studies have examined the effectiveness of various compounds combined with EDTA to improve their effectiveness against Gram-negative pathogenic bacteria. However, limited research has investigated the relationship between extracts from endophytic fungi and EDTA (Hamoud, Reichling & Wink, 2015; Liu et al., 2017; Pi et al., 2020). Therefore, this study aimed to classify the endophytic fungi isolated from medicinal plants and evaluate the antibacterial activities of endophytic fungal extracts alone and in combination with EDTA against selected Gram-negative bacteria.
Materials and Methods
Leaves samples
Leaves of Thai medicinal plants, including Capsicum annuum L., Elaeagnus latifolia L., Lantana camara L., Mansonia gagei Drumm., Orthosiphon aristatus (Blume) Miq., Oroxylum indicum L., Talinum paniculatum (Jacq.) Gaertn., and Terminalia bellirica (Gaertn.) Roxb., were collected from the Walailak University botanical garden in 2022. The voucher specimens of plants are deposited at the Walailak Herbarium, Walailak University, Thasala, Nakhon Si Thammarat, Thailand, listed in Table 1. For the further experiment, only healthy plant leaves were selected. Leaves that exhibited physical damage or signs of infection were not included in the study. Leaves were then stored in Ziploc plastic bags (Huang, Zimmerman & Arnold, 2018) and preserved on ice.
| Plant species | Family | Voucher number |
|---|---|---|
| Capsicum annuum L. | Solanaceae | 01569 |
| Elaeagnus latifolia Roxb. | Elaegnaceae | 01572 |
| Lantana camara L. | Verbenaceae | 01562 |
| Mansonia gagei Drumm. | Malvaceae | 01564 |
| Orthosiphon aristatus (Blume) Miq. | Lamiaceae | 01567 |
| Oroxylum indicum L. | Bignoniaceae | 01570 |
| Talinum paniculatum Jacq. | Talinaceae | 01574 |
| Terminalia bellirica Gaertn. | Combretaceae | 01566 |
Leaves preparation
The medical plant leaves were prepared according to the method by Phongpaichit et al. (2006) with minor modifications. The leaves were washed to remove any adhering epiphytes and subsequently exposed to a 70% ethanol solution for 5 min to disinfect the leaf surfaces. Cleaned leaves were cut into 0.5 cm pieces using a sterile hole puncher. The cut leaves were then subjected to surface disinfection by immersion in 95% ethanol for 1 min, followed by 3% sodium hypochlorite solution for 3 min. All samples were subsequently rinsed and dried.
Endophytic fungal isolation
Prepared leaves were placed onto Sabouraud dextrose agar (SDA) supplemented with chloramphenicol and incubated at 25 ± 2 °C for 1–3 days (Handayani et al., 2017). Fungal hyphae originating from the leaves and growing on the media were transferred to a new SDA plate. The fungal isolates were assessed for purity after incubation at 25 ± 2 °C to obtain pure cultures of endophytic fungi.
Endophytic fungi fermentation
The modified method of Phongpaichit et al. (2006) was performed to obtain the endophytic fungi extracts. Four pieces of mycelial agar plugs of endophytic fungi growth for 7 days (0.5 cm × 0.5 cm) were inoculated into culture flasks that had sabouraud dextrose broth (SDB). The culture was shaken at 200 rpm at 25 ± 2 °C for 14 days. The endophytic fungal culture was filtered to separate culture broth and mycelia. Cell-free supernatants (CFS) were concentrated by evaporation in a rotary vacuum evaporator. The evaporated extracts were dried using freeze-drying (FDU-2100; Eyela, Tokyo, Japan) at −80 °C for 36 h. The dried matter was weighed and stored at 4 °C for further analysis. The endophytic fungal extracts were dissolved in 50% dimethyl sulfoxide (DMSO). The endophytic fungal isolates are listed in Table 2.
| Medicinal plants | Endophytic fungal isolate |
|---|---|
| Capsicum annuum L. | CAE1, CAE2 |
| Elaeagnus latifolia Roxb. | ELE1 |
| Lantana camara L. | LCE1 |
| Mansonia gagei Drumm. | MGE1 |
| Orthosiphon aristatus (Blume) Miq. | OAE1 |
| Oroxylum indicum L. | OIE1 |
| Talinum paniculatum Jacq. | TPE1 |
| Terminalia bellirica Gaertn. | TBE1 |
Morphological identification of endophytic fungi
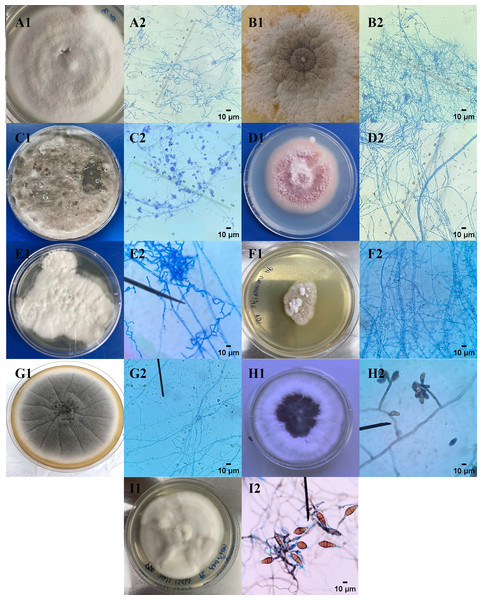
Endophytic fungi were identified by staining slides prepared from cultures with lactophenol cotton blue reagent. The stained slides were examined under a bright-field and phase contrast microscope (Sadananda, Govindappa & Ramachandra, 2014). Morphological characteristics, including hyphae, colony and medium color, surface texture, sporulation, production of acervuli, medium coloration, size, and coloration of conidia, were observed to identify the endophytic fungi (Barnett, 1960).
Bacterial strains and preparation
Pseudomonas aeruginosa ATCC27853 and Escherichia coli ATCC25922 were obtained from the American Type Culture Collection. All bacterial strains were stored in 20% glycerol stocks at −80 °C until use.
Cell lines and preparation
Human foreskin fibroblast cells (HFF-1, ATCC SCRC-1041) were obtained from Assoc. Prof. Rathapon Asasutjarit, Thammasat University Research Unit in Drug, Health Product Development and Application (DHP-DA), Department of Pharmaceutical Sciences, Faculty of Pharmacy, Thammasat University. HFF-1 cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 μg/mL streptomycin, and 100 IU/mL penicillin. The cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2 and were passaged every 3 days using 0.25% trypsin-EDTA solution to maintain exponential growth.
Agar well diffusion
Agar well diffusion assay was used to determine the antibacterial activities of endophytic fungal extracts (CLSI, 2020). The assay was performed in Mueller-Hinton agar (MHA) (Himedia, India). Overnight bacterial suspension was adjusted with Mueller-Hinton broth (MHB) to obtain a final concentration of 1 × 108 CFU/mL. Bacterial suspension was seeded to MHA, and a well of 6 mm was created. Endophytic fungal extracts were added to each well at a final concentration of 1,024 µg/mL. The plates were then incubated at 35 ± 2 °C for 16–18 h. Ciprofloxacin and DMSO (AppliChem GmbH, darmstadt, Germany) were used as positive and negative controls, respectively. The inhibition zone diameter was measured in millimeters using a vernier caliper.
Determination of minimal inhibitory concentration and minimal bactericidal concentration
Minimal inhibitory concentrations (MICs) and minimal bactericidal concentrations (MBCs) were determined using a modified broth microdilution method according to a modification of Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI, 2020). Bacterial suspensions were cultured until reaching the exponential growth phase, followed by adjusting the bacterial suspension to achieve a concentration of 1 × 106 CFU/mL. The crude extract concentrations were added to 96-well microtiter plates and diluted by two-fold-serial dilutions to obtain a final concentration between 512 and 2 µg/mL. The plates were incubated at 35 ± 2 °C for 16–20 h. MHB with the bacterial suspension was used as a positive control. The culture with extracts was used as a negative control. The MIC was determined as the minimal concentration of the extract present in the clear well of the microtiter plate. For MBC determination, aliquots from wells without visible growth in the MIC assay were plated onto agar media using the drop plate method (Herigstad, Hamilton & Heersink, 2001). The MBC was defined as the lowest extract concentration that resulted in no bacterial colonies on the agar plates. The assay was performed in triplicate.
Time-kill assay
Time-kill kinetics of the endophytic fungal extract against bacteria were evaluated with modifications (Sianglum et al., 2019). Briefly, crude extracts were prepared at concentrations corresponding to 1/4MIC, 1/2MIC, MIC, and 2MIC, respectively. Bacterial suspensions were then added to each well to provide a final inoculum density of 5 × 105 CFU/mL and incubated at 35 ± 2 °C for 0, 1, 2, 4, 6, 12, and 24 h. Treated and untreated bacterial aliquots were diluted ten-fold, and 10 µL of each dilution was plated onto agar media. Colony-forming units (CFU/mL) were enumerated after incubation. All experiments were performed in triplicate.
Determination of fractional inhibitory concentration index
The microdilution checkerboard assay was performed to evaluate the interaction between endophytic fungal extracts and the antimicrobials (Muroi & Kubo, 1996). Endophytic fungal extracts in combination with antimicrobials in different final concentrations ranged from 1/4MIC to 2MIC, and bacterial suspension was added to 96-well microtiter plates. The plates were incubated at 35 ± 2 °C overnight. The fractional inhibitory concentration (FIC) index formula was calculated according to the following formula: FIC index = FICA + FICB = (CA/MICA) + (CB/MICB), where MICA and MICB are the MICs of endophytic fungal extract and antimicrobial, respectively. CA and CB are the concentrations of the endophytic fungal extract and antimicrobial in combination. The synergistic effect indicated by the FIC index < 0.5, no interaction when the FIC index is between 0.5 and 4.0. The FIC index > 4 indicated an antagonistic effect (Norden, Wentzel & Keleti, 1979). At least two independent experiments were performed in duplicate.
Growth of bacteria in the presence of endophytic fungal extracts and antimicrobials
The bactericidal effect of selected endophytic fungal extracts, including TBE1, ELE1, OIE1, TPE1, CAE2, and TBE1 in combination with EDTA against P. aeruginosa ATCC27853, was evaluated using a drop plate method (Habeeb et al., 2007). Briefly, the exponential growth phase of the bacterial culture was diluted to approximately 106 CFU/mL and treated with a combination of endophytic fungal extracts and EDTA. The samples were then incubated for 12 and 24 h, respectively. The diluted samples were dropped onto nutrient agar plates for bacterial enumeration and then incubated at 35 ± 2 °C for 24 h. After incubation, the colonies were counted, and the results were expressed as the log CFU/mL against time. The experiments were performed in three biological replicates.
Molecular identification of endophytic fungi
The endophytic fungi that exhibited synergistic effects when combined with EDTA were selected for identification using molecular techniques (Raja et al., 2017). DNA amplification and sequencing of the internal transcribed spacer (ITS) region, which is widely used as a universal DNA barcode marker for fungi, exhibits a low level of intraspecific variation and a high level of interspecific variation were used for molecular identification of endophytic fungus (Crouch, Clarke & Hillman, 2005; Harnelly et al., 2022; He et al., 2017; Schoch et al., 2012). Fungal hyphae were lyophilized and ground before DNA extraction using the fungal genomic DNA kit (Geneaid, Taiwan), following the manufacturer’s protocol. The ITS region was amplified using the primers ITS5 5′ (GGA AGT AAA AGT CGT AACAAG G) 3′ and ITS4 5′ (TCC TCC GCT TAT TGA TAT GC) 3 by polymerase chain reaction (PCR) under the following conditions: 95 °C for 5 min, 31 cycles of 95 °C for 0.5 min, 57 °C for 0.5 min, and 72 °C for 1.4 min, and final extension with a 10 min at 72 °C.
Sequencing
PCR products were purified with a multiscreen filter plate (Millipore Corp, Burlington, MA, USA). A sequencing reaction was conducted using the PRISM BigDye Terminator v3.1 Cycle Sequencing Kit. Extension product-containing DNA samples were combined with Hi-Di formamide (Applied Biosystems, Foster City, USA), heated at 95 °C for 5 min, and then cooled on ice for 5 min. The prepared mixture was then analyzed with an ABI Prism 3730XL DNA Analyzer (Applied Biosystems, Foster City, USA).
Evaluation of the cytotoxicity
To determine the cytotoxic effect of endophytic fungal extracts on HFF-1 (ATCC, SCRC-1041) the 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay was performed (Mosmann, 1983). The 96-well microtiter plate contains HFF-1 cells at 5 × 103 cells/well in DMEM culture media (Gibco, Grand Island, NY, USA) supplemented with 10% FBS which were incubated at 35 ± 2 °C for 24 h in a 5% CO2 atmosphere. The endophytic fungal extracts at 0–1,000 µg/mL were added to the plates. The plates were incubated for 24 h. MTT solution at 0.5 mg/mL was added and incubated at 35 ± 2 °C in a humidified atmosphere with 5% CO2 for 2 h. The cells were suspended in 100 µL dimethyl sulfoxide (DMSO) after discarding the culture medium. Cellular formazan production, an indicator of metabolic activity and cell viability, was quantified by measuring the absorbance at 570 nm using a microplate reader. The resulting absorbance values were then used to calculate the percentage of viable cells compared to the untreated control. The cytotoxic activity of extracts was reported as half-maximum inhibitory concentration (IC50) value, which represents the concentration of the extract that inhibits cell growth by 50%. The viability percentages were calculated according to the following formula.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 9 software (GraphPad Holdings San Diego, CA, USA). Student’s t-test was used to compare treated and untreated groups directly. A p-value of less than 0.05 was considered statistically significant, indicating a significant difference between the compared groups.
Results
Identification of the endophytic fungal isolates
Morphological identification was accomplished through macroscopic and microscopic observation. The colony morphology of nine endophytic fungal isolates exhibited diverse shapes, edges, elevations, colors, and textures. Each fungal hyphae shape of nine endophytic fungi showed different characteristics. The characteristics of colonies and types of hyphae and conidia shape of endophytic fungi were summarized and explained in Table 3 and Fig. 1. The endophytic fungi from C. annuum, E. latifolia, L. camara, M. gagei, O. aristatus, O. indicum, T. paniculatum, and T. bellirica were classified into different genera, including Purpureocillium sp., Trichothecium sp., Exophiala sp., Fusarium sp., Scytalidium sp., Lecythophora sp., Acremonium sp., Curvularia sp., and Alternaria sp., respectively.
| Medicinal plants | Endophytic fungal | Macroscopic description | Microscopic description | Genus |
|---|---|---|---|---|
| Capsicum annuum | CAE1 | Colonies are fast growing, suede-like to floccose, vinaceous to violet-colored. | Septate hyaline hyphae, phialides with swollen bases, rough-walled conidiophore stipes. Conidia are ellipsoidal, smooth-walled to slightly roughened. | Purpureocillium sp. |
| CAE2 | Colonies are moderately fast-growing, flat, suede-like to powdery, white but becoming rosy or pink with age. | Septate hyaline hyphae, basipetal zig-zag chains of two-celled conidia with short conidiophores. | Trichothecium sp. | |
| Elaeagnus latifolia | ELE1 | Colonies are smooth, grey to black, mucoid, raised of aerial mycelium with age, and suede-like in texture. | Dematiaceous hyphae, conidia are annellidic and erect. | Exophiala sp. |
| Lantana camara | LCE1 | Colonies are rapidly growing, floccose, peach-coloured. Septate hyaline hyphae, conidiophores scattered. | Conidia slightly curved, fusiform with foot-cell, three to seven-septate. | Fusarium sp. |
| Mansonia gagei | MGE1 | Colonies are effuse, white to greyish, with a cream-coloured to deep ochraceous-yellow reverse. | Arthroconidia are darkly pigmented, flask-shaped phialides. Phialoconidia are one to three-celled and hyaline, brown, and are ovoid to ellipsoidal. | Scytalidium sp. |
| Orthosiphon aristatus | OAE1 | Colonies are flat, smooth, moist, pink to orange. | Septate hyaline hyphae, producing conidia laterally from small collarettes on the hyphae. Conidia are hyaline, smooth and thin walled, broadly ellipsoidal to cylindrical. | Lecythophora sp. |
| Oroxylum indicum | OIE1 | Colonies are slow-growing, suede-like to powdery, grey. | Septate hyaline hyphae, awl-shaped phialides producing single-celled, globose to cylindrical conidia. | Acremonium sp. |
| Talinum paniculatum | TPE1 | Colonies are fast-growing, suede-like to downy, blackish brown with a black reverse. | Septate dematiaceus hyphae, onidiophores erect, straight to flexuous, septate. Conidia are ellipsoidal, curved, pale brown, medium reddish brown to dark brown, and conidial wall smooth to verrucose. | Curvularia sp. |
| Terminalia bellirica | TBE1 | Colonies are fast growing, greyish to black, and are suede-like to floccose. | Septate dematiaceous hyphae branched acropetal chains of multicellular conidia, short conidiophores. Conidia are ellipsoidal, pale brown, smooth-walled, or verrucose. |
Alternaria sp. |
Figure 1: Macroscopic and microscopic images of endophytic fungi.
(1) Macroscopic and (2) microscopic morphological characteristics of endophytic fungi (A) CAE1, (B) CAE2, (C) ELE1, (D) LCE1, (E) MGE1, (F) OAE1, (G) OIE1, (H) TPE1, and (I) TBE1 were observed.Agar well diffusion assay
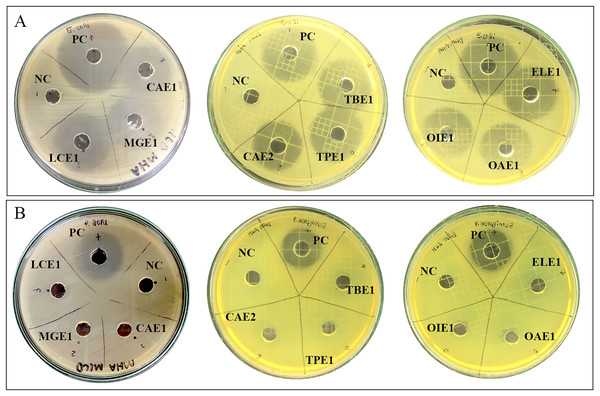
The preliminary antibacterial testing of test extracts against E. coli and P. aeruginosa was performed using agar well diffusion. Nine endophytic fungi cell-free supernatant at 1,024 µg/mL exhibited antibacterial activity against only E. coli. The diameter of inhibition zones of cell-free supernatants of endophytic fungi ranged from 28.20 mm to 31.15 mm, as represented in Table 4. Acremonium sp. showed the highest inhibition diameter zone with 31.15 mm against E. coli. Figure 2 shows the zones of inhibition produced by the cell-free supernatant of endophytic fungi against E. coli. No zones of inhibition were observed for the endophytic fungal extracts against P. aeruginosa.
| Medicinal plants | Endophytic fungi extracts (1,024 µg/mL) |
Inhibition zone (mm) | |
|---|---|---|---|
|
E. coli ATCC25922 |
P. aeruginosa ATCC27853 | ||
| C. annuum | Purpureocillium sp. | 29.40 | – |
| C. annuum | Trichothecium sp. | 28.20 | – |
| E. latifolia | Exophiala sp. | 28.60 | – |
| L. camara | Fusarium sp. | 31.00 | – |
| M. gagei | Scytalidium sp. | 29.30 | – |
| O. aristatus | Lecythophora sp. | 30.50 | – |
| O. indicum | Acremonium sp. | 31.15 | – |
| T. paniculatum | Curvularia sp. | 31.00 | – |
| T. bellirica | Alternaria sp. | 30.40 | – |
Note:
(-) indicates no inhibition activity.
Figure 2: Zone of inhibition of endophytic fungal extracts.
Extract of CAE1 (Purpureocillium sp.), MGE1 (Scytalidium sp.), LCE1 (Fusarium sp.), TBE1 (Alternaria sp.), TPE1 (Curvularia sp.), CAE2 (Trichothecium sp.), ELE1 (Exophiala sp.), OAE1 (Lecythophora sp.), and OIE1 (Acremonium sp.) against (A) E. coli and (B) P. aeruginosa. NC: Negative control 50% DMSO, and PC: Positive control ciprofloxacin.Minimum inhibitory concentration and minimum bactericidal concentration
The MIC values of endophytic fungal extracts ranged from 32 to 64 µg/mL against E. coli and from 512 to 2,048 µg/mL against P. aeruginosa (Table 5). The lower MIC values of the extracts against E. coli compared to P. aeruginosa suggest that the extracts exhibit stronger antibacterial activity against E. coli. Among the tested fungi, Acremonium sp. isolated from O. indicum showed the lowest MIC of 32 µg/mL against E. coli, while Fusarium sp. isolated from L. camara exhibited the highest antibacterial activity against P. aeruginosa, with a MIC value of 512 µg/mL. The MBC values for all endophytic fungal extracts were more than 512 µg/mL against both E. coli and P. aeruginosa.
| Medicinal plants |
Endophytic fungi |
P. aeruginosa ATCC27853 |
E. coli ATCC25922 |
||
|---|---|---|---|---|---|
| MIC (µg/mL) |
MBC (µg/mL) |
MIC (µg/mL) |
MBC (µg/mL) |
||
| C. annuum | Purpureocillium sp. | 2,048 | 4,096 | 64 | >512 |
| C. annuum | Trichothecium sp. | 2,048 | 4,096 | 64 | >512 |
| E. latifolia | Exophiala sp. | 2,048 | 4,096 | 64 | >512 |
| L. camara | Fusarium sp. | 512 | 4,096 | 64 | >512 |
| M. gagei | Scytalidium sp. | 2,048 | 4,096 | 64 | >512 |
| O. aristatus | Lecythophora sp. | 2,048 | 4,096 | 64 | >512 |
| O. indicum | Acremonium sp. | 2,048 | 4,096 | 32 | >512 |
| T. paniculatum | Curvularia sp. | 2,048 | 4,096 | 64 | >512 |
| T. bellirica | Alternaria sp. | 2,048 | 4,096 | 64 | >512 |
| EDTA | 375 | 48,000 | 6,000 | 12,000 | |
Time kill assay
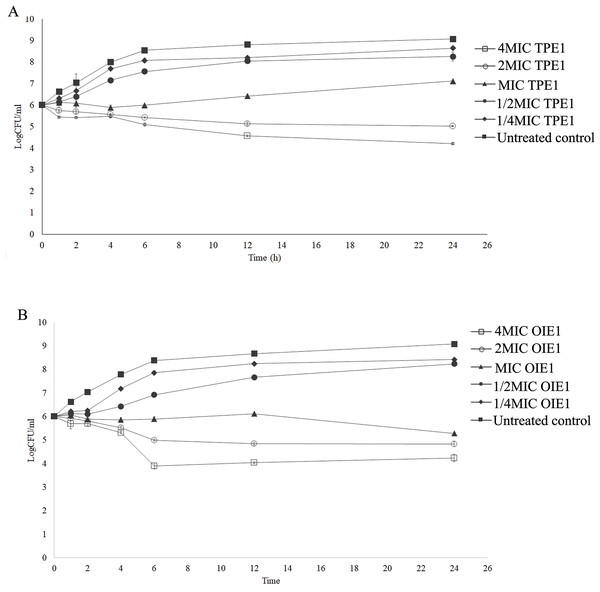
A time-kill assay was used to evaluate the bactericidal effects of endophytic fungal extracts exhibiting effective antibacterial activity against E. coli. As shown in Fig. 3A, the untreated E. coli group gradually increased in viable cell number before 6 h, after that the bacterial cell count remained relatively stable within 24 h. Similar growth patterns were observed in E. coli treated with 1/2MIC and 1/4MIC of TPE1 extract, identified as Curvularia sp. However, E. coli treated with 4MIC and 2MIC concentrations of TPE1 extracts, identified as Curvularia sp., exhibited a slight decrease in cell number within 24 h, with a reduction of more than 3 log CFU/mL compared to the untreated group, indicating bactericidal activity. MIC value of Curvularia sp. extracts demonstrated a bactericidal effect by reducing the viable cell count to 2 log CFU/mL at 24 h compared to the untreated group, suggesting a bacteriostatic effect (Fig. 3A). A similar bactericidal effect was observed in E. coli treated with 4MIC and 2MIC concentrations of OIE1 extract, identified as Acremonium sp., with a reduction in the bacterial count more than 3 log CFU/mL compared to the untreated group within 24 h (Fig. 3B). The results indicate that the antibacterial activity of Curvularia sp. and Acremonium sp. extracts are concentration-dependent, with higher concentrations of the extracts leading to a more reduction in the number of viable bacterial colonies over time.
Figure 3: Time kill curves of endophytic fungal extract against E. coli.
(A) OIE1 (Acremonium sp.) extract against E.coli. (B) TPE1 (Curvularia sp.) extract against E. coli. Data are presented as the mean ± SD.Effects of endophytic fungal extract and antibiotic combinations
To enhance the antibacterial activity of endophytic fungal extracts against P. aeruginosa, the combination of endophytic fungi with antimicrobials, including gentamicin, ciprofloxacin, levofloxacin, and EDTA, was investigated against P. aeruginosa. Table 6 revealed that three endophytic fungal extracts, including Purpureocillium sp., Scytalidium sp., and Fusarium sp., exhibited an indifferent effect when combined with EDTA. In contrast, synergism was observed in combinations between Lecythophora sp., Curvularia sp., Alternaria sp., Acremonium sp., Exophiala sp., and Trichothecium sp. extracts and EDTA against P. aeruginosa, as indicated by synergistic FIC index values of 0.375.
| Combination (Antimicrobials/fungal extracts) |
MIC (µg/mL) in combination | FIC index | Interpretation |
|---|---|---|---|
| GEN/Alternaria sp. | 0.25/2,048 | 1.00 | Indifference |
| CIP/Alternaria sp. | 0.25/1,024 | 1.25 | Indifference |
| LFX/Alternaria sp. | 0.5/512 | 1.13 | Indifference |
| EDTA/Purpureocillium sp. | 93.75/512 | 0.75 | Indifference |
| EDTA/Scytalidium sp. | 187.5/1,024 | 1.50 | Indifference |
| EDTA/Lecythophora sp. | 46.875/256 | 0.375 | Synergism |
| EDTA/Curvularia sp. | 46.875/256 | 0.375 | Synergism |
| EDTA/Fusarium sp. | 93.75/128 | 0.625 | Indifference |
| EDTA/Alternaria sp. | 46.875/256 | 0.375 | Synergism |
| EDTA/Acremonium sp. | 46.875/256 | 0.375 | Synergism |
| EDTA/Exophiala sp. | 46.875/256 | 0.375 | Synergism |
| EDTA/Trichothecium sp. | 46.875/512 | 0.375 | Synergism |
Bactericidal activity of the combination of endophytic fungi and EDTA
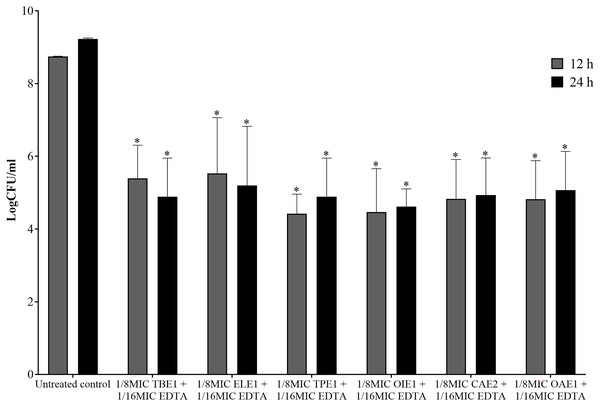
The combination of endophytic fungi and EDTA against P. aeruginosa, which demonstrated synergism, was selected to evaluate its bactericidal effect. A significant reduction in the number of viable P. aeruginosa cells was observed when treated with a combination of 1/8MIC endophytic fungal extracts and 1/16MIC EDTA at 12 and 24 h compared to the untreated control (p < 0.05, Student’s t-test). This combination decreased the number of viable bacterial cells by more than 3 log CFU/mL from the viable bacterial number of untreated controls at 12 and 24 h (Fig. 4). These results indicate that the combination of EDTA and endophytic fungal extracts exhibits synergistic interactions with bactericidal effects against P. aeruginosa.
Figure 4: The bactericidal activity of a combination between 1/8MIC of endophytic fungal and 1/16MIC of EDTA against P. aeruginosa.
TBE1 (Alternaria sp.), ELE1 (Exophiala sp.), TPE1 (Curvularia sp.), OIE1 (Acremonium sp.), CAE2 (Trichothecium sp.), and OAE1 (Lecythophora sp.) culture broth combine with EDTA. The significance of the sample compared to the control at p < 0.05 is indicated by asterisks (*).Molecular identification of effective endophytic fungi
Table 7 shows molecular identification by amplified ITS region and nucleotide BLAST search of each fungal endophytic fungi. A nucleotide BLAST search using ITS sequences revealed that TBE1, OIE1, and ELE1 were identified as Purpureocillium lilacinum. P. lilacinum is a common endophytic fungus found in the site where the medicinal plant in this study was collected. The organism has the ability to adapt to various plant hosts. As a result, the same endophytic fungi were found in different plant species within the same area (Huang et al., 2008). TPE1 endophytic fungi isolated from T. paniculatum, was found to be closely related to Fusarium verticillioides. CAE2 isolate was identified as Aspergillus aculeatus. OAE1 isolated from O. aristatus belonged to Acremonium sp.
| Endophytic fungal isolate | Identification | Identity (%) | Accession number |
|---|---|---|---|
| CAE2 | Aspergillus aculeatus | 99.12% | KJ958359.1 |
| ELE1 | Purpureocillium lilacinum | 99.31% | KJ863502.1 |
| OAE1 | Acremonium sp. | 97.80% | HQ607846.1 |
| OIE1 | Purpureocillium lilacinum | 98.30% | KF766523.1 |
| TBE1 | Purpureocillium lilacinum | 98.81% | KJ863502.1 |
| TPE1 | Fusarium verticillioides | 99.07% | KP003945.1 |
Cytotoxicity of endophytic fungal extracts
The cytotoxicity of the culture broth from endophytic fungi isolated from L. camara, O. indicum, T. bellirica, and T. paniculatum was evaluated against human foreskin fibroblasts cells, HFF-1, as shown in Table 8. The extracts which have IC50 ≤ 20 μg/mL had high cytotoxicity, IC50 > 20–100 μg/mL had moderately cytotoxicity, IC50 > 100–1,000 μg/mL had weakly cytotoxicity, IC50 > 1,000 μg/mL had inactive cytotoxicity (Nordin et al., 2018). The culture broth from endophytic fungi isolated from O. aristatus, O. indicum, and T. bellirica exhibited no cytotoxic effects, as their IC50 values exceeded 1,000 µg/mL. These results indicate that these endophytic fungal extracts are safe for use. In contrast, the endophytic fungal extracts from C. annuum and T. paniculatum exhibited low cytotoxicity against normal HFF-1 cells, with an IC50 value of 748.7 and 539.1 µg/mL.
| Medicinal plants | Endophytic fungi | IC50 (µg/mL) |
|---|---|---|
| C. annuum | A. aculeatus. | 748.7 |
| O. aristatus | Acremonium sp. | >1,000 |
| O. indicum | P. lilacinum | >1,000 |
| T. bellirica | P. lilacinum | >1,000 |
| T. paniculatum | F. verticillioides | 539.1 |
Discussion
In this study, nine endophytic fungi were isolated from eight medicinal plants, including C. annuum, E. latifolia, L. camara, M. gagei, O. aristatus, O. indicum, T. paniculatum, and T. bellirica. Genus of endophytic fungal isolates were identified by macroscopic and microscopic morphology. The identified genus included Purpureocillium sp., Trichothecium sp., Exophiala sp., Fusarium sp., Scytalidium sp., Lecythophora sp., Acremonium sp., Curvularia sp., and Alternaria sp. Medicinal plants serve as a vast habitat for microorganisms. Among these organisms, endophytes are microorganisms that reside within plant tissues without causing harm. Endophytes confer benefits upon their hosts by secreting bioactive compounds that enhance plant tolerance to both abiotic (non-living) and biotic (living) stresses (Kumar & Nautiyal, 2022). Endophytic fungi, particularly those found in medicinal plants, represent a significant source of bioactive compounds with potentially novel mechanisms of action. These compounds hold promise for developing highly effective and safe drugs (Bhardwaj & Agrawal, 2014).
The cell-free supernatant from nine endophytic fungi exhibited antibacterial activity against Gram-negative bacteria, which has no cytotoxicity to HFF-1 (human foreskin fibroblast) cells. The endophytic fungal extracts displayed moderate activity against E. coli and weak activity against P. aeruginosa. Taufiq & Darah (2019) reported that extracts from endophytic fungi isolated from medicinal plants, Ocimum sanctum L., had antibacterial activity against E. coli and P. aeruginosa. Penicillium griseofulvum isolated from Mentha pulegium L. showed broad antibacterial activity against Gram-positive and Gram-negative bacteria, including E. coli and P. aeruginosa (Zerroug et al., 2018). The endophytic fungal extracts showed potent antibacterial activity against E. coli. Extracts from endophytic fungi isolated from T. paniculatum and O. indicum, identified as Curvularia sp. and Acremonium sp., respectively, were particularly effective. Therefore, these extracts were selected for time-kill assay evaluation. Time-kill assays against E. coli demonstrated that extracts from endophytic fungi isolated from T. paniculatum and O. indicum exhibited bacteriostatic activity at the minimum inhibitory concentration (MIC) and bactericidal activity at 2MIC and 4MIC after 6 to 24 h of treatment. These extracts exhibited dose- and time-dependent antimicrobial activity, leading to unique time-kill curves for the tested bacteria.
The antimicrobial activity of endophytic fungi property is due to endophytic fungi can produce bioactive compounds. Alkaloids, terpenoids, steroids, polyketides, peptides, flavonoids, furandiones, quinols, perylene derivatives, and depsipeptides are groups of secondary metabolites that can be found in endophytic fungal extracts (Hashem et al., 2023). These compounds have different mechanisms of action against bacteria. Alkaloids can inhibit the growth of bacteria by disrupting cell respiration, intercalating with DNA, and inhibiting various enzymes involved in replication, transcription, and translation (Zielińska et al., 2019). Terpenoids target the cell membrane, adenosine triphosphate (ATP), quorum sensing (QS) system, and protein synthesis of bacteria (Huang et al., 2022). Flavonoids showed many modes of action against bacteria, including inhibiting the synthesis of nucleic acid, the function of the cytoplasmic membrane, energy metabolism enzymes, and biofilm formation (Xie et al., 2015). Aspergillus niger isolated from Opuntia ficusindica extracts contain compounds that have phenolic, alcoholic, and ketonic groups that lead to exhibited antibacterial activity against Gram-negative resistant bacteria (Elkady et al., 2022). Curvularia sp. and Acremonium sp. extracts may contain various bioactive compounds that could contribute to their potent antibacterial activity, potentially offering a solution to the growing problem of bacterial resistance. To enhance our understanding of the mechanism of action of these endophytic fungal extracts, it is essential to identify the specific compounds present and evaluate their antibacterial activity against clinical isolates or antibiotic-resistant E. coli strains.
Many studies reported that the antibacterial activity of endophytic fungi exhibited more excellent antibacterial activity against Gram-positive bacteria than Gram-negative bacteria (Sutjaritvorakul et al., 2011; Mishra et al., 2017; Qader et al., 2021; Sandrawati et al., 2023). This difference can be attributed to the presence of an outer membrane in Gram-negative bacteria. The outer membrane acts as a barrier, hindering the penetration of many antibacterial agents (Delcour, 2009). According to previous reports, Gram-positive bacteria were more sensitive than Gram-negative bacteria from endophytic fungi activities (Ababutain et al., 2021; Ghosh et al., 2018; Santra, Maity & Banerjee, 2022). These findings are likely due to the difference in the cell envelopes of Gram-positive and Gram-negative bacteria. Gram-negative bacteria possess an outer membrane rich in lipopolysaccharides, which act as a barrier. This complex outer membrane hinders the diffusion of hydrophobic compounds and small antimicrobial molecules, limiting their access to the cell membrane (Bassyouni et al., 2022).
This study investigated the combination of endophytic fungal extracts with EDTA to enhance the antibacterial activity of endophytic fungi against Gram-negative bacteria. EDTA enhances the activity of antimicrobial agents, including preservatives, antibiotics, and natural compounds, by disrupting the lipopolysaccharide layer in the outer membrane of Gram-negative bacteria (Lambert, Hanlon & Denyer, 2004). This disruption increases the permeability of the outer membrane, allowing other antimicrobial agents to penetrate more effectively. Our results indicated that the synergistic combination of endophytic fungal extracts and EDTA demonstrated enhanced antibacterial activity against P. aeruginosa. Among the endophytic fungi investigated, Aspergillus aculeatus CAE2, Purpureocillium lilacinum ELE1, Acremonium sp. OAE1, Fusarium verticillioides TPE1, P. lilacinum OIE1, and TBE1 exhibited significant potency when combined with EDTA. These fungal isolates were identified by sequencing their internal transcribed spacer (ITS) region, and sequence comparison using BLAST to NCBI database confirmed their taxonomic identification.
These results align with those reported by Umerska et al. (2018) who showed that combining antimicrobial peptides with EDTA enhanced their antibacterial activity. EDTA treatment disrupts Gram-negative bacteria’s outer membrane by rapidly releasing lipopolysaccharides and other cellular components. This effect is mediated by chelation of divalent cations. The loss of LPS can disrupt the structural integrity of the outer membrane, consequently increasing its permeability to various agents, which leads to an increase in the activity of antimicrobial agents against Gram-negative bacteria (Gray & Wilkinson, 1965; Leive, 1965; Umerska et al., 2018). The combination of EDTA with aminoglycoside antibiotics has demonstrated a synergistic effect against P. aeruginosa. EDTA enhances the activity of aminoglycoside antibiotics by chelating metal ions that compete with the antibiotics for cell wall receptors, facilitating antibiotic entry into the bacterial cell (Abd, Abu-Raghif & Al-Azzawi, 2011). Similarly, the combination of sanguinarine and EDTA exhibits a synergistic effect, enabling sanguinarine to more effectively inhibit multidrug-resistant Gram-negative bacteria. EDTA disrupts the outer membrane, allowing increased entry of sanguinarine into bacterial cells, where it inhibits growth by intercalating into DNA and double-stranded RNA (Hamoud, Reichling & Wink, 2015).
Consequently, it is hypothesized that the bioactive compounds in endophytic fungal extracts may also target DNA or RNA to inhibit the growth of P. aeruginosa. To confirm this assumption, further studies are needed to identify the mode of action of the compounds present in the extracts. The results obtained in this study showed that the endophytic fungi from medicinal plants in combination with EDTA have potent antibacterial activity against Gram-negative bacteria, which can be applied to develop novel treatment strategies for inhibiting Gram-negative resistant bacteria. Moreover, the combination of EDTA and endophytic fungal extracts can be applied as a food preservative.
Conclusions
Endophytic fungi isolated from various medicinal plants, including Mansonia gagei, Terminalia bellirica, Oroxylum indicum, Elaeagnus latifolia, Talinum paniculatum, and Capsicum annuum, demonstrated potent antibacterial activity against Gram-negative bacteria such as Escherichia coli. Additionally, the combination of endophytic fungi with EDTA exhibited excellent antibacterial activity against P. aeruginosa which are high-priority pathogens due to their widespread resistance to many commercially available antibiotics. These findings suggest that combinations of endophytic fungal extracts with EDTA could represent alternative strategies for treating infections caused by Gram-negative organisms. Further investigations are necessary to explore the antibacterial activity of these endophytic fungi/EDTA combinations in vivo to validate their potential therapeutic application.