Safety profile of EZH2 inhibitors for cancer: a systematic review and meta-analysis
- Published
- Accepted
- Received
- Academic Editor
- Hilal Ozdag Sevgili
- Subject Areas
- Clinical Trials, Drugs and Devices, Evidence Based Medicine, Hematology, Oncology
- Keywords
- EZH2 inhibitors, Safety profile, Meta-analysis, Systematic review, Cancer
- Copyright
- © 2025 Zhao et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. Safety profile of EZH2 inhibitors for cancer: a systematic review and meta-analysis. PeerJ 13:e18871 https://doi.org/10.7717/peerj.18871
Abstract
Objective
To evaluate the safety profiles of EZH2-targeted inhibitors in cancer treatment, focusing on treatment-related adverse events (TRAEs) across various clinical trials.
Methods
We conducted a systematic review and meta-analysis using data from clinical trials involving EZH2 inhibitors reported up to May 31, 2024. Databases searched included PubMed, Embase, CENTRAL (Cochrane Central Register of Controlled Trials), and ClinicalTrials.gov. Studies included were those involving patients treated with EZH2 inhibitors as monotherapy or in combination, specifically detailing the incidence of TRAEs. Data on all-grade TRAEs, grade 3 or higher TRAEs, and severe TRAEs were extracted and analyzed using random-effects models.
Results
Our systematic review and meta-analysis included 22 studies encompassing 1,002 patients who met the inclusion criteria. TRAEs were commonly observed during EZH2 inhibitor therapy, affecting 86% of patients (95% CI [79–94%]%; I2 = 89.5%). The incidence of grade 3 or higher TRAEs was 33% (95% CI [21–44%]; I2 = 93.5%), while severe TRAEs occurred in 15% of the cases (95% CI [9–22%]; I2 = 87.5%). The most frequently reported grade 3 or higher TRAEs in the pooled analysis were neutropenia (8%), thrombocytopenia (8%), and anemia (6%). Specifically, for tazemetostat, the most common grade 3 or higher TRAE was neutropenia (5%). For SHR2554, the most prevalent grade 3 or higher TRAEs were thrombocytopenia (17%), neutropenia (8%), and anemia (7%). Notably, treatment-related fatalities were rare, with only 0.9% of patients experiencing potentially fatal outcomes due to therapy.
Conclusion
EZH2 inhibitors demonstrate a manageable safety profile with a low incidence of severe TRAEs, emphasizing their potential as safe therapeutic options in cancer treatment. The low rate of severe TRAEs and the rare occurrences of treatment-related deaths support the continued clinical use and further investigation of EZH2 inhibitors.
Introduction
The targeting of Enhancer of Zeste Homolog 2 (EZH2) has emerged as a pivotal strategy in cancer treatment, driven by its central role in the epigenetic regulation of gene expression (Liu et al., 2023; Rosenthal, Munoz & Villasboas, 2023). As the catalytic subunit of the Polycomb Repressive Complex 2, EZH2 is instrumental in mediating trimethylation of histone H3 at lysine 27, leading to transcriptional repression of key tumor suppressor genes (Li et al., 2023; Romero et al., 2024). The clinical potential of EZH2 inhibitors has been demonstrated across various malignancies, with ongoing trials continuously exploring broader applications (Kaur, Shankar & Gupta, 2024; Liu & Yang, 2023). This surge in clinical utilization underscores the relevance of EZH2 as a therapeutic target, particularly in malignancies characterized by its overexpression or mutational activation.
The advent of EZH2 inhibitors, such as tazemetostat and valemetostat, has shown promising results in malignancies like lymphomas and solid tumors (Gounder et al., 2020; Sabour-Takanlou, Sabour-Takanlou & Biray-Avci, 2024; Zauderer et al., 2022). Tazemetostat was approved by FDA (Food and Drug Administration) for the treatment of advanced or metastatic epithelioid sarcoma in 2020 (Hoy, 2020). In the same year, the FDA extended the approval of tazemetostat for adult patients with relapsed or refractory (R/R) follicular lymphoma (Straining & Eighmy, 2022). Valemetostat was approved in Japan in September 2022 for treating patients with R/R adult T-cell leukemia/lymphoma (Dou et al., 2022). In China, SHR2554 is also undergoing multiple clinical trials (An et al., 2023).
Despite their efficacy, the clinical application of EZH2 inhibitors is accompanied by a range of adverse events (Izutsu et al., 2023; Morschhauser et al., 2020; Tachibana et al., 2024). These adverse effects vary widely, from mild and manageable to severe and potentially life-threatening, impacting patient quality of life and clinical outcomes. The complexity of these adverse event profiles necessitates a comprehensive analysis to better understand and manage the potential risks associated with EZH2-targeted therapies.
In response to this need, we conducted a systematic review and meta-analysis of treatment-related adverse events (TRAEs) associated with EZH2 inhibitors used in clinical trials. Our objectives were to collate and synthesize existing data on the safety profiles of these agents, and assess the incidence of specific TRAEs. This analysis would offer valuable insights for clinicians in optimizing treatment strategies and enhancing patient care.
Materials and Methods
Search strategy and selection criteria
We conducted a systematic review and meta-analysis to identify published clinical trials that employed EZH2-targeted inhibitors and reported TRAEs. Comprehensive searches were conducted in PubMed, Embase, CENTRAL, and ClinicalTrials.gov before May 31, 2024. The search terms used included “EZH2”, “inhibitor”, and “clinical trial” (see Table S1 for detailed search details). To ensure thorough coverage, references of relevant reviews and articles were manually reviewed.
Selection was based on the following criteria: (1) studies reporting on prospective clinical trials before May 31, 2024, (2) clinical trials of EZH2 inhibitors as monotherapy or in combination in patients with solid or hematological tumors, (3) clinical trials that provided detailed incidence or data on TRAEs, and (4) studies published in English. Exclusion was based on the following criteria: (1) studies with fewer than ten participants in the EZH2 inhibitor group, (2) trials using sequential therapies, (3) studies without clear TRAEs data, (4) publications reporting duplicate populations. For duplicated publications, we only included the most recent study or the study reporting the most complete profile of TRAEs.
Literature searches and data extraction were independently conducted by two reviewers (ZZ and XC), with discrepancies resolved through discussion or consultation with a senior author (HS).
This study adhered to the PRISMA guidelines (Page et al., 2021), and a prospective protocol was registered with INPLASY (202330028).
Data extraction
Data extracted included trial name (or author details), publication year, trial phase, type of cancer, name of the EZH2 inhibitor, dose of EZH2 inhibitor, number of patients receiving the therapy, and the number of patients experiencing at least one TRAEs. Specific data on all-grade TRAEs (Common Terminology Criteria for Adverse Events (CTCAE) grades 1 to 5) (Shah, 2022), Grade 3+ TRAEs (CTCAE grades 3 to 5), severe TRAEs, treatment-related deaths, TRAEs leading to dose reduction, TRAEs leading to therapy interruption, TRAEs leading to therapy discontinuation, and TRAEs leading to death were recorded. Adverse event terminology was standardized according to the Medical Dictionary for Regulatory Activities (MedDRA) (MedDRA, 2018).
Outcomes
According to the prespecified protocol, the primary outcomes were the overall incidences of TRAEs (defined as the number of patients experiencing at least one adverse event divided by the total number of patients), the profiles of all grade TRAEs, severe TRAEs, grades 3 or higher TRAEs, and treatment-related death. Additional analyses included the incidence of TRAEs leading to dose reduction, therapy interruption, and therapy discontinuation.
Statistical analysis
We applied random-effects models with logit transformation for meta-analysis of TRAEs incidences and TRAEs profiles. The effect size of all pooled results was represented by 95% CI with an upper limit and a lower limit. Subgroup analyses were performed using incidence with 95% confidence intervals, calculated through random-effects models.
Heterogeneity among studies was assessed using I2 statistics. All included studies (single-arm studies) were assessed by methodological index for non-randomized studies (MINORS) (Slim et al., 2003). The sensitivity analysis was performed by excluding each study one by one for the pooled results with high heterogeneity. Potential publication bias was evaluated through visual inspection of funnel plots. All statistical analyses were performed using STATA software, employing packages suitable for meta-analysis.
Protocol deviations
In the course of this systematic review and meta-analysis, several deviations from the initially registered protocol were necessary to accommodate new data and expand the scope of the analysis. Firstly, the cut-off time for including published clinical trials was extended to May 31, 2024. This adjustment was made to incorporate the most recent clinical trials involving EZH2 inhibitors, ensuring that our analysis reflects the latest developments and data in this rapidly evolving field. Secondly, we expanded our selection criteria to include combination therapies comprising three or more classes of therapeutic agents. This inclusion was driven by the growing clinical interest in more complex combination regimens that target multiple pathways, which may influence the safety profile and efficacy of EZH2-targeted treatments. These changes were made to enhance the comprehensiveness and relevance of our meta-analysis, allowing for a more detailed and current understanding of the TRAEs profiles associated with EZH2 inhibitors in cancer treatment.
Results
Study selection and characteristics
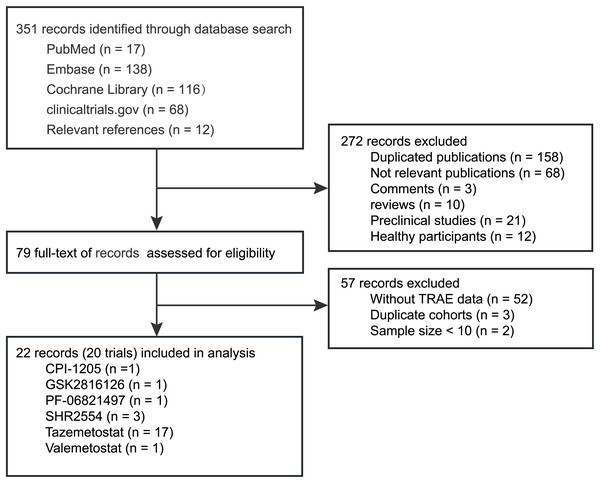
Figure 1 shows a flowchart of the study selection procedure. The electronic searches yielded 351 potentially relevant records, of which 79 potentially eligible records were assessed (Table S2). After further screening, we identified 28 studies reporting adverse events data of EZH2 inhibitors in cancer treatment (including treatment-emergent adverse events (TEAEs) and TRAEs) (Table 1). Finally, 22 eligible studies (20 trials) involving 1,002 patients, were included in the quantitative analysis (Abida et al., 2021; Chawla et al., 2021; Chi et al., 2022, 2023; Eisai, 2022; Feng et al., 2023; Gounder et al., 2020; Harb et al., 2018; Hussain et al., 2024; Italiano et al., 2018; Izutsu et al., 2023; Morschhauser et al., 2020; Palmieri et al., 2023; Palomba et al., 2022; Puram et al., 2023; Salles et al., 2023; Sarkozy et al., 2020; Schweizer et al., 2022; Song et al., 2024, 2022; Yap et al., 2019; Zauderer et al., 2022).
Figure 1: Flow chart of studies selection process.
| Phase | Trial ID | Cancer type | Involved EZH2 inhibitors | Dose | Sample size, n |
|---|---|---|---|---|---|
| 1/2 | NCT04104776A | Advanced HM and ST | CPI-0209 | 200/300/350 mg, qd, po, q28d | 117 |
| 1 | NCT02395601 | BCL | CPI-1205 | 200/400/800/1,600 mg, bid, po, q28d | 32 |
| 1 | NCT02082977 | Advanced HM and ST | GSK2816126 | 50 to 3,000 mg, biw, po, D1-21, q28d | 41 |
| 1 | NCT03460977 | R/R CRPC/SCLC/FL | PF-06821497 | dose escalation, bid, po | 57 |
| 1 | NCT03603951B | R/R mature lymphomas | SHR2554 | 50 to 800 mg, bid, po, q28d | 113 |
| 1 | NCT03603951B | R/R PTCL | SHR2554 | 350 mg, bid, po, q28d | 28 |
| 1/2 | NCT04407741 | Advanced ST and BCL | SHR2554 | 300 to 350 mg, bid, po, q21d | 33 |
| 1/3 | NCT04224493 | R/R FL | Tazemetostat | 400/600/800 mg, bid, po, q28d | 44 |
| 2 | NCT01897571C | R/R FL | Tazemetostat | 800 mg, bid, po, q28d | 99 |
| 1 | NCT01897571C | R/R DLBCL or ST | Tazemetostat | 100 to 1,600 mg, bid, po, q28d | 64 |
| 2 | NCT02601950 | INI1-Negative tumors or R/R synovial sarcoma |
Tazemetostat | 800 mg, bid, po, q28d | 62 |
| 1/3 | NCT04204941 | Advanced epithelioid sarcoma | Tazemetostat | 400/600/800 mg, bid, po | 16 |
| 2 | NCT03213665 | Tumors with alterations in EZH2 or SWI/SNF complex | Tazemetostat | 1,200 mg/m2, bid, po, q28d | 20 |
| 1/2 | NCT04179864 | Metastatic CRPC | Tazemetostat | Arm 1: escalated to 1,600 mg, bid, po | 14 |
| Arm 2: escalated to 800 mg, bid, po | 7 | ||||
| – | NCT02875548D | Advanced HM and ST | Tazemetostat | 800 mg, bid, po, q28d | 99 |
| 2 | NCT02860286 | R/R malignant mesothelioma | Tazemetostat | 800 mg, bid, po, q21d | 74 |
| 1/2 | NCT02889523 | DLBCL or FL | Tazemetostat | 400/600/800 mg, bid, po, q21d | 17 |
| 2 | NCT04762160E | R/R FL | Tazemetostat | 800 mg, bid, po, q28d | 5 |
| 2 | NCT03456726 | R/R BCL with EZH2 gene mutation | Tazemetostat | 800 mg, bid, po, q28d | 20 |
| 1 | NCT02601937 | R/R INI1-negative tumors or synovial sarcoma | Tazemetostat | 800 mg, bid, po, q28d | 46 |
| 800 mg, tid, po, q28d | 63 | ||||
| 1/2 | NCT03854474 | Advanced urothelial carcinoma | Tazemetostat | 800 mg, bid, po | 25 |
| 1 | NCT03009344E | R/R B-cell NHL | Tazemetostat | 800 mg, bid, po, q28d | 7 |
| 1 | NCT02220842 | R/R DLBCL | Tazemetostat | 800 mg, bid, po, q21d | 43 |
| 2 | NCT04705818 | Advanced MSS CRC | Tazemetostat | 800 mg, bid, po, q21d | 47 |
| 1 | NCT04624113 | Pembrolizumab- or nivolumab-resistant R/R HNSCC | Tazemetostat | 400/600/800 mg, bid, po, q21d | 12 |
| 1 | NCT02732275A | R/R NHL | Valemetostat | 150 to 300 mg, qd, po, q28d | 78 |
| 2 | NCT04703192A | R/R PTCL | Valemetostat | 200 mg, qd, po, q28d | 133 |
| 2 | NCT04102150 | R/R T-cell leukemia/lymphoma | Valemetostat | 200 mg, qd, po, q28d | 25 |
Note:
AThese studies reported only treatment-emergent adverse events, but no data were available on treatment-related adverse events; Bthese two records were from different subgroups in the same study, and there is no duplicate reporting of data; Cthese two records are phase 1 and phase 2 studies in the same study, and no duplicate patients are reported in their data; Dthe patients in this study were those who received treatment for more than 2 years in the NCT01897571 study; Esample size less than 10. A/D/ENot included in subsequent calculations; B/Cincluded in subsequent calculations. Abbreviation: BCL, B-cell lymphoma; CRC, colorectal cancer; CRPC, castration resistant prostate cancer; DLBCL, diffuse large B cell lymphoma; FL, follicular lymphoma; HL, Hodgkin lymphoma; HM, hematologic malignancies; HNSCC, head and neck squamous cell carcinoma; MSS, microsatellite stable; NHL, non-Hodgkin lymphoma; PTCL, peripheral T cell lymphoma; R/R, relapsed/refractory; SCLC, small cell lung cancer; ST, solid tumor.
Table S3 shows the baseline characteristics of the 22 studies (20 trials). Four trials (NCT04407741, NCT04224493, NCT04204941, and NCT04179864) are randomised controlled trials in phase II or phase III. However, the results of these phases have not yet been published. Cancer types tested in these studies included hematologic malignancies (n = 9), solid tumors (n = 7), and both (n = 6). Among the 1,002 patients included in the meta-analysis, 666 patients (15 studies) received tazemetostat, 174 patients (three studies) received SHR2554, 57 patients (one studies) received PF-06821497, 41 (one studies) received GSK2816126, 32 patients (one studies) received CPI-1205, and 25 patients (one studies) received valemetostat. For drug combinations, 744 patients (13 studies) received monotherapy, 33 patients (two studies) received tazemetostat with chemotherapy, 117 patients (four studies) received tazemetostat/SHR2554 with immunotherapy, 44 patients (one study) received tazemetostat with targeted therapy, 43 patients (one study) received tazemetostat with immunotherapy and targeted therapy, 14 patients (one study) received tazemetostat with endocrine therapy.
Quality assessment
Two independent authors (Z.Z. and X.C.) assessed the quality of each study included in the meta-analysis using MINORS. Evaluations were compared, and any inconsistencies between the review authors were discussed and resolved. The MINORS scores ranged from 12 to 16 out of a possible score of 16 (Table 2).
| Trial name | Year | MINORS total score |
1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|---|---|
| NCT02395601 | 2018 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02082977 | 2019 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03460977 | 2023 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03603951 | 2022 | 13 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03603951 | 2024 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04407741 | 2023 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04224493 | 2023 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT01897571 | 2020 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT01897571 | 2017 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02601950 | 2020 | 16 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04204941 | 2021 | 12 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03213665 | 2023 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04179864 | 2021 | 12 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02860286 | 2022 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02889523 | 2020 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03456726 | 2022 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02601937 | 2021 | 14 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT03854474 | 2024 | 13 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT02220842 | 2022 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04705818 | 2023 | 12 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04624113 | 2023 | 13 |  |
 |
 |
 |
 |
 |
 |
 |
| NCT04102150 | 2023 | 15 |  |
 |
 |
 |
 |
 |
 |
 |
Note:
Items 1–12 represent: 1, a clearly stated aim; 2, inclusion of consecutive patients; 3, prospective collection of data; 4, endpoints appropriate to the aim of the study; 5, unbiased assessment of the study endpoint; 6, follow-up period appropriate to the aim of the study; and 7, loss to follow-up less than 5%; 8, prospective calculation of the study size. An item scored 0 means not mentioned, one means reported but inadequate, and two means reported and adequate. The total score was 16 for self-controlled studies. Use red for 0, yellow for 1 and green for 2.
Any-grade TRAEs
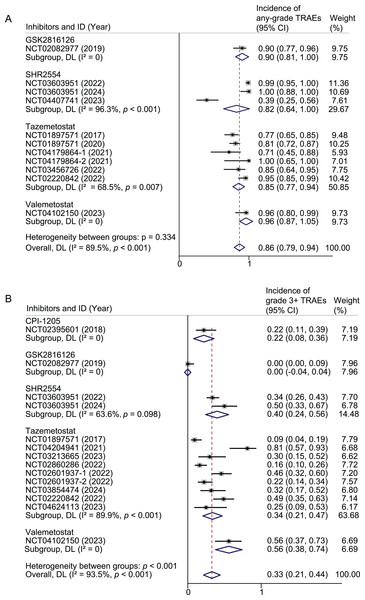
Any-grade TRAEs were reported in 418/487 patients of 10 studies. The pooled result of any-grade TRAEs was 86% (95% CI [79–94%]; I2 = 89.5%). Subgroup analyses were performed based on drug, drug combination, tumor type, and drug dose. For Tazemetostat, the pooled rates were 85% (95% CI [77–94%]; I2 = 68.5%) for all-grade TRAEs. In the case of SHR2554, the pooled rates were 82% (95% CI [64–100%]; I2 = 96.3%). The rate of any-grade TRAEs in GSK2816126 and valemetostat each reported in only one study, were 90% and 96%, respectively (Fig. 2A). When restricted to monotherapy studies, the pooled rates for tazemetostat, SHR2554, and valemetostat were 80%, 99%, and 96%, respectively (Fig. S1A). Further limiting the analysis to monotherapy studies using active doses, the pooled rates for tazemetostat, SHR2554, and valemetostat were 82%, 100%, and 96%, respectively (Fig. S2A).
Figure 2: Pooled results of any-grade TRAEs (A) and grade 3 or higher TRAEs (B) by inhibitor subgroup.
Weights and between-subgroup heterogeneity test are from random-effects model.When subgroup analysis was performed based on drug combination, the probability of any-grade TRAEs was 90%, 39%, 86%, and 95% for monotherapy, combination with immunotherapy, combination with endocrine therapy, and combination with targeted and immunotherapy, respectively (Fig. S3A). When analyzed by cancer type, the rates of any-grade TRAEs were 94%, 86%, and 70% in studies that included only hematologic tumors, only solid tumors, and both, respectively (Fig. S3B).
Grade 3 or higher TRAEs
Grade 3 or higher TRAEs were reported in 177/602 patients of 13 studies. The pooled result of grade 3 or higher TRAEs was 33% (95% CI [21–44%]; I2 = 93.5%). For tazemetostat, the pooled rates were 34% (95% CI [21–47%]; I2 = 89.9%) for grade 3 or higher TRAEs. In the case of SHR2554, the pooled rates were 40% (95% CI [24–56%]; I2 = 63.6%). The rate of grade 3 or higher TRAEs in CPI-1205, GSK2816126 and valemetostat each reported in only one study, were 22%, 0, and 56%, respectively (Fig. 2B). When restricted to monotherapy studies, the pooled rates for tazemetostat, SHR2554, and valemetostat were 23%, 40%, and 56%, respectively (Fig. S1B). Further limiting the analysis to monotherapy studies using active doses, the pooled rates for tazemetostat, SHR2554, and valemetostat were 27%, 50%, and 56%, respectively (Fig. S2B).
When subgroup analysis was performed based on drug combination, the pooled rates were 27%, 29%, 81%, and 49% for monotherapy, combination immunotherapy, combination chemotherapy, and combination targeted and immunotherapy, respectively (Fig. S4A). When analyzed by cancer type, the pooled rates were 41%, 35%, and 4% in studies that included only hematologic tumors, only solid tumors, and both, respectively (Fig. S4B).
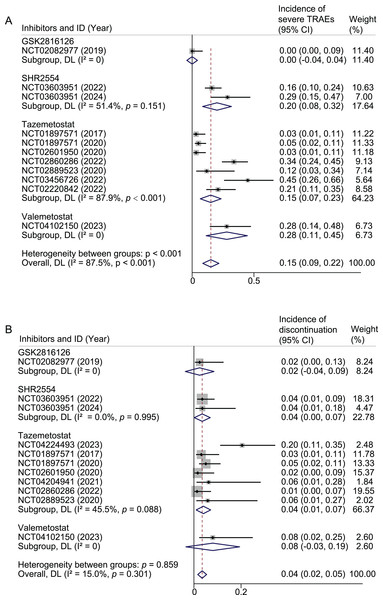
Severe TRAEs
Severe TRAEs were reported in 87/586 patients of 11 studies. The pooled result of severe TRAEs was 15% (95% CI [9–22%]; I2 = 87.5%). For tazemetostat, the pooled rates were 15% (95% CI [7–23%]; I2 = 87.9%) for severe TRAEs. In the case of SHR2554, the pooled rates were 20% (95% CI [8–32%]; I2 = 51.4%). The rate of severe TRAEs in GSK2816126 and valemetostat each reported in only one study, were 0% and 28%, respectively (Fig. 3A). When restricted to monotherapy studies, the pooled rates for tazemetostat, SHR2554, and valemetostat were 15%, 20%, and 28%, respectively (Fig. S1C). Further limiting the analysis to monotherapy studies using active doses, the pooled rates for tazemetostat, SHR2554, and valemetostat were 19%, 29%, and 28%, respectively (Fig. S2C).
Figure 3: Pooled results of severe TRAEs (A) and TRAEs leading to discontinuations by inhibitor subgroup.
Weights and between-subgroup heterogeneity test are from random-effects model.When subgroup analysis was performed based on drug combination, the pooled rates were 15%, 12%, and 21% for monotherapy, combination chemotherapy, and combination targeted and immunotherapy, respectively (Fig. S5A). When analyzed by cancer type, the pooled rates were 20%, 18%, and 1% in studies that included only hematologic tumors, only solid tumors, and both, respectively (Fig. S5B).
Compliance
Compliance was analyzed from three aspects: TRAEs leading to discontinuation, dose reduction, and interruption. TRAEs leading to discontinuation was reported in 28/583 patients of 11 studies. The pooled result of discontinuation was 4% (95% CI [2–5%]; I2 = 15.0%). For tazemetostat, the pooled rate was 4% (95% CI [1–7%]; I2 = 45.5%). In the case of SHR2554, the pooled rate was 4% (95% CI [0–7%]; I2 = 0). The rate in GSK2816126 and valemetostat each reported in only one study, were 2% and 8%, respectively (Fig. 3B).
TRAEs leading to dose reduction was reported in 53/587 patients of 11 studies. The pooled result of dose reduction was 8% (95% CI [4–13%]; I2 = 80.4%). For tazemetostat, the pooled rates were 10% (95% CI [3–16%]; I2 = 87.2%) for dose reduction. In the case of SHR2554, the pooled rates were 8% (95% CI [3–12%]; I2 = 0). The rate in GSK2816126 and valemetostat each reported in only one study, were 5% and 8%, respectively (Fig. S6A). TRAEs leading to interruption was reported in 175/505 patients of nine studies. The pooled result of interruption was 34% (95% CI [30–38%]; I2 = 88.8%). For tazemetostat, the pooled rate was 36% (95% CI [31–41%]; I2 = 93.5%). In the case of SHR2554, the pooled rate was 36% (95% CI [28–44%]; I2 = 0). The rate in GSK2816126 and valemetostat each reported in only one study, were 24% and 16%, respectively (Fig. S6B).
TRAEs leading to death
TRAEs leading to death was reported in 5/583 (0.9%) patients of 11 studies. Among them, three died due to infection, two died due to cardiopulmonary failure. In the study (NCT03603951) (Song et al., 2022), one patient died due to skin infection and toxic epidermal necrolysis and another one patient died because of respiratory failure. Both patients received SHR2554 350 mg twice daily. In the study (NCT02889523) (Sarkozy et al., 2020), one patient died because of infection-related death. The patient received tazemetostat (800 mg twice daily) with R-CHOP regimen every 21 days. In the study (NCT02220842), one died due to cardiac decompensation secondary to the event of hyponatremia, and one died due to septic shock. Both of them received tazemetostat (800 mg twice daily) every 21 days in combination with atezolizumab (1,200 mg) on day 1 of each cycle.
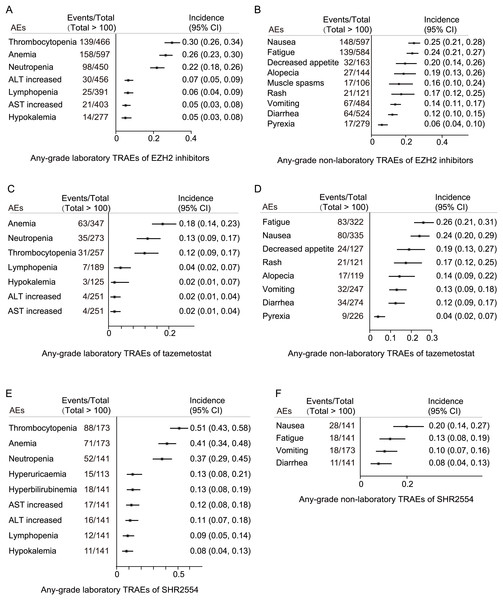
Specific any-grade TRAE spectrum
The most common laboratory any-grade TRAEs for EZH2 inhibitors were thrombocytopenia (139/466, 30%), anemia (158/597, 26%), neutropenia (98/450, 22%), ALT increased (30/456, 7%), lymphopenia (25/391, 5%), AST increased (21/403, 5%), and hypokalemia (14/277, 5%) (Fig. 4A). The most common non-laboratory any-grade TRAEs for EZH2 inhibitors were nausea (148/597, 25%), fatigue (139/584, 24%), decreased appetite (32/163, 20%), alopecia (27/144, 19%), muscle spasms (17/106, 16%), rash (21/121, 17%), vomiting (67/484, 14%), diarrhea (64/524, 12%), and pyrexia (17/279, 6%) (Fig. 4B).
Figure 4: Specific any-grade TRAE spectrum.
(A) Any-grade laboratory TRAE spectrum of EZH2 inhibitors; (B) any-grade non-laboratory TRAE spectrum of EZH2 inhibitors; (C) any-grade laboratory TRAE spectrum of tazemetostat; (D) any-grade non-laboratory TRAE spectrum of tazemetostat; (E) any-grade laboratory TRAE spectrum of SHR2554; (F) any-grade non-laboratory TRAE spectrum of SHR2554.For tazemetostat, the most common laboratory any-grade TRAEs were anemia (63/347, 18%), neutropenia (35/273, 13%), thrombocytopenia (31/257, 12%), lymphopenia (7/189, 4%), hypokalemia (3/125, 2%), ALT increased (4/251, 2%), and AST increased (4/251, 2%) (Fig. 4C). The most common non-laboratory any-grade TRAEs for tazemetostat were fatigue (83/322, 26%), nausea (80/335, 24%), decreased appetite (24/127, 19%), rash (21/121, 17%), alopecia (17/119, 14%), vomiting (32/247, 13%), diarrhea (34/274, 12%), and pyrexia (9/226, 4%) (Fig. 4D).
For SHR2554, the most common laboratory any-grade TRAEs were thrombocytopenia (88/173, 51%), anemia (71/173, 41%), neutropenia (52/141, 37%), hyperbilirubinemia (18/141, 37%), AST increased (17/141, 12%), ALT increased (16/141, 11%), lymphopenia (12/141, 9%), and hypokalemia (11/141, 8%) (Fig. 4E). The most common non-laboratory any-grade TRAEs for SHR2554 were nausea (28/141, 20%), fatigue (18/141, 13%), vomiting (18/173, 10%), and diarrhea (11/141, 8%) (Fig. 4F).
Specific grade 3 or higher TRAE spectrum
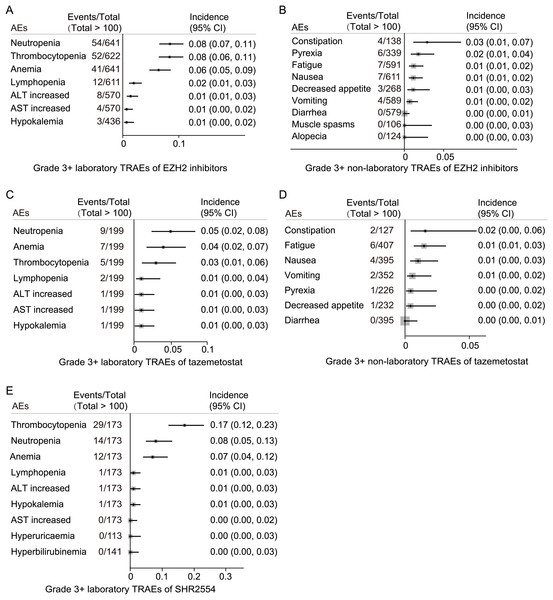
The most common laboratory grade 3 or higher TRAEs for EZH2 inhibitors were neutropenia (54/641, 8%), thrombocytopenia (52/622, 8%), anemia (41/641, 6%), lymphopenia (12/611, 2%), ALT increased (8/570, 1%), AST increased (4/570, 1%), and hypokalemia (3/436, 1%) (Fig. 5A). The most common grade 3 or higher non-laboratory TRAEs for EZH2 inhibitors were constipation (4/138, 3%), pyrexia (6/339, 6%), fatigue (7/591, 1%), nausea (7/611, 1%), decreased appetite (3/268, 1%), and vomiting (4/589, 1%) (Fig. 5B).
Figure 5: Specific grade 3 or higher TRAE spectrum.
(A) Grade 3 or higher laboratory TRAE spectrum of EZH2 inhibitors; (B) grade 3 or higher non-laboratory TRAE spectrum of EZH2 inhibitors; (C) grade 3 or higher laboratory TRAE spectrum of tazemetostat; (D) grade 3 or higher non-laboratory TRAE spectrum of tazemetostat; (E) grade 3 or higher laboratory TRAE spectrum of SHR2554.The most common laboratory grade 3 or higher TRAEs for tazemetostat were neutropenia (9/199, 5%), anemia (7/199, 4%), thrombocytopenia (5/199, 3%), lymphopenia (2/199, 1%), ALT increased (1/199, 1%), AST increased (1/199, 1%), and hypokalemia (1/199, 1%) (Fig. 5C). The incidence of grade 3 or higher non-laboratory TRAEs with tazemetostat is limited (all < 2%) (Fig. 5D).
For SHR2554, the most common laboratory grade 3 or higher TRAEs were thrombocytopenia (29/173, 17%), neutropenia (14/173, 8%), and anemia (12/173, 7%) (Fig. 5E). The incidence of grade 3 or higher non-laboratory TRAEs with SHR2554 was not reported.
Sensitivity analysis
Sensitivity analyses were conducted to evaluate the robustness of the pooled estimates for any-grade TRAEs (Fig. S7A), grade 3 or higher TRAEs (Fig. S7B), and TRAEs leading to discontinuation (Fig. S7C). The analyses involved sequentially omitting individual studies from the meta-analysis. The overall results remained stable and were not significantly altered by the exclusion of any single study, suggesting that our findings are robust and not unduly influenced by any particular study.
Risk of bias
To assess the presence of risk of bias in our meta-analysis, funnel plots were constructed for any-grade TRAEs (Fig. S8A), grade 3 or higher TRAEs (Fig. S8B), and TRAEs leading to discontinuation (Fig. S8C). The analysis of the funnel plots for any-grade TRAEs suggested potential publication bias in three studies: NCT01897571 (2017), NCT01897571 (2020), and NCT04407741 (2023). Similarly, the evaluation for grade 3 or higher TRAEs revealed potential biases in five studies: NCT01897571 (2017), NCT02601937-2 (2022), NCT02860286 (2022), NCT02395601 (2018), and NCT04204941 (2021). For TRAEs that led to therapy discontinuation, potential publication bias was observed in one study, NCT04224493 (2023). These findings indicate the need for cautious interpretation of the pooled results, particularly in the presence of indicated biases.
Discussion
This meta-analysis evaluated the safety profiles of EZH2-targeted inhibitors in cancer treatment, focusing particularly on TRAEs. Our extensive review included 22 studies with 1,002 patients, uncovering a high prevalence of any-grade TRAEs at 86%. Common any-grade TRAEs included thrombocytopenia, anemia, neutropenia, nausea, fatigue, and decreased appetite, each affecting more than 20% of patients (Figs. 2 and 4). Meanwhile, grade 3 or higher TRAEs occurred in 33% of the cohort, primarily as neutropenia, thrombocytopenia, and anemia (Figs. 2 and 4).
EZH2 plays a critical role in cancer progression through the epigenetic regulation of gene expression (Huang et al., 2023; Liu et al., 2023). Inhibitors targeting EZH2 are increasingly recognized for their therapeutic potential, especially in cancers characterized by overexpression or mutation of EZH2 (Grünewald et al., 2024; Wan et al., 2023). Our findings corroborate broader research that attests to the safety of these inhibitors (Marzochi et al., 2023).
Our subgroup analyses revealed variability in TRAEs across different inhibitor types, drug combinations, and cancer types. To explore which EZH2 inhibitor may be safer, we analyzed pooled data on TRAEs from monotherapy trials involving approved EZH2 inhibitors. The results suggest that, compared to SHR2554 and valemetostat, tazemetostat may have a lower incidence of any-grade TRAEs (80%), grade 3 or higher TRAEs (23%), and severe TRAEs (15%) (Fig. S7). However, due to the limited number of studies reporting adverse events for each inhibitor, definitive conclusions regarding the comparative safety profiles of these inhibitors cannot yet be drawn. Notably, the overall incidence of severe TRAEs at 15% indicates that EZH2 inhibitors are generally manageable and well-tolerated. Furthermore, treatment discontinuation due to adverse events was low, at only 4%.
In terms of drug combinations, most studies involved monotherapy. The rates of any-grade, grade 3 or higher, and severe TRAEs, were 90%, 27%, and 15%, respectively, in monotherapy settings (Figs. S3–S5). For rate of any-grade, one study reported a notably low incidence of any-grade TRAEs (39%) for SHR2254 combined with immunotherapy, though this study had a small sample size and potential risk of bias (NCT04407741) (Fig. S3A). Conversely, a high incidence of grade 3 or higher TRAEs was observed in a study evaluating tazemetostat combined with chemotherapy (NCT04204941) (Fig. S4A). Interestingly, a recent study revealed that the combination of valemetostat and irinotecan was not tolerated in patients with recurrent small-cell lung cancer, highlighting the potential for overlapping toxicity when EZH2 inhibitors are used in combination with chemotherapeutic agents (Choudhury et al., 2024). These findings underscore the importance of carefully selecting combination regimens to minimize treatment-related toxicities.
In terms of cancer types, it is noteworthy that studies involving both hematologic malignancies and solid tumors reported unexpectedly low rates of all-grade TRAEs, grade 3 or higher TRAEs, and severe TRAEs, at 70%, 4%, and 1%, respectively (Figs. S3–S5). For instance, one terminated study reported limited anticancer activity of GSK2816126, which contributed to the low TRAE rates observed (Yap et al., 2019). Another study (NCT01897571 (2017)) (Italiano et al., 2018) served as a first-in-human, open-label, phase 1 trial evaluating tazemetostat in cancer treatment. This trial was designed as a dose-escalation study, with dosages ranging from 100 to 1,600 mg administered twice daily. This dosing approach could explain the relatively low incidence of grade 3 or higher and severe TRAEs observed in the study. After excluding these two studies, our analysis revealed no significant differences in the rates of all-grade TRAEs, grade 3 or higher TRAEs, or severe TRAEs between hematologic malignancies and solid tumors treated with EZH2 inhibitors (94% vs. 86%, p = 0.610; 41% vs. 35%, p = 0.573; 20% vs. 18%, p = 0.904) (Figs. S9A–S9C).
Our study highlights the critical need for infection prevention, especially considering that EZH2 inhibitors can cause neutropenia and lymphopenia, which may predispose patients to infections. Out of the five patient deaths identified, three were potentially due to infectious diseases, underscoring the importance of vigilant monitoring and preventive measures against infection during treatment with EZH2 inhibitors.
In addition to assessing the safety profiles of EZH2 inhibitors, it is essential to evaluate their efficacy (Gold & Shilatifard, 2024). Research on EZH2 inhibitors has been most extensive in certain types of R/R lymphoma (Izutsu et al., 2023; Song et al., 2024, 2022). These types of lymphoma are common indications for all three approved inhibitors (Hoy, 2020; Keam, 2022; Song et al., 2024). In patients with R/R T-cell lymphoma treated with valemetostat at 200 mg per day, the objective response rate (ORR) was 44% (52/119) (Zinzani et al., 2024). In patients with R/R T-cell lymphoma treated with SHR2554 at 350 mg twice daily, the ORR was 62% (17/28) (Song et al., 2024). In patients with R/R follicular lymphoma treated with tazemetostat at 800 mg daily, the ORR was 55% (50/99) (Morschhauser et al., 2020). The efficacy of EZH2 inhibitors may be influenced by the EZH2 mutation status of the tumor. In trial of tazemetostat for patients with R/R follicular lymphoma, the ORR was 69% (31/45) in the EZH2-mutant cohort (95% CI [53–82]), compared to 35% (19/54) in the EZH2 wild-type cohort (95% CI [23–49]). Due to significant tumor heterogeneity and patient heterogeneity across studies, as well as the lack of direct comparisons between drugs, it is difficult to determine which inhibitor is most effective. However, in our review, tazemetostat appears to have a more favorable safety profile. Among the approved inhibitors (tazemetostat, valemetostat, and SHR2554), tazemetostat has been the most thoroughly studied (Orleni & Beumer, 2024). It has shown substantial efficacy in treating metastatic or relapsed epithelioid sarcoma, follicular lymphoma, and pleural mesothelioma (Grünewald et al., 2024; Morschhauser et al., 2020; Zauderer et al., 2022). For solid tumors, EZH2 inhibitors may be less effective. In one study, only 15% (9/62) of patients with advanced epithelioid sarcoma and loss of INI1/SMARCB1 responded to treatment (Gounder et al., 2020). However, these results are limited, and further studies are needed to confirm these findings.
While our results are robust, as confirmed by sensitivity analyses, the potential publication bias detected necessitates cautious interpretation of the data. This bias could arise from the preferential publication of studies with favorable safety outcomes or discrepancies in reporting adverse events.
Several limitations in this study warrant consideration. First, the inherent biases of included studies, particularly those related to the non-randomized design of many trials, may affect the generalizability of the findings. Non-randomized designs also limit the ability to identify the unique adverse events specifically attributable to EZH2 inhibitors and the potential overlapping toxicities in combination regimens. Second, the diversity in trial designs and populations, as well as variations in inhibitor type, dosage, and combination therapies, introduces heterogeneity that complicates direct comparisons across studies. Third, the disproportionate representation of tazemetostat in our dataset reflects its more advanced clinical development and greater number of published trials compared to other EZH2 inhibitors, such as SHR2554 and valemetostat. As a result, the meta-analysis findings may be more reflective of tazemetostat’s safety profile, potentially limiting the generalizability of the results to other inhibitors. These findings underscore the need for additional studies focusing on newer EZH2 inhibitors to provide a more balanced comparison in future meta-analyses. Moreover, we acknowledge that the inclusion of valemetostat, an EZH1/2 dual inhibitor, introduces complexity given its distinct mechanism of action compared to other EZH2-specific inhibitors. With only one study on valemetostat included in this analysis, its unique on-target and off-target toxicities could not be adequately evaluated. Future research should focus on further elucidating these differences. Finally, the presence of publication bias, as suggested by funnel plot asymmetry, indicates that the actual incidence of adverse events may be underreported, which could skew the perceived safety profile of these therapies.
Conclusions
Our meta-analysis provides the first pooled assessment of TRAE rates for approved and investigational EZH2 inhibitors, offering critical insights for clinicians and researchers. The identified differences in adverse event profiles between monotherapy and combination regimens, as well as between individual inhibitors, provide a foundation for optimizing future clinical trial designs and therapeutic strategies. EZH2 inhibitors maintain an acceptable safety profile. Their potential for serious adverse events is manageable, making them viable for cancer treatment. Future research should focus on refining dosing strategies, reducing adverse effects, and enhancing long-term safety and efficacy profiles of these potent epigenetic modifiers. As of May 31, 2024, our search identified 79 studies on EZH2 inhibitors in cancer treatment, with 51 still ongoing. These ongoing studies are expected to significantly enrich our understanding of the efficacy and safety of EZH2 inhibitors.
Supplemental Information
Analyzed data.
The raw data is in .dta format and can be accessed using the software STATA 15 or later versions. This software is available from https://www.stata.com/stata15/.