N. sphaeroides phycocyanin subunit Ns-α and Ns-β improve C. elegans antioxidative capacity via ROS-related regulation
- Published
- Accepted
- Received
- Academic Editor
- Francois van der Westhuizen
- Subject Areas
- Biochemistry, Bioengineering, Biotechnology, Cell Biology
- Keywords
- N. sphaeroides, Phycocyanin, Antioxidation, Protein, C. elegans
- Copyright
- © 2025 Wu et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. N. sphaeroides phycocyanin subunit Ns-α and Ns-β improve C. elegans antioxidative capacity via ROS-related regulation. PeerJ 13:e18917 https://doi.org/10.7717/peerj.18917
Abstract
Oxidative stress and damage to macromolecules due to free radicals such as reactive oxygen species (ROS) are commonly considered factors that can impair health. This study investigated the potential antioxidant properties of of two subunit proteins associated with the pigment-protein complex phycocyanin derived from Nostoc sphaeroides (Gexianmi). Bacterial expression vectors were separately constructed to induce the two engineering subunit proteins, Ns-α and Ns-β. These engineering proteins were then examined for their potential to enhance antioxidative capacity in Caenorhabditis elegans. Firstly, a proper concentration of the proteins Ns-α and Ns-β in vitro exhibited 2, 2-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical scavenging activity. Secondly, while there were no other observed effects on the nematodes, those treated with the proteins showed significant improvements in motility and reduced levels of lipofuscin compared to the control group. Furthermore, thirdly, the treated nematodes demonstrated increased resistance to oxidation, as evidenced by the higher survivals under oxidative conditions induced by 5 mM H2O2. Notably, the treated nematodes exhibited decline in endogenous ROS levels, and the redox-related genes, such as SOD-3 and CAT-1, were down-regulated following consumption of the engineering proteins. Taken together, these findings suggest that engineering proteins Ns-α and Ns-β improve the antioxidative capacity of C. elegans by modulating ROS-related regulation, making them potential modulators in responding to oxidative stressors.
Introduction
Individuals are exposed to various environments throughout their life, and environmental conditions generally modulate physiological and mental states and can even have substantial influence on health via multifaced channels (Finkel & Holbrook, 2000; Monzani et al., 2019; Romero et al., 2015). Hence short-or long-term stresses are aroused by exposure to unfavorable environmental conditions. There are specific stressors in vivo that potentially cause significant harm to the body through the activity of oxidative molecules, particularly, reactive oxygen species (ROS) (Romero et al., 2015; Labunskyy & Gladyshev, 2013). ROS are a group of chemically molecules with high reactivity derived from partial reduction of molecular oxygen in cells (Labunskyy & Gladyshev, 2013). Because they are specific by-products of metabolism, the endogenous redox mechanism is somewhat dependent on the presence of ROS-related regulation. These ROS are produced by organelles and enzymes, with mitochondria acting as the primary source, contributing to over 90% of the ROS generation within cells (Labunskyy & Gladyshev, 2013). Excessive levels of these molecules, including hydrogen peroxide (H2O2) and other oxygen-containing free radicals can be harmful to cellular health, resulting in oxidative stress and subsequent damage. For instance, ROS can trigger nucleic acid fragmentation, enzyme deactivation, polysaccharide depolymerization, lipid peroxidation, and other destructive biochemical processes that ultimately lead to cell aging and death (Labunskyy & Gladyshev, 2013; Finkel & Holbrook, 2000).
Persistent or long-term oxidative stresses are strongly associated with the initiation and progression of aging and various diseases, such as Parkinson’s disease (PD), Alzheimer’s disease (AD), and malignant tumors, which are becoming increasingly significant health concerns (Hemmati-Dinarvand et al., 2019; Monzani et al., 2019; Trist, Hare & Double, 2019; Aramouni et al., 2023). Aging is associated with an increased production of ROS and decrease of antioxidants in cells (Power, Jakeman & FitzGerald, 2013; Trist, Hare & Double, 2019). Under unfavorable conditions and without effective treatment, prevention of such diseases is hard to achieve during aging. PD is a neurodegenerative disease characterized with the loss of dopamine-producing neurons in the mid-brain that causes incapacitating symptoms including bradykinesia and muscular rigidity (Khan & Ali, 2018). Oxidative stress is found to be one critical factor responsible for the initiation and progression of PD, and patients with PD have high oxidative stress and lower antioxidant activity as evidenced by the biomarker activities of superoxide dismutase (SOD) and catalase (CTL) enzymes (Khan & Ali, 2018). In AD patients, biomarkers of oxidative stress have also been documented, including markers of protein, lipid, DNA and RNA oxidation (Butterfield et al., 2007). Oxidative stress occurs early in the course of AD, which supports its significant role in AD pathogenesis (Cheignon et al., 2018). Therefore, inhibition of excessive oxidation during aging for individuals is required via effective channels or treatment and is critical to prevent diseases in the aging population.
The effects of antioxidants in health promotion and disease prevention have been widely recognized, and findings suggest that antioxidant supplementation can slow the aging process and alleviate diseases linked to oxidative stress (Wang et al., 2017). Therefore, the integration of convenient and effective antioxidants into healthcare practices is crucial. Extensive research on natural antioxidant compounds, including polysaccharides and peptides, has been conducted in order to understand their mechanisms of action at the cellular and animal levels, as well as to enhance efficient extraction and large-scale production methods (Wang et al., 2017; Wu et al., 2015). These efforts are aimed at facilitating the incorporation of antioxidant products into pharmaceuticals, healthcare products, and diverse industrial applications. Of particular interest is the pigment-protein complex phycocyanin (PC) (Bannu et al., 2019; Liu, Qin & Li, 2022; Minato et al., 2021).
The cyanobacteria PC belongs to a class of light-harvesting proteins, referred as phycobiliproteins, which are multi-chain holo-proteins composed of apo-proteins (protein subunits) with covalently-bound phycobilins, open chain tetrapyrrole chromophores (Eisenberg et al., 2017; Eriksen, 2008; Guo et al., 2022; Liu, Qin & Li, 2022). Previous evidence has indicated that PC from cyanobacteria has various pharmacological activities, such as antioxidant properties, decreased inflammation, and boosting immunity (Bannu et al., 2019; Liu, Qin & Li, 2022). A kind of cyanobacteria source with medicinal and edible properties, Nostoc sphaeroides (Gexianmi), is rich in amino acids (aa), vitamins, polysaccharides, and other antioxidant compounds such as PC (Zhu et al., 2023; Xu et al., 2018). Along with changes in climate and environmental conditions, this natural resource is under threat and becoming rare (Chen et al., 2021). In addition, the isolation of active compounds including PC from N. sphaeroides generally involves complex procedures and is expensive. Hence, modern biotechnology must be leveraged for the comprehensive exploration and utilization of valuable resources.
In the context of the development of bioscience and biotechnology, there is great interest in antioxidant-rich proteins derived from specific edible species. However, to date, relevant research on N. sphaeroides is limited. In this study, two subunit protein genes, Ns-α and Ns-β, were respectively expressed in engineered Escherichia coli, and the derived proteins were subjected to the model organism C. elegans. Treated nematodes by the two engineering proteins both had significantly enhanced antioxidative capacity, and the functions were tightly linked to endogenous ROS modulation. The results introduce a novel basis for the efficient utilization of PC subunit protein resources and may potentially promote individuals’ health.
Materials and Methods
C. elegans, bacterial strain, and other reagents
Wild type Caenorhabditis elegans Bristol N2 and E. coli var strain, OP50, which initially originated from the Caenorhabditis Genetics Center (CGC), were provided by Professor Zhao’s laboratory, in the Key Laboratory of High Magnetic Field and Ion Beam Physical Biology, Chinese Academy of Sciences (Hefei, China). Fresh N. sphaeroides was initially collected from Zouma Town (29°49′N, 110°25′E) in the southwest part of Hubei province, Enshi City. The empty expression vector pCold I (simply referred as pC) was purchased from TaKaRa-Bio (Dalian, China). E. coli expression strain BL21 (DE3) harboring the constructed vectors pC-Ns-α (BL21-Nc-α) and pC-Ns-β (BL21-Nc-β) were used in this study, while the strain harboring the empty vector pC (BL21-C) was used as control bacteria in the treatments. The reagents (agar, peptone, and yeast extract) were purchased from Yuanye Biotechnology Company (Shanghai, China); the 5-Fluoro-2-deoxyuridine (FUDR) and 2,7-dichlorofluorescein diacetate (H2DCF-DA) were purchased from Sigma–Aldrich (St. Louis, MO, USA). All reagents were of analytical grade.
Vector construction
The full-length ORF sequences from the two genes, Ns-α and Ns-β, were cloned from N. sphaeroides (maintained in our laboratory) by primer-specific PCR using total DNA as the template. Two restriction sites (NdeI and EcoR1) were designed for the recombinant plasmid pC-Ns-α and pC-Ns-β construction, leading to the gene being under the control of cspA promoter (PcspA). The PCR system was as follows: 1 μL of template DNA, 5 μL of GoTaq G2 Green Master Mix, and 1 μL of the primer mixture, supplemented with ddH2O to a total volume of 10 μL. The reaction procedure consisted of an initial denaturation at 95 °C for 5 min, followed by denaturation at 94 °C for 30 s, annealing at 50 °C for 30 s, extension at 72 °C for 40 s, final extension at 72 °C for 5 min, and a final hold at 16 °C for 3 min. Thirty cycles were performed for the PCR. The primers used for the Ns-α and Ns-β genes were: Ns-α-F, GGCATATGACAAAAACACCTTTAACG, Ns-α-R, GAATTCACTCTAGCTTAGAGTAT TGA; Ns-β-F, GGCATATGGTTTTAGATGCATTTG, Ns-β-R, GAATTCTTAAGCTACTGC TGAAGCA.
Ns-α and Ns-β protein expression
Two gene-expression vectors, pC-Ns-α and pC-Ns-β, were both transformed into E. coli BL21 (DE3) cells and screened on the Luria-Bertani (LB)-Ampicillin (Amp, 100 μg/mL) solid medium. A single colony was inoculated into LB-Amp liquid medium and grown with shaking at 37 °C for up to 14~16 h. The culture was then inoculated at a 1:50 dilution into fresh LB-Amp medium and grown for about 4 h at 37 °C to reach an optical cell density of approximately 0.5~0.6 at A600. For the target engineering protein induction, after 1.0 mM (final concentration) inducer isopropylthio-β-D-galactoside (IPTG) was added, the cultures were induced at 16 °C for up to 4 h. Cells were collected by centrifugation at 5,000 rpm for 10 min and washed twice with washed buffer (20 mM NaCl, 50 mM Tris-HCl, pH 7.3). The bacteria that were resuspended in 5 mL of the homogenization buffer (3–4 mL/g wet cells) were homogenized by ultra-sonication. After centrifugation (12,000 rpm, 15 min), supernatant was used as a crude extract for further isolation by His-tag affinity resin (Takara-Bio, Kyoto, Japan). The details of the method for this procedure were based on the manufacturer’s instructions (Spriestersbach et al., 2015). Samples were subjected to SDS-PAGE and demineralization. Before further assays, the concentrations of the proteins were assessed using the Bradford method (Cheng et al., 2016; Hammond & Kruger, 1988).
In vitro activity evaluation by ABTS assay
2, 2-azino-bis 3-ethylbenzothiazoline-6-sulfonic acid (ABTS) assay was carried out referring to previous work from Wolosiak et al. (2021) with a little modulation. We mixed 7 mM ABTS and 2.45 mM K2S2O8 with equal volumes to generate radical ABTS•+. In dark incubation at least for 12 h, the solution was further diluted with buffers of pH 3.6 and 7.4 to at Abs734. The protein solutions (40 µL) and radical solution (4,000 µL) were mixed together for 6 min, after which the Abs734 was again tested. Bovine serum albumin (BSA) was used as a control and three replications were performed for each sample. Assays for each sample were carried out in triplicate to determine the mean level.
C. elegans culture and treatment
C. elegans N2 strain were maintained at 20 °C on nematode growth medium (NGM) agar plates seeded with E. coli var strain OP50, as previously described (Buchter et al., 2020). To obtain synchronous nematodes, the gravid adults were treated using a bleaching solution (NaOH (5M), NaClO (13%); 1:1) followed by three washing steps in liquid NGM. The remaining eggs were allowed to hatch either on fresh NGM agar plates seeded with OP50 for 3 days (synchronous L4 larvae) or in 1.5 mL S-medium for 12 h (synchronous L1 larvae). For the following treatments, the larvae were fed on the suspensions containing heat inactivated bacterial mixture, such as the OP50/BL21-Ns-α or OP50/BL21-Ns-β or OP50/BL21-C, and each suspension supplemented FUDR (50 μM) to prevent nematode spawning. In the suspensions, OP50 vs BL21-cells were controlled at ratios of 1:1 or 1:3, but the whole concentration of bacteria was referred to 1.0 × 109 cfu/mL. Following a 3-day treatment period, the nematodes matured into adults for subsequent assays.
Testing the body size of nematodes
To address whether the growth is affected by the two-protein treatment, the body size and stage-specific morphological characteristics were also tested. At depicted time points the nematodes in each group were transferred in 10 μL medium to a microscope slide and mixed with 10 μL of levamisole (20 mM). Images of individual nematodes were taken and analyzed to determine the length of each nematode using ImageJ (NIH, Bethesda, MD, USA).
H2O2 tolerance assay
For the H2O2 tolerance assay, the treated nematodes in each group (n ≥ 20) were respectively collected using M9 buffer and subjected to subsequent assays. Subsequently, the nematodes were exposed to 5 mM H2O2 in a 24-well plate, and their survival was scored at 20 min intervals under a dissecting microscopy. When treated nematodes did not respond to poking with a metal wire, they were judged to be dead. Consequently, survival was calculated as the surviving nematode ratio in the population. Each treatment was carried out in triplicate.
Endogenous ROS level
A relative fluorescence quantification method was referred to test the level of endogenous ROS of nematodes (Buchter et al., 2020). The nematodes from different groups were fed on each bacterial lawn for 24 h and collected with M9 buffer. Bacteria were removed by washing three times, and nematodes were resuspended in M9 buffer. Each 50 μL aliquot of the suspension was mixed with H2DCF-DA (50 μL) in a 48-well plate, leading to the ROS-specific fluorescent probe H2DCF-DA (2′,7′-dichlorodihydrofluorescein diacetate) a final concentration of 0.1 mM. Two control wells, such as that containing nematodes from each bacterial mixture lawn without H2DCF-DA or containing H2DCF-DA without nematodes were designed. Incubation was carried out at 25 °C for 1 h, and the nematodes were then transferred to slides, mounted with glycerol, and examined using a fluorescence microscope. ROS signal was subsequently imaged and quantified under a fluorescent microscope. The fluorescence was determined by subtracting the initial value from the final value for each well. Three independent assays were performed and for each replicate, ≥15 nematodes were assessed for the analysis.
Motility
To test the motility, L4 stage C. elegans for each treatment were cultured in S liquid medium supplemented by different bacterial suspension mixture at 20 °C shock conditions for 3 days. Subsequently, the nematodes were collected in a dish with M9 solution, and their body bends were counted every 10 s for three consecutive observations under light microscopy. Each group nematode analysis was replicated at least three times (n ≥ 20).
Lipofuscin accumulation
Treated and control group L4 stage nematodes were collected, washed with M9 buffer, anesthetized with 10 mM levamisole hydrochloride, and placed on a microscope slide. Spontaneous fluorescence was determined using fluorescence microscope (Eclipse Ni; Nikon, Tokyo, Japan; ImageJ; NIH, Bethesda, MD, USA). The treatment experiment for each sample type was repeated three times (n ≥ 10).
Total RNA extraction and quantitative real-time polymerase chain reaction
The nematodes fed different bacterial mixtures (the control group were the nematodes treated with OP50:BL-C, while the treatment groups were the nematodes treated with OP50:BL-Ns-α or Ns-β, and the ratio of the bacteria was all set as 1:1). Those sustained in the L3 stage were washed and collected in M9 buffer, and then the liquid nitrogen-treated nematodes were ground into powder for further total RNA isolation using Trizol reagent (Invitrogen, Waltham, MA, USA). Agarose gel electrophoresis was quickly performed to evaluate the total RNA quality. For each sample, after elimination of potential genomic DNA using gDNA eraser, 1.0 μg total RNA were subjected to RT-PCR using the First Strand cDNA Synthesis kit (PrimeScript™ FAST RT reagent Kit with gDNA Eraser, RR092A; TakaRa, Tokyo, Japan) according to the manufacturer’s instructions. For the first strand cDNA synthesis, a system of 20 μL containing each essential reagent such as Random 6-mers, dNTP Mixture, and RNA was prepared on ice. The cDNA was stored at −20 °C or evaluated subsequently by analyzed Actin expression using agarose gel electrophoresis. The quantitative real-time polymerase chain reaction (qRT-PCR) using the TB Green kit (TB Green® Premix Ex Taq™ II (Tli RNaseH Plus), RR420L; TaKaRa, Tokyo, Japan) and StepOne Plus real-time PCR system (Applied Biosystems, Waltham, MA, USA) were then performed in our lab. Reactions were initiated at 95 °C for 3 min, followed by 40 cycles of 95 °C for 15 s, 58 °C for 15 s, and 72 °C for 30 s to complete target fragments smaller than 350 bp in length, followed by melt curve analysis. At least three repeats were performed for each sample and each gene to characterize the mean level in the assay. Relative expression levels were calculated using the 2−ΔΔCT method. The control gene Actin was used as an internal reference to normalize gene expression data. The gene-specific primers were checked to test availability using BLAST and the detail sequences are shown in Table S2.
Nucleotide sequence
The referred genome was based on the sequence of N. sphaeroides ZMS-1 submitted to GenBank and can be accessed using the species name and accession number NZ_CP031941. The genes of C. elegans described in this study can be retrieved online from NCBI (https://www.ncbi.nlm.nih.gov/) or https://wormbase.org/.
Data statistics and analysis
Statistical analyses were based on the data given as mean ± SD using PASW Statistics (SPSSInc., Chicago, IL, USA). Statistical significance was determined by one-way ANOVA and t-tests. Differences were considered to be significant at a level of P < 0.05 (“*”) or P < 0.01(“**”).
Results and analysis
Engineering expression of Ns-α and Ns-β protein
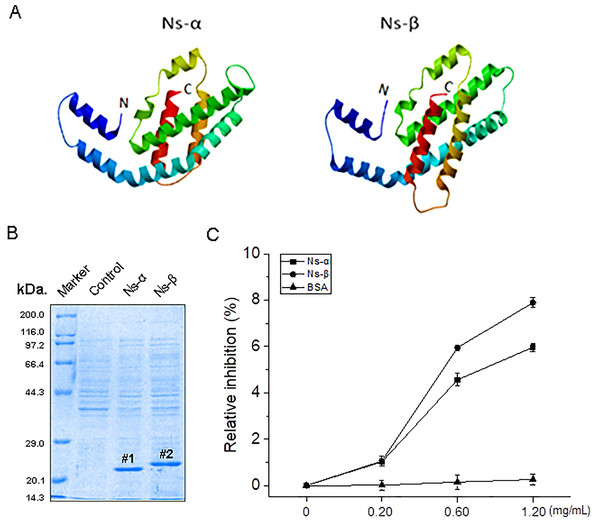
In the context of the reported genome, the coding sequences (CDS) of the Ns-α and Ns-β genes were identified, with lengths of 492 and 522 bp and resulting in proteins containing 163 and 173 aa residues, respectively (Table S1; Fig. S1). The cysteine residues in these proteins, particularly 85Cys in Ns-α and 85Cys and 154Cys in Ns-β, are probably crucial for binding PCB (Fig. S1), and might be conferred some potential. Despite low aa sequence identities, the two subunit proteins, Ns-α and Ns-β, are each comprised of seven typical α helices and exhibit structural similarities (Fig. 1A; Table S1).
Figure 1: Ns-α and Ns-β protein property and antioxidative activity in vitro.
(A) Advanced structure for the two proteins using Swiss-model, and the fully automated protein structure homology-modelling server (https://swissmodel.expasy.org/). The primary optical templates for Ns-α and Ns-β were identified as A0A367QAF2 and A0A2N6MG92, respectively. The protein Ns-α and Ns-β N and C terminals were simply marked and the helixes in the molecules were highlighted in different colour. (B) Isolation of the two engineering proteins, Ns-α and Ns-β, using a Ni-NAT affinity method. “#1 and #2” on the gel are marked for the accumulation of the two engineering proteins, and which molecular weights are referred to the protein marker on the left. (C) Antioxidative activity of Ns-α and Ns-β was assessed through an ABTS·− scavenging assay. Equal volumes (40 μL) of Ns-α and Ns-β at varying concentrations (0.2~1.2 mg/mL) were combined with a 4.0 mL prepared radical solution, and A734 was measured at specified time intervals. Each assay at different concentrations was repeated three times, with BSA serving as a negative control.To assess their activity, the recombinant expression vectors of Ns-α and Ns-β were constructed in the context of pC (Figs. S2A and S2B). Sequenced plasmids pC-Ns-α and pC-Ns-β were respectively transformed into E. coli BL21 (DE3) cells (the engineered strains were named BL21-α and BL21-β, and the control stain harboring the empty vector pC was called BL21-C). The target strains were treated with 1.0 mM IPTG inducer, and the engineering proteins were isolated using a metal Ni affinity method targeting the 6xHis-tag in the subunits (Fig. 1B). Subsequently, the two engineering proteins were subjected to oxidation assay. In vitro experiments demonstrated that, at concentrations of 0.60 mg/mL for Ns-α and Ns-β, the scavenging ratio of ABTS·+ reached approximately 4.6% and 6.0%, respectively. After increasing the engineering protein concentrations to 1.2 mg/mL, higher inhibition levels occurred, indicating a dose-dependent manner in the testing assays (Fig. 1C). Consequently, the engineering proteins Ns-α and Ns-β exhibited anti-oxidative properties.
Ns-α and Ns-β improved nematode movement
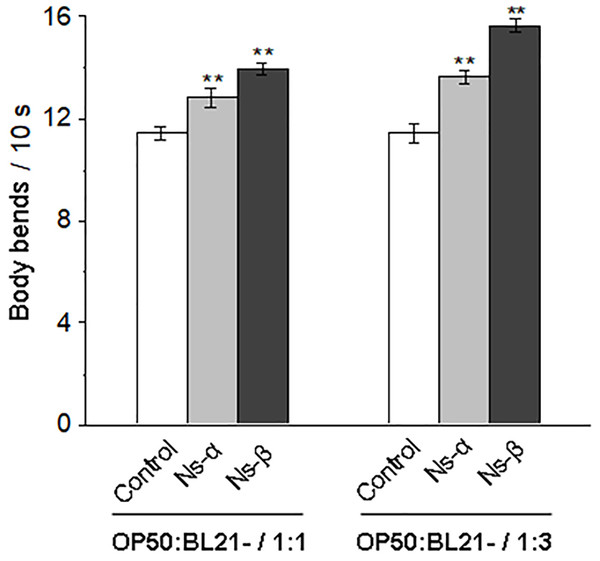
Although phycocyanin has health benefits (Yu et al., 2017; Eriksen, 2008), further evidence is needed to determine the specific roles of the subunit proteins α and β. For this, C. elegans N2 was treated with bacterial mixture suspension of OP50 and engineered bacteria expressing Ns-α and Ns-β (OP50/BL21-α, OP50/BL21-β), while control nematodes were treated with the suspension including the same OP50 and bacteria harboring empty vector (OP50/BL21-C). The ratio of OP50 vs BL21-α and BL21-β or BL21-C in the mixture suspensions were controlled by testing the bacteria concentration. During the treatment periods, no matter whether the ratio was 1:1 or 1:3, there were no significant differences in the body length of the synchronized nematodes compared to those in the control groups (Fig. S3, P > 0.05), indicating that other potential effects were present. Notably, the motility of nematodes treated with Ns-α and Ns-β was significantly improved. For example, when the ratio was 1:1, the mean number of body bends per unit time (10 s) increased by 12% and 22%, respectively, compared to the control group (Fig. 2). After we increased the ratio of engineered bacteria in the suspensions, the motility of the nematode was slightly enhanced by 15% and 26% compared to the control, respectively. If the target bacteria were not induced by IPTG, the motilities of the nematodes from different groups displayed similarities (Fig. S4), highlighting the role of engineering Ns-α and Ns-β.
Figure 2: Ns-α and Ns-β protein improve nematode motility.
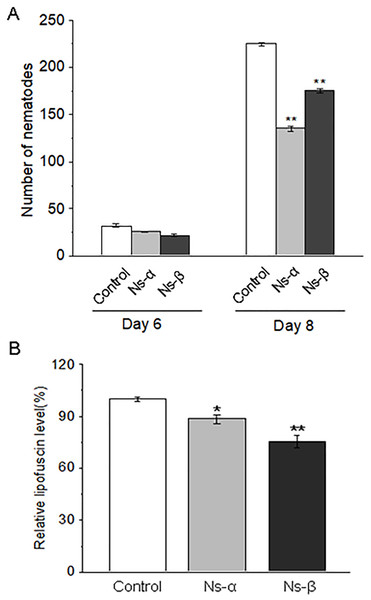
The mobility of nematodes treated with the engineered proteins Ns-α and Ns-β was significantly improved. Treatment 1:1 and 1:3 refered to the ratio of OP50 vs BL21-C (control), OP50 vs BL21-α (Ns-α) or OP50 vs BL21-β (Ns-β) in the bacterail suspensions, respectively. L4 stage C. elegans were cultured in S liquid medium for 3 days for each treatment, after which the nematodes were collected and their body bends were observed at 10 s intervals. For each sample n ≥ 20, and three replicates were conducted. Asterisks mean significant differences (**p < 0.01), using the t-tests.Reproduction is a particular period in a lifespan, and a precious reflection of individual physiology. The treated groups produced much fewer nematode posterities than the control group in the same period, suggesting that the intake of the two engineering proteins delayed the reproductive period of the nematodes (Fig. 3A). Under normal conditions, individual motility typically improves over time, while a decline in motility is often associated with aging. Lipofuscin, known as an aging pigment, can produce blue spontaneous fluorescence in related tissues (Di Guardo, 2015; Hohn & Grune, 2013). We found that the treated nematodes exhibited significantly lower levels of lipofuscin compared to the control group (Fig. 3B; Fig. S5). The physiological alterations in the nematodes supported the observed motility improvements during the testing periods. Therefore, it is reasonable to conclude that nematodes fed on the engineering proteins Ns-α and Ns-β were conferred enhanced motility.
Figure 3: The reproduction and lipofuscin level in the nematodes.
(A) The reproduction capacity of nematodes treated with the engineered proteins Ns-α and Ns-β was significantly inhibited. L4 stage C. elegans were cultured in S liquid medium for three days for each treatment, after which the nematodes were tested for the reproduction capacity by scoring the progeny in the following 3 or 5 days. For each sample n ≥ 15, and three replicates were conducted. (B) The lipofuscin levels in nematodes treated with the engineered proteins Ns-α and Ns-β were notably reduced. Mature nematodes were collected to assess spontaneous fluorescence. For each sample n ≥ 10, and three replicates were conducted. Asterisks mean significant differences (*p < 0.05, **p < 0.01), using the t-tests.Ns-α and Ns-β enhance nematode antioxidant capacity
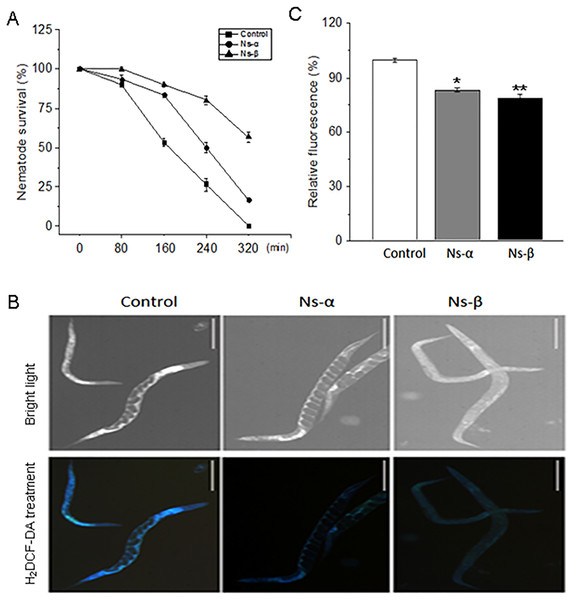
Environmental stressors, such as oxidative stress, have significant effects on individuals and impact the aging process and disease development (Finkel & Holbrook, 2000; Venkataraman, Khurana & Tai, 2013). Elevated oxidation is closely associated with aging and diseases. To directly evaluate the antioxidative property of Ns-α and Ns-β, nematodes were treated with bacterial mixture suspension (ratio of OP50/BL21-α or -β or -C was 1:1) for up to 3 days, and then collected and subjected to 5 mM H2O2 to simulate oxidative stress conditions. During the testing period, nematodes that fed on the two engineering proteins demonstrated more resistance to H2O2-induced oxidation compared to the control group. The survival rate of nematodes treated with Ns-α and Ns-β was approximately 2.0 and 4.0 times, respectively, of that of the control group at 240 min. Even after 320 min, a significant percentage of nematodes in the treated groups remained alive, while all nematodes in the control group had perished (Fig. 4A).
Figure 4: Ns-α and Ns-β increased the nematode antioxidative capacity.
(A) Intaking the Ns-α and Ns-β improved nematode motility. The collected nematodes were exposed to 5 mM H2O2 in a 48-well plate, and their survival was scored in intervals under a dissecting microscopy. For each sample n ≥ 20, and three replicates were conducted. (B and C) Endogenous ROS were clearly inhibited. The fluorescence indicated by H2DCF-DA treatment in the control group were signifcantly higher than in the Ns-α and Ns-β treated groups. Scale: 20 µm. Asterisks mean significant differences (*p < 0.05, **p < 0.01), using the t-tests.ROS, which are natural byproducts of oxidative metabolism, can cause cellular damage, contributing to aging and senescence (Labunskyy & Gladyshev, 2013). Nematodes that fed on Ns-α and Ns-β exhibited increased tolerance to H2O2 compared to the control group, suggesting a potential modulation of endogenous oxidation levels within the nematodes. To assess ROS levels in C. elegans, the fluorescent probe H2DCF-DA was used. Subsequently, the treated nematodes showed significant reductions in ROS levels compared to the control group (Figs. 4B and 4C), indicating that Ns-α and Ns-β facilitated the physiological modulation of ROS and enhanced the nematodes’ tolerance to H2O2-induced oxidation.
Nematode oxidative stress-related genes expression
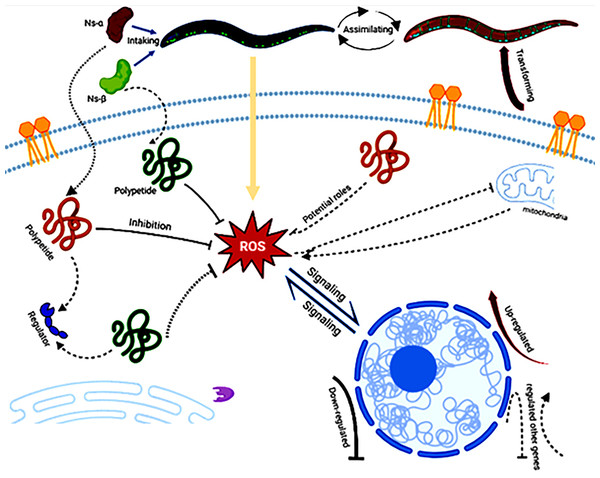
The consumption of Ns-α and Ns-β has been shown to enhance tolerance to oxidation in C. elegans and their involvement in complex mechanisms. On the nutritional scale, the aa in the Ns-α and Ns-β proteins should not be ignored as any protein has no nutritional value unless it is hydrolyzed by proteases and peptidases to aa, dipeptides, or tripeptides in the lumen of the small intestine (Wu, 2016; Power, Jakeman & FitzGerald, 2013). There were one or two Cys residues in the subunit proteins, which may support their antioxidative role via potential polypeptides intake or assimilation by the nematodes. The polypeptides interact with the ROS or other regulators via multi-channels, e.g., by modulating gene expression, hence leading to the transforming of nematodes (Fig. 5).
Figure 5: A hypothetical workflow of the engineering proteins Ns-α and Ns-β.
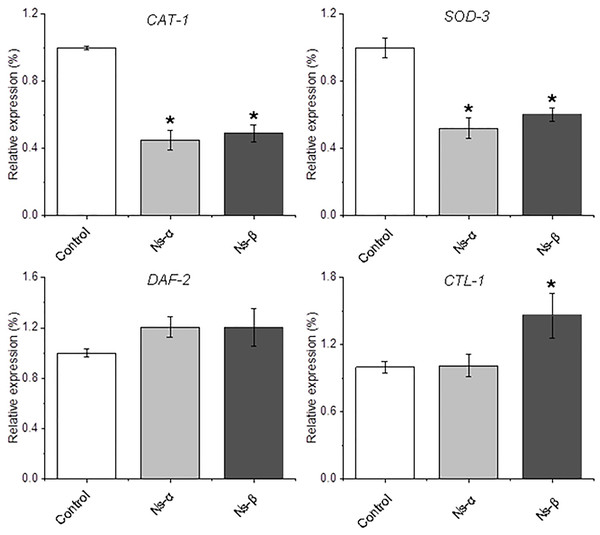
The engineered proteins may be ingested and absorbed by the treated nematodes. In vivo specific polypeptides from the digestion of Ns-α and Ns-β played roles in inhibiting reactive oxygen species (ROS) and interacting with various regulators or potential targets, thereby influencing ROS signaling, the expression of related genes, and transformation of the nematodes. Created in BioRender.To further investigate the antioxidative stress mechanism, total RNA was extracted from nematodes and analyzed using qRT-PCR. Genes related to oxidative stress and longevity in nematodes, such as SOD-3 (Hermeling et al., 2022; Wong, Haque & Chang, 2023), CAT-1 (Duerr et al., 1999), and DAF-2 (Kimura et al., 1997), were examined. The results revealed down-regulation of CAT-1, and SOD-3 in treated nematodes compared to the control, while SKN-1, a gene encoding a transcriptional regulator involved in oxidative stress and lifespan modulation (Kimura et al., 1997; Roy et al., 2022; Deng et al., 2020), was a little up-regulated in the treated nematodes (Fig. 6 and Fig. S6). The expression of other genes, for instance, CTL-1, was specifically and largely changed in the Ns-β treatment group but remained relatively stable in the Ns-α treatment group. SKN-1 and DAF-16 showed little alteration in both protein treatment groups compared with the control group (Song et al., 2014, 2020; Roy et al., 2022) (Fig. S6). These observed alterations in gene expression provide an initial molecular-level explanation for the antioxidant properties associated with Ns-α and Ns-β.
Figure 6: Relative expression of the redox regulation-related genes in nematodes.
The transcript levels of CAT-1, SOD-3, DAF-2, and CTL-1 in the two groups of nematodes treated with the two proteins showed either complete or partial changes when compared to the control group evidenced by quantitative reverse transcription polymerase chain reaction (qRT-PCR). The specific primer pairs corresponding to the genes listed in Table S1. Actin was used as an endogenous reference. Asterisks mean significant differences (*p < 0.05), using the t-tests.Discussion
N. sphaeroides (Gexianmi) is well known for its medicinal and edible properties (Chen et al., 2012, 2020b). Extraction of natural and beneficial components usually involves intricate procedures and relatively high costs. Changes in climate and environmental conditions are also an increasing threat. Most crucial compounds from the species require further exploration by novel channels. In this study, gene-specific expression vectors were constructed to derive the N. sphaeroides phycocyanin engineering α- and β-subunit proteins in bacteria (Fig. 1B). The engineering proteins Ns-α and Ns-β were fed on C. elegans via bacteria suspensions and demonstrated anti-oxidative stress properties, as the treated nematodes exhibited significantly improved motility and survival (Figs. 2 and 4). The recipient intake-assimilation-transformation of each exogenous nutrient is an essential cycle that involves a complex in vivo procedure. The alterations are closely linked to the nematode physiological status, highlighting the substantial benefits provided by these two engineering proteins. The clear decrease in lipofuscin and endogenous ROS levels in the treated nematodes (Figs. 3B and 4C) offers a plausible explanation for the observed differences in their responses.
Individuals encounter various and challenging environmental conditions through their life. When cells experience excessive oxidation or disruptions in redox regulation, aging can be accelerated and even lead to the development of diseases (Cheignon et al., 2018; Khan & Ali, 2018; Labunskyy & Gladyshev, 2013). Age-related chronic diseases associated with oxidative imbalances present significant challenges for individuals and can impose considerable burdens on both economic and social progress (Hemmati-Dinarvand et al., 2019; Monzani et al., 2019). Conditions such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) typically result in significant physiological changes and prolonged, multifaceted suffering for affected individuals (Aramouni et al., 2023; Butterfield et al., 2007; Monzani et al., 2019; Trist, Hare & Double, 2019). Therefore, it is crucial to maintain cellular oxidation equilibrium to delay aging and enhance overall well-being. In addition, physiological functions undergo various factors such as exercise capacity, metabolism, and levels of adipose tissue during aging (Hohn & Grune, 2013; Monzani et al., 2019). Accumulating evidence has indicated that adequate and appropriate supplementation of beneficial proteins is generally essential for maintaining health (Finkel & Holbrook, 2000; Liu, Qin & Li, 2022; Song et al., 2014). For instance, a notable improvement was observed in the radiologically induced intestinal damage in mice subjected to X-ray irradiation, which was attributed to the supplementation of phycocyanin (PC) (Lu et al., 2020). A recent summary has provided a comprehensive overview of the roles of PC derived from various species, with a specific emphasis on Spirulina platensis, in relation to various inflammatory conditions and aging-related diseases (Bannu et al., 2019; Liu, Qin & Li, 2022). Nevertheless, the majority of the treatments employed the PC complex, wherein the functions of the protein subunits remain somewhat ambiguous. The engineering protein Ns-α and Ns-β play a vital role in antioxidative stress, suggesting that the engineered components hold potential for practical applications.
Feeding on C. elegans with these engineering proteins via bacteria suspensions resulted in noticeable positive effects, such as increased motility and enhanced tolerance to oxidation compared to control nematodes (Figs. 2 and 4A). If the target protein were not supplemented via the previous bacteria strain without induction, the effects were not evident for the nematodes (Figs. S3 and S4), highlighting there were close association between the engineering proteins and alteration of the nematodes. The effects were significant for the nematodes. Most chances including the involved competition and defense from other stresses are conferred by motility (Roy et al., 2022). AD or PD generates severe neurological and locomotor impairment in the patients. Increasing evidence has suggested that PC to some extent plays roles in the oxidative stress response of neurons, reduce inflammation and then alleviate the damage caused by brain diseases (Bannu et al., 2019; Cheignon et al., 2018; Liu, Qin & Li, 2022; Trist, Hare & Double, 2019). As crucial elements in PC, to further explore the role of the subunit proteins is attractive. It might be reasonable to image more positive effects of the engineering proteins on the patient who was just lack in locomotion, because some studies has demonstrated that PC could effectively inhibit the formation of A53TαS amyloid, which was evidenced as hallmark in AD or PD (Butterfield et al., 2007; Finkel & Holbrook, 2000; Liu, Qin & Li, 2022; Trist, Hare & Double, 2019). Motility is closely linked to aging, and the improved motility observed in the treated nematodes suggests profound physiological alteration. Lipofuscin, an age-related pigment and aging marker, can hinder proteasome function, contributing to the aging process (Di Guardo, 2015). Interestingly, the levels of lipofuscin were clearly inhibited compared to the control group (Fig. 3B; Fig. S5), providing insights into the physiological status of the nematodes following treatment with the engineered proteins. Reproductive capacity means a fresh stage of life for an individual. We also found that the reproductive posterities of treated nematodes by the two proteins were reduced (Fig. 3A), suggesting that the aging of the nematodes was actually delayed. In this context, it is reasonable to understand the enhancement of motility in the nematodes at same periods.
Proteins are the most fundamental component of tissues in animals and human bodies. Dietary protein can prevent musculoskeletal disorders, particularly sarcopenia, which involves muscle loss in aging people. Recent research also indicates that dietary protein may help with osteoporosis (Ettinger, 2003; Hannafon & Cadogan, 2014). Certain bioactive proteins found in food not only provide nutrition but also promote bone formation. Additionally, bioactive peptides from food proteins show significant promise in combating cardiovascular disease, hypertension, and obesity (Shang et al., 2018; Wu, 2016). Dietary protein has no nutritional value unless it is hydrolyzed by proteases and peptidases to aa, dipeptides, or tripeptides in the lumen of the small intestine (Shang et al., 2018; Wu, 2016). Environmental stressors can lead to the accumulation of endogenous free radicals and excessive production of ROS, which can be significantly harmful to cellular macromolecules (Hemmati-Dinarvand et al., 2019; Power, Jakeman & FitzGerald, 2013). Antioxidant supplementation helps protect health by inhibiting ROS damage. In vivo H2O2 can be generated as a byproduct of oxidative protein folding in the endoplasmic reticulum, and homolytically cleaved in the presence of redox-active Fe(II) iron (Fenton reaction) to form highly reactive hydroxyl radicals (Labunskyy & Gladyshev, 2013; Tu & Weissman, 2002; Chen et al., 2020a). Nematodes treated with the two subunit proteins exhibited increased tolerance to oxidative environments (Fig. 4A), such as exposure to 5 mM H2O2, simulating unfavorable conditions. The results suggest a beneficial effect of nematode by consumption of the two engineering proteins. The special Cys residues in Ns-α and -β protein might facilitate the antioxidation role in vitro, because sulfhydryl (R-SH) in Cys takes on unique antioxidative stress activity and generally interacts with the radical species by hydrogen donation from the SH group (Power, Jakeman & FitzGerald, 2013). However, nematodes’ greater tolerance of H2O2 should not only result from the special Cys residues. There should be multifaced regulation or alteration in the treated nematodes, as in the hypothesized channel in Fig. 5. Vitamins, polyunsaturated fatty acids, polyphenols and glycosaminoglycans are most common antioxidants to inhibit aging and several aging-related diseases, including AD; by contrast, although protein intake is one of the major nutrients and plays critical roles in gut health, proteins and peptides are less directly used in the related studies (Bailey et al., 2015; Bougatef et al., 2020; Feng & Wang, 2012; Shang et al., 2018; Wu, 2016). The protein compounds are usually to be digested into amino acids and small peptides in body, including spirulina and PC, which in practice may indicate several shortcomings of such compounds. However, evidences showed that the antioxidative activity of bovine casein-derived peptide might benefit for AD prevention (Min et al., 2017). Polypeptides from the two engineering proteins’ digestion or the proteins themselves are probably involved in ROS modulation. Changes in endogenous ROS levels in the treated nematodes further support physiological status compared to the control group (Fig. 4).
Therefore, it can be concluded that the consumption of Ns-α and Ns-β aids in enhancing nematode tolerance to oxidative stress. Balance of ROS or redox in living organisms involves complex mechanisms. The expression of several genes associated with oxidation and aging in the treated nematodes, such as SOD-3, CAT-1, and DAF-2, showed differences compared to the control group (Fig. 6 and Fig. S6). Relatively, superoxide radicals are usually unstable and could be dismutated to H2O2 and molecular oxygen in very short-term by superoxide dismutases (SODs) (Labunskyy & Gladyshev, 2013). CAT-1 is the homolog of the mammalian vesicular monoamine transporter VMAT2 and plays a key role in dopamine release and packaging in C. elegans (Duerr et al., 1999). The down-regulation of SOD-3 and CAT-1 expression appeared to have fewer threats from ROS to treat in vivo, which was consistent with the reduced ROS levels in the treated nematodes. By contrast, the expressions of SKN-1 and DAF-16 were both up-regulated in the nematodes treated by the two engineering proteins. SKN-1, the C. elegans ortholog of mammalian Nrf, is a well-known transcription factor in longevity studies, and its activation is observed in several long-lived models. Evidence has indicated that DAF-16 could be inhibited by the SKN-1 in C. elegans stress responses (Deng et al., 2020). In this context, it is reasonable to understand that the two genes displayed a contrary expression pattern in the treatment groups, which implies the actual effect of both engineering proteins.
It was also notable that some genes displayed differentially in the treatments. DAF-2 encodes the sole ortholog of the mammalian insulin and IGF-1 receptors (IIRc), a single mutation in the gene can double the lifespan of C. elegans (Roy et al., 2022; Kimura et al., 1997). DAF-2 transcripts were up-regulated by the two engineering proteins (Fig. 6). Because the intake of the engineering protein Ns-α and Ns-β leads to enhanced motility and higher tolerance to H2O2 in the nematodes, it may be associated with DAF-2-involved regulation, because a previous study suggested that muscle DAF-2/IIRc plays a major role in the loss of worm motility observed in daf-2 mutants (Roy et al., 2022). Although it was difficult to distinguish roles of Ns-α and Ns-β in vivo, for the Ns-α and Ns-β treatment nematodes, the expressions of CTL-1 showed differently in the treated nematodes (Fig. 6). The result appeared to be consistent with the evidence that CTL-1 encodes a catalase downstream of superoxide dismutase in the detoxification pathway of ROS (Blaise et al., 2007). The comparable gene expression is largely due to the engineered proteins’ properties in the context of aa residues and specific advanced structure, which are believed to play a crucial role in the reaction or alteration in nematodes. To further understand the roles requires further exploration and assays, but our conclusion is that the consumption of Ns-α and Ns-β facilitates oxidative stress tolerance in nematodes.
Conclusion
Oxidative stress and the aging process can have detrimental impacts on individual health. When these two factors are combined over a long period, significant effects such as age-related diseases may arise. Therefore, preventing excessive oxidation and delaying the aging process could be an effective approach to promoting healthy aging in a population. N. sphaeroides is a valuable resource in both food and medicine. In this study, two engineered proteins from N. sphaeroides, Ns-α and Ns-β, were expressed and demonstrated significant roles in antioxidation in vitro. This was evidenced by the fact that nematodes treated with these proteins showed enhanced tolerance to 5 mM H2O2, reduced levels of endogenous ROS and changed in expression of some ROS-related genes. The roles of these engineered proteins suggest that, if further developed, they could serve as potential regulators for responding to oxidative stressors and maintaining health.