Nuclear eDNA metabarcoding primers for anthozoan coral biodiversity assessment
- Published
- Accepted
- Received
- Academic Editor
- Simon Man Kit Cheung
- Subject Areas
- Biodiversity, Ecology, Ecosystem Science, Marine Biology, Biological Oceanography
- Keywords
- Environmental DNA, Biodiversity, Antipatharia, Scleractinia, Octocorallia, Ribosomal DNA
- Copyright
- © 2024 McCartin et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2024. Nuclear eDNA metabarcoding primers for anthozoan coral biodiversity assessment. PeerJ 12:e18607 https://doi.org/10.7717/peerj.18607
Abstract
The distributions of anthozoan corals are undercharacterized due to their wide bathymetric ranges, occurrences in remote locales, and difficulties of identification from morphology alone. Environmental DNA (eDNA) sequencing promises to be a noninvasive strategy to complement conventional approaches for mapping and monitoring the distribution and biodiversity of coral communities. Primers for eDNA metabarcoding have been designed to amplify nuclear and mitochondrial DNA barcodes in shallow scleractinians and mitochondrial MutS in deep-sea octocorals. However, a comprehensive method for eDNA metabarcoding of all anthozoan corals, including black corals, has not been developed. We leveraged a sequence database of global coral collections, from shallow water to the deep sea, to design new PCR primers for coral eDNA sequencing that target the 28S rRNA gene (28S rDNA). We tested the performance of these primers by amplifying and sequencing eDNA from water samples collected in the Gulf of Mexico near mesophotic and deep-sea corals that were also imaged, sampled, and sequenced. Sequencing libraries produced using the primers were highly enriched in eDNA from octocorals, black corals and scleractinians, with up to 99.9% of the reads originating from these corals. Further, the 28S barcode amplified using the primers distinguished coral genera and species in many cases, like previously developed methods that target eDNA in only octocorals or scleractinians. We recovered amplicon sequencing variants (ASVs) identical to DNA barcodes derived from Sanger sequencing and genome skimming of corals sampled at the same field sites. This new eDNA metabarcoding strategy permits targeted eDNA sequencing of black corals, octocorals, and scleractinians at sites where they co-occur and expands our current toolkit for mapping and monitoring coral communities in shallow coral reefs and the deep sea.
Introduction
Anthozoan corals, including stony corals (Order: Scleractinia), black corals (Order: Antipatharia), and octocorals (Orders Malacalcyonacea and Scleralcyonacea), occur globally and are foundation species in shallow (less than 30 m depth), mesophotic (30 to 150 m), and deep-sea (>150 m) benthic ecosystems (Cordes et al., 2008; Slattery & Lesser, 2021). Mesophotic and deep-sea coral ecosystems are ecologically distinct from shallow reefs yet face similar threats, including invasive species, ocean warming, pollution, destructive fisheries, and other bottom damaging activities (Koslow et al., 2000; Fisher et al., 2014; Etnoyer et al., 2016; Rocha et al., 2018). Developing novel methods that can rapidly assess the distributions of coral communities with meaningful taxonomic resolution will expedite the assessment of coral biogeography, diversity, and resilience.
Environmental DNA (eDNA) sequencing complements conventional methods for assessing biodiversity and the distribution of invasive and ecologically important invertebrates in marine habitats, including corals (Everett & Park, 2018; West et al., 2020; Dunn et al., 2022, Nishitsuji et al., 2023). Water sampling for eDNA analysis is inherently nondestructive and thus is well suited for monitoring marine protected areas and areas where past disturbances have impacted deep-sea corals with slow growth and recovery rates. The strategy that is most often used for eDNA sequencing is eDNA metabarcoding, wherein a DNA barcoding gene is amplified and sequenced from an environmental sample. eDNA metabarcoding may be conducted using PCR primers that amplify DNA barcodes from specific taxa (e.g., Miya et al., 2015) or all metazoans (e.g., Leray et al., 2013). With a comprehensive reference database for a taxon of interest, primers can be designed to amplify taxonomically informative regions not yet amplified by existing eDNA metabarcoding protocols. Thus, taxonomically specific primers are necessary for generating sequencing data that can be used to assess coral diversity at a taxonomic resolution comparable to established methods that do not involve physical sampling (e.g., video transects).
Multiple primer sets have been designed to amplify taxonomically informative eDNA nuclear and mitochondrial DNA barcodes in shallow scleractinians (Brian, Davy & Wilkinson, 2019; Nichols & Marko, 2019; Alexander et al., 2020; Shinzato et al., 2021) and the mitochondrial MutS gene (mtMutS) in deep-sea octocorals (Everett & Park, 2018). Due to a slow rate of evolution in octocorals and scleractinians, typical mitochondrial barcoding genes (e.g., COI) are not taxonomically informative across all orders of anthozoan corals (McFadden et al., 2011). Furthermore, mtMutS is not present in black corals or scleractinians, precluding the use of this gene for comprehensive anthozoan metabarcoding (Shimpi & Bentlage, 2023). Nuclear ribosomal RNA (rRNA) genes (also referred to as rDNA) encode for the small (18S rRNA) and large (5S, 5.8S and 28S rRNA) subunits of the ribosome and are present in tandem repeats that occur in many copies in the eukaryotic nuclear genome. Due to their high copy number, like mitochondrial genes, nuclear rRNA genes have also been targeted for eDNA metabarcoding. In anthozoan corals, 28S has been sequenced in previous phylogenetic studies and has shown utility in discriminating taxa (e.g., Barbeitos, Romano & Lasker, 2010; Brugler, Opresko & France, 2013; McFadden et al., 2014; Cairns & Wirshing, 2015).
Here, we leveraged a database of sequences from global coral collections with broad bathymetric distributions (from shallow reefs to over 2,000 m depth) to design new eDNA primers for metabarcoding a taxonomically informative region of 28S in all anthozoan corals. We assessed the specificity of the primers to anthozoan corals and the ability of the amplified DNA barcode to discern taxonomic relationships both in silico and through the metabarcoding of field samples collected at mesophotic and deep-sea coral communities in the northern Gulf of Mexico. Furthermore, we investigated the utility of this novel nuclear eDNA metabarcoding sequencing approach for anthozoan coral biodiversity assessment by pairing eDNA sampling with video observation and sequencing coral specimens collected at the same field sites. Portions of this text were previously published as part of a preprint (McCartin et al., 2023).
Materials and Methods
Generating sequences for primer design
To generate 28S sequences for primer design, we (1) assembled sequences from an existing target-capture enrichment genomic dataset generated from global coral collections (first published in Quattrini et al. (2020)) and (2) generated new sequencing data by PCR amplifying and Sanger sequencing 28S from DNA isolated from mesophotic and deep-sea coral species collected in the northern Gulf of Mexico (GoMx).
Quattrini et al. (2020) conducted target-capture phylogenomic sequencing of anthozoans collected from the Atlantic, Indian, Pacific, and Southern Ocean basins. Partial contig assemblies from each specimen sequenced by Quattrini et al. (2020) were transformed into local BLAST (Altschul et al., 1990) databases, and BLASTN searches (Hexacorallia: -evalue 0.5; Octocorallia: -evalue 1e−5, -max_target_seqs 5 -max_hsps 5) were executed using 28S query sequences from a range of anthozoan species downloaded from GenBank. Out of the 28S blast results for each specimen, those with the highest bit scores, lowest e-values, and minimum match lengths of 3,000 nt. were selected. Sequences were aligned by their taxonomic group (Octocorallia, Antipatharia, and Scleractinia) using MAFFT online v7 (Katoh & Standley, 2013), visually adjusted, trimmed, and exported using AliView v1.26 (Larsson, 2014).
Mesophotic and deep-sea corals were collected from the Gulf of Mexico from depths of 53 to 1,850 m during remotely operated vehicle (ROV) dives from 2018 to 2021 (Table S1). Samples from the Flower Garden Banks National Marine Sanctuary were collected under permits FGBNMS-2017-007-A2 and FGBNMS-2019-003-A2 to S.H. DNA was extracted from these specimens using a salting-out protocol (dx.doi.org/10.17504/protocols.io.bypypvpw). An ~810 base pair (bp) sequence of 28S was amplified and Sanger-sequenced from 48 octocoral and black coral species using previously published primers (McFadden & Ofwegen, 2012). Further detail is provided in section 1.1 of the Supporting Information.
Primer design
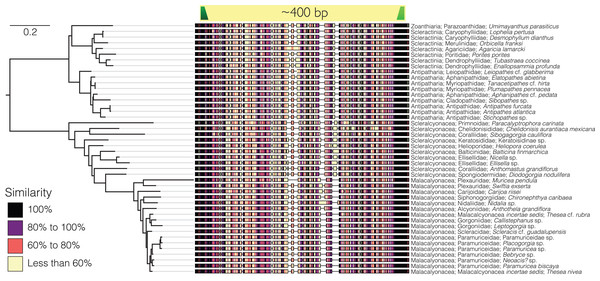
Sequences from the target-capture dataset (143 sequences) and the Sanger sequencing data generated from the GoMx samples (48 sequences) were aligned using Clustal Omega (Sievers et al., 2011) and the default parameters in Geneious Prime version 2022.1.1 (https://www.geneious.com). Primers were designed to amplify a ~400 bp variable region of the trimmed alignment (Fig. 1). Black corals and octocorals shared substantial sequence similarity at the forward primer sequence such that a single forward primer could be designed for both groups (Table 1). However, a second primer set needed to be designed specifically for scleractinians due to divergence in scleractinian sequences at the forward primer binding site as compared to octocorals and black corals.
Figure 1: Sequence alignment of the barcoding region of the 28S targeted for eDNA metabarcoding from a selection of zoantharian, octocoral, black coral, and scleractinian species.
Similarity across the aligned sequences reflects the percentage of sequences in the alignment that share a given nucleotide at each position, meaning that positions with high similarity scores across sequences in the alignment are more conserved. The scale bar represents the number of substitutions per site. The tree is rooted at the node representing the most recent common ancestor of hexacorals and octocorals. The dark green annotation represents the forward primer, and the light green annotation represents the reverse primer. The tree was modeled using IQ-TREE2 (Minh et al., 2020) without bootstrapping. The best substitution model was found with modelfinder (Kalyaanamoorthy et al., 2017) based on Bayesian Information Criterion. The model is a transition model where AC = CG and AT = GT and base frequencies were unequal (TIM3), with empirical base frequencies (+F), and rate heterogeneity modeled using a gamma function (+G4).| Primary target taxa | Primer sequences | Tm | %GC |
|---|---|---|---|
| Antipatharia and Octocorallia | Anth-28S-eDNA-F: 5′-CGTGAAACCGYTRRAAGGG–3′ | 55.7–62.3 °C | 56.3% |
| Anth-28S-eDNA-R: 5′-TTGGTCCGTGTTTCAAGACG–3′ | 59.9 °C | 50% | |
| Scleractinia | Scler-28S-eDNA-F: 5′-AKGGAAACGAATGGRCTMAG–3′ | 54.0–61.1 °C | 47.1% |
| Scler-28S-eDNA-R: 5′-TCCTTGGTCCGTGTTTCAAG–3′ | 59.3 °C | 50% |
Black coral and octocoral barcode sequences were aligned separately from scleractinian sequences using MUSCLE (Edgar, 2004) and the default parameters in Geneious. Primers for black corals and octocorals were designed to complement the 95% consensus sequence of the aligned sequences using Primer 3 (Untergasser et al., 2012; Version 2.3.7) in Geneious. Melting temperatures of candidate primers were calculated using the formula from SantaLucia (1998), with a 2 mM concentration of divalent salt cations (MgCl2), an oligo concentration of 50 nM, a 0.2 mM concentration of dNTPS, and a 50 mM concentration of monovalent salt cations. The minimum, optimal, and maximum primer lengths, melting temperatures, and GC% were 18, 20, and 22 bp; 55, 60, and 65 degrees C; and 35%, 50%, and 65%, respectively. A GC clamp of 1 bp was set, and the maximum polynucleotide run was limited to 3 bp. Primers were designed to the alignment of scleractinian barcodes using the same parameters as for black corals and octocorals. Forward and reverse primer pairs were chosen with a preference for primers with a C or G in three of the five nucleotides at the 3-prime ends of the forward primer sequences to increase specificity to the target (Andruszkiewicz et al., 2020).
We introduced ambiguities into the forward primer sequence for black corals and octocorals to complement single nucleotide polymorphisms (SNPs) in sequences of black corals and the primnoid octocorals Callogorgia gracilis and Paracalyptrophora carinata that occur in the northern Gulf of Mexico. Specifically, the degenerate bases Y (C or T), R (A or G) and R were included at the 11th, 13th, and 14th positions, respectively, in the forward primer. We introduced additional ambiguities into the forward primer sequence for scleractinians to complement SNPs present in the sequences of the deep-sea genus Enallopsammia, and the shallow Western Atlantic genera Orbicella and Montastraea. Specifically, the degenerate bases K (G or T), R (A or G) and M (A or C) were included at the 2nd, 15th, and 18th positions, respectively, in the forward primer. Primers with and without CS1 and CS2 Illumina linking adapters were synthesized by Eurofins Genomics using standard desalting. The 28S forward and reverse primer sequences were synthesized as mixtures with 50% of each nucleotide at each ambiguous position. We refer to the primers designed to target black coral and octocoral sequences as Anth-28S-eDNA, to reflect their broader diversity of targets and the primers designed to scleractinian sequences as Scler-28S-eDNA.
Compiling anthozoan coral 28S sequences for in silico analyses and taxonomic classification
To create a comprehensive reference database for in silico analyses of primer complementarity and taxonomic resolution, and for taxonomic classification of eDNA barcodes, we supplemented the barcode sequences used for primer design with (1) 28S sequences generated from low coverage whole-genome sequencing (genome skimming) data and (2) 28S sequences from GenBank. Genome skimming data were generated from specimens collected in the Gulf of Mexico following the methods described by Quattrini et al. (2024). Several of these specimens were also barcoded with Sanger sequencing to generate sequences for primer design. In Geneious, the barcoding region was extracted from consensus sequences generated from GoMx specimens in Quattrini et al. (2024). To retrieve 28S sequences from GenBank, GenBank was queried using the terms “Antipatharia”, “Octocorallia” or “Scleractinia” and “28S” or “large subunit”. The resulting sequences were aligned with sequences generated in this study using Clustal Omega with default parameters in Geneious, trimmed to the alignment, and extracted.
In silico analyses of primer complementarity and taxonomic resolution of the 28S rRNA barcode
To determine the complementarity of the designed primers to the compiled coral 28S barcode sequences, primers were queried against the barcode sequences while allowing two possible mismatches using the “Add Primers to Sequences” function in Geneious. For analysis, annotations of the primer matches to the 28S barcode reference sequences for the Anth-28S-eDNA and Scler-28S-eDNA primers were exported as .csv files and joined with metadata for each sequence using the tidyverse packages (Wickham et al., 2019) in R version 4.1.3 (R Core Team, 2022).
To determine the taxonomic resolution of the 28S barcodes amplified using the primers, predicted amplicons were extracted from the reference sequences using cutadapt with primer sequences set as required linked adapters with two possible mismatches to either the forward or reverse primer. 28S barcodes identified from the reference sequence database using the Anth-28S-eDNA primers (for Octocorallia and Antipatharia) and the Scler-28S-eDNA primers (for Scleractinia) were separately aligned in Geneious using MAFFT and the default settings. Pairwise percent identities were calculated between all 28S barcode sequences and exported as CSV files. The pairwise identity matrix was analyzed in R using the tidyverse packages and visualized using the pandas, seaborn, and matplotlib.pyplot Python v3 libraries.
To compare to 28S, we also determined the taxonomic resolution of mtMutS barcodes amplified with the primers described in Everett & Park (2018). Octocoral mtMutS sequences were downloaded from GenBank using the search terms “Octocorallia” and “mutS” or “msh1”. Predicted amplicons using the mtMutS primers were extracted from these sequences with cutadapt by setting the forward primer and the reverse complement of the reverse primer as linked adapters with a minimum overlap of 20 bp and a maximum of two allowed mismatches to either primer. Barcodes were aligned with MAFFT and pairwise identities were calculated as for the 28S barcodes. To minimize the influence of misidentified specimens, we only included sequences from samples identified to the species level.
In silico assessment of primer specificity to anthozoan coral 28S
To assess the specificity of the two primer sets to 28S sequences of anthozoan corals, we used cutadapt to identify potential PCR products from the SILVA ribosomal large subunit (LSU) dataset (version 138.1; https://www.arb-silva.de/), which is a curated reference database of prokaryotic and eukaryotic ribosomal RNA sequences. The primer sequences were set as required linked adapters and cutadapt was run with 0, 1 and 2 mismatches to either the forward or reverse primer (i.e., the “−e” flag was set to 0, 1 or 2). The minimum overlap was set to 19 bases (equal to the shortest primer sequence). Sequences that did not match were excluded from the output. Predicted amplicons were visualized by taxa and sequence length in R using ggplot2.
Testing the performance of the 28S primers with eDNA samples from the northern Gulf of Mexico
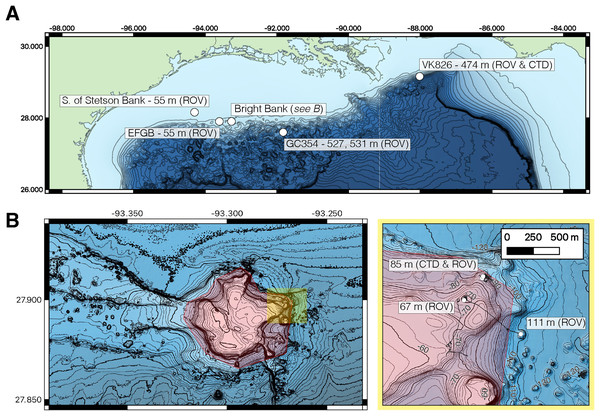
To test the performance of the 28S primers, we sequenced 50 field-collected eDNA samples from seven sites in the northern Gulf of Mexico at depths between 55 to 531 m (Fig. 2). Samples were collected using Niskin bottles during remotely operated vehicle (ROV) dives or Niskin bottle rosette casts (CTD casts) during expedition PS22-04 in August 2021 aboard the R/V Point Sur. We tested the Anth-28S-eDNA primers on water samples taken near mesophotic coral communities south of Stetson Bank, east of Bright Bank, and on Bright Bank within the Flower Garden Banks National Marine Sanctuary (FGBNMS). We also tested the Anth-28S-eDNA primers on water samples taken near deep-sea coral communities at Bureau of Ocean Energy and Management (BOEM) designated lease blocks GC354 and VK826. We tested the previously developed primers by Everett & Park (2018) that amplify mtMutS on the same samples. The Scler-28S-eDNA primers were tested on water samples collected from mesophotic coral communities at East Flower Garden Bank (EFGB), east of Bright Bank, and VK826. Details regarding field sample collection and steps taken to mitigate and monitor for contamination at the field sampling and laboratory steps are provided in the Supporting Information sections 1.2 and 1.3.
Figure 2: Maps of eDNA sampling sites in the northern Gulf of Mexico.
(A) Water sampling was conducted with Niskin bottles during remotely operated vehicle (ROV) dives and Niskin bottle rosette (CTD) casts at mesophotic and deep-sea sites (white points). Bathymetry data is from the Global Multi-Resolution Topography Data Synthesis (Ryan et al., 2009), and contours represent 100-m isobaths. EFGB stands for East Flower Garden Bank. (B) At Bright Bank and to its east, eDNA samples were collected at three proximate sites at differing depths. Navigational fixes during ROV dives are shown in black and intersect contour lines. The spatial extent of the highlighted area on the right is indicated as the yellow shaded area on the map of Bright Bank on the left panel. Bathymetry data is from the USGS Multibeam Mapping of Selected Areas of the Outer Continental Shelf, Northwestern Gulf of Mexico (Gardner & Beaudoin, 2005). Contours represent 10 and 2-m isobaths. Sampling sites at EFGB and at 67 and 85 m depth at Bright Bank were conducted within the boundaries of the Flower Garden Banks National Marine Sanctuary (highlighted in red).eDNA extraction and metabarcoding library preparation
DNA was extracted from frozen Sterivex filters using the Qiagen DNeasy Blood and Tissue Kit with a modified protocol for extraction from the filter capsule. This method was first described by Spens et al. (2017), and modifications were subsequently detailed by Govindarajan et al. (2022). DNA was eluted in 100 μL of Qiagen Buffer AE.
Samples collected from mesophotic sites were amplified via PCR using the Anth-28S-eDNA-F primer without the R ambiguity at the 14th nucleotide, since we did not expect to recover eDNA from deep-sea scleralcyonaceans from these samples. To amplify mtMutS using the primers designed by Everett & Park (2018) from samples collected at GC354 and VK826, we used a 50/50 mix of the published reverse primer and a second reverse primer (5′-CAGTCTTCTAAATTGCAACCGGGAGAATA-3′). This primer is complementary to sequences of coralliid octocorals that occur at deep-sea depths (M. Everett, 2018, personal communication).
Duplicate PCR reactions were performed for each eDNA extraction including sampling negative controls. The reactions consisted of 2 μL of DNA, 10 μL of 2X Platinum SuperFi II MasterMix (Thermo Fisher, Waltham, MA, USA), 2 μL of each of the forward and reverse primers with CS1/CS2 universal adapters diluted in molecular grade water to 10 μM (final reaction concentration 1.0 μM), 0.6 μL of 50 mM MgCl2 (final concentration 3.25 mM), and 3.4 μL of molecular-grade water. For the 28S rRNA primers, cycling conditions were as follows: initial denaturation at 98 °C for 30 s; then 15 cycles of denaturation at 98 °C for 10 s, the touchdown annealing step from 70 °C to 56 °C for 10 s, and elongation at 72 °C for 15 s; followed by 25 cycles of denaturation at 98 °C for 10 s, annealing at 55 °C for 10 s, and elongation at 72 °C for 15 s; and a final elongation at 72 °C for 5 min. For the mtMutS primers, 30 cycles with an annealing temperature of 55 °C following the touchdown cycles were performed to enhance amplification. Amplification success was assessed through electrophoresis by running 4 μL of each PCR product on a 1% agarose TBE gel stained with GelRed at 110V until the sample ran nearly to the entire gel length. Amplification success was scored based on the relative intensity of the band across samples and the visualization of any off-target products, as compared to the band corresponding to the predicted amplicon size. After visualization, the duplicate PCR products from each sample were pooled. Pooled PCR products were shipped to the Rush University Genomics and Microbiome Core Facility (GMCF) (Chicago, IL, USA) on dry ice for all subsequent library preparation steps and sequencing.
At GMCF, a second PCR was conducted to incorporate unique indices into the pooled PCR products from each sample. This PCR reaction was conducted in 10 μL reactions with 5 μL of 2X repliQa HiFi ToughMix (QuantaBio, Beverly, MA, USA), 1 μL of the PCR product, 2 μL of the unique barcode index from the Access Array Barcode Library for Illumina (Standard BioTools, South San Francisco, CA, USA), and 2 μL of molecular-grade water. Cycling conditions were as follows: initial denaturation at 98 °C for 2 min; then eight cycles of denaturation at 98 °C for 10 s, annealing at 60 °C 1 min, and elongation at 68 °C for 1 min. Indexed, pooled PCR products from each sample were combined, cleaned using a 0.6X ratio of AMPure beads (Beckman Coulter, Indianapolis, IN, USA), visualized on a TapeStation (Agilent Technologies, Santa Clara, CA, USA), and further size-selected using Blue Pippin (Sage Science, Beverly, MA, USA). The target fragment length was on average 541 bp, including the primers, universal adapters, and Access Array indices. Libraries were first pooled and sequenced with equal volumes of each sample (2 μL) on an Illumina MiniSeq (150 bp paired-end reads) with a 15% phiX spike-in. For subsequent sequencing on an Illumina MiSeq, the volumes of each library in the pool were adjusted based on the comparative number of reads each sample produced from the MiniSeq run. The goal was to normalize the number of reads produced across all samples. Volume-adjusted libraries were pooled and re-sequenced on an Illumina MiSeq with v3 chemistry (300 bp paired-end reads) and a 15% phiX spike-in.
Bioinformatics analysis
First, FASTQC (Andrews, 2010) and MultiQC (Ewels et al., 2016) were used to summarize read quality and counts across the samples. Primers anchored to the 5′ ends of the forward and reverse reads were trimmed from the sequences using cutadapt (Martin, 2011) with a minimum overlap of five bases and the default settings otherwise. The reverse complement of primers at the 3′ end was also trimmed if it was identified. Untrimmed read pairs were excluded from subsequent analysis. Sequences were processed using the DADA2 pipeline (Callahan et al., 2016) to infer error-corrected amplicon sequence variants (ASVs) from the sequencing data. For filtering and quality trimming, forward reads were trimmed to 250 bases in length, reverse reads were trimmed to 175 bases in length, and the maximum number of expected errors per read was set to 2. Denoising, wherein errors are inferred and corrected based on the error profile of the sequencing libraries, read pair merging and chimera identification and removal were conducted using the default settings. A remote BLASTN search was conducted to query the denoised ASVs against the entire GenBank nucleotide database. The hits were filtered from the resulting BLAST searches to identify denoised ASVs derived from anthozoan coral eDNA. ASVs were identified as derived from coral if the top hit (according to the lowest e-value) of the ASV was to a black coral, scleractinian or octocoral, and the ASV sequence was >90% identical to the subject sequence across its length. The ASV table output from DADA2 was then filtered to ASVs identified as corals and further curated using the LULU algorithm and a minimum match percent identity of 95% (Frøslev et al., 2017).
Taxonomic classification of coral amplicon sequence variants
From the sequences generated in this study and those downloaded from GenBank, a reference database of barcode sequences (N = 735; Table S2) of anthozoan corals, a zoantharian, several corallimorpharians, and an anemone was created for the taxonomic classification of 28S anthozoan coral ASVs using the assignTaxonomy and addSpecies functions in DADA2. The reference barcode sequences were included in a .fasta file with the required header format for the assignTaxonomy function. A second .fasta file was compiled from sequences of coral species recorded in the western North Atlantic with the required header format for the addSpecies function, which classifies ASVs to the species level if a 100% identical match to a sequence in the reference database is identified. We set the “allowMultiple” flag to TRUE in addSpecies, so that if an ASV was identical to two (or more) reference sequences, the species identities of all matching reference sequences would be reported. Sequences of corals not identified to the species level (e.g., Bebryce sp.) were only included in this file if it could be verified that they were collected from the western North Atlantic. The species distributions in the reference database were assessed using the Ocean Biodiversity Information System database (Ocean Biodiversity Information System (OBIS), 2023). Questionable occurrence records, for example, single occurrences of species in genera known otherwise exclusively from the Pacific, were not included. Both the .fasta files for assignTaxonomy and addSpecies were trimmed to the target barcode region amplified using the Anth-28S-eDNA and Scler-28S-eDNA primers using cutadapt with two mismatches to create separate reference files for each of these primer sets.
Another reference database was created for the taxonomic classification of octocoral mtMutS ASVs to compare to the 28S rRNA data. For the 5,068 MutS barcodes extracted from sequence data downloaded from GenBank (Table S3; discussed in “In Silico Analyses of Primer Complementarity and Taxonomic Resolution of the 28S rRNA Barcode”), the taxonomic hierarchy for each sequence was gleaned from WoRMS using the taxize package in R (Chamberlain & Szöcs, 2013), and a .fasta file with the taxonomy in the required header format was created for the assignTaxonomy function. Additional mtMutS sequences were used from mitochondrial genomes that were assembled and annotated using MitoFinder (Allio et al., 2020) from genome skimming data of corals from the northern Gulf of Mexico (Quattrini et al., 2024). Together, with an additional mtMutS sequence for Chelidonisis aurantiaca mexicana generated by Quattrini et al. (2013), these sequences were aligned, trimmed to the region amplified by the octocoral eDNA primers, and properly formatted to determine 100% matches to corals collected from the northern Gulf of Mexico using the addSpecies function.
For both datasets, 28S and mtMutS, ASVs were recovered with identical sequences to that of Thesea nivea. However, due to the uncertainty of the taxonomic placement of Thesea at the family level (Family: Malacalcyonacea incertae sedis), these ASVs were not classified beyond the order level. The taxonomic identity of these ASVs was manually assigned as Thesea nivea for analyses of the taxonomic composition of eDNA samples.
Metabarcoding data analysis and visualization
To visualize phylogenetic relationships, 28S barcode sequences were aligned using MAFFT and the default parameters in Geneious Prime. Maximum-likelihood phylogenetic trees were then created using IQ-Tree2 (Minh et al., 2020) with ModelFinder (Kalyaanamoorthy et al., 2017). ASV tables and taxonomic assignments generated from the bioinformatic pipeline were analyzed in R using the tidyverse packages. Correlation between the percentage of sequencing reads identified as coral and altitude from the seafloor was tested using Pearson’s correlation coefficient and the function corr.test in R. Bar plot visualizations of eDNA read abundances and their classifications to the genus level across eDNA samples were generated using ggplot2.
Results
In silico analyses of primer complementarity and specificity to anthozoan coral 28S
We analyzed the complementarity of the Anth-28S-eDNA and Scler-28S-eDNA primers to anthozoan coral sequences generated in this study and downloaded from GenBank (Table S2). The Anth-28S-eDNA primers were complementary with zero mismatches to all 68 black coral barcode sequences analyzed. These sequences represented all seven recognized black coral families and 22 accepted genera (according to WoRMS at the time of manuscript preparation). The Anth-28S-eDNA primers were complementary to the vast majority (84.7%, N = 409) of the 483 octocoral barcode sequences analyzed with zero mismatches and were complementary to sequences in 64 of 82 recognized octocoral families in 147 accepted genera with two or fewer mismatches. While the Anth-28S-eDNA primers were designed to an alignment of black coral and octocoral sequences, we also found that the primers were complementary to 165 of the 174 scleractinian barcodes analyzed with two or fewer mismatches, and most sequences (92) had zero mismatches. The Scler-28S-eDNA primers, which were designed to an alignment of scleractinian sequences, were complementary to a larger number of scleractinian sequences (165) with zero mismatches and complement sequences from 24 out of 36 recognized families and 75 accepted scleractinian genera.
We tested the specificity of the primers to anthozoan corals in silico by extracting barcodes with two or fewer mismatches to the forward or reverse primers from the entire SILVA large-subunit ribosomal RNA database. We found that with zero or one mismatch to the forward or reverse primer, off-target amplicons predicted using the Anth-28S-eDNA primers consisted largely of marine invertebrates. Besides anthozoans, sponges and hydrozoans had the highest number of predicted amplicons. Fungi sequences may be amplified using the primers if potential template sequences with two mismatches to the Anth-28S-eDNA primers are considered. However, the distribution of expected fungi amplicons was shorter (~200 bp) than that of marine invertebrates (Fig. S1). Thus, by visualizing the size distribution of the PCR product generated using the Anth-28S-eDNA primers (either through agarose gel or automated electrophoresis), it should be simple to assess the specificity of PCR reaction to corals and other marine invertebrates. When considering zero or one mismatch of the primers to potential template sequences, we found that the Scler-28S-eDNA primers were highly specific to hexacorals; amplicons were only predicted from hexacorals. When two mismatches in potential template sequences were considered, sponges were also predicted to be amplified using the Scler-28S-eDNA primers.
In silico analysis of the taxonomic resolution of the 28S barcode
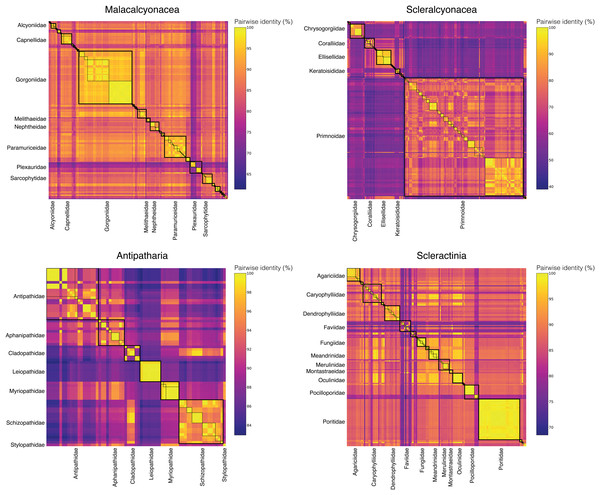
To assess the taxonomic resolution of the DNA barcode amplified with the Anth-28S-eDNA and Scler-28S-eDNA primer sets, we extracted and aligned predicted amplicons from anthozoan coral sequences generated in this study and downloaded from GenBank. We found that 28S DNA barcodes predicted using the primers were non-identical across all anthozoan families. Further, 28S barcodes were non-identical across all genera within 18 of 20 octocoral families, nine of 11 scleractinian families, and four of seven black coral families (Fig. 3).
Figure 3: Matrices of pairwise identities between 28S rRNA barcode sequences generated in this study and downloaded from GenBank.
Boxes with thick, solid black borders highlight comparisons within families, and boxes with thin, dashed black borders highlight comparisons within genera. Only families represented by the largest numbers of barcode sequences are labeled to highlight these comparisons and improve readability.To compare the taxonomic resolution of octocoral DNA barcodes amplified using the Anth-28S-eDNA primers and the mtMutS primers (Everett & Park, 2018), we analyzed the pairwise identities of predicted mtMutS barcodes in the same manner as for 28S. We found that mtMutS barcodes were identical between at least two sequences across genera within ~30% (N = 10) of the 34 families represented in the dataset (Figs. S2 and S3). The mtMutS barcode was, on average, less variable between sequences from different genera in the same family (average mean pairwise identity = 94.0%) than the 28S barcode (average mean pairwise identity = 89.0%).
Efficacy of metabarcoding coral 28S eDNA from field samples collected at the seafloor during ROV dives
We successfully amplified the 28S barcode from all field eDNA samples collected during ROV dives at the seafloor using the Anth-28S-eDNA primers. On average, sequencing yielded 50,859 ± 13,823 (1 SD) reads per sample and 26,808 ± 9,695 reads remained after primers were identified and trimmed from amplicon sequences. On average, 72.6 ± 33.1% of the filtered, denoised and non-chimeric sequencing reads generated from each sample taken at the seafloor during ROV dives were identified as coral. Up to 99.9% of reads were identified as coral in both mesophotic and deep-sea samples (Table S4). Other marine invertebrate ASVs recovered using the Anth-28S-eDNA primers included those with top BLAST hits to hydrozoans (16.9% of reads across all libraries), ctenophores (16.8%), sponges (2.3%), and anemones (<0.1%).
Using the Scler-28S-eDNA primers, a smaller proportion of samples collected at the seafloor during ROV dives amplified, reflecting the taxonomic specificity of this primer set to hexacorals. In samples that amplified using these primers (8 of 11), on average 51,322 ± 40,525 reads were produced and 21,237 ± 19,935 contained amplicons that included the primer sequences. Virtually all (on average 99.4 ± 1.5%) of the filtered, denoised and non-chimeric reads were identified as belonging to scleractinians and black corals.
Metabarcoding coral 28S eDNA using niskin bottles suspended above the seafloor
The distance above the seafloor at which a sample was taken during Niskin bottle rosette casts was significantly, negatively correlated with the percentage of coral sequencing reads produced in libraries using the Anth-28S-eDNA primers (Pearson’s r = −0.50, P-value = 0.018; Fig. S4). Compared to samples taken at the seafloor during ROV dives, across samples taken at altitudes greater than 5.8 m from the seafloor the average percentage of coral sequencing reads was just 0.8% and the remaining reads were derived from other taxa. Similarly, using the Scler-28S-eDNA primers, in sample duplicates taken ~20 m above the seafloor, no band on an agarose gel was visible after PCR and just one coral read was recovered.
Taxonomic composition of eDNA samples collected across sampling sites and depths in the Northern Gulf of Mexico
From metabarcoding eDNA field samples collected from mesophotic and deep-sea sites in the northern Gulf of Mexico, we recovered 112 coral ASVs using the Anth-28S-eDNA primers and 17 coral ASVs using the Scler-28S-eDNA primers (Table S5). Of the 112 coral ASVs recovered using the Anth-28S-eDNA primers, 73% (n = 82) were classified as octocorals, 24% (n = 27) were classified as black corals, and 3% were classified as scleractinians (n = 3). Of the 17 coral ASVs recovered using the Scler-28S-eDNA primers, 65% (n = 11) were classified as black corals and 35% (n = 6) were classified as scleractinians. The black corals detected using the Scler-28S-eDNA primers were a subset of those detected using the Anth-28S-eDNA primers. Likewise, the scleractinians detected using the Anth-28S-eDNA primers were a subset of those detected using the Scler-28S-eDNA primers. The proportions of ASVs classified to each group met our expectations for each primer set, given the taxonomic breakdown of the sequences to which they were designed.
Using the Anth-28S-eDNA primers, we recovered ASVs classified to both octocoral orders, Malacalcyonacea (n = 58, 71%) and Scleralcyonacea (n = 24, 29%). Among those ASVs classified to Malacalcyonacea, 69% (n = 40) were further classified to the family level and 62% (n = 36) were further classified to the genus level. Among those ASVs classified to Scleralcyonacea, 96% (n = 23) were further classified to the family level, and 21 of these ASVs were further classified to the genus level. Using the Anth-28S-eDNA and Scler-28S-eDNA primers, we recovered 38 ASVs classified to the order Antipatharia and 8 to Scleractinia. All but one (n = 37) of the black coral ASVs were classified to the family level, and nearly all (n = 36) of those ASVs were further classified to the genus level. Of the eight scleractinian ASVs, seven were classified further to the family and genus levels. Of all 129 coral ASVs, we found identical matches to 19 unique sequences in our reference database that we interpret as representing species-level detections.
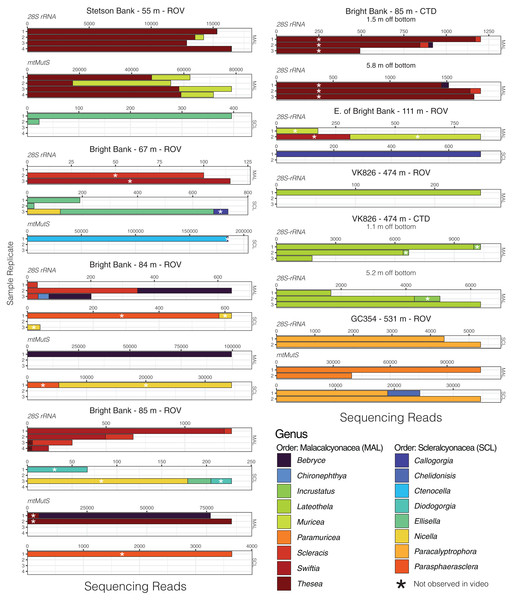
Given that most of the 28S ASVs were classified to the genus level, we were able to make meaningful comparisons of the taxonomic composition of eDNA samples across sites and depths and to observations made during ROV dives. Using eDNA sequencing, we detected coral genera observed in video and/or collected from the same field sites, and we also detected taxa that were not observed (Figs. 4 and 5). At Bright Bank and to its east, we recovered substantial numbers of sequencing reads from the octocoral genera Thesea (Family: incertae sedis) and Muricea (Family: Plexauridae), respectively. While neither taxon was observed during ROV dives at these sites, Muricea pendula and Thesea spp. are common mesophotic octocorals in the Gulf of Mexico, and we have observed high densities during ROV surveys at nearby sites. We detected ASVs classified to the genera Parasphaerasclera and Incrustatus, at Bright Bank and VK826, respectively, while neither of these genera were observed or are known from the Atlantic Ocean. It is possible that due to their small and cryptic morphologies, these octocorals have been overlooked. We did not detect eDNA from some species observed and sampled during ROV dives and subsequently sequenced for inclusion in our reference database. Notably, at VK826, we did not detect eDNA from a mushroom coral in the sub-family Anthomastinae. At Bright Bank at 67 m depth, we observed, sampled, and DNA barcoded a large colony of Plumapathes pennacea (Family: Myriopathidae); however, we did not detect eDNA that matched this species.
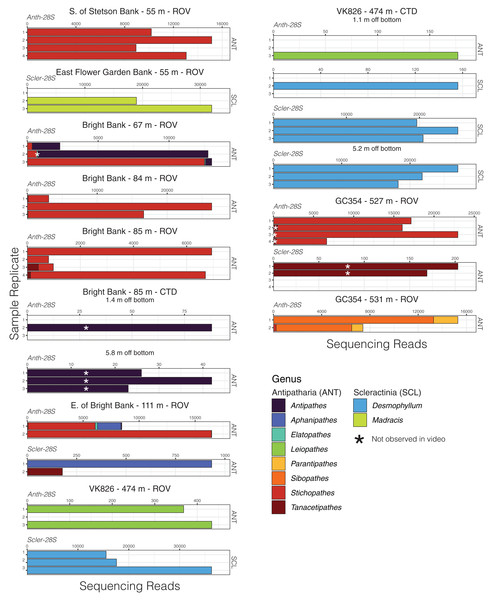
Figure 4: Octocoral eDNA sequence reads from replicate water samples taken during ROV dives and CTD casts near coral communities in the northern Gulf of Mexico.
Data were generated using the Anth-28S-eDNA and mtMutS primers for comparisons across samples that amplified using both primer sets, as indicated in each plot. Variable x-axis scales are used to aid in data visualization for all taxa.Figure 5: Black coral and scleractinian eDNA sequencing reads from replicate water samples taken during ROV dives and CTD casts near coral communities in the northern Gulf of Mexico.
Data were generated using both the Anth-28S-eDNA and Scler-28S-eDNA primers as indicated in each plot. Sequence libraries from East Flower Garden Bank were only generated using the Scler-28S-eDNA primers. Variable x-axis scales are used to aid data visualization.Comparison to data generated using mtMutS primers
Using the mtMutS primers, sequencing data was generated from all sample replicates collected at just two of the sampling locations, GC354 at 531 m and S. of Stetson Bank at 55 m. These same sample replicates produced the highest percentage of coral reads using the Anth-28S-eDNA primers and had the largest proportions of octocoral reads relative to black coral reads. We detected two scleralcyonacean octocoral genera not detected with the Anth-28S-eDNA primers, Ctenocella (Family: Ellisellidae) at Bright Bank and Paramuricea at GC354. Colonies with similar morphology to Ctenocella were observed at Bright Bank (although not sampled for confirmation of ID). Reads classified as Paramuricea were present in 28S data from GC354, but at an abundance (80 reads) lower than our cutoff threshold for deep-sea samples to control for contamination (100 reads). Sequence reads classified to Nicella and Ellisella (Family: Ellisellidae) were detected at two sites using the mtMutS primers where they were not detected using the 28S primers. eDNA sequence read abundances produced with the mtMutS and 28S primers also differed. For example, sequences classified to the family Paramuricea made up most of the sequence reads in sample duplicates from GC354 at 531 m using the mtMutS primers. However, using the 28S rRNA primers, sequences from Paracalyptrophora were the most numerous.
Discussion
The 28S eDNA metabarcoding primers are broadly complementary to black coral, octocoral, and scleractinian sequences
Here, we demonstrate that the Anth-28S-eDNA primers broadly complement black coral, octocoral, and scleractinian sequences. While 28S sequence data for several black coral genera, including the deepest genus Abyssopathes, were not available, we found that the primers perfectly matched all black coral sequences (across all seven families) that we tested. High sequence conservation at the forward and reverse primer binding sites in black coral sequences supports the utility of the primers for metabarcoding black coral species that lacked data at the time of this analysis. We expect that the primers are broadly applicable for metabarcoding black coral eDNA across all ocean depths.
The Anth-28S-eDNA primers were also broadly complementary to octocoral sequences with a few exceptions. Specifically, the primers were complementary to sequences of all malacalcyonacean octocorals with two or fewer mismatches except for sequences from Astrogorgia rubra and Pacifigorgia sp. The primers had one or two mismatches to five sequences in the genera Xenia, Hanah, Acrossota, and Pacifigorgia. We designed the forward primer with ambiguities to accommodate sequence variation in scleralcyonacean octocorals present at our field sites. Nevertheless, the forward primer had at least one mismatch to sequences of a sea pen (Pennatula sp.), two bamboo corals (Family: Keratoisididae), and 31 species in the family Primnoidae. Further, the forward primer had greater than two mismatches to Junceella fragilis, Dichotella gemmacea, and four primnoid species.
Alterations to the forward primer sequence are possible and recommended if primnoid species with mismatches to the primers are known or expected to occur at sampling locations. For example, octocorals of the genus Primnoa have an A rather than a G at the 10th nucleotide in the forward primer sequence. From samples taken near habitats where Primnoa may occur, substituting an R for the A at the 10th position would correct for this mismatch while maintaining a range of melting temperatures (54.9–62.3 °C) within 5 °C of the reverse primer.
The Anth-28S-eDNA primers will amplify many scleractinian species. However, we recommend using the Scler-28S-eDNA primers for eDNA metabarcoding from locales where scleractinians are known to occur. We found that these primers perfectly matched a greater proportion of the scleractinian sequences that we tested (94.8% compared to 55.8%), and that metabarcoding libraries produced using these primers were highly enriched in scleractinian eDNA, at both mesophotic and deep-sea sites. We expect that further testing these primers on samples collected at sites with a higher diversity of scleractinians will realize their predicted utility from the in silico analysis.
The 28S barcode resolves the identity of coral eDNA to the family level in all cases and to the genus level in most cases
Through in silico tests, we found that the 28S barcode amplified by the primers was non-identical between all sequences from different families and most sequences from different genera. Identical barcodes from corals in different genera were found in a minority of cases. Within the octocoral family Primnoidae, identical barcodes were found (1) between Candidella helminthophora and Parastenella pacifica and (2) between Pyrogorgia lemnos and Primnoa pacifica. Within Paramuriceidae, barcode sequences of Paramuricea sp. and Placogorgia sp. were identical. Within the scleractinian family Dendrophylliidae, barcode sequences of Cladopsammia gracilis and Tubastraea coccinea were identical, unfortunately precluding the identification of the invasive Tubastraea from Cladopsammia, two genera that are already challenging to distinguish morphologically (Hoeksema, Hiemstra & Vermeij, 2019). In Fungiidae, barcode sequences of Zoopilus echinatus, Polyphyllia and Sandolitha were identical, and barcode sequences of Halomitra sp. and Lobactis scutaria were also identical. Within the black coral family Antipathidae, a barcode sequence of Cirrhipathes anguina collected from Hawai’i (GenBank: FJ626243.1) and a sequence of Stichopathes sp. collected from the Gulf of Mexico were identical. The Stichopathes specimen is morphologically similar to Stichopathes pourtalesi and was collected from this species’ known depth range and habitat (Opresko, Nuttall & Hickerson, 2016). Within the deep-sea genus Schizopathidae, a sequence of Bathypathes sp. was identical to sequences of Stauropathes arctica. Within Cladopathidae, sequences of Cladopathes cf. plumosa and Trissopathes cf. tetracrada were identical.
If genera with identical barcodes do not occur in sympatry, then excluding genera that do not occur in the geographical range where an eDNA sample is taken can ameliorate the resulting uncertainties in identification at the genus level. Where genera with identical barcodes occur in sympatry (e.g., Tubastraea and Cladopsammia), genus level identifications may not be possible. That being said, some identicalities between sequences in different genera in these data, especially within the black corals, reflect incongruences between phylogenetic relationships and taxonomic nomenclature rather than the rate of evolution of the 28S barcode and its ability to distinguish taxonomic identity at the genus level. In black corals, phylogenetic evidence suggests that the genera Stichopathes and Cirrhipathes are polyphyletic (Bo et al., 2018; Quattrini et al., 2013). Likewise, Barrett et al. (2020) suggested that validations of the genera within Schizopathidae and Cladopathidae are necessary based on mitochondrial genome sequencing data. The existence of paraphyletic and polyphyletic taxa preclude the confident classification of ASVs derived from eDNA sequencing data. Unless a 100% identical match to a reference sequence is available, caution should be used when inferring occurrences from eDNA data within taxa where discrepancies between phylogenetic relationships and current taxonomy are known. Efforts focused on resolving the systematics of these anthozoan corals using phylogenomic methods will benefit biodiversity assessment and monitoring using eDNA sequencing. Whenever possible, voucher specimens deposited in accessible collections should accompany reference barcode sequences to confirm morphological identifications, especially considering our evolving understanding of coral taxonomy.
Metabarcoding libraries prepared using the 28S primers were enriched in coral eDNA
We found that on average the majority (72.6%) of sequences in libraries prepared using the Anth-28S-eDNA primers were from corals in samples collected at the seafloor during ROV dives. In some samples virtually all the sequencing reads were from corals. By producing sequencing libraries enriched with barcodes from coral eDNA, metabarcoding using the Anth-28S-eDNA primers is a cost-effective approach for discerning anthozoan biodiversity as compared to primers that broadly target marine invertebrates or eukaryotes. In addition, the Anth-28S-eDNA primers may be useful for targeting some other marine invertebrates, like ctenophores and medusozoans that are co-amplified with coral eDNA. Virtually all sequences in libraries prepared using the Scler-28S-eDNA primers were from hexacorals.
The levels of taxonomic specificity to anthozoan corals that we found using the Anth-28S-eDNA and Scler-28S-eDNA primer sets are comparable to those of previously published eDNA primer sets designed to coral DNA sequences. Among the multiple primer sets designed to scleractinian sequences, Shinzato et al. (2021) designed highly specific primers that target mitochondrial 12S and COI. Using their primers designed to 12S, they found that 83.27% to 98.98% of the sequencing reads in the sequencing libraries from field samples were scleractinian. Using their primers designed to COI, 55.78% to 96.92% of the sequencing reads in their libraries were scleractinian. Using the primers designed to octocoral mtMutS sequences (Everett & Park, 2018), we found that 100% of the sequencing reads were derived from octocorals in all samples that amplified. We cannot expect that the primers we designed to 28S rRNA have the same specificity as the mtMutS primers since nuclear 28S is present in all eukaryotic genomes. However, we are encouraged to find that in samples taken at the seafloor, most sequencing reads recovered using our 28S rRNA primers were from corals.
While the percentage of coral reads was overall quite high in samples collected at the seafloor during ROV dives, we found that the percentage of coral reads in samples collected using Niskin bottle rosette casts decreased precipitously with distance from the seafloor. While further systematic testing is necessary to constrain the vertical extent of benthic eDNA confidently, these results suggest that the efficacy of coral eDNA metabarcoding relies on proximity to the seafloor. Practically, accurate characterization of seafloor bathymetry and the use of altimeter sensors seem to be vital for successful coral eDNA capture using Niskin bottle rosettes in deep waters.
eDNA metabarcoding with the 28S primers detected coral genera and species in field samples
A high percentage of scleractinian, black coral, and scleralcyonacean octocoral ASVs that we detected in field samples were classified to the genus level: 87.5% for scleractinians, 94.7% for black corals, and 87.5% for scleralcyonacean octocorals. These high percentages of corals that were classified at the genus level is comparable to a study of shallow-water benthic fauna that paired eDNA sampling and sequencing using scleractinian-specific markers with collections of representative morphospecies across belt-transect surveys (West et al., 2022). When paired with systematic, regional sampling and sequencing of voucher specimens to generate reference DNA barcodes, it should be expected for marine invertebrates that a high percentage of ASVs from eDNA sequencing data will be classifiable to the genus or species levels, as observed in vertebrates (Gold et al., 2021).
The percentage of malacalcyonacean octocoral ASVs classified to the genus level (62%) was comparably lower than in other anthozoan coral orders. Among the malacalcyonacean ASVs unclassified beyond the order level was a clade of sequences (8) with a maximum pairwise identity of just 93.4% (BLASTN) to any sequence in GenBank. These ASVs were placed phylogenetically in a clade sister to a clade consisting of ASVs classified to the genera Lateothela, Chironephthya, and Nidalia (Fig. S5). We interpret that these eight ASVs unclassified beyond the order level represent a group of malacalcyonacean octocorals for which 28S sequencing data does not currently exist, highlighting the importance of compiling a comprehensive reference database for taxonomic classification to the family level or to genus or species. We expect that further sampling and sequencing efforts of Malacalcyonacea from the Western Atlantic will resolve the taxonomic identity of more ASVs classified in this diverse order.
Like several other studies, we found that coral eDNA sequencing data and video observations/collections are largely congruent, yet unique detections were gleaned using both methods (Gösser et al., 2023; Dugal et al., 2022; West et al., 2022; Everett & Park, 2018). Of particular interest were detections of small, cryptic octocoral genera like Parasphaerasclera, which could represent a new record of this genus in the region. On the contrary, we did not detect eDNA from some corals as well, including large colonies. For example, we did not detect eDNA from a colony of Plumapathes pennacea present at Bright Bank. This non-detection was particularly notable, due to the large size of this colony and its ecological importance as host to a trumpetfish, Aulostomus maculatus.
Recommendations for eDNA metabarcoding to study anthozoan corals
eDNA sequencing is being rapidly adopted to assess biodiversity in nearly all of Earth’s ecosystems, including the deepest depths of our oceans. While protocols and reference databases are well established for vertebrate taxa, such as fish, eDNA sequencing for corals is still in its infancy and will not reach its full potential until reference sequences are generated for more species and incongruencies between phylogenetics and current taxonomy are resolved. Nevertheless, with new molecular tools and the diminishing costs of amplicon and genomic sequencing, we are rapidly approaching a more comprehensive understanding of the distributions and diversity of these ecologically important and vulnerable animals.
Here, we demonstrate the utility of a new set of primers for metabarcoding eDNA from shallow, mesophotic, and deep-sea anthozoan corals that target a variable barcode region of 28S. We found that metabarcoding with these primers is a cost effective and taxonomically informative strategy to survey coral biodiversity with eDNA sequencing, and that 28S metabarcoding complements an existing method for metabarcoding mtMutS in octocorals. Like many other studies, we also found that eDNA metabarcoding complements rather than replaces direct observation (in this case video analysis) for biodiversity assessment. Combining direct observation with eDNA sequencing using taxonomically specific and informative primer sets, like 28S and mtMutS, will lead to more comprehensive coral biodiversity characterization, especially in underexplored regions of the ocean and in the deep sea.