Incidence and risk factors of hepatitis E virus infection in women with gynecological tumors in Eastern China
- Published
- Accepted
- Received
- Academic Editor
- Elliot Lefkowitz
- Subject Areas
- Virology, Epidemiology, Gynecology and Obstetrics, Infectious Diseases
- Keywords
- Hepatitis E virus, Gynecological tumor, Seroprevalence, Eastern China, Risk factors
- Copyright
- © 2024 Bai et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, reproduction and adaptation in any medium and for any purpose provided that it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Incidence and risk factors of hepatitis E virus infection in women with gynecological tumors in Eastern China. PeerJ 12:e18747 https://doi.org/10.7717/peerj.18747
Abstract
Background
Recently, there has been increasing interest in the exploration of the association between the hepatitis E virus (HEV) infection and malignancies; however, epidemiological data for HEV infection among women with a gynecological tumors (GT) are limited. Herein, we investigated the correlation between HEV and GT in Chinese women.
Methods
We recruited 452 women diagnosed with a primary GT and 452 healthy volunteers to investigate the possible routes and risk factors for HEV infection. The serum antibody levels of anti-HEV IgG and IgM were measured by enzyme-linked immunoassays once a year.
Results
After a median follow-up time of 5.4 years (range 4 to 7 years), the overall detection rate of anti-HEV antibodies in patients with GT and in controls were 69/452 (15.27%) and 23/452 (5.09%) (P = 0.001), respectively. The seroprevalence of anti-HEV IgG antibodies was significant higher in patients with GT (15.27%) than in healthy controls (5.09%) (P = 0.001). Moreover, 13 (2.88%) patients with GT were positive for IgM antibodies, while only 4 (0.88%) healthy controls tested positive for anti-HEV IgM antibodies (P = 0.028). The highest prevalence of HEV antibodies were detected in patients with ovarian borderline tumors (40%), followed by patients with ovarian cancer (20.54%) and endometrial cancer (18.46%). Multivariable analysis revealed that contact with dogs (OR, 1.88; 95% CI [1.10–3.22]; P = 0.015) and a history of anti-tumor chemotherapy (OR, 1.85; 95% CI [1.07–3.20]; P = 0.028) were independent risk factors for HEV infection.
Conclusion
Overall, the present study showed that patients with GT are more susceptible to HEV infection in Eastern China, particularly in patients with ovarian borderline tumors. Thus, effective strategies are needed to reduce HEV infection in patients with GT.
Introduction
The hepatitis E virus (HEV) is a single-stranded RNA virus, which is estimated to have infected nearly 20 million individuals worldwide (Ma et al., 2022). HEV has been classified into four major genotypes (HEV1-4) and 24 sub-types (Aslan & Balaban, 2020). HEV genotypes 3 and 4 can be transmitted from animals to humans via the fecal–oral route (Busara et al., 2024). HEV infection is usually a self-limiting disease. Sometimes they are completely non-specific symptoms, but often there are liver symptoms as well (Hoofnagle, Nelson & Purcell, 2012; Kamar et al., 2012). However, in immune-deficient patients, including patients with tumors or autoimmune diseases, HEV infection may cause liver failure and death (Elfert et al., 2018; Webb & Dalton, 2020).
A high incidence of HEV infection has been found in patients with cancer (Bai et al., 2018; Lin et al., 2023). In one study, Bai et al. (2018) demonstrated that nearly 26% of patients with cancer were seropositive for anti-HEV antibodies, which indicates either past or current HEV infection. This seroprevalence is considerably higher than the 13% positivity rate observed in the control group, a statistically significant difference suggesting that cancer patients may be at increased risk of HEV infection (Bai et al., 2018). Another study conducted by Chiu et al. (2022) reported a latent relationship between HEV and hematologic malignancies. In addition, Lin et al. (2023) analyzed the relationship between HEV infection and the risk for 17 types of cancer, finding a significant association between HEV infection and gastric cancer. Together, this evidence indicates that HEV infection may be a significant risk factor for malignancy.
Gynecological tumors (GT) include cancers that develop in the female reproductive system. According to the position of the cancer, GT can be classified as either external genital tumors, vaginal tumors, uterine tumors, ovarian tumors, or fallopian tube tumors, etc. GTs may further be classified as benign, malignant, or borderline. It is generally believed that the development of GT can be driven by genetic factors, pathogen infection, and physical and chemical factors. For example, human papillomavirus (HPV) is associated with tumor progression in HPV-associated cervical carcinoma (Senapati, Senapati & Dwibedi, 2016). Bai et al. (2018) also showed that patients with ovarian cancer were more to susceptible HEV infection, suggesting a potential association between HEV infection and ovarian tumors development.
There are many risk factors associated with HEV infection, including age, region of residence, and contact with infected animals, among other. Several epidemiological investigations of HEV infection in patients with malignancies have been conducted in recent years (Bai et al., 2018; Chiu et al., 2022). Further, other studies have shown that receiving blood transfusion and anti-tumor chemotherapy can also increase the risk of HEV infection (Bettinger et al., 2018; Boutrouille et al., 2007; Donald & Peter, 2018; Okumura et al., 2023). However, data regarding HEV infection in patients with benign GT is scarce, and the prevalence and potential risk factors for this virus in such patients are currently unknown. Thus, the aim of this study was to explore the risk of HEV infection in women with GT, and to clarify the potential risk factors for this patient group.
Methods
Ethics statement
This project was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University (QYFY WZLL 28350). All participants provided written informed consent to participate.
Study cohort and sociodemographic data
Between January 2016 and December 2019, 1,029 volunteers, including 543 women diagnosed with a primary GT and 486 healthy controls, were recruited to participate this study. Patients with GT ranged in age from 21–69 years old. Healthy controls were randomly invited from among women who participated in health screenings at the Affiliated Hospital of Qingdao University. Healthy controls are not diagnosed with any gynecological disorders when they were recruited. Participants who tested positive for anti-HEV antibodies or were treated with intravenous immunoglobulin (Ig) before blood collection were excluded. All volunteers were followed up until December 2023, and data regarding behavioral characteristics and patient survival were collected. The tests will be terminated when the volunteers infect with HEV, and the questionnaire will be given. The questionnaire of those negative for HEV antibodies was given on December 2023. Sociodemographic and lifestyle behavioral data were collected from participants using a structured questionnaire, as described by Wang et al. (2022). Clinical disease data (including tumor type, serum markers) were collected from medical records supplemented by the patients.
Sample collection and serological assay
Venous blood samples of ~5 mL were collected from volunteers once a year. After collection, blood samples were centrifuged at 3,000 rpm for 10 min at room temperature to collect serum. Serum samples were collected and stored at −80 °C until examination to ensure the integrity and reliability of the results (Wang et al., 2022). The ELISAs were completed within 3 months after blood collection.
Enzyme-linked immunosorbent assay (ELISA) kits (Wantai Bio, Beijing, China) were used to test for anti-HEV IgG and IgM antibodies. The sensitivity and specific of the ELISA are 98.5% and 99.1%, in accordance with the manufacturer’s instructions. Briefly, 100 μl sample diluent was pipetted into a single well of a 96-well plate, and supplemented with 10 μl serum. After incubation for 30 min at 37 °C, the well plates were washed five times. Subsequently, 100 μl of horseradish peroxidase-conjugated enzyme labeled HEV-Ag was added to each well, and incubated in the dark allowed for 30 min at 37 °C. After washing, chromogenic solution A (50 µL) and chromogenic solution B (50 µL) were added to the 96-well plate and incubated for 15 min. Termination solution (50 μL) was then added into the well to stop the reaction. The optical density (OD) values were measured at 450 nm using Labsystems Multiskan RC micro-plate reader. Positive and negative control sera were included in each plate. The cutoff value was calculated as the mean of negative controls plus 0.26. Results equal to or greater than the cutoff value were considered as positive.
Statistical analyses
All statistical analyses were performed using SPSS 22.0 (IBM, Armonk, NY, USA). The association between the anti-HEV antibody positive rate and socio-demographic and clinical data were analyzed by chi-square test or Fisher’s exact test. Data associated with HEV infection in univariate analysis (P ≤ 0.2) were included in a multivariate logistic regression analysis to define independent risk factors of HEV infection. The adjusted odds ratio (OR) and 95% confidence interval (CI) were calculated using logistic regression analysis. Results with a P-value of < 0.05 were considered significant.
Results
Epidemiological profile and risk factors for patients with GT and HEV infection
After a median follow-up time of 5.4 years (range 4 to 7 years), 452 women diagnosed with a primary GT and 452 healthy controls obtained complete follow-up data. The anti-HEV antibody presence was tested in these 904 participants (452 patients with GT and 452 controls). The overall incident rate of HEV infection in patients with GT and in controls was 69/452 (15.27%) and 23/452 (5.09%) (P = 0.001), respectively, representing a significantly higher level in GT patients (P = 0.001). In addition, 13 (2.88%) patients with GT were positive for IgM antibodies, while only 4 (0.88%) healthy controls were anti-HEV IgM antibody positive (P = 0.028) (Table 1). Univariate analysis showed that patients’ age, contact with dogs, source of drinking tap water, and history of anti-tumor chemotherapy were all associated with HEV seroprevalence in patients with GT. The detailed data are shown in Table 2. All socio-demographic and clinical treatment variables with P ≤ 0.2 on analysis (age, contact with dogs, contact with pigs, source of drinking water, and history of anti-tumor chemotherapy) were included in the subsequent multivariate analysis. This analysis revealed that contact with dogs (OR, 1.88; 95% CI [1.10–3.22]; P = 0.015) and a history of anti-tumor chemotherapy (OR, 1.85; 95% CI [1.07–3.20]; P = 0.028) were independent risk factors for HEV infection in patients with GT (Table 3). In additional, we tested the serum Glutamic Oxaloacetic transaminase (SGOT) and serum glutamic pyruvic transaminase (SGPT) values of volunteers who presented positive anti-HEV antibody. We found there was no difference in SGOT and SGPT values between each group of patients and the control group (Table 4).
| Sero-reaction | Patients with a GT (n = 452) | Healthy controls (n = 452) | Patients with a GT vs. Healthy controls | ||
|---|---|---|---|---|---|
| No. positive | % | No. positive | % | Pa | |
| IgG | 69 | 15.27 | 23 | 5.09 | 0.001 |
| IgM | 13 | 2.88 | 4 | 0.88 | 0.028 |
| IgG+/IgM+ | 13 | 2.88 | 4 | 0.88 | 0.028 |
| IgG+/IgM− | 56 | 12.39 | 22 | 4.87 | 0.001 |
| IgG−/IgM+ | 0 | 0 | 0 | 0 | 1 |
| Total | 69 | 15.27 | 23 | 5.09 | 0.001 |
Note:
| Characteristic | Patients with a gynecological tumor (n = 452) | Healthy controls (n = 452) | ||||||
|---|---|---|---|---|---|---|---|---|
| Prevalence of HEV infection | Prevalence of HEV infection | |||||||
| No. tested | No. positive | % | Pa | No. tested | No. positive | % | Pa | |
| Age (years) | ||||||||
| ≤30 | 95 | 10 | 10.53% | 0.008 | 65 | 6 | 9.23% | 0.267 |
| 31–50 | 109 | 9 | 8.26% | 119 | 8 | 6.72% | ||
| 50–70 | 188 | 35 | 18.62% | 233 | 12 | 5.15% | ||
| >71 | 60 | 15 | 25.00% | 35 | 0 | 0.00% | ||
| Residence area | ||||||||
| Urban | 251 | 42 | 16.73% | 0.332 | 240 | 15 | 6.25% | 0.629 |
| Rural | 201 | 27 | 13.43% | 212 | 11 | 5.19% | ||
| Contact with cats | ||||||||
| Yes | 141 | 24 | 17.02% | 0.485 | 118 | 8 | 6.78% | 0.577 |
| No | 311 | 45 | 14.47% | 334 | 18 | 5.39% | ||
| Contact with dogs | ||||||||
| Yes | 209 | 42 | 20.10% | 0.008 | 107 | 13 | 12.15% | 0.001 |
| No | 243 | 27 | 11.11% | 345 | 13 | 3.77% | ||
| Contact with pigs | ||||||||
| Yes | 74 | 15 | 20.27% | 0.191 | 89 | 6 | 6.74% | 0.655 |
| No | 378 | 54 | 14.29% | 363 | 20 | 5.51% | ||
| Consumption of raw/undercooked meat | ||||||||
| Yes | 107 | 19 | 17.76% | 0.412 | 75 | 6 | 8.00% | 0.360 |
| No | 345 | 50 | 14.49% | 377 | 20 | 5.31% | ||
| Consumption of raw vegetables | ||||||||
| Yes | 78 | 10 | 12.82% | 0.509 | 204 | 14 | 6.86% | 0.358 |
| No | 374 | 59 | 15.78% | 248 | 12 | 4.84% | ||
| Exposure to soil | ||||||||
| Yes | 269 | 41 | 15.24% | 0.986 | 144 | 10 | 6.94% | 0.457 |
| No | 183 | 28 | 15.30% | 308 | 16 | 5.19% | ||
| Source of drinking water | ||||||||
| Tap | 346 | 62 | 17.92% | 0.005 | 266 | 14 | 5.26% | 0.593 |
| River | 106 | 7 | 6.60% | 186 | 12 | 6.45% | ||
| Occupation | ||||||||
| Farmer | 278 | 44 | 15.83% | 0.675 | 296 | 20 | 6.76% | 0.206 |
| Worker | 174 | 25 | 14.37% | 156 | 6 | 3.85% | ||
| History of abortion | ||||||||
| Yes | 73 | 12 | 16.44% | 0.761 | 117 | 5 | 4.27% | 0.425 |
| No | 379 | 57 | 15.04% | 335 | 21 | 6.27% | ||
| History of chemotherapy | ||||||||
| Yes | 173 | 32 | 18.50% | 0.004 | ||||
| No | 279 | 37 | 1.46% | |||||
| History of blood transfusion | ||||||||
| Yes | 126 | 20 | 8.20% | 0.823 | ||||
| No | 326 | 49 | 13.31% | |||||
Note:
| Clinical diagnosis | No. tested | High SGOT | % | Pa | High SGPT | % | Pb |
|---|---|---|---|---|---|---|---|
| Gynecological tumor | 69 | 13 | 18.84 | 0.87 | 17 | 24.64 | 0.24 |
| Ovarian borderline tumor | 6 | 2 | 33.33 | 0.39 | 1 | 16.67 | 0.27 |
| Ovarian cancer | 23 | 5 | 21.74 | 0.71 | 7 | 30.43 | 0.35 |
| Endometrial cancer | 12 | 1 | 8.33 | 0.48 | 3 | 25 | 0.33 |
| Cervical squamous cell carcinoma | 15 | 3 | 20 | 0.84 | 3 | 20 | 0.44 |
| Ovarian mucinous cystadenoma | 3 | 1 | 33.33 | 0.51 | 2 | 66.67 | 0.085 |
| Uterine leiomyoma | 3 | 0 | 0 | 0.59 | 0 | 0 | 0.68 |
| Ovarian cystic mature teratoma | 7 | 1 | 14.229 | 0.62 | 1 | 14.29 | 0.68 |
Note:
As compared with 17.39% (4/23) higher SGOTa and 13.04% (3/23) higher SGPTb in controls, respectively.
HEV antibody prevalence in patients with different GT histological types
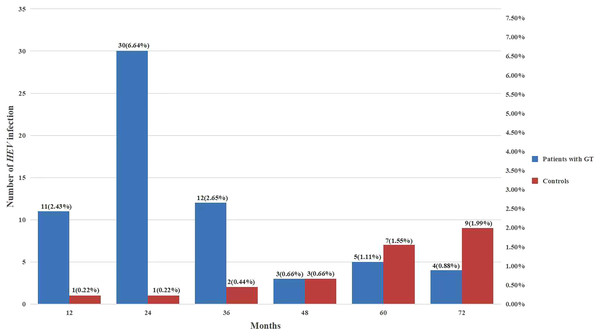
The levels of HEV exposure in patients with different GT histological types are presented in Table 5. The highest prevalence of HEV antibodies were detected in patients with ovarian borderline tumor (40%), followed by patients with ovarian cancer (20.54%) and endometrial cancer (18.46%) (P < 0.05). Overall, 37 cancer patients died during the study period, none of them infected whit HEV. In addition, among patients with GTs, nearly 80% of HEV infection cases were acquired within 3 years of diagnosis, while in healthy controls, the HEV infection rate did not present any obvious temporal characteristics (Fig. 1).
| Clinical diagnosis | No. tested | No. positive | % | Pa |
|---|---|---|---|---|
| Gynecological tumor | 452 | 69 | 15.27% | 0.001 |
| Ovarian borderline tumor | 15 | 6 | 40% | 0.001* |
| Ovarian cancer | 112 | 23 | 20.54% | 0.001 |
| Endometrial cancer | 65 | 12 | 18.46% | 0.001 |
| Cervical squamous cell carcinoma | 84 | 15 | 17.86% | 0.001 |
| Ovarian mucinous cystadenoma | 26 | 3 | 11.54% | 0.16* |
| Uterine leiomyoma | 41 | 3 | 7.32% | 0.47* |
| Ovarian cystic mature teratoma | 109 | 7 | 6.42% | 0.58 |
Figure 1: Comparison between HEV serostatus and follow-up time.
Discussion
Based on reports by the World Health Organization, viral hepatitis is responsible for approximately 1.45 million deaths globally each year (World Health Organization, 2016; Tjan, 2016). HEV infection has been shown to cause liver damage, accounting for ~3.3% of all viral hepatitis mortalities (Primadharsini, Nagashima & Okamoto, 2019). Thus, HEV infection is now recognized as a significant rising global burden. The HEV seroprevalence in patients with cancer has been increasingly explored in recent years including hepatocellular carcinoma (HCC) (Shen et al., 2023; Xu et al., 2017; Yin & Kan, 2023), gastric cancer, (Chiu et al., 2022; Webb & Dalton, 2020), and lung cancer (Okumura et al., 2023). In addition, in our previous study, we found a significantly higher seroprevalence of anti-HEV antibodies in patients with ovarian cancer than in controls (Bai et al., 2018). However, whether tumors promote HEV infection or potential routes for HEV infection in these patients group remains unclear. Thus, we conducted the present study to assess this situation. Our results showed that cancer could increase the risk of infection by HEV.
After 6 years of monitoring, we found a significantly higher detection rate of anti-HEV IgG antibodies in patients with GT (15.27%, 69/452) than in healthy controls (5.09%, 23/452) at the end of the follow-up period. These data suggested that patients with GT are more susceptible to HEV infection. Moreover, the seroprevalence of HEV in patients with malignancies was higher than that in patients with benign tumors, with particularly high rates observed in patients with ovarian and endometrial cancer. Patients with malignant tumors commonly show immune deficiencies, resulting in an inability to form effective responses against HEV (Lenglart et al., 2023; Lin et al., 2023; Yin & Kan, 2023). Moreover, anti-HEV antibodies were most commonly detected within 3 years of GT diagnosis, while the HEV infection rate in healthy women did not present any obvious temporal characteristics. Another interesting result of this study is our finding that patients with ovarian borderline tumors presented with the highest incidence rate of HEV, which suggested patients with ovarian borderline tumors were the most sensitive to HEV infection.
Previous studies have indicated that the HEV infection rate in healthy individuals may increase with age, due to an overall lifetime exposure to HEV among older people (Ouyang et al., 2024). However, one study showed that in cancer patients, HEV seroprevalence was significantly higher in young patients (Lin et al., 2023). Some researchers have speculated that the change in immune function and hormonal levels caused by aging may account for this discrepancy. In our study, we found that patients with GTs older than 70 years had the highest incidence for HEV infection, while in healthy controls, younger participants had a greater risk of HEV exposure. The possible reason for this phenomenon is that women with GTs are immunodeficient, and older patients maybe have more fragile immune system to against HEV infections. Overall, this study revealed a potential correlation between HEV infection and age in GTs patients; however, further studies are needed to confirm the potential mechanisms.
The fecal–oral route is an important mode of transmission for HEV. Contaminated drinking water, contact with pigs and cats, and exposure to feces are the most common risk factors for HEV infection (Michelle et al., 2013). In immunocompromised patients, HEV can also be transmitted from blood products (Hoofnagle, Nelson & Purcell, 2012). Kogias et al. (2023) demonstrated that in hemodialysis patients, HEV infection was significantly associated with area of residence and contact with pork. However, the risk factors for HEV infection among patients with malignant tumors have not been well demonstrated. In our study, multivariate analysis showed that contact with dogs was an independent risk factor for HEV infection in women with GTs and healthy controls. This result is consistent with a study conducted by Bai et al. (2018), in which cancer patients in contact with dogs at home harbored the highest HEV seroprevalence. In addition, one survey including nearly 4,500 dogs in Southwestern China identified anti-HEV antibodies in 36.55% of stray city dogs. This data suggests that a high HEV seroprevalence in dogs and humans exposed from dogs should be considered an urgent public health concern (Zeng et al., 2017). Although patients with GTs can be infected with HEV through contact with dogs, little attention has been paid to this phenomenon. Therefore, it will be necessary to further investigate this risk factor for HEV infection in cancer patients, particularly those with GTs, in order to reduce the transmission of HEV.
Chemotherapy was identified as another risk factor for HEV infection in patients with GT in our study. This result is in agreement with other studies, which identified acute HEV infection in some tumor patients during anti-tumor chemotherapy (Bettinger et al., 2018; Lenglart et al., 2023). Adjuvant chemotherapy combined with targeted therapy is an important treatment strategy for gynecologic cancer. For example, the combination of paclitaxel and carboplatin is the first-line clinical treatment for gynecologic malignancies. However, this management strategy commonly causes liver injury in patients. Moreover, the combination of paclitaxel and carboplatin adjuvant chemotherapy is usually used for patients within 2 years after diagnosis. Therefore, it is reasonable to propose chemotherapy may increase the risk of HEV infection.
This study has some limitations which should be mentioned. First, the sample size was relatively small and therefore is not representative of the entire Chinese population. Second, we did not conduct HEV RNA tests to exclude false positives caused by the use of ELISA diagnostic equipment; thus, the influence of false positivity caused by the methodology is uncertain. Third, there might have been cases who did not detect anti-HEV antibodies when they had an HEV infection due to the difference in time between HEV infection and sample collection.
Conclusions
The results of the present study show that patients with GTs are more susceptible to HEV infection, especially in patients with ovarian borderline tumors. Contact with dogs and treatment with chemotherapy are independent risk factors for this virus infection. Thus, effective strategies are urgently needed to reduce HEV infection in patients with GT.