Efficacy of sacubitril/valsartan on improving clinical symptoms in patients with acute myocardial infarction complicated with heart failure: a retrospective study
- Published
- Accepted
- Received
- Academic Editor
- Xin Zhang
- Subject Areas
- Cardiology, Evidence Based Medicine, Public Health
- Keywords
- Sacubitril/Valsartan, Angiotensin-converting enzyme inhibitors, Acute myocardial infarction, Heart failure, Cardiovascular pharmacotherapy, Comparative efficacy, Observational study
- Copyright
- © 2025 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Efficacy of sacubitril/valsartan on improving clinical symptoms in patients with acute myocardial infarction complicated with heart failure: a retrospective study. PeerJ 13:e18873 https://doi.org/10.7717/peerj.18873
Abstract
Background
Acute myocardial infarction (AMI) significantly contributes to the progression of heart failure (HF). Standard treatment for HF has long been angiotensin-converting enzyme inhibitors (ACEIs), targeting the renin-angiotensin-aldosterone system (RAAS). Recent developments in HF management introduced sacubitril/valsartan (S/V), a novel angiotensin receptor-neprilysin inhibitor (ARNI), showing promising results in global trials. This study aimed to assess the efficacy of early S/V application compared to angiotensin-converting enzyme inhibitors (ACEIs) in reducing NT-proBNP levels and improving clinical outcomes, specifically focusing on dyspnea symptomatology, in Chinese patients with AMI complicated by HF.
Methods
This single-center, mixed methods study was conducted at Tangshan Gongren Hospital from January to December 2021, including 88 patients diagnosed with AMI and HF. Patients were divided into two groups: 31 received S/V, while 57 were treated with ACEIs. Data collection encompassed baseline demographic, clinical, and biochemical variables, NT-proBNP levels, blood pressure measurements, and dyspnea symptom severity. Follow-up assessments were conducted 1 year post-discharge to evaluate NT-proBNP levels, and symptom progression. Statistical analyses, including t-tests, Wilcoxon rank-sum tests, and chi-square tests, were performed to compare outcomes between the two groups.
Results
At baseline, no significant differences were observed between the two groups in terms of demographic, lifestyle, and medical history. Although patients in the S/V group presented with more severe baseline renal impairment and cardiac dysfunction, there was no significant difference in NT-proBNP levels from admission to discharge. 1-year follow-up showed a trend towards reduced NT-proBNP levels in the S/V group, though this difference did not reach statistical significance. All patients in both groups reported improvements in dyspnea at discharge and at follow-up, with no significant inter-group difference. Notably, the S/V group demonstrated a more significant reduction in both systolic and diastolic blood pressure from admission to discharge compared to the ACEIs group.
Conclusions
This study found that S/V had similar effects to ACEIs in reducing NT-proBNP levels among Chinese patients with AMI complicated by HF, though S/V was associated with greater reductions in blood pressure. These findings suggest that while S/V may offer additional benefits in blood pressure management, its impact on cardiac biomarkers in acute settings may not significantly differ from ACEIs. Given the study’s limitations, including its single-center design, small sample size, and baseline differences. Further multi-center, randomized controlled trials are warranted to validate these findings and explore tailored treatment strategies for AMI patients with concurrent HF.
Introduction
Acute myocardial infarction (AMI) is a serious coronary heart disease caused by diminished blood supply to the myocardium and leads to significant morbidity and mortality worldwide (Bauersachs et al., 2019; Mechanic, Gavin & Grossman, 2023). Not only does AMI accelerate the progression toward heart failure (HF), but it also heightens the risk of developing persistent complications (Roger, 2013; Vernon et al., 2019). Approximately 25% of patients with ST-segment elevation AMI are likely to develop HF (Kelly et al., 2011; Danchin et al., 2020), with up to 13% of these patients experiencing HF within 3 years post-event (Sulo et al., 2016).
Pharmacological interventions for HF, primarily angiotensin-converting enzyme inhibitors (ACEIs), target the renin-angiotensin-aldosterone system (RAAS), which plays a crucial role in left ventricular remodeling post-AMI (Heart Failure Group of Chinese Society of Cardiology of Chinese Medical Association, Chinese Heart Failure Association of Chinese Medical Doctor Association, Editorial Board of Chinese Journal of Cardiology, 2018). This mechanism blocks the conversion of angiotensin I to angiotensin II, reducing vascular resistance, lowering blood pressure, and alleviating cardiac workload (Ferrari, 2013). Recent advancements in the understanding of AMI and HF pathophysiology have led to the development of innovative therapeutic agents such as sacubitril/valsartan (S/V), a leading drug in the class of angiotensin receptor-neprilysin inhibitors (ARNIs). Sacubitril prolongs the natriuretic peptides by inhibiting neprilysin, an enzyme that degrades vasoactive peptides, while valsartan blocks the angiotensin II type 1 receptor, together offering a synergistic effect that inhibits RAAS further and promotes natriuresis and vasodilation (Mangiafico et al., 2013; Wang et al., 2015; Sauer et al., 2019).
Several large-scale trials have shown the efficacy of sacubitril/valsartan (S/V) in heart failure management. The Prospective Comparison of ARNI with ACEIs to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial involving over 8,000 patients with reduced ejection fraction (HFrEF) demonstrated that S/V reduced all-cause mortality by 16% and cardiovascular death by 20% compared to enalapril, making it the first therapy to outperform ACE inhibitors (McMurray et al., 2014). The PIONEER-HF trial (Angiotensin–Neprilysin Inhibition in Acute Decompensated Heart Failure) confirmed the early use of S/V in hospitalized patients with acute HF, evidencing a greater reduction in N-terminal pro–B-type natriuretic peptide (NT-proBNP) levels compared to enalapril (Velazquez et al., 2019). Similarly, the PARAMOUNT-HF trail reaffirmed S/V’s benefits in improving ventricular remodeling and reducing HF symptoms. However, the PARADISE-MI trial revealed no significant differences between S/V and ramipril in post-AMI patients (Pfeffer et al., 2021). Additionally, various systematic reviews and meta-analyses have consistently demonstrated that S/V significantly decreases NT-proBNP levels and reduces the incidence of major adverse cardiovascular events across diverse patient populations, including those with AMI, HF, or heart failure following to AMI (HF-AMI) (Zhao, Zeng & Shen, 2021; Liu et al., 2022; Gao et al., 2023).
Despite the established efficacy of S/V, clinical evidence remains limited regarding its use among Chinese patients with AMI complicated by HF. While advancements in treatment have been recognized, the mortality risk continues to be substantial for HF patients with a history of AMI (Gerber et al., 2016), as current strategies predominantly focus on AMI management, potentially neglecting the progression of HF. Additionally, demographic shifts toward an aging population have exacerbated the prevalence of HF following AMI (Roger, 2013; Salari et al., 2023). As the incidence of HF post-AMI increases, identifying the most effective treatment approaches becomes imperative for enhancing patient outcomes and alleviating the burden on healthcare systems. We hypothesized that S/V is more effective than ACEIs in improving clinical outcomes in Chinese patients with AMI complicated by HF, as measured by reductions in NT-proBNP levels and improvements in dyspnea symptoms. Thus, this study aims to investigate the early application of S/V in AMI patients with concurrent HF, offering insights into optimizing clinical care and reducing the healthcare burden.
Materials and Methods
Study design
This single-center, mixed-method clinical study was conducted at Tangshan Gongren Hospital from January 2021 to December 2021. We retrospectively enrolled patients who were diagnosed with AMI complicated with HF at admission, and immediate treatment outcomes were recorded at discharge. A total of 1 year post-discharge, a follow-up assessment was performed to systematically record the incidence of AMI or HF recurrence and to document any subsequent hospital readmissions. According to the guidelines from the Chinese Society of Cardiology of Chinese Medical Association, AMI is defined as a myocardial injury due to ischemia with elevated troponin and typical symptoms or ECG changes, while HF is a syndrome caused by heart abnormalities, leading to shortness of breath, fatigue, and fluid retention (Cardiovascular Disease Prevention and Rehabilitation Committee of Chinese Association of Rehabilitation Medicine et al., 2020). All patients were treated with either S/V or ACEIs. The initial ACEIs dosage was 2.5 mg once daily, adjusted based on blood pressure and tolerability, with a target optimal dose of 10 mg daily. The S/V treatment began at 25 mg twice daily, with increments of 50 mg every 2–4 weeks up to a maximum of 200 mg twice daily, depending on the patient’s blood pressure and overall health. Patients were informed about dosage adjustments and instructed on when and how to modify the dosage based on their blood pressure and physical condition. Medication was initiated once the patient’s hemodynamics stabilized post-admission.
The inclusion criteria were age >18 years and meeting the diagnostic criteria for AMI complicated with AHF. Exclusion criteria included the severe cardiovascular diseases, renal failure, hypotension, cardiogenic shock, malignant arrhythmias, or infections (bacteria, viruses, or other pathogens) at the time of the AMI, as these could compromise treatment efficacy. Exclusion criteria were applied at admission to ensure that participants did not present with conditions or infections that could impact treatment outcomes. All participants were informed about the study and provided with written informed consent. The study design was reviewed and approved by the Ethics Committee of Tangshan Gongren Hospital (the approval number is GRYY-LL-2020-54), and it complied with the Helsinki Declaration.
Data collection
Baseline characteristics were collected through structured interviews and medical record reviews, encompassing demographic information, lifestyle habits, and medical history. Complete blood count was derived from laboratory analysis of blood samples at admission. Information specific to the AMI, including diagnostic imaging reports, was systematically extracted from patient records. Biochemical data were measured using standardized laboratory procedures from blood samples obtained at admission. Cardiac function-associated indexes were assessed via echocardiography performed within the first day after hemodialysis treatment, utilizing advanced imaging equipment in accordance with established protocols and professional associations. Hospitalization data were extracted from hospital records and billing systems. Follow-up assessments, including tracking of changes in symptomatology, were conducted through scheduled clinic visits or telephone interviews at regular intervals over 1 year following discharge.
Outcome measurement
In evaluating the therapeutic efficacy of S/V vs. ACEIs in patients with AMI complicated by HF, this study assessed two primary endpoints: the level of NT-proBNP and the clinical symptomatology, with a particular focus on dyspnea. NT-proBNP, a biomarker released in response to increased heart wall stress, is a critical diagnostic and management tool for HF, with concentrations measured in picograms per milliliter (pg/mL). Clinical symptomatology was assessed through patient self-reports of dyspnea, particularly significant while lying flat or following physical activity and improving when sitting up or using a high pillow—key measures of therapeutic efficacy in heart failure. Improvement in dyspnea is directly correlated with enhanced patient quality of life and functional status, reflecting the alleviation of one of the most distressing symptoms of HF. Symptom improvement was thus measured by the change in reported dyspnea severity from baseline to follow-up that was conducted 1 year after the patients were discharged from the hospital.
Covariates measurement
Demographic factors included age (in years), and gender (male or female). Body mass index, an indicator of body fat, was calculated using weight in kilograms divided by the height in meters squared. Lifestyle factors such as smoking and drinking were determined as either present or absent based on patient self-report. Medical history variables, specifically diabetes and hypertension, were identified through patient medical records or self-reported histories. For complete blood count parameters, red blood cells, white blood cells, and platelets were counted per microliter of blood, whereas hemoglobin concentration was measured in grams per deciliter. Biochemical data, including levels of triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein, creatinine levels, and blood glucose, were measured through blood samples and reported in millimoles per liter. Cardiac function was assessed through echocardiographics with measurements of left ventricular ejection fraction expressed as a percentage and left ventricular end-diastolic dimension in millimeters. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded in millimeters of mercury (mm Hg) at admission, discharge, and 1-year follow-up.
Statistical analyses
All data analyses were conducted using Stata 17.0, and graphical visualization was performed using GraphPad Prism 8.0.2. Prior to statistical testing, Levene’s Test and Shapiro–Wilk test were employed to assess whether the two groups exhibited equal variances and whether the data followed a normal distribution. Two-sample t-tests were utilized to compare baseline, pre- and post-medication, and follow-up differences between groups when both Levene’s test and Shapiro–Wilk test indicated P-values greater than 0.05. For self-matching data, Paired samples t-tests were employed for within-group differences. When normality assumptions were violated or variances were unequal, the Wilcoxon rank-sum test was applied for between-group differences, and the Wilcoxon signed-rank test for within-group differences. Analysis of categorical variables was conducted using the Chi-square tests. Descriptive statistics for continuous variables with normal distribution and equal variances are presented as means ± SD. For variables with a non-normal distribution or unequal, medians and interquartile ranges M (P25–P75) are used. Categorical variables are reported as percentages and numbers (%). A P-value of <0.05 was considered statistically significant (two-tailed).
Results
Baseline characteristics
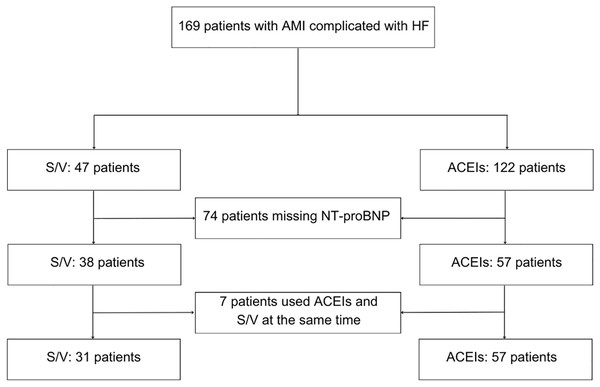
During January and December 2021, the hospital treated 376 patients diagnosed with AMI complicated with HF. After exclusions due to incomplete medication data, missing NT-proBNP levels, and concurrent use of both ACEIs and S/V, 88 patients remained eligible for final analysis and were followed up 1-year post-discharge (Fig. 1). As shown in Table 1, the study is comprised of 31 patients in the S/V group with an average age of 62.29 years (SD 13.54), and 57 patients in the ACEIs group with an average age of 62.74 years (SD 11.76). The gender distribution showed 16.13% female in the S/V group vs. 35.09% in the ACEIs group. At baseline, which is also the admission to the hospital, demographic characteristics, lifestyle factors (smoking and drinking), medical history (diabetes and hypertension), and complete blood count parameters (red blood cells, white blood cells, hemoglobin, and platelets) showed no significant differences between the groups. AMI sites were similarly distributed, with anterior myocardial infarction presented in 48.39% of the S/V group and 33.33% of the ACEIs group (P = 0.166) and inferior myocardial infarction in 16.13% of the S/V group and 12.28% of the ACEIs group (P = 0.615).
Figure 1: Flowchart.
| Data categories | S/V (n = 31) |
ACEIs (n = 57) |
Z/t/ | P-value |
|---|---|---|---|---|
| Demographic | ||||
| Age (years) | 62.29 ± 13.54 | 62.74 ± 11.76 | 0.16b | 0.872 |
| Female (%) | 5 (16.13) | 20 (35.09) | 3.55c | 0.060 |
| Married (%) | 30 (96.77) | 56 (98.25) | 0.20c | 0.658 |
| BMI (kg/m2) | 26.14 ± 3.32 | 25.65 ± 3.66 | −0.62b | 0.537 |
| Lifestyles | ||||
| Smoking (%) | 12 (38.71) | 17 (29.82) | 0.72c | 0.397 |
| Drinking (%) | 10 (32.26) | 13 (22.81) | 0.93c | 0.335 |
| Medical history | ||||
| Diabetes (%)d | 11 (35.48) | 20 (35.09) | 0.02c | 0.884 |
| Hypertension (%) | 15 (48.39) | 39 (68.42) | 3.40c | 0.065 |
| Complete blood count | ||||
| Red Blood Cells (mcL) | 4.51 ± 0.53 | 4.48 ± 0.59 | −0.27b | 0.791 |
| White Blood Cells (mcL) | 8.23 (6.98–11.14) | 8.67 (7.4–11.19) | 0.69a | 0.487 |
| Hemoglobin (gm/dL) | 139.48 ± 16.41 | 135.89 ± 18.69 | −0.90b | 0.372 |
| Platelets (mcL) | 233.45 ± 61.19 | 230.60 ± 54.29 | −0.23b | 0.822 |
| Myocardial infarction | ||||
| Anterior myocardial infarction (%) | 15 (48.39) | 19 (33.33) | 2.89c | 0.235 |
| Inferior myocardial infarction (%) | 5 (16.13) | 7 (12.28) | ||
| Unclassified (%) | 11 (35.48) | 31 (54.39) | ||
| Biochemical data | ||||
| Triglycerides (mmol/L)d | 1.53 (1.01–2.15) | 1.67 (1.23–2.51) | 1.08a | 0.281 |
| Total cholesterol (mmol/L)d | 4.86 ± 1.31 | 4.77 ± 1.20 | −0.31b | 0.756 |
| High-Density Lipoprotein (mmol/L)d | 0.83 (0.73–1.03) | 0.95 (0.80–1.11) | 1.89a | 0.058 |
| Low-Density Lipoprotein (mmol/L)d | 2.92 (2.33–3.56) | 2.85 (2.43–3.22) | −0.45a | 0.651 |
| Creatinine (mmol/L) | 78.05 (68.47–97) | 68.02 (56.41–81.19) | −2.69a | 0.0072* |
| Blood glucose (mmol/L)d | 6.32 (5.26–7.75) | 6.03 (5.03–7.32) | −0.98a | 0.328 |
| Cardiac function-associated indexes | ||||
| Ejection fraction, EF (%)e | 51 (43–55) | 58 (55–64.5) | 4.00a | 0.0001* |
| Left ventricular end-diastolic dimension (mm)e | 57 (47–59) | 48 (45–50.5) | −2.91a | 0.0036* |
| NT-proBNP (pg/mL) | 1,780 (252–5,850) | 566 (189–2,590) | −1.86a | 0.063 |
| Blood pressure | ||||
| SBP (mm Hg) | 122.77 ± 18.94 | 134.28 ± 18.08 | 2.80b | 0.0062* |
| DBP (mm Hg) | 76.90 ± 13.04 | 80.72 ± 11.77 | 1.40b | 0.165 |
| Hospitalization | ||||
| Hospitalization times (days)d | 9 (7–14) | 8 (7–9) | −2.54a | 0.011 |
Biochemical data, including triglycerides, total cholesterol, high-density lipoprotein, low-density lipoprotein, and blood glucose levels, were comparable across both groups. Notably, the S/V group exhibited significantly higher creatinine levels (median 78.05, IQR 68.47–97 mmol/L) than the ACEIs group (median 68.02, IQR 56.41–81.19 mmol/L), indicating greater renal impairment in the S/V group at baseline (P < 0.01).
In addition to the creatinine level, cardiac assessments revealed that the S/V group had a lower left ventricular ejection fraction compared to the ACEIs group (median 51 IQR (43–55) vs. median 58 IQR (55–64.5)%; P < 0.001), and a larger left ventricular end-diastolic dimension (median 57 IQR (47–59) vs. median 48 IQR (45–50.5) mm; P < 0.01). Although the difference in NT-proBNP levels between the two groups was insignificant (P = 0.063), the median NT-proBNP in the S/V group reached 1,780 pg/mL. All the cardiac function-associated indexes indicated more severe cardiac dysfunction in the S/V group at baseline.
NT-proBNP levels between two groups at admission, discharge, and follow-up
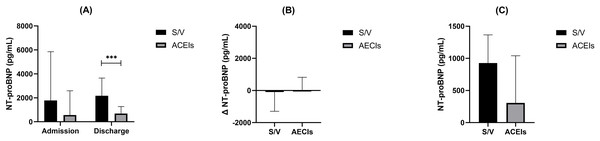
NT-proBNP, a crucial biomarker for heart failure assessment, was analyzed at various stages of patient care. Initially, at admission, there was no significant difference in NT-proBNP levels between the S/V and ACEIs groups (P = 0.063). However, at discharge, a significant difference emerged, with the S/V group showing higher median NT-proBNP levels at 2,170 pg/mL (IQR 714–3,650) compared to the ACEIs group at 690 pg/mL (IQR 328–1,270) (P = 0.0002).
Within-group comparisons from admission to discharge revealed no significant changes in NT-proBNP levels for either the S/V group (P = 0.624) or the ACEIs group (P = 0.231) despite the observed rise in median levels for both groups (S/V: from 1,780 pg/mL (IQR 252–5,850) to 2,170 pg/mL (IQR 714–3,650); ACEIs: from 566 pg/mL (IQR 189–2,590) to 690 pg/mL (IQR 328–1,270)) (Fig. 2A).
Figure 2: The between and within comparison of NT-proBNP levels at admission, discharge, and follow-up.
(A) Comparison of NT-proBNP levels at admission and discharge for S/V and ACEIs groups. (B) Change in NT-proBNP levels from admission to discharge for S/V and ACEIs groups. (C) NT-proBNP Levels at 1-Year follow-up for S/V and ACEIs groups. *** indicates a P-value of less than 0.001 between the two groups.Furthermore, the change in NT-proBNP levels (Δ NT-proBNP) did not differ significantly between the two groups (Fig. 2B). At 1-year follow-up, a trend towards lower NT-proBNP levels was noted in the S/V group, although it did not reach statistical significance (P = 0.052) (Fig. 2C).
Symptom improvement
During hospitalization, all patients in the S/V group (100%, n = 30) reported improvement in dyspnea symptoms, compared to 94.74% (n = 54) in the ACEIs group. Although the S/V group had a slightly higher percentage of symptom improvement, the difference was not statistically significant (P = 0.201), suggesting that both treatment modalities were effective in providing symptomatic relief. Upon 1-year follow-up after discharge, all patients in both groups who were available for assessment (S/V n = 13; ACEIs n = 29) reported continued improvement in dyspnea symptoms (Table 2).
| S/V | ACEIs | P-value | ||
|---|---|---|---|---|
| N | 30 | 54 | ||
| Improvement in symptoms of difficulty breathing at admission (%) | 30 (100) | 54 (94.74) | 1.6353 | 0.201 |
| N | 13 | 19 | N/A | N/A |
| Improvement in symptoms of difficulty breathing at discharge (%) | 13 (100) | 29 (100) | N/A | N/A |
Blood pressure response to S/V and ACEIs at admission, discharge, and follow-up
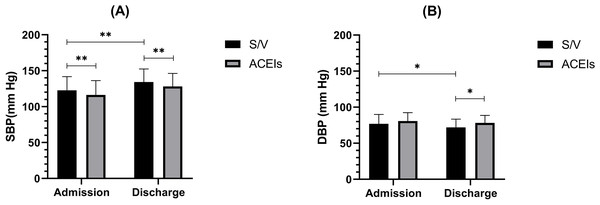
At admission, DBP showed no significant difference between the groups (P = 0.166), while the SBP in the S/V group was significantly lower than in the ACEIs group (122.77 ± 18.94 vs. 134.28 ± 18.08 mm Hg; P < 0.01). By discharge, the S/V group’s mean DBP decreased to 71.97 ± 11.39 mmHg, which was significantly lower than the ACEIs group’s mean of 78.21 ± 10.31 mmHg (P < 0.05). Similarly, the SBP of S/V group was also significantly lower compared to the ACEIs group (116.13 ± 19.97 vs. 128.14 ± 18.07 mmHg; P < 0.01). Both groups demonstrated a significant reduction in SBP from admission to discharge, with the S/V group showing a larger decrease (Fig. 3A). For DBP, the S/V group exhibited a significant reduction by the end of hospitalization (76.90 ± 13.04 vs. 71.97 ± 11.39 mm Hg; P < 0.05), whereas the ACEIs group showed no significant change (Fig. 3B).
Figure 3: The between and within comparison of blood pressure at admission, discharge, and follow-up.
(A) Systolic blood pressure at admission and discharge for S/V and ACEIs groups. (B) Diastolic blood pressure at admission and discharge for S/V and ACEIs groups. * indicates a P-value of less than 0.05 between the two groups. ** indicates a P-value of less than 0.01 between the two groups.Discussion
This single-center retrospective clinical study aims to investigate the clinical efficacy of S/V compared to ACEIs in patients with AMI complicated by HF. Pivotal trials such as PARADIGM-HF and PIONEER-HF have underscored the significant role of S/V in heart failure management. However, the results of our study did not demonstrate a statistically significant change in NT-proBNP levels from admission to discharge within both treatment groups and a significant improvement in dyspnea symptoms between the two groups, suggesting that the specific clinical benefit of S/V over ACEIs may not be as pronounced in this particular subset of patients with AMI complicated by HF.
NT-proBNP is a well-established biomarker that reflects the cardiac physiological status, particularly ventricular walls stress (Velazquez et al., 2019). The increased level of NT-proBNP is indicative of both the presence and the prognosis of HF (Januzzi et al., 2006). A study involving 600 patients presenting with dyspnea in the emergency department found that NT-proBNP testing demonstrated high sensitive and specific for diagnosing acute congestive heart failure (CHF) (Januzzi et al., 2005). Previous studies investigating the efficacy of S/V across various heart failure contexts, such as acute decompensated HF, HF with preserved ejection fraction (LVEF > 40%), and chronic HF, consistently reported significant reductions in NT-proBNP. Contrary to prior findings, our study aligned with a recent double-blind, randomized clinical trial involving 335 patients with advanced HF and reduced ejection fraction, which found no significant difference in reducing NT-proBNP levels between the S/V and valsartan treatments over 24 weeks. In this trial, S/V did not significantly improve the clinical outcomes measured by days alive, out of hospital, and free from HF events compared to valsartan (Mann et al., 2022). Similarly, the PARADISE-MI trial also noted no significant benefits of S/V over ramipril in patients post-AMI, with minor differences in cardiovascular outcomes (Pfeffer et al., 2021). The consistent findings across trials suggest a limited role of S/V in certain HF contexts.
One possible explanation for the conflicting result is that patient with AMI complicated by HF, represent a distinct pathophysiological state compared to those with chronic HF. The acute phase of myocardial infarction introduces a complex clinical scenario where the biomarkers and clinical endpoints may not respond to interventions in the same manner as in stable, chronic conditions. Furthermore, the timing of the intervention post-AMI is another factor to consider. The post-AMI pathophysiological state is complex, involving various compensatory mechanisms. The impact of RAAS inhibition via ACEIs or neprilysin inhibition via S/V may vary depending on the timing of the intervention in relation to the AMI event.
Our study’s findings also suggest a regional clinical practice trend where physicians may preferentially prescribe S/V to patients with more severe clinical profiles. This is evidenced by the S/V group’s baseline characteristics, including higher creatinine and NT-proBNP levels, lower left ventricular ejection fraction, and greater left ventricular end-diastolic dimension. This tendency might come from the perceived or demonstrated efficacy of S/V in critically ill patients, especially those with HF with reduced ejection fraction, as seen in prior successful studies. However, a study on the prognostic significance of baseline NT-proBNP found that, although it predicted HF outcomes, it did not affect the treatment efficacy of S/V, which consistently reduced NT-proBNP levels regardless of patient characteristics (Cunningham et al., 2020). Thus, the lack of significant differences in NT-proBNP level reduction between S/V and ACEIs in our study may not be entirely attributable to differences in baseline characteristics. The regional inclination to administer S/V to patients in poorer conditions also highlights the practical complexities and nuanced decision-making in real-world clinical settings, which is an aspect that randomized clinical trials may not fully capture.
While our study did not demonstrate a significant difference in NT-proBNP reduction between the S/V and ACEIs groups, ARNI’s role in HF management extends beyond its hemodynamic effects. Studies have shown ARNI can positively influence cardiac remodeling by reducing fibrosis and improving ventricular function in heart failure patients (Sardu et al., 2022). Post-AMI, cardiac remodeling is driven by processes such as myofibroblast phenoconversion, in which fibroblasts transition into myofibroblasts, contributing to fibrosis. Exosomal microRNAs, such as miR-92a, play a critical role in this transition (Wang et al., 2020). Additionally, exosomal epigenetic effectors influence fibroblast activation, potentially contributing to pathological remodeling (Gall & Dupont, 2019). Though our study did not examine these molecular pathways, ARNI’s ability to modulate these processes may explain its broader therapeutic effects in heart failure. Thus, while the immediate clinical benefits of S/V may appear limited, its impact on long-term remodeling through these molecular mechanisms highlights the need for further investigation.
Furthermore, it is important to note that the S/V group exhibited significant reductions in both SBP and DBP, whereas the ACEIs group did not. This finding aligns with broader research indicating S/V’s effectiveness in lowering blood pressure, as evidenced by animal studies and clinical trials, including a phase 2 trial where S/V significantly reduced SBP in heart failure patients with preserved ejection fraction (Solomon et al., 2012) and a retrospective study in China showing positive effects on blood pressure and cardiac function in hypertensive patients with heart failure (Guan et al., 2023). Despite these findings, the clinical significance of blood pressure reduction in the context of ACS remains unclear. Elevated blood pressure increases cardiac workload and promotes pathological myocardial changes, which may contribute to systolic and diastolic dysfunction (Oh & Cho, 2020). By effectively lowering blood pressure, S/V might help mitigate these risks in a broader heart failure population, though its direct impact in acute settings such as post-AMI may not be as pronounced or immediately relevant to clinical outcomes. Therefore, while S/V shows potential in managing hypertension associated with heart failure, its specific role in ACS requires cautious interpretation and further study.
Our research, while providing valuable insights into the treatment of AMI complicated by HF, does contain several limitations. First, the non-randomized design and baseline difference in renal function and cardiac dysfunction between groups introduce selection bias, potentially affecting the outcomes. Second, as a single-center study, our findings may have limited generalizability beyond the specific Chinese patient population and practices observed at our institution. Furthermore, our study was limited by a relatively small sample size, with 88 patients included at baseline and only 19 followed up 1 year later. This small cohort size may reduce the statistical power and the robustness of our findings. Additionally, the infrequent follow-up—only once, 1 year post-discharge—may not sufficiently capture the variability and progression of symptoms over shorter intervals. Another limitation is the disparity in group sizes, with 31 patients in the S/V group and 57 in the ACEI group. However, this is mitigated by the real-time nature of our data collection. A further limitation is the subjective nature of patient-reported clinical symptoms, which may not accurately reflect their true clinical status. Therefore, future research should aim to include larger, multi-center trials with longer follow-up periods to robustly assess the long-term effectiveness of S/V vs ACEIs across varied clinical environments. Moreover, studies designed to stratify patients based on baseline characteristics should be conducted to provide insight into personalized medicine approaches.
Conclusion
This study hypothesized that S/V would be more effective than ACEIs in managing AMI complicated by HF, as measured by changes in NT-proBNP levels and improvements in clinical symptoms. Although no significant differences were observed in NT-proBNP reduction between S/V and ACEIs, S/V was associated with a reduction in blood pressure. Given the constraints of our study, including a small sample size and potential confounding variables, these findings should be regarded as preliminary. To substantiate these initial observations, we advocate for future research in the form of larger, multi-center, randomized controlled trials to confirm these findings and explore the identification of patient subgroups that may benefit more distinctly from one treatment over the other.
Supplemental Information
Raw data.
Variable and value labels, metadata, and formatting details. Required Software: Stata (any version supporting the specific.dta format; newer versions are backward-compatible).
Data analysis script.
Plain-text commands to execute commands for data analysis and manipulation. This can be opened with any text editor but is designed to be run in Stata software. Required Software: Stata (any version supporting.do scripts).