LTR retrotransposon-derived novel lncRNA2 enhances cold tolerance in Moso bamboo by modulating antioxidant activity and photosynthetic efficiency
- Published
- Accepted
- Received
- Academic Editor
- Nikolaos Nikoloudakis
- Subject Areas
- Agricultural Science, Molecular Biology, Plant Science
- Keywords
- LTR retrotransposon, Phyllostachys edulis, lncRNA, TElncRNA, Cold stress
- Copyright
- © 2025 Zhao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. LTR retrotransposon-derived novel lncRNA2 enhances cold tolerance in Moso bamboo by modulating antioxidant activity and photosynthetic efficiency. PeerJ 13:e19056 https://doi.org/10.7717/peerj.19056
Abstract
In Moso bamboo, the mechanism of long terminal repeat (LTR) retrotransposon-derived long non-coding RNA (TElncRNA) in response to cold stress remains unclear. In this study, several Pe-TElncRNAs were identified from Moso bamboo transcriptome data. qRT-PCR analysis showed that the expression of a novel Pe-TElncRNA2 in Moso bamboo seedlings reached its highest level at 8 hours of cold treatment at 4 °C and was significantly higher in the stems compared to the leaves, roots, and buds. Furthermore, cellular localization analysis revealed that Pe-TElncRNA2 expression was significantly higher in the cytoplasm than in the nucleus. Pe-TElncRNA2 overexpression in Moso bamboo protoplasts showed that Pe-TElncRNA2 positively regulated the expression of FZR2, NOT3, ABCG44 and AGD6 genes. Further validation of this lncRNA in Arabidopsis thaliana enhanced antioxidant activities, as evidenced by increased superoxide dismutase (SOD) activity and proline content, as well as maximum photochemical efficiency PS II in dark-adapted leaves (Fv/Fm), in the transgenic plants compared to the wild-type controls. Conversely, malondialdehyde (MDA) content, a lipid peroxidation marker (a marker of oxidative stress), was significantly reduced in the transgenic plants. Notably, the expression levels of both Pe-TElncRNA2 and the genes that were regulated by this lncRNA were upregulated in the transgenic plants after two days of cold stress treatment. These findings elucidate the critical role of LTR retrotransposon-derived lncRNAs in mediating cold stress responses in Moso bamboo.
Introduction
Moso bamboo (Phyllostachys edulis), renowned for its rapid growth, is a versatile resource with applications in construction, textiles, biofuels, and food. Its potential to address food, energy, and climate challenges has garnered significant attention (Chen et al., 2022; Dlamini et al., 2022; Ramakrishnan et al., 2020). While Moso bamboo dominates China’s bamboo cultivation landscape, accounting for approximately 73.76% of its bamboo-growing areas, its productivity is hindered by abiotic stresses (Wang et al., 2022; Zhu et al., 2024). Among different stresses, cold stress induces oxidative stress and reduced cellular metabolism, ultimately inhibiting plant growth, including Moso bamboo (Kim et al., 2024; Wang et al., 2022). For example, during the rapid growth period, cold conditions affect internode length by slowing the growth of the shoots compared to warm conditions. Cell division-related genes (such as PeCYC1BAT, PeCYCB2;4, PeCDC20.1, PeMAD2, PeTPX2, and PeTCX2) and cell elongation-related genes (such as PeEXPA1) were upregulated with increasing temperature, suggesting that cold temperatures inhibit cell division-related genes during the rapid growth period in Moso bamboo (Chen et al., 2022).
In addition to identifying both cold resistance and cold susceptible genes (Chen et al., 2022; Wang et al., 2022), several studies have also identified genes in Moso bamboo, including PeDREB1A, PeDREB2A, PeLEAs, PeAAAP, PeAQPs, PeTIFY, PeIQD, PeDi19-4, and PeZEP, that exhibit differential expression in response to various abiotic stresses (Huang et al., 2016a; Huang et al., 2016b; Liu et al., 2017; Liu et al., 2020; Lou et al., 2017; Sun et al., 2016; Wu et al., 2015; Wu et al., 2018; Wu et al., 2016; Wu et al., 2017). Additionally, non-coding RNAs have been shown to regulate gene expression in response to abiotic stresses (Ding et al., 2022; Yu, Ding & Zhou, 2023). These findings collectively underscore the complexity of Moso bamboo’s adaptive mechanisms to abiotic stresses. Although significant progress has been made in understanding the molecular responses of Moso bamboo to various abiotic stresses, the role of long non-coding RNAs (lncRNAs) in response to cold stress remains elusive. Therefore, a deeper understanding of cold stress response is still essential.
LncRNAs, which are more than 200 nucleotides long and have no translation potential, regulate many biological processes and function as either cis or trans regulators (Zhou, Zheng & Wu, 2024). They also activate chromatin remodeling, modulate alternative splicing, and influence post-transcriptional regulation. LncRNAs are associated with various agricultural traits and offer opportunities to be utilized as biomarkers and in breeding applications, being influential without protein production (Gonzales et al., 2024). LncRNAs exhibit differential expression in response to stress and regulate cold stress through various mechanisms (Zhao et al., 2024).
For instance, COLD INDUCED lncRNA 1 (CIL1) acts as a positive regulator of cold stress in Arabidopsis thaliana by regulating cold response genes, maintaining reactive oxygen species (ROS) activity, and glucose metabolism (Liu et al., 2022). In Arabidopsis, the lncRNA COOLAIR inhibits the expression of the FLC gene under cold stress via the reduction of H3K36 methylation and trimethylation of histone H3 lysine 27 (H3K27me3). This inhibition, in Vitis vinifera L, confers cold tolerance by regulating hormone signal transduction, secondary metabolite biosynthesis, sucrose metabolism pathways, and various transcription factors, including CBF, WRKY, and NACs. Additionally, in Cassava, lncRNAs like CRIR1 interact with the MeCSP5 gene (cold shock protein 5 encoding gene) to help confer cold tolerance, while in Ammopiptanthus nanus, the lncRNA TCONS00065739 targets miR530, contributing to cold stress adaptation by regulating the TZP gene (Jha et al., 2023). In cotton, the nucleus-localized lncRNA973 enhances salt tolerance by modulating reactive oxygen species (ROS), whereas its knockdown reduces tolerance, leading to plant wilting and leaf yellowing under salt stress (Zhang et al., 2019).
However, the role of transposon-derived lncRNAs (TElncRNAs) in response to cold stress remains largely unknown. Transposable elements (TEs), known as mobile DNA, occupy a significant portion of eukaryotic genomes, contribute to genome evolution, and regulate gene expression (Gebrie, 2023; Ramakrishnan et al., 2022). TEs provide cis- regulatory regions, such as promoters and enhancers, that influence both TE-encoded and host gene expression. Many miRNAs and lncRNAs are derived from TEs and play crucial roles in regulatory functions, including target mRNA binding (Gebrie, 2023). Additionally, TEs often exhibit tissue-specific functions, contributing to stress tolerance regulation. For instance, tissue-specific and stress-induced TE-lncRNAs (long intergenic noncoding RNAs) have been identified under salt, abscisic acid (ABA), and cold treatments in Arabidopsis, rice, and maize. Notably, these TE-lncRNAs are predominantly derived from retrotransposons rather than DNA transposons (Wang et al., 2017). Furthermore, TE-lncRNAs, due to their transposon-derived nature, are recognized as transposons themselves and are consequently subjected to similar epigenetic silencing mechanisms. These lncRNAs, like transposons, have the potential to move and propagate within the genome (Kornienko et al., 2023).
In Moso bamboo, several lncRNAs have been identified that are associated with abiotic stress, nitrogen metabolism, and secondary cell wall biosynthesis (Ding et al., 2024; Ding et al., 2022; Wang et al., 2021; Yuan et al., 2022). Moso bamboo’s genome comprises over 63% TEs and thus heavily relies on TE genetic components for its development and stress tolerance. TEs are known for their crucial roles in genome modification (Guo et al., 2019; Liufu et al., 2023; Zhao et al., 2018). In our previous study, we identified differentially methylated and expressed TE-lncRNAs under cold, heat, UV, and salt stress conditions (Ding et al., 2024). Despite this, the role of lncRNAs derived from long terminal repeat (LTR) retrotransposons (TElncRNAs) in response to cold stress remains largely unexplored.
To address this gap, our study investigated the function of a novel LTR retrotransposon-derived lncRNA, Pe-TElncRNA2, under cold stress in Moso bamboo. Pe-TElncRNA2 is 616 bp in length and consists of two exons. It was overexpressed in Moso bamboo protoplasts and Arabidopsis thaliana. For the functional analysis of Pe-TElncRNA2, various experimental approaches were employed, including quantitative real-time PCR (qRT-PCR) for gene expression analysis and assays to evaluate antioxidant enzyme activity. Our results show that Pe-TElncRNA2 modulates gene-specific expression, enhances antioxidant activities, and improves cold tolerance in the transgenic Arabidopsis. These findings suggest that Pe-TElncRNA2 is a promising candidate for developing cold-resistant crops.
Materials & Methods
Plant materials and growth conditions
Moso bamboo seeds from a single maternal plant and Arabidopsis thaliana seeds (Columbia ecotype) were used in this study. The Moso bamboo seeds were collected from the same plant located in Lingchuan County, Guilin City, Guangxi Province, China. All seedlings were cultivated in a controlled greenhouse under a 16-hour light/8-hour dark photoperiod at 25 ° C/22 °C (day/night) and 60% relative humidity.
Cold stress treatment
For cold stress treatment, five-leaf-stage Moso bamboo seedlings were subjected to 4 °C for 8, 16, 24, and 32 h in a plant incubator. For Arabidopsis, four-week-old wild-type and transgenic Arabidopsis plants were subjected to 4 °C for 2 and 4 days in a plant incubator. After the respective cold stress treatments, five mature leaves from Moso bamboo seedlings and Arabidopsis leaves were collected, immediately frozen in liquid nitrogen, and stored at −80 °C for subsequent RNA extraction. Three biological replicates of each stress treatment were used for both species.
Identification of Pe-TElncRNA2
Pe-TElncRNA2 and its target genes (PeFZR2, PeNOT3, PeABCG44, and PeAGD6) were identified from Moso bamboo transcriptome data generated under cold stress conditions (Ding et al., 2022). Notably, all these transcripts exhibited differential expression under cold stress. To further characterize Pe-TElncRNA2, we employed several bioinformatic tools, including the Coding-Non-Coding Index (CNCI) (Sun et al., 2013), the Coding Potential Calculator 2 (CPC2) (Kong et al., 2007), the Pfam protein families database (Finn et al., 2016), and PLEK, a predictor of long non-coding RNAs and messenger RNAs based on an improved k-mer scheme (Li, Zhang & Zhou, 2014) to analyze the translation potential of Pe-TElncRNA2. Additionally, NCBI BLASTN was used to determine Pe-TElncRNA2 sequence similarity to known plant transcripts.
RNA extraction, cDNA synthesis, and qRT-PCR analysis
Total RNA was extracted from Moso bamboo and Arabidopsis samples using the SteadyPure Universal RNA Extraction Kit II (Accurate Biology, Changsha, China), following the manufacturer’s protocol. The cDNA synthesis was performed using the Hifair® II 1st Strand cDNA Synthesis SuperMix for qPCR (gDNA digester plus) (Yeasen Biotechnology, Shanghai, China) according to the manufacturer’s instructions. Quantitative real-time PCR (qRT-PCR) amplification was conducted on a CFX96 Touch Real-Time PCR System (Bio-Rad) using the Hieff® qPCR SYBR® Green Master Mix (Yeasen Biotechnology, Shanghai, China). Gene-specific primers for Pe-TElncRNA2, its target genes, and their Arabidopsis homologues were designed using Primer 5.0 software. AtACTIN2 and PeNTB served as reference genes for Arabidopsis and Moso bamboo, respectively, while PeACT and PeEFlα were used as reference genes for cellular localization studies in Moso bamboo. Relative gene expression levels were calculated using the 2−ΔΔCt method. The primer names and their sequences are listed in Table S1.
Nuclear and cytoplasmic protein extraction from Moso bamboo leaves
For the cellular localization analysis of Pe-TElncRNA2, Moso bamboo leaves were cut into the smallest possible pieces. Nuclear and cytoplasmic proteins were extracted using a Nuclear and Cytoplasmic Protein Extraction Kit (Beyotime Biotechnology, Shanghai, China) and phenylmethylsulfonyl fluoride (PMSF). The cut leaf tissue was homogenized at a ratio of 3:20, and the extraction process was carried out according to the manufacturer’s instructions. The extracted proteins were stored at −80 °C for future use. RNA extraction, cDNA synthesis, and qRT-PCR analysis were performed as previously described. PeACT and PeEF1A were used as reference markers for nuclear and cytoplasmic localization, respectively. The primer names and their sequences are listed in Table S1. The formulae used to calculate the relative expression levels in the nucleus and cytoplasm are as follows: Nuclear % =2−ΔCT nucleus/(2−CT cytoplasm + 2−CT nucleus), Proton % = 1 − Nuclear %.
Plasmid construction for Pe-TElncRNA2 overexpression
To overexpress Pe-TElncRNA2 in Moso bamboo protoplasts and Arabidopsis, the full-length cDNA of Pe-TElncRNA2 (containing only exons) was amplified by PCR using 2 ×Hieff Canace® Plus PCR Master Mix (with Dye) (Yesen, Shanghai, China) and a pair of specific primers: Pe-TElncRNA2-full length-F and Pe-TElncRNA2-full length-R. The amplified Pe-TElncRNA2 fragments were then cloned into two different vectors: pUBQ10, which is driven by the Ubiquitin (UBQ) promoter, and pER8, which is driven by the cauliflower mosaic virus (CaMV) 35S promoter. The resulting constructs, pUBQ10-Pe-TElncRNA2 and pER8-Pe-TElncRNA2, were then used for genetic transformation of Moso bamboo protoplasts and Arabidopsis, respectively. The primer names and their sequences are listed in Table S1.
Moso bamboo protoplast genetic transformation
Moso bamboo protoplast isolation and the polyethylene glycol (PEG)-mediated genetic transformation methods were conducted as described in our previous study (Yu, Ding & Zhou, 2023). Briefly, 21-day-old Moso bamboo leaf sheaths were cut into the smallest possible pieces and incubated in an enzyme solution for 4 h. Following centrifugation, 2 − 3 × 104 protoplasts were resuspended in 200 µL of MMG solution (4 mM MES-KOH (pH 5.7), 0.4 M mannitol, and 15 mM MgCl2). Next, a mixture of 10 µg pUBQ10-Pe-TElncRNA2 and 100 µL protoplasts was incubated with PEG solution (40% PEG4000, 0.8 M mannitol, and 1 M CaCl2) for 4 min. Subsequently, W5 solution (4 mM MES-KOH (pH 5.7), 0.5 M mannitol, and 20 mM KCl) was added to the sample, and the protoplasts were then incubated and harvested. Transfection efficiency of the pUBQ10-Pe-TElncRNA2 construct in Moso bamboo protoplasts was assessed by confocal laser scanning microscopy (CLSM, Zeiss LSM510; Zeiss, Oberkochen, Germany) after 12–16 h post-transfection.
The experiment was divided into two groups: the protoplasts with the pUBQ10 empty vector, and the protoplasts with the pUBQ10-Pe-TElncRNA2 construct. To induce cold stress, the protoplasts from all three groups were incubated at 4 °C for 4 h in a plant incubator. Subsequently, the protoplasts were centrifuged at 150 g for 2 min, and the supernatant was discarded. RNA extraction, cDNA synthesis, and qRT-PCR analysis were performed immediately as previously described. Three biological replicates were used for gene expression analyses in each group.
Arabidopsis floral dip transformation
Arabidopsis transgenic plants were generated using the Agrobacterium-mediated floral dip genetic transformation method (Zhang et al., 2006). In this process, the pER8-Pe-TElncRNA2 construct was introduced into Arabidopsis plants by dipping fully blossomed inflorescences for 30 s in an Agrobacterium suspension containing the construct. The suspension included a 5% sucrose solution and 0.03% (vol/vol) Silwet L-77. The transgenic T1 seeds were harvested from plants grown on half-strength Murashige and Skoog (MS) medium supplemented with 35 mg/L hygromycin. PCR amplification using Pe-TElncRNA2-specific primers was employed to screen for the transgenic plants.
Measurement of proline content
To assess antioxidant activities in Arabidopsis transgenic plants under cold stress conditions, proline content was measured using kits from Suzhou Comin Biotechnology Co., Ltd., Suzhou, China, following the manufacturer’s instructions. Briefly, after the cold stress treatment, fresh Arabidopsis transgenic leaves (0.05 g) from each collected sample were homogenized in an ice bath using a TGrinder tissue grinder (China). The proline extraction was then performed, and the absorbance data were recorded. Finally, the proline content was calculated according to the supplier’s instructions.
Superoxide dismutase activity analysis
Superoxide dismutase (SOD) activity was assessed using an activity assay kit from Suzhou Comin Biotechnology Co., Ltd., Suzhou, China, following the manufacturer’s instructions. Briefly, after the cold stress treatment, fresh Arabidopsis transgenic leaves (0.05 g) from each collected sample were homogenized in one mL of extraction buffer. The homogenates were then centrifuged at 12,000 rpm at 4 °C for 10 min to obtain a crude enzyme extract, and the supernatant was used for SOD enzyme activity analysis. The absorbance data at λ = 560 nm were recorded. SOD enzyme activity was defined as a unit of enzyme activity (U/mL) when the percentage of inhibition in the reaction system reached 50%.
Determination of malondialdehyde content
To evaluate the oxidative stress level in Arabidopsis transgenic plants under cold stress conditions, malondialdehyde (MDA) content—a lipid peroxidation marker and indicator of oxidative stress—was measured using a kit from Suzhou Comin Biotechnology Co., Ltd., Suzhou, China, according to the manufacturer’s instructions. Briefly, after the cold stress treatment, fresh Arabidopsis transgenic leaves (0.05 g) from each collected sample were homogenized in an ice bath using a TGrinder tissue grinder (Tiangen, Beijing, China). The MDA extraction was then carried out following the supplier’s instructions. The corresponding absorbance data were recorded, and the MDA content was calculated.
Determination of photosynthetic efficiency
To assess photosynthetic efficiency in Arabidopsis transgenic plants under cold stress conditions, the maximum photochemical efficiency PS II (Fv/Fm) was measured in dark-adapted leaves using the Multi-Function Plant Efficiency Analyser (M-PEA). After dark-adapting the leaves for 20 min, the fluorescence parameters were measured. In this process, F0 represents the initial fluorescence, Fm is the maximum fluorescence, and Fv/Fm is calculated as (Fm − F0)/Fm.
Statistical analysis
Pearson correlation coefficients were calculated to assess the relationship between the relative expression levels of Pe-TElncRNA2 and its target genes. The data are presented as mean values of three replicates ± standard deviation. Fisher’s least significant difference (LSD) and Duncan’s multiple range test (DMRT) were used for multiple comparisons among the samples, with analysis performed using GraphPad Prism 8.0 (GraphPad Software, San Diego, CA, USA, http://www.graphpad.com). Treatment means followed by different lowercase letters are significantly different at p < 0.05.
Results
Structural analysis of Pe-TElncRNA2
Our previous studies revealed that approximately 12.4% of lncRNAs in Moso bamboo originated from TEs, primarily from the Ty1/copia and Ty3/gypsy LTR retrotransposon superfamilies. While TE-lncRNAs and non-TE-lncRNAs exhibited similar length distributions and expression patterns, TE-lncRNAs displayed stress-specific expression profiles, often downregulated under cold stress, and were predicted to target multiple genes. Moreover, epigenetic regulation, particularly promoter and genic region methylation, was found to suppress lncRNA expression (Ding et al., 2024; Ding et al., 2022). Building upon these findings, the current study delved deeper into the functional characterization of a novel LTR retrotransposon-derived lncRNA, Pe-TElncRNA2, and its target genes within the context of cold stress in Moso bamboo, utilizing the same transcriptome dataset (Ding et al., 2022).
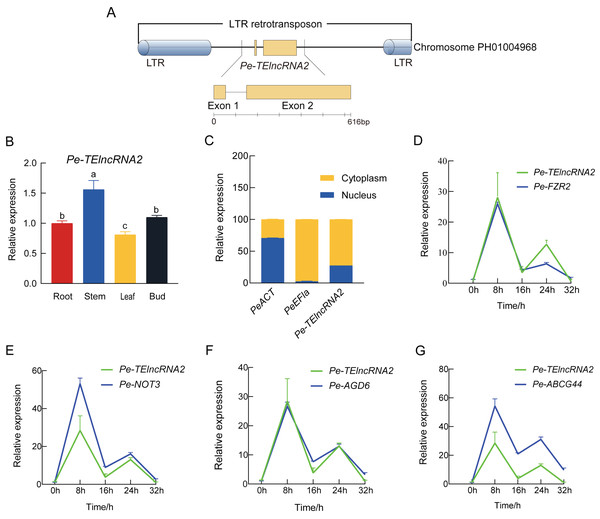
Pe-TElncRNA2 is a 616 bp full-length lncRNA composed of two exons and lacking protein-coding potential, as confirmed by CNCI, Pfam, and PLEK analyses. While CPC2 predicted a low coding probability (0.005) and Fickett score (0.411), the overall evidence strongly supports its classification as a lncRNA. It is located within an LTR retrotransposon on Chromosome PH01004968, spanning positions 16,954–17,569 (Fig. 1A). BLASTN analysis has further confirmed that Pe-TElncRNA2 is a novel lncRNA with no sequence similarity to any known plant transcripts.
Figure 1: Structural analysis of Pe-TElncRNA2, its expression profile, and its target genes in Moso bamboo.
(A) The genomic location of the 616 bp full-length Pe-TElncRNA2 within an LTR retrotransposon on chromosome PH01004968. The exons, lacking coding potential, are indicated by green rectangles. (B) Tissue-specific expression of Pe-TElncRNA2 in the root, stem, leaf, and bud. (C) Cellular localization of Pe-TElncRNA2 and the reference genes (PeACT and PeEF1α) in the cytoplasm and nucleus. (D-G) Expression patterns of Pe-TElncRNA2 and four genes, such as PeFZR2, PeNOT3, PeABCG44, and PeAGD6, in the leaves under cold stress. Under cold treatment, Pe-TElncRNA2 and four genes exhibited a biphasic expression pattern, peaking first at 8 h, then declining, reaching a second peak at 24 h, and decreasing again.The expression profiles of Pe-TElncRNA2 and its related genes
Based on the transcriptome dataset, Pe-TElncRNA2 was upregulated under cold stress. To further investigate, Pe-TElncRNA2 was analyzed in Moso bamboo seedlings using qRT-PCR. Pe-TElncRNA2 expression peaked at 8 h of cold treatment (4 °C) and was significantly higher in the stems compared to the leaves, roots, and buds (Fig. 1B). Further cellular localization analysis showed that Pe-TElncRNA2 expression was significantly higher in the cytoplasm, with 72.53% expression in the cytoplasm and 27.47% in the nucleus. Conversely, the reference gene PeEF1α was expressed entirely in the cytoplasm, while PeACT was significantly higher in the nucleus than in the cytoplasm (Fig. 1C). These results indicate that Pe-TElncRNA2 mainly plays a role in the cytoplasm.
Further analysis revealed that Pe-TElncRNA2 acts as a cold-induced regulator of four genes: fizzy-related 2 protein (FZR2), negative regulator of transcription subunit 3 (NOT3), ABC transporter G family member 44 (ABCG44), and ADP-ribosylation factor GTPase-activating protein (AGD6). To investigate their expression dynamics, Pe-TElncRNA2 and its target genes (PeFZR2, PeNOT3, PeABCG44, and PeAGD6) were analyzed using qRT-PCR in Moso bamboo seedlings subjected to cold treatment for 8, 16, 24, and 32 h. The expression patterns of the target genes exhibited a strong correlation with that of Pe-TElncRNA2 (Figs. 1D–1G). Pearson correlation analysis further revealed correlation coefficients greater than 0.8 between Pe-TElncRNA2 and its target genes (Fig. S1). The expression patterns of Pe-TElncRNA2 and its regulated genes exhibited a biphasic response to cold stress, with significant upregulation at 8 and 24 h. The expression levels decreased between these peak times and further declined with extended cold exposure (Figs. 1D–1G). These findings suggest that Pe-TElncRNA2 is involved in the cold stress response of Moso bamboo by regulating the expression of these genes.
Pe-TElncRNA2 overexpression in Moso bamboo protoplasts
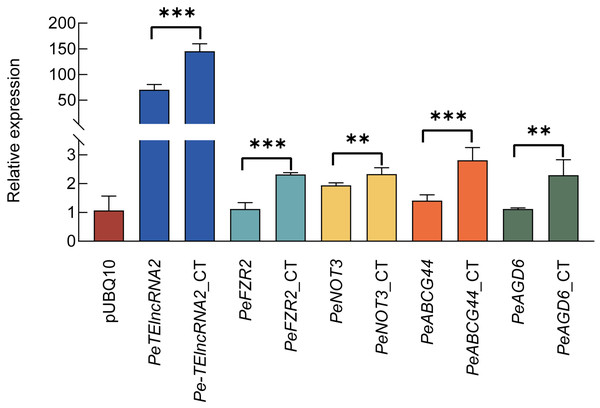
To investigate the regulatory function of Pe-TElncRNA2 on these four genes under cold stress, a pUBQ10-Pe-TElncRNA2 overexpression vector (Fig. S2A) was constructed and transfected into Moso bamboo protoplasts using the polyethylene glycol (PEG)-mediated method. This achieved a transgenesis success rate of up to 50% transformation efficiency. The transfected protoplasts were subsequently subjected to a 4-hour cold treatment. Pe-TElncRNA2 expression was significantly elevated in the pUBQ10-Pe-TElncRNA2-transfected protoplasts compared to the control, pUBQ10 empty vector-transfected protoplasts, under cold stress conditions (Fig. S2B). Concurrently, the expression levels of the target genes (PeFZR2, PeNOT3, PeABCG44, and PeAGD6) were significantly elevated in the pUBQ10-Pe-TElncRNA2-transfected protoplasts compared to the control under cold stress conditions (Fig. 2). These findings suggest that Pe-TElncRNA2 positively regulates these four genes in response to cold stress in Moso bamboo.
Figure 2: Relative expression levels of Pe-TElncRNA2 and four genes in the pUBQ10-Pe-TElncRNA2 -transfected Moso bamboo protoplasts.
The expression levels of Pe-TElncRNA2 (A) PeFZR2 (B) PeNOT3 (C) PeABCG44 (D) and PeAGD6 (E) in the pUBQ 10-Pe-TElncRNA2 -transfected protoplasts under cold stress conditions after 4 h of treatment. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences. CT indicates cold stress conditions after 4 h of treatment. The empty vector pUBQ10 and pUBQ10-Pe-TElncRNA2 -transfected protoplasts under normal conditions served as controls.Pe-TElncRNA2 overexpression in Arabidopsis
To investigate the phenotypic effects of Pe-TElncRNA2, its full-length cDNA (only exons) was cloned into the overexpression vector pER8. The resulting construct (pER8-Pe-TElncRNA2) was introduced into Arabidopsis via Agrobacterium-mediated floral dip transformation (Zhang et al., 2006). Three independent transgenic Arabidopsis lines were generated through PCR screening (Fig. S3). Under cold stress, the transgenic plants exhibited less water loss and shrinkage and were greener compared to the wild-type plants after 2 days of treatment. Nonetheless, both transgenic and wild-type plants exhibited similar phenotypic responses, including leaf base purpling and leaf crumpling due to water loss, when subjected to 4-day cold treatments (Fig. 3). However, Pe-TElncRNA2 expression levels were significantly higher in the transgenic plants compared to the wild-type plants after 2 days of cold treatment, but there was no significant difference after 4 days of cold treatment (Fig. 4). These results suggest that Pe-TElncRNA2 plays an important role in the early cold stress response of Arabidopsis.
Figure 3: Phenotypic comparison between the wild-type and transgenic Arabidopsis under cold stress.
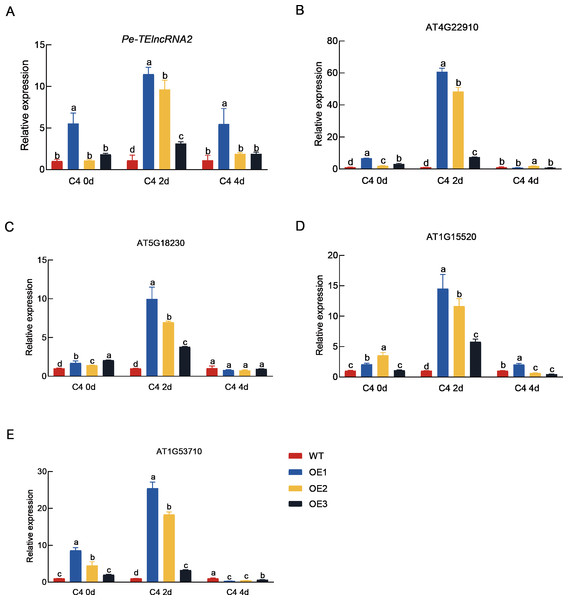
Representative images show the wild-type (WT) and transgenic Arabidopsis plants overexpressing Pe-TElncRNA2 , subjected to 2-day (C4-2d) and 4-day (C4-4d) cold treatments. Transgenic plants exhibited increased plant vigor, as indicated by reduced water loss and yellowing, compared to WT under cold stress conditions.Figure 4: Expression analysis of Pe-TElncRNA2. and four homologous genes in the wild-type and transgenic Arabidopsis under cold stress.
Relative expression levels of Pe-TElncRNA2 (A), AT4G22910 (B), AT5G18230 (C), AT1G1552 0 (D), and AT1G53710 (E) in the transgenic Arabidopsis lines (OE1, OE2, and OE3) overexpressing Pe-TElncRNA2 , exposed to 2-day (C4-2d) and 4-day (C4-4d) cold treatments. The four genes, including AT4G22910 (AtFZR2), AT5G18230 (AtNOT3), AT5G18230 (AtABCG44), and AT1G53710 (AtAGD6), are homologous to PeFZR2, PeNOT3, PeABCG44, and PeAGD6, respectively. AtACTIN2 was used as the reference gene, and the samples with 0-hour cold treatment served as control. The data represent the mean values of three replicates ± standard deviation. Fisher’s least significant differences (LSD) and Duncan’s multiple range test (DMRT) were used for multiple comparisons among all the samples. Different lowercase letters indicate significant differences at p < 0.05.Concurrently, the expression levels of the genes (AT4G22910 (AtFZR2), AT5G18230 (AtNOT3), AT5G18230 (AtABCG44), and AT1G53710 (AtAGD6)), which are homologous to PeFZR2, PeNOT3, PeABCG44, and PeAGD6, respectively were also significantly higher in the transgenic plants compared to the wild-type plants after 2 days of cold treatment. The result suggest that Pe-TElncRNA2 also regulates Moso bamboo’s homologous genes in Arabidopsis, contributing to early cold stress responses.
Antioxidant activities and photosynthetic efficiency
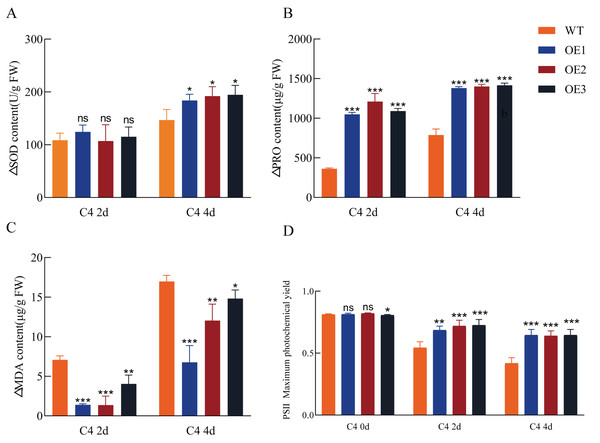
To assess antioxidant capacity and photosynthetic efficiency, SOD activity, proline content, MDA content, and maximum photochemical efficiency (Fv/Fm) in dark-adapted leaves were measured in both transgenic and wild-type Arabidopsis plants following cold treatment. Compared to the wild-type controls, the transgenic plants exhibited enhanced antioxidant capacity and photosynthetic efficiency under cold stress (Fig. 5). SOD activity and proline content were significantly increased in the transgenic plants, while MDA content, a lipid peroxidation marker (a marker of oxidative stress), was reduced compared to the wild-type. Additionally, the Fv/Fm ratio significantly decreased in both wild-type and transgenic plants under cold stress; however, the transgenic plants maintained higher Fv/Fm values, indicating improved photoprotection under cold stress conditions. These results suggest that Pe-TElncRNA2 overexpression enhances cold stress tolerance in Arabidopsis.
Figure 5: Antioxidant enzyme activity, osmolyte accumulation, lipid peroxidation, and photosynthetic efficiency in transgenic and wild-type (WT) Arabidopsis under cold stress.
Superoxide dismutase (SOD) activity (A), proline content (B), malondialdehyde (MDA) content (C), and chlorophyll content (D). Increased levels of SOD and proline, along with an increased maximum quantum efficiency of photosystem II (PSII) (Fv/Fm ratio) and decreased MDA content in the leaves, indicate an enhanced cold stress response. OE1, OE2, and OE3 are the transgenic lines overexpressing Pe-TElncRNA2. FW represents the fresh weight of leaf tissues. The data represent the mean values of three replicates ± standard deviation. Fisher’s least significant differences (LSD) and Duncan’s multiple range test (DMRT) were used for multiple comparisons among all the samples. *p < 0.05, **p < 0.01, and ***p < 0.001 indicate statistically significant differences.Discussion
Identification of a novel TElncRNA2 in Moso bamboo
Moso bamboo is a high-yielding, fast-growing crop with significant economic value in food and construction. However, its cultivation is largely limited to tropical and subtropical regions due to its sensitivity to cold temperatures (Chen et al., 2022). Additionally, a significant negative correlation between monthly precipitation and internode diameter and thickness has been observed, particularly during the colder months of December and January (Zhang et al., 2024). This correlation affects primary thickening growth and, consequently, internode size, which impacts its global production and utilization.
Cold stress inhibits cell division-related genes (PeCYC1BAT, PeCYCB2;4, PeCDC20.1, PeMAD2, PeTPX2, and PeTCX2) and cell elongation-related genes (PeEXPA1) during the rapid growth period in Moso bamboo (Chen et al., 2022). Given the importance of cold stress tolerance, several studies have identified cold-related genes in Moso bamboo, including PeEREBP, PeHSF, PeMYB, PeNAC, PeWRKY, and PeLEA (Huang et al., 2022; Liu et al., 2020; Wang et al., 2022). However, most of these genes have yet to be validated. Additionally, non-coding RNAs, including lncRNAs, play a role in regulating stress responses in Moso bamboo (Ding et al., 2022; Yu, Ding & Zhou, 2023), underscoring the complexity of its stress response mechanisms. Despite this, the role of lncRNAs in response to cold stress remains elusive, particularly the function of transposon-derived lncRNAs (TElncRNAs), which is still largely unknown.
Therefore, this study provides the first evidence of the role of TElncRNAs in cold stress response in Moso bamboo. We identified a novel TElncRNA, Pe-TElncRNA2, located within an LTR retrotransposon on chromosome PH01004968 (Fig. 1A). This lncRNA, which is 616 bp in length and composed of two exons, has no translation potential and lacks evolutionary conservation, as confirmed by CNCI, Pfam, and PLEK analyses. Previous studies have demonstrated that lncRNAs constitute 22.9%, 49.7%, and 51.5% of the total transcriptome in Arabidopsis, rice, and maize, respectively. Retrotransposons play a crucial role in shaping plant stress responses by influencing the epigenome. For example, Arabidopsis, rice, and maize predominantly generate stress-induced TE-lncRNAs from retrotransposons (Wang et al., 2017).
In Moso bamboo, TElncRNAs also predominantly originate from LTR retrotransposons and exhibit stress-specific expression patterns. Typically, these lncRNAs are downregulated under cold and salt stress conditions, while upregulation is observed under heat stress. For instance, Pe-TElncRNA3 plays a crucial role in the heat stress response by regulating downstream genes (PH02Gene25732, PH02Gene25729, and PH02Gene03426) (Ding et al., 2024). In contrast, our study found that Pe-TElncRNA2, also derived from an LTR retrotransposon, is upregulated under cold stress in Moso bamboo (Figs. 1B–1G). This highlights a broader trend in which retrotransposons contribute to stress adaptation through the regulation of lncRNAs.
Additionally, lncRNA expression varies across different tissues and developmental stages. For instance, AtR8 and BoNR8 lncRNAs are predominantly expressed in the root and root elongation zone epidermis of Arabidopsis, respectively (Wu et al., 2019; Wu et al., 2012). In contrast, Pe-TElncRNA2 is specifically upregulated in the stem under cold stress conditions, suggesting that lncRNA tissue-specific expression patterns can vary across different organs in Moso bamboo.
Transient overexpression of Pe-TElncRNA2 in Moso bamboo
The subcellular localization of lncRNAs can significantly influence their function. For example, nuclear lncRNAs often participate in pre-rRNA transcriptional regulation (Xing et al., 2017), while cytoplasmic lncRNAs modulate mRNA stability, translation, signaling pathways, and interact with RNA-binding proteins to regulate gene expression (Statello et al., 2021). In this study, Pe-TElncRNA2 was predominantly localized in the cytoplasm (Fig. 1C and Fig. S2B), suggesting its potential role in mRNA stability, translation, and post-transcriptional regulation.
Due to the absence of a stable transformation system for Moso bamboo, we employed protoplast transformation to overexpress Pe-TElncRNA2. Given the current limitations, protoplasts offer a viable alternative for assessing gene functionality Moso bamboo (Yu, Ding & Zhou, 2023). This approach allowed us to verify its association with downstream genes through transient expression without altering Moso bamboo’s genome. Notably, the upregulation of Pe-TElncRNA2 was associated with the concurrent upregulation of its regulating genes (PeFZR2, PeNOT3, PeABCG44, and PeAGD6) (Fig. 2). This observation is in line with our previous study, where upregulation of Pe-lncRNA1 coincided with the upregulation of its regulating genes (such as PH02Gene33364, PH02Gene38550, PH02Gene43330, PH02Gene19065, PH02Gene05460, PH02Gene26812, PH02Gene35897, and PH02Gene50461) under UV-B stress (Yu, Ding & Zhou, 2023). Our previous study demonstrated that, without genome integration, LTR retrotransposons increase their copies under heat stress (Papolu et al., 2021). This finding aligns with the current study, where LTR retrotransposon-derived Pe-TElncRNA2 is expressed under cold stress conditions. This supports the hypothesis that TE-lncRNAs, as transposons, have the potential to move and propagate within the genome (Kornienko et al., 2023).
Stable overexpression of Pe-TElncRNA2 in Arabidopsis
Given the potential regulatory role of Pe-TElncRNA2 in cold stress response in Moso bamboo, we validated its stable function by overexpressing it in Arabidopsis. Consistent with our protoplast transient expression results, the stable overexpression of Pe-TElncRNA2 in Arabidopsis demonstrated similar results (Fig. 4). The transgenic plants exhibited reduced water loss, maintained leaf color, and showed increased expression of Pe-TElncRNA2 and its regulating genes, AtFZR2, AtNOT3, AtABCG44, and AtAGD6. These findings suggest that Pe-TElncRNA2 positively regulates homologous genes in both Moso bamboo and Arabidopsis, thereby contributing to early cold stress responses. This observation aligns with our previous study, where Pe-lncRNA1 upregulation also positively regulated homologous genes in both species under UV-B stress (Yu, Ding & Zhou, 2023).
Pe-TElncRNA2f-modulated antioxidant activities and photosynthetic efficiency
To elucidate the mechanisms underlying Pe-TElncRNA2-mediated cold tolerance, we assessed antioxidant capacity by measuring SOD activity, proline content, and MDA levels, as well as photosynthetic efficiency through Fv/Fm measurements (Fig. 5). The transgenic plants exhibited significantly increased SOD activity and proline content, while MDA levels were reduced compared to the wild-type controls. Moreover, the transgenic plants maintained higher Fv/Fm values, indicating improved photoprotection under cold stress conditions. These findings align with previous studies demonstrating that enhanced antioxidant capacity and photosynthetic efficiency contribute to cold stress tolerance (Ghosh et al., 2024; Xu et al., 2023).
Pe-TElncRNA2-induced regulation of antioxidant enzyme genes contributes to this enhanced antioxidant capacity. Previous studies have demonstrated that lncRNAs can modulate antioxidant responses. For instance, lncRNA CIL1 maintains ROS homeostasis through cold response genes (Liu et al., 2022), while lncRNA973 enhances salt tolerance by regulating ROS scavenging (Zhang et al., 2019). Moreover, lncRNA and mRNA co-expression plays a critical role in signal transduction and stress tolerance (Cui et al., 2019; Tan et al., 2020). For example, the lncRNA MtCIR2 and its target genes MtCBF/DREB1s regulate freezing tolerance in Medicago truncatula. MtCIR2 overexpression upregulates the expression of these target genes, while mutant MtCIR2 downregulates them (Zhao et al., 2023).
In this study, Pe-TElncRNA2 overexpression upregulates the expression of its downstream genes. The coordinated expression of Pe-TElncRNA2 and its downstream genes contributes to enhanced cold tolerance by modulating antioxidant activity and photosynthetic efficiency. Given that Pe-TElncRNA2 originates from an LTR retrotransposon, it is reasonable to conclude that this may act as a cis-regulatory element, such as a promoter or enhancer, impacting the expression of antioxidant enzyme genes (Gebrie, 2023). These findings suggest that Pe-TElncRNA2 acts as a key regulator in a complex regulatory network that promotes cold stress resilience in Moso bamboo.
Conclusion
This study uncovered the critical role of a novel LTR retrotransposon-derived lncRNA, Pe-TElncRNA2, in enhancing cold tolerance in Moso bamboo. The results demonstrated that Pe-TElncRNA2 was predominantly localized in the cytoplasm, highly expressed in the stem, and positively regulated the expression of key genes involved in antioxidant defense and photosynthesis. Overexpression of Pe-TElncRNA2 in Arabidopsis conferred increased cold tolerance, characterized by reduced oxidative stress and improved photosynthetic efficiency. These findings collectively suggest that Pe-TElncRNA2 functions as a crucial regulator in the complex network of cold stress responses in plants. Understanding the molecular mechanisms underlying Pe-TElncRNA2 function may provide valuable insights for developing cold-tolerant crop varieties.
Supplemental Information
Pearson correlation coefficients between the relative expression levels of Pe-TElncRNA2 and four genes
Correlation coefficients between Pe-TElncRNA2 and PeFZR2 (A), PeNOT3 (B), PeABCG44 (C), and PeAGD6 (D).
Construction of the pUBQ10-Pe-TElncRNA2 overexpression vector and its expression in Moso bamboo protoplasts
(A) Map of the pUBQ10-Pe-TElncRNA2 vector. (B) Transient overexpression of Pe-TElncRNA2 in Moso bamboo protoplasts under cold stress conditions, visualized by fluorescence microscopy (GFP: green fluorescent protein, green channel; Brightfield: grey channel; Merged: overlay). Scale bar = 20 µm. The empty vector pUBQ10 served as a control.
Generation of transgenic Arabidopsis lines
Representative images of the wild-type (WT) and three independent transgenic Arabidopsis lines (OE1, OE2, OE3) overexpressing Pe-TElncRNA2 .