Advances and insights for DKK3 in non-cancerous diseases: a systematic review
- Published
- Accepted
- Received
- Academic Editor
- Rohit Upadhyay
- Subject Areas
- Cell Biology, Cardiology, Geriatrics, Oncology, Urology
- Keywords
- DKK3, Non-tumor-related diseases, Biomarker, Wnt pathway, Review
- Copyright
- © 2025 Sun et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Advances and insights for DKK3 in non-cancerous diseases: a systematic review. PeerJ 13:e18935 https://doi.org/10.7717/peerj.18935
Abstract
This review delves into the role of Dickkopf-3 (DKK3), a secreted glycoprotein and member of the Dickkopf family, in non-malignant diseases. DKK3 is particularly known for its regulatory effects on the Wnt signaling pathway, a critical mediator in various biological processes including cell proliferation, differentiation, and migration. Our review highlights DKK3’s influence in disorders of the cardiovascular, respiratory, renal, and muscular systems, where it contributes to disease progression by modulating these key biological processes. As an emerging biomarker, DKK3’s levels have been found to correlate with various disease states, underscoring its potential diagnostic and therapeutic implications.
Introduction
The Dickkopf (DKK) family, which includes four secreted proteins (DKK1, DKK2, DKK3, and DKK4) in vertebrates, is recognized as a pivotal group of inhibitors of the Wnt signaling pathway, known collectively as the Wnt inhibitor family. These proteins regulate Wnt/β-catenin signaling by interacting with and impeding the function of the Wnt co-receptor low-density lipoprotein receptor-related protein 5/6 (LRP5/6) (Tamai et al., 2000). Notably, DKK3, with its cysteine-rich domain 2 (CRD2), exhibits a higher binding affinity for LRP6, setting it apart from other DKK family members in terms of structural diversity, activity, and physicochemical properties (Sadeghi et al., 2019).
DKK3 plays a critical role in the pathogenesis of various diseases through its regulation of the Wnt signaling pathway (Arnold et al., 2021; Cen et al., 2023; Guo & Qin, 2015; Katase et al., 2013). The Wnt signaling pathway is a key regulator of biological processes such as cell proliferation, differentiation, and migration (Logan & Nusse, 2004; Nusse & Clevers, 2017). In cardiovascular diseases, DKK3 modulates the Wnt/β-catenin signaling pathway, influencing myocardial cell proliferation and fibrosis (Zeng et al., 2021). In kidney diseases, DKK3 acts as a pro-fibrotic glycoprotein released by stressed renal tubular epithelial cells, promoting renal fibrosis (Federico et al., 2016). Furthermore, the role of DKK3 in neurological disorders has gained increasing attention, with its upregulation in Alzheimer’s disease potentially contributing to neurodegeneration through effects on neuronal survival and synaptic formation (Martin Flores et al., 2024).
DKK3’s widespread expression was demonstrated by Inoue et al. (2017), who found DKK3 mRNA in various adult mouse organs, including the brain, retina, heart, gastrointestinal tract, adrenal glands, prostate, and ovaries. The cytoplasmic localization of DKK3 protein in adrenal medullary cells and its immunoreactive presence in the stomach and intestines suggest that DKK3 plays multifaceted roles in multiple organ systems (Fujita et al., 2020; Inoue et al., 2017). In hair follicle stem cells, DKK3, among other Wnt inhibitors, is expressed to inhibit medial bulge cells, thus enhancing differentiation capacity and maintaining stem cell potency during the hair cycle’s quiescent and active phases (Lim et al., 2016). The knockdown of DKK3 has been shown to boost the generation of induced pluripotent stem cells without affecting the colony-forming ability of embryonic stem cells, underscoring its role in organ regeneration (Arnold et al., 2021). Leonard et al. (2017) identified Dkk3b, an intracellular product of the DKK3 locus, which sequesters cytoplasmic unphosphorylated β-catenin, preventing its nuclear translocation and playing a crucial role in early developmental processes in mice. DKK3 also maintains prostate structural integrity in mice by modulating TGF-β/Smad signaling and is critical for genital development and growth (Romero et al., 2013).
DKK3’s involvement in a spectrum of diseases, both oncological and non-oncological, is well-documented. Emerging research is shedding light on DKK3’s role in non-oncological conditions, further defining its function as a Wnt signaling inhibitor (Poorebrahim et al., 2017; Suchitha et al., 2023).
While the majority of DKK3 research has focused on its association with tumor-related diseases and the WNT signaling pathway, there is a growing body of evidence that highlights DKK3’s significance in non-neoplastic diseases. A comprehensive review of DKK3’s regulatory mechanisms and its role in various diseases is essential for a holistic understanding of DKK3 as a gene. This understanding will not only elucidate the molecular underpinnings of DKK3 in non-oncological diseases but also pave the way for the development of innovative therapeutics and expand its applications in related fields.
Survey methodology
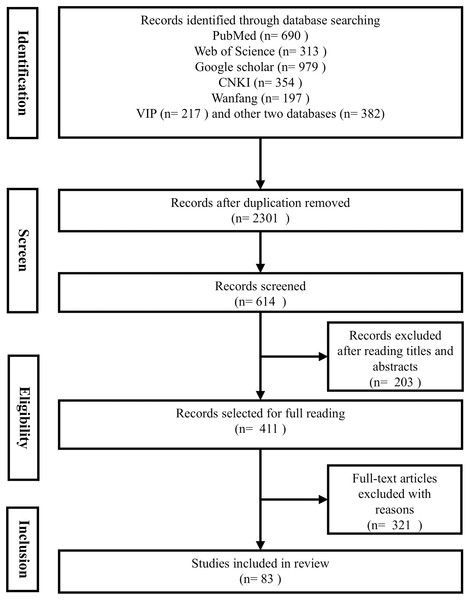
This systematic search was conducted across eight reputable databases, including PubMed, Google Scholar, Embase, and Web of Science, to ensure a comprehensive coverage of the literature. Our search strategy was designed to encompass articles from the inception of each database up to 30th August 2024, focusing on studies published in English to maintain consistency and accessibility. We employed a set of keywords were utilized in the search: “DKK3” or “Dickkopf-related protein 3” in combination with “non-tumor diseases”, “non-cancer diseases”, “diseases”, or “disorders”. This approach was carefully crafted to align with the journal’s focus on non-cancerous diseases and to capture the broad spectrum of DKK3-related research (see Fig. 1).
Figure 1: PRISMA flow diagram.
The identified articles were systematically compiled using Endnote X9 citation manager (Clarivate, Philadelphia, PA, USA), which facilitated the organization and management of our bibliography. For each article, key information such as Digital Object Identifier (DOI), titles, authors, and publication years was meticulously recorded to enable thorough analysis and future reference.
It is important to acknowledge that despite the rigor of our search strategy, there may be potential limitations regarding residual confounding. For instance, the heterogeneity of study designs and variations in patient populations across different studies could introduce biases that are not fully accounted for in our analysis. Additionally, the reliance on published literature may lead to publication bias, where negative or inconclusive results are less likely to be reported. We have taken steps to mitigate these issues by critically appraising the quality of the included studies to assess the robustness of our findings to these potential biases.
Relationship between DKK3 and cardiovascular/respiratory diseases
Aging-related muscle atrophy is a common complication in patients with chronic heart failure (CHF). Clinical studies suggest that probiotics may enhance functional capacity in CHF by modulating Wnt signaling to mitigate muscle atrophy. Moreover, changes in the Short Physical Performance Battery (SPPB) score in CHF patients are closely associated with DKK3 levels (Karim et al., 2022). A meta-analysis examining the role of probiotics in the prevention of myocardial infarction (MI) has uncovered a significant relationship between hand grip strength (HGS), a measure of physical performance, and Wnt pathway biomarkers. Notably, this study has shed light on the inverse relationship between the SPPB scores and DKK3 protein levels (Taslim et al., 2023). Piek et al. (2020), using bioinformatics techniques, found that heart failure biomarkers showed high cardiac specificity for natriuretic peptides (>99%), while DKK3 had limited diagnostic and prognostic utility (44%). Curcumin, isolated from rhizome of turmeric, upregulates DKK3 and exerts a cardioprotective effect in chronic heart failure (Cao et al., 2018). In addition to its association with age-related macular degeneration (AMD), Tom et al. (2020) identified DKK3 as a potential biomarker for AMD treatment in an in vivo model. The gene directly associated with AMD is HTRA1, and since DKK3 is its substrate, it can be utilized as a biomarker for monitoring AMD progression and treatment efficacy (Tom et al., 2020).
The Bruneck Study, a population-based prospective cohort investigation initiated in 1985 within the northeastern Italian district of Bruneck, is dedicated to elucidating the risk factors associated with cardiovascular diseases (CVD), encompassing coronary heart disease, cerebrovascular accidents, and other pertinent conditions (Tsimikas et al., 2009; Willeit & Kiechl, 1993; Xu et al., 1993). The Bruneck prospective study, which included 684 participants in 2000 and 574 in 2005, found that plasma DKK3 levels were inversely associated with carotid intima-media thickness and the 5-year progression of carotid atherosclerosis. Mouse models confirmed that DKK3 stimulates endothelial cell migration and upregulates JNK and c-jun phosphorylation, suggesting a protective role in endothelial migration and atherosclerosis repair. These findings highlight the therapeutic potential of DKK3 in vascular disease (Yu et al., 2017). A study of 88 healthy volunteers and 280 patients with coronary artery disease (CAD) demonstrated a significant association between serum DKK3 levels and an increased risk of CAD (OR = 1.131, 95% CI = [1.091–1.173], P < 0.001) and acute coronary syndromes (ACS) (OR = 1.201, 95% CI = [1.134–1.271], P < 0.001) (Xu et al., 2024). No significant correlation was observed between serum DKK3 levels and coronary stenosis of <50%, while a negative correlation was noted with severe stenosis (≥50%) (Wang et al., 2020).
Cardiac regeneration involves the de-differentiation and proliferation of cardiomyocytes. Studies in zebrafish have shown that cardiac injury triggers the secretion of Wnt inhibitors, including Dkk1 and Dkk3, in tissues near the injury site. Experimental overexpression of Dkk1, which inhibits Wnt signaling, enhances cardiomyocyte proliferation and regeneration. Dkk3, another Wnt inhibitor, may exert similar effects (Peng et al., 2021). DKK3 overexpression mitigates angiotensin II–induced myocardial hypertrophy and fibrosis by modulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways (Zhai et al., 2018). In myocardial fibrosis, DKK3 attenuates Smad3 activation and the expression of fibrosis-related genes by upregulating Smad7 in cardiac fibroblasts (Zeng et al., 2021). DKK3 regulates hypertension by promoting vascular endothelial growth factor (VEGF) expression and activating the VEGF/Akt/endothelial nitric oxide synthase (eNOS) hypotensive pathway in animal models (Busceti et al., 2023).
Fewer studies have examined the role of DKK3 in the respiratory system compared with its role in cardiovascular disease (Qaisar et al., 2020; Thöni & Mayer, 2021). Ventilator-induced lung injury (VILI) is primarily driven by inflammation, apoptosis, and oxidative stress, with the Wnt/β-catenin pathway modulating these processes to reduce lung injury (Chen et al., 2023). In an in vitro model of lipopolysaccharide-induced pneumoconiosis, nuclear respiratory factor 1 (NRF1) was shown to mitigate inflammatory injury through regulation of the DKK3 and GSK-3β/β-catenin pathway. DKK3 may also serve as a protective factor in sepsis-associated lung injury (Kang et al., 2023).
Relationship between DKK3 and diseases of the renal system
The relationship between DKK3 and the kidney has garnered increasing attention in recent years. Tubular atrophy and interstitial fibrosis are hallmarks of progressive chronic kidney disease (CKD). DKK3, a pro-fibrotic glycoprotein released by stressed renal tubular epithelial cells, promotes renal fibrosis (Federico et al., 2016; González et al., 2022). In a CKD mouse model, N6-methyladenosine (m6A) modification upregulated DKK3, activating the Wnt/β-catenin pathway and increasing mitochondrial fission factor (MFF) expression, which led to mitochondrial dysfunction, oxidative stress, and progression of renal failure (Song et al., 2024).
Urinary DKK3 (uDKK3) levels are closely linked to the degree of tubular atrophy and interstitial fibrosis, correlating with stages of chronic kidney disease (CKD), and may serve as a therapeutic target and diagnostic marker for renal fibrosis (Dziamałek-Macioszczyk et al., 2023; Federico et al., 2016; González et al., 2022; Xing et al., 2023). Elevated urinary DKK3 is associated with a high risk of acute kidney injury (AKI) and long-term renal dysfunction following cardiac surgery, with uDKK3/lactate concentrations >471 pg/mg indicating increased risk of AKI (Schunk et al., 2019). In a cohort of patients with CKD, urinary DKK3/creatinine levels correlated with tubulointerstitial fibrosis and predicted estimated glomerular filtration rate (eGFR) decline over 6–12 months (Zewinger et al., 2018). In pediatric patients, uDKK3 predicted early AKI, mortality in the intensive care unit, and distinguished sepsis-associated AKI from other forms of AKI (Hu et al., 2023). Urinary DKK3/creatinine ratios have shown utility in predicting post-surgical AKI risk in CKD patients undergoing cardiovascular surgery and are an independent predictor of contrast-induced AKI (Roscigno et al., 2021; Seibert et al., 2021). Studies in patients with autosomal dominant polycystic kidney disease (ADPKD) confirmed the association of urinary DKK3 levels with disease severity (Arjune et al., 2024). Additionally, urinary DKK3 may help identify peritoneal dialysis patients at high risk for declining renal function and predict transplant kidney function up to 36 months post-transplant (Schuster et al., 2022). Torigoe et al. (2021), in examining the link between urinary DKK3 levels and the deterioration of residual renal function in peritoneal dialysis patients, found that 24-h urinary DKK3 excretion (β = 0.44, P = 0.0015) and renal Kt/V (β = 0.38, P = 0.0059) were independently associated with the annual decline in renal Kt/V on multivariate analysis (González et al., 2022).
Urinary DKK3 (uDKK3) levels were not independently associated with eGFR or albuminuria in hypertensive, non-diabetic CKD patients. However, higher uDKK3 levels appeared to be associated with a greater risk of cardiovascular events, incident end-stage renal disease (ESKD), acute kidney injury (AKI), ≥30% eGFR decline, and mortality, but these associations were not independent of eGFR and albuminuria (Peschard et al., 2024). In a cohort of 31 patients with pharmacologically treated hypertension, uDKK3 levels were inversely correlated with eGFR changes (r = −0.3714, P = 0.0397), with higher uDKK3 levels linked to greater eGFR loss over 24 months (Schäfer et al., 2023). In contrast, a large cohort study of 8,420 patients found that plasma DKK3 (sDKK3) was associated with cardiovascular risk factors but not independently linked to cardiovascular or chronic kidney disease progression (Piek et al., 2021).
Relationship between DKK3 and disorders of the osteomuscular system
DKK3 has a protective role in osteoarthritis (OA) by inhibiting NF-κB activation and reducing IL-1α-driven extracellular matrix-associated matrix metalloproteinase-13 (MMP-13) expression (Conde et al., 2021). DKK3 also impairs muscle stem cell differentiation, hindering muscle regeneration in vivo. In obese and type 2 diabetic (T2D) mice, myofiber-specific ablation of Baf60c impairs muscle regeneration and contraction, with significant upregulation of DKK3. DKK3-mediated paracrine signaling plays a key role in regulating muscle regeneration (Xu et al., 2023). A study demonstrated that DKK3 induces nuclear import of β-catenin in young mice, enhancing its interaction with FoxO3, which activates transcription of E3 ubiquitin ligases Fbxo32 and Trim63, contributing to muscle atrophy (Yin et al., 2018). DKK3 has potential as a diagnostic marker and therapeutic target for age-associated muscular dystrophy. DKK family genes are expressed in adult mouse muscle, with high DKK3 expression in the quadriceps and low expression in the gastrocnemius and soleus (de Wilde et al., 2010). RNA sequencing of pigeon skeletal muscle at various developmental stages identified 20 candidate mRNAs, including DKK3, with enrichment analysis highlighting the involvement of the PI3K/AKT/mTOR, AMPK, FAK, and thyroid hormone pathways in muscle growth (Ding et al., 2021). Diaphragm dysfunction is central to many respiratory diseases, and a study of patients with chronic obstructive pulmonary disease (COPD), asthma, and pulmonary tuberculosis found a strong correlation between DKK3 levels, collateral bone mass index, and elevated markers of inflammation, oxidative stress, and muscle damage. DKK3 may serve as a marker for accelerated hypoxia phenotypes in older adults with respiratory diseases (Qaisar et al., 2020).
Dickkopf proteins, including DKK3, contribute to the pathogenesis of osteoporosis by inhibiting Wnt signaling. DKK3, a regulator of canonical Wnt signaling, is required for clonal self-renewal in the G0 phase, a key mechanism in stem cell maintenance with implications for cancer and degenerative diseases (Subramaniam et al., 2014). DKK3 also regulates bone regeneration during fracture healing, influencing the rate and quality of recovery. In the early stages of fracture healing, DKK3 is transiently expressed in fibrochondrocytes in the superficial periosteal region, alongside markers such as smooth muscle α-actin and Col3.6, but its expression diminishes in later stages (Mori et al., 2016).
Temporomandibular joints adapt to static loading through cartilage thickening, with transgenic mice expressing green fluorescent proteins (GFP) showing DKK3 activity in the development of connective tissues, including bone and cartilage (Utreja et al., 2016). Temporal gene expression analysis reveals that DKK3 may inhibit osteogenesis but not chondrogenesis, indicating a regulatory role in endochondral bone formation. DKK3 has been identified as a key gene with a novel function in this process (Aslan et al., 2006).
Relationship between DKK and non-neoplastic diseases of the nervous system
DKK3 expression is elevated in the early stages of Alzheimer’s disease (AD) and may contribute to neurodegeneration by affecting neuronal survival and synapse formation. DKK3 is linked to the loss of excitatory synapses via inhibition of Wnt/GSK3β signaling, while activation of inhibitory synapses through the Wnt/JNK pathway is implicated in AD pathogenesis (Martin Flores et al., 2024; Naskar et al., 2022). In Parkinson’s disease (PD), the production of midbrain dopaminergic (mdDA) neurons, including those in the substantia nigra, is regulated by WNT/β-catenin signaling. Dkk3 plays a crucial role in the activation and maintenance of LMX1A (LIM homeobox transcription factor 1α) and PITX3 (paired-like homeodomain transcription factor 3) expression within a specific subset of mesencephalic dopaminergic (mdDA) precursors in mice. Treatment of pluripotent stem cells with recombinant DKK3 and WNT1 proteins during differentiation enhances the generation of mdDA neurons that exhibit molecular characteristics of the substantia nigra (SNc) dopaminergic cells. Soluble DKK3 proteins may represent a promising new therapeutic agent for enhancing the directed differentiation of pluripotent stem cells into mesolimbic dopaminergic neurons (Fukusumi et al., 2015).
A prospective study of 200 patients with acute ischemic stroke (AIS) identified DKK3 levels (threshold: 93.0 pg/mL) as a potential biomarker for early neurological deterioration, with low DKK3 levels independently associated with increased in-hospital mortality (Zhou et al., 2024). In the China Acute Ischemic Stroke Antihypertensive Trial, analysis of serum DKK3 levels in 3,344 patients revealed a cumulative incidence of the primary outcome (death and vascular events within 3 months). The patients in the third quintile of serum Dkk-3 had the lowest cumulative incidence rate of primary outcome compared to those in the other four quintiles of serum Dkk-3 ranging from 2.54% to 6.73% across quintiles (log-rank P = 0.004), suggesting DKK3 as a prognostic biomarker for ischemic stroke (Zhu et al., 2020). In cerebral hemorrhage, DKK3 improves neurological outcomes by reducing JNK/AP-1-mediated inflammation (Xu et al., 2020). DKK3 also protects neurons and astrocytes from toxic insults via VEGF induction, and in a middle cerebral artery occlusion model, DKK3 mitigated oxidative stress in astrocytes through VEGF (Busceti et al., 2018). Additionally, DKK3 acts as a Wnt/β-catenin signaling inhibitor, playing a regulatory role in cerebral ischemia. Caffo et al. (2023) subjected animals to 1 h of ischemia followed by reperfusion at intervals of 1, 6, 12, and 24 h. Notably, Dkk3 expression was elevated in animals with the most extended reperfusion period. Interestingly, they found that downregulation of Dkk3 using curcumin resulted in a reduction of apoptosis and a concurrent enhancement of neurogenesis. The seemingly opposing effects of DKK3 highlight the complexity of its role in neuroprotection and underscore the need for further research to elucidate the precise mechanisms underlying its actions in cerebral ischemia (Caffo et al., 2023).
Relationship of DKK3 to the immune system, endocrine and other metabolic diseases
DKK3 acts as a negative regulator of insulin resistance, hepatic steatosis, and associated inflammatory responses. In mice, DKK3, in combination with apoptosis signal-regulating kinase 1 (ASK1) and chondroitin, inhibited P38/JNK pathway activation by suppressing ASK1, thereby mitigating fatty liver disease (Xie et al., 2016). Additionally, the Meg3/miR-217/Dkk3 axis promotes adipogenesis and angiogenesis in 3T3-L1 preadipocytes by activating the VEGF signaling pathway and inhibiting Wnt/β-catenin signaling (Huang et al., 2019).
Wnt pathway regulation plays a critical role in both normal physiological responses and pathological inflammatory diseases, with DKK family proteins functioning as immunomodulators in tissue injury and repair. In FoxO4-deficient CD4+ T cells, recombinant DKK3 proteins reduce lymphoid enhancer binding factor-1 (LEF-1) expression and increase IFN-γ levels, influencing Th1 cell differentiation (Chen et al., 2022). Experimental autoimmune encephalomyelitis (EAE) is induced by injecting MOG33–55 peptide. The deletion or neutralization of DKK3 exacerbates EAE, characterized by increased IFN-γ-producing T cells in the CNS, highlighting DKK3’s role in modulating local T cell responses (Meister et al., 2015). DKK3 is also expressed in tolerant CD8+ T cells, and its deletion or blockade restores CD8+ T cell proliferation and IL-2 production in response to self-antigens, underscoring its role in immune tolerance (Papatriantafyllou et al., 2012). In the B-cell compartment, DKK3 limits the survival and proliferation of B1 cells, reducing autoimmunity in systemic lupus erythematosus models, positioning DKK3 as a key immune regulator (Ludwig et al., 2015).
Extension of the DKK3 oncology therapeutic area to non-oncology diseases
The DKK3 gene is linked to miRNA interactions, with its polymorphisms and promoter methylation regulating cancer cell proliferation. DKK3 serves as both a biomarker and therapeutic target across various cancers (Hamzehzadeh et al., 2018). A recent studies by Mohammadpour et al. (2016) demonstrates that DKK3 blocks downstream signaling of Wnt and EGFR, highlighting its potential in cancer treatment.
Adenoviral vectors, leveraging the natural infectivity of adenoviruses, serve as gene delivery tools for gene therapy. Adenovirus (Ad)-REIC vectors, inducing REIC/DKK3 overexpression, have demonstrated significant therapeutic effects across various cancers. These systems enhance adenovirus productivity while reducing normal cell death, exhibiting potent anticancer effects (Mori et al., 2017; Putranto et al., 2017; Sawahara et al., 2016; Sugimoto et al., 2012). Ad-REIC gene therapy not only inhibits tumor growth but also induces immune infiltration, stimulating T cells, natural killer cells, and major histocompatibility complex (MHC) class I expression, providing both direct and immune-modulatory effects (Liu et al., 2023; Suzawa et al., 2017). However, immunogenicity and toxicity concerns remain. In adriamycin-resistant bladder cancer cells (KK47/ADM), the Ad-REIC system downregulates P-glycoprotein via the JNK pathway, inducing apoptosis and reversing multidrug resistance (Hirata et al., 2012). DKK3-targeted approaches, including recombinant proteins and adenoviral systems, show promise for therapeutic intervention in various diseases. DKK3 has shown potential as a therapeutic target not only in oncology but also in non-cancerous diseases, and it is increasingly gaining attention in these fields. For example, preclinical studies in cardiovascular and kidney diseases have demonstrated therapeutic potential through the modulation of DKK3 expression using recombinant DKK3 protein or adenoviral systems. These findings support the role of DKK3 as a promising therapeutic target in the treatment of these conditions.
DKK3, as a key regulator of the Wnt signaling pathway, shows significant potential in the treatment strategies for various non-cancerous diseases. For example, in cardiovascular diseases, DKK3 alleviates angiotensin II-induced cardiac hypertrophy and fibrosis by modulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways (Kang et al., 2023; Zhai et al., 2018). In kidney diseases, upregulation of DKK3 is closely associated with the progression of renal fibrosis (Federico et al., 2016; González et al., 2022; Song et al., 2024). Therefore, therapeutic strategies targeting DKK3 may help reduce renal fibrosis and protect kidney function. Additionally, DKK3’s role in neurodegenerative diseases suggests its potential application in treatment strategies, particularly in Alzheimer’s disease and Parkinson’s disease, where modulation of DKK3 may contribute to the protection of neuronal cells and synapses (Martin Flores et al., 2024; Naskar et al., 2022).
Conclusion and prospective
DKK3 has emerged as a potential biomarker for various diseases, particularly in the context of kidney diseases. The measurement of urinary DKK3 (uDKK3) levels has been shown to correlate with the degree of tubular atrophy and interstitial fibrosis, making it a promising diagnostic marker for renal fibrosis and chronic kidney disease (CKD) progression (Dziamałek-Macioszczyk et al., 2023; Federico et al., 2016; González et al., 2022; Xing et al., 2023). Additionally, uDKK3 levels have been associated with an increased risk of acute kidney injury (AKI) and long-term renal dysfunction following cardiac surgery, highlighting its potential use in early diagnosis and risk assessment (Schunk et al., 2019). In the context of neurological disorders, serum DKK3 levels have been identified as a potential biomarker for early neurological deterioration in acute ischemic stroke (AIS) patients, with low DKK3 levels being associated with increased in-hospital mortality (Zhai et al., 2018).
Therapeutic strategies targeting DKK3 are being explored for various conditions. In the realm of cardiovascular diseases, overexpression of DKK3 has been shown to mitigate angiotensin II–induced myocardial hypertrophy and fibrosis by modulating the ADAM17/ACE2 and GSK-3β/β-catenin pathways (Zhai et al., 2018).
Although the role of DKK3 in cancer has been extensively studied, its mechanisms in non-cancerous diseases remain to be fully elucidated (Al Shareef et al., 2018). In the tumor microenvironment, DKK3 generally acts as an inhibitor of the Wnt signaling pathway, influencing tumor cell proliferation and survival (Moon et al., 2004). Dickkopf-3 is a key inhibitor of the Wnt signaling pathway, with most studies focusing on its role in oncological conditions, including breast, gastrointestinal, gonadal, and intracranial tumors, where it regulates tumorigenesis and progression. Beyond cancer, DKK3 plays a pivotal role in embryogenesis and organ development, and emerging research highlights its involvement in non-tumor disorders, including cardiovascular, respiratory, renal, and muscular diseases. Elevated DKK3 levels have been associated with various pathological states, suggesting its potential as a biomarker for diagnosis and prognosis, particularly in acute kidney injury (AKI) and chronic kidney disease (CKD) progression.
DKK3 not only demonstrates potential as a therapeutic target in oncology but is also gaining increasing attention in the context of non-cancerous diseases. For example, the use of recombinant DKK3 protein or adenoviral systems to modulate DKK3 expression has shown therapeutic potential in preclinical studies of cardiovascular and kidney diseases. These findings support the exploration of DKK3 as a therapeutic target for treating a range of diseases. Although the potential of DKK3 as a biomarker and therapeutic target is increasingly supported by research, caution is needed when interpreting these findings. Differences in study design, heterogeneity of patient populations, and potential publication bias may all influence the conclusions drawn from the literature. Furthermore, the role of DKK3 in different diseases may involve multiple signaling pathways, which may intersect and interact, adding complexity to the research. To further elucidate the role of DKK3 in various diseases, future research should adopt more targeted approaches. This includes detailed analysis of DKK3 expression patterns in specific disease states, as well as in-depth studies of its cellular and molecular mechanisms. Additionally, more prospective studies are needed to validate the clinical utility of DKK3 as a biomarker and explore its potential as a therapeutic target.