UPLC-MS metabolite profiling and antioxidant activity of Sanghuangporus sanghuang extract
- Published
- Accepted
- Received
- Academic Editor
- Viktor Brygadyrenko
- Subject Areas
- Agricultural Science, Biochemistry, Food Science and Technology, Plant Science
- Keywords
- Sanghuangporous sanghuang, Ethanol extract, Antioxidant, Bioactive compounds, UPLC-MS
- Copyright
- © 2025 Wang et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2025. UPLC-MS metabolite profiling and antioxidant activity of Sanghuangporus sanghuang extract. PeerJ 13:e18758 https://doi.org/10.7717/peerj.18758
Abstract
Background
The objective of the present study is to examine the total phenolic and flavonoid content of an ethanol extract of Sanghuangporus sanghuang and to evaluate its phytochemical properties, antioxidant activity, and capacity to protect DNA from damage. This pharmaceutical/food resource mushroom may serve as a novel substitute functional food for health-conscious consumers, given its promising source of phenolics and flavonoids.
Methods
S. sanghuang ethanol extract (SEE) was evaluated for total phenolic and flavonoid contents, while UPLC-MS analysis was used for terpenoids, phenylpropanoid, flavonoids, steroidal, phenols identification, and function prediction. Antioxidant and anti-DNA damage activities were tested in vitro using ferric reducing antioxidant power (FRAP), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis-3-ethylbenzotiazolin-6-sulfonic acid (ABTS), and DNA damage protection assay.
Results and Conclusion
Total phenolic content (TPC) in SEE was 385.38 ± 1.36 mg GA/g extract, while total flavonoid content (TFC) was 298.22 ± 2.38 mg QE/g extract. The extracts exhibited high antioxidant and free radical scavenging activities with relatively stronger free radical scavenging activity. A total of 491 metabolites were investigated by Ultra-performance liquid chromatography-mass spectrometry (UPLC-MS). Most of the top 20 compounds were predicted to have various functions like antioxidant, anti-cancer and anti-inflammatory. This study highlighted S. sanghuang was a beneficial source of phenolics and flavonoids. It contains potential natural antioxidant that could be used as a lead contender in the development of antioxidant medicines for the treatment of a wide range of oxidative stress-related illnesses.
Introduction
Sanghuangporus sanghuang (S. sanghuang) is a valuable medicinal fungus, which belongs to Basidiomycota • Hymenomycetes • Aphyllophorales • Hymenochaetaceae • Sanghuangporus (Li et al., 2020a; Feng et al., 2024). It has been used as a traditional Chinese medicine for at least 2000 years and recent research showed that S. sanghuang have various bioactivities, such as anti-oxidative, anti-inflammatory, anti-carcinogenesis, anti-fungal and immunomodulatory activities, as well as antidiabetic, hepatoprotective and neuroprotective effects (Lin et al., 2017; Dong et al., 2023; Gafforov et al., 2023a; Lu et al., 2024; Zhong et al., 2024).
Previous phytochemical studies have shown that S. sanghuang mainly contains polysaccharides, flavonoids, triterpenoids, polyphenols and alkaloids (Zuo et al., 2021; Gafforov et al., 2023b; Yuan et al., 2023). Besides these well-known compounds, there are also numerous unknown metabolites to be studied. Given the complex nature and potential benefits of natural products, there is increasing interest in the use of Chinese herbal medicines. In fact, the use of herbal medicines has significantly increased in recent years (Hao & Liu, 2022; Gafforov et al., 2023a; Sevindik et al., 2024).
The extraction of bioactive compounds from fruiting bodies using various solvents is a key focus of research (Li et al., 2020b; Gafforov et al., 2023b). Utilizing the low-cost technology to extract molecules from fruiting bodies, is a suitable strategy for producing food additives and nutraceutical products (Zuo et al., 2021; Gu, Hao & Xiao, 2022). The present study thus aims to explore the total phenolic and flavonoid present in ethanol extract of S. sanghuang, as well as to assess its phytochemical properties, antioxidant activity, and its capacity to protect DNA. UPLC-MS analysis was also conducted to find out functional groups and active compounds in the ethanol extract of S. sanghuang.
Materials and Methods
Preparation of the S. sanghuang ethanol extract (SEE)
S. sanghuang was authenticated by the Institute of Microbiology, Chinese Academy of Sciences, Beijing, China. The fruiting body was dried and ground. The powder of fruiting body was extracted three times by maceration with 80% ethanol (1:10 w/v) at 25 °C, each time for 2 h. The supernatant from the extraction was filtered directly through Whatman No. 1 filter paper. Three extractions were combined and freeze-dried in FreeZone (Labconco, Kansas City, MO, USA) and stored at 4 °C.
Determination of total phenolic content (TPC)
The total phenolic content (TPC) of SEE was determined by spectrophotometric method (Singleton, Orthofer & Lamuela-Raventos, 1999). A total of 1 mg of SEE was dissolved in 1 mL of methanol. Then, 0.5 mL of SEE solution was combined with 1 mL of Folin-Ciocalteu reagent (1:10 v/v). After 5 min, Na2CO3 (7% w/v) was added to the mixture, and the volume was increased to 10 mL with water before shaking vigorously. Gallic acid was used as a standard compound, and a calibration curve was created based on concentrations of 20, 40, 60, 80, and 100 μg/mL. Following 1 h of incubation at 25 °C, absorbance was measured at 760 nm with a UV-VIS spectrophotometer (Shimadzu, Kyoto, Japan). Total phenolic content was quantified in gallic acid equivalents (mg GA/g of dw).
Determination of total flavonoid content (TFC)
The total flavonoid content (TFC) of SEE was determined using the AlCl3 colourimetric method (Chang et al., 2002). SEE was diluted with methanol to 1 mg/mL, and quercetin was used as a standard compound by constructing a calibration curve with concentrations of 20, 40, 60, 80, and 100 µg/mL. 2 mL of diluted SEE or quercetin was combined with 0.1 mL (10% w/v) AlCl3 and 0.1 mL (0.1 mmol/L) CH3COOK. Following a 30 min incubation at 25 °C, absorbance was measured at 415 nm. The total flavonoid concentration of the extracts was expressed as mg quercetin equivalent per gram dry weight of extracts (mg Q/g of dw).
UPLC-MS analyses of SEE
Chromatography was performed using an ACQUITY UPLC I-Class HF system (Waters, Milford, MA, USA). ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 um; Waters, Milford, MA, USA) was used as a separation column. The elution solution consisted of deionized water contains 0.1% formic acid (A) and acetonitrile (B) and separation was achieved using the following gradient: 0.01 min, 5% B; 2 min, 5% B; 4 min, 30% B; 8 min, 50% B; 10 min, 80% B; 14 min, 100% B; 15 min, 100% B; 15.1 min, 5% and 16 min, 5% B. The rate was set at 0.35 mL/min, and the injection volume was 5 μL. Mass spectrometric experiments were performed using Obritrap-QE (Thermo Fisher Scientific, Watham, MA, USA) in negative mode.
Ferric reducing antioxidant power (FRAP) assay
Total antioxidant activity (TAA) of SEE was measured using the classic Fe3+ reducing power test (Chu, Chang & Hsu, 2000). SEE and standard (1 mL) were combined with 5 mL of PBS (0.2 mol/L, pH 6.6) and 1% K3[Fe(CN)6] (5 mL). The mixture was incubated at 50 °C for half an hour. Then, 5 mL of TCA (10%) was joined and centrifuged for 10 min. Lastly, 5 mL of supernatant was collected and combined with 5 mL of ddH2O and 1 mL of FeCl3 (0.1%). The absorbance was measured at lmax = 700 nm by a UV-spectrometer.
DPPH assay
The 1,1-diphenyl-2-picrylhydrazyl (DPPH) test was performed to assess antioxidant activity of SEE (Chen et al., 2020). A total of 0.1 mL of DPPH in methanol (0.1 mmol/L) was combined with 0.1 mL of SEE solutions (10, 20, 30, 40, 50 μg/mL each). After 1 h of incubation at room temperature in the dark, the absorbance at 517 nm was measured with a UV-spectrometer.
ABTS scavenging assay
The 2,2′-azino-bis-3-ethylbenzotiazolin-6-sulfonic acid (ABTS) scavenging assay uses the radical cation decolorization technique (Nejma et al., 2017). K2S2O8 (2.45 mmol/L) and ABTS (7 mmol/L), were produced in ddH2O and combined in a ratio of 1:1 before being incubated in the dark for 24–48 h. After diluting the ABTS at a ratio of 1:25, 300 μL of SEE was introduced to 3.0 mL of the ABTS+ solution. Subsequently, the absorbance of this uniform mixture was assessed at a wavelength of 745 nm.
DNA damage protection activity
The ability of SEE to protect DNA from the harmful effects of hydroxyl radicals was evaluated using a modified DNA nicking assay (Wu & Dhanasekaran, 2020). A final volume of 50 μL reactant solution, consisting of 10 μL vector DNA (0.1 μg/μL), 25 μL Fenton’s testing agent (0.2 mmol/L FeCl3, 0.1 mmol/L Vitamin C and 0.03 mol/L H2O2), and 15 μL SEE (1 mg/mL), was prepared. The formula mix was then incubated at a temperature of 37 °C for a duration of 45 min. A total of 6 μL loading buffer was then added into the mixture. DNA cleavage and protective efficacy were assessed by 1% agarose gel electrophoresis.
Data preprocessing
The original LC-MS data were processed using Progenesis QI V2.3 software (Nonlinear Dynamics, Newcastle, UK) to perform baseline filtering, peak identification, integral, retention time correction, peak alignment, and normalization. Using the LuMet-TCM, Animal_DB, and Herb databases, compounds were identified using exact mass-to-charge ratios (M/z), secondary fragments, and isotopic distributions. Qualitative compounds found in the QI search database are preserved as the original ingredient if their overall score exceeds 40 points and their secondary matching score exceeds 50 points. To produce a qualitative and quantitative result data matrix, set the entire content of the relative peak area of the metabolites to 100%.
Statistical analysis
All analyses were conducted using SPSS 17.0 for Windows (SPSS, Chicago, IL, USA). Data were presented as mean ± standard deviation (SD). The statistical significance of the difference between the SEE and Control was assessed by the Student’s t-test. Differences with p values <0.05 were considered statistically significant.
Results
Total phenolic and flavonoid contents of SEE
SEE was analyzed to assess the content of phenolics and flavonoids, as shown in Table 1. Total phenolic content (TPC) in SEE amounting to 385.38 ± 1.36 mg GA/g Extract. Total flavonoid content (TFC) in SEE amounting to 298.22 ± 2.38 mg QE/g Extract. A larger concentration of these phytochemicals in SEE increased its radical scavenging action.
| Phenolic and flavonoid contents | |
| TPC (mg GA/g Extract) | 385.38 ± 1.36 |
| TFC (mg QE/g Extract) | 298.22 ± 2.38 |
| Antioxidant activity | |
| Total antioxidant activity (µmol/g Extract) | 380.46 ± 2.12 |
| Free radical scavenging activity | |
| DPPH radical scavenging activity (% inhibition) | 72.09 ± 1.27 |
| ABTS radical scavenging activity (% inhibition) | 57.25 ± 1.03 |
Ferric-reducing/antioxidant power (FRAP) assay
The antioxidant function of SEE was assessed by measuring their capability to reduce iron from Fe3+ to the Fe2+ state, absorbance was directly proportional to reducing capability of the extract. In this study, SEE exhibited strong antioxidant capacity of 380.46 ± 2.12 µmol/g extract (Table 1), which suggested that SEE possesses the ability to interact with free radicals, transforming them into a stable and non-reactive state. It was indicated that flavonoids and phenolic acids in SEE act as free radical scavengers by donating electrons or hydrogen, thereby endowing SEE with reducing power function.
DPPH radical scavenging activity
DPPH assay revealed the existence of phenolic and flavonoid components in phytoextracts, serving as a prevalent method to examine the antioxidant characteristics of phytoextracts. Our findings demonstrated significant antioxidant activities in SEE. DPPH activity values for SEE as presented in Table 1. The SEE was able to reduce DPPH radicals with a percentage of 72.09 ± 1.27% at the concentration of 1 mg/mL.
ABTS radical scavenging activity
SEE showed an inhibition of 57.25 ± 1.03% at the concentration of 10 µg/mL (Table 1). The significant impact suggested that SEE is an effective suppressor of free radicals. The elevated scavenging ability of SEE was associated with increased levels of flavonoids, polyphenols, and proanthocyanins. This further substantiated that SEE has the potential to eliminate free radicals. SEE might serve as a preferable alternative for inhibiting the sequential reaction in lipid peroxidation and could also be considered as health supplements.
Inhibition of fenton’s reagent induced strand breaks in plasmid DNA
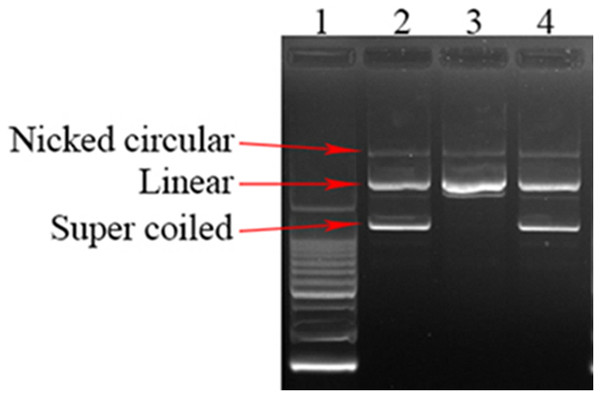
In this study, SEE was examined for their protective ability against oxidative damage to plasmid DNA (pGEM-T) caused by Fenton’s reagent (Fig. 1). Result showed that SEE have significant antioxidant activity. The inclusion of SEE in conjunction with Fenton’s reagent demonstrated the existence of super coiled plasmid DNA, akin to the native pGEM-T plasmid, indicating a diminished impact of DNA damage induced by Fenton’s reagent.
Figure 1: DNA damage protecting activity of SEE.
Lane1: Ladder 200 bp; Lane2: Plasmid DNA; Lane3: Plasmid DNA + fenton’s reagent; Lane4: Plasmid DNA + Fenton’s reagent + SEE.Identify metabolites of SEE
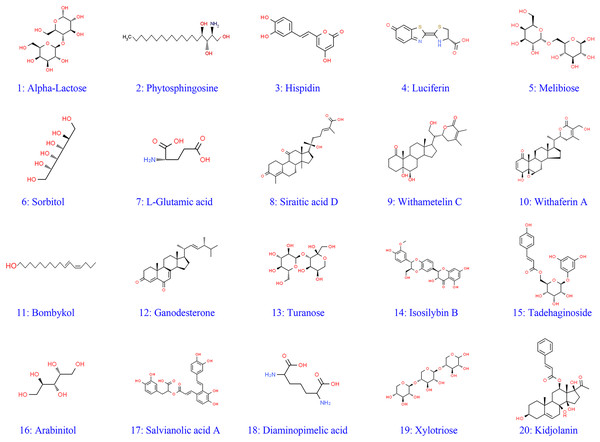
A total of 491 metabolites were identified based on their MS/MS spectra (Table S1). Mass spectra of all identified compounds were matched with LuMet-TCM and Herb databases. The top 20 compounds were shown in Table 2. Most of the top 20 compounds were identified as phenolics and flavonoids. In addition, two compounds that were not reported in fungi extracts yet have been identified (siraitic acid D and withametelin C). Extraordinary, the structures of the most present were determined by comparing the retention time, MS, and MS data with the references (Fig. 2).
| No. | Peak area ratio (%) | Retention time (min) | m/z | Mass error (ppm) | Formula | Metabolites |
|---|---|---|---|---|---|---|
| 51 | 22.56 | 0.72 | 387.11 | −0.47 | C12H22O11 | Alpha-Lactose |
| 25 | 8.67 | 9.6 | 318.30 | −0.88 | C18H39NO3 | Phytosphingosine |
| 43 | 8.56 | 5.02 | 245.05 | −0.57 | C13H10O5 | Hispidin |
| 9 | 6.08 | 5.49 | 281.00 | −1.05 | C11H8N2O3S2 | Luciferin |
| 30 | 4.60 | 0.73 | 360.15 | −0.99 | C12H22O11 | Melibiose |
| 17 | 4.34 | 0.72 | 181.07 | −3.91 | C6H14O6 | Sorbitol |
| 29 | 3.37 | 0.71 | 148.06 | −1.06 | C5H9NO4 | L-Glutamic acid |
| 164 | 2.86 | 9.38 | 479.28 | −1.02 | C28H40O5 | Siraitic acid D1 |
| 179 | 1.90 | 8.5 | 473.29 | 0.43 | C28H42O6 | Withametelin C1 |
| 38 | 1.09 | 7.9 | 453.26 | −0.33 | C28H38O6 | Withaferin A |
| 445 | 1.06 | 12.42 | 256.26 | −1.43 | C16H30O | Bombykol |
| 411 | 0.98 | 13.55 | 409.31 | −1.03 | C28H40O2 | Ganodesterone |
| 187 | 0.95 | 0.73 | 325.11 | −1.35 | C12H22O11 | Turanose |
| 134 | 0.92 | 6.94 | 463.10 | 0.59 | C25H22O10 | Isosilybin B |
| 159 | 0.88 | 4.92 | 473.08 | −0.66 | C21H22O10 | Tadehaginoside |
| 451 | 0.86 | 0.73 | 175.06 | −0.34 | C5H12O5 | Arabinitol |
| 239 | 0.84 | 5.74 | 495.13 | −2.19 | C26H22O10 | Salvianolic acid A |
| 22 | 0.80 | 0.73 | 191.10 | −1.23 | C7H14N2O4 | Diaminopimelic acid |
| 176 | 0.71 | 0.71 | 432.17 | −1.42 | C15H26O13 | Xylotriose |
| 142 | 0.68 | 7.97 | 555.26 | −4.42 | C30H38O7 | Kidjolanin |
Note:
Figure 2: Structures of identified metabolites 1–20.
Classification of SEE
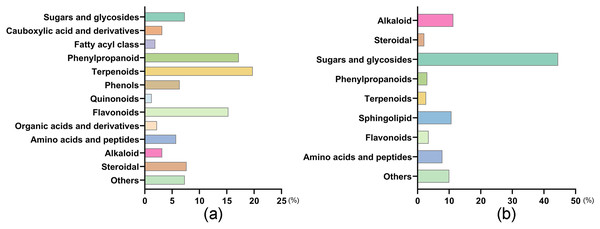
UPLC-MS analysis of SEE, component identification, and function prediction were performed. In terms of the number of components, the compounds with the highest numbers were terpenoids (19.75%), phenylpropanoid (17.20%), flavonoids (15.29%), steroidal (7.64%), and phenols (6.37%) (Fig. 3A). In terms of compositional content, the most abundant compounds were sugars and glycosides (44.48%), alkaloid (11.31%), sphingolipid (10.73%), amino acids and peptides (7.85%), flavonoids (3.51%), and phenylpropanoids (3.08%) (Fig. 3B).
Figure 3: Distribution of number and composition of SEE.
(A) The number of components of SEE. (B) The compositional content of SEE.Discussion
Natural products and derivatives account for more than half of all clinically used medications worldwide (Bhattacharya, 2017; Krupodorova et al., 2024). Mushrooms have been utilized for their nutritional and medicinal properties for centuries. They have constituted a significant therapeutic raw material in folk medicine (Muszyńska et al., 2018). These edible species value stems from their bioactive phytochemical components, such as phenolics, flavonoids, terpenes, tocopherols, and carotenoids, which are collectively termed as antioxidants (Chen et al., 2018; Mehta, Rayalam & Wang, 2018; Sevindik, Mohammed & Uysal, 2023). Oxidative stress, which is frequently generated by an excess of free radicals, has been linked to a variety of degenerative illnesses, including cancer, ischemic heart disease, atherosclerosis, diabetes, and neurological disorders (Wang & Kang, 2020; Uysal et al., 2023). The biological role that antioxidant play in medicinal fungus is very important, numerous studies have shown that medicinal fungus possess a wide range of pharmacological properties, such as antibacterial, anti-inflammatory, anti-cancer, and anthelmintic properties (Lo et al., 2013; Marín et al., 2023; El-Chaghaby et al., 2024; Mohammed et al., 2024). Thus, natural compounds generated from medicinal fungus, including extracts, provide several prospects for novel medication development.
S. sanghuang is a medicinal fungus rich in bioactive phytochemical components. Phenolic and flavonoid compounds are the main active ingredient in the alcoholic extract of S. sanghuang (Seephonkai et al., 2024). They are known for their radical scavenging activity, that helps to reduce oxidative stress (Yuen & Gohel, 2008; Huang et al., 2020; Zuo et al., 2021; Dong et al., 2023). For example, S. sanghuang isolation compounds 9–12 showed good cellular antioxidant activities (Zhang et al., 2021). Sanghuang extract can reduce inflammatory cell infiltration and decreased the MPO activity and the MDA level, simultaneous increase the SOD, CAT and GSH-Px activities in lung tissue homogenates (Su et al., 2019). Thus, S. sanghuang composition (food and supplements) can act as protective medicament and provide numerous advantages for health.
Antioxidant-rich medicinal fungus extracts, characterized by their DPPH free radical scavenging ability, can donate hydrogen to lipid peroxyl or hydroperoxyl radicals, which are pivotal in the propagation of lipid autoxidation. It also produces non-radicals that disrupt lipid peroxidation chain reaction (Sowndhararajan & Kang, 2013; Korkmaz, Dayangaç & Sevindik, 2021). Free radicals have the potential to facilitate DNA damage, thereby significantly decreasing the carcinogenic, mutagenic, and cytotoxic effects of reactive oxygen species (ROS) (Fischer, Seo & Efferth, 2018). The findings from our research compellingly demonstrated that SEE mitigated oxidative stress and protected the DNA from the harmful effects of hydroxyl radicals generated by Fenton’s reagent. The shielding effect of SEE against DNA damage induced by hydroxyl radicals might be ascribed to the presence of various flavonoids and phenolic compounds. Similar protective effect on DNA were observed in various plant extracts (Salar, Purewal & Sandhu, 2017). For example, Yen, Hung & Hsieh (2000) reported that water extracts of Hsian-tsao could lessen DNA damage brought on by UV-C, and it was more effective against UV-C than H2O2-induced DNA damage. Hsieh et al. (2018) demonstrated that Lycium barbarum extract exhibit a higher level of preventive action against UVB-induced growth arrest in ARPE-19 cells, protect cells from UVB-induced apoptosis. Furthermore, it demonstrated a dose-responsive reduction in the activation of γH2AX, a sensor of DNA damage in ARPE-19 cells. Consequently, there is a great deal of medical potential for the study of active chemicals in pricey medicinal supplies.
As a result, the findings offer the reader with a more appropriate method for evaluating active components in valued medicinal fungus. It provides a possible avenue for future study and development in the investigation and quality assurance of S. sanghuang, as well as the discovery of bioactive compounds.
Conclusions
Finally, the results of this investigation provide insight into the ethanol extract of S. sanghuang. This investigation confirmed that S. sanghuang was a good source of phenolics, flavonoids, and terpenoids. This is reflected in its potent antioxidant properties. According to the findings, this pharmaceutical/food resource mushroom has a possible natural antioxidant that might be employed as a lead candidate in the development of antioxidant medications for the treatment of a variety of oxidative stress-related disorders.